Abstract
Background
Transient Receptor Potential Mucolipin 1 (TRPML1) serves as a pivotal reactive oxygen species (ROS) sensor in cells, which is implicated in the regulation of autophagy. However, its function in melanocyte autophagy under oxidative stress remains elusive.
Methods
The expression and ion channel function of TRPML1 were investigated using immunofluorescence and calcium imaging in primary human melanocytes (MCs). After activating TRPML1 with MLSA1 (TRPML1 agonist), autophagy‐related molecules were investigated via western blot. ROS level, apoptosis‐ and autophagy‐related molecules were investigated after pretreatment with MLSA1. After interference with TRPML1 expression, mitochondrial structures were visualized by electron microscopy with hydrogen peroxide (H2O2)treatment.
Results
TRPML1 was expressed and functionally active in primary human MCs, and its activation promotes elevated expression of LC3‐II and reduced apoptosis and ROS levels under oxidative stress. TRPML1 downregulation caused mitochondrial swelling and disruption of cristae structures under oxidative stress in primary human MCs.
Conclusions
TRPML1 might mediate lysosomal autophagy in primary human MCs under oxidative stress, participating in mechanisms that maintain the oxidative and antioxidant systems in balance.
Keywords: autophagy, melanocyte, oxidative stress, transient receptor potential mucolipin 1, reactive oxygen species
1. INTRODUCTION
Transient Receptor Potential Mucolipin 1 (TRPML1) mediates the flux of Ca2+, Fe2+, and Zn2+ from the lysosome to the cytoplasm. 1 TRPML1 serves as a reactive oxygen species (ROS) sensor in numerous cells, its opening converts the stimulatory signal into a Ca2+ signal, which could promote transcription factor EB (TFEB) activation and lysosomal transport, and then stimulate cellular autophagy. The autophagy functions to clear the damaged organelles (e.g., mitochondria) and prevent damaged mitochondria from further ROS generation, thus interrupting the ROS‐induced positive feedback process. 2 , 3 , 4 At present, the roles of TRPML1 in primary human melanocytes (MCs) have not been clarified.
The pigment melanin is essential for shielding the MCs from the damage induced by ultraviolet light and other environmental stresses. 5 Considering a series of catalytic reactions involving the key rate‐limiting enzymes (e.g., tyrosinase) through melanin biosynthesis, during which the MCs are more prone to stimulate the intracellular production of ROS. 6 Environmental stimuli such as ultraviolet light, vaccination, and mental stress could also promote ROS formation. 7 MCs have sophisticated antioxidant systems including antioxidant metabolites (e.g., uric acid, ascorbic acid, glutathione, vitamin E), enzymes (e.g., superoxide dismutases, catalase and glutathione systems), and autophagy. 8 When the balance of oxidative and antioxidant systems is broken, it is easy to cause MC damage.
Autophagy is an evolutionarily conserved biological function that could degrade misfolded proteins, impaired organelles, foreign microorganisms, etc. 9 It is involved in the removal of damaged organelles under oxidative stress and prevents excessive accumulation of ROS. 10 Defects in autophagy‐related gene (ATG) 7 are associated with decreased melanin content, melanogenesis dysfunction, premature senescence, and increased levels of apoptosis in MCs, which indicates that autophagy is crucial for maintaining the normal biological functions of human MCs. 11
Given that TRPML1 acts as a key ROS sensor in cells and is involved in the regulation of autophagy, it may participate in the regulation of autophagy under oxidative stress in MCs. 4 This would be one of the mechanisms in maintaining the balance of oxidative and antioxidant systems.
2. MATERIALS AND METHODS
2.1. Skin specimen and cell culture
Primary human MCs were isolated from abandoned foreskin samples obtained from healthy male children after circumcision in the Department of Urology, Hangzhou Third People's Hospital. Primary human MCs were cultured in an F‐12 medium (Gibco, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Gibco), 20 ng/mL basic fibroblast growth factor (bFGF; Pepro‐Tech, Rocky Hill, NJ), 20 µg/mL isobutyl methylxanthine (IBMX; Sigma‐Aldrich, St Louis, MO) and antibiotics. 12 All experiment protocols were approved by the Ethics Committee of Hangzhou Third People's Hospital and performed in accordance with the Declaration of Helsinki. Informed consent was obtained before surgeries.
