Abstract
The study aimed to evaluate the effect of an intervention on the prevalence and severity of incontinence‐associated dermatitis (IAD) in six hospitals in one state in Australia. This quasi‐experimental pre‐and post‐study, conducted in 18 wards, was part of a larger implementation science study on incontinence‐associated dermatitis. Skin and incontinence assessments were conducted on patients during February and March 2020 (pre‐intervention) and July and August 2021 (post‐intervention). The intervention comprised continence assessment and management, an education brochure for patients, family and caregivers on IAD, the Ghent Global IAD Categorisation Tool (GLOBIAD) and a skin care regime with patient skin protection measures (three‐in‐one barrier cream cloths, minimisation of bed protection layers, use of appropriate continence aid). A total of 1897 patients were assessed (pre‐intervention = 964, post‐intervention = 933). A total of 343 (35.6%) pre‐intervention patients and 351 (37.6%) post‐intervention patients had incontinence. The prevalence of hospital‐acquired IAD was 6.71% in the pre‐intervention group and 4.27% in the post‐intervention group; a reduction of 36.3% (p = 0.159) despite higher patient acuity, prevalence of double incontinence and the COVID‐19 pandemic in the post‐intervention group compared with the pre‐intervention group. Our multisite best practice IAD prevention and treatment intervention was able to reduce the prevalence and severity of hospital‐acquired IAD, suggesting enduring effectiveness of the intervention.
Keywords: faecal incontinence, irritant dermatitis, pressure ulcer, prevalence, urinary incontinence
1. INTRODUCTION
Incontinence‐associated dermatitis (IAD) is a painful skin condition linked to hospital‐acquired pressure injuries (PI). It is often overlooked and misdiagnosed, resulting in delayed and costly treatment, while causing significant patient discomfort. In various studies, the prevalence of IAD in hospital patients has been reported to range from 1.44% to 21.3%, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 but is known to be under‐reported largely due to misdiagnosis and variation in IAD data collection methods. 11
IAD causes physical, social and emotional impact on patients. 12 A recent qualitative study of 10 patients with IAD and a family caregiver of a patient with IAD in Australia found it to be not only a debilitating painful condition but a condition that left patients desperate for relief. 13 Patients' IAD were aggravated by leaking pads and reduced mobility or being bed bound. They described the experience of having IAD as characterised by a painful, burning, itching sensation. Some patients were unable to resist the temptation to scratch themselves to relieve their discomfort, resulting in some cases of bleeding sores.
The surveillance of IAD is widely accepted as a crucial step towards patient safety and has become a recommended component of PI prevention programmes and audits. 14 , 15 IAD is a concern in both acute hospitals and long‐term aged care settings as it can lead to the development of PIs. 14 The World Health Organization's International Classification of Diseases, 11th edition (ICD‐11) code for IAD (section EK02. 22, Irritant contact dermatitis due to incontinence) 16 has been recently established globally, signifying this condition as a global health priority. Despite the best efforts of the World Health Organization to change the terminology for IAD to a global language of irritant contact dermatitis due to incontinence, 17 , 18 , 19 in Australia, this has not yet been adopted and the 10th edition (ICD‐10‐AM [Australia Modified]) classification system is still used (L22, Diaper dermatitis). Identifying the risks for, and occurrence of, IAD at an organisational level has the potential to redirect resources to reduce the impact of IAD for both the patient and the organisation.
As part of a large implementation science study (here on referred to as the IMBED study, which stands for ‘A novel implementation of best available evidence into practice for incontinence‐associated dermatitis [IMBED])’, 20 we developed, implemented and tested an evidence‐based healthcare bundle 14 to prevent and manage IAD in one state in Australia. This paper presents quantitative findings on the impact of the intervention on the prevalence of IAD.
2. METHODS
2.1. Design
The IMBED study was a quasi‐experimental, pre‐ and post‐translational research study to determine, primarily, the prevalence of IAD, using an implementation science approach—the Promoting Action on Research Implementation in Health Services (PARIHS) framework . 21 Owing to the COVID‐19 pandemic, this study consisted of a pre‐intervention period of 12 months (October 2019 to September 2020) and a post‐intervention period of 15 months (May 2021–July 2022), separated by the implementation of the IMBED multifaceted initiative. This study is reported according to the modified Consolidated Standards of Reporting Trials (CONSORT) statement 22 and the Template for Intervention Description and Replication (TIDieR) checklist for interventions. 23
2.2. Setting and participants
The study was undertaken across six hospitals (four major referral hospitals, one regional and one rural hospital) in five health districts (three metropolitan, two rural) across New South Wales (NSW), Australia. Eighteen wards participated (three per hospital), comprising three 28‐bed wards from the four metropolitan hospitals and three 15‐bed wards from the two regional hospitals (426 beds in total), specialising in subacute and rehabilitation medicine, acute geriatrics, palliative care, respiratory and gastroenterology, general medical, surgical and intensive care. No long‐term care facilities such as nursing homes were included in the study.
2.3. Intervention
The intervention was multifaceted and comprised of clinician and patient facing approaches (Table 1).
