Abstract
Adoptive cell therapies involve infusing engineered immune cells into cancer patients to recognize and eliminate tumor cells. Adoptive cell therapy, as a form of living drug, has undergone explosive growth over the past decade. The recognition of tumor antigens by the T-cell receptor (TCR) is one of the natural mechanisms that the immune system used to eliminate tumor cells. TCR-T cell therapy, which involves introducing exogenous TCRs into patients’ T cells, is a novel cell therapy strategy. TCR-T cell therapy can target the entire proteome of cancer cells. Engineering T cells with exogenous TCRs to help patients combat cancer has achieved success in clinical trials, particularly in treating solid tumors. In this review, we examine the progress of TCR-T cell therapy over the past five years. This includes the discovery of new tumor antigens, protein engineering techniques for TCR, reprogramming strategies for TCR-T cell therapy, clinical studies on TCR-T cell therapy, and the advancement of TCR-T cell therapy in China. We also propose several potential directions for the future development of TCR-T cell therapy.
Keywords: TCR, pMHC, TCR-T cell therapy, tumor antigen, catch bond, dynamic binding
Introduction
The immune system is the body’s natural defense system that recognizes and kills tumor cells [1]. Although the immune system may fail to eliminate tumor cells due to the exhaustion of immune cells [2], a suppressive tumor microenvironment [ 3, 4], and multiple tumor escape strategies [ 5, 6], we can enhance the immune system through protein engineering or cell engineering to combat cancer. In the last two decades, we have witnessed a boom in basic immunology, particularly in the understanding of the checkpoint mechanism of T-cell activation and the design of chimeric antigen receptors (CARs). Meanwhile, mechanisms related to tumor immunology, therapeutic targets, and molecular design have been successfully translated into approved immunotherapy, saving many cancer patients. The discovery of the immune checkpoint mechanism and the development of T-cell engineering have made immunotherapy one of the key pillars in the contemporary treatment of cancer [ 7– 10].
Immunotherapy can take many forms, but the majority of clinical success comes from antibody blockade treatment and adoptive cell transfer therapy (ACT). Antibody blockade treatment includes immune checkpoint blockade (ICB), such as anti-PD-1 and anti-CTLA-4 treatments [ 9, 11– 14]. Over six anti-PD-1 and anti-CTLA-4 drugs have been approved by the FDA [15]. For adoptive cell therapy, chimeric antigen receptor T-cell (CAR-T) therapy has achieved significant success, including the complete cure of leukemia patients [16]. CAR-T therapy involves transducing T cells with artificial receptors that can recognize surface antigens on tumor cells and deliver activation signals to the T cells. More than six CAR-T therapies have been approved for B-cell lymphoma, but no CAR-T therapy has been approved for solid tumors [17]. Both checkpoint blockade and CAR-T cell therapy have cured many, but not all, cancer patients [ 18, 19]. As of now, there are no approved adoptive cell therapies (including CAR-T, CAR-NK, etc.) for solid tumors [ 20– 22].
T cells play important roles in killing tumors. The immune system naturally uses T cell receptors (TCRs) to recognize tumor antigens and eradicate tumor cells. The TCR molecule has evolved over 450 million years as an elegant system to balance antigen recognition, cross-reactivity, and sensitive signaling [ 23– 26]. It is not surprising that people have been considering using TCR to enhance the immune system for over 30 years [ 27, 28]. One idea is to isolate a potent TCR that can effectively eliminate tumor cells and then administer the TCR to patients by modifying their autologous T cells. TCR-T cell therapy is the name of the treatment. Rosenberg and his colleagues were pioneers in the first clinical trial of TCR-T cell therapy for the treatment of melanoma. They observed a positive response in two patients [29]. Based on TCR research, in the 2010s, significant success has been achieved in the development of TCR-T cell therapy for solid tumors [21].
We are on the eve of TCR-T cell therapy potentially being approved for treating solid tumors. CAR-T cell therapy has achieved tremendous success in treating liquid tumors but has shown limited efficacy in treating solid tumors [ 30– 32]. However, solid tumors account for 95% of all cancer patients [33]. Since the antigenic peptide is derived from protein degradation, TCRs have access to the entire proteome as potential targets. TCR-T therapy might be the solution for patients with solid tumors and those who do not respond to checkpoint blockade.
There are numerous reviews available on the antigen targets, clinical trials, engineering strategies, and the history of TCR-T cell therapy. In this paper, we primarily focus on the recent advancements in TCR-T cell therapy, particularly within the last five years, including the discovery of novel targets, innovative protein engineering methods, ongoing and completed clinical trials, and the evolving trends of TCR-T cell therapy in China. We also propose several potential directions for the advancement of TCR-T cell therapy in the next decade.
The Structure and Signaling of TCR
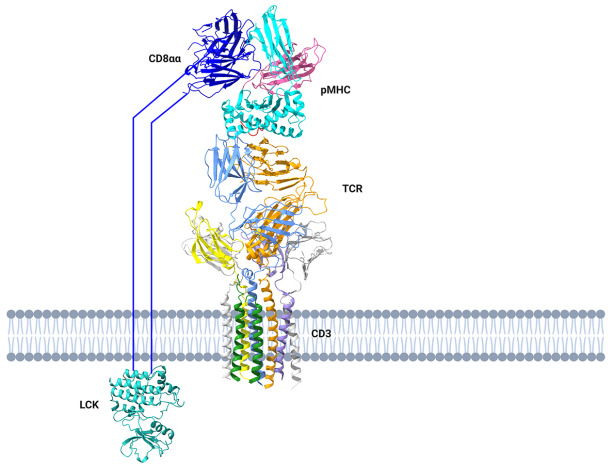
T cells are crucial components of adaptive immunity, and the TCR serves as the gatekeeper of T cell functions. There are 2 populations of T lymphocytes, αβ T and γδ T cells, that can be distinguished by the expression of either an αβ TCR or a γδ TCR respectively. The αβ TCR is a heterodimer consisting of an α chain and a β chain, and it is always associated with the CD3 complex. The CD3 complex consists of CD3γ, CD3δ, two CD3ε, and two CD3ζ chains. The TCR-CD3 complex is an octameric membrane protein machinery [ 34– 36]. Similarly, the γδ TCR is assembled from a γ chain and a δ chain, but the structure of γδ TCR-CD3 is different from the former. Its CD3 subunits are arranged differently, the FG loop is much shorter, the disulfide-bond between the ligand binding subunits is placed differently and CD3 glycosylation is different [37].The ligand for the TCR is the peptide-major histocompatibility complex (pMHC). The peptides, particularly MHC class I-restricted antigenic peptides, are produced from the breakdown of proteins expressed in tumor cells or somatic cells infected by pathogens. Therefore, T cells and TCRs are important systems for recognizing tumor cells or pathogens by detecting the proteome of target cells [ 38– 42]. The structural basis of TCR-CD3 assembly has recently been elucidated, but it remains unclear whether there is a conformational change in the TCR-CD3 complex before and after pMHC ligation [ 34– 36, 43, 44] In addition to its antigen recognition function, TCR-CD3 can be phosphorylated by kinases such as Lck and Fyn after pMHC binding, initiating T cell effector functions and differentiation [ 45– 48] ( Figure 1).
Figure 1 .
The structural assembly of pMHC-TCR-CD3 complex and TCR signaling cascade
The fully assembled pMHC-TCR-CD3 complex has been solved. CD8 binds to the pMHC and recruits LCK to phosphorylate CD3 and initiate TCR downstream signaling cascade.
Tumor Antigens
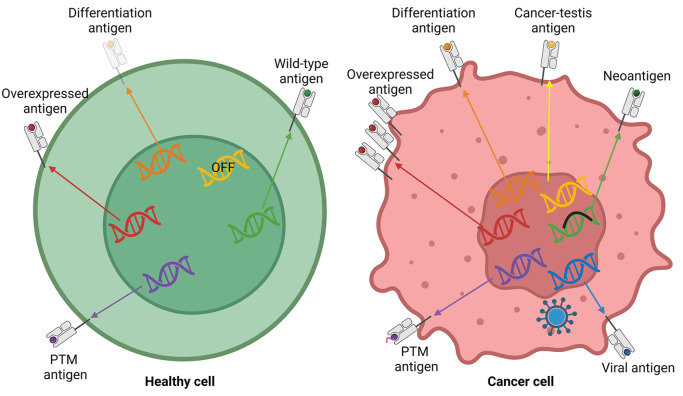
There is no doubt that tumor antigens, as therapeutic targets, are one of the most important components in TCR-T cell therapy. In the last 40 years, numerous tumor-associated or tumor-specific antigens have been identified. They are classified into several groups, including overexpressed tumor antigens, differentiation antigens, cancer-testis antigens, neoantigens, and viral antigens [49]. An ideal tumor antigen should be specific to the tumor to enhance effectiveness and minimize toxicity ( Figure 2). In recent years, numerous new antigens and challenging-to-target antigens have been discovered or studied to enhance our comprehension of tumor antigens.