2.2. Reagents and cell transfection
Hydrogen peroxide(H2O2;Sigma‐Aldrich) was diluted with phosphate balanced solution (PBS). To establish the cellular oxidative stress model, the cells were incubated with H2O2 at a concentration of 200 µM for 12 h and 24 h. H2O2 concentrations ranging from 100 µM to 500 µM were used to explore the influence of oxidative stress on TRPML1 expression. MLSA1 (TRPML1 agonist; Abcam, Cambridge, UK) was dissolved in dimethylsulfoxide (DMSO). To evaluate TRPML1 channel activity in primary human MCs, MLSA1 was used at concentrations ranging from 5 µM to 20 µM. Primary human MCs were transfected with negative control (siNC) and TRPML1 siRNAs (synthesized by Genomeditech, Shanghai, China). Knockdown efficiency was confirmed by western blot (WB). 13
2.3. WB
Whole‐cell extracts were lysed in RIPA buffer (Beyotime) containing phenylmethanesulfonylfluoride (Beyotime). Equal amounts of protein were resolved on 4–20% SDS‐PAGE gels (ACE, Wuhan, China) and transferred to a nitrocellulose membrane (Pall, New York). After blocking with 5% skimmed milk (Becton, Dickinson, and Company, New Jersey), the membranes were incubated overnight (4°C) with the primary antibodies as follows: anti‐LC3A/B rabbit polyclonal (Abcam), anti‐TRPML1/MG‐2 rabbit polyclonal (Abcam), anti‐SQSTM1/p62 mouse monoclonal (Cell Signaling Technology), anti‐Beclin1 rabbit monoclonal (Abcam) and Anti‐GAPDH mouse monoclonal (Proteintech, Wuhan, China). After washing, the membranes were incubated with the secondary antibodies, including IRDye® 680RD‐ or IRDye® 800CW‐ coupled secondary antibodies (LI‐COR Biosciences, Lincoln) for 1 h at room temperature. Signal densities of the visualized proteins (LI‐COR Biosciences) were quantified using the Image J software (National Institutes of Health, Bethesda, MD)
2.4. Immunofluorescence
Cells were fixed with 4% paraformaldehyde (Beyotime) for 20 min and then incubated with the following primary antibodies: anti‐TRPML1/MG‐2 rabbit polyclonal (Abcam), anti‐MelanA mouse monoclonal (Abcam) and anti‐Lysosome‐associated membrane protein 1(LAMP1) mouse monoclonal (Cell Signaling Technology) overnight (4°C). The primary antibodies were visualized using goat anti‐mouse Alexa Fluor 555 conjugate and goat anti‐rabbit Alexa Fluor 488 conjugate antibodies (Abcam). DAPI (Haoke, Hangzhou, China) was used to stain the nucleus. Fluorescence signals were analyzed by recording stained images using laser confocal microscopy (Zeiss, Oberkochen, Germany). Finally, Image J was utilized to evaluate the colocalization coefficients and Pearson's_Rr between TRPML1 and LAMP1 immunosignals. 14
2.5. Transmission electron microscopy
After treatment, the cells were washed in PBS and fixed in 2.5% glutaraldehyde. Samples were post‐fixed with 1% osmium acid for 1 h at 4°C, stained with 2.0% aqueous uranyl acetate at 4°C overnight, followed by ethanol dehydration, and embedded in Spurr's plastic resin. Ultrathin sections were analyzed and imaged using a Hitachi H‐7650 transmission electron microscope (Hitachi, Tokyo, Japan).
2.6. Apoptosis assay
After corresponding treatments, cells were detected by the Annexin V‐FITC/PI apoptosis kit (AP101, Multisciences, Hangzhou, China) following the manufacturer's instructions and identified in the flow cytometer (Attune NxT, Thermo Scientific, MA) within 1 h. The analysis was performed with the Flow Jo software. Each apoptosis assay was performed in triplicate.
