ABSTRACT
Objectives:
Lumbar spondylolysis is a common condition; nonetheless, its cause in patients with spastic cerebral palsy (CP) remains unknown. Furthermore, examination of children with CP may not accurately capture complaints, thus causing diseases to be overlooked. Understanding the clinical features and gait patterns of lumbar spondylolysis in CP can aid in diagnosis. This study aimed to identify the clinical features and specific gait patterns of lumbar spondylolysis in ambulatory children with CP.
Methods:
Seventy-three children with CP were divided into two groups according to the presence or absence of lumbar spondylolysis on X-ray and magnetic resonance imaging. Three-dimensional gait analysis (3DGA) was performed to evaluate the kinematic data of the lower limbs.
Results:
Eight participants (11.4%) had lumbar spondylolysis primarily affecting the L5 vertebra. The lumbar spondylolysis group had a higher body weight and Body Mass Index, along with a smaller left popliteal angle on the spastic side. In 3DGA, detailed kinematic data indicated significant group differences in the mean angles of hip internal rotation (39.6° vs. 20.2°) during an entire gait cycle. The gait profile score was 19.7° in the lumbar spondylolysis group and 14.9° in the spinal uninvolved group; the difference in gait profile score between the two groups showed a minimal clinically important difference of 2.75.
Conclusions:
The overall gait profile score revealed that the gait of the lumbar spondylolysis group was deteriorated. Excessive internal rotation of the hip during gait might be a contributing factor to lumbar spondylolysis in children with CP.
Keywords: cerebral palsy, gait analysis, lumbar spondylolysis, popliteal angle
INTRODUCTION
The overexertion theory of bone damage recognizes that some participants may exceed tolerance limits in sports and other activities. This mechanism is considered the most common cause of spondylolysis.1,2) However, there are sporadic reports of spondylolysis in patients with cerebral palsy (CP) both with and without a history of sports participation.3) In cases of athetotic CP, twisting of the trunk and abnormal changes in muscle tone have been reported to cause spondylolysis.4) However, there is a lack of recent studies on spastic-type lumbar spondylolysis, with the most recent report dating back to 1993.5) The current study aimed to investigate the epidemiological and clinical features of spondylolysis in ambulatory children with spastic CP. In addition, given the absence of reports focusing on spondylolysis in CP that investigate gait patterns, the secondary objective of this study was to detect specific gait patterns in children with spastic CP who developed lumbar spondylolysis.
MATERIALS AND METHODS
This study was approved by the institutional review board of Hokkaido Medical Center for Child Health and Rehabilitation where the surgeries and measurements were performed (approval no.: 42–26; approval date: February 8, 2022). Informed consent was obtained from the patients in written form.
This study included 73 children with spastic CP (mean age, 10.4 years), and all participants received treatment at the same institution. At the time of hospitalization, informed consent was obtained from all patients for the academic use of their data, such as the type of treatments, treatment progress, and any other details acquired during their treatments; no identifiable information of the participants is included in this article. The children were categorized into two groups based on the presence or absence of lumbar spondylolysis, as determined by three orthopedic surgeons (R.F., H.F., Y.Y.) using X-ray and magnetic resonance imaging data. The Gross Motor Function Classification System (GMFCS)6) was used to classify the motor function of the children, with levels I–III indicating ambulatory status. The following variables of interest were assessed or recorded: age, sex, type of paralysis [spastic diplegia (SD) or spastic hemiplegia (SH)], height, weight, Body Mass Index (BMI), Functional Mobility Scale (FMS),7) presence of back pain, perinatal information (days of gestation and birth weight), right and left range of motion of hip joints (Thomas test, abduction, internal and external rotation), knee joint parameters (angle of extension, popliteal angle), various spinal and pelvic parameters from X-ray data (lumbar lordosis, pelvic inclination, pelvic tilt, sacral slope, pelvic incidence of lumbar lordosis), and the age of initial walking. These variables were compared between the two groups.
