Abstract
Viral infections which induce strong T-cell responses are often characterized by a period of transient immunodeficiency associated with the failure of host T cells to proliferate in response to mitogens or to mount memory recall responses to other antigens. During acute infections, most of the activated, proliferating virus-specific T cells are sensitized to undergo apoptosis on strong T-cell receptor (TCR) stimulation, but it has not been known why memory T cells not specific for the virus fail to proliferate on exposure to their cognate antigen. Using a lymphocytic choriomeningitis virus (LCMV) infection model in which LCMV-immune Thy 1.1+ splenocytes are adoptively transferred into Thy 1.2+ LCMV carrier mice, we demonstrate here that T cells clearly defined as not specific for the virus are sensitized to undergo activation-induced cell death on TCR stimulation in vitro. This bystander sensitization was in part dependent on the expression of Fas ligand (FasL) on the activated virus-specific cells and gamma interferon (IFN-γ) receptor expression on the bystander T cells. We propose that FasL from highly activated antiviral T cells may sensitize IFN-γ-conditioned T cells not specific for the virus to undergo apoptosis rather than to proliferate on encountering antigen. This may in part explain the failure of memory T cells to respond to recall antigens during acute and persistent viral infections.
In 1908 von Pirquet reported that individuals acutely infected with measles virus failed to mount a delayed-type hypersensitivity reaction to tuberculin even though they had been previously immunized (35). After resolution of the infection, responsiveness to this memory recall antigen returned. Similar types of transient immune deficiencies have subsequently been reported during many viral infections, including those caused by Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus (HIV) in humans and by lymphocytic choriomeningitis virus (LCMV) in the mouse model (7, 21, 25, 27). In addition to the failure to respond to memory recall antigens, T cells from infected individuals fail to proliferate in response to signals delivered via the T-cell receptor (TCR) by mitogenic lectins, superantigens, or anti-CD3 antibody. In several infections, this transient immune deficiency has been correlated with the induction of apoptosis in the highly activated T cells, which express CD95 (Fas) and CD95 ligand (FasL) (1, 7, 21, 25).
Apoptosis by activation-induced cell death (AICD) is now a well-characterized feature of activated T-cell populations that become strongly signaled through their TCR. The interaction of Fas with FasL after TCR stimulation causes Fas to associate with the intracytoplasmic Fas-activated death domain, which initiates the apoptotic pathway (16). Recent work has shown that most of the T cells proliferating in response to viral infections are highly activated virus-specific cells, which would be expected to eventually undergo AICD on appropriate signalling (9, 23). This would explain the failure of the virus-induced T-cell population to respond to nonspecific T-cell mitogens, but this observation does not explain why memory T cells should have deficient responses to their specific cognate antigens. These deficient memory responses might be a consequence of a dilution of memory cells by the proliferating virus-specific cells, of a redistribution of memory T cells such that they are no longer present in the peripheral blood or spleen populations used for testing their function, or of the action of immunosuppressive factors which inhibit their ability to function. Alternatively, as shown here, these non-virus-specific T cells may become sensitized in a bystander fashion to undergo AICD on receptor stimulation.
The distinctions between virus-specific and nonvirus-specific T cells can be blurred because many memory T cells can be stimulated during viral infections by cross-reactive interactions (29). We have therefore developed models to clearly identify, in the same systems, T cells that are specific and those that are not specific for viral antigens (38). In this way, one can ask whether bystander events occurring as a consequence of the T-cell response to viral infections alter the activity of T cells whose receptors are not being triggered by the virus. We have used the highly defined LCMV infection of the mouse to explore these processes. The acute LCMV infection is associated with a marked expansion of the CD8 T-cell population, and most of the activated T cells during the LCMV infection are virus specific (23, 25, 29, 38). Between 6 and 12 days postinfection, the host undergoes a transient but severe state of immune deficiency characterized by impaired proliferative response in vitro and by the induction of apoptosis in those T cells (25). This immune deficiency, however, is not found in mice harboring persistent LCMV infection as a consequence of congenital infection. Those persistently infected “LCMV carrier” mice clonally delete their LCMV-specific T cells but can make normal T-cell responses to other antigens (11, 14). If T cells from Thy 1.1+ LCMV-immune mice are transferred into the tolerant Thy 1.2+ carrier mice, they proliferate in response to the viral antigens present, but the host cells, which cannot respond to LCMV antigens, do not increase in number (11, 12, 38). This model can therefore be used to ask how a vigorous antiviral T-cell response can affect the biology of T cells defined as being virus nonspecific but nevertheless coexisting in this milieu.
Here we show that a potent antiviral T-cell response causes T cells not participating in the immune response to undergo AICD rather than to proliferate on TCR stimulation. This bystander sensitization to AICD may explain the immunosuppression that follows infections with many viruses.
MATERIALS AND METHODS
Mice.
Conventionally housed male and female C57BL/6, B6Smn.C3H-Faslgld, B6.MLR-Faslpr, and B6.PLThy1a/C mice were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 HY-transgenic mice, whose transgenic TCR recognizes the male-specific antigen HY in the context of H2-Db, were a gift from B. J. Fowlkes (National Institutes of Health, Bethesda, Md.) (26). 129 SV/EV mice, homozygous for a targeted mutation that disrupts the murine gamma interferon (IFN-γ) gene, were a gift from M. Aguet (University of Zurich, Zurich, Switzerland) (22). HY transgenic and IFN-γ receptor knockout mice strains were bred and maintained under microisolator conditions in the University of Massachusetts Medical School Department of Animal Medicine. Persistently infected LCMV carrier C57BL/6, B6.MLR-Faslpr, and IFN-γ receptor knockout mice were bred in the Biocontainment Suite at the University of Massachusetts Medical School Department of Animal Medicine. All mice examined were major histocompatibility complex compatible, and except for the IFN-γ receptor knockout 129/SV mice, all were congenic strains on the C57BL/6 background.
Virus.
The LCMV Armstrong strain was propagated in baby hamster kidney (BHK21) cells. Unless otherwise noted, mice were injected intraperitoneally i.p. with 4 × 104 PFU of virus.
Antibody and complement depletion.
Spleens were isolated and ground between the frosted ends of two glass microscope slides. The cell suspension was then passed through a fine nylon mesh to obtain a single-cell suspension. Erythrocytes were lysed by briefly suspending the spleen cell pellet in a 0.84% NH4Cl solution. To enrich for T cells prior to in vitro culture, granulocytes and B cells were eliminated by treatment with antibody J11d (2) and complement. After counting, 5 × 107 spleen leukocytes were treated with 50 μl of J11d (ammonium sulfate-precipitated ascites) for 45 min at 4°C and then washed twice with complete RPMI 1640. The cells were incubated at 37°C for 45 min in a humidified 5% CO2 incubator with 2 ml of a 1:10 dilution of rabbit C′ (Pel-Freez Clinical Systems, Brown Deer, Wis.). They were washed twice in complete RPMI 1640 and recounted. In vitro T-cell depletions were performed as above, except that 25 μl of either anti-Thy1.1 or anti-Thy1.2 antibody (Pharmingen, San Diego, Calif.) was used instead of J11d.
