Abstract
This review describes the clinical pharmacology of the major drugs used for the treatment of patients with inflammatory bowel disease (IBD). Pharmacokinetics, drug metabolism, mechanism of action, efficacy, and safety profile are discussed. Some small molecules were developed to act systemically (eg, ozanimod) or locally (eg, aminosalicylates) and thus have disparate pharmacokinetic properties. In addition, locally acting compounds have been optimized to mitigate systemic exposure—eg, budesonide, which undergoes extensive first-pass metabolism—thereby reducing systemic bioavailability and side effects. Other small molecules such as thiopurines are precursors of their active metabolites and differences in genotype or phenotype of metabolizing enzymes may affect efficacy and safety, requiring therapeutic drug monitoring (TDM). Monoclonal antibodies (MAs) are large molecules administered parenterally, and their pharmacokinetics may be influenced not only by the general immunoglobulin (Ig) G metabolism and recycling pathways but also by antigen properties such as antigen distribution and antigen concentration. In addition, antibody structure, host factors, concurrent medications, and immunogenicity may contribute to the substantial inter- and intrapatient variability in drug exposure and response observed for MAs. Current guidelines recommend reactive TDM of tumor necrosis factor antagonists at the time of loss of response. Evidence for proactive TDM and for the role of TDM for biologics with a different mechanism of action is emerging. Although small molecules offer potential benefits over biologics with oral administration and lack of immunogenicity, there may be risk for more systemic side effects due to off-target binding. Understanding drug metabolism, pharmacokinetic characteristics, and mechanism of action are important in selecting the right drug at the right time at the right dose for patients with IBD.
Keywords: Crohn’s disease, ulcerative colitis, drug metabolism, pharmacokinetics, pharmacodynamics, therapeutic drug monitoring
INTRODUCTION
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders of the gastrointestinal tract. IBD is a result of a dysregulated mucosal immune response to intestinal microflora in a genetically predisposed host.1, 2 In the United States, more than 1.4 million people suffer from IBD.3 Conventional therapy for IBD includes glucocorticoids, aminosalicylates, and immunomodulators. Differences in genotype and/or enzymatic activity of thiopurine methyltransferase have been shown to influence the efficacy and safety of thiopurines. The advent of biologic therapies such as tumor necrosis factor (TNF) antagonists, integrin inhibitors, and interleukin (IL)-12/23 inhibitors dramatically changed the way patients who are refractory to conventional therapy are treated. Although these biologics have been shown to be efficacious, substantial inter- and intrapatient variability exists in drug exposure and response to therapy. Therapeutic drug monitoring (TDM) with measurement of drug concentrations in serum has been proposed to optimize therapy with biologics. Congruently, this has created a need for effective nonimmunogenic oral therapies with less variability in drug pharmacokinetics. Small molecule therapies with novel targets, eg, janus kinase inhibitors and sphingosine-1-phosphate receptor modulators, are emerging as future therapies. Here, the clinical pharmacology of old and new drugs used to treat IBD is reviewed.
GLUCOCORTICOIDS
Glucocorticoids are employed in a number of inflammatory, autoimmune, and neoplastic diseases. Glucocorticoids are inhibitors of cytokine secretion and T-cell activation.4 Exogenous glucocorticoids are transported in the blood predominantly bound to corticosteroid-binding globulin and, to a lesser extent, to albumin. Free glucocorticoids are able to diffuse passively across plasma membranes and interact with a cytosolic receptor expressed in virtually all tissues.4 Glucocorticoids bind to glucocorticoid receptors in the cytoplasm, which then dimerize and translocate to the nucleus, where they bind to glucocorticoid response elements on glucocorticoid-responsive genes. Glucocorticoids may increase the transcription of genes coding for anti-inflammatory proteins or repress inflammatory gene expression.4, 5
Prednisone
Prednisone is a synthetic glucocorticoid derived from cortisone. Prednisolone is the pharmacologically active species. Absorption occurs in the upper jejunum, and maximum plasma concentrations are reached after 1 to 3 hours.6, 7 Plasma concentrations of glucocorticoids vary considerably after oral ingestion of identical doses by normal subjects and patients with IBD. Some studies have found normal absorption compared with healthy controls, whereas other studies have found reduced peak plasma levels of prednisolone in IBD patients.8 The liver is responsible for about 70% of corticosteroid metabolism.7 The distribution and clearance of prednisolone are dependent on its dose and plasma concentrations. At 20 mg, clearance was approximately 0.15 to 0.21 L/h/kg bodyweight, volume of distribution varied between 0.4 and 0.7 L/kg, and the biologically active half-life was independent of dose between 2 and 4 hours.9 Dose-dependent renal clearance was about 2.1 L/h for prednisone and 1.8 L/h for prednisolone.10 At a dose of 100 mg, both the clearance and volume of distribution increased by about 2-fold.9
Oral systemic corticosteroids have been used to induce remission in patients with active IBD for more than 50 years but are problematic due to unacceptable side effects. Systemic corticosteroids can cause ophthalmic (cataracts, glaucoma), dermatologic (skin thinning, striae, acne), metabolic (altered fat distribution, diabetes mellitus), gastrointestinal (dyspepsia, peptic ulceration, pancreatitis), musculoskeletal (avascular necrosis, osteopenia, osteoporosis, fractures), central nervous system (psychological, sleep disturbance), and cardiovascular (hypertension, congestive heart failure) adverse effects and adrenal suppression and opportunistic infections.11 Additionally, mortality rates with prolonged corticosteroid therapy have also been shown to increase when compared with anti-TNF-directed therapy for inflammatory bowel disease.12 This has prompted the development of glucocorticoids with enhanced topical anti-inflammatory activity and low systemic bioavailability.
Budesonide
Budesonide is an oral synthetic corticosteroid with topical potency approximately 5 times that of prednisone and limited systemic bioavailability. Budesonide is released in the proximal jejunum (Entocort; target pH ≥5.5), ileum (Budenofalk; target pH ≥6.4), or homogenously through the ascending, transverse, and descending colon (MMX, Uceris; target pH ≥7).13 The differences in the time delay before drug absorption (lag time) and time to reach a peak plasma concentration are consistent with the earlier and more proximal release of Entocort compared with Budenofalk. The apical enterocyte drug transporter P-glycoprotein 170 (protein for the MDR1 gene) facilitates its absorption to be rapidly metabolized via the cytochrome P450 isoenzymes CYP3A4 and CYP3A5 expressed in the liver and in intestinal epithelial cells.13 About 90% of budesonide undergoes first-pass metabolism in the liver, with a resultant low systemic bioavailability (10%–15%), minimizing its systemic effects.14 Eighty-eight percent of the systemically available budesonide is bound to plasma proteins.15 The products of budesonide metabolism, 16α-hydoxyprednisolone and 6β-hydroxybudesonide, have negligible corticosteroid activity and are primarily cleared by the kidneys.13, 14 Budesonide includes the asymmetric 16α and 17α-acetyl groups, resulting in a 1:1 mixture of 2 epimers labeled as 22R and 22S.16, 17 Both epimers have similar terminal half-life of 2.7 ± 0.6 hours.16
The pharmacokinetics of budesonide-controlled ileal release capsules was evaluated in children (age, 12.4 ± 1.8 years; weight, 39.8 ± 6.1 kg) and adults (age, 33.2 ± 12.6 years; weight, 64.3 ± 13.3 kg) with active CD.18 The systemic exposure and systemic bioavailability after oral administration of 9 mg budesonide were similar in children and adults with active CD. After 1 week of once-daily oral administration of budesonide, systemic exposure, area under the plasma concentration–time curve was 41 nmol/L × h in children and 35 nmol/L × h in adults. Systemic availability was on average 9% in children and 11% in adults. The mean maximal concentration was 6.0 ± 3.4 nmol/L in children and 4.0 ± 2.1 nmol/L in adults. The time to maximum plasma concentration was 4.7 ± 2.6 hours in children and 4.3 ± 1.5 hours in adults.
In a Cochrane review, budesonide was shown to be more effective than placebo for induction of remission in active CD.19 After 8 weeks of treatment with budesonide 9 mg daily, patients were almost twice as likely to enter remission than those on placebo. Although short-term efficacy with budesonide was lower than with conventional steroids, particularly in those with severe disease or more extensive colonic involvement, the likelihood of adverse events and adrenal suppression with budesonide was lower.
In the CORE II study in patients with mild to moderate UC, 9 mg of budesonide MMX once daily for 8 weeks provided a statistically significant increase in the combined clinical and endoscopic remission rate compared with placebo (17.4% vs 4.5%) and improved rates of histological healing and symptom resolution compared with placebo, (16.5% vs 6.7% and 23.9% vs 11.2%, respectively).20
In a pooled safety analysis of budesonide for the induction of remission in mild to moderate UC, budesonide administered for up to 8 weeks demonstrated a favorable safety and tolerability profile.21 Budesonide was not more likely than placebo to induce potential glucocorticoid adverse effects such as mood changes, sleep changes, acne, insomnia, moon face, fluid retention, hirsutism, flushing, and striae rubrae. There was no apparent dose-related increase in the incidence of infections. Although budesonide does appear to suppress morning cortisol to an extent, adrenal suppression is less likely than with prednisone.
AMINOSALICYLATES
Sulfasalazine consists of 1 molecule of 5-amino salicylic acid (ASA) azo-bound to a molecule of the sulfa antibiotic sulfapyridine. The therapeutic action of sulfasalazine in IBD resides with the 5-ASA moiety.22 5-ASA interacts with pathways of inflammation and apoptosis. 5-ASA interferes with TNF-alpha, transforming growth factor beta, and nuclear factor κB. It is a strong scavenger for reactive oxygen species. Aminosalicylates also alter fecal bacteria profiles and exert anti-inflammatory activity by inhibition of leukocyte motility.23
Standard, non-delayed-release preparations of 5-ASA are efficiently absorbed in the proximal small bowel. It is quickly metabolized by N-acetyl-transferase 1 to N-acetyl-5-ASA within intestinal epithelial cells and the liver. It is excreted through the urine as either unmetabolized 5-ASA or N-acetyl-5-ASA. The metabolite N-acetyl-5-ASA has no active anti-inflammatory properties.23 Two strategies have been developed to improve delivery of oral 5-ASA to the site of disease: coating the free 5-ASA with polymers that dissolve slowly and release the drug gradually (coated with a pH-dependent resin or encapsulated in semipermeable ethyl cellulose microgranules) and formulation of the drug as an azo-conjugate that is poorly bioavailable until bacterial azo-reductases in the colon split the azo bond and release the active 5-ASA.22
Plasma concentrations and urinary excretion of 5-ASA and N-acetyl-5-ASA were compared in healthy volunteers and patients with CD or UC. There was great variation in the pharmacokinetic parameters within each group, but no significant differences were noted between the groups.24 Systemic absorption of the 5-ASA and acetyl-5-ASA was low with a single oral dose regimen. Only about 20% of the 5-ASA given was absorbed, with more than 80% of the drug available in the terminal ileum and colon for therapeutic activity. There was a significantly higher plasma concentration and urine excretion of both 5-ASA and acetyl-5-ASA with a multiple-dose regimen.25
The pharmacokinetic profiles of children and adolescents with UC receiving a 5-ASA preparation were shown to be similar to historical adult data.26, 27 Steady-state plasma concentrations for 5-ASA were attained by day 5. For 30, 60, and 100 mg/kg/d doses, mean percentages of 5-ASA absorbed were 29.4%, 27.0%, and 22.1%, respectively. For a 70-kg individual, typical estimates of the central volume of distribution were 109 L for 5-ASA. The typical value of 5-ASA apparent renal clearance was estimated to be 1.15 L/h, and apparent metabolic clearance was estimated to be 85.6 L/h.