TABLE 1.
Study intervention.
| Intervention component | Description | |
|---|---|---|
| 1 | Education |
Five modules 1. Continence Assessment and Management 2. IAD Aetiology and Risk 3. IAD Classification and Diagnosis 4. IAD Prevention and Management 5. Case Studies The modules were developed by an expert working group with knowledge and expertise in IAD. They were reviewed by two national experts in the field of IAD. All staff within the six facilities received education on the intervention and regime. The education underwent iterative refinement, incorporating clinicians' feedback and recurring intervals to reinforce the intervention. |
| 2 | Incontinence assessment and management |
Step 1. Incontinence Assessment and Management in a Hospital Setting Flow Chart (a validated flow chart with guiding interventions and referral measures) Step 2. Three‐Day Incontinence Record Chart Step 3. Continence Product Chart |
| 3 | Education of patients, family and caregivers | Patient, family and caregiver education brochure on IAD |
| 4 | Practice guidelines and categorisation tool |
|
| 5 | Patient skin protection |
|
2.4. Standard care
Standard care varied across each hospital, with hospitals either implementing some, but not all, components of the best practice guidelines, or none that aligned with best practice. The latter included not assessing for incontinence status with a screening tool, not conducting daily skin assessments, not categorising IAD with a validated tool, using multiple bed protection layers under the patient, using an inappropriately sized aid or aid with inappropriate absorbency and applying thick layers of barrier cream onto the patient's skin impairing the microclimate.
2.5. Outcome measures
The primary outcome measures were the prevalence of incontinence and IAD at the time of assessment. Incontinence was defined as when any urine or faeces was not passed into the toilet. IAD was categorised using the GLOBIAD. 24
2.6. Measures
Data were collected using two data collection tools that were used in previous clinical audits (hereafter known as ‘audit’) conducted in one of the participating Local Health Districts (LHDs). 3 , 25 The research officer (RO) completed the data collection as part of the audit at the participating sites:
An IAD and incontinence audit tool focused on assessing patient incontinence and skin, used in a previous audit conducted in one of the participating LHDs. 3 , 25 Data collected included level of incontinence soiling, stool quality and frequency, stoma, mobility status, incontinence products used (including cleansers, moisturisers, pads), other skin injuries (including pressure injuries, skin tears and other moisture‐associated skin damage [not IAD]).
A Baseline Data Collection Form, recording basic patient health and demographic data, such as patient primary and secondary diagnosis, type of admission, skin tone and outcomes, including length of stay (LoS). Patient demographics (age, gender, diagnosis, comorbidities) and outcomes (LoS, reason for discharge) were also collected by the RO from the electronic medical record.
2.7. Procedures
Following relevant ethics and site governance approvals, an RO was employed at each site. ROs were trained by the investigators in the study procedures including use of the GLOBIAD categorisation tool 24 and diagnosis of IAD. Over two‐month periods in each phase (February–March 2020 [pre‐intervention] and July–August 2021 [post‐intervention]) (Figure 1). A public health emergency for COVID‐19 was declared in March 2020. 26 One random study ward at each hospital was assigned by the programme manager to be audited each week. All patients present on the ward were eligible to be in the audited sample. All patients on the study wards were assessed for incontinence and underwent a skin assessment as part of the audit. The local site investigator (the skin integrity lead at the facility or LHD) assisted the RO with the first audit and provided real‐time training and guidance.
FIGURE 1.

Schema of clinical audit activities.
All audits were conducted with the cooperation of the ward staff, the clinical nurse educator (CNE) and the nurse unit manager (NUM). The CNEs provided onsite support and assistance to the RO to ensure the study activities were carried out successfully. The programme manager provided the RO notice 1 week in advance of the random ward to be audited. The RO would then notify the NUM and CNE of the chosen ward of the forthcoming audit and arrange a mutually convenient day and time.
Each patient, including those who were verbally or cognitively impaired, was approached individually by the RO, informed of the purpose and processes of the audit and verbal consent was obtained. Patients were able to opt out of the study by signing an opt‐out form. A head‐to‐toe skin inspection was conducted using the methods outlined in Barakat‐Johnson et al.'s study 27 and patients were assessed for incontinence.
All patient data were de‐identified when recorded and entered directly into a secure web‐based platform, Research Electronic Data Capture (REDCap) 28 database, hosted by the University of Sydney.
2.8. Ethical considerations
The study was conducted in accordance with the National Health and Medical Research Council's (NHMRC) National Statement on Ethical Conduct in Human Research. 29 Ethical approval for study procedures was obtained by the local health district hospital research ethics committee (approval number: HERCC/EXCOR\19–05; X19‐0121 & 2019/ETH08742).
The study employed an opt‐out consent procedure as skin assessments are part of normal nursing care and the intervention addressed the implementation of best evidence‐based practice. A participant information sheet with an opt‐out form attached to it containing the purpose, procedure and confidentially was provided to each participant, who could verbally indicate they would like to opt out and sign the opt‐out form attached to the participant information sheet. Patients who were not present at the time of the audit or were deemed too ill were not audited.
