Abstract
This preliminary study explored potential serum biomarkers for predicting the onset of milk fever (MF), a bovine parturient disease with hypocalcemia. We conducted two-dimensional gas chromatography mass spectrometry-based metabolomics in 8 and 17 pregnant Holstein cows that did and did not develop MF 3 weeks later, respectively. In principal component analysis (PCA) applied to a dataset containing 1,498 metabolites, serum metabolites exhibited highly similar chemical profiles between cows with and without MF. PCA with a limited dataset of metabolites containing fatty acids, which had significantly different values between the groups and/or correlation coefficients of >0.5 for the serum calcium concentration, distinguished the two groups. These suggest the possibility of developing serum biomarkers for predicting bovine MF.
Keywords: cow, gas chromatograph-mass spectrometry, milk fever, serum metabolomics
Milk fever (MF) is a bovine parturient disease that primarily affects older, high-producing dairy cows that have undergone three or more lactations [9]. This disease is characterized by severe hypocalcemia (serum calcium concentration <1.5 mmol/L, <6.0 mg/dL), resulting in flaccid paresis due to neuromuscular dysfunction, ataxia, recumbency, and depression, sometimes with loss of consciousness [3, 15]. Hypocalcemia disrupts ruminal and abomasal motility, leading to body fat mobilization and an increased risk of various parturient and metabolic disorders including dystocia, ketosis, and abomasal displacement [5, 15]. Efforts have been made to prevent MF because of its significant impact on the well-being and economic performance of dairy cows. To ensure effective preventive measures, it is crucial to identify predictive indicators of MF before parturition. Our recent study showed that the prepartum concentrations of serum bone biomarkers are related to the degree of hypocalcemia and risk of MF in dairy cows [19]. In particular, cows with MF exhibited lower serum activity of alkaline phosphatase isoenzyme 3 (ALP3), a specific biomarker for osteoblast function and bone mineralization, during the 3 weeks before parturition. Interestingly, ALP3 activity had a moderate positive association with serum calcium concentration on the day of parturition [19]. These findings suggest that osteoblast activity may be reduced in cows with MF during the prepartum period and that prepartum ALP3 activity has great potential as a predictive indicator for cows to develop MF.
Metabolomics has been utilized in dairy cows to examine the changes in the metabolome associated with liver function and calcium metabolism during the parturient period [2, 18]. In contrast, metabolomics studies characterizing the MF and predictive indicators of the risk of MF prior to parturition are lacking. We hypothesized that metabolomic analysis of cow’s serum samples will reveal useful biomarkers for predicting MF. In the present study, we performed two-dimensional gas chromatography mass spectrometry (GC×GC/MS)-based metabolomics analysis using prepartum serum samples from a previous study [19]. Considering the variation of ALP3 between MF and normal groups, we aim to identify serum indicators that can enhance our ability to predict the risk of MF in cows during the crucial 3-week prepartum period.
This study analyzed serum samples obtained from 25 late-pregnant Holstein cows approximately 3 weeks (18 to 23 days, average 20.7 days) before parturition (Supplementary Table 1). These samples were originally collected for a previous study [19] and were kept at −80°C without a freeze–thaw cycle. The experimental procedures of animal care and blood sampling were complied with the Guide for the Care and Use of Agricultural Animals of Obihiro University of Agriculture and Veterinary Medicine (approval #19-49). The 25 Holstein cows were multiparous and had been housed at the university farm of Obihiro University of Agriculture and Veterinary Medicine. The cows were dried-off for approximately 60 days and kept in a free-stall barn with an outside paddock until 3 to 4 weeks before the expected parturition date. They were moved to another free-stall barn with outside paddock where all late-pregnant cows were kept. Then they were kept in individual pens between 1 day before and 5 days after parturition. During the 3 weeks leading up to the expected parturition date, all cows had free access to water, hay, and a total mixed ration. Among the multiparous cows, eight cows (two cows in their second lactation and six cows in their third or fifth lactation) were diagnosed with MF within a few hours after parturition. The diagnosis was based on the presence of any of the following eight characteristic clinical signs of MF: astasia, anorexia, cold extremities, flaccid tail, tachycardia (>80 beats/min), tachypnea (>36 breaths/min), decreased rectal temperature (<38.0°C), and coma [17].