2.7. Ca2+ imaging
The cells were washed with Hank's balanced salt solution (HBSS; Beyotime) followed by incubation in HBSS supplemented with 5 µM Fluo‐3AM and 0.05% Pluronic F‐127 (both from Beyotime) away from light at 37°C for 1 h. Continuous acquisition of Fluo‐3AM fluorescence signals was captured via a cooled digital CMOS camera within 35 s (ORCA‐Flash 4.0, Hamamatsu Photonics KK, Japan). Specifically, during 0–20 s, 10 µM MLSA1 was applied via the fast exchange perfusion system (ALA‐VM8; ALA Scientific Instruments, New York, USA). Then, between 20 and 35 s, 10 µM ionomycin (Beyotime) was applied to stimulate the cells as the positive control. The effect of MLSA1‐induced Ca2+ mobilization was indicated via the curve of Fluo‐3AM fluorescence density in responsive cells. 15 Image J software was utilized to analyze fluorescence intensity.
2.8. Measurement of intracellular ROS levels
After corresponding treatments, the average level of intracellular ROS in all experimental cells was detected with DCFH‐DA (Beyotime). Cells were washed with F‐12 medium (Gibco) followed by incubation in F‐12 medium supplemented with 10 µM DCFH‐DA (Beyotime) away from light at 37°C for 20 min. Fluorescence microscopy was adjusted to ISO800, with 500 ms exposure time to detect the fluorescence intensity for each group. 16
2.9. Statistical analysis
All data reported in this study were obtained from independent experiments performed in triplicate. The data are presented as the mean value ± standard deviations (SD). Two‐tailed Student's t‐test were used for data comparisons. GraphPad Prism 9.3 software (GraphPad Software, San Diego, CA) was used to perform statistical analyses.
3. RESULTS
3.1. TRPML1 expression and function in primary human MCs
To confirm the expression of the TRPML1 and its ion channel function in primary human MCs. Double immunofluorescence staining was performed with TRPML1 antibody (green) and an MC marker (MelanA, red), and nuclei were labeled with DAPI (blue). The results demonstrated that the expression of TRPML1 in primary human MCs (Figure 1A). Concurrently, we explored the subcellular localization of TRPML1 in primary human MCs. In this way, we marked lysosomes with LAMP1 and explored the overlap degree between LAMP1 (red) and TRPML1 (green) immunosignals by immunofluorescence. The degree of overlap was quantified by calculating both the Pearson's correlation coefficient and the overlap coefficient across the red and green fluorescent channels. The colocalization of TRPML1 with lysosomes was evidenced by a Pearson's correlation coefficient of 0.7031 ± 0.006 and an overlap coefficient of 0.774 ± 0.001, derived from three independent experimental replicates. These findings indicate that TRPML1 channels are partially colocalized with lysosomes (Figure 1B). To detect the ion channel function of TRPML1 in primary human MCs, alterations in Ca2+ were measured in the cytoplasm by Ca2+ Imaging. Continuous acquisition of Fluo‐3AM fluorescence signals were captured via a cooled digital CMOS camera within 35 s. Specifically, during 0–20 s, 10 µM MLSA1 (TRPML1 agonist) was applied via the fast exchange perfusion system. Then, between 20 and 35 s, 10 µM ionomycin was applied to stimulate the cells as the positive control. The effect of MLSA1‐induced Ca2+ mobilization was indicated via the curve of Fluo‐3AM fluorescence density in responsive cells. The concentration of Ca2+ increased (Figure 1C) under treatment with 10 µM MLSA1. Taken together, these results provided evidence that TRPML1 was expressed in primary human MCs with ion channel activity.
FIGURE 1.
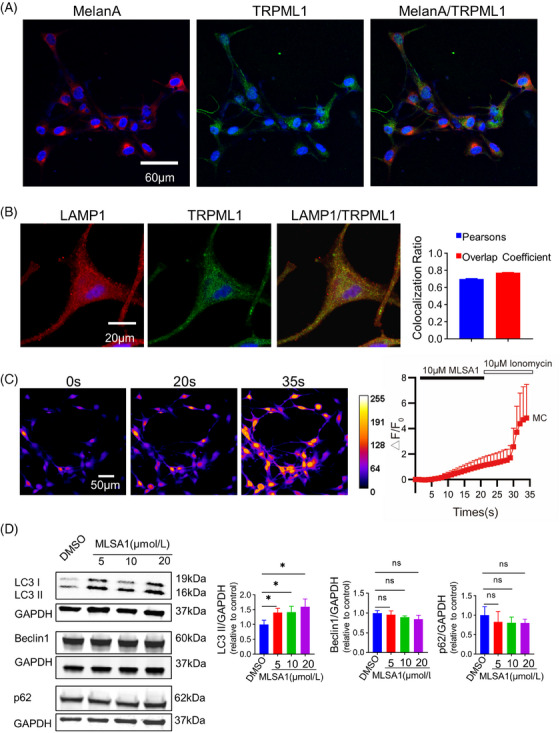
TRPML1 expression and function in primary human MCs. (A): Representative images of immunofluorescence staining for TRPML1 in primary human MCs marked with MelanA. (B): Representative images of immunofluorescence staining for TRPML1 and LAMP1. Pearson's correlation coefficient and overlap coefficient assessed between red and green fluorescent channels. (C): Ca2+ imaging of TRPML1. Average relative fluorescence intensity curve of TRPML1 activated by MLSA1 and ionomycin (n = 60). (D): Detection of p62, LC3‐I, LC3‐II, and Beclin1 protein levels by WB. F: relative fluorescence intensity; F0: initial relative fluorescence intensity; △F = F‐F0; *: P < 0.05, ns: not significant.