Different formulations of 5-ASA have been widely used in the clinical management of mild to moderate UC. 5-ASAs are highly effective for inducing remission and preventing relapse in UC. Evidence suggests that doses of ≥2.0 g/d have greater efficacy, although doses >2.5 g/d do not appear to lead to high remission rates.28 Combined oral and topical 5-ASA therapy appears to be superior to oral 5-ASAs for induction of remission in mildly to moderately active UC.29 There is no evidence to suggest that oral 5-ASA preparations are superior to placebo for the maintenance of medically induced remission in patients with CD.30
Reported adverse events were mild to moderate in intensity. Adverse effects include gastrointestinal symptoms (eg, flatulence, abdominal pain, nausea, vomiting, and diarrhea), headache, skin rash, and worsening ulcerative colitis.30, 31
CALCINEURIN INHIBITORS
Cyclosporine and tacrolimus are immunosuppressive agents. Cyclosporine is a cyclic endecapeptide, whereas tacrolimus is a macrocyclic lactone, but they act in a similar manner. Their main mechanism of action involves inhibition of calcineurin. Upon entering enterocytes, both drugs are metabolized by gastrointestinal CYP3A isozymes, predominantly CYP3A4 and CYP3A5. Both drugs bind extensively to erythrocytes, and only an unbound drug is capable of entering lympho cytes and exerting its main immunosuppressive effects. Cyclosporine and tacrolimus are extensively metabolized, with less than 1% and 0.5%, respectively, of the parent drug appearing unchanged in the urine and feces. Cyclosporine and tacrolimus metabolites are eliminated via the biliary route, with only 2%–3% undergoing renal elimination.32
Cyclosporine
Cyclosporine is a neutral, lipophilic cyclic peptide produced by the fungus Tolypocladium inflatum gams.33 Cyclosporine binds to the cytoplasmic protein cyclophilin. The cyclosporine/cyclophilin complex inhibits the cytoplasmic phosphatase calcineurin, which is an enzyme essential for activating the cytosolic component of the nuclear factor of activated T cells (NFAT). The cytosolic component of NFAT is unable to enter the nucleus and associate with the nuclear component of NFAT. Therefore, the NFAT complex is unable to be formed, which is responsible for transcription of mRNA encoding for IL-2 and its receptor. Additionally, cyclosporine indirectly inhibits B-cell activating factors and interferon γ by T-helper cells.
Cyclosporine is available as a liquid oral preparation, an oral gelatin capsule, a micro-emulsion, and an intravenous concentration. Maximum absorption of cyclosporine after an oral liquid dose occurs at ~4 h, and the bioavailability of cyclosporine displays considerable inter- and intrapatient variability, which ranges from 12% to 35%.34, 35 Oral gelatin bioavailability is equivalent to the oral liquid solution.36 Cyclosporine absorption from the small bowel follows 0-order kinetics and is a function of contact time. The absorption of the lipophilic cyclosporine formulation is markedly influenced by the presence and concentration of bile acids, the length of the small bowel, the rate of gastric emptying and gastrointestinal motility, and metabolism of cyclosporine by CYP3A isozymes of the gastrointestinal mucosa.37, 38 The oral cyclosporine micro-emulsion formulation creates micelles, which are absorbed in the small bowel without the presence of bile.
Pharmacokinetic studies in healthy volunteers demonstrate an increased bioavailability of the micro-emulsion formulation of cyclosporin.39 The pharmacokinetics of the cyclosporine micro-emulsion was characterized in patients (age 16–64 years) with CD (29 patients) and UC (29 patients).38 The pharmacokinetic parameters of the cyclosporine micro-emulsion were broadly similar to that previously measured in healthy volunteers: average peak plasma drug concentration 620% vs baseline, time to peak plasma drug concentration of 86.5 minutes, elimination half-life of 115 minutes. The average peak plasma drug concentration and area under the concentration–time curve to 12 hours tended to increase linearly with the dose, whereas the time to average peak plasma drug concentration and elimination half-life did not appear to change with the dose. The average peak plasma drug concentration was about 22% higher in ulcerative colitis, which may be secondary to different anatomical areas affected by the inflammatory process.
Tissue concentrations of cyclosporine in the colon are among the highest of any organ in the body. The pharmacokinetic parameters and colonic tissue concentrations of cyclosporine after oral and intravenous (IV) administration were compared. The colonic tissue concentration of cyclosporine after IV administration was 10-fold higher than that for oral dosing.40
High-dose cyclosporine is used as a rapidly acting “rescue therapy” for a short period of time as a “bridge” to other agents that appear to be safer for long-term use but are limited by a slower onset of action.37 In a controlled multicenter trial in 20 patients with severe UC refractory to steroid therapy, 9 of 11 patients (82%) treated with cyclosporine had a response within a mean of 7 days, leading to discharge from the hospital without colectomy.41 No patient in the placebo group had response. Five patients in the placebo group elected to start cyclosporine therapy with response. Cyclosporine and infliximab show similar response rates for induction of remission in patients with severe flare-ups of UC refractory to corticosteroids (90%), with no difference between these drugs in terms of the colectomy rate after 3 or 12 months.42
Cyclosporine is not efficacious as a long-term low-dose therapy and is potentially toxic at high doses. Renal toxicity, hypertension, lymphoma, infection, seizure, paresthesia, tremor, headache, gingival hyperplasia, hypertrichosis, and anaphylaxis can be associated with cyclosporine therapy. The incidence of adverse events is high, at 0.94 adverse events/patient, but adverse events are often dose related and resolve after dose reduction or discontinuation of cyclosporine.37
Tacrolimus
Tacrolimus is a macrolide agent isolated from the fungus Streptomices tsukubaensis.43 Tacrolimus binds to an intracellular T-lymphocyte protein called FK binding protein. The tacrolimus–FK binding protein complex prevents activation of calcineurin, which interrupts signal transduction in T-lymphocytes, leading to inhibition of transcription of genes for interleukins, TNF-alpha, and interferon gamma. Tacrolimus has a more potent inhibitory effect (10- to 100-fold) on activated T cells compared with cyclosporine.44, 45 Additionally, tacrolimus has a more predictable oral bioavailability and a somewhat better side effect profile compared with cyclosporine.46
Tacrolimus has a rapid onset of action, with peak blood or plasma concentrations reached in 0.5 to 6 hours.47 Approximately 25% of the oral dose is bioavailable. The mean half-life is 12 hours.45
Although the number of studies assessing its efficacy in UC are limited, tacrolimus has been recommended in steroid-refractory active UC. Tacrolimus has been shown to be effective in inducing short-term clinical response in active UC patients, with a durable effect of preventing colectomy without increased risk of severe adverse events.44 The current evidence on the role of tacrolimus in the management of CD is poor.
In randomized controlled trials, the rate of overall adverse effects is more common with tacrolimus compared with placebo (relative risk, 2.01). However, the risk of serious adverse effects was not increased with tacrolimus.44 In a long-term retrospective single-center study in patients with steroid-dependent or steroid-refractory IBD, tacrolimus therapy appeared to be safe, with side effects including a temporary rise of creatinine (7.6%), tremor or paresthesia (9.4%), hyperkalemia (1.9%), hypertension (1.9%), and opportunistic infections (3.8%).48
ANTIMETABOLITES
Thiopurines
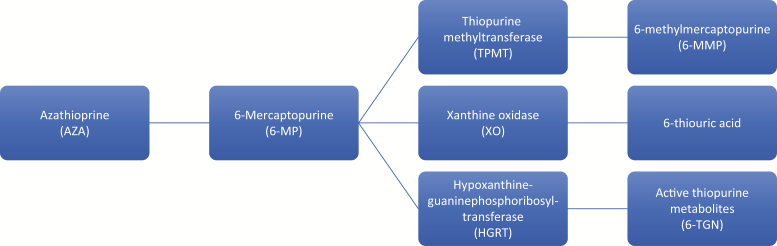
Azathioprine (AZA) is a prodrug that is converted to 6-meracaptopurine (6-MP) in an enzymatic and nonenzymatic reaction by sulfhydryl-containing substances such as glutathione.22 6-MP can enter 3 known metabolic pathways (Fig. 1). The action of hypoxanthine-guanine phosphoribosyl-transferase (HGRT) converts 6-MP to 6-thioguanine nucleotides (6-TGN), the putative active metabolites. Two of the pathways are catalyzed by thiopurine methyltransferase (TPMT) and xanthine oxidase (XO), leading to the inactive metabolites 6-methylmercaptopurine (6-MMP) and 6-thiouric acid, respectively. More 6-MP is shunted into the HGRT pathway with low TPMT activity.
FIGURE 1.
Azathioprine metabolism.