Each frozen serum sample was thawed on ice for 60 min, and a 100 µL aliquot was transferred into a glass tube. For deproteinization, 400 µL methanol was added to each tube, and then the mixture was centrifuged at 600 × g for 10 min at room temperature (approximately 22 ± 2°C). The resulting supernatant was carefully transferred into a new glass tube and completely dried using a centrifugal vacuum evaporator (Tomy Kogyo Co., Ltd., Tokyo, Japan) without additional heating. To derivatize the dried samples, 50 µL O-methylhydroxylamine (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) in pyridine (Tokyo Chemical Industry Co., Ltd.) (20 mg/mL) was added to the sample and incubated at 80°C for 15 min for oximation. Subsequently, 50 µL N-methyl-N (trimethylsilyl) trifluoroacetamide (GL Science Inc., Tokyo, Japan) was added to the sample and incubated at 80°C for 15 min for trimethylsilyl (TMS) derivatization. These samples were used for analysis within 24 hr after derivatization.
The GC×GC time-of-flight MS analyses were performed using LECO’s Pegasus 4D instrument (LECO Corp., St. Joseph, MI, USA) equipped with a multipurpose autosampler (GERSTEL GmbH & Co. KG, Mülheim an der Ruhr, NRW, Germany). The derivatized serum samples (2 µL aliquots) were analyzed using a split mode (split ratio of 20). The order of sample injections into the instrument followed the cow’s ID shown in Supplementary Table 1, but did not correspond to the categories between the MF and normal groups. The GC incorporated two different polarity columns: DB-5MS (38 m length × 0.25 mm i.d. × 0.25 µm df; Agilent Technologies, Inc., Santa Clara, CA, USA) and Rtx-200 (1.4 m length × 0.18 mm i.d. × 0.25 µm df; Restek Corporation, Bellefonte, PA, USA) as the secondary column via a thermal modulator. Before secondary column separation, a dual-stage quad-jet thermal modulator released cold and hot nitrogen gas at 1.5 sec intervals to trap and refocus compounds eluted from the primary column. The carrier gas (pure helium) flowed at a rate of 1.5 mL/min. The primary column oven temperature was initially held at 40°C for 1 min, was increased at a rate of 6°C/min, and finally held at 290°C for 6 min. The secondary column oven temperature was programmed with a +10°C offset from the primary column oven temperature. The modulation offset was 25°C. The mass spectrometer was operated using electron impact ionization, with the electron energy set to 70 eV and the ion source temperature set to 250°C. Spectra were acquired with the mass range of 35 to 800 m/z at an acquisition rate of 200 spectra/sec and solvent delay of 400 sec. To ensure reproducibility, all of the 25 samples were analyzed in triplicate, producing 75 GC×GC/MS results.
ChromaTOF software version. 4.72.0.0 (LECO Corp.) was used to analyze raw instrument data in the following steps. The software identified peaks from the total ion chromatogram. Each peak underwent chromatographic deconvolution, the raw data were aligned, and the Wiley 11 and NIST 11 libraries were used for chemical identification with the similarity threshold set at 80% according to the previous report [11]. This was followed by generation of a data sheet containing information for each peak, including the retention time, peak area, and similarity to compounds from the libraries. In the present study, all statistical analyses including t-test, calculation of fold change and false discovery rate, generation of volcano plot, and principal component analysis (PCA) score plots were carried out using MetaboAnalyst 6.0 (browser version available at www.metaboanalyst.ca). The data were normalized using auto-scaling. The false discovery rate was set to 0.1 for the analysis parameters, while all other parameters were run with default settings. The Fisher ratio (FR) was calculated using Microsoft Excel (Microsoft Japan, Tokyo, Japan). The correlation coefficients between the spot areas of compounds and the calcium concentration were calculated and box plots were drawn using JMP version 12.0.0 (SAS Institute Inc., Cary, NC, USA).
In this study, groups of cows were established as follows. The MF group included the 8 cows that developed MF after parturition, and the normal group was made up of the remaining 17 cows that did not develop MF. The serum calcium concentration in the MF and normal groups ranged from 3.6 to 5.6 mg/dL (average, 4.8 mg/dL) and from 6.3 to 10.3 mg/dL (average, 7.7 mg/dL), respectively, within 12 hr of parturition (Supplementary Table 1).