Gait Analysis
Kinematic data of the lower limbs were evaluated, and the Gait Profile Score (GPS)7) and Gait Variable Score (GVS)8) were calculated and compared. Multiple instrumented three-dimensional gait analysis (3DGA) data were used to assess prior gait function by utilizing quantitative scoring systems (Movement Analysis Profile [MAP], including the GVS and GPS).7,8,9) 3DGA data were collected using an MXF-20 motion capture camera (Vicon; Oxford, UK) with two force plates (AMTI; Watertown, MA, USA). Kinematic and kinetic data were calculated using Plug-in Gait software (Vicon). The MAP visually represents kinematic data with simple bar charts, improving readability over conventional graphs. The constituents of MAP were GVS and GPS, as described by Baker et al.8) Both GVS and GPS were measured in degrees. On a GVS chart, higher bars indicated greater deviation from control in each joint motion, whereas higher bars on GPS represented decreased function in the overall gait pattern. The GVS was calculated as the root mean square difference of the individual kinematic data, which consisted of nine kinematic parameters: pelvic tilt, pelvic obliquity, pelvic rotation, hip flexion, hip abduction, hip rotation, knee flexion, ankle dorsiflexion, and foot rotation. These parameters were compared against a control dataset collected from 38 normative Australian children (aged <18 years) from 2005 to 2007.8) We defined a change of 1.6° on the GPS as the minimal clinically important difference (MCID).10)
Statistical Analysis
The Mann–Whitney U-test was used for statistical analysis of the interval data, and Fisher’s exact probability test was used to analyze the data in a cross-contingency table. Statistical significance was set at P<0.05. Non-parametric tests were utilized in this study because of the small sample size, the presence of outliers, and the qualitative nature of data, which led to concerns about the normality of the data.
RESULTS
The GMFCS of the 73 participants was I in 23 cases, II in 41 cases, and III in 9 cases. The L5 vertebra was involved in seven of the eight affected cases, and the L4 was involved in one. Six L5 cases and one L4 case exhibited bilateral separation, whereas one L5 case displayed separation on the left side. The group with lumbar spondylolysis had a higher body weight (47.9 kg vs 31.3 kg; P=0.043) and BMI (22.5 kg/m2 vs 17.8 kg/m2; P=0.031) along with a smaller left popliteal angle (18.1° vs 35.2°; P=0.025) compared to the group without lumbar spondylolysis. Two patients (25%) in the lumbar spondylolysis group had lower back pain, whereas none of the patients without lumbar spondylolysis reported such symptoms (P=0.0001). No significant inter-group difference was observed for other variables (Tables 1 and 2).
Table 1. Patient background.
| Characteristic | Lumbar spondylolysis | Without lumbar spondylolysis | P value |
| Number | 8 | 65 | |
| GMFCS (I/II/III) | 3/5/0 | 20/36/9 | 0.132 |
| Age, years | 12.9 | 10 | 0.141 |
| Sex (male/female) | 5/3 | 35/30 | 0.648 |
| Type of paralysis (SD/SH) | 5/3 | 48/17 | 0.945 |
| Height, cm | 141.6 | 129.8 | 0.129 |
| Weight, kg | 47.9 | 31.3 | 0.043* |
| BMI, kg/m2 | 22.5 | 17.8 | 0.031* |
| FMS 5 m | 5.125 | 5.11 | 0.77 |
| 50 m | 5.00 | 4.94 | 0.976 |
| 500 m | 4.75 | 4.58 | 0.831 |
| Back pain (yes/no) | 2/6 | 0/65 | 0.0001* |
| Gestation, days | 232 | 229 | 0.696 |
| Birth weight, g | 2034.9 | 1935 | 0.832 |
*P<0.05.
Data given as number or mean.
Table 2. Range of motion of the knee joint and hip joint.
| Measurement | Lumbar spondylolysis | Without lumbar spondylolysis | P value |
| Hip range of motion (°) | |||
| Thomas (right) | 4.5 | 4.8 | 0.926 |
| Thomas (left) | 4.4 | 4.8 | 0.847 |
| Abduction (right) | 47.4 | 43.8 | 0.395 |
| Abduction (left) | 48 | 44.2 | 0.357 |
| Internal rotation (right) | 65 | 62.4 | 0.483 |
| Internal rotation (left) | 65 | 64.5 | 0.64 |
| External rotation (right) | 30 | 36.3 | 0.293 |
| External rotation (left) | 30 | 35.4 | 0.26 |
| Knee joint range of motion (°) | |||
| Extension angle (right) | −0.63 | −0.74 | 0.701 |
| Extension angle (left) | 0 | −0.49 | 0.921 |
| Popliteal angle (right) | 28 | 34.5 | 0.444 |
| Popliteal angle (left) | 18.1 | 35.2 | 0.025* |
| X-ray spine and pelvis parameters (°) | |||
| Lumbar lordosis | 25.9 | 20.2 | 0.204 |
| Pelvic inclination | 46 | 41.7 | 0.395 |
| Pelvic tilt | 22.4 | 20.1 | 0.679 |
| Sacral slope | 30.1 | 21.7 | 0.27 |
| Pelvic incidence of lumbar lordosis | 18.3 | 18.6 | 0.837 |
*P<0.05.