After administration of AZA and 6-MP, the 6-TGN accumulate intracellularly, causing inhibition of the pathways of purine nucleotide metabolism and DNA synthesis and repair. The bioavailability of azathioprine ranges from 27% to 83%, and the bioavailability of 6-MP ranges from 5% to 37%. Erythrocyte 6-TGN concentration gradually rises to a plateau after 2–4 weeks of oral azathioprine. The molecular basis for its therapeutic effects are not known, but antiproliferative or functionally inhibitory actions on cells of the immune system may underlie its immunosuppressive actions.22
The TPMT gene is located on the short arm of chromosome 6, and at least 10 variant alleles for TPMT have been associated with decreased enzyme activity.49 TPMT activity is inherited in a monogenic codominant trait. TPMT enzyme activity is measured by radioimmunoassay; 0.3% of the population have homozygous low activity, 11.1% have heterozygous or intermediate activity, and 88.6% have homozygous high or normal activity. Patients with low or intermediate TPMT enzyme activity divert 6-MP away from the 6-MMP metabolite and toward 6-TGN, which is associated with dose-related toxicity and leukopenia. Current guidelines recommend routine TPMT testing (enzymatic activity or genotype) to guide thiopurine dosing.50 Individuals with 2 nonfunctional TPMT alleles are at 100% risk for life-threatening myelosuppression, but only 30%–60% of patients who are heterozygous for TPMT are unable to tolerate full doses of AZA or 6-MP.51 Patients with normal TPMT activity are treated with standard doses of AZA or 6-MP. Patients with intermediate TPMT activity have their dose of AZA or 6-MP reduced by 50%, and patients with low TPMT activity are not treated due to high mortality from leukopenia and sepsis.49 Monitoring thiopurine metabolite levels may be of significant value in detecting nonadherence to treatment, the need for dose adjustment, preferential 6-MMP metabolism, pharmacological resistance to thiopurines, or refractoriness to thiopurines.52
In a pooled analysis of 6 studies (489 participants) comparing azathioprine (1.0 to 2.5 mg/kg/d) and placebo, azathioprine was superior to placebo for maintenance of remission in quiescent CD over a 6–18-month period, but the overall quality of the evidence supporting this outcome was low due to sparse data and unclear risk of bias.53 Azathioprine therapy appears to be more effective than placebo for maintenance of remission in UC. Thiopurines may be effective as maintenance therapy for patients who have failed or cannot tolerate mesalazine or sulfasalazine and for patients who require repeated courses of steroids.54 In contrast, a multicenter Pediatric Inflammatory Bowel Disease Network (PIBDNet) cohort study showed thiopurines to be less effective in maintaining remission for pediatric CD compared with past rates.55 The therapeutic role of thiopurine monotherapy therefore remains controversial due to the relatively slow onset of action and potential risk for adverse events such as gastric intolerance, flu-like symptoms, pancreatitis, hepatitis, rash, myelotoxicity, and lymphoma. Up to 28% of patients have adverse reactions, about 9% of patients are resistant to thiopurine therapy, and as many as one-third of patients discontinue thiopurines for either of these reasons.52 It is noteworthy that the use of thiopurine monotherapy or TNF antagonist monotherapy was associated with an increased risk of lymphoma compared with exposure to either medication and that this risk was higher with combination therapy than with either of these treatments alone (incidence rate per 1000 person-years, 0.95 for combination therapy vs 0.54 for thiopurine monotherapy and 0.41 for anti-TNF monotherapy).56
Methotrexate
Methotrexate is a folic acid analogue with inhibitory activity against many of the enzymes in the metabolic pathway of folic acid.57 Methotrexate and its polyglutamate metabolites potently inhibit dihydrofolate reductase, leading to accumulation and polyglutamation of dihydrofolate and oxidized folates, impaired production of tetrahydrofolates, and inhibition of enzymes downstream of dihydrofolate reductase further impairing folate-dependent synthetic reactions. Dihydrofolate reductase is critical for regenerating the fully reduced folate co-factors that are required for reactions involving transfer of 1-carbon fragments, such as the production of thymidylate and purines.22 The immune-modulating and anti-inflammatory effects of methotrexate include antiproliferative effects on leukocytes, decreased immunoglobulin production, decreased eicosanoid production, decreased production of proinflammatory cytokines, and local release of adenosine at sites of inflammation.
Methotrexate absorption from the small intestine is dose dependent and a saturable process.57 Bioavailability decreases in a roughly linear fashion with increasing doses. Bioavailability ranges from 50 to 90%. Oral absorption is uncertain in patients with disease of the digestive tract, whereas intramuscular and subcutaneous methotrexate exhibit near complete bioavailability. Subcutaneous injections have better patient acceptability than intramuscular injections and have similar bioavailability. Absorption after intramuscular administration is rapid, with maximum serum concentrations in approximately 1 hour. The distribution has been described with 2 or 3 compartment models. Tissue concentrations of methotrexate are highest in the liver, kidney, and intestines. The terminal elimination phase has a half-life of 44–55 hours. Methotrexate is metabolized by a hepatic mixed function oxidase to 7-OH-MTX, and excretion is predominantly renal.
Compared with adults, the pharmacokinetics of methotrexate are less predictable in children, with oral absorption varying from 23% to 95% after 20-mg/m2 doses.58 There is much interindividual variability in the peak and time to maximum plasma concentration, varying from 0.67 to 4 hours. Intramuscular absorption is generally complete. Peak serum concentrations are attained within 30–60 minutes.
Moderate-quality evidence indicates that intramuscular methotrexate at a dose of 15–25 mg/wk is superior to placebo for maintenance of remission of CD. Low-dose oral methotrexate (12.5–15 mg/wk) does not appear to be effective for maintenance of remission in CD.59 There is no good evidence for the use of methotrexate in UC. However, emerging evidence shows a role for oral low-dose methotrexate as combination therapy to reduce the risk of immunogenicity to TNF antagonists.60
Although there are no placebo-controlled, randomized trials of methotrexate for pediatric CD, the use of methotrexate in this patient population is increasing as monotherapy and as combination therapy with anti-TNF biologics, due to its more favorable safety profile relative to thiopurines.61 Data from 10 predominately retrospective studies showed that children treated with methotrexate once weekly had remission rates of 25–53% at 1 year.58 A retrospective longitudinal study conducted at a single tertiary referral center in 32 pediatric patients with UC found that clinical response or remission was achieved in 72%, 63%, and 50% of patients at 3, 6, and 12 months, respectively.62
Methotrexate is teratogenic and should be avoided in women of childbearing potential. Adverse effects of methotrexate include hepatocellular liver disease, nausea/vomiting (15%), fatigue (2%–6%), stomatitis (6%), myelosuppression (4.5%), and pulmonary toxicity (1%–7%).63–65 Elevated liver enzymes usually improve after temporary discontinuation or dose reduction of methotrexate. Liver toxicity may be related to the underlying disease, and the risk of liver disease with long-term methotrexate therapy in IBD is not well known.63 Hepatic and gastrointestinal adverse events are higher in patients who do not receive folic acid supplementation (74% vs 38%).64
MONOCLONAL ANTIBODIES
Monoclonal antibodies are large molecules with insufficient resistance against the hostile proteolytic gastrointestinal milieu and very limited permeation through the lipophilic intestinal wall. The distribution of monoclonal antibodies is restricted to the blood stream and extracellular spaces because of their high molecular weight and hydrophilicity.66 Due to their large size, renal clearance of monoclonal antibodies is almost nonexistent. Monoclonal antibodies are cleared from the circulation primarily by catabolism. Catabolism may be dependent on rates of extracellular degradation via proteolysis, rates of recycling through interaction with the Brambell or neonatal Fc receptor (FcRn), and rates of receptor-mediated antibody endocytosis.67
All approved monoclonal antibodies are of the IgG class. Antigen properties such as antigen distribution (soluble vs membrane associated) and antigen concentration can influence monoclonal antibody pharmacokinetics. Additionally, pharmacokinetics can be affected by monoclonal antibody structure and engineering, host factors, concurrent medications, and immunogenicity.68
Antibodies against soluble antigens, such as circulating cytokines, typically show a linear pharmacokinetic profile characterized by a 2-compartment linear model with a short distribution phase and a more prolonged elimination phase as a result of the nonspecific clearance by the reticuloendothelial system (RES) and the interaction with FcRn.68 Clearance of antibodies through the cells of the RES is mediated by Fc gamma receptors.69 Antibody salvage and recirculation is mediated by FcRn. Antibodies bind tightly to FcRn with pH-dependent affinity inside the acidic environment of endosomes and are protected from proteolysis. When the IgG-FcRn complex is returned to the cell surface, the antibody is released back into circulation at physiologic pH.69
In contrast to soluble antigens, membrane-associated antigens can enhance antibody clearance through a target-mediated process by internalization in the cell of the antigen–antibody complex. Total clearance is a result of a specific antigen-mediated pathway and a nonspecific linear clearance pathway mediated by the RES.68 After internalization, the antigen–antibody complex is degraded in lysosomes; this pathway is often referred to as the “antigen sink.” In contrast to the nonspecific clearance pathway, the target-mediated pathway is saturable due to finite amounts of antigen.70 Clearance is dependent on antigen concentration and distribution, antigen internalization rate, and antigen turnover rate. Some monoclonal antibodies show high clearance at lower antibody doses and a decrease in clearance, approaching the nonspecific clearance, with higher antibody doses because of saturation of the antigen sink.68
Owing to the different elimination and recycling pathways and various patient-, disease-, and product-related factors, there is substantial inter- and intra-individual variability in the pharmacokinetics of monoclonal antibodies.69
Antibodies Targeting Soluble Antigens
Ustekinumab
Ustekinumab is a fully human monoclonal antibody directed against the p40 subunit of interleukin IL-12 and IL-23.71 Ustekinumab is administered intravenously for induction and subcutaneously for maintenance therapy. In patients with CD, following the recommended intravenous induction dose, mean peak serum ustekinumab concentration was 125.2 ± 33.6 μg/mL, with steady-state concentration (mean trough concentration, 2.51 ± 2.06 μg/mL) achieved by the start of the second maintenance dose.72 The total volume of distribution at steady state was 4.62 L, clearance was 0.19 L/d (95% confidence interval [CI], 0.185–0.197), and the estimated median terminal half-life was approximately 19 days in patients with CD. The half-life was approximately 3 weeks, likely secondary to the salvage effect of the FcRn.
The phase 3 development program for ustekinumab for the treatment of moderately to severely active CD consisted of two 8-week induction trials (UNITI-1 and UNITI-2) and one 44-week maintenance trial (IM-UNITI).73 Intravenous ustekinumab induction regimens (either 130 mg or approximately 6 mg/kg body weight) showed significantly higher response rates at week 6 than for placebo (in UNITI-1, 34.3%, 33.7%, 21.5%, respectively; in UNITI-2, 51.7%, 55.5%, 28.7%, respectively). At week 44, subcutaneous ustekinumab maintained remission in patients who had a clinical response to induction therapy (53.1% receiving ustekinumab every 8 weeks, 48.8% receiving ustekinumab every 12 weeks, and 35.9% receiving placebo). Rates of antibody formation to ustekinumab were low (0.2%–2.3%). The efficacy date for pediatrics is limited to case reports.74, 75
In the UNITI trials, an association was observed between serum ustekinumab levels and remission.73 During induction, the highest rates of response and remission were observed with the dose of 6 mg/kg, which was also associated with higher blood levels of ustekinumab. During the maintenance trial, efficacy and exposure–response data seemed to favor administration every 8 weeks over administration every 12 weeks. Trough concentrations of ustekinumab of 0.8 or greater were associated with maintenance of clinical remission.76
The rates of overall adverse events and serious adverse events were similar across treatment groups in the induction and maintenance trials.77 Patients receiving 130 mg of ustekinumab, 6 mg/kg of ustekinumab, and placebo had overall adverse events of 64.6%, 65.9%, and 64.9% and serious adverse events of 4.9%, 7.2%, and 6.1%, respectively, in UNITI-1. Corresponding rates of overall adverse events were 50.0%, 55.6%, and 54.4%, and corresponding rates of serious adverse events were 4.7%, 2.9%, and 5.8%, respectively, in UNITI-2. At week 44 of IM-UNITI, the percentages of patients with an adverse event receiving 90 mg of ustekinumab every 8 weeks, 90 mg of ustekinumab every 12 weeks, and placebo were 81.7%, 80.3%, and 83.5%, respectively. The percentages of serious adverse events were 9.9%, 12.1%, and 15.0%, respectively. The adverse events observed were consistent with the long-term safety data for ustekinumab in patients with psoriasis, including infection, nonmelanoma skin cancer, other malignancies, and major adverse cardiovascular events.78 There was no apparent relationship between dose and safety.77
Brazikumab
Selective blockage of IL-23 may increase safety by allowing the normal IL-12-mediated Th1 response required in the immune response to intracellular pathogens while conferring the same efficacy as with p40 antibodies.79 Brazikumab is an intravenous fully human IgG2 monoclonal antibody that selectively binds the p19 subunit on IL-23 with no impact on IL-12. To date, limited information exists the on inter- and intra-individual variability in pharmacokinetics and exposure–response relationship of brazikumab in patients with IBD. In a phase 2a trial in patients with moderate to severe CD previously treated with a TNF antigonist with primary or secondary nonresponse or intolerance, patients were randomized to receive 700 mg of brazikimab at weeks 0 and 4.80 At week 8, clinical response occurred in 49.2% of patients receiving brazikimab compared with 26.7% of patients receiving placebo. Clinical remission at week 8 occurred in 27.1% of patients receiving brazikimab compared with 15.0% of patients receiving placebo. Brazikimab was well tolerated. Rates of treatment-emergent adverse events and serious adverse events in patients taking brazikimab were similar to those of patients taking placebo (67.8% vs 68.3%; 8.5 vs 8.3%).