To explore the roles of TRPML1 in autophagy. We monitored fluctuations in LC3, p62, and Beclin1 protein levels in primary human MCs after treatment with DMSO or MLSA1 (5 µM, 10 µM, and 20 µM) for 24 h, respectively. As shown in Figure 1D, in primary human MCs treated with MLSA1, the expression of p62 and Beclin1 did not alter considerably, but the level of LC3‐II increased significantly. Statistically, there was no difference in LC3‐II expression among the 5 µM, 10 µM and 20 µM MLSA1 treated groups. These findings suggested that autophagy may be influenced by TRPML1 activation in primary human MCs. However, whether TRPML1 participates in autophagy induction under oxidative stress in primary human MCs still needs to be elucidated.
3.2. The protective role of TRPML1 in primary human MCs under oxidative stress
We then attempted to figure out the roles of TRPML1 in primary human MCs under oxidative stress, primary human MCs were treated with H2O2 in a dose‐dependent manner (0∼500 µM) for 24 h, and TRPML1 expression levels were detected by WB. TRPML1 levels were found to be higher in the 100 µM and 200 µM groups (Figure 2A). While no statistically significant difference was observed in 300 µM, 400 µM, and 500 µM groups.
FIGURE 2.
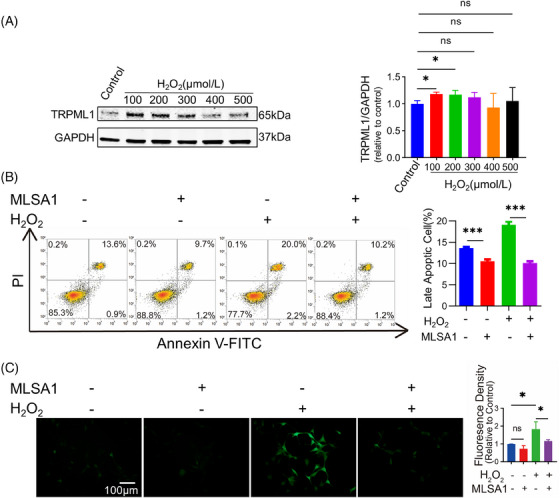
Modulation of Cellular Apoptosis and Intracellular ROS Levels through TRPML1 Activation Under Oxidative Stress in Primary Human MCs. (A): Detection of TRPML1 protein levels by WB. (B): Cell apoptosis rates detected by Annexin V‐FITC/PI staining. (C): Detection of intracellular ROS levels by redox‐sensitive dye DCFH‐DA. *: P < 0.05, ***: P < 0.001, ns: not significant.
Herein, 200 µM H2O2 was used to establish a MC oxidative stress model in subsequent experiments. We went on to identify the potential function of TRPML1 under oxidative stress by determining apoptotic rates, intracellular ROS levels, and autophagy‐related proteins. Primary human MCs were pretreated with MLSA1 (10 µM) for 2 h and then incubated with 200 µM H2O2 for 24 h. Apoptotic rates were examined by Annexin V‐FITC/PI staining in the flow cytometer. The results revealed that pretreatment with MLSA1 significantly decreased the number of late apoptotic cells induced by H2O2 (Figure 2B). The average level of intracellular ROS in all experimental cells was evaluated by using redox‐sensitive dye DCFH‐DA. Fluorescence microscopy was adjusted to ISO800, with 500 ms exposure time to detect the fluorescence intensity for each group. Statistical analysis of the fluorescence density proved that pretreated with MLSA1 (10 µM) for 2 h could effectively inhibit the increase of intracellular ROS level caused by H2O2 (Figure 2C). Taken together, pretreatment with MLSA1 (10 µM) significantly decreased oxidative stress damage and increased cell viability levels after incubation with H2O2 (200 µM).