Data are given as mean values.
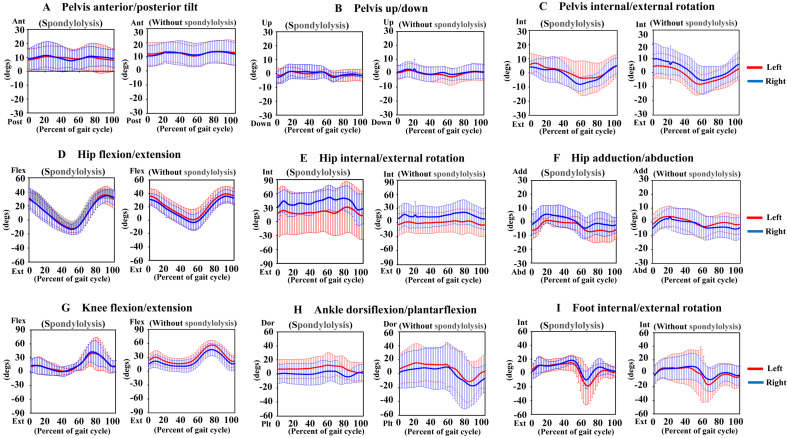
The gait analysis included 5 patients with CP (3 boys and 2 girls; mean age, 7.4 years) in the lumbar spondylolysis group and 20 patients with CP (10 boys and 10 girls; mean age, 8.0 years) in the group without lumbar spondylolysis (Table 3). The mean angle of hip internal rotation (lumbar spondylolysis group, 39.6°; group without lumbar spondylolysis, 20.2°; P=0.046) during one gait cycle demonstrated significant group differences (Table 4, Fig. 1). The GPS was 19.7° in the lumbar spondylolysis group and 14.9° in the group without lumbar spondylolysis. The observed gait difference corresponded to an MCID of 2.75 in GPS (Table 5). There was no significant inter-group difference in other gait parameters, including pelvis anterior/posterior tilt, pelvis vertical movement, pelvis internal/external rotation, hip flexion/extension, hip adduction/abduction rotation, knee flexion, plantar dorsiflexion of the ankle joint, and internal/external rotation of the foot.
Table 3. Patient background for cases in which gait analysis was possible.
| Characteristic | Lumbar spondylolysis | Without lumbar spondylolysis | P value |
| Number | 5 | 20 | |
| GMFCS (I/II/III) | 1/4/0 | 5/13/2 | 0.164 |
| Age, years | 7.4 | 8 | 0.71 |
| Sex (male/female) | 3/2 | 10/10 | 0.65 |
| Height, cm | 126.9 | 124.4 | 0.753 |
| Weight, kg | 27.6 | 33.2 | 0.312 |
| BMI, kg/m2 | 20.2 | 17.2 | 0.06 |
| Back pain (yes/no) | 4/1 | 0/20 | 0.0001* |
*P<0.05.
Data given as number or mean.
Table 4. Gait variable scores (average angle during one gait cycle).
| Measurement | Lumbar spondylolysis | Without lumbar spondylolysis | P value | ||||||
| Left | Right | Average | Left | Right | Average | ||||
| Pelvis | Anterior/posterior tilt (°) | 7.6 | 7.3 | 7.45 | 6.1 | 6.4 | 6.25 | 0.428 | |
| Up/down (°) | 3.9 | 4.3 | 4.1 | 4.9 | 4.8 | 4.85 | 0.13 | ||
| Internal/external rotation (°) | 8.3 | 5.9 | 7.1 | 7.5 | 8.8 | 8.15 | 0.857 | ||
| Hip | Flexion/extension (°) | 11.3 | 12 | 11.65 | 11.4 | 10.5 | 10.95 | 0.793 | |
| Internal/external rotation (°) | 40.7 | 38.5 | 39.6 | 18.6 | 21.8 | 20.2 | 0.046* | ||
| Adduction/abduction (°) | 6.3 | 6.8 | 6.55 | 6.7 | 7.2 | 6.95 | 0.138 | ||
| Knee | Flexion/extension (°) | 18.1 | 16.3 | 17.2 | 15.6 | 14.7 | 15.15 | 0.81 | |
| Ankle | Dorsiflexion/plantarflexion (°) | 13.6 | 9.4 | 11.5 | 16.7 | 13.5 | 15.1 | 0.526 | |
| Foot | Internal/external rotation (°) | 20.1 | 13.5 | 16.8 | 16.3 | 13.9 | 15.1 | 0.661 | |
*P<0.05.