Risankizumab
Risankizumab is also an intravenous humanized IL-23p19 inhibitor. To date, limited information exists the on inter- and intra-individual variability in pharmacokinetics and exposure–response relationship of risankizumab in patients with IBD. In a randomized, double-blind, placebo-controlled phase 2 study, patients with moderate to severe CD were randomized to receive 200 mg of risankizumab, 600 mg of risankizumab, or placebo at weeks 0, 4, and 8.71 Clinical remission was achieved in 24.4% and 36.6% of patients receiving 200 mg and 600 mg of risankizumab, respectively, compared with 15.4% of patients receiving placebo. Risankizumab showed a favorable safety profile, with no association between drug dose and incidence of adverse events. Rates of adverse events were 78% and 76% in patients receiving 200 mg and 600 mg of risankizumab, respectively, compared with 82% of patients receiving placebo. The most common serious adverse event was worsening of underlying CD.
Antibodies Targeting Membrane-Associated Antigens
Natalizumab
Natalizumab is an intravenous recombinant monoclonal antibody (95% human and 5% murine) against the cell adhesion molecule α4-integrin. Natalizumab targets the shared α4-integrins of α4β7 and α4β1, whose ligands are, respectively, mucosal addressin cell adhesion molecule (MAdCAM-1), expressed on the intestinal microvasculature, and vascular cell adhesion molecule–1 (VCAM-1), expressed on the intestinal microvasculature, endothelial cells in the brain, and microvessels in the central nervous system.81 T-cell infiltration in the gut is dependent upon interactions between surface-expressed α4β7 integrins and MAdCAM-1.82 Inhibition of the α4β7 interaction with MAdCAM-1 results in blocking of extravasation of leukocytes to the inflamed gut tissue. Preventing α4β1 integrin binding to VCAM-1 may result in decreased immune surveillance within the central nervous system and increase the risk of developing progressive multifocal leukoencephalopathy (PML).83
Natalizumab appears to demonstrate linear kinetics over a wide range of doses and body weights.84 Ghosh et al. evaluated 244 patients with active CD randomized to 1 of 4 treatment regimens: natalizumab as a single 3-mg/kg intravenous infusion followed by a placebo infusion 4 weeks later or 2 infusions of 3 mg/kg, 6 mg/kg, or placebo.85 Mean natalizumab maximum concentrations were 85.1 and 83.8 µg/mL after initial doses in the two 3-mg/kg groups and 147 µg/mL in the 6-mg/kg group. Mean half-lives ranged from 5.5 to 6.7 days. In adolescent patients (11–17 years of age; n = 38) with moderate to severe CD, the apparent elimination half-life of natalizumab appeared shorter (≤4.6 days).86 In a pilot study of treatment of active UC with natalizumab, the mean serum half-life was 3.8 days.87
The ENACT-1 trial showed that there was a small and nonsignificant improvement in response and remission rate in patients with moderately to severely active CD taking natalizumab for induction compared with placebo.88 Although the ENACT-1 trial failed to demonstrate that induction treatment with natalizumab was superior to placebo, the ENACT-2 trial showed that among the group of patients who had a response to natalizumab, there were significantly increased rates of sustained response and remission through week 60 if natalizumab treatment was continued rather than stopped. The ENCORE trial showed that natalizumab induced response and remission by week 8 that was sustained through week 12.89 One patient who received 3 doses of natalizumab in combination with azathioprine during the ENACT-1 study, 9 doses of placebo in combination with azathioprine during the ENACT-2 study, and 5 doses of natalizumab in monotherapy during an open-label extension study after early discontinuation from the ENACT-2 study died from PML.88 The benefits of natalizumab for patients with Crohn’s disease should be carefully weighed against the potential risk for PML.
Vedolizumab
Unlike natalizumab, vedolizumab specifically targets the heterodimer α4β7 and does not inhibit binding of α4β1 to VCAM-1. Vedolizumab is administered intravenously. Vedolizumab pharmacokinetics demonstrate approximately dose-proportional pharmacokinetics and maximally saturated α4β7 receptors over the tested dose range.90 Following infusion, serum concentrations increased with increasing dose. Following the fourth and final dose on day 85, serum concentrations were shown to decline monoexponentially until the concentration reached 1–10 μg/mL. Then serum concentrations fell nonlinearly. The mean elimination half-life was 15–22 days across the dose range tested.
The GEMINI 1 trial demonstrated that vedolizumab was more effective than placebo as induction and maintenance therapy for UC.91 In the GEMINI 2 trial, vedolizumab-treated patients were more likely than patients receiving placebo to have remission, but not a CDAI-100 response at week 6 in patients with active CD. Patients who had response to induction therapy were more likely to be in remission at week 52 with continued vedolizumab treatment than those who had switched to placebo.92 Post hoc analyses showed that vedolizumab had increased efficacy over placebo in CD patients irrespective of TNF antagonist treatment history, but rates of response and remission were numerically higher in patients who were naïve to TNF antagonists.93
The exposure–efficacy relationship of vedolizumab in the patients with UC from GEMINI 1 was evaluated.94 Quartile analyses revealed a positive exposure–response relationship for clinical remission, clinical response, and mucosal healing for vedolizumab induction therapy in UC. Induction trough concentrations of less than approximately 17 μg/mL were associated with a clinical remission similar to that of placebo. Further research is needed to confirm the causality of this exposure–efficacy association and the potential role for TDM.
Safety data for >4000 person-years of vedolizumab exposure demonstrate that vedolizumab treatment is well tolerated in patients with moderately to severely active UC or CD.95 Infusion-related reactions are rare (≤5% of patients). There was not an overall increase in the risk of infection or serious and opportunistic infection with vedolizumab exposure. The rate of malignancy (0.1/100 person-years) was consistent with that observed in patients with IBD. No cases of PML were observed.
Antibodies Targeting Soluble and Membrane-Associated Antigens
Infliximab
The structure of infliximab consists of a murine-variable region grafted into a human IgG1 κ scaffold.96 Infliximab is administered intravenously. A 2-compartment model with 0-order elimination describes infliximab concentration–time data. The volume of distribution is low (4.5–6 L) and represents the intravascular space. Clearance of infliximab is low and ranges between 0.230 and 0.407 L/d, resulting in a half-life between 11 and 19 days. Interindividual variability in clearance is substantial and is influenced by ADA status, concomitant immunomodulator use, the degree of systemic inflammation, serum albumin concentration, and body weight.97 The association between infliximab drug exposure and clinical, biochemical, and endoscopic outcomes has been described.97
In the ACCENT 1 trial, patients with CD who responded to an initial dose of infliximab were more likely to be in remission at 30 and 54 weeks, to discontinue corticosteroids, and to maintain their response if infliximab treatment was maintained.98 The ACCENT II trial demonstrated efficacy in maintaining closure of draining fistulas for patients with fistulizing CD.99 In patients with moderately to severely active UC, infliximab induction and maintenance infusions were superior to placebo in achieving clinical response and remission, mucosal healing, and corticosteroid-sparing effects.100 Despite its efficacy, around 10%–30% of patients do not respond to the initial treatment, and 23%–46% of patients lose response over time.101
The use of TDM for infliximab is well established. Low infliximab exposure is associated with loss of response to therapy, and current guidelines recommend performing reactive TDM, targeting a trough concentration of ≥5 μg/mL for infliximab to guide whether escalation of therapy may be beneficial compared with switching therapy in patients with active IBD.50 This approach of infliximab dose intensification to achieve adequate infliximab trough concentration has been shown to be associated with endoscopic healing.102
Potential adverse effects of infliximab include infusion reactions, serious opportunistic infections, malignancies/lymphomas, and demyelinating and lupus-like disorders. The TREAT registry is a large, prospective, observational registry designed to examine the long-term safety outcomes of various treatment regimens, including infliximab.103 Mean follow-up was 5.2 years. Three percent of infliximab infusions were associated with an infusion reaction, and 0.047% were serious reactions. An increased risk of infection was observed, but moderate to severe CD was the strongest predictor of infection (hazard ratio [HR], 2.24), followed by treatment with narcotics (HR, 1.98), prednisone (HR, 1.57), and infliximab (HR, 1.43). Infliximab has been associated with new onset or exacerbation of clinical symptoms or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis and lupus-like syndrome.104 The overall incidence rates of solid tumors, nonmelanoma skin cancer, and lymphoma were similar in infliximab-treated patients and those who received other treatments for CD.103 The rare and usually fetal occurrence of hepatosplenic T-cell lymphoma has been reported in IBD patients receiving either thiopurine monotherapy or combination therapy with infliximab and/or adalimumab.105 There were no reported cases of hepatosplenic T-cell lymphoma in patients with IBD who received only anti-TNF therapy. There have been 36 cases involving IBD patients. Most patients were between the ages of 15 and 40 years, and the vast majority were male. The estimated risk in men younger than age 35 years on concomitant thiopurine and anti-TNF therapy is 1:3534.
Adalimumab
Adalimumab is a subcutaneous, recombinant, fully human IgG1 antibody that binds with a high affinity and specificity to soluble TNF-alpha and neutralizes its biological function by blocking its interaction with TNF receptors. In healthy volunteers who received a single dose of adalimumab 40 mg subcutaneously, mean values for peak serum concentration and time to achieve peak serum concentration were 4.7 μg/mL and 131 hours (5.5 days). Absolute bioavailability after a single 40-mg subcutaneous dose of the drug was 64%.106 In general, the pharmacokinetic profile of adalimumab in patients with CD appears to be similar to that in patients with rheumatoid arthritis (RA).106
In patients with moderate to severe CD naïve to anti-TNF therapy, adalimumab was superior to placebo for induction of remission in the CLASSIC-1 trial107 and superior to placebo for long-term maintenance of remission in the CLASSIC II trial and the CHARM trial.108, 109 The efficacy of adalimumab to induce and maintain clinical remission for children with CD was also demonstrated in the IMAgINE-1 trial.110 Adalimumab was more effective than placebo in inducing and maintaining clinical remission in patients with moderate to severe UC in the ULTRA 2 trial.111
Current guidelines recommend an adalimumab trough concentration threshold ≥7.5 μg/mL during maintenance therapy at the time of loss of response, before switching to a drug out of class, because only a small proportion of patients may not be in remission at this trough concentration (approximately 10%).50 Indirect evidence shows that there may be a benefit to targeting higher trough concentrations in individual patients.