Analytical platform using GC×GC/MS was checked by a quality-control sample that were prepared by mixing 100 µL serum samples of 15 normal cows. The reproducibility was confirmed by coefficient of variation lower than 30% in 562 of the 747 compounds detected, which was an accepted tolerance for GC/MS [8]. Thus, we examined the 25 serum samples in the GC×GC/MS system in triplicate. Figure 1A and 1B show representative contour plots of the GC×GC/MS total ion chromatograms of the serum metabolites from the MF and normal groups, respectively. Each spot indicates a trimethylsilyl-derivatized compound that was characterized by both the first-dimensional and second-dimensional retention times, resulting in a large number of detectable serum metabolites using the nontarget GC×GC/MS approach.
Fig. 1.
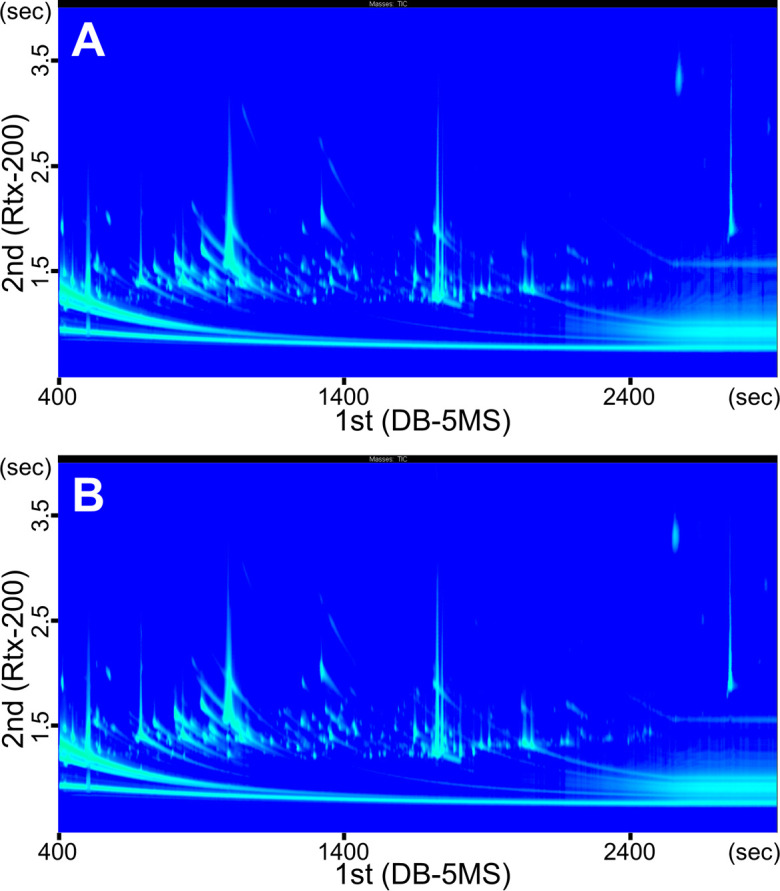
Two-dimensional gas chromatography mass spectrometry (GC×GC/MS) total ion chromatograms of the trimethylsilyl-derivatized serum compounds from the milk fever (MF) and normal groups. The figure shows representative two-dimensional contour plots of GC×GC/MS total ion chromatograms of trimethylsilyl-derivatized metabolites in serum samples obtained 3 weeks before parturition from pregnant cows that were further diagnosed (A) with and (B) without MF after parturition. The x- and y-axes show the primary and secondary column retention time (sec), respectively.
By aligning the 75 raw data using ChromaTOF software with the chromatographic deconvolution algorithm [12], 4,416 compounds whose signal-to-noise ratios were over 100 were detected in the 25 serum samples. If the peak area value of a compound was 0 in only one of the triplicate GC×GC/MS analyses of each sample, it was replaced with the average of the other two peak area values. If the peak area value was 0 in two of the triplicate analyses, the non-0 value was replaced with 0. In addition, the FR was calculated for each compound. The FR reflects the variation between different classes based on the detected signal, divided by the sum of the within-class variation in the signal [14]. Compounds with an FR <10 were excluded from subsequent analyses. Finally, any compound with three or fewer samples containing non-0 values was excluded, resulting that 1,498 compounds were obtained as significant compounds corresponding to serum metabolites from 75 GC×GC/MS results (Supplementary Table 2). Although all samples exhibited >700 compounds, only 225 of the 1,498 compounds were consistently detected in all samples. The MF group had 716 to 813 compounds and the normal group had 721 to 823 compounds, indicating no significant difference in the number of serum metabolites between the groups 3 weeks before parturition (P=0.132).
