Abstract
Objectives:
Sepsis is associated with significant mortality. Telehealth may improve the quality of early sepsis care, but the use and impact of telehealth applications for sepsis remain unclear. We aim to describe the telehealth interventions that have been used to facilitate sepsis care, and to summarize the reported effect of telehealth on sepsis outcomes.
Data Sources:
We identified articles reporting telehealth use for sepsis using an English-language search of PubMed, CINAHL Plus (EBSCO), Academic Search Ultimate (EBSCO), APA PsycINFO (EBSCO), Public Health (ProQuest), and Web of Science databases with no restrictions on publication date.
Study Selection:
Included studies described the use of telehealth as an intervention for treating sepsis. Only comparative effectiveness analyses were included.
Data Extraction and Synthesis:
Following the PRISMA-ScR guidelines, two investigators independently selected articles for inclusion and abstracted data. A random-effects subgroup analysis was conducted on patient survival treated with and without telehealth.
Results:
A total of fifteen studies were included, involving 188,418 patients with sepsis. Thirteen studies used observational study designs, and the most common telehealth applications were provider-to-provider telehealth consultation and intensive care unit telehealth. Clinical and methodological heterogeneity was significantly high. Telehealth use was associated with higher survival, especially in settings with low control group survival. The effect of telehealth on other care processes and outcomes were more varied and likely dependent on hospital-level factors.
Conclusions:
Telehealth has been used in diverse applications for sepsis care, and it may improve patient outcomes in certain contexts. Additional interventional trials and cost-based analyses would clarify the causal role of telehealth in improving sepsis outcomes.
Keywords: sepsis, telehealth, teleICU/teleED, survival, patient outcomes, processes of care
Summary:
Telesepsis effectiveness depends on various hospital-level factors. Telesepsis may promote survival in hospitals with low baseline survival, but it has been evaluated mostly through observational studies; additional interventional trials and cost-based analyses would be valuable.
Introduction
Sepsis is a leading cause of critical illness and death worldwide, with estimates suggesting 31.5 million sepsis cases and 5.3 million deaths occur annually. 1,2 Sepsis is also a leading cause of death in U.S. hospitals, 3 now comprising 30% to 50% of hospital deaths. 4,5 Standardization of early sepsis care has been shown to improve survival and between 2009-2017 annual sepsis survival rate increased by 9%. 6
Early intervention and appropriate treatment are essential components of high-quality sepsis care. 7,8 The Surviving Sepsis Campaign (SSC), a multidisciplinary collaborative, publishes recommendations for sepsis care consisting of early recognition, prompt administration of antibiotics, and early resuscitation. 7,9-12 Adherence with SSC bundle guidelines has been associated with reduced intensive care unit (ICU) length-of-stay and improved hospital survival. 13,14 Unfortunately, SSC bundle adherence remains low, especially amongst rural sepsis patients in low-volume hospitals, where prior work has shown that bundle adherence is significantly lower than in higher-volume hospitals. 15-19
While management of sepsis patients requires no specialized equipment or procedural capabilities, low-volume emergency departments (EDs) have a 38% lower sepsis survival compared to high-volume EDs. 20-22 A similar trend is observed outside of EDs: hospital wards and ICUs that treat more than 500 severe sepsis cases annually have survival rates that are 36% greater compared to those that treat fewer than 50 cases. 23 Furthermore, rural sepsis patients who are transferred between hospitals have 9% lower survival compared to those who are not transferred, and even those who bypass a rural hospital to seek care in a higher volume facility have a 6% lower survival. 22,24,25 Some theorize that low rates of guideline-adherent care may be attributable to delays in sepsis recognition, provider education, and access to sepsis specialty care. 26-28
One promising strategy that has been proposed to improve the quality of emergency sepsis care is telehealth, 29-32 which we defined as technology-enabled communication between telehealth providers and bedside providers and/or patients for care-related purposes. Since its inception in the early 1960s, 33 telehealth has been used in EDs, ICUs, and hospital wards. Telehealth can be used for remote monitoring, nursing care, or physician consultation. 34 Telehealth could potentially be used to improve sepsis care and patient outcomes, yet it is unclear how, where, and for whom it is used, and how its use may affect clinical outcomes. We therefore conducted this systematic review to evaluate the current landscape of studies that have applied “tele-sepsis” and to characterize the types of telehealth that have been used for sepsis care. The objectives of this systematic review were to 1) describe the global literature focused on the use of telehealth in sepsis patients, 2) summarize the effect of telehealth on processes of care and clinical outcomes, and 3) provide guidance for the direction of future studies.
Methods
We originally conducted a scoping review according to the guidelines in Munn et al., 35 the Joanna Briggs Institutes Reviewer’s Manual, 36 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR)37 (eTable 1). In the context of the scoping review, we sought to answer the following questions:
How has telehealth been used to impact care for sepsis patients?
What are the characteristics, contexts, and key findings of studies (including patient outcomes) on the use of telehealth in the treatment of sepsis?
What are the conceptual and methodological gaps in research concerning the use of telehealth in sepsis care?