Figure 2 .
The generation of different categories of tumor antigens
Overexpressed tumor antigens are derived from overexpression of certain proteins. Differentiation antigens are derived from differentiation-related proteins. Cancer-testis antigens are derived from proteins which are only expressed in tumor and testis. Neoantigens are derived from mutated proteins. Viral antigens are derived from virus-encoded proteins. Post-translation modified antigens are derived from post-translation modified proteins. Adapted from [49].
Overexpressed tumor antigen
Overexpressed tumor antigens are highly expressed in tumors but are expressed at low levels in healthy tissue. Overexpressed tumor antigens could be targeted by TCR-T cells to eliminate tumor cells, but they may also cause on-target, off-tumor toxicity in healthy tissue. Overexpressed tumor antigens, such as Wilms’ tumor-1 (WT1) and preferentially expressed antigen of melanoma (PRAME), are important therapeutic targets and have been tested in multiple TCR-T clinical trials [50]. WT1 is ranked as the No. 1 tumor antigen by the National Cancer Institute [51]. WT1 in the context of HLA-A*24:02 has been targeted in a clinical trial for acute myeloblastic leukemia (AML) using TCR-T cell therapy [50]. In clinical trials, TCR-T cells targeting WT1 have shown an objective response rate (ORR) ranging from 0% to 40% [ 21, 50, 52].
Recently, more overexpressed tumor antigens have been discovered, and their potential in TCR-T cell therapies has been preliminarily tested. Laminin subunit gamma 2 (LAMC2) has been identified as an overexpressed tumor antigen in pancreatic ductal adenocarcinoma (PDAC). ‘LAMC2203-211’ is an HLA-A2-restricted epitope. TCR-T cell therapy targeting LAMC2 has been shown to control tumor growth in a PDAC xenograft model [53]. CCCTC-binding factor (CTCFL) and Claudin-6 (CLDN6) are overexpressed in ovarian cancer and 20 times less expressed in healthy tissues. Different HLA alleles-restricted epitopes of CTCFL or CLDN6 have been identified. Specific TCRs were isolated from allogeneic HLA donors, and TCR-T cell therapy targeting CTCFL has been developed. These TCRs could recognize tumor cells from ovarian cancer patients [54]. The immunoglobulin J chain is overexpressed in multiple myeloma, and TCRs targeting HLA-A*01, A*24, A*03, or A*11-restricted J chain peptides have been discovered. TCR-T cell therapy could eliminate 99% of multiple myeloma cells in a preclinical in vivo model [55]. Human telomerase reverse transcriptase (hTERT) is overexpressed in 90% of tumors. A TCR clone, named Radium-4, targeting an HLA-DP04-restricted hTERT peptide, has been isolated from pancreatic cancer patients who were vaccinated with a long hTERT peptide. Radium-4 TCR-T cell therapy demonstrated efficient killing of tumor cells in a xenograft mouse model and improved the survival rate. The Radium-4 TCR-T cell therapy has the potential to reach 75% of the population and shows promise in the treatment of solid tumors [56]. Overall, overexpressed tumor antigens are promising therapeutic targets, and further research is needed to address the on-target, off-tumor issue, which emphasizes the importance of this category of tumor antigens.
Differentiation antigen
Certain proteins are specifically expressed in particular cells to facilitate the differentiation of tissues and organs. The proteins are tumor-associated but not tumor-specific, as healthy tissues from the same lineage would also exhibit relatively high expression of the proteins. For example, both melanoma cells and skin cells express proteins that are important for the differentiation of skin cells, such as MART-1 and gp100 [ 57, 58]. Targeting differentiation antigens could lead to on-target, off-tumor toxicity, with the severity varying [ 49, 59]. Recently, new differentiation antigens have been discovered, which could potentially serve as new targets for TCR-T cell therapies. Alpha-fetoprotein is associated with liver cancer. HLA-A2-restricted alpha-fetoprotein epitope has been targeted by TCR-T cell therapy in an NSG mouse model [60]. Leukemia-associated minor H antigen HA-1 has been targeted by TCR-T cell therapy [61].
Cancer-testis antigen
Cancer-testis antigens (CTAs) are highly expressed in tumor cells, testis, and placental tissues, but are barely expressed in other healthy tissues [62]. Therefore, CTAs are a group of promising therapeutic targets with a lower risk of on-target, off-tumor toxicity. The majority of TCR-T cell therapies target CTAs, particularly NY-ESO-1 and the MAGE family. Recently, CTAs such as NY-ESO-1 and MAGE have shown early-stage success in clinical trials. An anti NY-ESO-1 TCR-T cell therapy clinical trial was performed in patients with metastatic melanoma or metastatic synovial cell sarcoma refractory to all standard treatments. Objective clinical responses were observed in 4 of 6 patients with synovial cell sarcoma and 5 of 11 patients with melanoma bearing tumors expressing NY-ESO-1, which indicated that TCR-based gene therapies directed against NY-ESO-1 represent a new and effective therapeutic approach for patients with melanoma and synovial cell sarcoma [63]. HLA-A*02:01 transgenic mice were used to identify TCRs recognizing the NY-ESO-1 157-165 epitope [64]. TCR-T cell therapy targeting HLA-A2-NY-ESO-1 has achieved an objective response in a phase I clinical trial [ 65, 66]. One trial was conducted in non-small cell lung cancer, and two out of four patients showed clinical responses [65]. Another trial utilizing high-affinity TCR targeting HLA-A2-NY-ESO-1 in multiple myeloma achieved a 44% objective response at one year after the therapy [66]. By using peripheral blood mononuclear cells (PBMC) from an allogeneic donor, high-avidity TCRs have been isolated to target the MAGE-A4 epitope restricted by HLA-A2 [67]. TCR-T cell therapy targeting HLA-A*24:02-MAGE-A4 143-151 in recurrent esophageal cancer has been in clinical trials [68]. Preferentially expressed antigen of melanoma (PRAME) is another important CTA. It is expressed in medulloblastoma, and TCR-T cells targeting HLA-A*02-restricted PRAME epitopes could suppress tumor growth in a xenograft mouse model [69].
Neoantigen
The development of a tumor is often associated with gene mutations. Some mutations can result in the expression of proteins, which are then further processed into peptides. The antigenic peptide containing the point mutation could be presented on the surface of tumor cells and referred to as a neoantigen. Neoantigens could be highly immunogenic because their sequences differ from the wild-type peptides and are not presented in the thymus for negative selection. Some neoantigens are shared among different patients and are termed public neoantigens. Public neoantigens are promising therapeutic targets because they are highly tumor-specific and shared by a significant number of patients [70]. The KRAS-G12V neoantigen, one of the most extensively studied public neoantigens, has been effectively targeted by TCR-T cell therapy [71]. The FMS-related receptor tyrosine kinase (FLT3)-D835Y mutation is a gain-of-function mutation found in acute myeloid leukemia (AML). The HLA-A*02:01-restricted FLT3-D835Y epitope has recently been discovered and targeted by TCR-T cell therapy for AML [72]. The coenzyme A synthase (COASY) - S55Y was identified as a novel neoantigen in multiple myeloma [73]. The QYSPVQATF peptide was identified as the HLA-A*24:02-restricted epitope sequence from the COASY-S55Y protein. TCR-T cell therapy targeting HLA-A*24:02-COASY-S55Y exhibited an objective response. The MyD88 L265P mutation, a driver mutation in B-cell lymphoma, has been effectively targeted by TCR-T cell therapy [74]. The HLA-B*07:02-restricted MyD88 L265P epitope could elicit a strong TCR-T cell response in a xenograft model. HLA-A*02:01-restricted neoantigen KIAA1429-D1358E was identified in head and neck squamous cell carcinoma, and specific TCR-T cell therapy has been developed [75]. Neoantigens derived from the fusion gene CBFB-MYH11 in AML have been targeted by TCR-T cell therapy [76]. An HLA-B*40:01-restricted epitope has been discovered and could be effectively targeted in a xenograft model.
