Abstract
A 69‐year‐old ex‐smoker Japanese man presented with a left mediastinal lymph node and left upper lobe tumour. Bronchoscopic biopsy specimens from the enlarged left mediastinal lymph node and left upper lobe tumour revealed small cell lung carcinoma (SCLC). He was treated with first‐line chemotherapy with carboplatin, etoposide, and atezolizumab for four courses and subsequent atezolizumab maintenance therapy. However, his left upper lobe lung tumour only increased in size, and left upper lobectomy revealed combined SCLC (adenocarcinoma and chondrosarcoma‐like features). Four months after lobectomy, liver metastasis of chondrosarcoma‐like features (similar to pathological findings of the left upper lobe tumour) were observed. Combined SCLC, including sarcomatous components, is rare and poorly responds to chemotherapy. The metastases of combined SCLC in this patient were of only one type of histological component, making diagnosis and treatment difficult. If treatment for SCLC responds inadequately, considering combined SCLC and actively re‐examining histological diagnosis is necessary.
Keywords: chondrosarcoma‐like features, combined small cell lung carcinoma, lobectomy, lymph node metastasis, small cell lung carcinoma
We report a case of combined small cell lung carcinoma (c‐SCLC) with adenocarcinoma and chondrosarcoma‐like features and mediastinal lymph node metastasis of only SCLC components. The diagnosis was complex and finally achieved due to a left upper lobectomy.
INTRODUCTION
Small cell lung carcinoma (SCLC) has an extremely poor prognosis among lung cancers and requires early diagnosis and therapeutic intervention.
Among SCLC, combined SCLC (c‐SCLC), which include components of non‐small cell carcinoma, such as adenocarcinoma and large‐cell neuroendocrine carcinoma, and combinations of sarcomatous components, are rare. 1 In addition, the metastatic pattern of c‐SCLC may metastasize with only one component, 2 complicating the diagnosis and treatment.
Here, we report a case of c‐SCLC (adenocarcinoma and chondrosarcoma‐like features) with mediastinal lymph node metastasis of only SCLC components finally diagnosed due to a left upper lobectomy.
CASE REPORT
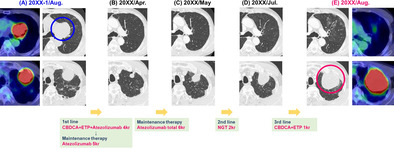
A 69‐year‐old Japanese man visited our hospital in July 2022 because of haemoptysis. He was an ex‐smoker with no history of dust exposure or medically significant family history. Chest computed tomography (CT) showed an enlarged left mediastinal lymph node (65 × 64 mm) and a left upper lobe tumour (50 × 46 mm) (Figure 1A). Laboratory data showed no elevated levels of tumour markers associated with lung cancer and other specific findings (Table 1). Bronchoscopic examination of the left mediastinal lymph node and left upper lobe tumour revealed SCLC (Figure 2A). 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) showed high uptake lesions in the left mediastinal lymph node, left upper lobe tumour, and right sixth rib (Figure 1A), and head magnetic resonance imaging showed no brain metastases; therefore, he was diagnosed with extensive‐stage SCLC (cT3N2M1b‐clinical Stage IVA). After four courses of first‐line chemotherapy with carboplatin, etoposide, and atezolizumab started in August 2022, subsequent atezolizumab maintenance therapy was initiated in December 2022. After six courses of atezolizumab maintenance therapy, chest CT in May 2023 showed controlled left mediastinal lymph node metastasis with re‐increased upper lobe tumour (Figure 1B,C). The left upper lobe tumour did not respond to second‐line chemotherapy with nogitecan from May 2023 to June 2023 and third‐line chemotherapy with carboplatin and etoposide in July 2023 (Figure 1D,E). Bronchoscopic examination for the left upper lobe tumour in August 2023 failed to obtain appropriate sample for pathological examination, and the results of CT‐guided biopsy from the left S3 showed atypical cells growing in a round or spindle shape with myxomatous stroma that were positive for vimentin, indicative of mesenchymal tumour. FDG‐PET revealed a high uptake of FDG only in the left upper lobe tumour (Figure 1E), and left upper lobectomy was performed. The lung tumour exhibited a mixture of adenocarcinoma (immunostaining; positive for TTF‐1 and CD56 and negative for synaptophysin) and chondrosarcoma‐like features (immunostaining; positive for vimentin, S100, and sox9) (Figure 2B); thus, he was finally diagnosed our patient with c‐SCLC. In January 2024, follow‐up CT revealed a new nodular shadow in the right lobe of the liver, and histopathological findings showed chondrosarcoma‐like features, similar to the left upper lobe lung tumour (Figure 2C). Therefore, fourth‐line chemotherapy with carboplatin and nab‐paclitaxel was initiated and the patient is on treatment at the time of reporting.