Upon completing the scoping review, we determined that there were enough studies and overlap in study outcomes to conduct a systematic review, and that the diversity of telehealth use in sepsis was low. Thereafter, we also sought to answer the question, “how has telehealth affected processes of care and clinical outcomes in sepsis care?”. We followed PRISMA guidelines in conducting the systematic review. 38 We registered the protocol in the Open Science Framework on May 25, 2022. 39
Search Strategy
The search strategy was developed by the research team in collaboration with a public health librarian (N.T.) experienced in systematic review methods, combining search terms of 1) sepsis and 2) telehealth (detailed search strategy available in eTable 2). On May 27, 2022, we searched Academic Search Ultimate (EBSCO), APA PsycINFO (EBSCO), CINAHL Plus (EBSCO), Public Health (ProQuest), PubMed, and Web of Science for peer-reviewed studies published in English. We did not apply other limits. We included all comparative effectiveness study designs (e.g., cohort, case-control, interventional studies), but case reports, uncontrolled case series, editorials, and opinion pieces were excluded. We searched through grey literature (i.e., abstracts, pre-prints) using the TRIP database, BASE Search Engine, and MedRxiv. Google Scholar was also searched for studies using the same terms as the original search in order to identify studies that may have been missed. Additionally, we reviewed reference lists of the included studies by hand. We reviewed the meeting proceedings of the Society of Critical Care Medicine and the American Telemedicine Association from 2017-2022 for relevant abstracts. We excluded abstracts that were subsequently published as peer-reviewed manuscripts to avoid “double-counting” samples. We contacted authors if there was missing or unpublished referenced data.
Study Selection
The analysis included studies that reported on the treatment of sepsis patients with the use of telehealth. Each title, abstract, and the full-text manuscript were independently screened by two reviewers (K.J.T., C.W.) using Rayyan (Rayyan Systems Inc, Cambridge, MA), a web-based application designed to facilitate the screening process for researchers working on systematic reviews. 40 We used the following inclusion criteria:
Use of telehealth as an intervention
Sepsis was diagnosed and/or treated using telehealth
Surveillance for the presence of sepsis was included if it is done using telehealth
The study was original comparative effectiveness research (no case studies/series, reviews, protocols, editorials, or opinion pieces)
Disagreements were resolved through discussion with a third reviewer (N.M.M.).
Data Abstraction
We used a structured data abstraction form to characterize the features of the included studies. Two reviewers (K.J.T, C.W.) independently abstracted all data, and the risk of bias was assessed using the Newcastle-Ottawa scale for non-randomized studies and the Cochrane Risk of Bias tool for randomized trials. 41,42 We considered studies to be of low risk of bias if they received a score of six or greater on Newcastle-Ottawa’s nine-point scale. 43 The following data were abstracted: publication information (i.e., title, authors, year of publication), study characteristics (i.e., type of study, outcomes studied), study population characteristics (i.e., sample size, age, sex/gender, race/ethnicity, rurality, control group survival), telehealth characteristics (i.e., synchronous vs. asynchronous, users, hospital unit), and key findings. Patient outcomes (survival, transfer) and care processes (antibiotic timeliness, bundle adherence, lactate measurement) were also abstracted, and odds ratios (ORs) were calculated between patients treated without and with telemedicine if not reported in the paper. Each study’s reported outcomes were classified into measurement domains as described by the Rural Telehealth and Healthcare System Readiness Committee (National Quality Forum) for telehealth measure development for: 1) Access to Care and Technology, 2) Costs, Business Models, and Logistics, 3) Experience, 4) Effectiveness, and 5) Equity. 44
Subgroup Analysis and Weighted-Regression
We determined after review that the clinical and methodological heterogeneity was too high to pool study outcomes in a formal meta-analysis. However, we reasoned that an explanatory analysis may explain the heterogeneity and still provide insights into the use of telehealth for sepsis care. We qualitatively evaluated patient survival data after separating the studies into three subgroups related to study methodology (randomized, cohort, and before-after). After conducting the analysis, we explored a hypothesis post hoc that the survival effect associated with telehealth was related to the severity of illness. To test that hypothesis, we performed a weighted regression with an explanatory variable of control-group survival using OpenMetaAnalyst (Agency for Healthcare Research and Quality, Rockville, MD).45 We modeled the treatment effect based on the β of the telehealth effect size from adjusted models, and pooling was performed using a random effect weighted regression.
Results
The original search yielded 365 unique studies, of which 15 studies met the inclusion criteria and were included in this systematic review. 46-60 Figure 1 describes the search results, and Table 1 summarizes the characteristics of the included studies. Most of the studies used observational designs: cohort studies (n=9) with concurrent controls, before-after quasi-experimental studies (n=4), and randomized trials (n=2), with a total enrollment across all studies of 188,418 participants (Table 1). Twelve (80%) of our studies had a low risk of bias, according to the Cochrane Risk of Bias Tool (eFigure 1) and Newcastle-Ottawa Scale (eTable 3).
Figure 1. PRISMA Study Selection Flow Diagram.
Table 1. Characteristics of included studies.