In parallel, our study aimed to elucidate the influence of TRPML1 channel activation on the modulation of autophagy‐related proteins under oxidative stress. To achieve this, we pre‐incubated MC with 10 µM MLSA1 for 2 h. Post‐pretreatment, primary human MCs were exposed to 200 µM H2O2 for two distinct durations: 12 h and 24 h, respectively. Afterwards, autophagy‐related proteins were identified. Our results revealed that p62 and Beclin1 levels were not significantly altered (Figure 3A,B). However, LC3‐II was upregulated after exposure to H2O2 (Figure 3A,B). Interestingly, LC3‐II was also upregulated after pretreatment with MLSA1 for 12 h (Figure 3A), while no significant difference was observed after pretreatment with MLSA1 for 24 h (Figure 3B). Our results corroborated that TRPML1 activation could significantly enhance the expression of the autophagy‐related protein LC3‐II and decrease the proportion of late apoptotic cells and intracellular ROS levels under oxidative stress.
FIGURE 3.
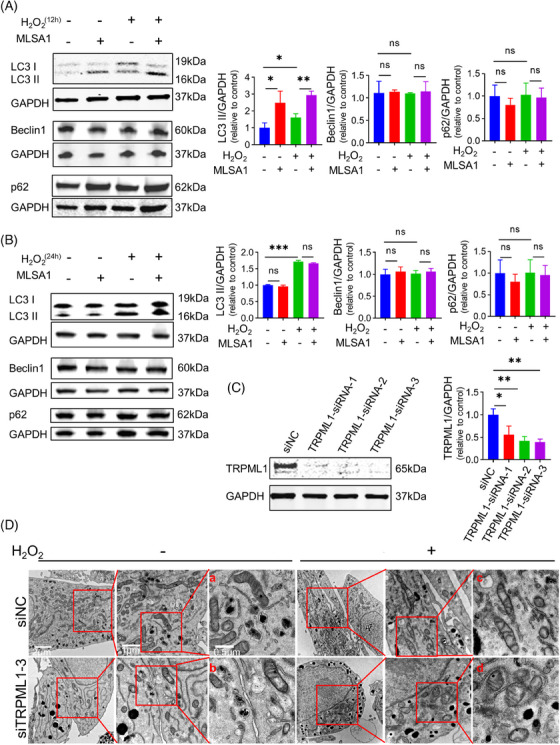
The removal of damaged mitochondria facilitated by activation of TRPML1 in primary human MCs. (A): Detection of of p62, LC3‐I, LC3‐II, and Beclin1 protein levels after stimulated with H2O2 for 12 h by WB. (B): Detection of of p62, LC3‐I, LC3‐II, and Beclin1 protein levels after stimulated with H2O2 for 24 h by WB. (C): Immunoblotting assays of TRPML1 in primary human MCs transfected with TRPML1 siRNAs. (D): Representative TEM images of mitochondria in transfected primary human MCs after treated with H2O2. siNC: siRNA control of primary human MCs; TRPML1‐siRNA: siRNA against TRPML1. *: P < 0.05, **: P < 0.01, ***: P < 0.001, ns: not significant.
Furthermore, TRPML1 was partially down‐regulated using siRNA in MC for the ensuing experiments. We screened the best interference fragment from three TRPML1‐siRNA, and the results showed that TRPML1‐siRNA‐3 had the highest efficiency (Figure 3C). Thus, TRPML1‐siRNA‐3 was selected for the subsequent experiments. TEM images of mitochondria in transfected primary human MCs treated with 200 µM H2O2 12 h shown that mitochondrial morphology was largely intact in the primary human MCs transfected in the siNC group (Figure 3D‐a). However, minor mitochondria were damaged in the siNC group treated with H2O2 for 12 h (Figure 3D‐c), as well as in the TRPML1‐siRNA‐3 transfected group in the absence of H2O2 (Figure 3D‐b). Notably, mitochondrial ultrastructural damage was severe in the TRPML1‐siRNA‐3 transfected group when treated with H2O2 (Figure 3D‐d). Irrespective of whether primary human MCs was stimulated with H2O2 or not, ultrastructural transmission electron microscopy uncovered that increased mitochondrial swelling and disruption of cristae after TRPML1‐siRNA‐3 treatment (Figure 3D‐a vs. Figure 3D‐b, Figure 3D‐c vs. Figure 3D‐d).