Adalimumab has generally been safe and well tolerated in global clinical trials in patients with CD.112 The safety profile was similar to that of other TNF antagonists in CD populations and comparable to other approved indications for adalimumab. Serious infection (5.8%) was the most frequently reported serious adverse event. There were low incidence rates of opportunistic infections (2%), malignancies including lymphoma and nonmelanoma skin cancer (1.3%), demyelinating disorders (0.2%), and lupus-like syndrome (0.2%).
Certolizumab Pegol
Certolizumab pegol is a humanized Fab’ fragment linked to polyethylene glycol (PEG) resulting in high-affinity binding to TNF-alpha, without needing an Fc region. Certolizumab pegol is administered by subcutaneous injection, with a resulting bioavailability of 80% compared with intravenous injection. PEGylation has improved its pharmacokinetic profile and allowed for an increased half-life of 2 weeks.113 The apparent volume of distribution is 8.33 L, and the clearance is 0.527 L/d.114 The antidrug antibody concentration (2.5–214 units/mL) was found to increase the median clearance by 142%–174%. The interindividual variability of certolizumab pegol clearance was 19.6% when accounting for time-varying C-reactive protein, albumin, and body weight, which influence certolizumab clearance.114
In the PRECISE 1 trial, there was a statistically significant clinical response in the certolizumab pegol group compared with the placebo group at week 6 (37% vs 26%) and at both weeks 6 and 26 (22% vs 12%) in patients with moderate to severe CD and a baseline C-reactive protein concentration of at least 10 mg/L.115 There was no significant improvement in remission rates. In the PRECISE 2 trial, patients with moderate to severe CD who responded to induction therapy at week 6 were more likely to have a maintained response (62% vs 34%) and remission (48% vs 29%) at week 26 with continued certolizumab treatment than with placebo.116 A long-term, open-label extension of PRECISE 1 and 2 called PRECISE 3 was a 7-year prospective clinical study demonstrating that some patients can be successfully treated for as long as 7 years without adjustment in therapy.117
An exposure–response relationship was found in patients with CD on certolizumab pegol, with higher certolizumab pegol concentrations at weeks 2, 4, and 6 associated with clinical response and remission. Approximate certolizumab pegol concentrations of at least 36.1 μg/mL at week 6 and 14.8 μg/mL at week 12 were associated with week 6 and 26 outcomes.118
In the PRECISE 1 trial, the rate of serious adverse events was 10% in patients treated with certolizumab pegol and 7% in patients treated with placebo.115 Serious infection occurred in 2% of patients who received certolizumab pegol vs <1% of patients in the placebo group. Injection site reactions were low. Cancer developed in 2 patients in the certolizumab pegol group and 2 patients in the placebo group. In the PRECISE 2 trial, certolizumab pegol was associated with a safety profile consistent with profiles in previous studies.116 Certolizumab pegol was well tolerated in the 7-year prospective clinical study.117 There were no new safety signals. The event rates of serious infections (4.37 new cases per 100 patient-years) and malignancies (1.06 new cases per 100 patient-years) were low.
Golimumab
Golimumab is an injectable fully human IgG1 antibody that has a higher affinity for soluble human TNF-alpha. Golimumab exhibits approximately dose-proportional pharmacokinetic behavior at doses ranging from 50 mg to 400 mg.119 Steady state is reached approximately 8 weeks after golimumab maintenance is commenced (week 14 of golimumab) regardless of induction dose. Factors associated with golimumab exposure were body weight, antibody-to-golimumab status, serum albumin, alkaline phosphatase, fecal markers, C-reactive protein, and pancolitis.
Overall, clinical outcomes and pharmacokinetic disposition in a small pediatric study population were found to be comparable to the historical reference adult UC population.120, 121 However, subgroup analysis showed that serum golimumab concentrations were lower among pediatric patients with body weight <45 kg who received the body surface area (BSA)–adjusted dose regimen relative to both the pediatric patients with body weight ≥45 kg and the historical reference adult population on the fixed-dose regimen. This suggests the potential need for a higher BSA-adjusted dose regimen to attain similar exposures of golimumab compared with the historical reference adult population.
PURSUIT-SC suggested that treatment with golimumab was superior to placebo in inducing clinical response, remission, and mucosal healing and increasing quality of life in patients with moderate to severe UC121; 51.0% and 54.9% of patients in the golimumab 200/100 mg and golimumab 400/200 groups were in clinical response, vs 30.3% of patients in the placebo group. The PURSUIT-M demonstrated that golimumab therapy maintained clinical response through week 54.122 Golimumab every 4 weeks maintained clinical benefit and reduced corticosteroid use through 2 years in a long-term extension trial.123
An exposure–response relationship has been described between serum golimumab concentrations and rates of clinical response. When assessed by serum golimumab quartiles, patients in the lowest quartile showed lower rates of clinical response, clinical remission, and mucosal healing, with rates of success sometimes approaching those observed in patients assigned to placebo during induction and maintenance.119 Patients in the highest serum golimumab concentration quartiles had greater rates of clinical response and clinical remission when compared with those in the lower quartiles at week 6.121 Optimal golimumab trough concentration thresholds are sparse but emerging. Detrez et al. found a cutoff of 2.6 μg/mL at week 6 to be associated with partial clinical response at week 14 (90% specificity, 56% sensitivity).124 Adedokun et al. suggested that serum golimumab concentrations of 2.5 μg/mL at week 6 and 1.4 μg/mL at week 44 are desirable concentrations targets for attainment of optimal clinical outcomes.119
Golimumab induction therapy had an adverse event profile similar to that of placebo.121 The incidence of serious adverse events was also similar for golimumab- and placebo-treated patients (3.0% vs 6.1%). Injection site reactions were uncommon. One patient had a demyelinating disorder reported after completion of golimumab induction on 400/200 mg. There was 1 death from peritonitis and sepsis after surgical complications related to an ischiorectal abscess repair and subsequent bowel perforation after surgery. Adverse events of special interest during the maintenance trial were consistent with the known safety profile of golimumab in other indications, such as the incidence of serious infections, tuberculosis, malignancy, and antibodies to golimumab.122
JANUS KINASE INHIBITORS
Janus kinases are located at the cytoplasmic tail of various cytokine receptors and are activated upon receptor–ligand interaction. Janus kinase (JAK) activation results in autophosphorylation and phosphorylation of the cytokine receptor chains. This forms a binding site for the signal transducers and activator of transcription (STAT) molecules. The activated STAT molecules translocate to the nucleus, where they bind DNA and modulate transcription. There are 4 JAK subtypes: JAK1, JAK2, JAK 3, and tyrosine kinase 2 (TYK2).125 JAK inhibitors target cytokine signaling by preventing phosphorylation of JAKs associated with the cytokine receptor. JAK inhibitors are orally delivered small molecules with a molecular weight of less than 900 Da, which allows diffusion across cell membranes. Small molecules offer potential benefits over biologics with a shorter half-life and lack of immunogenicity.126
Tofacitinab
Tofacitinab is an oral, small molecule JAK inhibitor that inhibits all JAKs but preferentially inhibits JAK1 and JAK3. Tofacitinib was shown to be a well-absorbed drug with a predicted gut availability of 93%. Unchanged parent tofacitinib was the primary circulating species in plasma (approximately 70%), with all metabolites accounting for <10% of total drug-related activity. Clearance pathways of tofacitinib included approximately 30% renal and 70% hepatic metabolism, with most attributable to CYP3A4 turnover.127
The pharmacokinetics and relationships of tofacitinab exposure to efficacy end points were recently characterized using exposure–response modeling of data from the phase 3 maintenance study in moderately to severely active UC.128 The pharmacokinetics was linear, with a mean estimated clearance of 26.3 L/h and a volume of distribution of 115.8 L. The half-life of tofacitinib was 3 hours. Age, sex, body weight, and disease severity at baseline did not have a clinically meaningful effect on oral clearance and average plasma tofacitinib concentration. The incremental efficacy of 10 mg twice daily relative to 5 mg twice daily appeared to be more pronounced in patients who were not in remission at maintenance baseline.129
The efficacy of tofacitinib was investigated in 3 phase 3, randomized, double-blind placebo-controlled trials (OCTAVE Induction 1, OCTAVE Induction 2, OCTAVE Sustain) in patients with moderately to severely active ulcerative colitis.128 Tofacitinib was found to be more effective at inducing and maintaining clinical remission than placebo. Remission at 8 weeks occurred in 18.5% and 16.6% of the patients in the tofacitinib group vs 8.2% and 3.6% in the placebo group in the OCTAVE Induction 1 and 2 trials, respectively. Remission at 52 weeks occurred in 34.3% and 40.6% of patients taking 5 mg and 10 mg of tofacitnib, respectively, vs 11.1% of patients taking placebo. Mucosal healing and sustained and glucocorticoid-free remission occurred significantly more in the tofacitinib group than placebo as well. The efficacy of tofacitinib was also investigated in 2 randomized, double-blind, placebo-controlled, multicenter phase 2b studies in patients with moderately to severely active CD.130 Primary efficacy end points were not significantly different from placebo.
In the OCTAVE trials, infections occurred at higher rates with tofacitinib than with placebo, and most infections were mild to moderate in severity.128 In the OCTAVE Induction 1 and 2 trials, infections of any severity occurred in 23.3% and 18.2%, respectively, in the 10-mg tofacitinib groups and 15.6% and 15.2%, respectively, in the placebo groups. In the OCTAVE Sustain trial, infections occurred in 35.9%, 39.8%, and 24.2% of patients in the 5-mg tofacitinib, 10-mg tofacitinib, and placebo groups, respectively. A higher rate of herpes zoster infection was observed in patients taking 10 mg of tofacitinib (3 and 2 patients, respectively, in the Induction trials and 10 patients in the Sustain trial), but no cases of herpes zoster infection were serious adverse events or resulted in discontinuation. Across all 3 trials, lipid levels increased with tofacitinib, and 5 patients had adjudicated cardiovascular events. Intestinal perforation occurred in 1 patient on 10 mg of tofacinib. Nonmelanoma skin cancer occurred in 2 patients on tofacinib in the Induction trials and 3 patients on tofacinib in the Sustain trial. All the patients with nonmelanoma skin cancer had previous exposure to thiopurines.
In the phase 2b studies in patients with CD, most adverse effects were either gastrointestinal adverse effects such as nausea and flare of CD or infections.130 There were 2 cases of nonserious herpes zoster in patients on tofacitinib 10 mg twice daily in the maintenance study. The proportions of patients with serious adverse events were numerically higher in the maintenance study (10%–13.1%) compared with those in the induction study (3.3%–11.6%). No cases of cardiovascular events were confirmed. Intestinal perforation occurred in 1 patient on tofacinib 5 mg twice daily in the maintenance study. There was 1 case of breast cancer in a patient in the tofacitinib 10 mg twice daily induction treatment group, confirmed by adjudication.