Adoptive transfer of immune spleen cells to LCMV carrier mice.
A total of 2 × 107 to 4 × 107 spleen leukocytes from LCMV-immune B6.PL Thy1a/Cy mice (Thy 1.1+) were injected intravenously via the retro-orbital sinus into C57BL/6 LCMV carrier mice (Thy 1.2+). These unfractionated donor cell populations consisted of about 10% CD8 T cells and 20% CD4 T cells. At 6 days after adoptive transfer, spleen cell populations were examined for reconstitution with donor Thy 1.1+ T cells. As shown previously (38), few (<1%) donor T cells were observed in spleen lymphocytes of recipient mice injected with LCMV-naive donor T cells whereas about 5% (with some variation) of lymphocytes were donor T cells in mice inoculated with LCMV-immune Thy 1.1+ cells. By day 6, the great majority (>70%) of donor T cells in the recipients were CD8+ cells and more than 80% of the donor CD8 and CD4 T cells expressed high levels of the activation/memory marker CD44. In the host Thy 1.2+ T-cell populations from mice reconstituted with naive donor cells, about one-third of the host CD8 T cells were defined as CD44+ and the proportion of CD44+ host CD8 T cells declined slightly after the adoptive transfer (38). Similar trends were seen with the CD4 T-cell population (data not shown). Spleens from recipient mice were harvested at 6 days posttransfer, subjected to J11d antibody and complement depletion, and then stimulated with plate-bound anti-CD3 (see above). Prior to culture, the T-cell-enriched populations originating from Thy 1.2+ LCMV carrier mice reconstituted with naive donor cells were 44 to 55% Thy 1.2+; cultures originating from Thy 1.2+ LCMV carrier mice reconstituted with Thy 1.1+ LCMV-immune donor cells ranged from 33 to 42% host Thy 1.2+ cells and 4 to 13% donor Thy1.1+ cells.
Flow cytometry.
After being stained, cells were analyzed or sorted by flow cytometry using a FACSTAR apparatus (Becton-Dickinson, San Jose, Calif.). Data analysis was performed using the program Cell Quest (Becton-Dickinson). Flow cytometry for the expression of Fas was done using phycoerythrin-conjugated hamster anti-mouse Fas immunoglobulin G monoclonal antibody clone Jo2, purchased from Pharmingen.
Detection of transgenic T cells.
Spleen cells from HY-TCR-transgenic mice were prepared as described above, and 106 cells were incubated with normal rat serum for 20 min at 4°C. The cells were then incubated with the monoclonal antibody T3.70 (a kind gift from Hung-sia Teh, University of British Columbia, Vancouver, Canada) for 30 min on ice (26). The cells were then washed twice and blocked again with normal rat serum at 4°C for 20 min. They were stained with anti-CD8-phycoerythrin (Gibco/BRL, Grand Island, N.Y.) and rabbit anti-mouse immunoglobulin G1-fluorescein isothiocyanate (Cappel, Aurora, Ohio) for 25 min at 4°C. They were then washed twice and fixed with 2% paraformaldehyde.
TdT-mediated dUTP biotin nick end labeling (TUNEL) flow cytometry.
Forty- eight-well plates (Costar) were coated overnight at 4°C with a 1:25 dilution of anti-CD3 (Pharmingen). The next day, the plates were washed twice with phosphate-buffered saline (PBS), and 106 T-cell-enriched spleen cells were added to each well. After 2 days of in vitro culture in the presence of plate-bound anti-CD3, apoptotic cells were detected by flow cytometry using the method of Ralph Budd (University of Vermont College of Medicine) adapted from reference 34. Surface staining of approximately 106 lymphocytes was performed as described above, and the cells were then fixed with 1% paraformaldehyde for 15 min at 4°C. After being washed with PBS, the cells were permeabilized by treatment with 70% ethanol for 15 min at 4°C. The permeabilized cells were then washed twice with cold PBS and suspended for 1 h at 37°C in reaction buffer containing 1× terminal deoxyribonucleotidyltransferase (TdT) buffer, 2.5 mM cobalt chloride, 10 U of TdT, and 0.5 nmol of biotin-dUTP (Boehringer-Mannheim, Indianapolis, Ind.). After being washed twice, the cells were stained with streptavidin-TRICOLOR (Caltag, San Francisco, Calif.) for 22 min at 4°C and fixed in 1% paraformaldehyde.
Proliferation assay.
After 2 days of in vitro culture with plate-bound anti-CD3, 2 × 105 cells in 200 μl of RPMI-MLC medium were placed in 96-well U-bottom microtiter plates (Falcon). Each well was then pulsed with 50 μl of complete RPMI medium containing 5 × 10−5 M 2-mercaptoethanol and 1 μCi of [3H]thymidine (Amersham) and incubated for 6 h at 37°C in a humidified 5% CO2 incubator. The plates were then harvested onto fiberglass filter mats, and [3H]thymidine incorporation was determined using a Betaplate scintillation counter (Wallac, Gaithersburg, Md.).
RESULTS
Bystander sensitization of T cells to AICD during an antiviral T-cell response.