Viral antigen
Some viral infections may progress into cancer. Hepatitis B virus (HBV) infection is responsible for over 60% of hepatocellular carcinoma cases in Asia [77]. Human papillomavirus (HPV) infection may lead to the development of cervical cancer, with 99.7% of cervical cancer cases being caused by HPV infection [78]. The Epstein-Barr virus (EBV) may lead to the development of nasopharyngeal carcinoma (NPC), and 82% of EBV-associated malignancies are NPC [79]. Viral proteins can serve as effective therapeutic targets for TCR-T cell therapy. HBV-specific TCR-T cell therapies have been extensively reviewed [ 80, 81]. A high-affinity TCR targeting HLA-A*02:01-HBs 371-379 has been isolated and was able to eliminate hepatocellular carcinoma in a xenograft model [82]. A phase I clinical trial showed a decrease in circulating HBsAg and HBV DNA after TCR-T cell therapy infusion targeting the HLA-A*02:01-HBs183-191 or HLA-Cw0801-epitope [83]. Immunosuppressive drug-resistant HBV-specific TCR-T cell therapies could effectively eliminate circulating tumor cells (CTC) [84]. HLA-A*11:01-restricted epitopes from HPV E6 and E7 proteins have been identified, and specific TCRs have been discovered. TCR-T cells were able to inhibit the growth of cervical tumor cells in vivo [ 85, 86]. The HLA-A*02:01-restricted E7 peptide has also been targeted by TCR-T cell therapy. A potent TCR was isolated from a uterine cervix biopsy and demonstrated CD8-independent killing of HPV-16-positive cervical tumors in a xenograft model [87]. Peptides derived from CMV pp65 and presented by HLA-A*02:01, HLA-A*11:01, or HLA-A*24:02 have been identified, and specific TCRs have been discovered. Clinical trials using TCR-T cells to treat patients with cytomegalovirus (CMV) reactivation after hematopoietic stem cell transplantation showed a complete response and mild adverse effects [88]. Another study utilized circular mRNA to express CMV antigen and prime specific T cells. The study also investigated the use of circular mRNA to express specific TCR to target and kill HLA-A*02:01-CMVpp65-positive tumor cells [89]. The EBV antigen LMP2-derived peptide, which is presented by HLA-A*01:01, has been targeted by TCR-T cell therapy [90].
Post-translational modified antigen
Protein post-translational modifications (PTMs) are present in multiple autoimmune diseases and tumors. Protein phosphorylation, sumoylation, and acetylation may offer new targets for TCR-T cell therapy [91]. An antigen discovery pipeline called Protein Modification Integrated Search Engine (PROMISE) could help identify thousands of new cancer-specific PTM antigens [92]. PTM antigens could elicit a higher T-cell response compared to unmodified antigens do [93]. Therefore, targeting PTM antigens could be an important focus for future TCR-T cell therapy. More work is needed to identify tumor-specific PTM antigens. The mechanism of PTM antigen production may also offer new therapeutic targets for other strategies.
Human endogenous retrovirus (HERV)-derived antigen
HERV could be expressed in tumor cells and produce tumor-specific antigens. HERV-E is expressed in renal cell carcinomas but not in normal kidneys. HLA-A2 and HLA-A11-restricted HERV-E epitopes have been utilized to identify specific T cells [ 94– 96].
Discovery and Engineering of TCR for Cell Therapy
Most tumor antigens consist of self-peptides and are tolerated by wild-type TCRs. As a result, isolating high-potency TCRs from peripheral T cells is challenging. Protein engineering is commonly utilized to modify wild-type TCRs and produce high-potency TCRs. For a very long time, high-affinity maturation was the only methodology adopted in the protein engineering of TCR, based on the assumption that TCR potency is correlated to binding affinity [97]. However, high affinity is not necessarily correlated with TCR potency [98]. On one hand, high-affinity maturation may pose a risk of off-target toxicity. High-affinity-matured cancer-testis tumor antigen MAGE-A3-specific TCR had cross-reactivity with the cardiac antigen TITIN and caused fatal effects in the TCR-T cell therapy [ 99– 101]. On the other hand, high-affinity of TCR may also lead to severe on-target off-tumor effect. In this clinical trials, Of the 9 patients, 3 patients developed severe neurologic toxicity and 2 of them died due to necrotizing leukoencephalopathy with extensive white matter defects [102].
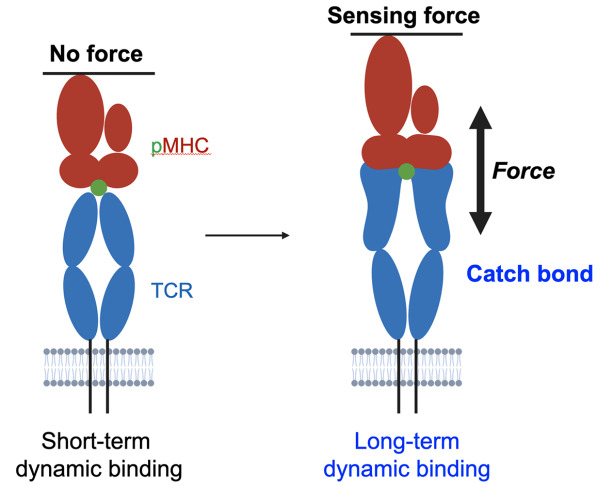
Recently, TCR has been identified as a mechano-sensor capable of detecting force transmitted from the internal cytoskeleton [ 103– 112]. Under force, TCR-T cells can form transient hydrogen bonds and/or salt bridges with pMHC to prolong the dynamic binding [ 103, 113]. The mechanism is also known as a catch bond. Moreover, the duration of the dynamic binding between TCR-T cells and pMHC is found to be associated with TCR signaling strength. Therefore, the potency of TCR can be enhanced through protein engineering to increase the duration of dynamic binding, independent of affinity maturation ( Figure 3). By employing dynamic binding engineering, individuals could acquire TCRs with high potency and low affinity [114]. The low-affinity phenotype could help prevent off-target toxicity toward healthy tissue. Thus, engineering catch bonds or prolonging dynamic binding could generate high-potency and low-toxicity TCRs [114]. The protein engineering of TCR based on the dynamic binding theory may represent a new and promising direction for TCR-T cell therapy in the future ( Figure 4).
Figure 3 .
TCR is a mechano-sensing receptor
In the absence of force, the TCR forms short-term dynamic bindings with pMHC. When a force of 10-15 pN is applied to the TCR, it can form transient hydrogen bonds and/or salt bridges with specific pMHC to create long-term dynamic binding, also known as catch bond. Adapted from [103].
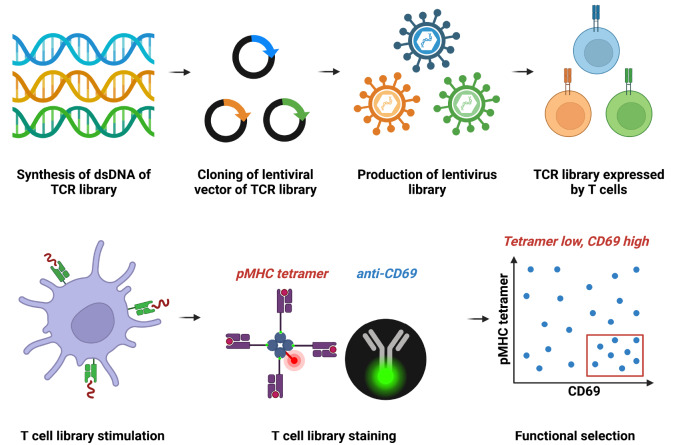
Figure 4 .
The workflow of dynamic binding engineering of TCR
The TCR library is designed and packaged as a lentiviral library for infecting SKW-3 T cells. The TCR-T cell library is activated when antigen-presenting cells are pulsed with specific antigenic peptides. The activated TCR-T cell library is stained with both pMHC tetramer and anti-CD69 antibody, each tagged with the appropriate fluorescent marker. High-potency and low-affinity TCR variants are enriched based on high levels of anti-CD69 staining and low levels of pMHC tetramer staining.
Strategies to Improve TCR-T Cell Therapy beyond TCR Engineering
The success of TCR-T cell therapy depends on tumor-specific antigens, potent TCRs, and the presence of healthy, long-lived T cells. In combination with other strategies, TCR-T cells could be further enhanced to improve metabolic fitness and memory phenotype percentage, while reducing exhaustion.
Gene editing is a powerful molecular tool for modifying TCR-T cells. A study utilizing zinc finger nuclease technology successfully transferred HLA-A*02:01-NY-ESO-1-specific TCR into the TCR α locus, resulting in high-level expression of TCR and a high percentage of stem memory and central memory phenotype [115]. CRISPR/Cas9, the most popular gene editing tool, has been extensively applied in TCR-T cell therapy. CRISPR-Cas9 gene editing could enhance the consistency of therapeutic efficacy. Endogenous TCR could be replaced by transgenic TCR to improve the correct pairing of TCR [ 116, 117]. For example, the simultaneous knockout of TRAC and TRBC using CRISPR-Cas9 and the concomitant overexpression of transgenic TCR could further enhance the expression and function of anti-tumor TCR, including structurally modified TCR [117]. CRISPR-Cas9 knockout of TCRβ and overexpression of αβ TCR or γδ TCR could enhance the eradication of tumor cells. NY-ESO-1-specific TCR can be integrated into the TCR locus using CRISPR/Cas9 [ 118, 119]. In a clinical trial, the knockout of TCRα ( TRAC) and TCRβ ( TRBC) could reduce TCR mispairing. Further removal of programmed cell death protein 1 (PD-1; PDCD1) could enhance anti-tumor immunity. The TCR-T cells edited with CRISPR-Cas9 could persist for 9 months [120].
