Abstract
Kadsuraols A–C (1–3), which are tetrahydrocyclobutaphenanthrofuranone-type lignans with a new carbon skeleton comprising a four-membered ring across C-1′–C-8, have been isolated from the roots of Kadsura longipedunculata. Their structures and absolute configurations were unambiguously determined using nuclear magnetic resonance, X-ray diffraction crystallography, DP4+ calculations, and computed and experimental electronic circular dichroism spectra. Kadsuraol C (3) exhibited hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity. The compounds showed no cytotoxicity at 10 μM in a zone assay.
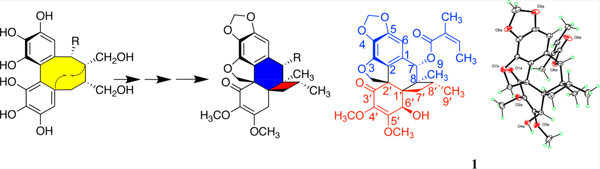
Graphical Abstract
Chronic liver disease caused by xenobiotics and viral infection is a leading cause of liver cirrhosis and liver cancer,1 which is the second most common cancer in the world. In 2018, the World Health Organisation (WHO) reported that ~788 000 people die from primary liver cancer every year. It is also reported that, from 1999 to 2015, deaths from liver cancer in the United States increased by 60% while deaths from other cancers decreased by 26%. Levels of both hepatitis testing and treatment are still extremely low in most regions of the world, reaching only 1 or 2 out of every 10 people in need.2 Traditional medicines and small molecule drugs still play a vital role in controlling liver disease.
Dibenzocyclooctadiene lignans are the primary hepatoprotective active ingredients of Schisandra chinensis and Kadsura longipedunculata, which are known as “liver tonic” in Asian herbal medicine for chronic liver disease and poor liver function. Interestingly, two liver therapies have been designed using schisandrin C, which is a dibenzocyclooctadiene lignan isolated from S. chinensis and K. longipedunculata, as a model. These include diphenyl dimethyl bicarboxylate (DDB)3 and bicyclol.4 Clinical trials found that bicyclol is effective in improving abnormal liver function, inhibiting the replication of HBV in chronic hepatitis B patients, as well as inducing differentiation of human hepatocarcinoma cells (HepG2 and Bel-7402 cells) without side effects being reported.5 These results implicate the possibility of dibenzocyclooctadiene lignans as promising chemopreventive agents active in the control of liver carcinogenesis.
Here, we report three dibenzocyclooctadiene lignans that contain a new ring system. We isolated kadsuraols A–C (1–3) from K. longipedunculata and describe their structural elucidation and bioactivity, as well as a putative biosynthesis pathway toward their formation (see Figure 1). Kadsuraol C (3) exhibited hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells with a cell survival rate of 48.22% at 10 μM, which is as active as the positive control bicyclol (48.77%). Compounds 1–3 showed no cytotoxicity in a zone assay at a concentration of 10 μM of each compound per disk. Preliminary structure–activity relationships for 1–3 are briefly discussed.
Figure 1.