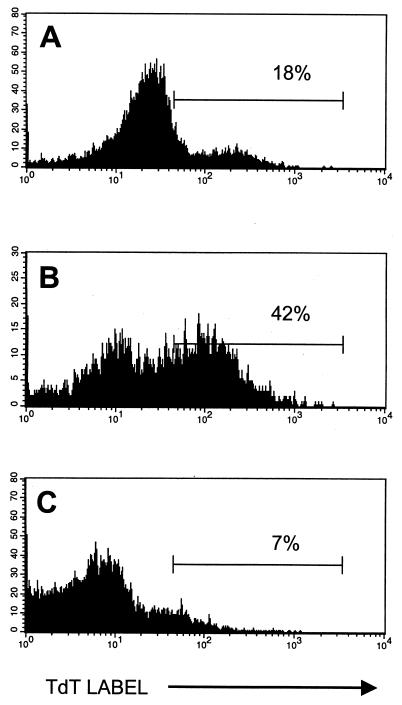
Transfer of Thy 1.1+ T cells from LCMV-immune mice into Thy 1.2+ persistently infected LCMV carrier mice results in a proliferation of donor cells and a partial and sometimes complete clearance of infectious virus (11, 12, 38). We have shown that the potent antiviral T-cell response mounted by the LCMV-specific memory T cells after transfer did not result in any detectable increase in the number, size, or activation antigen status of the LCMV-tolerant host CD8 T cells (38). Here we questioned whether, on TCR stimulation, the proliferation of those host T cells was impaired and whether they underwent AICD. Thus, after adoptive transfer of Thy 1.1+ LCMV-immune spleen cells into Thy 1.2+ LCMV carrier mice, splenocytes were harvested, enriched for T cells, and cultured with plate-bound anti-CD3, and after 2 days TUNEL flow cytometry was performed. As shown in Fig. 1, anti-CD3 treatment resulted in the apoptosis of CD8+ T cells from mice 8 days after LCMV infection, consistent with the earlier report that these highly activated T cells are very susceptible to AICD (25). Here we use the TUNEL stain exclusively as an indicator of apoptosis, but we have documented apoptosis in anti-CD3-stimulated day 8 T-cell populations previously by several other techniques, including DNA ladder analysis and propidium iodide/light scatter/forward-scatter flow cytometric analyses (24, 25). Conversely, CD8+ cells from LCMV carrier mice underwent very little apoptosis during the 2 days in culture with anti-CD3; T cells from uninfected control mice behaved similarly to those from carrier mice (data not shown but documented by us in reference 19). This result is consistent with the fact that naive or resting T cells will proliferate on TCR cross-linking. In LCMV carrier mice adoptively reconstituted with LCMV-immune spleen cells, the activated donor CD8+ T cells, much like T cells from acutely infected mice, underwent apoptotic cell death after exposure to anti-CD3. In contrast to the CD8+ T cells taken from the unmanipulated LCMV carrier mice, the CD8+ host T cells present in the adoptively reconstituted mice also underwent apoptotic cell death after TCR stimulation. This result, which was obtained in over 10 separate experiments, strongly supports the hypothesis that CD8+ T cells not specific for the virus are sensitized during a potent antiviral T-cell response to undergo AICD after TCR stimulation.
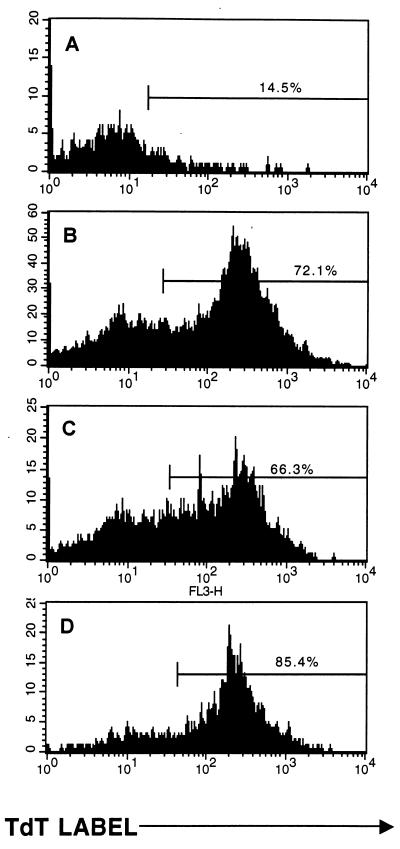
FIG. 1.
CD8+ host cells in LCMV carrier C57BL/6 mice become sensitized to undergo apoptosis after adoptive transfer of LCMV-immune C57BL/6 mouse spleen cells. Splenocytes enriched for T cells by treatment with J11d monoclonal antibody and complement were cultured with plate-bound anti-CD3 for 48 h and tested for apoptosis by the TUNEL assay. T-cell preparations were from untreated LCMV carrier mice, which are similar to uninfected control mice, as shown in reference 19 (A), or from normal mice 8 days after acute LCMV infection (B). In panels C and D, T-cell-enriched splenocytes were taken from Thy 1.2+ LCMV-carrier mice 6 days after transfer of 4 × 107 LCMV-immune Thy 1.1+ spleen cells. By gating on host Thy 1.2+ (C) or donor Thy 1.1+ (D) cells, apoptosis in each population was determined by TUNEL stain.
To determine whether these host CD8+ T cells were capable of proliferating in response to anti-CD3, [3H]thymidine incorporation assays were performed. The results of a representative experiment are shown in Table 1. Spleen cells from both C57BL/6 mice and LCMV carrier mice proliferated well after culture with anti-CD3. However, splenocytes taken from mice on day 8 after LCMV infection or from LCMV carrier mice 6 days after adoptive transfer of LCMV-immune spleen cells incorporated very little [3H]thymidine, consistent with the fact that these cells were undergoing apoptosis rather than proliferating. In 10 individual experiments, the reduction in [3H]thymidine incorporation between LCMV carrier mice (either unreconstituted or reconstituted with naive cells) and immune-cell adoptively reconstituted LCMV carrier mice ranged from a high of 242-fold to a low of 3-fold, with an average of 60-fold. Because most of the T cells put into culture were of host origin, this suggests that there was a marked inhibition of proliferation of the host T cells.
TABLE 1.
Inhibition of proliferation of T cells from LCMV carrier mice adoptively reconstituted with LCMV-immune splenocytes
| Mouse | Proliferationa |
|---|---|
| C57BL/6 | 200 ± 10 |
| C57BL/6 8 days after LCMV infection | 16 ± 0.5 |
| LCMV carrier | 164 ± 18 |
| Adoptively reconstituted carrier | 1.0 ± 0.0 |
Numbers represent 103 mean cpm ± standard deviation after a 6-h pulse with 1 μCi of [3H]thymidine after 2 days of culture with plate-bound anti-CD3 antibody.
To determine which cell populations in the transferred donor leukocyte preparations were required to induce bystander sensitization to AICD, LCMV carrier mice were reconstituted with LCMV-immune spleen cells, either treated in vitro with antibody and complement to deplete Thy 1+ T cells or treated with complement alone. Spleen cells from unmanipulated LCMV carrier mice survived (Fig. 2A) and proliferated (61,917 ± 14,639 cpm), whereas splenocytes taken from carrier mice reconstituted with complement-treated LCMV-immune spleen cells underwent significantly more apoptosis (Fig. 2B) and proliferated less than did unmanipulated carriers (30,993 ± 2,379 cpm). In contrast, spleen cells taken from mice reconstituted with spleen cells treated with anti-Thy1.2 plus complement survived (Fig. 2C) and incorporated nearly as much [3H]thymidine (53,864 ± 8,939 cpm) as did the unmanipulated carriers. This result suggests that the donor T cells are required to sensitize the host T cells to AICD.