2.9. Statistical analysis
Data were exported as an Excel file and then analysed using IBM SPSS Statistics Version 26. 30 The sample was summarised descriptively, by pre‐ and post‐cohorts. Any substantive imbalances on key prognostic factors between cohorts were identified. An uncontrolled analysis of the association between the intervention and the primary outcome of IAD was initially conducted using a Z‐test for binomial proportions, assuming no commonalities within hospitals. This structure was tested for model fit against a 2‐level data hierarchy, with anticipated commonalities of patients (Level 1) within hospitals (Level 2). A multilevel logistic regression analysis was conducted, including the intervention variable, the incontinence type variable and the propensity score for covariates.
This approach ensured that the minimum criteria of 10 events per variable, as recommended by simulation studies, 31 were maintained. Significance levels, odds ratios (OR) and associated 95% confidence intervals (CI) were reported for all constituent components of the model. The intraclass correlation coefficient (ICC) for the regression model was also determined. To investigate the extent of the influence of each participating hospital on the outcome of interest, sensitivity studies on the primary outcome were conducted with each hospital in turn removed from the dataset, comprising multilevel multiple logistic regression analyses of IAD prevalence as in the main analysis. The constituent variables and propensity score were defined as in the main analysis.
Severity of IAD prevalence and length of patient stay in hospital were secondary outcomes and were reported descriptively. Length of patient stay in hospital was assessed in an unadjusted model comparing values across patient cohorts using independent samples t‐testing.
3. RESULTS
A total of 964 patients participated in the pre‐intervention audit and had their skin and continence status assessed, with 343 deemed incontinent. In the post‐intervention audit, 933 patients had their skin and continence status assessed, with 351 deemed incontinent (Figure 2). Incontinent patient characteristics in the two cohorts were broadly similar. The post‐intervention implementation cohort appeared to have inferior health profiles including higher proportions of bed‐bound patients, those who were doubly incontinent and greater length of hospital stay. (Table 2).
FIGURE 2.
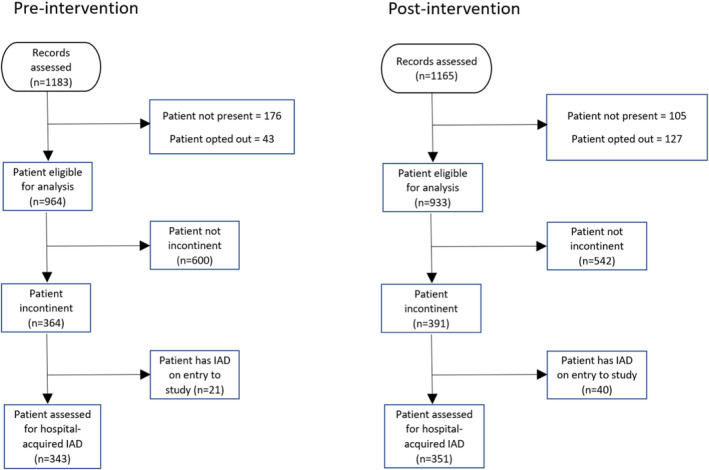
Modified CONSORT diagram. 19
TABLE 2.
Key patient characteristics pre‐ and post‐intervention bundle.
| Variable | Pre‐intervention | Post‐intervention | ||||
|---|---|---|---|---|---|---|
| No IAD | IAD | All patients | No IAD | IAD | All patients | |
| Sex | ||||||
| Female | 173 (54.1%) | 14 (60.9%) | 187 (54.5%) | 182 (54.5%) | 6 (40.0%) | 188 (53.6%) |
| Male | 147 (45.9%) | 9 (39.1%) | 156 (45.5%) | 154 (45.8%) | 9 (60.0%) | 163 (46.4%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Mean age (SD; range) | 80.4 (11.4; 44–103) | 77.8 (15.0; 48–98) | 80.2 (11.7; 44–103) | 79.5 (11.6; 33–101) | 79.9 (9.3; 60–93) | 79.5 (11.5; 33–101) |
| Incontinence status | ||||||
| Faecal only | 49 (15.3%) | 4 (17.4%) | 53 (15.5%) | 54 (16.1%) | 1 (6.7%) | 55 (15.7%) |
| Urinary only | 113 (35.3%) | 4 (17.4%) | 117 (34.1%) | 90 (26.8%) | 3 (20.0%) | 93 (26.5%) |
| Urinary and faecal | 158 (49.4%) | 15 (62.5%) | 173 (50.4%) | 192 (57.1%) | 11 (73.3%) | 203 (57.8%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Specialities | ||||||
| Stroke/rehabilitation | 72 (22.5%) | 5 (21.7%) | 77 (22.4%) | 66 (19.6%) | 1 (6.7%) | 67 (19.1%) |
| Aged care/geriatrics acute | 134 (41.8%) | 13 (56.5%) | 147 (42.9%) | 131 (39.0%) | 8 (53.3%) | 139 (39.6%) |
| Medical/general medical | 68 (21.3%) | 2 (8.7%) | 70 (20.4%) | 89 (26.5%) | 2 (13.3%) | 91 (25.9%) |