Fig. 1.
Kinematic results for gait analysis of lumbar spondylolysis group and those without lumbar spondylolysis. (A) Pelvis anterior/posterior tilt, (B) pelvis up/down, (C) pelvis internal/external rotation, (D) hip flexion/extension, (E) hip internal/external rotation, (F) hip adduction/abduction, (G) knee flexion/extension, (H) ankle dorsiflexion/plantarflexion, (I) foot internal/external rotation. The (+) and (−) represent the measured values for each range of motion on the vertical axis.
Table 5. Gait profile score as deviation index.
| Lumbar spondylolysis | Without lumbar spondylolysis | ||||||
| Left | Right | Overall | Left | Right | Overall | ||
| Gait profile score (°) | 19.1 | 17.2 | 19.7 | 14 | 13.6 | 14.9 | |
DISCUSSION
According to previous reports, the prevalence of lumbar spondylolysis is about 1.6%–2.5% in typically developed children.3,11) However, in children with CP aged 0–15 years, including both spastic and athetotic types, the prevalence was notably higher, reaching 5.5%.3) In terms of the prevalence categorized by the type of paralysis, Sekiya3) reported a prevalence of 9.5% for the athetotic type and 2% for the spastic type. Harada et al.5) reported a higher prevalence of 21.4% for the spastic type, although the older age of the subjects (3–39 years) may have influenced this finding. In the present study, the prevalence of lumbar spondylolysis was 11.0%. Compared to typically developed children, lumbar spondylolysis was higher in children with CP, including both the spastic and athetotic types.
In a report on the prevalence of back pain based on the paralysis type, Nakamura et al.12) reported that back pain was experienced by about 70% of patients with athetotic CP aged 20–63 years. By contrast, lower back pain was experienced by 2.9% of patients with spastic-type CP; however, the age range of participants was different, spanning 2–24 years. Furthermore, Nakamura et al.12) reported that the prevalence of lumbar spondylolysis in patients with lower back pain was 58.6%. Among the patients in the present study who had spondylolysis, 25% complained of lower back pain. Although this figure is somewhat lower than that of the previous report, it underscores the importance of considering lumbar spondylolysis when children with CP complain of lower back pain. Notably, individuals without spondylolysis in this study did not report back pain.
The findings of this study regarding the affected vertebrae align with those of previous reports, indicating that the L5 vertebra was the most commonly affected in healthy, spastic, and athetotic children with CP (Table 6).5,12) This study also identified a single vertebral body with spondylolysis. Generally, the frequency of multiple lumbar spondylolysis cases is relatively low, constituting only 1.2%. Although the cause of lumbar spondylolysis is unknown, repeated trauma is believed to be a contributing factor. This belief is further supported by the higher prevalence of lumbar spondylolysis among men, likely because of increased participation in sports and heavy labor.13) Yanagisawa et al.14) reported that healthy girls aged 8–18 years with lumbar spondylolysis had significantly lower BMI than girls without lumbar spondylolysis. Their report suggested that healthy children may be more active in sports and other activities, resulting in overexertion and bone damage that may result in spondylolysis.14) By contrast, our study revealed that the children with lumbar spondylolysis had a higher BMI than those without the condition, implying a distinct mechanism of lumbar spondylolysis in children with CP. In typically developed children, lumbar spondylolysis is attributed to sports and other activities. Previous studies have suggested that increased intervertebral shear forces and intervertebral joint pressure caused by extension and rotation of the lumbar spine are responsible for the lumbar spondylolysis.1,2,15‒17) In the current study, it is important to note that BMI was within the normal range (18–25 kg/m2), even in the lumbar spondylolysis group, and we cannot necessarily say that a high BMI is a real risk factor. As shown by Yanagisawa et al.,14) in general, children with lumbar spondylolysis tend to be more active and have a lower BMI. We emphasize that a high BMI is not a risk factor; rather, the pathogenesis of lumbar spondylolysis with CP may differ from that of general spondylolysis.