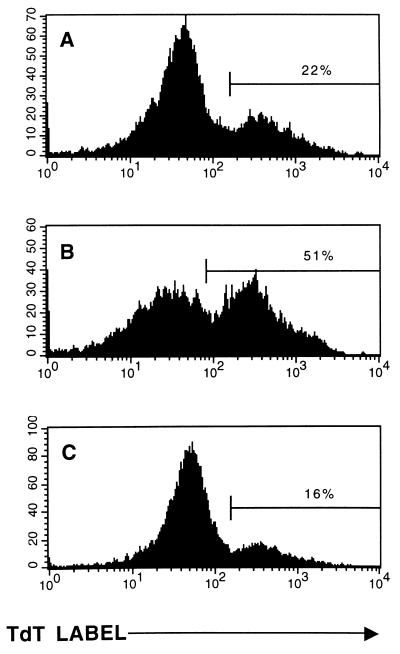
FIG. 2.
Donor T cells are required to induce susceptibility to AICD. TUNEL flow assays were performed on splenocytes from unmanipulated Thy 1.2+ LCMV carrier C57BL/6 mice (A), LCMV carrier C57BL/6 mice adoptively reconstituted with 2.5 × 107 complement-treated LCMV-immune Thy 1.2+ C57BL/6 mouse spleen cells (B), or LCMV carrier C57BL/6 mice adoptively reconstituted with 2.5 × 107 LCMV-immune spleen cells depleted of T cells by treatment with anti-Thy 1.2 antibody and complement (C). Histograms represent the incorporation of the TdT label on the gated T-cell populations.
Naive splenocytes transferred into LCMV carrier mice do not substantially expand in number or effect the clearance of virus (11, 12, 38). Splenocytes from LCMV carrier mice reconstituted with 3 × 107 LCMV-naive spleen cells proliferated to the same extent as did splenocytes from unmanipulated LCMV carrier mice and displayed no evidence of sensitization to AICD after anti-CD3 stimulation (data not shown). Thus, LCMV carrier mice and LCMV carrier mice that received naive spleen cells were used interchangeably as controls. It should also be noted that after the 2 days in culture with anti-CD3, the level of CD8 expression was often dim, making it sometimes difficult to determine which cells were CD8dim versus CD8−. As a result, host and donor cell populations were distinguished based on expression of the strongly staining Thy 1 allotypic markers in most of the following experiments.
Exogenous accessory cells cannot rescue host T cells from AICD.
The proliferation of T cells in vitro often requires the presence of accessory cells such as antigen-presenting cells (APCs) in the culture (9). It has also been suggested that the inability of T cells to proliferate during acute viral infections may be the result of an APC defect (5, 6, 10, 27). To determine if bystander sensitization to AICD was the result of the absence of some vital APC-derived positive signal that was required for the proliferation of host T cells in vitro, exogenous APCs were added to culture wells containing splenocytes taken from the adoptively reconstituted LCMV carrier mice. These exogenous APCs were T-depleted spleen cells from uninfected C57BL/6 mice. T cells from unmanipulated LCMV carrier mice proliferated vigorously (174,583 ± 36,381 cpm) and did not undergo apoptosis (Fig. 3A), whereas spleen cells taken from LCMV carrier mice previously reconstituted with Thy 1.1+ LCMV-immune splenocytes underwent apoptosis (Fig. 3B) and proliferated very poorly (2,224 ± 91 cpm). The addition of T-depleted normal spleen cells as accessory cells failed to rescue the host T cells from apoptotic death (Fig. 3C) or to substantially increase their [H3]thymidine incorporation (4,490 ± 261 cpm). This result was seen in two other experiments and demonstrates that the lack of a vital APC-derived signal could not explain the observed immune suppression after in vitro stimulation with anti-CD3.
FIG. 3.
Exogenous APCs do not rescue host T cells from AICD. TUNEL flow assays were performed on splenocytes from unmanipulated LCMV carrier C57BL/6 mice (A), LCMV carrier C57BL/6 mice adoptively reconstituted with 3 × 107 LCMV-immune spleen cells (B), or LCMV carrier C57BL/6 mice adoptively reconstituted with 3 × 107 LCMV-immune Thy 1.1+ spleen cells and then cultured 1:1 in the presence of T-cell-depleted spleen cells from uninfected C57BL/6 mice (C). Histograms represent the incorporation of the TdT label in the host Thy 1.2+ cells.
Activated accessory cells do not suppress proliferative T-cell responses under the test conditions.
It has been reported that in some systems, adherent accessory cells from virus-infected hosts may inhibit the proliferation of T cells (13), and while we have found similar types of inhibition under certain high-cell-density culture conditions, significant levels of inhibition did not occur under the culture conditions of this study. Table 2 shows that mixtures of unfractionated or T-cell-depleted spleen leukocytes from adoptively reconstituted LCMV carrier mice with control leukocytes or with leukocytes from unreconstituted LCMV carrier mice caused very low or modest levels of inhibition of proliferation, other than that accounted for by the 1:2 dilution occurring as a consequence of the mixture. This is consistent with two earlier reports which showed that day 8 splenocytes from acutely LCMV-infected mice did not inhibit the proliferation of day 0 splenocytes (25, 28). These experiments and those in the section above argue that the bystander sensitization to apoptosis and proliferative inhibition is not, under the conditions of these assays, a function of non-T accessory cells and argue that a sensitization to apoptosis may be occurring in vivo before the cells are put in culture.
TABLE 2.
Proliferation of T cells after coculture with splenocytes from adoptively reconstituted LCMV carrier mice
| Bystander cellsa | 103 Bystander cpm | Reconstituted carrier cellsb | 103 Reconstituted cpm | 103 Coculture cpmc |
|---|---|---|---|---|
| C57BL/6 | 121 ± 21 | Anti-Thy + C′ | 3.0 ± .3 | 59 ± 4.1 |
| C57BL/6 | 110 ± 16 | Thy− (FACS) | 3.5 ± .5 | 54 ± 5.7 |
| C57BL/6 | 176 ± 6.3 | Unseparated | 2.2 ± .0 | 46 ± 0.5 |
| C57BL/6 | 119 ± 18 | Unseparated | 4.7 ± .2 | 21 ± 0.9 |
| B6 carrier | 99 ± 13 | Anti-Thy + C′ | 4.6 ± .6 | 112 ± 9.1 |
| B6 carrier | 55 ± 9.7 | Thy− (FACS) | 3.5 ± .5 | 43 ± 9.9 |
| B6 carrier | 169 ± 20 | Unseparated | 3.0 ± .3 | 34 ± 0.2 |
| B6 carrier | 232 ± 9.6 | Unseparated | 35 ± 4.7 | 96 ± 4.2 |
Bystander cell spleen leukocytes from normal C57BL/6 or LCMV carrier C57BL/6 (B6 carrier) mice were placed into culture in the presence of anti-CD3 for 2 days and pulsed with [3H]thymidine, as described in Materials and Methods.