| Gastroenterology and respiratory | 17 (5.3%) | 2 (8.7%) | 19 (5.5%) | 14 (4.2%) | 1 (6.7%) | 15 (4.3%) |
| ICU/palliative care | 16 (5.0%) | 0 (0%) | 16 (4.7%) | 9 (2.7%) | 0 (0%) | 9 (2.6%) |
| General surgical | 13 (4.1%) | 1 (4.3%) | 14 (4.1%) | 27 (8.0%) | 3 (20.0%) | 30 (8.5%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Mobility | ||||||
| Full mobility | 33 (10.3%) | 1 (4.3%) | 34 (9.9%) | 30 (8.9%) | 2 (13.3%) | 32 (9.1%) |
| Restricted mobility | 175 (54.7%) | 11 (47.8%) | 186 (54.2%) | 167 (49.7%) | 4 (26.7%) | 171 (48.7%) |
| Bed bound | 107 (33.4%) | 11 (47.8%) | 118 (34.4%) | 139 (41.4%) | 9 (60.0%) | 148 (42.2%) |
| Unknown | 5 (1.6%) | 0 (0%) | 5 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Urinary catheter status | ||||||
| No urinary catheter in situ | 272 (85.0%) | 19 (82.6%) | 291 (84.8%) | 270 (80.4%) | 15 (100%) | 285 (81.2%) |
| Urinary catheter in situ | 42 (13.1%) | 4 (17.4%) | 46 (13.4%) | 46 (13.7%) | 0 (0%) | 46 (13.9%) |
| Unknown | 6 (1.9%) | 0 (0%) | 6 (1.8%) | 20 (6.0%) | 0 (0%) | 20 (5.7%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Faecal containment device | ||||||
| No faecal containment device | 174 (54.4%) | 19 (82.6%) | 193 (56.3%) | 189 (56.3%) | 7 (46.7%) | 196 (55.8%) |
| Faecal containment device | 2 (0.6%) | 0 (0%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 144 (45.0%) | 4 (17.4%) | 148 (43.1%) | 147 (43.8%) | 8 (53.3%) | 155 (44.2%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Soiling | ||||||
| High | 47 (14.7%) | 6 (26.1%) | 53 (15.5%) | 50 (14.9%) | 5 (33.3%) | 55 (15.7%) |
| Medium | 143 (44.7%) | 11 (47.8%) | 154 (44.9%) | 100 (29.8%) | 3 (20.0%) | 103 (29.3%) |
| Low | 76 (23.8%) | 4 (17.4%) | 80 (23.3%) | 70 (20.8%) | 4 (26.7%) | 74 (21.1%) |
| Unknown | 54 (16.9%) | 2 (8.7%) | 56 (16.3%) | 116 (34.5%) | 3 (20.0%) | 119 (33.9%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Stool quality/frequency | ||||||
| Formed | 30 (9.4%) | 1 (4.3%) | 31 (9.0%) | 24 (7.1%) | 1 (6.7%) | 25 (7.1%) |
| Semi‐formed | 106 (33.1%) | 9 (39.1%) | 115 (33.5%) | 127 (37.8%) | 6 (40.0%) | 133 (37.9%) |
| Liquid | 23 (7.2%) | 7 (30.4%) | 30 (8.7%) | 15 (4.5%) | 1 (6.7%) | 16 (4.6%) |
| Unknown | 161 (50.3%) | 6 (26.1%) | 167 (48.8%) | 170 (50.5%) | 7 (46.7%) | 177 (50.4%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Stoma (urinary) | ||||||
| No | 319 (99.7%) | 23 (100%) | 342 (99.7%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Yes | 1 (0.3%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Stoma (faecal) | ||||||
| No | 317 (99.1%) | 23 (100%) | 340 (99.1%) | 329 (97.9%) | 15 (100%) | 344 (98.0%) |
| Yes | 3 (0.9%) | 0 (0%) | 3 (0.9%) | 7 (2.1%) | 0 (0.0%) | 7 (2.0%) |
| Total | 320 (100%) | 23 (100%) | 343 (100%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Primary diagnosis | ||||||
| Diseases and disorders of the nervous system | 70 (21.9%) | 5 (21.7%) | 75 (21.9%) | 76 (22.6%) | 3 (20.0%) | 79 (22.5%) |
| Diseases and disorders of the respiratory system | 23 (7.2%) | 2 (8.7%) | 25 (7.3%) | 31 (9.2%) | 1 (6.7%) | 32 (9.1%) |
| Diseases and disorders of the digestive system | 13 (4.1%) | 1 (4.3%) | 14 (4.1%) | 21 (6.3%) | 0 (0%) | 21 (6.0%) |
| Diseases and disorders of the musculoskeletal system and connective tissue | 43 (13.4%) | 4 (17.4%) | 47 (13.7%) | 41 (12.2%) | 4 (26.7%) | 45 (12.8%) |
| Diseases and disorders of the kidney and urinary tract | 21 (6.6%) | 0 (0.0%) | 21 (6.1%) | 24 (7.1%) | 0 (0.0%) | 24 (6.8%) |
| Factors influencing health status and other contacts with health services |
73 (22.8%) 0 (0%) |
8 (34.8%) 0 (0%) |
81 (23.6%) 0 (0%) |
74 (22.0%) 2 (0.6%) |
5 (33.3%) 0 (0%) |
79 (22.5%) 2 (0.6%) |
| Other a | 49 (15.3%) | 3 (13.0%) | 52 (15.2%) | 65 (19.3%) | 2 (13.3%) | 66 (18.8%) |