ED (emergency department), ICU (intensive care unit). The telehealth provider type is the healthcare provider responsible for administering telehealth. Tele-ICU and Tele-ED are the use of telehealth to diagnose/treat sepsis patients from an off-site telehealth center in ICUs and EDs, respectively.
| Author, Year | Patient Sample |
Study Design | Type of Intervention | Mode of Telemedicine |
Setting of Telemedicine Use |
Telehealth Provider-Type |
|---|---|---|---|---|---|---|
| Agarwal et al., 2016 49 | 14 | Cohort | Tele-ED consulting through a telehealth cart | Real-time video | ED | Physicians |
| Campbell et al., 2017 50 | 114 | Cohort | Tele-ED Consulting | Real-time video | ED | Physicians |
| Davis et al., 2022 51 | 566 | Before-After Cohort | Virtual sepsis surveillance + telehealth | Audio | Acute Care Units (excluding ICUs) | Physicians and Nurses |
| Deisz et al., 2019 52 | 196 | Before-After Cohort | Tele-ICU Physician Daily Rounds | Real-time video | ICU | Physician |
| Fortis et al., 2018 53 | 25,260 | Before-After Cohort | Tele-ICU Intervention | Real-time video | ICU | Physicians and Nurses |
| Ilko et al., 2019 54 | 150,845 | Cohort | Tele-ICU Intervention | Real-time video | ED and ICU | Physicians and Nurses |
| Machado et al., 2018 47 | 314 | Cohort | Introduction of a telemedicine eICU cart | Real-time video | ED | Physician |
| Marx et al., 2022 55 | 748 | Stepped-Wedge Cluster Randomized Controlled Trial | Telemedicine consultation inpatient-outpatient network | Real-time video | ICU | Physician |
| Mohr et al., 2021 46 | 655 | Cohort | Tele-ED Consulting | Real-time video | ED | Physicians |
| Mohr et al., 2022 66 | 1,191 | Cohort | Tele-ED Consulting | Real-time video | ED | Physicians |
| Powell et al., 2022 56 | 1,753 | Cohort | In Situ Simulation Training on Telehealth | Real-time video | ED | Physicians and Nurses |
| Rincon et al., 2011 48 | 5,437 | Cohort | Tele-ICU Intervention | Real-time video | ICU | Physicians and Nurses |
| Scott et al., 2020 57 | NR | Before-After Cohort | Nursing Review of Automated Surveillance Output | Audio | ICU | Nurse |
| Steinman et al., 2015 58 | 633 | Cohort | Tele-ICU & Tele-ED | Real-time video | ED and ICU | Physicians |
| Taylor et al. 2021 59 | 692 | Pragmatic Randomized Control Trial | Post-discharge care of sepsis patients | Audio | Direct-to-consumer | Nurse |
Telehealth Characteristics
Telehealth applications were diverse, with care being provided to patients in emergency departments (EDs), 46,47,49-51,54,56,58,60 intensive care units (ICUs), 48,52-54,57,58 and in post-discharge recovery at home. 59 Most applications used high-definition audio-video real-time connections through either a portable cart or permanently installed telehealth hardware in hospitals. 46-50,52-56,58,60 Most telehealth programs fell into two categories: 1) provider-to-provider on-demand telehealth consultations for patients with suspected sepsis and 2) real-time tele-ICU monitoring for patients on inpatient units (ICUs or wards) by nurses or physicians (Table 1). Many of the reports were small demonstration projects, with only a single originating site and a single telehealth provider site, but three studies reported on interventions that had been scaled using a hub-and-spoke model, many of which had spokes in rural facilities. 46,50,60
Several studies reported results from provider-to-provider ED-based teleconsultations, with three coming from a single rural ED network.46,50,60 In these studies, staff in rural hospitals use an on-demand consultation service with a board-certified emergency physician and experienced ED nurse for advice on diagnosis, treatment, and help with inter-hospital transfer. This intervention was part of a mature network that also provided care for patients with cardiac emergencies, trauma, and stroke, but sepsis-specific pathways were used.
Powell et al. 56 combined a similar ED-based telehealth consultation service with an educational intervention to examine the role of in situ simulations in promoting telehealth use in sepsis patients. Although clinical outcomes were measured, the goal of the educational program was to increase network use.
The other major category of studies used tele-ICU technology, which allows for a physician or nurse-directed real-time telemonitoring of patients using high-definition video and telemetry in an inpatient setting. Three studies 48,51,59 used this style of surveillance systems, often in conjunction with algorithms to identify clinical decompensation, trigger formal screening, and notify staff in suspected sepsis cases, and one had a health care provider that conducted a virtual consultation when indicated.51 Another program used a tele-ICU nurse team to work collaboratively with bedside teams to verify the completion of bundle-adherent care by time targets for qualifying sepsis patients. 48
Taylor et al. 59 reported the use of direct-to-consumer telehealth post-discharge from a sepsis hospitalization. The study implemented a program called STAR (Sepsis Transition And Recovery), a nurse-led post-discharge sepsis telehealth program designed to identify and address treatable complications of recovery that commonly lead to readmissions and preventable post-discharge morbidity
Outcome Reporting
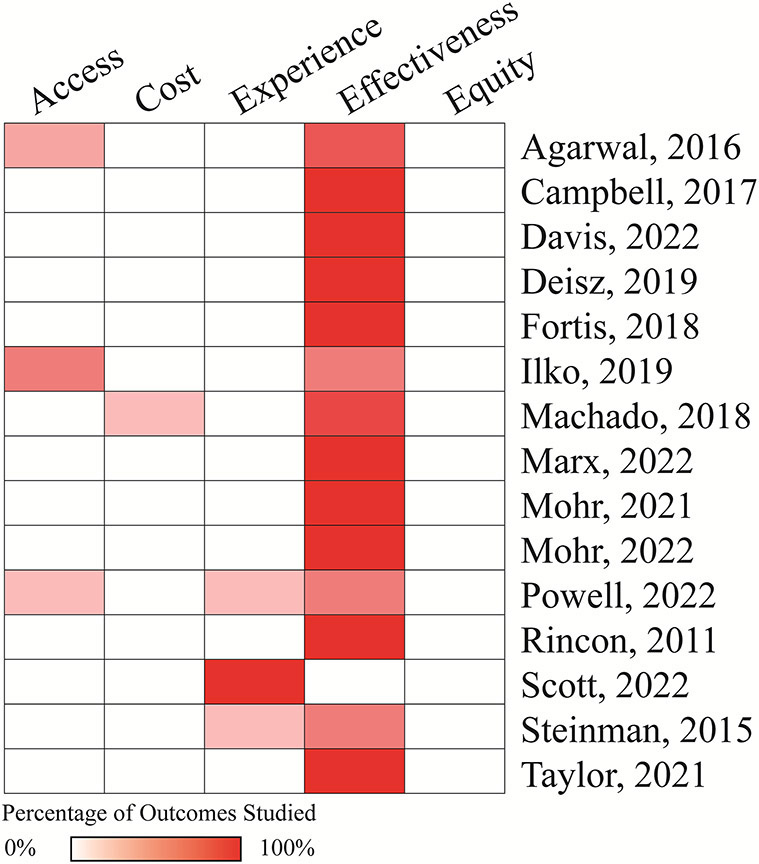
The most commonly studied outcomes, using the telehealth measure domains, were effectiveness outcomes, including survival and SSC bundle adherence (Figure 2). The summary of all measured outcomes is available in eTable 4 and eTable 5. The most frequently reported outcomes, including survival, SSC 3-hour bundle adherence, and antibiotic timeliness, are described further below.