Filgotinib
Filgotinib is an oral second-generation selective JAK-1 inhibitor with 30-fold selectivity for JAK1– over JAK 2–dependent signaling, and 50 times selectivity for JAK 1 over JAK3. Filgotinib dosing leads to the formation of a metabolite, resulting from the loss of the cyclopropyl carboxylic acid group. This metabolite is active and exhibits a similar JAK1 selectivity profile as the parent compound, albeit substantially less potent. The formation of this metabolite is mediated via carboxylesterases.131 The pharmacokinetics of filgotinib was described from two phase 1 randomized, double-blind, placebo-controlled clinical trials in healthy male adults (aged 40–60 years; body mass index, 18–30 kg/m2) and one phase 2a proof-of-concept study in patients with rheumatoid arthritis.131 The decrease in plasma concentration was biphasic. The apparent total filgotinib and metabolite clearances were 3.97 L/h and 1.04 L/h, respectively. The apparent intercompartmental filgotinib clearance was 2.02 L/h. The apparent volumes of distribution of the central filgotinib, peripheral filgotinib, and metabolite compartment were 3.08 L, 4.72 L, and 4.36 L, respectively. The between-subject variability was low to moderate. Its active metabolite was detected within 30 minutes and reached a maximum 3–5 hours postdose. The average elimation half-life was 6 hours. After repeated dosing, the active metabolite half-life ranged between 22 and 27 hours. Filgotinib and its metabolites are predominantly eliminated in urine (>80%).132
In the FITZROY trial, filgotinib induced clinical remission in significantly more patients with moderate to severe CD compared with placebo.133 In patients who had prior nonresponse to TNF-alpha inhibitors, 47% of patients in the filgotinib group achieved clinical remission at week 10 compared with 23% of patients in the placebo group. In those who were anti-TNF-alpha inhibitor–naïve patients, 60% of patients in the filgotinib group achieved clinical remission at week 10, compared with 13% of patients in the placebo group.
Filgotinib had an acceptable safety profile.133 In the pooled analysis, the proportion of patients experiencing at least 1 treatment-emergent adverse event was 75% with filgotinib vs 67% with placebo. Nine percent of patients taking filgotinib and 4% of patients taking placebo experienced serious treatment-emergent adverse events. Three percent of patients on filgotinib vs no patients in the poor placebo group reported serious infections, including herpes zoster infection.
Upadacitinib
Upadacitinib is an oral selective JAK 1 inhibitor. Upadacitinib is a nonsensitive substrate for cytochrome P450 (CYP) 3A, and in vitro studies suggest that CYP2D6 may be a minor contributor to its metabolism. Approximately 20% of upadacitinib dose is eliminated unchanged in urine.134 Upadacitnib pharmacokinetics was described using a 2-compartment model with first-order absorption and elimination in 107 healthy subjects and in 466 adult subjects with rheumatoid arthritis from three phase 1 and two phase 2b 12-week rheumatoid arthritis trials.135 Pharmacokinetic parameter estimates in healthy volunteers using reference covariate values (male, body weight of 74 kg, and creatinine clearance of 107 mL/min) were as follows: apparent clearance 39.7 L/h, apparent volume of distribution of the central compartment 146 L, steady-state volume of distribution 210 L, apparent volume of distribution in the peripheral compartment 64.3 L, absorption lag time 0.48 hours, mean absorption time 0.08 hours, and intersubject variability 3.2 L/h. Differences in body weight, sex, or renal function did not result in clincially relevant effects on upadacitinib exposures.
The efficacy of upadacitnib was assessed in the Celest Study, a multicenter, randomized, double-blind placebo-controlled study of adults with moderately to severely active CD who had inadequate response or intolerance to a TNF antagonist.136 Signficantly more patients on 6 mg twice daily (27%) vs placebo (11%) achieved clinical remission. A signficant dose–response releationship for endoscopic remission was observed on upadacitinib vs placebo.
The safety profile for upadacitnib was as expected with a JAK inhibitor in this population.136 One patient on upadacitinib developed herpes zoster. Two patients on upadacitinib had adjudicated myocardial infection. There were 2 gastrointestinal perforation events and 1 case of nonmelanoma skin cancer in patients on upadacitinib.
SPHINGOSINE-1-PHOSPHATE RECEPTOR MODULATOR
Sphingolipids serve as signaling molecules involved in cell proliferation, viability, motility, and migration and lymphocyte trafficking. Sphingosine derives from catabolism of endogenous cellular sphingolipids, and sphingosine-1-phosphate (S1P) is the 1-phosphorylated form of sphingosine. The S1P receptor is expressed by lymphocytes, dendritic cells, cardiomyocytes, and vascular endothelial cells and is involved in immune surveillance, immune cell trafficking and differentiation, cardiac function, and endothelial barrier integrity.137, 138 These activities are mediated through interaction with 5 G-protein coupled receptors, S1P1-5 receptors. S1P signaling via S1P1 controls lymphocyte egress from lymph nodes into lymph. Binding of S1P receptor modulators to the S1P receptors decreases the level of circulating blood lymphocytes, which results in a reduced inflammatory response.137 S1P receptors also regulate vascular function and play a key role in maintaining endothelial cell junctions. S1P1 is strongly expressed in the colonic vasculature in UC.139
Ozanimod
Ozanimod is an oral selective modulator of mainly S1P1R and, to a lesser extent, S1P5R receptors.138 Ozanimod is metabolized to form RP101988 and RP101075 via 2 parallel metabolic pathways. Alcohol dehydrogenase and aldehyde dehydrogenase are believed to catalyze a 2-step conversion of ozanimod to RP101988, and cytochrome P450 (CYP) 3A is the primary human enzyme converting ozanimod to RP101075. Ozanimod is eliminated primarily via biotransformation, followed by biliary excretion, and renal elimination is limited.140 The pharmacokinetics of ozanimod (for age 18–55 years, BMI 18–30 kg/m2) was described to be linear, with dose-proportional increases in exposure and low to moderate interindividual variability.138 The volume of distribution was high (73–101 L/kg) and likely reflects extensive distribution of ozanimod into tissues. The oral clearance is 204–227 L/h, and the elimination half-life is approximately 17–21 hours.
Ozanimod produced a robust dose-dependent reduction in total peripheral lymphocytes with a median decrease of 65%–68% observed after 28 days of dosing at 1 and 1.5 mg/d, respectively. The pharmacodynamics effects of ozanimod showed selectivity for lymphocyte subtypes, with greater effects on CD4+ CCR7+ and CD8+ CCR7+ T cells and a lesser effect on effector memory cells than central memory cells.
Treatment efficacy for ozanimod was shown in the phase 2 TOUCHSTONE trial in moderate to severe UC.141 Clinical response occurred in 57% of those receiving 1 mg of ozanimod and 54% of those receiving 0.5 mg, as compared with 37% of those receiving placebo at week 8. Clinical remission occurred in 16% of the patients who received 1 mg of ozanimod and in 14% of those who received 0.5 mg of ozanimod, as compared with 6% of those who received placebo at week 8.
In a phase 1 study characterizing the safety of ozanimod in 88 healthy volunteers, ozanimod was shown to be generally well tolerated up to a maximum single dose of 3 mg and multiple doses of 2 mg/d, with no severe adverse events and no dose-liming toxicities.138 A dose-dependent negative chronotropic effect was observed following the first dose secondary to S1P receptor desensitization on atrial myocytes. The use of a gradual dose-escalating regimen over several days may attenuate ozanimod-induced reductions in heart rate.
DISCUSSION
Here we provide a comprehensive summary of the drug metabolism, including pharmacokinetic parameters, efficacy, and safety of the drugs currently used for the treatment of IBD. Despite these treatment options, few patients with IBD remain in sustained clinical remission. There is great interindividual variability in the pharmacokinetics and efficacy of the drugs used. The utility of TDM is increasingly being recognized to provide optimized and individualized care. TDM of thiopurines based on TPMT genotyping/phenotyping and metabolite levels can improve patient outcomes by predicting adverse effects and therapeutic response. TDM for biologics can be used to achieve the suggested trough concentration thresholds associated with improved clinical and endoscopic response. Knowledge of patient and disease factors that influence drug clearance and of the minimal level of exposure that is needed to achieve clinically important outcomes for biologics is important to optimizing available drugs in clinical practice. For example, pharmacokinetic processes may be different in the pediatric population than in adults, leading to underexposure with standard dosing. Promising agents are in the pipeline to treat IBD, many of which are orally delivered small molecules with less variability in exposure and antigenicity. Unfortunately, not all patients may respond favorably to all drugs, and with the growing therapeutic armamentarium, stratification based on disease mechanism may be required to guide patients to the right treatment. Research is needed in the field of pharmacogenetics to help predict whether an individual patient will respond to a particular drug. Furthermore, state of the art biomarker and translational research can help elucidate the mechanism of action of compounds to potentially direct patients more rapidly to efficient therapies.
Conflicts of interest: A.H. has no conflicts of interest. W.J.S. reports grants and personal fees from Prometheus Laboratories, grants and personal fees from AbbVie, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Takeda, grants and personal fees from Atlantic Pharmaceuticals, grants and personal fees from Janssen, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Genentech, grants and personal fees from Nutrition Science Partners, personal fees from Kyowa Hakko Kirin, personal fees from Millennium Pharmaceuticals, personal fees from Celgene Cellular Therapeutics, personal fees from Santarus, personal fees from Salix Pharmaceuticals, personal fees from Catabasis Pharmaceuticals, personal fees from Vertex Pharmaceuticals, personal fees from Warner Chilcott, personal fees from Gilead Sciences, personal fees from Cosmo Pharmaceuticals, personal fees from Ferring Pharmaceuticals, personal fees from Sigmoid Biotechnologies, personal fees from Tillotts Pharma, personal fees from Am Pharma BV, personal fees from Dr. August Wolff, personal fees from Avaxia Biologics, personal fees from Zyngenia, personal fees from Ironwood Pharmaceuticals, personal fees from Index Pharmaceuticals, personal fees from Nestle, personal fees from Lexicon Pharmaceuticals, personal fees from UCB Pharma, personal fees from Orexigen, personal fees from Luitpold Pharmaceuticals, personal fees from Baxter Healthcare, personal fees from Ferring Research Institute, personal fees from Amgen, personal fees from Novo Nordisk, personal fees from Mesoblast Inc., personal fees from Shire, personal fees from Ardelyx Inc., personal fees from Actavis, personal fees from Seattle Genetics, personal fees from MedImmune (AstraZeneca), personal fees from Actogenix NV, personal fees from Lipid Therapeutics Gmbh, personal fees from Eisai, personal fees from Qu Biologics, personal fees from Toray Industries Inc., personal fees from Teva Pharmaceuticals, personal fees from Eli Lilly, personal fees from Chiasma, personal fees from TiGenix, personal fees from Adherion Therapeutics, personal fees from Immune Pharmaceuticals, personal fees from Celgene, personal fees from Arena Pharmaceuticals, personal fees from Ambrx Inc., personal fees from Akros Pharma, personal fees from Vascular Biogenics, personal fees from Theradiag, personal fees from Forward Pharma, personal fees from Regeneron, personal fees from Galapagos, personal fees from Seres Health, personal fees from Ritter Pharmaceuticals, personal fees from Theravance, personal fees from Palatin, personal fees from Biogen, personal fees from Western University (owner of Robarts Clinical Trials), and grants, personal fees, and nonfinancial support from Abbvie. N.V.C. has received consultancy fees from Janssen and Takeda outside of the submitted work.