TCR-T cell therapy could be enhanced by overexpressing additional T cell effector molecules, such as receptors, cytokines, and chemokines, to activate the TCR-T cells. Co-transduction of the CD3 complex with TCR could further enhance T cell functions by increasing sensitivity to low amounts of antigens and facilitating faster infiltration of tumors [ 121, 122]. The TNF and STAT3 signaling pathways are associated with the response to TCR-T cell therapy. Prefusion serum levels of IL-15, CX3CL1, and Flt-3L are higher in responding patients compared to nonresponders, suggesting that overexpression of TNF, pSTAT3, IL-15, CX3CL1, Flt-3L, and other cytokines may help boost TCR-T cell response [123]. Overexpression of c-Jun has been found to improve the proliferation and longevity of TCR-T cells. TCR-T cells overexpressing c-Jun and targeting hepatocellular carcinoma could improve the survival of mice with tumor growth [124]. The fusion of the TRAF-binding motif from 4-1BB ICD at the C-terminal of CD3z could enhance the persistence and expansion of TCR-T cells both in vitro and in vivo [125]. The induced expression of IL-12 in the PDCD1 locus could enhance the function of NY-ESO-1-specific TCR-T cells and eliminate established tumors in a xenograft mouse model, with greater TCR-T cell expansion potential [126]. Overexpression of IL-7 and CCL-19 could promote long-term memory formation in TCR-T cells, enhance the antitumor efficacy of TCR-T cells, and elicit a better response when combined with anti-PD-1 treatment [127]. The insertion of the intracellular domain of CD28 or 4-1BB into CD3z has been shown to enhance the proliferation and persistence of TCR-T cells, and improve the antitumor activity of TCR-T cells in a xenograft mouse model [128]. TCR-T cells secreting an anti-PD-1 antibody have been tested in a clinical trial aimed at treating EBV-related head and neck squamous cell carcinoma [129]. Applying a logic switch in TCR-T cell therapy may enhance the effectiveness and safety of T cell therapy. Multiple studies have reviewed the synergistic effects of combining CAR-T and TCR-T [130]. Both CAR and transgenic TCR could be co-expressed effectively in the same T cell. The dual function of CAR and TCR could prevent tumor escape and enhance signaling.
During the preparation of TCR-T cells, supplementing with other proteins or metabolites may reprogram the T cell phenotype and improve the anti-tumor efficacy. Metabolism-related pathways can be targeted to enhance the function and persistence of TCR-T cells. Aging and senescent TCR-T cells can be treated with spermidine to restore autophagic flux and reduce the expression of PD-1, TIM-3, and LAG-3. Spermidine treatment further enhances the functions of TCR-T cells and suppresses tumor growth in a xenograft mouse model [131]. Manipulating mTOR signaling could enhance the fitness and cytotoxicity of TCR-T cells [132]. TCR-T cells supplemented with a p38 inhibitor, IL-7, and IL-15, but not IL-2, could further increase T cell proliferation, leading to a largely naïve phenotype and sustained expression of effector molecules such as granzyme B and IFN-γ [133].
T cell-derived induced pluripotent stem cells can be utilized for the production of TCR-T cells. iPSC-derived T cells could enhance the off-the-shelf and universal application of TCR-T cell therapy. It could solve the problem of the short lifetime typically experienced by CTLs [134]. Melanoma-specific TCR or neoantigen-specific TCR has been introduced into T cell-derived iPSCs, and the production of TCR-iPSCs could be rapid and of high quality [ 135, 136]. γδTCR has also been used in the bispecific T cell engager (BiTE) format for solid tumors. The γδ TCR induced phosphoantigen-dependent killing of tumor cells by αβ TCR in a melanoma xenograft model [137].
TCR-T Cell Therapy in Clinical Trials
The published and ongoing TCR-T cell clinical trials have been extensively reviewed [21]. TCR-T cell therapies that have demonstrated clinical effectiveness in solid tumors have also been evaluated [138]. Due to the natural process of T cell signaling, most adverse effects in TCR-T cell therapies are manageable [49]. As of now, the FDA has not approved any TCR-T cell therapy. The first TCR-based therapy to reach the market, Tebentafusp, was approved by the FDA in early 2022 from Immunocore. Tebentafusp is a BiTE that targets HLA-A*02:01-gp100 and CD3 to connect tumor cells and T cells. Tebentafusp was tested in a phase 3 clinical trial for metastatic uveal melanoma and achieved a 73% survival rate at one year, compared to a 59% survival rate in the control group. Tebentafusp has achieved a milestone victory for TCR therapy against solid tumors [139]
Afami-cel from Adaptimmune is the leading TCR-T cell therapy for solid tumors. Afami-cel is targeting HLA-A*02:01-MAGE-A4. The phase 1 clinical trial included 38 patients with relapsed/refractory metastatic solid tumors, such as head and neck cancer, ovarian cancer, and synovial sarcoma. The overall response rate (ORR) for synovial sarcoma was 7/16 (44%) [140]. The phase 2 trial of Afami-cel has commenced, focusing on advanced/metastatic synovial sarcoma or myxoid/round cell liposarcoma. This two-cohort, open-label trial is named SPEARHEAD-1. The overall survival (OS) data for cohort 1 of advanced synovial sarcoma has been published. The median OS was 15.4 months. The probability of 24-month OS was 40%. Patients with advanced synovial sarcoma in the SPEARHEAD-1 trial have shown promising survival [141]. In relation to cytokine release syndrome (CRS), data from cohort 1 and cohort 2 indicated that 72.2% of patients with synovial sarcoma in the trial experienced CRS, with 50% experiencing Grade 1 CRS. A high level of serum IL-6 was associated with CRS, suggesting the use of tocilizumab to control the CRS [142]. Therefore, CRS was common in Afami-cel but most of patients are with moderate CRS. The SPEARHEAD-1 trial has been also shown to control metastases of refractory melanoma in combination with radiotherapy and checkpoint blocking [143]. The duration of OS was 18.4 weeks for the patient and the patient progressed at 28 weeks, but the tumor was controlled after radiotherapy and checkpoint inhibitors. More research should be done to explore the combination of TCR-T cell therapy with radiotherapy, chemotherapy, and ICB.
HLA-A*02:01-restricted NY-ESO-1 is the most commonly targeted antigen and has been tested in 38 cases [21]. One trial is the Lete-cel or IGNYTE-ESO trial, which aims to target NY-ESO-1 in synovial sarcoma and myxoid/round cell liposarcoma [144]. NY-ESO-1-targeting TCR-T cells have achieved a response rate of 55% in melanoma and 61% in synovial cell sarcoma [145]. A high-affinity TCR targeting NY-ESO-1 also achieved a 36% response rate [ 138, 146, 147]. C259 is an affinity-matured TCR that recognizes HLA-A*02:01-NY-ESO-1. In one synovial sarcoma trial, C259 TCR-T cell therapy achieved a 50% OS at 6 months. All patients had circulating C259 TCR-T cells for at least 6 months. The persistent cells mostly exhibit a central memory and stem-cell memory phenotype [146]. The detailed mechanism of response and resistance to C259 TCR-T cell therapy, tested in synovial sarcoma, showed that 1 out of 42 patients had a complete response and 14 out of 42 had a partial response. The expansion of TCR-T cells after infusion was correlated with the response. C259 TCR-T cells trafficked to the tumor microenvironment in some patients and were able to maintain effector functions [147].
TCR targeting the HLA-C*08:02-restricted KRAS-G12D neoantigen achieved a 72% overall partial response in a patient with progressive metastatic pancreatic cancer. The transgenic TCR-T cells constituted 2% of all the peripheral circulating T cells in the blood 6 months after the treatment [148]. TCR targeting HLA-A*02:01-restricted p53-R175H has resulted in a 55% reduction in tumor size in a patient with chemorefractory breast cancer. The response lasted 6 months, and the transgenic TCR-T cell therapy showed better infused cell immunophenotyping and extended persistence compared to tumor-infiltrating lymphocyte (TIL) therapy [149]. Two clinical trials have been conducted to target HPV16 E6 or E7 proteins [ 150, 151]. A phase I/II trial with 12 patients achieved objective tumor response in two patients and complete regression in one patient with lung metastasis [151]. The first-in-human, phase 1 clinical trial targeting HPV-16 E7 was conducted for HPV-associated epithelial cancers. Tumor regression was observed in 6 out of 12 patients with osteosarcoma. Some resistance occurred due to defects in antigen presentation pathways and interferon response pathways [150].