Figure 2. Telehealth outcome domain heatmap.
A heatmap classifying study outcomes reported according to the RTHSRC telehealth domains. The percentage of studied outcomes within a study (number of outcomes in each domain evaluated by the study/total number of outcomes evaluated by the study) is correlated to the redness of the cell.
Three studies reported outcomes on patient and provider experiences, largely reporting that both providers and patients were comfortable with telehealth use. 49,52,56 Other observations included providers’ views that offloading surveillance and documentation activities reduce alert fatigue,57 and that telehealth led to cultural changes that improved the quality of sepsis care.58
Only one study47 reported on cost-effectiveness of their telehealth intervention, observing no difference in costs between telehealth and non-telehealth groups (US$19,713 vs. US$24,364, p=0.274).
Care Processes and Patient Outcomes
The odd ratios (ORs) of care processes and patient outcomes following telehealth use are listed in Table 2. Generally, survival was increased among sepsis patients treated with telehealth, with ORs ranging from 0.81 [95% CI 0.46, 1.41] to 3.59 [95% CI 2.33, 5.55]. Though survival OR point estimates were increased in nine out of ten studies, survival was only increased statistically significantly in three studies. 51,52,58 The effect of telehealth on interhospital transfer was highly varied, likely in part due to hospital-level factors and the lack of adjustment among analyses. Interhospital transfer OR ranged from 0.57 [95% CI 0.12, 2.72] to 77.57 [95% CI 51.55, 116.73]. Telehealth increased antibiotic timeliness and appropriateness in five out of seven studies with ORs ranging from 0.86 [CI 95% 0.42, 1.77] to 6.82 [CI 95% 1.27, 56.61]. Telehealth increased bundle adherence in a majority of studies, with 3-hour, 6-hour, and complete bundle adherence having ORs ranging from 0.59 [CI 95% 0.29, 1.22] to 3.33 [CI 95% 0.86, 12.92]; 0.67 [CI 95% 0.08, 5.82] to 20.00 [CI 95% 2.24, 178.92]; and 0.45 [CI 95% 0.02, 11.60] to 17.27 [CI 95% 6.64, 44.90], respectively. Only one study reported a significant increase in 3-hour lactate measurement, all studies together having ORs ranging from 1.18 [CI 95% 0.29, 4.86] to 7.85 [CI 95% 2.64, 23.34]. Two out of three studies reported a significant increase in 6-hour lactate measurement, all studies together having ORs ranging from 0.94 [CI 95% 0.15, 5.80] to 23.89 [CI 95% 1.26, 453.00].
Table 2. aOR of study outcomes following telemedicine.
aOR (adjusted odds ratio), RR (relative risk), N/A (not applicable). We defined antibiotic timeliness according to the underlying studies as broad-spectrum antibiotics (according to the SSC guidelines) administered within three hours of sepsis onset. Unadjusted results are in red, adjusted results are in black. *refers to the general transfer rate among hospitals with and without critical care teleconsultation service.