Supported by: N.V.C. holds a Career Development Award (545474) from the Crohn’s and Colitis Foundation (CCF).
REFERENCES
- 1. Fiocchi C. Inflammatory bowel disease pathogenesis: where are we?J Gastroenterol Hepatol. 2015;30(Suppl 1):12–18. [DOI] [PubMed] [Google Scholar]
- 2. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015;169:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long MD, Hutfless S, Kappelman MDet al. Challenges in designing a national surveillance program for inflammatory bowel disease in the United States. Inflamm Bowel Dis. 2014;20:398–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Iudicibus S, Franca R, Martelossi Set al. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17:1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998;94:557–572. [DOI] [PubMed] [Google Scholar]
- 6. Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1990;19:126–146. [DOI] [PubMed] [Google Scholar]
- 7. Thiesen A, Thomson AB. Review article: older systemic and newer topical glucocorticosteroids and the gastrointestinal tract. Aliment Pharmacol Ther. 1996;10:487–496. [DOI] [PubMed] [Google Scholar]
- 8. Schölmerich J. Review article: systemic and topical steroids in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):66–74. [DOI] [PubMed] [Google Scholar]
- 9. Tanner A, Bochner F, Caffin Jet al. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther. 1979;25:571–578. [DOI] [PubMed] [Google Scholar]
- 10. Rose JQ, Yurchak AM, Jusko WJ. Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm. 1981;9:389–417. [DOI] [PubMed] [Google Scholar]
- 11. Bonovas S, Nikolopoulos GK, Lytras Tet al. Comparative safety of systemic and low-bioavailability steroids in inflammatory bowel disease: systematic review and network meta-analysis. Br J Clin Pharmacol. 2018;84:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis JD, Scott FI, Brensinger CMet al. Increased mortality rates with prolonged corticosteroid therapy when compared with antitumor necrosis factor-α-directed therapy for inflammatory bowel disease. Am J Gastroenterol. 2018;113:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdalla MI, Herfarth H. Budesonide for the treatment of ulcerative colitis. Expert Opin Pharmacother. 2016;17:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryrfeldt A, Andersson P, Edsbäcker Set al. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86–95. [PubMed] [Google Scholar]
- 15. Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2011;7:419–428. [DOI] [PubMed] [Google Scholar]
- 16. Ryrfeldt A, Edsbäcker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther. 1984;35:525–530. [DOI] [PubMed] [Google Scholar]
- 17. Dahlberg E, Thalén A, Brattsand Ret al. Correlation between chemical structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoids. Mol Pharmacol. 1984;25:70–78. [PubMed] [Google Scholar]
- 18. Lundin PD, Edsbäcker S, Bergstrand Met al. Pharmacokinetics of budesonide controlled ileal release capsules in children and adults with active Crohn’s disease. Aliment Pharmacol Ther. 2003;17:85–92. [DOI] [PubMed] [Google Scholar]
- 19. Rezaie A, Kuenzig ME, Benchimol EIet al. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015:CD000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Travis SP, Danese S, Kupcinskas Let al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014;63:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lichtenstein GR, Travis S, Danese Set al. Budesonide MMX for the induction of remission of mild to moderate ulcerative colitis: a pooled safety analysis. J Crohns Colitis. 2015;9:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Targan SR, Shanahan F, Karp LC.. Inflammatory Bowel Disease From Bench to Bedside. Dordrecht, Netherlands: Springer; 2003. [Google Scholar]
- 23. Campregher C, Gasche C. Aminosalicylates. Best Pract Res Clin Gastroenterol. 2011;25:535–546. [DOI] [PubMed] [Google Scholar]
- 24. Norlander B, Gotthard R, Ström M. Steady-state pharmacokinetics of enteric coated 5-amino-salicylic acid tablets in healthy volunteers and in patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther. 1991;5:291–300. [DOI] [PubMed] [Google Scholar]
- 25. Gionchetti P, Campieri M, Belluzzi Aet al. Bioavailability of single and multiple doses of a new oral formulation of 5-ASA in patients with inflammatory bowel disease and healthy volunteers. Aliment Pharmacol Ther. 1994;8:535–540. [DOI] [PubMed] [Google Scholar]
- 26. Tolia V, Massoud N, Klotz U. Oral 5-aminosalicyclic acid in children with colonic chronic inflammatory bowel disease: clinical and pharmacokinetic experience. J Pediatr Gastroenterol Nutr. 1989;8:333–338. [DOI] [PubMed] [Google Scholar]
- 27. Cuffari C, Pierce D, Korczowski Bet al. Randomized clinical trial: pharmacokinetics and safety of multimatrix mesalamine for treatment of pediatric ulcerative colitis. Drug Des Devel Ther. 2016;10:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ford AC, Achkar JP, Khan KJet al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:601–616. [DOI] [PubMed] [Google Scholar]
- 29. Ford AC, Khan KJ, Achkar JPet al. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:167–176; author reply 177. [DOI] [PubMed] [Google Scholar]
- 30. Akobeng AK, Zhang D, Gordon Met al. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;9:CD003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Parker CE, Feagan BGet al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016:CD000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbarino JM, Staatz CE, Venkataramanan Ret al. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013;23:563–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandborn WJ. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1:48–63. [Google Scholar]
- 34. Kahan BD. Cyclosporine. N Engl J Med. 1989;321:1725–38. [DOI] [PubMed] [Google Scholar]
- 35. Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986;11:107–132. [DOI] [PubMed] [Google Scholar]
- 36. Min DI, Hwang GC, Bergstrom Set al. Bioavailability and patient acceptance of cyclosporine soft gelatin capsules in renal allograft recipients. Ann Pharmacother. 1992;26:175–179. [DOI] [PubMed] [Google Scholar]
- 37. Sandborn W. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1:48–63. [Google Scholar]
- 38. Latteri M, Angeloni G, Silveri NGet al. Pharmacokinetics of cyclosporin microemulsion in patients with inflammatory bowel disease. Clin Pharmacokinet. 2001;40:473–483. [DOI] [PubMed] [Google Scholar]
- 39. Friman S, Bäckman L. A new microemulsion formulation of cyclosporin: pharmacokinetic and clinical features. Clin Pharmacokinet. 1996;30:181–193. [DOI] [PubMed] [Google Scholar]
- 40. Sandborn WJ, Strong RM, Forland SCet al. The pharmacokinetics and colonic tissue concentrations of cyclosporine after i.v., oral, and enema administration. J Clin Pharmacol. 1991;31:76–80. [DOI] [PubMed] [Google Scholar]
- 41. Lichtiger S, Present DH, Kornbluth Aet al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. [DOI] [PubMed] [Google Scholar]
- 42. Chang KH, Burke JP, Coffey JC. Infliximab versus cyclosporine as rescue therapy in acute severe steroid-refractory ulcerative colitis: a systematic review and meta-analysis. Int J Colorectal Dis. 2013;28:287–293. [DOI] [PubMed] [Google Scholar]
- 43. McSharry K, Dalzell AM, Leiper Ket al. Systematic review: the role of tacrolimus in the management of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:1282–1294. [DOI] [PubMed] [Google Scholar]
- 44. Komaki Y, Komaki F, Ido Aet al. Efficacy and safety of tacrolimus therapy for active ulcerative colitis; a systematic review and meta-analysis. J Crohns Colitis. 2016;10:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Inoue T, Kakimoto K, Higuchi K.. New Insights Into Inflammatory Bowel Disease. London, UK: InTech. 2016. [Google Scholar]
- 46. Chow DK, Leong RW. The use of tacrolimus in the treatment of inflammatory bowel disease. Expert Opin Drug Saf. 2007;6:479–485. [DOI] [PubMed] [Google Scholar]
- 47. Venkataramanan R, Swaminathan A, Prasad Tet al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–430. [DOI] [PubMed] [Google Scholar]
- 48. Baumgart DC, Pintoffl JP, Sturm Aet al. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease–a long-term follow-up. Am J Gastroenterol. 2006;101:1048–1056. [DOI] [PubMed] [Google Scholar]
- 49. Sandborn WJ. Pharmacogenomics and IBD: TPMT and thiopurines. Inflamm Bowel Dis. 2004;10(Suppl 1):S35–S37. [DOI] [PubMed] [Google Scholar]
- 50. Feuerstein JD, Nguyen GC, Kupfer SSet al. American Gastroenterological Association Institute Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 51. Relling MV, Gardner EE, Sandborn WJet al. Clinical Pharmacogenetics Implementation Consortium . Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moon W, Loftus EV Jr. Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43:863–883. [DOI] [PubMed] [Google Scholar]
- 53. Chande N, Patton PH, Tsoulis DJet al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015:CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Timmer A, Patton PH, Chande Net al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016:CD000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boyle BM, Kappelman MD, Colletti RBet al. Routine use of thiopurines in maintaining remission in pediatric Crohn’s disease. World J Gastroenterol. 2014;20:9185–9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lemaitre M, Kirchgesner J, Rudnichi Aet al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Egan LJ, Sandborn WJ. Methotrexate for inflammatory bowel disease: pharmacology and preliminary results. Mayo Clin Proc. 1996;71:69–80. [DOI] [PubMed] [Google Scholar]
- 58. Scherkenbach LA, Stumpf JL. Methotrexate for the management of Crohn’s disease in children. Ann Pharmacother. 2016;50:60–69. [DOI] [PubMed] [Google Scholar]
- 59. Patel V, Wang Y, MacDonald JKet al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014:CD006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soon SY, Ansari A, Yaneza Met al. Experience with the use of low-dose methotrexate for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:921–926. [DOI] [PubMed] [Google Scholar]
- 61. Herfarth HH, Kappelman MD, Long MDet al. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aloi M, Di Nardo G, Conte Fet al. Methotrexate in paediatric ulcerative colitis: a retrospective survey at a single tertiary referral centre. Aliment Pharmacol Ther. 2010;32:1017–1022. [DOI] [PubMed] [Google Scholar]
- 63. Gabbani T, Deiana S, Lunardi Set al. Safety profile of methotrexate in inflammatory bowel disease. Expert Opin Drug Saf. 2016;15:1427–1437. [DOI] [PubMed] [Google Scholar]
- 64. Saibeni S, Bollani S, Losco Aet al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123–127. [DOI] [PubMed] [Google Scholar]
- 65. Saravanan V, Kelly CA. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology (Oxford). 2004;43:143–147. [DOI] [PubMed] [Google Scholar]
- 66. Deng R, Jin F, Prabhu Set al. Monoclonal antibodies: what are the pharmacokinetic and pharmacodynamic considerations for drug development?Expert Opin Drug Metab Toxicol. 2012;8:141–160. [DOI] [PubMed] [Google Scholar]
- 67. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. [DOI] [PubMed] [Google Scholar]
- 68. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–88. [DOI] [PubMed] [Google Scholar]
- 69. Ordás I, Mould DR, Feagan BGet al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635–646. [DOI] [PubMed] [Google Scholar]
- 70. Keizer RJ, Huitema AD, Schellens JHet al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. [DOI] [PubMed] [Google Scholar]