Table 6. Levels of lumbar spondylolysis in the present study and previous reports.
| Vertebrae | This study | Harada et al.5) | Nakamura et al.12) |
| L5 | 7 | 18 | 10 |
| L4, L5 | 0 | 0 | 1 |
| L4 | 1 | 0 | 3 |
| L3, L4, L5 | 0 | 0 | 2 |
| L2, L3 | 0 | 0 | 1 |
Data given as number.
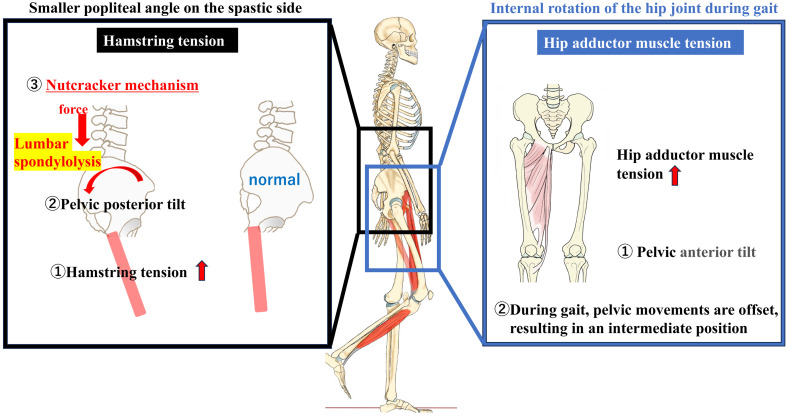
Based on these results, we studied the cause of lumbar spondylolysis in children with spastic CP and found a smaller popliteal angle in the group with spondylolysis than in the group without spondylolysis. Previous reports have suggested that a smaller popliteal angle results in high hamstring tension and pelvic posterior tilt.18,19) 3DGA is regarded as a common modality for evaluating postoperative outcomes and assisting in surgical decision-making in ambulatory patients with CP.10) This is the first study to use 3DGA to investigate the relationship between lumbar spondylolysis and gait patterns in children with CP. We found that the gait patterns of children with lumbar spondylolysis were characterized by excessive internal hip rotation. The physical findings and the results of the 3DGA suggest that posterior pelvic tilt caused by decreased popliteal angle (increased hamstring tension) and internal hip rotation during gait are associated with the development of lumbar spondylolysis in children with CP.
Although lumbar lordosis and pelvic anterior tilt have generally been associated with lumbar spondylolysis, an alternate theory suggests that patients with a posterior pelvic tilt, as observed in this study, may develop lumbar spondylolysis via a different mechanism. This theory is linked to the “nutcracker” mechanism, involving impingement of the posterior component (articular process), as described by Ogilvie and Sherman.20) Repeated impingement may cause lumbar spondylolysis in children with CP.
In addition, although we have discussed posterior pelvic tilt caused by decreased knee popliteal angle, gait analysis revealed no significant difference between the two groups in “movement of the pelvis.” In general, during gait, the tension of the hip adductor muscles, which are antigravity muscles, increases, especially in children with CP, as does the internal hip rotation and pelvic anterior tilt.21) The lack of a significant difference in “pelvic movement” in this study may be attributed to an offset of the pelvic posterior tilt because of hamstring tension and pelvic anterior tilt associated with hip internal rotation by the hip adductor muscle. The movement of hip internal rotation during gait may be a compensatory movement for the pelvic posterior tilt.
Figure 2 shows the mechanism of lumbar spondylolysis based on the results of this study and those from previous reports. We reconsidered the mechanism of lumbar spondylolysis, and it is assumed that in children with CP, tension in the hamstrings and hip adductor muscles increases as part of a pathological condition. We hypothesized that the tension in the hamstrings causes a posterior pelvic tilt, leading to the nutcracker mechanism, and that the tension in the hip adductor muscles, which are antigravity muscles, also increases during walking, further increasing the tension in the hamstrings and increasing the likelihood of lumbar spondylolysis. The conventional theory of Harada et al.5) is that hip flexion, knee flexion, and pelvic anterior tilt are common in the crouched position. The results of this study suggest that in children with CP who can walk, a pelvic posterior tilt and nutcracker mechanism may occur, which is different from the conventional mechanism of lumbar spondylolysis and is a new conclusion of this study. Pelvic movements may be offset during gait, resulting in an intermediate pelvic position.