Spleen leukocytes from LCMV-carrier mice that had been adoptively reconstituted for 6 days with splenocytes from LCMV-immune mice were isolated, processed as described in Materials and Methods, and either tested directly (unseparated) or after depletion of T cells with either monoclonal antibody to Thy 1.2 + complement (C′) or by staining with monoclonal antibody to Thy 1 and removal of the T cells by fluorescence activated cell sorting. These cells were examined for proliferation identically to the bystander cells.
Cocultured cells were 1:1 mixtures of the bystander and reconstituted carrier populations, dispensed at total cell concentrations equal to the totals of the separated cell populations. Thus, the cocultures have the same total number of cells as the individual bystander or reconstituted carrier cultures; consequently, there is a 1:2 dilution of the component cell numbers in the cocultures.
Roles of donor and host T cells in the proliferative responses.
The experiments described above showed that depletion of donor T cells in the LCMV-immune splenocyte populations before adoptive transfers into carrier mice eliminated the ability of donor cell populations to sensitize host T cells to proliferative inhibition and to apoptosis. Experiments were done to test if donor T cells were required within the in vitro proliferative assay that occurred after the adoptive reconstitution. Donor T cells were depleted from the reconstituted T-cell population by staining them with anti-Thy 1.1 and sorting them away from the remaining cells. Spleen cells from unmanipulated LCMV-carrier mice proliferated strongly after anti-CD3 treatment (116,029 ± 3,387 cpm), whereas splenocytes from reconstituted LCMV carrier mice proliferated poorly (28,762 ± 2,515). Removal of the Thy 1.1+ donor cells from the population increased the proliferation only slightly (41,170 ± 7,485). Flow TUNEL data for apoptotic cells in this experiment showed 33% apoptotic cells in the cultures of the unreconstituted mice but over 50% apoptotic cells in the reconstituted cell cultures, with or without Thy 1.1 cell depletion (results not shown). Similar patterns of proliferation and TUNEL results were also noted in reconstituted mice whose cells were treated with anti-Thy1.1 antibody and complement to deplete donor cells, but data are presented only for the sorted cells, whose depletions were the most quantitative. These experiments indicate that even though donor T cells were required in vivo to bring about this sensitized state, the continued presence of highly activated donor T cells in the cultures was not required for sensitivity of the host cells to proliferative inhibition and to apoptosis ex vivo.
Experiments were also performed to determine if host Thy 1.2+ cells purified by cell sorting were refractory to proliferation in response to anti-CD3. Table 3 lists five experiments showing that highly purified (>92% pure) host Thy 1.2+ cells from unreconstituted or naive cell-reconstituted carrier mice proliferated substantially better than did Thy 1.2+ host cells purified from carrier mice reconstituted with LCMV-immune splenocytes. This inhibition in proliferation of purified host cells was not as great that seen in the presence of donor T and activated accessory cells (Table 1), but the proliferative response of the purified host T cells was clearly weakened by their previous in vivo exposure to a T-cell response to a viral infection.
TABLE 3.
Impaired proliferation of purified bystander LCMV carrier host T cells from Thy 1.2+ LCMV carrier mice adoptively reconstituted with LCMV-immune Thy 1.1+ splenocytesa
| Exptb | Reconstitution of carrier mice | Host cell | 103 Host cell cpm ± SD |
|---|---|---|---|
| 1 | None | Thy 1.2+ | 123 ± 18 |
| LCMV-immune cells | Thy 1.2+ | 54 ± 5.9 | |
| 2 | None | Thy 1.2+ | 56 ± 3.9 |
| LCMV-immune cells | Thy 1.2+ | 35 ± 5.9 | |
| 3 | Naive cells | Thy 1.2+ | 158 ± 9.1 |
| LCMV-immune cells | Thy 1.2+ | 113 ± 6.1 | |
| 4 | Naive cells | Thy 1.2+ | 127 ± 7.8 |
| LCMV-immune cells | Thy 1.2+ | 53 ± 8.9 | |
| Naive cells | CD8+ Thy 1.2+ | 98 ± 3.4 | |
| LCMV-immune cells | CD8+ Thy 1.2+ | 36 ± 14 | |
| 5 | Naive cells | Thy 1.2+ | 98 ± 16 |
| LCMV-immune cells | Thy 1.2+ | 54 ± 9.8 | |
| Naive cells | CD8+ Thy 1.2+ | 62 ± 10 | |
| LCMV-immune cells | CD8+ Thy 1.2+ | 34 ± 12 |
Spleen leukocytes from LCMV carrier Thy 1.2+ C57BL/6 mice, either untreated or reconstituted with naive or LCMV-immune Thy 1.1+ C57BL/6 spleen leukocytes, were purified by flow cytometry, stimulated with anti-CD3 antibody, and examined for proliferation by the uptake of [3H]thymidine.
Experiments 1 and 2 used the method described in Materials and Methods. Experiments 3 to 5 used a slightly different method, in which 105 responder cells from the treated mice were placed into 96-well flat-bottom microtiter plates along with 6 × 105 spleen leukocytes from control C57BL/6 mice depleted of T cells with anti-Thy 1.2 and complement, as feeder/accessory cells. [3H]thymidine was added directly to those wells after 2 days of stimulation.
Role of IFN-γ in bystander sensitization to AICD.
We have recently reported that T cells derived from mice genetically deficient in the IFN-γ receptor are relatively resistant to AICD after acute LCMV infection (19). To ascertain what role, if any, IFN-γ played in bystander sensitization of host T cells to AICD, LCMV-immune Thy 1.1+ spleen cells were adoptively transferred into Thy 1.2+ LCMV carrier mice lacking the IFN-γ receptor. Under these conditions, the host T cells would be unable to respond to the IFN-γ that was produced by the activated proliferating donor cells. The results of these adoptive-transfer experiments are shown in Fig. 4 and Table 4. T cells from persistently infected IFN-γ receptor knockout mice and T cells from IFN-γ receptor knockout mice reconstituted with LCMV-naive splenocytes were relatively resistant to AICD (Fig. 4A and B). In IFN-γ receptor knockout mice adoptively reconstituted with LCMV-immune Thy 1.1+ spleen cells (Fig. 4C), the extent of apoptosis was slightly greater than that seen in IFN-γ receptor knockout carrier mice reconstituted with LCMV-naive spleen cells (42 and 32%, respectively) but not nearly as extensive as that seen in immune cell-reconstituted persistently infected normal mice capable of responding to IFN-γ (42 and 71%, respectively [Fig. 4D]). The [3H]thymidine incorporations from three separate reconstitution experiments involving persistently infected IFN-γ receptor knock-out mice are shown in Table 4. In agreement with the TUNEL data, the inhibition of proliferation after reconstitution was not as profound when the host T cells cannot respond to IFN-γ. This result suggests that IFN-γ may play a role in sensitizing host cells to undergo apoptosis after TCR ligation. Some sensitization, however, clearly can take place in the absence of IFN-γ.