| Not recorded | 28 (8.8%) | 0 (0%) | 28 (8.2%) | 5 (1.5%) | 0 (0%) | 5 (1.4%) |
| Total | 320 (100%) | 23 (100%) | 343 (100.0%) | 336 (100%) | 15 (100%) | 351 (100%) |
| Hospital days (n = 349 post) | 19.8 (22.5; 1–140) | 28.7 (25.4; 4–90) | 20.3 (22.8; 1–140) | 24.9 (39.9; 0–388) | 16.1 (10.9; 6–46) | 24.5 (39.1; 0–388) |
| Length of stay (n = 347 post) | 34.0 (29.6; 1–149) | 44.5 (34.9; 7–124) | 34.7 (30.0; 1–149) | 41.7 (54.4; 2–482) | 24.6 (18.9; 7–78) | 41.0 (53.5; 2–482) |
= Includes the following primary diagnosis codes: Diseases and disorders of the ear, nose and throat, Diseases and disorders of the circulatory system, Diseases and disorders of the hepatobiliary system and pancreas, Diseases and disorders of the skin, subcutaneous tissue and breast, Endocrine, nutritional and metabolic diseases and disorders, Diseases and disorders of the female reproductive system, Diseases and disorders of blood, blood forming organs; immunological disorders, Neoplastic disorders (haemotological and solid neoplasms), Infectious and parasitic diseases, systemic or unspecified sites, Injuries, poisonings and toxic effects of drugs; multiple trauma, Injuries, poisonings and toxic effects of drugs, General issues unrelated to principal diagnosis.
3.1. Incontinence‐associated dermatitis prevalence rates
Twenty‐three of 343 patients (6.71%) had hospital‐acquired IAD in the pre‐intervention group and 15 out of 351 (4.27%) in the post‐intervention group, a reduction of 36.3%. The difference in proportions of IAD cases between the two pre‐ and post‐cohorts was 2.43 percentage points (95% confidence interval [CI] −0.96 to 5.82, p = 0.159). The odds of IAD in post‐implementation intervention cohort compared to the pre‐implementation intervention cohort was 0.621 (95% CI 0.318 to 1.21). Characteristics of observed IAD prevalence are summarised in Table 3.
TABLE 3.
Incontinence‐associated dermatitis characteristics in the pre‐ and post‐implementation cohorts.
| Pre‐implementation | Post‐implementation | |
|---|---|---|
| Hospital‐acquired IAD | 23 (6.8%) | 15 (4.3%) |
| IAD category a (n = 23 pre; n = 15 post) | ||
| 1A | 16 (69.6%) | 11 (73.3%) |
| 1B | 3 (13.0%) | 1 (6.7%) |
| 2A | 2 (8.7%) | 3 (20.0%) |
| Unknown/unable to ascertain | 2 (8.7%) | 0 (0.0%) |
| IAD principal location (n = 23 pre; n = 15 post) | ||
| Buttocks | 7 (30.4%) | 4 (26.7%) |
| Gluteal fold/cleft | 2 (8.7%) | 4 (26.7%) |
| Groin | 10 (43.5%) | 6 (40.0%) |
| Sacrum | 1 (4.3%) | 1 (6.7%) |
| Thighs | 2 (8.7%) | 0 (0.0%) |
| Unknown/unable to ascertain | 1 (4.3%) | 0 (0.0%) |
| Associated infection | 8 (2.3%) | 3 (0.9%) |
| Skin tear b | 24 (7.0%) | 25 (7.1%) |
| Other skin injury b | 58 (16.9%) | 69 (19.7%) |
Based on the Ghent Global IAD Categorisation Tool (GLOBIAD).
Denominator is incontinence.
3.2. Hierarchical regression model
The following variables were selected for inclusion in the propensity score: ward type, patient mobility, patient age, patient sex, patient outcome (i.e., whether died, discharged or transferred within/between hospitals), length of stay in hospital. A multilevel multiple logistic regression of the intervention status on these variables revealed LoS in hospital to be significantly associated with intervention status (p = 0.031) and surgical ward type to be significantly associated with intervention status (p = 0.017). Propensity scores representing the probability of a case belonging to the post‐intervention implementation group were generated, with a mean score of 0.503 (SD 0.086) and ranging from 0.249 to 0.900 (Table 4). The score was included in a multilevel, multiple logistic regression model alongside the key prognostic factors of intervention status and incontinence type. All parameters from this model are summarised in Table 4.
TABLE 4.