FIGURE 1.
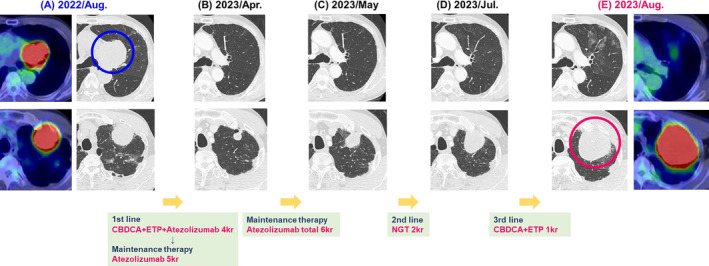
Computed tomography images. (A) Before chemotherapy (August 2022). FDG‐PET showed high uptake of FDG in the left mediastinal lymph node and the left apical tumour. (B) After four courses of chemotherapy with carboplatin, etoposide, and atezolizumab, and then after five courses of atezolizumab maintenance therapy, both tumours shrunk in size. (C) After a total of six courses of atezolizumab maintenance therapy, only the left upper lung tumour was observed to have increased in May 2023. (D) After two courses of second‐line chemotherapy with nogitecan, the left upper lung tumour was further noted to have increased in July 2023. (E) After one course of third‐line chemotherapy with carboplatin and etoposide, the left upper lung tumour was noted to have increased and showed high uptake of FDG in August 2023. CBDCA, carboplatin; ETP, etoposide; FDG‐PET, 18F‐fluorodeoxyglucose positron emission tomography; NGT, nogitecan.
TABLE 1.
Laboratory findings on admission.
| Blood cell counts | Blood chemistry | Serology | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 6800 | /μL | TP | 7.1 | g/dL | NSE (≦16.3) | 12.5 | Ng/mL |
| Neutrophils | 84.0 | % | Alb | 4.1 | g/dL | Pro‐GRP (≦81.0) | 38.0 | pg/mL |
| Lymphocytes | 10.6 | % | AST | 19 | IU/L | CEA (≦3.5) | 2.6 | ng/mL |
| Eosinophils | 0.6 | % | ALT | 15 | IU/L | CYFRA (≦2.1) | 1.3 | ng/mL |
| Monocytes | 4.4 | % | LDH | 235 | IU/L | Coagulation | ||
| Basophils | 0.4 | % | BUN | 14.0 | mg/dL | PT | 11.9 | s |
| RBC | 277× 104 | /μL | Cre | 0.94 | mg/dL | PT‐% | 96.6 | % |
| Hb | 9.5 | g/dL | Na | 141 | mmol/L | INR | 1.00 | |
| Ht | 28.6 | % | K | 4.5 | mmol/L | APTT | 28.8 | s |
| Platelets | 38.7× 104 | /μL | Cl | 106 | mmol/L | |||
| CRP | 0.2 | mg/dL | ||||||
Abbreviations: Alb, albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CEA, carcinoembryonic antigen; Cre, creatinine; CRP, C‐reactive protein; CYFRA, cytokeratin 19 fragment; Hb, haemoglobin; Ht, haematocrit; INR international normalized ratio; LDH, lactate dehydrogenase; NSE, neuron specific enolase; Pro‐GRP, pro‐gastrin‐releasing peptide; PT, prothrombin time; RBC, red blood cell; TP, total protein; WBC, white blood cell.
FIGURE 2.