Structures of kadsuraols A–C (1–3) and key core structural features (4 and 5).
Kadsuraol A (1), obtained as yellow crystals, EtOH); mp 154–157 °C; (c = 1, MeOH); had the molecular formula C27H30O9 as determined by positive HRESIMS (m/z 521.1784 [M + Na]+, calcd. 521.1782) and 13C NMR data, indicating 13 indices of hydrogen deficiency. Its UV bands (218 and 270 nm) and IR absorptions (1712 and 1616 cm−1) indicated the presence of carbonyl and benzene functionalities. The 1H NMR data (Table S7 in the Supporting Information (SI)) displayed an aromatic proton singlet at δH 6.61, two singlets characteristic of a methylenedioxy group at δH 6.00 and 5.93 (br s), an olefinic proton signal at δH 5.86 (br q, J = 7.0 Hz), an AB quartet at δH 4.59 and 4.54 (d, J = 10.0 Hz), and two enolic methoxy singlets at δH 4.09 (3H) and 3.68 (3H). In the highfield region, a methylene (δH 1.90, dd, J = 11.0 and 13.0 Hz, and 1.48, dd, J = 7.0 and 13.0 Hz, respectively), a methine (δH 1.59, m), and four methyl groups [δH 1.84 and 0.99 (each 3H, d, J = 7.0 Hz), 1.71 and 1.12 (each 3H, s)] were observed. The 13C NMR spectrum revealed 27 carbon resonances. The 1H and 13C NMR data (Table S8 in the Supporting Information), and the HSQC spectrum exhibited the presence of an α,β-unsaturated carbonyl carbon (δC 193.9), an ester carbonyl carbon (δC 167.7), a pentasubstituted aromatic moiety (δC 102.9, 124.3, 124.9, 130.5, 142.2, and 150.3), four olefinic carbons (δC 128.3, 135.3, 137.0, and 160.6), a methylenedioxy carbon (δC 101.5), an oxymethylene carbon (δC 81.9), two oxymethine carbons (δC 72.7 and 75.6), two O-methyl groups (δC 59.6 and 60.3), three quaternary carbons (δC 56.6, 45.7, and 46.2), a methylene group (δC 31.8), a methine group (δC 29.9), and four methyl groups (δC 20.2, 16.9, 15.5, and 14.1).
Analysis of the 1D and 2D NMR data assigned a C18 skeleton comprising two C6·C3 constituent units for 1. The HMBC cross-peaks from H-6′ to C-1′, C-2′, and C-7′, H-7 to C-2 and C-6, H2-7′ to C-1′, C-2′, C-6′, C-8′, and C-9′, H3-9 to C-8′, C-7, and C-8, and especially H3-9 and H-7 to C-1′ (green arrows in Figure 2) revealed the presence of a bicyclo[4,2,0]-octene moiety comprising C-2, C-1, C-7, C-8, C-8′, C-7′, C-1′, and C-2′. In addition, the HMBC crosspeaks of H-7 with C-6, and H-6′ with C-4′ and C-5′ indicated the presence of the tetrahydrophenanthren-4(1H)-one moiety. The characteristic isolated AB doublet of doublets at δH 4.59 and 4.54 in the 1H NMR spectrum, a quaternary C atom at δC 56.6 in the 13C NMR spectrum, and the HMBC crosspeaks from H-20α to C-3′, C-2, and C-3, and H-20β to C-3′ confirmed the presence of a 2H-spiro[benzofuran-3,1′-cyclohexan]-3′-en-2′-one structural moiety in 1 (see Figure 2, as well as Figure S10 in the Supporting Information). The methylenedioxy group was connected to C-4 and C-5 based on the HMBC crosspeaks from H2-19 to C-4 and C-5. The two methoxy groups were assigned to C-4′ and C-5′ by the HMBC crosspeaks from 4′-OCH3 to C-4′ and from 5′-OCH3 to C-5′, respectively. In addition, the HMBC correlations from H3-5″ to C-1′′, from H3-4″ to C-2′′ and C-3′′, from H-3′′ to C-1′′, and from H-7 to C-1′′ indicated the presence of an angeloxy group located at C-7. Thus, the 2D structure of 1 was established as shown in Figure 1.
Figure 2.

Key HMBC correlations of 1.
The ROESY correlations of H-7 with H-6 and H-8′ (see Figure 3, as well as Figure S11 in the Supporting Information) permitted the tentative assignment of an α-orientation to the angelate group. The ROESY correlations of H-20β with H-7′β, H-7′α with H-6′, and H-6′ with H3-9 and H3-9′ (see Figure 3 and Figure S11) support that HO-6′ and the four-membered ring were β-oriented, and H3C-9′ and H3C-9 were α-oriented.
Figure 3.