Multilevel multiple logistic regression model parameters (main analysis).
| Variable | p‐value | OR | 95% CI for OR |
|---|---|---|---|
| Intervention status: Post‐intervention (reference = pre‐intervention) | 0.086 | 0.546 | (0.274, 1.09) |
| Incontinence status: More than one type of incontinence (reference = singly incontinent) | 0.064 | 1.95 | (0.962, 3.96) |
| Propensity score on covariates | 0.392 | 1.22 | (0.774, 1.93) |
The ICC for this model was 7.30% (95% CI 5.66% to 52.2%), which justified the hierarchical model assumption.
3.3. Secondary outcome: Length of stay in hospital
The mean LoS in hospital for incontinent patients was 34.7 days (SD 30.0 days) in the pre‐intervention implementation cohort and 41.0 days (SD 30.1 days) in the post‐intervention implementation cohort. The difference of 6.28 days was statistically significant (t 546 = 1.90; p = 0.029).
For patients with IAD, LoS was also significantly associated with intervention status. The mean LoS for patients in the pre‐intervention cohort with IAD was 44.5 days (SD 34.2 days). The mean LoS for patients in the post‐intervention cohort was 24.6 days (SD 18.2 days). The difference of 19.9 days was statistically significant (t 35 = 2.27; p = 0.030).
3.4. Secondary outcome: Severity of IAD
Most IAD cases in both cohorts were category 1A (69.6% in pre‐implementation and 73.3% in post‐implementation intervention periods). Hence, there was no substantive difference in the severity of cases in the two patient cohorts.
3.5. Intervention adherence
Over a 7‐week post‐intervention period, intervention fidelity across the six facilities was recorded. Mean adherence to each component of the evidence‐based healthcare bundle were as follows: (a) clinician use of Incontinence Assessment and Management Chart: 68%; (b) appropriate number of layers under incontinent patients: 87%; (c) appropriate continence aid worn by patient: 94%; (d) completion of 3 Day Continence Record Chart: 53%; and (e) use of barrier cream cloths: 90%. This indicates that intervention fidelity was high for nearly all components of the intervention after implementation.
4. DISCUSSION
Despite the higher rates of incontinence and double incontinence (the odds of IAD in double incontinent patients were about double the odds of IAD in singly incontinent patients [over both cohorts]) found in the post‐intervention group (41.9% and 57.8%, respectively) compared with the pre‐intervention group (37.8% and 50.4%, respectively), it could be reasonably anticipated that the post‐intervention group would exhibit higher rates of IAD. However, there was a lower prevalence of IAD in the post‐intervention group (albeit not significantly lower). This is also despite patients in the post‐intervention cohort having higher acuity and having longer LoS in hospital than those in the pre‐intervention cohort. Similar results have been found in other studies that have implemented structured skin care regimes as part of preventing and managing IAD. For example, Zhang and colleagues 32 implemented a structured skin care protocol for preventing and treating IAD in critically ill patients and examined its effectiveness in reducing the incidence and severity of IAD. They found that the incidence of IAD reduced from 35.9% in the control phase to 17.7% in the intervention phase and the severity of IAD also decreased and IAD developed later in the intervention group than in the control group. A study of acute care patients in Singapore 33 conducted an open‐label cluster randomised trial by comparing the effectiveness of a combined regimen of 1 specialised skin cleansers with disposable body wipes and 2 either an acrylic terpolymer or zinc oxide skin protectant versus the control which was disposable body wipes and a zinc oxide protectant. The authors were unable to establish statistical significance for the treatment groups, however, patients in the treatment groups were 1.5 times more likely to experience IAD healing within 7 days compared with the control group. As outlined in the IAD best practice guidelines, 14 using a bundled intervention may be more effective than an individual approach to the prevention of IAD. A recent study in China using a structured skin care protocol guided by IAD guidelines in critical care settings was able to reduce the IAD incidence and severity of IAD from 35.9% to 17.7%. 32 Several other studies have attempted to decrease the development of IAD with evidence‐based interventions involving regimes combining skin care cleansing and protection with the majority decreasing the development and/or severity of IAD. 33 , 34 , 35 , 36
This study found that IAD acquired within hospitals can be reduced when using evidence‐based strategies such as those in IMBED. While there was an improvement from pre‐ (6.71%) to post‐intervention (4.27%) in our study, it did not achieve statistical significance at the conventional 5% significance level (p = 0.159). This lack of statistical significance might be attributed to the already low baseline rates of IAD in the pre‐intervention group, making it challenging to demonstrate significant improvements when starting from a relatively favourable position. Clinicians in the pre‐ and post‐implementation cohorts had implemented strategies aimed at reducing the incidence of IAD. It also suggests that a proactive approach was employed in both phases. However, the post‐intervention group still experienced less IAD despite the existence of these measures in the pre‐intervention group, and inferior health profiles in the post‐intervention group, who also experienced the COVID pandemic.