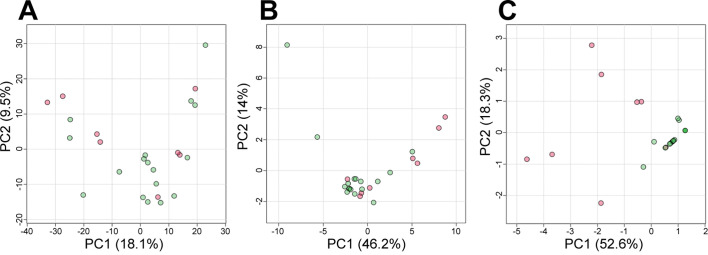
Following statistical analyses used median of peak area calculated from triplicated results in each serum sample and we implemented the following strategies to identify specific compounds in the serum of the MF group (Supplementary Fig. 1). To examine the variation in serum metabolites between the MF and normal groups, we conducted unsupervised PCA using a dataset of the 1,498 compounds. The PCA score plot did not distinctly separate the 25 cows into MF and normal groups along the first and second PCs, which explained only 27.6% of the total variation in the serum metabolic profiles (Fig. 2A). This result suggests that most of the serum metabolites varied by factors unrelated to MF among the 25 pregnant cows before parturition.
Fig. 2.
Nontarget metabolomics analyses of serum metabolites between the milk fever (MF) and normal groups. (A) principal component analysis (PCA) score plot of 1,498 metabolites (shown in Supplementary Table 2) in 75 Two-dimensional gas chromatography mass spectrometry (GC×GC/MS) results of serum samples obtained 3 weeks before parturition from pregnant cows that developed MF (MF group, n=8, median of triplicate data) and did not develop MF (normal group, n=17, median of triplicate data) after parturition. The red and green dots represent the MF and normal groups, respectively. PC1 and PC2 explain 18.1% and 9.5% of the total variance, respectively. (B) PCA score plot based on 35 metabolites with high correlation coefficients between the serum calcium concentration (Supplementary Table 3) in the 75 GC×GC/MS results. PC1 and PC2 explain 46.2% and 14% of the total variance, respectively. (C) PCA score plot based on 5 metabolites with statistically significant differences between the two groups and two-fold larger and half-fold smaller area in the MF group than in the normal group (Supplementary Table 4). PC1 and PC2 explain 52.6% and 18.3% of the total variance, respectively. MF, milk fever; PCA, principal component analysis; GC×GC/MS, two-dimensional gas chromatography mass spectrometry
Considering our hypothesis that some serum metabolites vary between the MF and normal groups, we next calculated correlation coefficients between the spot area of each compound in the dataset (Supplementary Table 2) and the calcium concentration (Supplementary Table 1). As a result, we could obtain a dataset of 35 compounds with moderate to high correlation coefficients of more than 0.5 or less than −0.5 with the cows’ serum calcium concentrations within 12 hr after parturition (Supplementary Table 3). We conducted PCA using the dataset of 35 compounds. The PCA score plot showed a moderate separation between the MF and normal groups (Fig. 2B). This result suggests that some specific serum metabolites were already distinctly different between the two groups 3 weeks before parturition.
Then we examined differences of the average spot area of 35 compounds between the two groups using a t-test and also calculated the fold change by dividing an average area of each compound in MF group by that of normal group (Supplementary Fig. 2). Based on statistically significant differences and two-fold larger or half-fold smaller between the two groups, 5 serum metabolites were selected from the 35 compounds (Supplementary Table 4). In PCA using a subset of the 5 serum metabolites, the plots of normal cows on the PCA score plots were relatively close, indicating that samples from the Normal group were very homogeneous (Fig. 2C). Notably, the first two principal components (PC1 and PC2) jointly accounted for 70.9% of the total variance in this PCA. This value was higher than those of other PCAs, accounting for 27.6% and 60.2% of the variance using the 1,498 and 35 compounds, respectively.