The Development of TCR-T Cell Therapy in China
The first phase 1 clinical trial of TCR-T cell therapy in China targeting HLA-A2-NY-ESO-1 in soft tissue sarcoma has been concluded. The TCR is affinity-matured and targets HLA-A*02:01-NY-ESO-1. None of the 12 patients experienced serious adverse effects. The OS rate is 41.7%, and the median progression-free survival (PFS) is 7.2 months [152]. In another clinical trial, patients who underwent hematopoietic stem cell transplantation and experienced CMV reactivation were treated by targeting CMV epitopes. The single-arm, open-label, phase I trial included 6 patients, and no severe adverse effects were observed. Four out of 6 patients received a response within 1 month. The transgenic TCR-T cells persist for 1 to 4 months. Therefore, CMV-specific TCR-T cell therapies are effective and safe treatments for CMV reactivation after hematopoietic stem cell transplantation [153]. Another CMV-specific TCR-T cell therapy in a phase I clinical trial conducted earlier involved 7 patients and resulted in mild CRS. Six patients achieved a complete response, and the transgenic TCR-T cells could be detected three months after the treatment [154]. As of January 2024, there are 68 TCR-T cell therapies in clinical trials in China. We hope to see more clinical trials of TCR-T cell therapies to be published in the near future.
Perspectives
Given that most tumor antigens are self-peptides, TCR engineering is almost inevitable in order to acquire high-potency TCRs. However, high-affinity maturation may result in off-target toxicity. A method like dynamic binding engineering, which can ensure both high efficacy and no toxicity, is the direction of future development. Dynamic binding engineering should be further optimized to ensure the availability of an easy-to-follow protocol for engineering any TCR without structural guidance.
In addition to TCR engineering, another challenge of TCR-T cell therapy is the cost. Autologous T cell engineering and adoptive cell transfer are challenging to optimize further and reduce the cost. While universal TCR-T cell therapy using donors’ T cells and off-the-shelf products may be one direction to consider, another important option is to perform T cell engineering in situ. TCR could be delivered by adenovirus, retrovirus, lentivirus, mRNA electroporation, and transposons [155]. An important area of focus would be exploring the possibility of injecting TCR mRNA in vivo to enable in situ engineering of T cells.
Early studies on TCR-T cells mainly focused on CD8+ T cells. Currently, the role of CD4+ T cells are also being considered. CD4+ T cells recognize antigenic peptides presented by MHC-II molecules. Unlike CD8+ T cells, which mainly exert cytotoxic effects, the function of CD4+ T cells is mainly to regulate the adaptive immune system, enhance the function of CD8+ T cells, and induce long-term memory of T cells [156]. Although most of the isolated TCRs with high affinity for tumor antigens are MHC-I restricted and function best in the presence of CD8 co-receptors, studies have shown that these TCRs can function in CD4+ T cells in the absence of CD8 co-receptors. Tumor-specific CD4+ T cells can be produced by MHC-I molecular restriction TCR, and the tumor-killing ability of antigen-specific CD8+ T cells can be enhanced. The reason for this phenomenon may be related to the secretion of multiple immune factors by CD4+ T cells [157]. In addition, the use of antigen-specific CD4+ T cells to treat tumor patients alone has also achieved good results [158]. These results suggest that attention should be paid to the role of CD4+ T cells in TCR-T cell therapy.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the start-up funding from the Center for Excellence in Molecular Cell Science at the Chinese Academy of Science (to X.Z.), the National Natural Science Foundation of China (No. 82350107), and the Shanghai Pujiang Program (No. 23PJ1414300).
References
- 1.Yang Y, Yang F, Huang Z, Li Y, Shi H, Sun Q, Ma Y, et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front Immunol. . 2023;14:1199173. doi: 10.3389/fimmu.2023.1199173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. . 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heintzman DR, Fisher EL, Rathmell JC. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol Immunol. . 2022;19:316–326. doi: 10.1038/s41423-021-00833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell. . 2017;31:311–325. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aktar N, Yueting C, Abbas M, Zafar H, Paiva-Santos AC, Zhang Q, Chen T, et al. Understanding of immune escape mechanisms and advances in cancer immunotherapy. J Oncol. . 2022;2022:1–13. doi: 10.1155/2022/8901326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Lu Q, Zhou H, Liu J, Nadorp B, Lasry A, Sun Z, et al. A membrane-associated MHC-I inhibitory axis for cancer immune evasion. Cell. 2023, 186: 3903-3920.e21 . [DOI] [PMC free article] [PubMed]
- 7.Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. . 2020;27:87–97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Fernández C, Saz A, Fornaguera C, Borrós S. Cancer immunotherapies revisited: state of the art of conventional treatments and next-generation nanomedicines. Cancer Gene Ther. . 2021;28:935–946. doi: 10.1038/s41417-021-00333-5. [DOI] [PubMed] [Google Scholar]
- 9.Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. . 2022;21:509–528. doi: 10.1038/s41573-021-00345-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. . 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. . 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. . 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieder T, Eigentler T, Brenner E, Röcken M. Immune checkpoint blockade therapy. J Allergy Clin Immunol. . 2018;142:1403–1414. doi: 10.1016/j.jaci.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. . 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. . 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Newitt VN. The incredible story of Emily Whitehead & CAR T-cell therapy. Oncology Times 2022, 44: 19–21
- 17.Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. . 2023;20:359–371. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol. . 2019;12:59. doi: 10.1186/s13045-019-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Ni Y, Liang X, Lin Y, An B, He X, Zhao X. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front Immunol. . 2022;13:915094. doi: 10.3389/fimmu.2022.915094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: state of the art and perspectives. Sci Adv. . 2023;9:eadf3700. doi: 10.1126/sciadv.adf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Li J, Zheng H, Yang S, Hua Y, Huang N, Kleeff J, et al. Adoptive cellular immunotherapy for solid neoplasms beyond CAR-T. Mol Cancer. . 2023;22:28. doi: 10.1186/s12943-023-01735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong L, Jiménez-Cortegana C, Tay AHM, Wickström S, Galluzzi L, Lundqvist A. NK cells and solid tumors: therapeutic potential and persisting obstacles. Mol Cancer. . 2022;21:206. doi: 10.1186/s12943-022-01672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat Commun. . 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Criscitiello MF, Ohta Y, Saltis M, McKinney EC, Flajnik MF. Evolutionarily conserved TCR binding sites, identification of T cells in primary lymphoid tissues, and surprising trans-rearrangements in nurse shark. J Immunol. . 2010;184:6950–6960. doi: 10.4049/jimmunol.0902774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauta PR, Nayak B, Das S. Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol Lett. . 2012;148:23–33. doi: 10.1016/j.imlet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Barnaba V. T cell memory in infection, cancer, and autoimmunity. Front Immunol. . 2022;12:811968. doi: 10.3389/fimmu.2021.811968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DembiĆ Z, Haas W, Weiss S, McCubrey J, Kiefer H, von Boehmer H, Steinmetz M. Transfer of specificity by murine α and β T-cell receptor genes. Nature. . 1986;320:232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Liu Z, Wei W, Li Y. TCR engineered T cells for solid tumor immunotherapy. Exp Hematol Oncol. . 2022;11:38. doi: 10.1186/s40164-022-00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. . 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. . 2024;21:47–66. doi: 10.1038/s41571-023-00832-4. [DOI] [PubMed] [Google Scholar]
- 31.Yan T, Zhu L, Chen J. Current advances and challenges in CAR T-cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp Hematol Oncol. . 2023;12:14. doi: 10.1186/s40164-023-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, Shomali N, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. . 2021;12:81. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022, 2: 1–9
- 34.Dong D, Zheng L, Lin J, Zhang B, Zhu Y, Li N, Xie S, et al. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. . 2019;573:546–552. doi: 10.1038/s41586-019-1537-0. [DOI] [PubMed] [Google Scholar]