| OR [95% CI] following telemedicine implementation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year | Survival | Interhosital Transfer |
Antibiotic timeliness |
3-hr Bundle Adherence |
6-hr Bundle Adherence |
Complete bundle adherence |
3-hr lactate measurement |
6-hr lactate measurement |
| Cohort Studies | ||||||||
| Campbell et al., 2017 50 | 1.83 [0.36, 9.25] | 7.79 [2.89, 21.05] | 2.65 [0.99, 7.09] | 1.18 [0.29, 4.86] | ||||
| Ilko et al., 2019 54 | 0.69 [0.54, 0.88] * | |||||||
| Mohr et al., 2021 46 | 7.14 [3.48, 14.66] | 4.01 [1.16, 13.87] | 3.05 [1.42, 6.57] | 17.27 [6.64, 44.90] | 7.85 [2.64, 23.34] | |||
| Mohr et al., 2022 66 | 1.96 [0.49, 7.89] | 77.57 [51.55, 116.73] | 0.86 [0.42, 1.77] | 0.59 [0.29, 1.22] | 0.67 [0.08, 5.82] | 0.45 [0.02, 11.60] | 1.30 (0.55 - 3.06) | 0.94 [0.15, 5.80] |
| Machado et al., 2018 47 | 0.81 [0.46, 1.41] | 0.57 [0.12, 2.72] | 1.85 [1.02, 3.38] | 9.97 [1.32, 75.24] | ||||
| Powell et al., 2022 56 | 1.04 [0.56, 1.93] | 11.17 [6.54, 19.06] | 0.93 [0.52, 1.66] | 2.47 [0.34, 18.09] | ||||
| Steinman et al., 2015 58 | 3.59 [2.33, 5.55] | |||||||
| Before-After Studies | ||||||||
| Davis et al., 2022 51 | 1.60 [1.08, 2.37] | |||||||
| Deisz et al., 2019 52 | 2.86 [1.04, 7.83] | 3.31 [0.13, 86.07] | 1.37 [0.25, 7.39] | 20.00 [2.24, 178.92] | 23.89 [1.26, 453.00] | |||
| Fortis et al., 2018 53 | 1.04 [0.91, 1.19] | RR 0.70 [0.46-1.07] | ||||||
| Randomized Studies | ||||||||
| Marx et al., 2022 55 | 1.29 [0.70, 2.41] | 2.903 [2.01, 4.19]. | 6.82 [1.27, 56.61] | 3.33 [0.86, 12.92] | 14.245 [3.12, 85.424] | 7.739 [2.38, 28.03] | ||
| Taylor et al. 2021 59 | 1.61 [0.85, 3.02] | |||||||
We discovered significant clinical and methodological heterogeneity within our studies, which prevented us from pooling the data in a formal meta-analysis. Instead, we performed an explanatory analysis to uncover the reasons for this heterogeneity. We accounted for some heterogeneity in study methodology by focusing on studies that reported on provider-to-provider on-demand video consultations in EDs and ICUs as their telehealth intervention. We also focused on patient survival as an outcome (n = 28,708 pooled patients), as it was the most frequently reported outcome. We assessed the risk of publication bias to be low because the funnel plots were symmetric (eFigure 2). We compared cohort, randomized, and before-after studies. The cohort studies, which had a high risk of bias, seemed to demonstrate the highest, and most variable telehealth effect sizes (Table 2).
We also noticed that studies with lower baseline survival tended to demonstrate larger telehealth effect sizes for improving survival. We thus hypothesized that baseline illness severity may be an effect modifier. Focusing on studies that reported on provider-to-provider on-demand telehealth consultations as an intervention, we performed weighted meta-regression using control group survival as our sole predictor. We observed that those studies with the highest control group survival had the smallest association between telehealth use and survival (β 0.18 lower log-odds survival per 10% increase in control group survival, 95 % CI −0.12 to −0.24) (Figure 3).
Figure 3. Weighted-regression of effect of telehealth on survival, related to baseline sepsis survival.
The horizontal axis reports the control group survival (percentage), and the vertical axis is the β, from the adjusted effect size of telehealth on survival, from each of the underlying studies. The size of each point is proportional to the sample size and weighting in our regression model, and the linear weighted regression line is represented on the graph.
Discussion
Telehealth has been used to augment sepsis care, especially in settings where sepsis specialty care may not be readily available. Of the studies included in this systematic review, most were published in the past five years, suggesting increased recent interest in this area. Our systematic review found that telehealth has been used in diverse settings for the management of patients in EDs and ICUs, surveillance of inpatient services, and post-discharge monitoring, and current comparative effectiveness research has primarily focused on clinical effectiveness for process and clinical outcomes. Telehealth use was typically associated with improved sepsis survival and care processes, but these effects were variable. Ultimately, the effectiveness of telemedicine is likely be dependent on hospital-level factors.
Our results confirm broader findings from Totten, et al.61 that non-sepsis telehealth applications are diverse. Part of the reason for that diversity of application may be that telehealth is being used to solve very different clinical problems related to sepsis care. It also highlights that the heterogeneous treatment effects we observed may reflect the underlying resources and operations of the systems in which telehealth is deployed. Although we were unable to determine why some studies had poor control group outcomes, baseline survival is likely a surrogate for underlying health system performance, patient selection within health systems, training and experience of local care teams, or other factors that may make telehealth a more effective alternative. Similar results have been found in tele-stroke, where telehealth has previously been shown to have the greatest effect in rural areas with the fewest available local resources.62 One of our included references observed that the effect of telehealth was greatest in the smallest rural EDs with the lowest sepsis volume most often staffed by advanced practice providers, which aligns closely with the findings of our weighted meta-regression analysis.60
Telehealth has many potential mechanisms that could affect outcomes. Several studies highlighted that telehealth contributes to the education of local providers. 63 This has previously been cited as a secondary benefit of patient-focused teleconsultation, and it is one of the ways that care evolution can propagate through professional networks. Other studies focused on surveillance to improve early detection or expert consultation for treatment recommendations. 34 Early detection is a significant barrier to early treatment, and it may contribute to higher mortality for inpatients developing sepsis than those who present with sepsis present on admission. The barriers of early diagnosis could have affected the observed effects in provider-to-provider teleconsultation in other studies because local sepsis recognition may be required prior to telehealth activation.