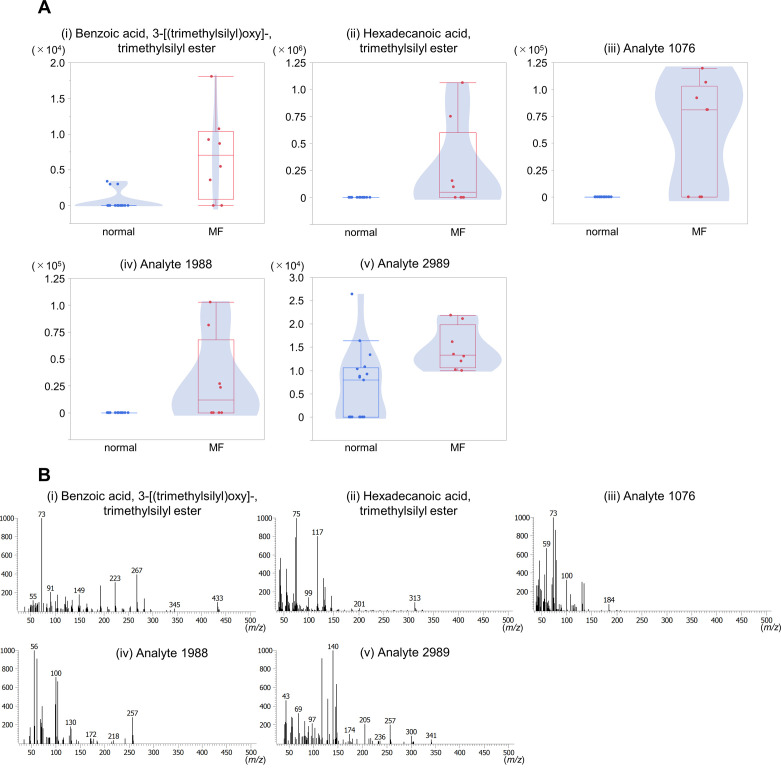
Figure 3A compares the peak areas of the 5 compounds between the MF and normal groups. All 5 serum metabolites were significantly higher in the MF group than in the normal group. Two compounds were benzoic acid and hexadecenoic acid. However, we encountered challenges in identifying the molecular species of 3 out of the 5 compounds. Unfortunately, MS libraries did not have the mass spectra with high similarity to those of the three compounds (Fig. 3B). These suggests that benzoic acid and hexadecenoic acid may be putative metabolites that varied in cows with risk of the MF 3 weeks before parturition. To improve our understanding of pathophysiology for MF in cows, further investigations are required to determine the molecular species of these unidentified compounds by analyzing their accurate mass and data of nuclear magnetic resonance.
Fig. 3.
Comparison of peak areas of the 5 metabolites that exhibited significant variation between the milk fever and normal groups and their correlations with serum calcium concentration. (A) Box-and-whisker plots showing the median, interquartile range, and maximum and minimum values. The y-axis indicates the peak area of 5 metabolites. (B) Mass spectra of the 5 metabolites from the representative samples. (i) Benzoic acid, 3-[(trimethylsilyl) oxy]-, trimethylsilyl ester. (ii) Hexadecanoic acid, trimethylsilyl ester. (iii) Analyte 1076. (iv) Analyte 1988. (v) Analyte 2989.
There is a limited reports on utilizing GC×GC/MS for exploring biomarkers in veterinary medicine. The comprehensive analysis of all serum compounds presents in a given biological sample poses a challenge for instrumental analysis. In conventional GC, where derivatized serum compounds are separated in a capillary column, the co-elution of several derivatized serum compounds with the same retention time often becomes problematic. However, the use of GC×GC can overcome this issue. In GC×GC, effluents from the first-dimensional (1D) column are trapped in a temperature-controlled modulator for a few seconds and then injected into the second dimension (2D) column [7]. During the second process, derivatized serum compounds that still co-elute at the end of the 1D separation, due to similar boiling points, can be separated based on their different polarities in the 2D phase. As a result, GC×GC/MS can detect ten times more serum compounds than conventional GC/MS. We propose that GC×GC/MS is useful for investigating serum metabolic profiles with complex mixtures of derivatized serum compounds, aiding in the examination of metabolic syndromes, including MF.