- 35.Saotome K, Dudgeon D, Colotti K, Moore MJ, Jones J, Zhou Y, Rafique A, et al. Structural analysis of cancer-relevant TCR-CD3 and peptide-MHC complexes by cryoEM. Nat Commun. . 2023;14:2401. doi: 10.1038/s41467-023-37532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sušac L, Vuong MT, Thomas C, von Bülow S, O’Brien-Ball C, Santos AM, Fernandes RA, et al. Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC. Cell. . 2022;185:3201–3213.e19. doi: 10.1016/j.cell.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morath A, Schamel WW. αβ and γδ T cell receptors: similar but different. J Leukoc Biol. . 2020;107:1045–1055. doi: 10.1002/JLB.2MR1219-233R. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger AM, Stopfer LE, Ahn R, Sanders EA, Sandel DA, Freed-Pastor WA, Rideout Iii WM, et al. Deciphering the immunopeptidome in vivo reveals new tumour antigens. Nature. . 2022;607:149–155. doi: 10.1038/s41586-022-04839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adiko AC, Babdor J, Gutiérrez-Martínez E, Guermonprez P, Saveanu L. Intracellular transport routes for MHC I and their relevance for antigen cross-presentation. Front Immunol. . 2015;6:335. doi: 10.3389/fimmu.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Verteuil D, Granados DP, Thibault P, Perreault C. Origin and plasticity of MHC I-associated self peptides. Autoimmun Rev. . 2012;11:627–635. doi: 10.1016/j.autrev.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Apcher S, Prado Martins R, Fåhraeus R. The source of MHC class I presented peptides and its implications. Curr Opin Immunol. . 2016;40:117–122. doi: 10.1016/j.coi.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Neefjes J, Jongsma MLM, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. . 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Zhu Y, Li X, Gao W, Zhen Z, Dong D, Huang B, et al. Cholesterol inhibits TCR signaling by directly restricting TCR-CD3 core tunnel motility. Mol Cell. . 2022;82:1278–1287.e5. doi: 10.1016/j.molcel.2022.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Notti RQ, Yi F, Heissel S, Molina H, Klebanoff CA, Walz T. The resting state of the human T-cell receptor. bioRxiv. 2023, doi: 10.1101/2023.08.22.554360
- 45.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. . 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 46.Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, Lindquist JA, et al. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J Immunol. . 2007;179:4635–4644. doi: 10.4049/jimmunol.179.7.4635. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Guo X, Shi X, Li C, Wu W, Yan C, Wang H, et al. Ionic CD3-Lck interaction regulates the initiation of T-cell receptor signaling. Proc Natl Acad Sci USA. . 2017;114:E5891–E5899. doi: 10.1073/pnas.1701990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartl FA, Beck-Garcìa E, Woessner NM, Flachsmann LJ, Cárdenas RMHV, Brandl SM, Taromi S, et al. Noncanonical binding of Lck to CD3ε promotes TCR signaling and CAR function. Nat Immunol. . 2020;21:902–913. doi: 10.1038/s41590-020-0732-3. [DOI] [PubMed] [Google Scholar]
- 49.Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: current strategies, challenges, and prospects. Front Immunol. . 2022;13:835762. doi: 10.3389/fimmu.2022.835762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tawara I, Kageyama S, Miyahara Y, Fujiwara H, Nishida T, Akatsuka Y, Ikeda H, et al. Safety and persistence of WT1-specific T-cell receptor gene−transduced lymphocytes in patients with AML and MDS. Blood. . 2017;130:1985–1994. doi: 10.1182/blood-2017-06-791202. [DOI] [PubMed] [Google Scholar]
- 51.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. . 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, Voillet V, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. . 2019;25:1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Zhang T, Li P, Gai J, Chen S, Espinoza G, Kung HC, et al. Engineered TCR T-cell therapy targeting mass spectrometry-identified natural epitope in PDAC. Cancer Lett. . 2023;573:216366. doi: 10.1016/j.canlet.2023.216366. [DOI] [PubMed] [Google Scholar]
- 54.van Amerongen RA, Tuit S, Wouters AK, van de Meent M, Siekman SL, Meeuwsen MH, Wachsmann TLA, et al. PRAME and CTCFL-reactive TCRs for the treatment of ovarian cancer. Front Immunol. . 2023;14:1121973. doi: 10.3389/fimmu.2023.1121973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meeuwsen MH, Wouters AK, Wachsmann TLA, Hagedoorn RS, Kester MGD, Remst DFG, van der Steen DM, et al. Broadly applicable TCR-based therapy for multiple myeloma targeting the immunoglobulin J chain. J Hematol Oncol. . 2023;16:16. doi: 10.1186/s13045-023-01408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillard P, Köksal H, Maggadottir SM, Winge-Main A, Pollmann S, Menard M, Myhre MR, et al. Targeting telomerase with an HLA class II-restricted TCR for cancer immunotherapy. Mol Ther. . 2021;29:1199–1213. doi: 10.1016/j.ymthe.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoashi T, Watabe H, Muller J, Yamaguchi Y, Vieira WD, Hearing VJ. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J Biol Chem. . 2005;280:14006–14016. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. . 2009;35:193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med. . 2016;22:26–36. doi: 10.1038/nm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li Q, Liu H, et al. Identification of α‐fetoprotein‐specific T‐cell receptors for hepatocellular carcinoma immunotherapy. Hepatology. . 2018;68:574–589. doi: 10.1002/hep.29844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dossa RG, Cunningham T, Sommermeyer D, Medina-Rodriguez I, Biernacki MA, Foster K, Bleakley M. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood. . 2018;131:108–120. doi: 10.1182/blood-2017-07-791608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan C, Qu H, Wang X, Sobhani N, Wang L, Liu S, Xiong W, et al. Cancer/testis antigens: from serology to mRNA cancer vaccine. Semin Cancer Biol. . 2021;76:218–231. doi: 10.1016/j.semcancer.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. . 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Sun M, Wang J, Zeng B, Cao X, Han Y, Tan S, et al. Identification of NY-ESO-1157–165 specific murine T cell receptors with distinct recognition pattern for tumor immunotherapy. Front Immunol. . 2021;12:644520. doi: 10.3389/fimmu.2021.644520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Y, Tian X, Wang J, Qiao D, Liu X, Xiao L, Liang W, et al. Treatment of metastatic non‑small cell lung cancer with NY‑ESO‑1 specific TCR engineered‑T cells in a phase I clinical trial: a case report. Oncol Lett. . 2018;16:6998–7007. doi: 10.3892/ol.2018.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stadtmauer EA, Faitg TH, Lowther DE, Badros AZ, Chagin K, Dengel K, Iyengar M, et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. . 2019;3:2022–2034. doi: 10.1182/bloodadvances.2019000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davari K, Holland T, Prassmayer L, Longinotti G, Ganley KP, Pechilis LJ, Diaconu I, et al. Development of a CD8 co-receptor independent T-cell receptor specific for tumor-associated antigen MAGE-A4 for next generation T-cell-based immunotherapy. J Immunother Cancer. . 2021;9:e002035. doi: 10.1136/jitc-2020-002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kageyama S, Ikeda H, Miyahara Y, Imai N, Ishihara M, Saito K, Sugino S, et al. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced Lymphocytes in patients with recurrent esophageal cancer. Clin Cancer Res. . 2015;21:2268–2277. doi: 10.1158/1078-0432.CCR-14-1559. [DOI] [PubMed] [Google Scholar]
- 69.Tsukamoto M, Imai K, Ishimoto T, Komohara Y, Umezaki N, Yamao T, Kitano Y, et al. Abstract 3140: infiltrating macrophage-derived TNF-α promotes PD-L1 expression, leading to poor prognosis of patients with pancreatic cancer. Cancer Res. . 2018;78:3140. doi: 10.1158/1538-7445.AM2018-3140. [DOI] [Google Scholar]
- 70.Pearlman AH, Hwang MS, Konig MF, Hsiue EHC, Douglass J, DiNapoli SR, Mog BJ, et al. Targeting public neoantigens for cancer immunotherapy. Nat Cancer. . 2021;2:487–497. doi: 10.1038/s43018-021-00210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu D, Chen Y, Jiang M, Wang J, Li Y, Ma K, Sun W, et al. KRAS G12V neoantigen specific T cell receptor for adoptive T cell therapy against tumors. Nat Commun. . 2023;14:6389. doi: 10.1038/s41467-023-42010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannakopoulou E, Lehander M, Virding Culleton S, Yang W, Li Y, Karpanen T, Yoshizato T, et al. A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo . Nat Cancer. . 2023;4:1474–1490. doi: 10.1038/s43018-023-00642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okada M, Shimizu K, Nakazato H, Yamasaki S, Fujii S. Detection of mutant antigen-specific T cell receptors against multiple myeloma for T cell engineering. Mol Ther Methods Clin Dev. . 2023;29:541–555. doi: 10.1016/j.omtm.2023.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Çınar Ö, Brzezicha B, Grunert C, Kloetzel PM, Beier C, Peuker CA, Keller U, et al. High-affinity T-cell receptor specific for MyD88 L265P mutation for adoptive T-cell therapy of B-cell malignancies. J Immunother Cancer. . 2021;9:e002410. doi: 10.1136/jitc-2021-002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei T, Leisegang M, Xia M, Kiyotani K, Li N, Zeng C, Deng C, et al. Generation of neoantigen-specific T cells for adoptive cell transfer for treating head and neck squamous cell carcinoma. Oncoimmunology. . 2021;10:1929726. doi: 10.1080/2162402X.2021.1929726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biernacki MA, Foster KA, Woodward KB, Coon ME, Cummings C, Cunningham TM, Dossa RG, et al. CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia. J Clin Invest. . 