It was surprising that we observed highly variable improvement in guideline adherence or antibiotic timeliness, although the point estimates for both of these outcomes suggest that care may actually be different in patients whose care was supplemented with telehealth.64 These two process outcomes were narrowly focused, however, and there may be other ways, such as through guidance on airway management, hemodynamic evaluation and optimization, interhospital transfer, and management of underlying comorbidities where telehealth recommendations may not be captured in SSC guideline measurement. Future work may explore the social relationship between telehealth and local providers to better define the ways in which telehealth recommendations reflect guidelines, are implemented, and contribute to changes in local care pathways. The highly variable bundle adherence and antibiotic timeliness rates also raise questions about the mechanism by which telehealth is associated with improved survival.
Our systematic review highlighted some key areas of opportunity for future investigation. Most published comparative effectiveness research is derived from observational cohort studies. Quasi-experimental or randomized studies are clearly needed to better elucidate the causal role of telehealth in improving patient outcomes within high-risk groups and to better understand the heterogeneity of treatment effects. The other theme we identified is that the outcomes included in sepsis telehealth studies are narrow. Few studies include long-term clinical or patient-centered outcomes (e.g., quality of functional recovery, the ability of a family to visit patients closer to home). Robust large-scale cost and cost-effectiveness studies have not been performed, even though these are often cited as benefits of telehealth use. A study is currently underway to evaluate the economic effect of telehealth in rural ED-based sepsis care.65
Limitations
Our systematic review has several limitations. First, the number of published studies evaluating telehealth in sepsis care is limited and most are published by only a few research groups. This could mean that other groups have developed telehealth interventions that have not been reported in the scientific literature, but the certainty of our conclusions is limited by the underlying data. Clinical and statistical heterogeneity remains a limitation because the systems and interventions reported differ and are likely only partially explained by baseline survival. Due to the high variance in the studies’ findings, the certainty of our outcomes from the subgroup and regression analyses are low, which impacts the strength of our conclusions. Finally, our subgroup analyses and weighted regression are subject to the limitations of the underlying observational study designs, which risk being confounded by indication.
Conclusion
Telehealth has been used by a variety of groups in diverse ways to improve sepsis care. Telehealth use for sepsis care may be associated with improved survival in some settings, with the greatest effect in studies with the lowest baseline survival. Future work would benefit from interventional studies, long-term post-discharge and patient-centered outcomes, and cost-based analyses, because the causal effect of novel care delivery programs on clinical outcomes and resource utilization has policy relevance and is needed to optimize the ways in which these programs are used.
Supplementary Material
KEY POINTS.
Question:
How has telemedicine been used to treat sepsis, and how does its use affect sepsis patient outcomes?
Findings:
Telehealth for sepsis has been implemented in diverse ways, but it has been evaluated mostly through observational studies. Telehealth was associated with higher survival in settings with low control group survival. However, its effect on other care processes and outcomes is highly variable.
Meaning:
Telehealth likely benefits sepsis patients, but interventional trials and cost-based analyses would be valuable.
Funding:
K.J.T. was supported by a Daniel and Carly Rochkind research grant and a Banneker/Key scholarship, both provided by the University of Maryland Honor College. N.M.M was supported by a grant from the Agency for Healthcare Research and Quality (AHRQ, grant K08HS025753) and the Health Resources and Services Administration (HRSA, award U3GRH40003). The views expressed in this publication are solely those of the authors and do not reflect the official views of the funders or the U.S government.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Vincent J, Marshall J, Namendys-Silva S, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5). doi: 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 3.Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009: statistical brief #122. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed June 20, 2022. http://www.ncbi.nlm.nih.gov/books/NBK65391/ [Google Scholar]
- 4.Rhee C, Dantes R, Epstein L, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017;318(13):1241. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu V, Escobar GJ, Greene JD, et al. Hospital Deaths in Patients With Sepsis From 2 Independent Cohorts. JAMA. 2014;312(1):90. doi: 10.1001/jama.2014.5804 [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019— results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239. doi: 10.1186/s13054-020-02950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307 [DOI] [PubMed] [Google Scholar]
- 8.Kent N, Fields W. Early recognition of sepsis in the emergency department: an evidence-based project. J Emerg Nurs. 2012;38(2):139–143. doi: 10.1016/j.jen.2010.07.022 [DOI] [PubMed] [Google Scholar]
- 9.Rivers EP. Point: adherence to early goal-directed therapy: does it really matter? Yes. After a decade, the scientific proof speaks for itself. Chest. 2010;138(3):476–480; discussion 484-485. doi: 10.1378/chest.10-1405 [DOI] [PubMed] [Google Scholar]