- 71. Feagan BG, Sandborn WJ, D’Haens Get al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709. [DOI] [PubMed] [Google Scholar]
- 72. Stelara(R) [package insert]. Horsham, PA: Janssen Biotech I; 2016. [Google Scholar]
- 73. Feagan BG, Sandborn WJ, Gasink Cet al. UNITI–IM-UNITI Study Group . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 74. Bishop C, Simon H, Suskind Det al. Ustekinumab in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr. 2016;63:348–351. [DOI] [PubMed] [Google Scholar]
- 75. Rinawi F, Rosenbach Y, Assa Aet al. Ustekinumab for resistant pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2016;62:e34–e35. [DOI] [PubMed] [Google Scholar]
- 76. Adedokun OJ, Xu Z, Gasink Cet al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154(6):1660–1671. [DOI] [PubMed] [Google Scholar]
- 77. Sandborn WJ, Gasink C, Gao LLet al. CERTIFI Study Group . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 78. Papp KA, Griffiths CE, Gordon Ket al. PHOENIX 1 Investigators; PHOENIX 2 Investigators; ACCEPT Investigators . Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844–854. [DOI] [PubMed] [Google Scholar]
- 79. Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn’s disease. Gastroenterol Clin North Am. 2017;46:603–626. [DOI] [PubMed] [Google Scholar]
- 80. Sands BE, Chen J, Feagan BGet al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 81. Danese S, De la Rue SA, Gasbarrini A. Antibody to alpha4beta7 integrin for ulcerative colitis. N Engl J Med. 2005;353:1180–1181; author reply 1180. [DOI] [PubMed] [Google Scholar]
- 82. Lin L, Liu X, Wang Det al. Efficacy and safety of antiintegrin antibody for inflammatory bowel disease: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Van Assche G, Van Ranst M, Sciot Ret al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. [DOI] [PubMed] [Google Scholar]
- 84. Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn’s disease. Ann Pharmacother. 2005;39:1833–1843. [DOI] [PubMed] [Google Scholar]
- 85. Van Deventer SJ, Rutgeerts P, Rask-Madsen Jet al. Pharmacokinetics (PK) and pharmacodynamics (PD) of natalizumab in active Crohn’s disease patients (abstract T1212). Gastroenterology. 2002;122(suppl 4):A–434. [Google Scholar]
- 86. Grundy J, Pan W-J, Kugathasan Set al. Pharmacokinetics and pharmacodynamics of natalizumab in a phase 2 study of adolescent patients with Crohn’s disease: 147. J Pediatr Gastroenterol Nutr. 2005;41:538. [Google Scholar]
- 87. Gordon FH, Hamilton MI, Donoghue Set al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther. 2002;16:699–705. [DOI] [PubMed] [Google Scholar]
- 88. Sandborn WJ, Colombel JF, Enns Ret al. International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group . Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. [DOI] [PubMed] [Google Scholar]
- 89. Targan SR, Feagan BG, Fedorak RNet al. International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group . Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE trial. Gastroenterology. 2007;132:1672–1683. [DOI] [PubMed] [Google Scholar]
- 90. Parikh A, Leach T, Wyant Tet al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470–1479. [DOI] [PubMed] [Google Scholar]
- 91. Feagan BG, Rubin DT, Danese Set al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15:229–239.e5. [DOI] [PubMed] [Google Scholar]
- 92. Sandborn WJ, Feagan BG, Rutgeerts Pet al. GEMINI 2 Study Group . Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 93. Sands BE, Sandborn WJ, Van Assche Get al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23:97–106. [DOI] [PubMed] [Google Scholar]
- 94. Rosario M, French JL, Dirks NLet al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11:921–929. [DOI] [PubMed] [Google Scholar]
- 95. Colombel JF, Sands BE, Rutgeerts Pet al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Knight DM, Trinh H, Le Jet al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. [DOI] [PubMed] [Google Scholar]
- 97. Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. In press. [DOI] [PubMed] [Google Scholar]
- 98. Hanauer SB, Feagan BG, Lichtenstein GRet al. ACCENT I Study Group . Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 99. Sands BE, Anderson FH, Bernstein CNet al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 100. Rutgeerts P, Sandborn WJ, Feagan BGet al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 101. Roda G, Jharap B, Neeraj Net al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Paul S, Del Tedesco E, Marotte Het al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576. [DOI] [PubMed] [Google Scholar]
- 103. Lichtenstein GR, Feagan BG, Cohen RDet al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reddy JG, Loftus EV Jr. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am. 2006;35:837–855. [DOI] [PubMed] [Google Scholar]
- 105. Kotlyar DS, Osterman MT, Diamond RHet al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41.e1. [DOI] [PubMed] [Google Scholar]
- 106. Plosker GL, Lyseng-Williamson KA. Adalimumab: in Crohn’s disease. Biodrugs. 2007;21:125–132; discussion 133. [DOI] [PubMed] [Google Scholar]
- 107. Dashwood MR, Noertersheuser P, Kirchengast Met al. Altered endothelin-1 binding following balloon angioplasty of pig coronary arteries: effect of the ETA receptor antagonist, LU 135252. Cardiovasc Res. 1999;43:445–456. [DOI] [PubMed] [Google Scholar]
- 108. Sandborn WJ, Hanauer SB, Rutgeerts Pet al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Colombel JF, Sandborn WJ, Rutgeerts Pet al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 110. Hyams JS, Griffiths A, Markowitz Jet al. Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology. 2012;143:365–374.e2. [DOI] [PubMed] [Google Scholar]
- 111. Sandborn W, van Assche G, Reinisch W. Adalimumab in the treatment of moderate-to-severe ulcerative colitis: ULTRA 2 trial results. Gastroenterol Hepatol (N Y). 2013;9:317–320. [PMC free article] [PubMed] [Google Scholar]
- 112. Colombel JF, Sandborn WJ, Panaccione Ret al. Adalimumab safety in global clinical trials of patients with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1308–1319. [DOI] [PubMed] [Google Scholar]
- 113. Tun GS, Lobo AJ. Evaluation of pharmacokinetics and pharmacodynamics and clinical efficacy of certolizumab pegol for Crohn’s disease. Expert Opin Drug Metab Toxicol. 2015;11:317–327. [DOI] [PubMed] [Google Scholar]
- 114. Vande Casteele N, Mould DR, Coarse Jet al. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn’s disease. Clin Pharmacokinet. 2017;56:1513–1523. [DOI] [PubMed] [Google Scholar]
- 115. Sandborn WJ, Feagan BG, Stoinov Set al. PRECISE 1 Study Investigators . Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 116. Schreiber S, Khaliq-Kareemi M, Lawrance ICet al. PRECISE 2 Study Investigators . Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. [DOI] [PubMed] [Google Scholar]
- 117. Sandborn WJ, Lee SD, Randall Cet al. Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn’s disease: 7-year results from the PRECISE 3 Study. Aliment Pharmacol Ther. 2014;40:903–916. [DOI] [PubMed] [Google Scholar]
- 118. Vande Casteele N, Feagan BG, Vermeire Set al. Exposure-response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn’s disease. Aliment Pharmacol Ther. 2018;47:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Adedokun OJ, Xu Z, Marano CWet al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis. 2017;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hyams JS, Chan D, Adedokun OJet al. Subcutaneous golimumab in pediatric ulcerative colitis: pharmacokinetics and clinical benefit. Inflamm Bowel Dis. 2017;23:2227–2237. [DOI] [PubMed] [Google Scholar]
- 121. Sandborn WJ, Feagan BG, Marano Cet al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95; quiz e14–e85. [DOI] [PubMed] [Google Scholar]
- 122. Sandborn WJ, Feagan BG, Marano Cet al. PURSUIT-Maintenance Study Group . Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.e1. [DOI] [PubMed] [Google Scholar]
- 123. Gibson PR, Feagan BG, Sandborn WJet al. Maintenance of efficacy and continuing safety of golimumab for active ulcerative colitis: PURSUIT-SC maintenance study extension through 1 year. Clin Transl Gastroenterol. 2016;7:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Detrez I, Dreesen E, Van Stappen Tet al. Variability in golimumab exposure: a ‘real-life’ observational study in active ulcerative colitis. J Crohns Colitis. 2016;10:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. De Vries LCS, Wildenberg ME, De Jonge WJet al. The future of Janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2017;11:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Boland BS, Vermeire S. Janus kinase antagonists and other novel small molecules for the treatment of Crohn’s disease. Gastroenterol Clin North Am. 2017;46:627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dowty ME, Lin J, Ryder TFet al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab Dispos. 2014;42:759–773. [DOI] [PubMed] [Google Scholar]
- 128. Sandborn WJ, Su C, Sands BEet al. OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators . Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 129. Mukherjee A, D'Haens GR, Sandborn WJet al. Pharmacokinetics and exposure-response of tofacitinib in a phase 3 maintenance study in ulcerative colitis patients. Gastroenterology. 2017;152:S595–S596. [Google Scholar]
- 130. Panés J, Sandborn WJ, Schreiber Set al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Namour F, Diderichsen PM, Cox Eet al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet. 2015;54:859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Namour F, Desrivot J, Van der Aa Aet al. Clinical confirmation that the selective JAK1 inhibitor filgotinib (GLPG0634) has a low liability for drug-drug interactions. Drug Metab Lett. 2016;10:38–48. [DOI] [PubMed] [Google Scholar]
- 133. Vermeire S, Schreiber S, Petryka Ret al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389:266–275. [DOI] [PubMed] [Google Scholar]
- 134. Mohamed MF, Camp HS, Jiang Pet al. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin Pharmacokinet. 2016;55:1547–1558. [DOI] [PubMed] [Google Scholar]
- 135. Klunder B, Mohamed MF, Othman AA. Population pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I and II clinical trials. Clin Pharmacokinet. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sandborn WJ, Feagan BG, Panes Jet al. Safety and efficacy of ABT-494 (Upadacitinib), an oral Jak1 inhibitor, as induction therapy in patients with Crohn’s disease: results from celest. Gastroenterology. 152:S1308–S1309. [Google Scholar]
- 137. Nielsen OH, Li Y, Johansson-Lindbom Bet al. Sphingosine-1-phosphate signaling in inflammatory bowel disease. Trends Mol Med. 2017;23:362–374. [DOI] [PubMed] [Google Scholar]
- 138. Tran JQ, Hartung JP, Peach RJet al. Results from the first-in-human study with ozanimod, a novel, selective sphingosine-1-phosphate receptor modulator. J Clin Pharmacol. 2017;57:988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Montrose DC, Scherl EJ, Bosworth BPet al. S1p(1) localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J Lipid Res. 2013;54:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tran JQ, Hartung JP, Olson ADet al. Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc study. Clin Pharmacol Drug Dev. 2018;7:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sandborn WJ, Feagan BG, Wolf DCet al. TOUCHSTONE Study Group . Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–1762. [DOI] [PubMed] [Google Scholar]