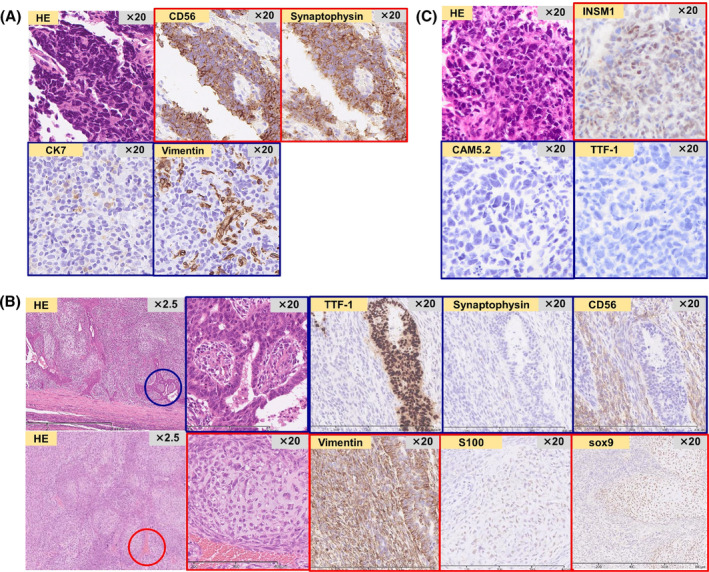
Pathological findings. (A) The mediastinal lymph node and left upper lobe mass exhibited pathological findings of small cell carcinoma on transbronchial biopsy and endobronchial ultrasound‐guided transbronchial lung biopsy. (B) The left upper lung lobectomy tissue exhibited findings of a mixture of adenocarcinoma (immunostaining; positive for TTF‐1 and CD56 and negative for synaptophysin) and chondrosarcoma‐like features (immunostaining; positive for vimentin, S100, and sox9). He was finally diagnosed with combined small cell lung carcinoma, in which the small cell lung carcinoma component disappeared after chemotherapy. (C) Four months after left upper lung lobectomy, the liver metastatic tumour exhibited pathological findings of chondrosarcoma‐like features, similar to those of the left upper lobe lung tumour.
DISCUSSION
We present a case of c‐SCLC (adenocarcinoma and chondrosarcoma‐like features) that was diagnosed differently from the initial diagnosis of SCLC after to lobectomy.
C‐SCLC is a special type of SCLC that contains components of non‐small cell carcinoma and is rare, accounting for 2%–5% of all SCLC. 3 The histological type associated with c‐SCLC are mostly large‐cell neuroendocrine carcinoma in 80% and adenocarcinoma in 17% of cases. Sarcoma‐like components similar to those in this patient are rare. 1 No established treatment exists for c‐SCLC, and chemotherapy similar to that for SCLC is generally administered. 4 Consequently, this patient underwent general chemotherapy for SCLC; however, the left upper lung tumour only increased. In cases of c‐SCLC, including sarcoma‐like features, patients who underwent surgical resection in addition to chemotherapy had a relatively long survival, 5 thus surgical lobectomy was considered in this patient (Table 2).
TABLE 2.
Published case reports of combined small cell carcinoma with sarcomatous histological components.
| Case | Age/sex | Staging | Sarcoma type | Other pathology | Treatment | Prognosis | Authors/published years |
|---|---|---|---|---|---|---|---|
| 1 | 67/M |
pT3N0M0 pStage IIB |
Chondrosarcoma |
LCNEC SCC |
Surgical treatment CBDCA+ETP |
>2 years | Takayama et al./2010 |
| 2 | 73/M |
pT2aN0M1a pStage IVA |
Chondrosarcoma |
Adenocarcinoma SCC |
Surgical treatment CBDCA+ETP |
17 months | Noma et al./2015 |
| 3 | 75/M |
cT2aN1M1a cStage IVA |
Rhabdomyosarcoma | – | CDDP+ETP | 12 months | Yamane et al./2017 |
| 4 | 50/M |
pT4N0M0 pStage IIIA |
Rhabdomyosarcoma Chondrosarcoma |
Adenocarcinoma | Surgical treatment | >4 years | Ueda et al./2022 |
| 5 | 69/M |
cT3N2M1b cStage IVA |
Chondrosarcoma‐like features | Adenocarcinoma |
CBDCA+ETP + Atezolizumab Surgical treatment |
– | Present case/2024 |
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; ETP, etoposide; LCNEC, lung‐cell neuroendocrine carcinoma; SCC, squamous cell carcinoma.