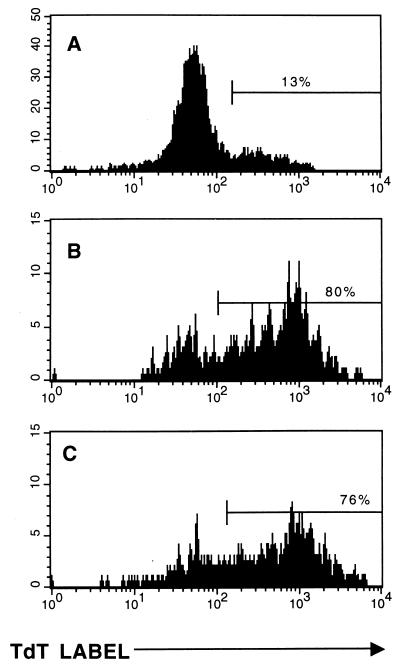
FIG. 4.
Bystander sensitization to apoptosis is inhibited in IFN-γ receptor knockout mice. TUNEL flow assays were performed on splenocytes from unmanipulated IFN-γ receptor knockout LCMV carrier 129/SV mice (A), IFN-γ receptor knockout 129/SV LCMV carrier mice reconstituted with 3 × 107 LCMV-naive C57BL/6 mouse spleen cells (B), IFN-γ receptor knockout LCMV carrier 129/SV carrier mice reconstituted with 3 × 107 LCMV-immune Thy 1.1+ C57BL/6 spleen cells (C), or wild-type LCMV carrier C57BL/6 mice reconstituted with 3 × 107 LCMV-immune C57BL/6 mouse spleen cells (D). LCMV carrier normal 129/SV mice were not available for this experiment, but we have shown previously that T cells from these mice become highly sensitized to apoptosis during the acute LCMV infection (19). Histograms represent the incorporation of the TdT label in the host Thy 1.2+ T cells.
TABLE 4.
T-cell proliferation after adoptive reconstitution of LCMV carrier mice lacking the IFN-γ receptor
| Expt | Reconstitutiona | Proliferationb |
|---|---|---|
| 1 | None | 196 ± 18 |
| LCMV naive | 116 ± 13 | |
| LCMV immune | 36 ± 1.8 | |
| Controlc | 2.2 ± 0.1 | |
| 2 | None | NDd |
| LCMV naive | 115 ± 0.8 | |
| LCMV immune | 47 ± 21 | |
| Controlc | 4.7 ± 0.9 | |
| 3 | None | 154 ± 17 |
| LCMV naive | 100 ± 15 | |
| LCMV immune | 46 ± 8.9 | |
| Controlc | 11 ± 2.2 |
IFN-γ receptor knockout LCMV carrier 129/SV mice were reconstituted with 3 × 107 of the indicated C57BL/6 spleen cells.
Numbers represent 103 mean cpm ± standard deviation after a 6-h pulse with 1 μCi of [3H]thymidine after 2 days of culture with plate-bound anti-CD3.
Wild-type LCMV carrier C57BL/6 mice reconstituted with 3 × 107 of the same LCMV-immune spleen cells used to reconstitute persistently infected IFN-γ receptor knockout mice. Wild-type LCMV carrier normal 129/SV controls were not available for this experiment, but we have documented the high sensitivity of T cells from acutely LCMV-infected 129/SV mice elsewhere (19).
ND, not done.
Role of FasL in bystander sensitization to apoptosis.
Fas and FasL play key roles in regulating T-cell apoptosis. To see what, if any, role this receptor-ligand pair plays in inducing bystander AICD, the gld mouse, which harbors a point mutation (Phe to Leu) in the carboxy terminus of the FasL molecule (20, 31), ablating its ability to induce apoptosis in Fas-expressing cells, was used as a source of LCMV-immune T cells. In these experiments Thy 1.2+ LCMV carrier mice were adoptively reconstituted with splenocytes from Thy 1.2+ LCMV-immune gld mice (Table 5; experiments 1 to 3); we have shown that gld mice have normal numbers of memory cytotoxic T lymphocytes as detected by the limiting-dilution assay (19). Reconstitution of these cells in this Thy 1.2-syngeneic system was demonstrated by an increase in the percentage of CD8 splenocytes expressing activation markers (large CD44high CD62Llow-expressing cells) 6 days postinfection. To further confirm that adoptively transferred T cells from gld mice can otherwise function properly in vivo, 3 × 107 splenocytes from naive or LCMV-immune C57BL/6 mice or gld mice were transferred intravenously into 6-week-old C57BL/6 mice infected 1 day previously with 5 × 104 PFU of LCMV intraperitoneally. The next day, spleens were harvested and the titer of LCMV plaques was determined; a reduction in PFU has been shown in this system to be a function of MHC-restricted CD8 T cells (39). Splenocytes from LCMV-immune C57BL/6 and gld mice each led to similar 1-log-unit reductions in viral titers (log10 PFU/spleen after reconstitution with splenocytes from naive C57BL/6, 4.4 ± 0.1; immune C57BL/6, 3.2 ± 0.1; naive gld, 4.2 ± 0.4; immune gld, 3.3 ± 0.0), arguing that transferred gld-immune T cells functioned properly in vivo to clear the virus. However, when LCMV carrier mice were reconstituted with spleen cells from LCMV-immune gld mice, the inhibition in [H3]thymidine incorporation was significantly lower than that seen when FasL+/+ LCMV-immune spleen cells were used for reconstitution. In Table 5 experiment 1, there appeared to be some effect of the immune gld cells, but it was much smaller than that seen with normal immune cells in the experiment in Table 1 and in experiments 2 to 4 in Table 5. In one experiment, Thy 1.1+ LCMV carrier mice, which were of very limited availability, were reconstituted with cells from LCMV-immune Thy 1.2+ gld mice (Table 5, experiment 4) and the proliferation was substantially lower in mice reconstituted with LCMV-immune normal C57BL/6 mouse control cells than with LCMV-immune gld cells. Figure 5 shows the results of one of three similar TUNEL experiments, indicating that T cells in adoptively reconstituted carrier mice were not substantially sensitized to undergo apoptosis if reconstituted with LCMV-immune gld mouse splenocytes. These data suggest that expression of FasL on the activated donor cells is important for the sensitization of host T cells for antiproliferative and apoptotic effects.
TABLE 5.