2020;130:5127–5141. doi: 10.1172/JCI137723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. . 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 78.Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. . 2020;40:602–608. doi: 10.1080/01443615.2019.1634030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su ZY, Siak PY, Leong CO, Cheah SC. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front Microbiol. . 2023;14:1116143. doi: 10.3389/fmicb.2023.1116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertoletti A, Tan AT. HBV as a target for CAR or TCR-T cell therapy. Curr Opin Immunol. . 2020;66:35–41. doi: 10.1016/j.coi.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Bertoletti A, Brunetto M, Maini MK, Bonino F, Qasim W, Stauss H. T cell receptor-therapy in HBV-related hepatocellular carcinoma. Oncoimmunology. . 2015;4:e1008354. doi: 10.1080/2162402X.2015.1008354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Q, Tian Y, Li Y, Zhang W, Cai W, Liu Y, Ren Y, et al. In vivo therapeutic effects of affinity-improved-TCR engineered T-cells on HBV-related hepatocellular carcinoma . J Immunother Cancer. . 2020;8:e001748. doi: 10.1136/jitc-2020-001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, Wu J, et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. . 2021;15:1402–1412. doi: 10.1007/s12072-021-10250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin M, Bhakdi SC, Tan D, Lee JJX, Tai DWM, Pavesi A, Wai LE, et al. Lytic efficiency of immunosuppressive drug-resistant armoured T cells against circulating HBV-related HCC in whole blood. Immunother Adv. . 2023;3:ltad015. doi: 10.1093/immadv/ltad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong C, Huang L, Kou H, Wang C, Zeng X, Sun H, Liu S, et al. Identification of novel HLA-A*11:01-restricted HPV16 E6/E7 epitopes and T-cell receptors for HPV-related cancer immunotherapy. J Immunother Cancer. . 2022;10:e004790. doi: 10.1136/jitc-2022-004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang J, Xia M, Zhang L, Chen X, Zhao Y, Zeng C, Yang H, et al. Rapid generation of genetically engineered T cells for the treatment of virus‐related cancers. Cancer Sci. . 2022;113:3686–3697. doi: 10.1111/cas.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin BY, Campbell TE, Draper LM, Stevanović S, Weissbrich B, Yu Z, Restifo NP, et al. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight. . 2018;3:e99488. doi: 10.1172/jci.insight.99488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu G, Chen H, Cao X, Jia L, Rui W, Zheng H, Huang D, et al. Efficacy of pp65‐specific TCR‐T cell therapy in treating cytomegalovirus infection after hematopoietic stem cell transplantation. Am J Hematol. . 2022;97:1453–1463. doi: 10.1002/ajh.26708. [DOI] [PubMed] [Google Scholar]
- 89.Shen L, Yang J, Zuo C, Xu J, Ma L, He Q, Zhou X, et al. Circular mRNA-based TCR-T offers a safe and effective therapeutic strategy for treatment of cytomegalovirus infection. Mol Ther. . 2023;32:168–184. doi: 10.1016/j.ymthe.2023.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huisman W, Gille I, van der Maarel LE, Hageman L, Morton LT, de Jong RCM, Heemskerk MHM, et al. Identification of functional HLA-A*01:01–restricted Epstein-Barr latent membrane protein 2-specific T-cell receptors. J Infect Dis. . 2022;226:833–842. doi: 10.1093/infdis/jiaa512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang D, Duan Z, Yuan P, Ding C, Dai X, Chen G, Wu D. How does TCR-T cell therapy exhibit a superior anti-tumor efficacy. Biochem Biophys Res Commun. . 2023;687:149209. doi: 10.1016/j.bbrc.2023.149209. [DOI] [PubMed] [Google Scholar]
- 92.Kacen A, Javitt A, Kramer MP, Morgenstern D, Tsaban T, Shmueli MD, Teo GC, et al. Post-translational modifications reshape the antigenic landscape of the MHC I immunopeptidome in tumors. Nat Biotechnol. . 2023;41:239–251. doi: 10.1038/s41587-022-01464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. . 2001;22:443–449. doi: 10.1016/S1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 94.Camp F, Slansky J. Implications of antigen selection on T cell-based immunotherapy. Pharmaceuticals. . 2021;14:993. doi: 10.3390/ph14100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cherkasova E, Scrivani C, Doh S, Weisman Q, Takahashi Y, Harashima N, Yokoyama H, et al. Detection of an immunogenic HERV-E envelope with selective expression in clear cell kidney cancer. Cancer Res. . 2016;76:2177–2185. doi: 10.1158/0008-5472.CAN-15-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, Hanada K, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. . 2008;118:1584. doi: 10.1172/JCI34409C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. . 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 98.Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. . 2001;2:229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 99.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. . 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raman MCC, Rizkallah PJ, Simmons R, Donnellan Z, Dukes J, Bossi G, Le Provost GS, et al. Direct molecular mimicry enables off-target cardiovascular toxicity by an enhanced affinity TCR designed for cancer immunotherapy. Sci Rep. . 2016;6:18851. doi: 10.1038/srep18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, et al. Identification of a titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. . 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J ImmunoTher. . 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu B, Chen W, Evavold BD, Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. . 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu B, Kolawole EM, Evavold BD. Mechanobiology of T cell activation: to catch a bond. Annu Rev Cell Dev Biol. . 2021;37:65–87. doi: 10.1146/annurev-cellbio-120219-055100. [DOI] [PubMed] [Google Scholar]
- 105.Hong J, Ge C, Jothikumar P, Yuan Z, Liu B, Bai K, Li K, et al. A TCR mechanotransduction signaling loop induces negative selection in the thymus. Nat Immunol. . 2018;19:1379–1390. doi: 10.1038/s41590-018-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi HK, Cong P, Ge C, Natarajan A, Liu B, Zhang Y, Li K, et al. Catch bond models may explain how force amplifies TCR signaling and antigen discrimination. Nat Commun. . 2023;14:2616. doi: 10.1038/s41467-023-38267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu P, Zhang T, Liu B, Fei P, Cui L, Qin R, Zhu H, et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol Cell. . 2019;73:1015–1027.e7. doi: 10.1016/j.molcel.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feng Y, Reinherz EL, Lang MJ. αβ T cell receptor mechanosensing forces out serial engagement. Trends Immunol. . 2018;39:596–609. doi: 10.1016/j.it.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng Y, Brazin KN, Kobayashi E, Mallis RJ, Reinherz EL, Lang MJ. Mechanosensing drives acuity of αβ T-cell recognition. Proc Natl Acad Sci USA. . 2017;114:E8204–E8213. doi: 10.1073/pnas.1703559114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Judokusumo E, Tabdanov E, Kumari S, Dustin ML, Kam LC. Mechanosensing in T lymphocyte activation. Biophys J. . 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu C, Chen W, Lou J, Rittase W, Li K. Mechanosensing through immunoreceptors. Nat Immunol. . 2019;20:1269–1278. doi: 10.1038/s41590-019-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu C. Mechanosensing through immunoreceptors. Biophys J. 2022, 121: 4a . [DOI] [PMC free article] [PubMed]
- 113.Sibener LV, Fernandes RA, Kolawole EM, Carbone CB, Liu F, McAffee D, Birnbaum ME, et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell. . 2018;174:672–687.e27. doi: 10.1016/j.cell.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao X, Kolawole EM, Chan W, Feng Y, Yang X, Gee MH, Jude KM, et al. Tuning T cell receptor sensitivity through catch bond engineering. Science. . 2022;376:eabl5282. doi: 10.1126/science.abl5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mastaglio S, Genovese P, Magnani Z, Ruggiero E, Landoni E, Camisa B, Schiroli G, et al. NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood. . 2017;130:606–618. doi: 10.1182/blood-2016-08-732636. [DOI] [PubMed] [Google Scholar]
- 116.Legut M, Dolton G, Mian AA, Ottmann OG, Sewell AK. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood. . 2018;131:311–322. doi: 10.1182/blood-2017-05-787598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morton LT, Reijmers RM, Wouters AK, Kweekel C, Remst DFG, Pothast CR, Falkenburg JHF, et al. Simultaneous deletion of endogenous TCRαβ for TCR gene therapy creates an improved and safe cellular therapeutic. Mol Ther. . 2020;28:64–74. doi: 10.1016/j.ymthe.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, Hiatt J, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. . 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao Q, Dong X, Xu Q, Zhu L, Wang F, Hou Y, Chao C. Therapeutic potential of CRISPR/Cas9 gene editing in engineered T‐cell therapy. Cancer Med. . 2019;8:4254–4264. doi: 10.1002/cam4.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. . 2020;367:eaba7365. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmadi M, King JW, Xue SA, Voisine C, Holler A, Wright GP, Waxman J, et al. CD3 limits the efficacy of TCR gene therapy in vivo . Blood. . 2011;118:3528–3537. doi: 10.1182/blood-2011-04-346338. [DOI] [PubMed] [Google Scholar]