Optimized conformation and the key ROESY correlations (indicated by blue arrows) of 1.
Quantum chemical calculations of the ECD spectrum were conducted for the (7S, 8S, 1′S, 2′S, 6′R, 8′R) stereoisomer of 1 (see Figure S1 in the Supporting Information) using time-dependent density functional theory (TDDFT) at the B3LYP/6-311+G(2d,p) level in methanol. The absolute configuration of 1 was defined by comparison of the experimental and calculated ECD spectra of 1 and its enantiomer (see Figure 4). Except for the 325 nm region, the calculated and experimental spectra agreed well, thus defining the absolute configuration of 1 as (7S, 8S, 1′S, 2′S, 6′R, 8′R).
Figure 4.

Experimental ECD spectrum of 1 (black) compared to (left image) calculated averaged Boltzmann-weighted ECD spectra of conformers 1a1, 1a2, 1a3, 1a5, and 1a6 (red) of the (7S, 8S, 1′S, 2′S, 6′R, 8′R) diastereomer of 1; and (right image) calculated averaged ECD spectrum of the enantiomer of 1 in MeOH (red). The σ-value (artificial line broadening) was set to 0.21 eV.
Crystallization of 1 from MeOH:H2O (10:1) afforded yellow crystals of the hemihydrate suitable for X-ray crystal structure studies. Data at T = 100 K with Mo Kα radiation yielded R = 0.053 and a Flack parameter x = 0.2(2), in agreement with the (7S, 8S, 1′S, 2′S, 6′R, 8′R) absolute configuration. In the crystalline hemihydrate, there are eight independent (unrelated by symmetry) molecules of 1, one of which is shown in Figure 5, and four water molecules. The eight molecules differ somewhat in conformation, particularly for the two methoxy groups, and one of the eight exhibits disorder.
Figure 5.

(A) X-ray ORTEP drawing of 1, revealing (B) an unusual crystal packing with eight structures in the unit cell.
In addition, 1H and 13C NMR chemical shift calculations were performed to assign the absolute configuration of compounds 1 (7S, 8S, 1′S, 2′S, 6′R, 8′R), and three other diastereomers labeled 1′ (7S, 8S, 1′S, 2′R, 6′R, 8′R), 1″ (7R, 8S, 1′S, 2′R, 6′R, 8′R) and 1‴ (7S, 8S, 1′S, 2′R, 6′S, 8′R) considering the positions that are readily epimerizable such as allylic alcohols, and protons near to carbonyl moieties and esters. We used the gauge independent atomic orbital (GIAO)6–8 method at the PCM/mPW1PW91/6-311+G(d,p) level of theory8 for DP4+ calculations.9 The computed chemical shifts of 1, 1′, 1″, and 1‴ were compared with the experimental values utilizing total absolute deviation (TAD), mean absolute error (MAE), and DP4+ probability analyses; all three methods have been successfully applied in addressing stereochemical assignment of isomeric compounds.10,11 The TAD and MAE values for 1H NMR and 13C NMR chemical shifts were in accordance, and clearly noted 1 as the most probable stereoisomer (see Figure 6). DP4+ analysis also predicted the structure of 1 to match the experimental results with 100% probability (Figure S4 in the Supporting Information). Considering the relative configuration assigned via the X-ray diffraction data, the comparison of experimental and ab initio computed ECD spectra, and the DP4+ analysis, the absolute configuration of 1 was unequivocally defined as (7S, 8S, 1′S, 2′S, 6′R, 8′R).
Figure 6.