T-cell proliferation after adoptive reconstitution of LCMV carrier mice with spleen cells from LCMV-immune or naive gld mice
| Expt | Reconstitutiona | Proliferationb |
|---|---|---|
| 1 | LCMV-naive gld | 222 ± 11 |
| LCMV-immune gld | 53 ± 4.4 | |
| 2 | None | 212 ± 17 |
| LCMV-immune C57BL/6 | 2.5 ± 15 | |
| LCMV-immune gld | 141 ± 3.3 | |
| 3 | None | 174 ± 36 |
| LCMV-immune C57BL/6 | 2.2 ± 0.1 | |
| LCMV-immune gld | 179 ± 12 | |
| 4 | LCMV-immune C57BL/6 | 5.9 ± 2.0 |
| LCMV-immune gld | 41 ± 5.1 |
LCMV carrier mice were reconstituted with 3 × 107 to 4 × 107 of the indicated C57BL/6 or congenic gld mutant spleen cells.
Numbers represent 103 mean cpm ± standard deviation after a 6-h pulse with 1 μCi of [3H]thymidine after 2 days of culture with plate-bound anti-CD3 antibody.
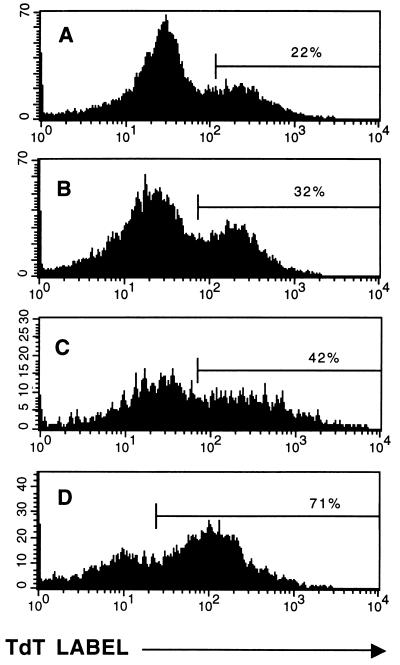
FIG. 5.
Immune splenocytes from C57BL/6 congenic gld mice are inefficient at sensitizing cells from adoptively reconstituted LCMV carrier mice to apoptosis on anti-CD3 stimulation. LCMV carrier C57BL/6 Thy 1.2+ mice were either unreconstituted (A) or reconstituted with splenocytes from LCMV-immune control C57BL/6 (B) or LCMV-immune gld (C) Thy1.2+ mice, in a method similar to that used in Fig. 1. This figure shows the results for the total T-cell populations from these mice, since both host and donor cells were Thy 1.2+.
Similar types of experiments were done using LCMV carrier lpr mice, which have a mutation in Fas (36, 37), as recipients for normal LCMV-immune T cells. There was no induced inhibition of host T-cell proliferative responses (data not shown), but these experiments were uninterpretable because T cells from lpr mice are in general resistant to AICD (19) and because fluorescence analyses indicated that Thy 1.1+ donor cells did not reconstitute in the Thy 1.2+ lpr mice; this may be due to the reported elevations in the levels of lpr mouse FasL (37), which may interfere with the expansion of activated T cells in a manner similar to the phenomenon that we are describing here.
Inhibition of proliferation in LCMV-infected HY transgenic mice.
To examine the effects of a more typical acute viral infection on T cells known not to be specific for the virus, HY-specific transgenic T-cell C57BL/6 mice were used. These mice have a “leaky” transgenic T-cell repertoire in that they contain a considerable array of T cells that do not express both the α and β chains of the HY-specific TCR; about 30% of the CD8 T cells express both receptors and have HY specificity. We have shown that LCMV induces in these mice a strong T-cell response that does not elicit the proliferation of the transgenic T cells, which are detectable by the monoclonal antibody T3.70 (38). By 8 days postinfection, 15 to 25% of the CD8 T cells express the HY-specific transgene (37), yet [3H]thymidine incorporation assays showed that these total T-cell populations proliferated extremely poorly in response to anti-CD3. Splenocytes from uninfected HY transgenic mice proliferated well after culture with anti-CD3 (cpm ranging in three experiments from 111,218 ± 21,424 to 257,934 ± 363). In contrast, splenocytes from HY transgenic mice 8 days after LCMV infection proliferated very poorly, from a low of 424 ± 23 cpm up to a maximum of only 5,463 ± 77 cpm after culture with anti-CD3. Predictively, TUNEL stains showed high levels of cell death in these cultures; however, HY+ cells could not be distinguished from the rest of the T cells because they could not be detected after anti-CD3 stimulation, either because of cell loss by apoptosis or because of receptor downregulation. Nevertheless, the extremely impaired uptake of the radiolabel, the low recovery of cells after stimulation, and the high frequency of TUNEL staining in the remaining cells indicates that the “bystander” HY+ cells died in these cultures.
DISCUSSION
Here we show that T cells clearly defined as being not virus specific fail, during a potent antiviral immune response, to proliferate on TCR ligation but instead undergo apoptosis. This bystander sensitization to AICD occurred as a consequence of an ongoing virus-specific T-cell response and appeared to be dependent on the expression of FasL on the activated proliferating cells, since sensitization did not occur when the virus-specific T cells lacked expression of FasL. This might indicate that FasL on the virus-specific cells interacts with Fas on the bystander cells before, during, or after receptor engagement and directs them into a death pathway. The importance of Fas could not be easily assessed in the adoptive-transfer model because donor T cells failed to repopulate in Fas-mutant lpr mice, perhaps because splenocytes from lpr mice have upregulated FasL, which can be toxic for activated T cells and therefore may have prevented their repopulation (37). Experiments with IFN-γ receptor knockout mice suggest that the ability of the non-virus-specific T cells to respond to IFN-γ plays a significant role in inducing this susceptible state. T cells from IFN-γ receptor-deficient mice are themselves somewhat resistant to AICD (19), and we show here that this deficiency could not be overcome by the presence of wild-type activated T cells. IFN-γ is reported to upregulate Fas expression, and this may in part explain its role in sensitizing cells to apoptosis (18, 19).
Other factors may also be involved in this bystander sensitization to apoptosis, including interleukin-2 (IL-2), which would be produced by the virus-specific activated T cells. After exposure to high concentrations of IL-2, murine Th1 clones or lymph node T cells undergo apoptosis on TCR ligation (15). The data presented here do not directly address the role of IL-2 in bystander sensitization to AICD, but we have previously shown in the LCMV system that in vitro exposure to IL-2 greatly augments the sensitization of T cells to AICD (25). It is also noteworthy that IL-2 can induce the synthesis of IFN-γ, which itself may regulate apoptosis (19).