4. DISCUSSION
Transient receptor potential channel is a family of non‐selective cation that abundantly expressed in skin cells such as MCs, keratinocytes, hair follicles, neurons, etc. 17 Intracellular Ca2+ signals could further influence physiological processes such as cell proliferation, differentiation, and migration. 18 In recent years, a growing number of studies have shown that TRP channels, excessive or deficient channel activity, are involved in pathological skin conditions such as, pruritus, vitiligo, dermatitis and chronic pain. 19 Both exogenous and endogenous ROS can activate TRPML1 20 and then rapidly initiate autophagy through the calmodulin (CaM)—AMP—activated protein kinase (AMPK) 21 and calcineurin (CaN)—TFEB 22 pathways. In addition, it is also involved in synaptotagmins 7‐dependent lysosomal exocytosis to promote autophagic flux. 22 These mechanisms ultimately promote the clearance of damaged mitochondria within cells and prevent excessive accumulation of ROS. At the same time, the negative feedback mechanism involved in mechanistic target of rapamycin complex 1 (mTORC1) can prevent cell death caused by excessive autophagy. 23
At present, TRPML1 roles in normal MCs have not been clarified. TRPML1 participates in autophagy inhibition to suppress cancer metastasis by evoking the ROS‐mediated TP53/p53 pathway in melanoma. 24 TRPML1 is also found to be required in melanoma cells to negatively regulate the MAPK pathway and mTORC1 signaling. 25 A mutation in TRPML3 results in the varitint–waddler (Va) phenotype. Va mice are deaf, exhibit circling behavior due to vestibular defects, and have variegated/dilute coat color as a result of pigmentation defects. MCs are neural‐crest derived cells that produce melanin, the primary determinant of skin color. 26 In primary neurons, TRPML1 function loss leads to neurodegeneration, participating in Alzheimer's disease pathogenesis. 27
Herein, we validated the expression of TRPML1 and ion channel activity in primary human MCs. According to previous researches, ROS can activate TRPML1, which then promotes autophagy to scavenge mitochondria and other damaged organelles during oxidative stress. 28 Prior studies have established that TRPML1‐deficient mouse neurons exhibited defects in autophagy and an accumulation of misfolded proteins. 29 Damaged mitochondria can prompt TRPML1 activation by ROS and orchestrate lysosomal adaptation to clear damaged mitochondria via autophagy, known as mitophagy. 30 In our research, autolysosomes in TRPML1‐knockdown MCs were fewer compared to untreated MCs, with swollen mitochondria accompanied by disorganized cristae structure. This observation suggested that TRPML1 might play a pivotal role in autophagy and mitochondrial homeostasis of normal MCs under oxidative stress. Mitochondrial structure disorders in MCs treated with TRPML1‐siRNA‐3 were also suspected of mitochondrial calcium concentration dysregulation. TRPML1 lysosomal calcium release mediated the direct transfer of calcium into mitochondria. 31 Dysfunction in TRPML1 has been associated with several mitochondrial defects. 32 Fibroblasts in mucolipidosis type IV (MLIV) exhibit disrupted mitochondria‐lysosome contact site dynamics and defective contact‐dependent calcium transfer. 33
In addition, it was noted that H2O2 could upregulate the expression level of TRPML1. We speculated that when the body exposed to an external pressure environment, the cells expressed more TRPML1 to detect ROS and respond to sudden external stimuli. In H2O2‐treated primary human MCs, the cells were pretreated with TRPML1 agonist, and our results corroborated that TRPML1 activation could significantly enhance the expression of the autophagy‐related protein LC3‐II and decrease the proportion of late apoptotic. Based on these results, we concluded that TRPML1 might act as a sensor of ROS in primary human MCs. Moreover, its opening could trigger autophagy to clear misfolded proteins and restore impaired organelles. 34 All these could be pivotal for MCs structural stability and function under extreme conditions.