- 10.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 11.ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40(11):1623–1633. doi: 10.1007/s00134-014-3496-0 [DOI] [PubMed] [Google Scholar]
- 14.Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med. 2015;41(9):1620–1628. doi: 10.1007/s00134-015-3906-y [DOI] [PubMed] [Google Scholar]
- 15.Miller RR, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82. doi: 10.1164/rccm.201212-2199OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374. doi: 10.1097/CCM.0b013e3181cb0cdc [DOI] [PubMed] [Google Scholar]
- 17.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924. doi: 10.1016/S1473-3099(12)70239-6 [DOI] [PubMed] [Google Scholar]
- 18.Venkatesh AK, Slesinger T, Whittle J, et al. Preliminary performance on the new CMS Sepsis-1 National Quality Measure: early insights from the Emergency Quality Network (E-QUAL). Ann Emerg Med. 2018;71(1):10–15.e1. doi: 10.1016/j.annemergmed.2017.06.032 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Ma X dong, Kang X hui, et al. Association of annual hospital septic shock case volume and hospital mortality. Crit Care. 2022;26(1):161. doi: 10.1186/s13054-022-04035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen YL, Wallace DJ, Yordanov Y, et al. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148(1):79–92. doi: 10.1378/chest.14-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocher KE, Haggins AN, Sabbatini AK, Sauser K, Sharp AL. Emergency department hospitalization volume and mortality in the United States. Ann Emerg Med. 2014;64(5):446–457.e6. doi: 10.1016/j.annemergmed.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 22.Faine BA, Noack JM, Wong T, et al. Interhospital transfer delays appropriate treatment for patients with severe sepsis and septic shock: a retrospective cohort study. Crit Care Med. 2015;43(12):2589–2596. doi: 10.1097/CCM.0000000000001301 [DOI] [PubMed] [Google Scholar]
- 23.Gaieski DF, Edwards JM, Kallan MJ, Mikkelsen ME, Goyal M, Carr BG. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190(6):665–674. doi: 10.1164/rccm.201402-0289OC [DOI] [PubMed] [Google Scholar]
- 24.Mohr NM, Harland KK, Shane DM, et al. Rural patients with severe sepsis or septic shock who bypass rural hospitals have increased mortality: an instrumental variables approach. Crit Care Med. 2017;45(1):85–93. doi: 10.1097/CCM.0000000000002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohr NM, Harland KK, Shane DM, Ahmed A, Fuller BM, Torner JC. Inter-hospital transfer is associated with increased mortality and costs in severe sepsis and septic shock: an instrumental variables approach. J Crit Care. 2016;36:187–194. doi: 10.1016/j.jcrc.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yealy DM, Huang DT, Delaney A, et al. Recognizing and managing sepsis: what needs to be done? BMC Medicine. 2015;13(1):98. doi: 10.1186/s12916-015-0335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohr NM, Collier J, Hassebroek E, Groth H. Characterizing critical care physician staffing in rural America: a description of Iowa intensive care unit staffing. J Crit Care. 2014;29(2):194–198. doi: 10.1016/j.jcrc.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 28.Ferrer R, Martínez ML, Gomà G, et al. Improved empirical antibiotic treatment of sepsis after an educational intervention: the ABISS-Edusepsis study. Crit Care. 2018;22(1):167. doi: 10.1186/s13054-018-2091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricci MA, Caputo M, Amour J, et al. Telemedicine reduces discrepancies in rural trauma care. Telemed J E Health. 2003;9(1):3–11. doi: 10.1089/153056203763317602 [DOI] [PubMed] [Google Scholar]
- 30.Heath B, Salerno R, Hopkins A, Hertzig J, Caputo M. Pediatric critical care telemedicine in rural underserved emergency departments. Pediatr Crit Care Med. 2009;10(5):588–591. doi: 10.1097/PCC.0b013e3181a63eac [DOI] [PubMed] [Google Scholar]
- 31.Hicks LL, Boles KE, Hudson ST, et al. Using telemedicine to avoid transfer of rural emergency department patients. J Rural Health. 2001;17(3):220–228. doi: 10.1111/j.1748-0361.2001.tb00959.x [DOI] [PubMed] [Google Scholar]
- 32.Mohr NM, Harland KK, Chrischilles EA, Bell A, Shane DM, Ward MM. Emergency department telemedicine is used for more severely injured rural trauma patients, but does not decrease transfer: a cohort study. Acad Emerg Med. 2017;24(2):177–185. doi: 10.1111/acem.13120 [DOI] [PubMed] [Google Scholar]
- 33.Nesbitt Thomas S.. The evolution of telehealth: where have we been and where are we going? In: The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press. [PubMed] [Google Scholar]
- 34.Mohr NM, Hurst EK, MacKinney AC, Nash EC, Carr BG, Skow B. Telemedicine for early treatment of sepsis. In: Koenig MA, ed. Telemedicine in the ICU. Springer International Publishing; 2019:255–280. doi: 10.1007/978-3-030-11569-2_15 [DOI] [Google Scholar]
- 35.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters MD, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: scoping reviews. JBI manual for evidence synthesis, JBI. 2020;2020. doi: 10.46658/JBIMES-20-12 [DOI] [PubMed] [Google Scholar]
- 37.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 38.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Published online March 29, 2021:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu K, Wymore C, Tchangalova N, Mohr N. The use of telemedicine for treating sepsis patients: a scoping review protocol. Published online August 17, 2022. doi: 10.17605/OSF.IO/D3H24 [DOI] [Google Scholar]
- 40.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Monnin K, Terian E, Yaegar LH, et al. Low tidal volume ventilation for emergency department patients: a systematic review and meta-analysis on practice patterns and clinical impact. Crit Care Med. 2022;50(6):986–998. doi: 10.1097/CCM.0000000000005459 [DOI] [PubMed] [Google Scholar]