Metabolomics has been used to investigate the underlying causes of diseases and understand physiological states based on comprehensive profiles [1]. Given the need to detect a large number of serum metabolites for comprehensive nontarget metabolomics, GC×GC/MS with two distinct polarity columns provides advantages over conventional GC with a column that is limited by peak capacity [6, 13]. In the present study, GC×GC/MS-based serum metabolic fingerprints of 25 pregnant cows 3 weeks before parturition revealed that specific examination of certain compounds, including carboxylic acids, enabled us to distinguish between the MF and normal groups, while the overall profiles of the serum metabolites showed minimal variation between these two groups. A previous study showed that the serum concentrations of lactate, tumor necrosis factor, serum amyloid A, and haptoglobin were higher in cows with than without MF 4 weeks before parturition [20]. These findings strongly suggest that the serum levels of certain metabolites are altered in cows before the onset of MF. Nonetheless, however, we were unable to identify the molecular species of most serum metabolites that exhibited variation between the two groups because of minimal similarity with the MS libraries. By identifying such unknown MF-associated metabolites, we may gain an understanding of the pathophysiology underlying the onset of MF and develop efficient preventive strategies.
In this study, two approaches were used to investigate significant serum metabolites that exhibited variation in pregnant cows prior to the onset of MF. The first involved selection of compounds with high correlation coefficients between the serum calcium concentrations and the content of each compound detected from GC×GC/MS data of serum metabolites from the 25 cows for PCA. The second involved application of t-tests and fold-change analyses to each compound between the MF and normal groups to identify compounds suitable for PCA. Remarkably, both of these approaches identified carboxylic acids, among the extensive pool of more than 1,000 compounds. A previous report indicated that the serum profiles of fatty acids in pregnant cows change at least 1 week before parturition [16]. Fatty acids are also considered indicators of homeostasis disorders [4, 10]. Fatty acids may serve as biomarkers for predicting MF onset in cows. Further studies are needed to elucidate the potential pathological mechanisms underlying MF and the relationship between the pathogenesis and fatty acids.
Conflicts of Interest
We declare that we have no competing interests.
Supplementary Material
Acknowledgments
This work was funded by JSPS under KAKENHI Grant numbers 18K05988 (N.Y.). R.U. was supported by a Grant-in-Aid for JSPS Fellows.
REFERENCES
- 1.Ashrafian H, Sounderajah V, Glen R, Ebbels T, Blaise BJ, Kalra D, Kultima K, Spjuth O, Tenori L, Salek RM, Kale N, Haug K, Schober D, Rocca-Serra P, O’Donovan C, Steinbeck C, Cano I, de Atauri P, Cascante M. 2021. Metabolomics: The Stethoscope for the Twenty-First Century. Med Princ Pract 30: 301–310. doi: 10.1159/000513545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basoglu A, Baspinar N, Tenori L, Licari C, Gulersoy E. 2020. Nuclear magnetic resonance (NMR)-based metabolome profile evaluation in dairy cows with and without displaced abomasum. Vet Q 40: 1–15. doi: 10.1080/01652176.2019.1707907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai C, Kong Y, Wu D, Wang J. 2018. Changes of macrominerals and calcitropic hormones in serum of periparturient dairy cows subject to subclinical hypocalcaemia. J Dairy Res 85: 12–15. doi: 10.1017/S0022029918000031 [DOI] [PubMed] [Google Scholar]
- 4.Caron JP, Gandy JC, Brown JL, Sordillo LM. 2018. Docosahexaenoic acid-derived oxidized lipid metabolites modulate the inflammatory response of lipolysaccharide-stimulated macrophages. Prostaglandins Other Lipid Mediat 136: 76–83. doi: 10.1016/j.prostaglandins.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Curtis CR, Erb HN, Sniffen CJ, Smith RD, Powers PA, Smith MC, White ME, Hillman RB, Pearson EJ. 1983. Association of parturient hypocalcemia with eight periparturient disorders in Holstein cows. J Am Vet Med Assoc 183: 559–561. [PubMed] [Google Scholar]