One of the main strengths of this study was that the IMBED intervention was simple and consequently able to be translated to a variety of hospital settings. Components of the intervention included, for example, use of barrier cream cloths, minimisation of bed protection layers, use of an appropriate continence aid, use of continence assessment and management tools, staff education and patient education resources. Another strength of this study was that the reduction in IAD prevalence in the post‐intervention cohort was achieved despite data collection occurring during the COVID‐19 pandemic. The health system was navigating the COVID‐19 pandemic with competing priorities for nursing staff which may have negatively impacted on patient treatment during this period. For example, patient care was more challenging, such as the additional infection control measures. Although the effect cannot be quantified, it further strengthens the practical implication of the pre‐post improvements demonstrated in the study. Further, the data are of high quality and have been subject to extensive checking of pre‐admission records at each individual hospital to ensure that no IAD case in either cohort was erroneously assigned to be study‐acquired. Engagement of clinical staff in most included institutions was good. The amount of missing data was low, and zero or negligible on all outcome variables or variables of key prognostic interest.
5. CONCLUSION
IAD is a significant concern in healthcare service provision, especially among individuals with incontinence, and can lead to patient safety issues in hospitals. Our study used an implementation science approach 21 to assess the effect of a multisite IAD prevention/treatment intervention on reducing the prevalence and severity of IAD resulting in a decrease in IAD prevalence. The post‐intervention group, despite having a higher prevalence of double incontinence, exhibited lower rates of IAD, suggesting enduring effectiveness of the intervention.
FUNDING INFORMATION
This study was supported by the New South Wales Ministry of Health Translational Research Grant Scheme, Round 4 [TRGS Application: H19/53776].
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We are indebted to the members of the IMBED Clinical Expert Group, Project Steering Committee, Post‐Research Implementation Advisory Committee and the research officers for their collaboration. We especially thank the Executive Sponsor, Ivanka Komusanac, for her ongoing support and leadership, as well as Dr. Katja Heuer and Willoughby Hay. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Barakat‐Johnson M, Stephenson J, Lai M, et al. Impact of an evidence‐based bundle on incontinence‐associated dermatitis prevalence in hospital patients: A quasi‐experimental translational study. Int Wound J. 2024;21(6):e14936. doi: 10.1111/iwj.14936
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Kayser SA, Phipps L, VanGilder CA, Lachenbruch C. Examining prevalence and risk factors of incontinence‐associated dermatitis using the international pressure ulcer prevalence survey. J Wound Ostomy Cont Nurs. 2019;46(4):285‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansen E, Bakken LN, Duvaland E, et al. Incontinence‐associated dermatitis (IAD): prevalence and associated factors in 4 hospitals in Southeast Norway. J Wound Ostomy Cont Nurs. 2018;45(6):527‐531. [DOI] [PubMed] [Google Scholar]
- 3. Barakat‐Johnson M, Barnett C, Lai M, Wand T, White K. Incontinence, incontinence‐associated dermatitis, and pressure injuries in a Health District in Australia: a mixed‐methods study. J Wound Ostomy Cont Nurs. 2018;45(4):349‐355. [DOI] [PubMed] [Google Scholar]
- 4. Gray M, Giuliano KK. Incontinence‐associated dermatitis, characteristics and relationship to pressure injury: a multisite epidemiologic analysis. J Wound Ostomy Continence Nurs. 2018;45(1):63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei M, Yang D, Wu L, Chen W, Chen Y, Fu Q. The prevalence of incontinence‐associated dermatitis in hospitalized patients in China: a systematic review and meta‐analysis. Adv Skin Wound Care. 2020;33(10):1‐7. [DOI] [PubMed] [Google Scholar]
- 6. Yüceler Kaçmaz H, Kaplan Ö, Kaplan A, Şahin MG, Cetinkaya A, Avci A. Incontinence‐associated dermatitis: prevalence in intensive care units and knowledge, attitudes, and practices of nurses. J Nurs Care Qual. 2023;38(4):354‐360. [DOI] [PubMed] [Google Scholar]
- 7. de Castro DLV, da Silva EL, Onaga LS, Nogueira PC, Furlan PC, de Gouveia Santos VLC. The prevalence of skin lesions and associated factors in hospitalised adult patients with cancer. J Wound Care. 2022;31(8):660‐668. [DOI] [PubMed] [Google Scholar]
- 8. Grden CRB, Martins AR, Cabral LPA, et al. Incontinence associated dermatitis in elderly people admitted to a university hospital. Rev Bras Enferm. 2020;73(Suppl 3):e20190374. [DOI] [PubMed] [Google Scholar]
- 9. Borges EL, Moraes JT, Otoni Spira JA, et al. Prevalence and Management of Urinary Incontinence in a Brazilian hospital: a prospective, descriptive study. Wound Manag Prev. 2019;65(12):12‐20. [PubMed] [Google Scholar]
- 10. Hödl M, Blanař V, Amir Y, Lohrmann C. Association between incontinence, incontinence‐associated dermatitis and pressure injuries: a multisite study among hospitalised patients 65 years or older. Australas J Dermatol. 2020;61(1):e144‐e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beeckman D. A decade of research on incontinence‐associated dermatitis (IAD): evidence, knowledge gaps and next steps. J Tissue Viability. 2017;26(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 12. Beeckman D, Smet S, van den Bussche K. Incontinence‐associated dermatitis: why do we need a core outcome set for clinical research? Wounds Int. 2018;9(2):21‐25. [Google Scholar]
- 13. Barakat‐Johnson M, Lai M, Basjarahil S, et al. “I feel like a baby wearing a nappy…It's embarrassing”: a qualitative study of patients' experience of incontinence and incontinence‐associated dermatitis in hospital settings. J Wound Care. 2024; In press. [Google Scholar]
- 14. Beeckman D, Campbell J, Campbell K, et al. Incontinence‐associated dermatitis: moving prevention forward. Wounds Int. 2015. [Google Scholar]
- 15. European Pressure Ulcer Advisory Panel . National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. 2019.