- 44.National Quality Forum. Rural Telehealth and Healthcare System Readiness Measurement Framework. Accessed August 11, 2022. https://www.qualityforum.org/Publications/2021/11/Rural_Telehealth_and_Healthcare_System_Readiness_Measurement_Framework_-_Final_Report.aspx [Google Scholar]
- 45.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9(1):80. doi: 10.1186/1471-2288-9-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohr NM, Campbell KD, Swanson MB, Ullrich F, Merchant KA, Ward MM. Provider-to-provider telemedicine improves adherence to sepsis bundle care in community emergency departments. J Telemed Telecare. 2021;27(8):518–526. doi: 10.1177/1357633X19896667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machado SM, Wilson EH, Elliott JO, Jordan K. Impact of a telemedicine eICU cart on sepsis management in a community hospital emergency department. J Telemed Telecare. 2018;24(3):202–208. doi: 10.1177/1357633X17691862 [DOI] [PubMed] [Google Scholar]
- 48.Rincon TA, Bourke G, Seiver A. Standardizing sepsis screening and management via a tele-ICU program improves patient care. Telemed E Health. 2011;17(7):560–564. doi: 10.1089/tmj.2010.0225 [DOI] [PubMed] [Google Scholar]
- 49.Agarwal AK, Gaieski DF, Perman SM, et al. Telemedicine REsuscitation and Arrest Trial (TREAT): A feasibility study of real-time provider-to-provider telemedicine for the care of critically ill patients. Heliyon. 2016;2(4):e00099. doi: 10.1016/j.heliyon.2016.e00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell Kalyn, Vakkalanka Priyanka, Wittrock Amy, et al. Telemedicine is associated with improved antibiotic appropriateness in rural emergency departments. Presented at: SEARCH 2017: National Telehealth Research Symposium; 2017. [Google Scholar]
- 51.Davis C, Faruki A, Breyer D, et al. The case for virtual sepsis surveillance and intervention. Telemed E Health. 2022;28(1):102–106. doi: 10.1089/tmj.2020.0513 [DOI] [PubMed] [Google Scholar]
- 52.Deisz R, Rademacher S, Gilger K, et al. Additional telemedicine rounds as a successful performance-improvement strategy for sepsis management: Observational multicenter study. J Med Internet Res. 2019;21(1):e11161. doi: 10.2196/11161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fortis S, Sarrazin MV, Beck BF, Panos RJ, Reisinger HS. ICU telemedicine reduces interhospital ICU transfers in the veterans health administration. Chest. 2018;154(1):69–76. doi: 10.1016/j.chest.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 54.Ilko SA, Vakkalanka JP, Ahmed A, Harland KK, Mohr NM. Central venous access capability and critical care telemedicine decreases inter-hospital transfer among severe sepsis patients: A mixed methods design. Crit Care Med. 2019;47(5):659. doi: 10.1097/CCM.0000000000003686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marx G, Greiner W, Juhra C, et al. An innovative telemedical network to improve infectious disease management in critically Ill patients and outpatients (TELnet@NRW): Stepped-wedge cluster randomized controlled trial. J Med Internet Res. 2022;24(3):e34098. doi: 10.2196/34098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell ES, Bond WF, Barker LT, et al. In situ simulation for adoption of new technology to improve sepsis care in rural emergency departments. J Patient Saf. 2022;18(4):302–309. doi: 10.1097/PTS.0000000000000923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott B, Hassell A, Davis C, Huntley N, Pierce R. 1333: Impact of a system-wide virtual sepsis response program on the volume of actionable alerts. Crit Care Med. 2020;48(1):643–643. doi: 10.1097/01.ccm.0000645248.03328.20 [DOI] [Google Scholar]
- 58.Steinman M, Morbeck RA, Pires PV, et al. Impact of telemedicine in hospital culture and its consequences on quality of care and safety. Einstein (São Paulo). 2015;13:580–586. doi: 10.1590/S1679-45082015GS2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor SP, Murphy S, Rios A, et al. Effect of a multicomponent sepsis transition and recovery program on mortality and readmissions after sepsis: the improving morbidity during post-acute care transitions for sepsis randomized clinical trial*. Crit Care Med. 2021;50(3):469–479. doi: 10.1097/CCM.0000000000005300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohr NM, Okoro U, Harland KK, et al. Outcomes associated with rural emergency department provider-to-provider telehealth for sepsis care: a multicenter cohort study. Annals of Emergency Medicine. Published online October 2022:S019606442200525X. doi: 10.1016/j.annemergmed.2022.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Totten AM, Womack DM, Eden KB, et al. Telehealth: Mapping the Evidence for Patient Outcomes From Systematic Reviews. Agency for Healthcare Research and Quality Accessed August 20, 2022. https://www.ncbi.nlm.nih.gov/books/NBK379320/ [PubMed] [Google Scholar]
- 62.Zhang D, Wang G, Zhu W, et al. Expansion of telestroke services improves quality of care provided in super rural areas. Health Affairs. 2018;37(12):2005–2013. doi: 10.1377/hlthaff.2018.05089 [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Merchant KAS, Mohr NM, Wittrock AJ, Bell AL, Ward MM. Real-time learning through telemedicine enhances professional training in rural emergency departments. Telemed E Health. 2021;27(4):441–447. doi: 10.1089/tmj.2020.0042 [DOI] [PubMed] [Google Scholar]
- 64.Badawi O, Hassan E. Telemedicine and the patient with sepsis. Crit Care Clin. 2015;31(2):291–304. doi: 10.1016/j.ccc.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 65.Mohr NM, Schuette AR, Ullrich F, et al. An economic and health outcome evaluation of telehealth in rural sepsis care: a comparative effectiveness study. J Comp Eff Res. Published online May 24, 2022. doi: 10.2217/cer-2022-0019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.