Fig. 2.
The mechanism of lumbar spondylolysis in children with CP. It is assumed that in children with CP, tension in the hamstring and hip adductor muscles increases as part of a pathological condition. We hypothesized that tension in the hamstrings causes posterior pelvic tilt, leading to the nutcracker mechanism and facilitating lumbar spondylolysis. Figure elements prepared using IllustAC (https://www.ac-illust.com/).
This study has several limitations. First, this was a retrospective study with a short follow-up period and a limited number of patients. This may be the reason why only the left side showed a significant difference in knee popliteal angle. On the right side, this angle was 28° in the group with lumbar spondylolysis and 34.5° in the group without lumbar spondylolysis, which, although not statistically significant, suggested an apparent trend. We attributed the lack of a significant difference for this result to the small sample size of this study. Although the data from this study alone cannot elucidate all the mechanisms of lumbar spondylolysis, all patients with a significant difference in the popliteal angle of the left knee also had lumbar spondylolysis on the left side. There were no cases of only right-sided lumbar spondylolysis. As described above, we believe that one mechanism of lumbar spondylolysis in children with CP is increased hamstring tension, which leads to a posterior pelvic tilt and nutcracker mechanism. Based on this hypothesis, we believe that the left-sided decrease in the popliteal angle is consistent with the results of all cases of left-sided spondylolysis. It is also possible that in bilateral cases, lumbar spondylolysis occurred on the left side and then on the right side, resulting in lumbar spondylolysis; however, this mechanism is not clear from the results of this study alone.
Other limitations include the possibility of selection bias caused by small sample size and restricted group allocation (GMFCS I and II). In addition, gait analysis was not performed on all patients. Despite these limitations, this study is the first to perform gait analysis in children with CP who have lumbar spondylolysis, aiming to explore dynamic causes. This approach suggests a cause of lumbar spondylolysis that differs from those proposed previously.
CONCLUSION
We investigated the clinical features of lumbar spondylolysis in children with spastic CP who could walk. The incidence of spastic CP spondylolysis was approximately 11%, with 25% of these patients experiencing lower back pain. The most frequently affected vertebra was L5. Notably, patients with spastic CP were characterized by a high body weight and BMI, along with a smaller left popliteal angle on the spastic side. Gait analysis suggested that excessive internal rotation of the hip joint during gait might contribute to lumbar spondylolysis in children with CP. According to the study results, if a child with CP exhibits the aforementioned gait pattern, it may warrant suspicion of lumbar spondylolysis, prompting the need for diagnostic imaging tests.
ACKNOWLEDGMENTS
We thank Editage (www.editage.com) for English language editing. We also gratefully acknowledge the work of the past and present members of our laboratory.