5. CONCLUSION
In summary, this study determined that TRPML1 was expressed in normal primary MCs with ion channel activity. It might be an essential component of the antioxidant system and play an essential role in oxidative stress induced damage. Increased ROS levels may activate TRPML1 to facilitate lysosomal Ca2+ release and scavenge excess ROS via autophagy process, during which the misfolded proteins could be removed and damaged organelles could be restored in MCs.
ROS is a double‐edged sword in MCs. When ROS level is low, it can promote growth and metabolism. However, when the oxidative‐antioxidant system in MCs is out of balance, mitochondria could be damaged and cause the generation of excessive ROS which could further impair the function of organelles such as endoplasmic reticulum, mitochondria itself, and lysosomes. 35 Due to the dysfunction of autophagy in MCs, the damaged organelles cannot be restored in time. Cellular homeostasis could hardly be restored then. Eventually, these MCs are recognized and killed by the immune system, leading to vitiligo. 36 Thus, we plan to examine the TRPML1 channel expression and function in vitiligo MCs in the future research.
This study had some limitations that need to be taken into account. This work did not investigate how to trigger autophagy and lysosomal biogenesis following TRPML1 activation, and its downstream signaling pathways deserves further investigation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
All informed consent was obtained before surgeries. The experiment protocols were approved by the Ethics Committee of Hangzhou Third People's Hospital, Hangzhou, China, reference number 2020KA001, in accordance with the Declaration of Helsinki. Informed consent was obtained before surgeries.
ACKNOWLEDGMENTS
We appreciat Dr. Heng Zhang for Ca2+ Imaging technical guidance. This study was granted by the Basic Public Welfare Research Project of Zhejiang (LY23H110001), Medical Science and Technology Project of Zhejiang Province (2023KY189), National Natural Science Foundation of China (82303998), Science and Technology Major Project of Zhejiang Province and the State Administration of Traditional Chinese Medicine (No: GZY‐ZJ‐KJ‐23035), and Health Science and Technology Major Project of Hangzhou (No: Z20220040).
Chen Y, Xie B, Hu Y, et al. Transient receptor potential mucolipin 1 circumvents oxidative stress in primary human melanocytes. Skin Res Technol. 2024;30:e13772. 10.1111/srt.13772
Yi Chen and Bo Xie authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study that are included in this article are available upon request to the corresponding author.
REFERENCES
- 1. Paola SD, Scotto‐Rosato A, Medina DL. TRPML1: The Ca(2+)retaker of the lysosome. Cell calcium. 2018;69:112‐121. [DOI] [PubMed] [Google Scholar]
- 2. Wong C‐O, Li R, Montell C, et al. Drosophila TRPML is required for TORC1 activation. Curr Biol. 2012;22:1616‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Y‐T, Xie L‐P, Hua Y, et al. Tanshinone I inhibits oxidative stress‐induced cardiomyocyte injury by modulating Nrf2 signaling. Front Pharmacol. 2021;12:644116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xuan Y, Yang Y, Xiang L, et al. The role of oxidative stress in the pathogenesis of vitiligo: a culprit for melanocyte death. Oxid Med Cell Longev. 2022;2022:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Slominski RM, Sarna T, Płonka PM, et al. Melanoma, melanin, and melanogenesis: the Yin and Yang relationship. Front Oncol. 2022;12:842496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denat L, Kadekaro AL, Marrot L, et al. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol. 2014;134:1512‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev. 2021;41:1138‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Y, Tonissen KF, Trapani GD. Modulating skin colour:role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci Rep. 2021;41:20210427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glick D, Barth S, Macleod KF. Autophagy:cellular and molecular mechanisms. J Pathol. 2010;221:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Cheng X, Yu L, et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiao Z, Xu Z, Xiao Q, et al. Dysfunction of ATG7‐dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress‐induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong WS, Hu DN, Qian GP, et al. Ratio of size of recipient and donor areas in treatment of vitiligo by autologous cultured melanocyte transplantation. Br J Dermatol. 2011;165:520‐525. [DOI] [PubMed] [Google Scholar]