- 16. World Health Organization . EK02.22 Irritant contact dermatitis due to incontinence 2023. Available from: https://icd.who.int/browse/2024-01/mms/en#/http://id.who.int/icd/entity/326384712
- 17. McNichol L, Bliss DZ, Gray M. Moisture‐associated skin damage: expanding practice based on the newest ICD‐10‐CM codes for irritant contact dermatitis associated with digestive secretions and fecal or urinary effluent from an abdominal stoma or enterocutaneous fistula. J Wound Ostomy Continence Nurs. 2022;49(3):235‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray M, Bliss DZ, McNichol L. Moisture‐associated skin damage: expanding and updating practice based on the newest ICD‐10‐CM codes. J Wound Ostomy Continence Nurs. 2022;49(2):143‐151. [DOI] [PubMed] [Google Scholar]
- 19. Bliss DZ, McNichol L, Cartwright D, Gray M. Practice alert: new ICD‐10 codes for MASD. J Wound Ostomy Continence Nurs. 2022;49(1):15‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barakat‐Johnson M, Basjarahil S, Campbell J, et al. Implementing best available evidence into practice for incontinence‐associated dermatitis in Australia: a multisite multimethod study protocol. J Tissue Viability. 2020;30:67‐77. [DOI] [PubMed] [Google Scholar]
- 21. Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care. 1998;7(3):149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D. The CG. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Br Med J. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 24. Beeckman D, van den Bussche K, Alves P, et al. The Ghent Global IAD Categorisation Tool (GLOBIAD). Skin Integrity Research Group ‐ Ghent University; 2017. [Google Scholar]
- 25. Barakat‐Johnson M, Lai M, Barnett C, et al. Hospital‐acquired pressure injuries: are they accurately reported? A prospective descriptive study in a large tertiary hospital in Australia. J Tissue Viability. 2018;27(4):203‐210. [DOI] [PubMed] [Google Scholar]
- 26. Parliament of Australia . COVID‐19: a chronology of state and territory government announcements (up until 30 June 2020). https://www.aph.gov.au/About_Parliament/Parliamentary_departments/Parliamentary_Library/pubs/rp/rp2021/Chronologies/COVID‐19StateTerritoryGovernmentAnnouncements
- 27. Barakat‐Johnson M, Lai M, Wand T, Coyer F, White K. Cultivating incontinence‐associated dermatitis prevention practices in an Australian local Health District: a quasi‐experimental study. Ostomy Wound Manage. 2018;64(12):16‐28. [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The National Health and Medical Research Council, Australian Research Council, Universities Australia . National Statement on Ethical Conduct in Human Research (Updated 2018). Commonwealth of Australia; 2007. [Google Scholar]
- 30. IBM Corp . IBM SPSS Statistics for Windows, Version 26.0. IBM Corp; 2019. [Google Scholar]
- 31. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373‐1379. [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Wang X, Zhao X, Zhang Y. A structured skin care protocol for preventing and treating incontinence‐associated dermatitis in critically ill patients. Adv Skin Wound Care. 2022;35(6):335‐342. [DOI] [PubMed] [Google Scholar]
- 33. Glass GF Jr, Goh CCK, Cheong RQ, Ong ZL, Khong PCB, Chan EY. Effectiveness of skin cleanser and protectant regimen on incontinence‐associated dermatitis outcomes in acute care patients: a cluster randomised trial. Int Wound J. 2021;18(6):862‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kon Y, Ichikawa‐Shigeta Y, Iuchi T, et al. Effects of a skin barrier cream on management of incontinence‐associated dermatitis in older women: a cluster randomized controlled trial. J Wound Ostomy Continence Nurs. 2017;44(5):481‐486. [DOI] [PubMed] [Google Scholar]
- 35. Coyer F, Gardner A, Doubrovsky A. An interventional skin care protocol (InSPiRE) to reduce incontinence‐associated dermatitis in critically ill patients in the intensive care unit: a before and after study. Intensive Crit Care Nurs. 2017;40:1‐10. [DOI] [PubMed] [Google Scholar]
- 36. Beeckman D, van Damme N, Schoonhoven L, et al. Interventions for preventing and treating incontinence‐associated dermatitis in adults. Cochrane Database Syst Rev. 2016;11(11):Cd011627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