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Sairyo K,Katoh S,Komatsubara S,Terai T,Yasui N,Goel V, Vadapalli S, Biyani A, Ebraheim N: Spondylolysis fracture angle in children and adolescents on CT indicates the facture producing force vector: a biomechanical rationale. Internet J Spine Surg 2004;1. http://ispub.com/IJSS/1/2/3373. [Google Scholar]
- 2.Terai T,Sairyo K,Goel VK,Ebraheim N,Biyani A,Faizan A,Sakai T,Yasui N: Spondylolysis originates in the ventral aspect of the pars interarticularis: a clinical and biomechanical study. J Bone Joint Surg Br 2010;92-B:1123–1127. 10.1302/0301-620X.92B8.22883 [DOI] [PubMed] [Google Scholar]
- 3.Sekiya A: The spondylolysis and spondylolisthesis in cerebral palsied children: a view of the etiologic factor of spondylolysis [in Japanese]. TWMUJ 1974;44:841–850. [Google Scholar]
- 4.Peter JC,Hoffman EB,Arens LJ: Spondylolysis and spondylolisthesis after five-level lumbosacral laminectomy for selective posterior rhizotomy in cerebral palsy. Childs Nerv Syst 1993;9:285–288. 10.1007/BF00306275 [DOI] [PubMed] [Google Scholar]
- 5.Harada T,Ebara S,Anwar MM,Kajiura I,Oshita S,Hiroshima K,Ono K: The lumbar spine in spastic diplegia. A radiographic study. J Bone Joint Surg Br 1993;75-B:534–537. 10.1302/0301-620X.75B4.8331105 [DOI] [PubMed] [Google Scholar]
- 6.Palisano R,Rosenbaum P,Walter S,Russell D,Wood E,Galuppi B: Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–223. 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 7.Harvey A,Graham HK,Morris ME,Baker R,Wolfe R: The Functional Mobility Scale: ability to detect change following single event multilevel surgery. Dev Med Child Neurol 2007;49:603–607. 10.1111/j.1469-8749.2007.00603.x [DOI] [PubMed] [Google Scholar]
- 8.Baker R,McGinley JL,Schwartz M,Thomason P,Rodda J,Graham HK: The minimal clinically important difference for the Gait Profile Score. Gait Posture 2012;35:612–615. 10.1016/j.gaitpost.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 9.Baker R,McGinley JL,Schwartz MH,Beynon S,Rozumalski A,Graham HK,Tirosh O: The gait profile score and movement analysis profile. Gait Posture 2009;30:265–269. 10.1016/j.gaitpost.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 10.Fujita H,Fusagawa H,Nishibu H,Nosaka T,Matsuyama T,Iba K,Yamashita T: Motion analysis and surgical results of anterior transfer of flexor hallucis longus for equinovarus gait in children with hemiplegia. J Orthop Sci 2021;26:441–447. 10.1016/j.jos.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Zippel H,Runge H: [Morbid anatomy and pathogenesis of spondylolysis and spondylolysthesis in childhood (author’s transl)]. Z Orthop Ihre Grenzgeb 1976;114:189–201. [PubMed] [Google Scholar]
- 12.Nakamura E,Uchida K,Yamada H,Sakai N,Kawasaki M,Nanamori K,Nakamura T: Low back pain in athetoid cerebral palsy due to lumber spondylolyses [in Japanese]. Seikeigeka to Saigai Geka 2004;53:307–312. 10.5035/nishiseisai.53.307 [DOI] [Google Scholar]
- 13.Ravichandran G: Multiple lumbar spondylolyses. Spine 1980;5:552–557. 10.1097/00007632-198011000-00011 [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa R,Tsukagoshi Y,Nakashima Y,Hagino N,Wakatsuki M,Tomaru Y,Morita M,Minamoto Y: Analysis of the physique of adolescents with lumbar spondylolysis [in Japanese]. Jpn J Clin Sports Med 2018;26:242–246. [Google Scholar]
- 15.Murphy KP: The adult with cerebral palsy. Orthop Clin North Am 2010;41:595–605. 10.1016/j.ocl.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Mihara H,Onari K,Cheng BC,David SM,Zdeblick TA: The biomechanical effects of spondylolysis and its treatment. Spine 2003;28:235–238. 10.1097/01.BRS.0000042226.59713.0E [DOI] [PubMed] [Google Scholar]
- 17.Sakamaki T,Katoh S,Sairyo K: Normal and spondylolytic pediatric spine movements with reference to instantaneous axis of rotation. Spine 2002;27:141–145. 10.1097/00007632-200201150-00004 [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki R,Yokoyama G,Kawabata S,Suzuki T: Lumbar extension during stoop lifting is delayed by the load and hamstring tightness. J Phys Ther Sci 2014;26:57–61. 10.1589/jpts.26.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jandre Reis FJ,Macedo AR: Influence of hamstring tightness in pelvic, lumbar and trunk range of motion in low back pain and asymptomatic volunteers during forward bending. Asian Spine J 2015;9:535–540. 10.4184/asj.2015.9.4.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogilvie JW,Sherman J: Spondylolysis in Scheuermann’s disease. Spine 1987;12:251–253. 10.1097/00007632-198704000-00010 [DOI] [PubMed] [Google Scholar]
- 21.Dobson F,Morris ME,Baker R,Graham HK: Gait classification in children with cerebral palsy: a systematic review. Gait Posture 2007;25:140–152. 10.1016/j.gaitpost.2006.01.003 [DOI] [PubMed] [Google Scholar]