- 13. Hämäläinen L, Kärkkäinen E, Takabe P, et al. Hyaluronan metabolism enhanced during epidermal differentiation is suppressed by vitamin C. Br J Dermatol. 2018;179:651‐661. [DOI] [PubMed] [Google Scholar]
- 14. Pastorek L, Sobol M, Hozák P. Colocalization coefficients evaluating the distribution of molecular targets in microscopy methods based on pointed patterns. Histochem Cell Biol. 2016;146:391‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aierken A, Xie Y‐K, Dong W, et al. Rational design of a modality‐specific inhibitor of TRPM8 channel against oxaliplatin‐induced cold allodynia. Adv Sci (Weinh). 2021;8:e2101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong J, He J, Zhu J, et al. Lactylation‐driven METTL3‐mediated RNA m6A modification promotes immunosuppression of tumor‐infiltrating myeloid cells. Mol Cell. 2022;82:1660‐1677. e10.e10. [DOI] [PubMed] [Google Scholar]
- 17. Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 2016;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie B, Li X‐Y. Inflammatory mediators causing cutaneous chronic itch in some diseases via transient receptor potential channel subfamily V member 1 and subfamily A member 1. J Dermatol. 2019;46:177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang P, Zhang W, Chen X, et al. TRPM2 mediates mitochondria‐dependent apoptosis of melanocytes under oxidative stress. Free Radic Biol Med. 2018;126:259‐268. [DOI] [PubMed] [Google Scholar]
- 21. Wen Z, Jin K, Shen Y, et al. N‐myristoyltransferase deficiency impairs activation of kinase AMPK and promotes synovial tissue inflammation. Nat Immunol. 2019;20:313‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medina DL, Paola SD, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abuammar H, Bhattacharjee A, Simon‐Vecsei Z, et al. Ion channels and pumps in autophagy: a reciprocal relationship. Cells.3537, 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xing Y, Wei X, Liu Y, et al. Autophagy inhibition mediated by MCOLN1/TRPML1 suppresses cancer metastasis via regulating a ROS‐driven TP53/p53 pathway. Autophagy. 2022;18:1932‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abrahamian C, Grimm C. Endolysosomal cation channels and MITF in melanocytes and melanoma. Biomolecules.1021, 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu H, Delling M, Li L, et al. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint‐waddler mice. Proc Natl Acad Sci U S A. 2007;104:18321‐18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Somogyi A, Kirkham ED, Lloyd‐Evans E, et al. The synthetic TRPML1 agonist ML‐SA1 rescues Alzheimer‐related alterations of the endosomal‐autophagic‐lysosomal system. J Cell Sci. 2023;136:259875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang X, Yu L, Xu H. Lysosome calcium in ROS regulation of autophagy. Autophagy. 2016;12:1954‐1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curcio‐Morelli C, Charles FA, Micsenyi MC, et al. Macroautophagy is defective in mucolipin‐1‐deficient mouse neurons. Neurobiol Dis. 2010;40:370‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clement D, Goodridge JP, Grimm C, et al. TRP channels as interior designers: remodeling the endolysosomal compartment in natural killer cells. Front Immunol. 2020;11:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peruzzo R, Costa R, Bachmann M, et al. Mitochondrial metabolism, contact sites and cellular calcium signaling: implications for tumorigenesis. Cancers (Basel).2574, 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eichelsdoerfer JL, Evans JA, Slaugenhaupt SA, et al. Zinc dyshomeostasis is linked with the loss of mucolipidosis IV‐associated TRPML1 ion channel. J Biol Chem. 2010;285:34304‐34308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng W, Wong YC, Krainc D. Mitochondria‐lysosome contacts regulate mitochondrial Ca(2+) dynamics via lysosomal TRPML1. Proc Natl Acad Sci U S A. 2020;117:19266‐19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang P, Xu M, Wu Y, et al. Multiple facets of TRPML1 in autophagy. Cell calcium. 2020;88:102196. [DOI] [PubMed] [Google Scholar]
- 35. Emanuelli M, Sartini D, Molinelli E, et al. The double‐edged sword of oxidative stress in skin damage and melanoma: from physiopathology to therapeutical approaches. Antioxidants (Basel). 2022;11:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie B, Song X. The impaired unfolded protein‐premelanosome protein and transient receptor potential channels‐autophagy axes in apoptotic melanocytes in vitiligo. Pigment Cell Melanoma Res. 2022;35:6‐17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study that are included in this article are available upon request to the corresponding author.