During acute infections of humans and animals with many viruses, there is a transient period in which the ability to mount a T-cell response to other antigens is severely compromised (27, 35). The results detailed here suggest a mechanism by which those T cells not responding to the virus become sensitized, in a bystander fashion, to undergo AICD on receptor triggering. This period when the T cells are susceptible to AICD is transient, because the ability to respond to recall antigens returns after the acute phase of the antiviral immune response has passed. Careful quantification of memory cytotoxic T-lymphocyte precursors, however, has recently revealed that acute viral infections can cause significant and permanent reductions in the total number of T cells specific to viruses from previous infections (30). In addition, studies have shown that the total numbers of T cells not specific for LCMV decline by 30 to 50% during an acute LCMV infection, and such reductions are greatest in bystander T cells expressing the CD44 activation/memory marker (38; J. M. McNally, C. C. Zarozinski, and R. M. Welsh, FASEB J. 13:982, 1999, abstract). Thus, T cells not specific for a virus may become destabilized, possibly in the FasL-rich environment, and undergo some attrition even in the absence of high-affinity receptor stimulation, but in the presence of strong ligands they may quickly undergo apoptosis. TNF-TNF receptor interactions can sometimes substitute for Fas-FasL interactions in the AICD of CD8 T cells (16), and it would be interesting to explore the role of that receptor-ligand pair in this system. It is noteworthy that after clearance of virus, the overall T-cell numbers decline normally in an environment lacking Fas or FasL (17, 24), but this is probably due to a distinctly different mechanism from AICD and is probably related to removal of growth and survival factors for activated lymphocytes.
Bystander sensitization to apoptosis may also be a mechanism for immune deficiencies associated with persistent viral infections and autoimmune conditions involving chronic T-cell responses. In HIV infection, the immune response is chronic, and the T cells are in an environment in which there is a continual T-cell response threatening attrition of other cells. The T cells undergoing apoptosis in HIV- and SIV-infected lymph nodes are predominantly uninfected bystander cells (4). Previous work has shown that CD8+ T cells from HIV-infected asymptomatic individuals respond poorly to anti-CD3 stimulation in vitro and undergo apoptosis (8, 33) and that CD8+ T cells derived from asymptomatic HIV-positive individuals were in a “preapoptotic” state in vivo and could undergo apoptosis after overnight culture in media (17). Our work presented here is consistent with those results and indicates in a defined experimental system that uninfected bystander cells that are clearly not virus specific can become destabilized in the environment of an activated T-cell response.
ACKNOWLEDGMENTS
We thank Barbara Fournier and Tammy Krumpoch for running the FACS samples and Ralph Budd for the TUNEL protocol.
This work was supported by USPHS research grant AI17672 to R.M.W. and training grant AI07349 to C.C.Z.
REFERENCES
- 1.Akbar A N, Borthwick N, Salmon M, Gombert W, Bofill M, Shamsadeen N, Pilling D, Pett S, Grundy J E, Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;179:427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce J, Symington F W, McKearn T J, Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981;127:2496–2501. [PubMed] [Google Scholar]
- 3.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel R M. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Monks T, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV and SIV infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 5.Fugier-Viver I, Severt-Delprat C, Rivailler P, Rissoan M, Liu Y, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD8+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groux H, Torpier G, Monté D, Mouton Y, Carpon A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruters R A, Terpsta F G, De Jong R, von Nossel C J M, van Lier R A W, Miedema F. Selective loss of T cell functions in different stages of HIV infection. Eur J Immunol. 1990;20:1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Steinman R M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984;160:1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs R P, Cole G A. Lymphocytic choriomeningitis virus-induced immunosuppression: a virus-induced macrophage defect. J Immunol. 1976;117:1004–1009. [PubMed] [Google Scholar]
- 11.Jamieson B D, Ahmed R. T-cell tolerance: exposure to virus in utero does not cause a permanent deletion of specific T cells. Proc Natl Acad Sci USA. 1988;85:2265–2268. doi: 10.1073/pnas.85.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson B D, Somasundaram T, Ahmed R. Abrogation of tolerance to a chronic viral infection. J Immunol. 1991;147:3521–3529. [PubMed] [Google Scholar]
- 13.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cumo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity to measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 14.King C, Jamieson B D, Reddy K, Bali N, Concepcion R J, Ahmed R. Viral infection of the thymus. J Virol. 1992;66:3155–3160. doi: 10.1128/jvi.66.5.3155-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenardo M J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–851. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 16.Lenardo M J. The molecular regulation of lymphocyte apoptosis. Semin Immunol. 1997;9:1–5. doi: 10.1006/smim.1996.0050. [DOI] [PubMed] [Google Scholar]
- 17.Lewis D E, Ng Tang D S, Adu-Oppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 18.Liu Y, Janeway C A. Interferon γ plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman B L, Welsh R M. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol. 1998;72:7815–7821. doi: 10.1128/jvi.72.10.7815-7821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch D H, Watson M L, Alderson M R, Baum P R, Miller R E, Tough T, Gibson M, Davis-Smith T, Smith C A, Hunter K, Bhat D, Din W, Goodwin R G, Seldin M F. The mouse Fas-ligand gene is mutated in gld mice as is part of a TNF family gene cluster. Immunity. 1994;1:131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 21.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P M, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 22.Muller U, Steinhoff U, Reiss L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 23.Murali-Krishna K, Altman J D, Suresh M, Sourdive D, Zajac A J, Miller J, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a re-evaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 24.Razvi E S, Jiang Z, Woda B A, Welsh R M. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am J Pathol. 1995;147:79–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Razvi E S, Welsh R M. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993;67:5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 27.Rouse B T, Horohov D W. Immunosuppression during viral infections. Rev Infect Dis. 1986;8:850–873. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saron M, Shidani B, Nahori M A, Guillon J, Truffa-Bachi P. Lymphocytic choriomeningitis virus-induced immunodepression: inherent defect in B and T lymphocytes. J Virol. 1990;64:4076–4083. doi: 10.1128/jvi.64.9.4076-4083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selin L K, Vergilis K, Welsh R M, Nahill S R. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte (CTL) memory by heterologous viral infections. J Exp Med. 1996;183:2489–2500. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahasi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Generalized lymphoproliferative disease in mice caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 32.Tough D L, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 33.van Nossel C J M, Gruters R A, Terpstra F G, Schellekens P T A, van Lier R A W, Miedema F. Functional and phenotypic evidence for a selective loss of memory T cells in different stages of HIV infection. J Clin Investig. 1990;86:293–299. doi: 10.1172/JCI114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent M S, Roessner K, Lynch D, Wilson D, Cooper S M, Tschopp J, Sigal L H, Budd R C. Apoptosis of Fashigh CD4+ synovial T cell by Borrelia-reactive Fas-ligandhigh γδ T cells in lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Pirquet C. Das Verhalten der kutanen Tuberkulin-Reakton wahrend der Masern. Dtsch Med Wochenschr. 1908;34:1297–1300. [Google Scholar]
- 36.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe-Fukunaga R, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarozinski C C, Welsh R M. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinkernagel R M, Welsh R M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976;117:1495–1502. [PubMed] [Google Scholar]