- 122.Watanabe K, Nishikawa H. Engineering strategies for broad application of TCR-T- and CAR-T-cell therapies. IntImmunol. 2021, 33: 551–562 . [DOI] [PubMed]
- 123.Nowicki TS, Peters CW, Quiros C, Kidd CK, Kawakami M, Klomhaus AM, Baselga-Carretero I, et al. Infusion product TNFα, Th2, and STAT3 activities are associated with clinical responses to transgenic T-cell receptor cell therapy. Cancer Immunol Res. 2023, 11: 1589–1597 . [DOI] [PMC free article] [PubMed]
- 124.Hussein MS, Li Q, Mao R, Peng Y, He Y. TCR T cells overexpressing c-Jun have better functionality with improved tumor infiltration and persistence in hepatocellular carcinoma. Front Immunol. . 2023;14:1114770. doi: 10.3389/fimmu.2023.1114770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lah S, Kim S, Kang I, Kim H, Hupperetz C, Jung H, Choi HR, et al. Engineering second-generation TCR-T cells by site-specific integration of TRAF-binding motifs into the CD247 locus . J Immunother Cancer. . 2023;11:e005519. doi: 10.1136/jitc-2022-005519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S, Park CI, Lee S, Choi HR, Kim CH. Reprogramming of IL-12 secretion in the PDCD1 locus improves the anti-tumor activity of NY-ESO-1 TCR-T cells. Front Immunol. . 2023;14:1062365. doi: 10.3389/fimmu.2023.1062365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tokunaga Y, Sasaki T, Goto S, Adachi K, Sakoda Y, Tamada K. Enhanced antitumor responses of tumor antigen-specific TCR T cells genetically engineered to produce IL7 and CCL19. Mol Cancer Ther. . 2022;21:138–148. doi: 10.1158/1535-7163.MCT-21-0400. [DOI] [PubMed] [Google Scholar]
- 128.Miyao K, Terakura S, Okuno S, Julamanee J, Watanabe K, Hamana H, Kishi H, et al. Introduction of genetically modified CD3ζ improves proliferation and persistence of antigen-specific CTLs. Cancer Immunol Res. . 2018;6:733–744. doi: 10.1158/2326-6066.CIR-17-0538. [DOI] [PubMed] [Google Scholar]
- 129.Grimes JM, Carvajal RD, Muranski P. Cellular therapy for the treatment of solid tumors. Transfus Apher Sci. . 2021;60:103056. doi: 10.1016/j.transci.2021.103056. [DOI] [PubMed] [Google Scholar]
- 130.Teppert K, Wang X, Anders K, Evaristo C, Lock D, Künkele A. Joining forces for cancer treatment: from “TCR versus CAR” to “TCR and CAR”. Int J Mol Sci. . 2022;23:14563. doi: 10.3390/ijms232314563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang C, Sun Y, Li S, Shen L, Teng X, Xiao Y, Zhou P, et al. Autophagic flux restoration of senescent T cells improves antitumor activity of TCR engineered T cells. Clin Trans Imm. . 2022;11:e1419. doi: 10.1002/cti2.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mao W. Overcoming current challenges to T-cell receptor therapy via metabolic targeting to increase antitumor efficacy, durability, and tolerability. Front Immunol. . 2022;13:1056622. doi: 10.3389/fimmu.2022.1056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen S, Zhang J, Shen M, Han X, Li S, Hu C, Wang W, et al. p38 inhibition enhances TCR-T cell function and antagonizes the immunosuppressive activity of TGF-β. Int Immunopharmacol. . 2021;98:107848. doi: 10.1016/j.intimp.2021.107848. [DOI] [PubMed] [Google Scholar]
- 134.Lu F, Ma XJN, Jin WL, Luo Y, Li X. Neoantigen specific T cells derived from T cell-derived induced pluripotent stem cells for the treatment of hepatocellular carcinoma: potential and challenges. Front Immunol. . 2021;12:690565. doi: 10.3389/fimmu.2021.690565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kawamoto H, Masuda K, Nagano S, Maeda T. Cloning and expansion of antigen-specific T cells using iPS cell technology: development of “off-the-shelf” T cells for the use in allogeneic transfusion settings. Int J Hematol. . 2018;107:271–277. doi: 10.1007/s12185-018-2399-1. [DOI] [PubMed] [Google Scholar]
- 136.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Cell. . 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 137.van Diest E, Hernández López P, Meringa AD, Vyborova A, Karaiskaki F, Heijhuurs S, Gumathi Bormin J, et al. Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J Immunother Cancer. . 2021;9:e003850. doi: 10.1136/jitc-2021-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Norberg SM, Hinrichs CS. Engineered T cell therapy for viral and non-viral epithelial cancers. Cancer Cell. . 2023;41:58–69. doi: 10.1016/j.ccell.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, Sullivan RJ, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. . 2021;385:1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 140.Hong DS, Van Tine BA, Biswas S, McAlpine C, Johnson ML, Olszanski AJ, Clarke JM, et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial. Nat Med. . 2023;29:104–114. doi: 10.1038/s41591-022-02128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Tine BA, Ganjoo KN, Blay JY, Valverde C, Araujo DM, Abdul Razak AR, Cesne AL, et al. The SPEARHEAD-1 trial of afamitresgene autoleucel (afami-cel [formerly ADP-A2M4]): analysis of overall survival in advanced synovial sarcoma. J Clin Oncol. 2023, 41: 11563
- 142.D’Angelo SP, Ganjoo KN, Blay JY, Valverde CM, Araujo DM, Abdul Razak AR, Le Cesne A, et al. The SPEARHEAD-1 trial of afamitresgene autoleucel (afami-cel [formerly ADP-A2M4]): pooled analysis of cytokine release syndrome across cohorts in patients with advanced synovial sarcoma. J Clin Oncol. 2023, 41: e14531
- 143.He K, Hong DS, Ke D, Kebriaei P, Wang T, Danesi H, Bertolet G, et al. Durable control of metastases in an HLA-A2+ patient with refractory melanoma after low-dose radiotherapy in combination with MAGE-A4 T cell therapy: a case report. Melanoma Res. . 2023;33:332–337. doi: 10.1097/CMR.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.D’Angelo SP, Noujaim JC, Thistlethwaite F, Razak ARA, Stacchiotti S, Chow WA, Haanen JBAG, et al. Demetri, IGNYTE-ESO: a master protocol to assess safety and activity of letetresgene autoleucel (lete-celGSK3377794) in HLA-A*02+ patients with synovial sarcoma or myxoid/round cell liposarcoma (Substudies 1 and 2). J Clin Oncol 2021, 39: TPS11582
- 145.Robbins PF, Kassim SH, Tran TLN, Crystal JS, Morgan RA, Feldman SA, Yang JC, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1–reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. . 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D′Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 c259T cells in synovial sarcoma. Cancer Discov. . 2018;8:944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ramachandran I, Lowther DE, Dryer-Minnerly R, Wang R, Fayngerts S, Nunez D, Betts G, et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J Immunother Cancer. . 2019;7:276. doi: 10.1186/s40425-019-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP, Leung A, et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med. . 2022;386:2112–2119. doi: 10.1056/NEJMoa2119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim SP, Vale NR, Zacharakis N, Krishna S, Yu Z, Gasmi B, Gartner JJ, et al. Adoptive cellular therapy with autologous tumor-infiltrating lymphocytes and T-cell receptor–engineered T cells targeting common p53 neoantigens in human solid tumors. Cancer Immunol Res. . 2022;10:932–946. doi: 10.1158/2326-6066.CIR-22-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, Warner AC, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. . 2021;27:419–425. doi: 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Doran SL, Stevanović S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, Faquin WC, et al. T-cell receptor gene therapy for human papillomavirus–associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. . 2019;37:2759–2768. doi: 10.1200/JCO.18.02424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pan Q, Weng D, Liu J, Han Z, Ou Y, Xu B, Peng R, et al. Phase 1 clinical trial to assess safety and efficacy of NY-ESO-1-specific TCR T cells in HLA-A*02:01 patients with advanced soft tissue sarcoma. Cell Rep Med. . 2023;4:101133. doi: 10.1016/j.xcrm.2023.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ma C, Chen P, Du J, Wang L, Lu N, Sun J, Qilong X, et al. Adoptive transfer of CMV-specific TCR-T cells for the treatment of CMV infection after haploidentical hematopoietic stem cell transplantation. J Immunother Cancer. . 2024;12:e007735. doi: 10.1136/jitc-2023-007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao X, Liu G, Zhang J, Zhao Y, Chen H, Zheng H, Rui W, et al. A novel CMV-specific TCR-T cell therapy is effective and safe for refractory CMV infection after allogeneic hematopoietic stem cell transplantation. Blood. . 2021;138:3848. doi: 10.1182/blood-2021-146446. [DOI] [Google Scholar]
- 155.Manfredi F, Cianciotti BC, Potenza A, Tassi E, Noviello M, Biondi A, Ciceri F, et al. TCR redirected T cells for cancer treatment: achievements, hurdles, and goals. Front Immunol. . 2020;11:1689. doi: 10.3389/fimmu.2020.01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018, 10: a030338 . [DOI] [PMC free article] [PubMed]
- 157.Liang Y, Xu Q, Liu S, Li J, Wang F, Li Z, Liao L, et al. Single-cell transcriptomics reveals killing mechanisms of antitumor cytotoxic CD4+ TCR-T cells. Front Immunol. . 2022;13:939940. doi: 10.3389/fimmu.2022.939940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu YC, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, Yao X, et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II–restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. . 2017;35:3322–3329. doi: 10.1200/JCO.2017.74.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]