- 6.Dallüge J, Beens J, Brinkman UAT. 2003. Comprehensive two-dimensional gas chromatography: a powerful and versatile analytical tool. J Chromatogr A 1000: 69–108. doi: 10.1016/S0021-9673(03)00242-5 [DOI] [PubMed] [Google Scholar]
- 7.Dimandja JMD, Clouden GC, Colón I, Focant JF, Cabey WV, Parry RC. 2003. Standardized test mixture for the characterization of comprehensive two-dimensional gas chromatography columns: the Phillips mix. J Chromatogr A 1019: 261–272. doi: 10.1016/j.chroma.2003.09.027 [DOI] [PubMed] [Google Scholar]
- 8.Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, Brown M, Knowles JD, Halsall A, Haselden JN, Nicholls AW, Wilson ID, Kell DB, Goodacre R. Human Serum Metabolome (HUSERMET) Consortium.2011. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 6: 1060–1083. doi: 10.1038/nprot.2011.335 [DOI] [PubMed] [Google Scholar]
- 9.Goff JP. 2014. Calcium and magnesium disorders. Vet Clin North Am Food Anim Pract 30: 359–381, vivi.doi: 10.1016/j.cvfa.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Hammami H, Vandenplas J, Vanrobays ML, Rekik B, Bastin C, Gengler N. 2015. Genetic analysis of heat stress effects on yield traits, udder health, and fatty acids of Walloon Holstein cows. J Dairy Sci 98: 4956–4968. doi: 10.3168/jds.2014-9148 [DOI] [PubMed] [Google Scholar]
- 11.Izadmanesh Y, Garreta-Lara E, Ghasemi JB, Lacorte S, Matamoros V, Tauler R. 2017. Chemometric analysis of comprehensive two dimensional gas chromatography-mass spectrometry metabolomics data. J Chromatogr A 1488: 113–125. doi: 10.1016/j.chroma.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 12.Mohler RE, Dombek KM, Hoggard JC, Young ET, Synovec RE. 2006. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry analysis of metabolites in fermenting and respiring yeast cells. Anal Chem 78: 2700–2709. doi: 10.1021/ac052106o [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondello L, Tranchida PQ, Dugo P, Dugo G. 2008. Comprehensive two-dimensional gas chromatography-mass spectrometry: a review. Mass Spectrom Rev 27: 101–124. doi: 10.1002/mas.20158 [DOI] [PubMed] [Google Scholar]
- 14.Pierce KM, Hoggard JC, Hope JL, Rainey PM, Hoofnagle AN, Jack RM, Wright BW, Synovec RE. 2006. Fisher ratio method applied to third-order separation data to identify significant chemical components of metabolite extracts. Anal Chem 78: 5068–5075. doi: 10.1021/ac0602625 [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez EM, Arís A, Bach A. 2017. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J Dairy Sci 100: 7427–7434. doi: 10.3168/jds.2016-12210 [DOI] [PubMed] [Google Scholar]
- 16.Rukkwamsuk T, Geelen MJH, Kruip TAM, Wensing T. 2000. Interrelation of fatty acid composition in adipose tissue, serum, and liver of dairy cows during the development of fatty liver postpartum. J Dairy Sci 83: 52–59. doi: 10.3168/jds.S0022-0302(00)74854-5 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Sasaki K, Sato Y, Devkota B, Furuhama K, Yamagishi N. 2013. Response of Holstein cows with milk fever to first treatment using two calcium regimens: a retrospective clinical study. J Vet Med Sci 75: 373–376. doi: 10.1292/jvms.12-0352 [DOI] [PubMed] [Google Scholar]
- 18.Schären M, Riefke B, Slopianka M, Keck M, Gruendemann S, Wichard J, Brunner N, Klein S, Snedec T, Theinert KB, Pietsch F, Rachidi F, Köller G, Bannert E, Spilke J, Starke A. 2021. Aspects of transition cow metabolomics-Part III: Alterations in the metabolome of liver and blood throughout the transition period in cows with different liver metabotypes. J Dairy Sci 104: 9245–9262. doi: 10.3168/jds.2020-19056 [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi N, Kawashima C. 2022. Prepartum measurement of serum biomarkers reflecting osteoclastic and osteoblastic bone metabolism for predicting the risk of milk fever in dairy cows. J Dairy Res 89: 1–9. doi: 10.1017/S0022029922000218 [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Dervishi E, Ametaj BN. 2018. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res Vet Sci 117: 167–177. doi: 10.1016/j.rvsc.2017.12.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.