In this patient, after the diagnosis of SCLC, chemotherapy for SCLC was performed, and the left mediastinal lymph node shrank; however, only the left upper lobe tumour grew, leading to the diagnosis of c‐SCLC. Furthermore, within a short period after left upper lung lobectomy, a liver metastasis of only chondrosarcoma‐like features (similar to the left upper lobe tumour) was newly revealed. To our knowledge, this patient is the first case report of metastasis with chondrosarcoma‐like component after SCLC chemotherapy for c‐SCLC reported in the literature. In general, metastases from lung cancer have no fixed tendency in histological types, however, the histological types of lymph node metastases from c‐SCLC include metastases with combined components. Moreover, some cases show metastasis with only one type of histological component, such as adenocarcinoma and SCLC. 2 This patient was initially diagnosed with SCLC because the histology of the left mediastinal lymph node revealed SCLC on the first bronchoscopy, and he was treated with chemotherapy for SCLC. However, only the left upper lung tumour increased in size, and only adenocarcinoma and chondrosarcoma‐like tissues remained in the final histological findings. As a differential diagnosis for this patient, the transformation from SCLC to adenocarcinoma was also considered because SCLC and adenocarcinoma reportedly develop from a common stem cell and the stem cells might mutually transform during the treatment, but this transformation is extremely rare. 6 Moreover, the clinical course of this patient being that the mediastinal lymph node and left upper lobe tumour temporarily decreased but substantially re‐enlarged during chemotherapy for SCLC, might importantly indicate that the left mediastinal lymph node tumour might be metastasized solely from the SCLC component of the c‐SCLC. In addition, this patient exhibited liver metastasis of only the chondrosarcoma‐like component similar to the left upper lobe lung tumour within a short period after lobectomy; therefore, even after c‐SCLC treatment, the chondrosarcoma‐like component might progress and metastasize.
Herein, we present a case of c‐SCLC with mediastinal lymph node metastasis of only SCLC components and liver metastasis with chondrosarcoma‐like components that occurred shortly after chemotherapy and lobectomy. If SCLC treatment achieves inadequate response or rapid residual disease progression, considering c‐SCLC and actively re‐examining the histological diagnosis is crucial.
AUTHOR CONTRIBUTIONS
T.K. wrote and drafted the manuscript. K. Yamasaki and K. Yatera critically revised the manuscript. All the authors have read and approved the final version of the manuscript.
FUNDING INFORMATION
The authors do not have any financial support to declare.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and the accompanying images.
ACKNOWLEDGMENTS
We thank Editage (www.editage.com) for English‐language editing.
Kawaguchi T, Yamasaki K, Shigemi S, Dosaka H, Hirosawa R, Nagasawa T, et al. A combined small cell lung carcinoma patient with only small cell carcinoma components in mediastinal lymph node metastasis and chondrosarcoma‐like components in liver metastasis. Respirology Case Reports. 2024;12(6):e01416. 10.1002/rcr2.1416
Associate Editor: Kazuhisa Takahashi
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Wallace AS, Arya M, Frazier SR, Westgate S, Wang Z, Doll D. Combined small‐cell lung carcinoma: an institutional experience. Thorac Cancer. 2014;5:57–62. 10.1111/1759-7714.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukui T, Tsuta K, Furuta K, Watanabe S, Asamura H, Ohe Y, et al. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci. 2007;98:1714–1719. 10.1111/j.1349-7006.2007.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14:113–119. 10.1016/j.cllc.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 4. Lantuejoul S, Fernandez‐Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9:2233–2244. 10.21037/tlcr-20-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noma D, Morohoshi T, Adachi H, Natsume I, Ookouchi M, Tsuura Y, et al. A resected case of combined small cell lung carcinoma with carcinosarcoma. Pathol Int. 2015;65:332–334. 10.1111/pin.12269 [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Jia Q, Tang X, Yan L, Zhu B. Transformation to lung adenocarcinoma from complete remission‐experienced SCLC. J Thorac Oncol. 2020;15:e1–e3. 10.1016/j.jtho.2019.07.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