Total absolute deviation (TAD), mean absolute error (MAE), and DP4+ probability analyses (sarotti-nmr.weebly.com) for 1 and three of its diastereomers (Gibbs free energies at the PCM/mPW1PW91/6-311+G(d,p) level were used for the analysis).
Kadsuraol B (2) white powder, (c = 1, MeOH); Kadsuraol C (3) white powder, (c = 1, MeOH); IR (KBr) νmax 3430, 2959, 1712, 1654, 1616, 1459, 1419, 1395, 1354, 1331, 1236, 1190, 1115, 1109, 1058, 1020, 964, 937, 903, 846, 786, 756 cm−1; 2 and 3 were assigned molecular formulas of C29H28O9 and C24H26O9, respectively, on the basis of the sodium adduct HRESIMS ions at m/z 543.1626 and 481.1469 and 13C NMR data. The HSQC overlay (see Figures S17 and S25 in the Supporting Information) showed 2 and 3 were similar to 1 except for the C-7 substituents. The 9-angelate group in 1 was replaced by a benzoate and an acetoxy group in 2 and 3, respectively. The structures of 2 and 3 were confirmed via comparison of 1H, 13C NMR, and ECD data with those of 1 (Tables S7 and S8 in the Supporting Information).
Kadsuraols A–C (1–3) are likely biosynthesized via the phenylpropenoid pathway (see Scheme 1). Thus, one-electron oxidation of 3,4,5-trihydroxycinnamyl alcohol (6) would lead to formation of the C-8 radical 7. Dirigent protein guided C– C radical coupling12 would afford the 8,8′-linked quinonemethide 8 and subsequently the bis-phenylpropanol 9 via reduction. Phenol-oxidative formation of the biphenyl bond would lead to the dibenzocyclooctadiene intermediate 10 that may be susceptible to further oxidation and generation of the unique cyclobutane moiety in 12 via the diradical 11. Various well-established functional group manipulations would transform 12 into the target pentacyclic architecture 15 via intermediates 13 and 14. The appropriate acylations would then convert 15 into the novel kadsuraols A–C (1–3). The putative biosynthesis pathway of compounds 1–3 is presented in Scheme 1.
Scheme 1.

Putative Biosynthesis Pathway Toward the Formation of 1–3
Compounds 1–3 were evaluated for their in vitro hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 (human hepatocellular liver carcinoma cell line) cells,13 using the liver protectant bicyclol as the positive control. Compound 3, when added into the resuscitated HepG2 cells incubated with APAP for 48 h, effected a cell survival rate of 48.22%, which is highly comparable to the positive control bicyclol (48.77%) at 10 μM, while compounds 1 and 2 were inactive (see Table S1 in the Supporting Information). These findings implied a simple SAR, that the nature of the C-7 chain is crucial for the hepatoprotective activity, which is likely associated with the length and/or the size of compound 3. Compounds 1–3 did not show activity in a differential cytotoxicity zone assay used for discovery of new anticancer leads13 which includes 11 tumor cell lines, indicating that compound 3 is a noncytotoxic hepatoprotective molecule.
In conclusion, three unique tetrahydrocyclobutaphenanthrofuranone-type lignans containing a four-membered ring across C-1′–C-8 were identified. This is the first report of the natural occurrence and structural assignment of pentacyclic lignans containing a cyclobutane structural moiety. Herein, we showed that NMR and DP4+ calculations arrived at the same structure as X-ray, revealing that the DP4+ approach could be used as a reliable primary approach to the assignment of structure and stereochemistry for highly complex natural products. Kadsuraol C (3) showed noncytotoxic hepatoprotective effects against APAP-induced toxicity in HepG2 cells comparable to a clinically used control.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported financially by the CAMS Initiative for Innovative Medicine (No. CAMS-I2M-1-010). Supercomputer support is acknowledged from NSF (No. MRI 1338056) and the Mississippi Center for Supercomputer Research. This investigation was supported in part by Grant No. P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH), and was performed in part in a facility constructed with support from the Research Facilities Improvements Program (No. C06RR14503) from the National Institutes of Health (NIH) National Center for Research Resources. We also thank NCCIH (R01AT007318), CSC, The Cooper Family, and The Abney Foundation for Financial Support.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b02207.
Full details of experimental procedures, copies of NMR (1D and 2D), ECD and MS spectra of 1–3, NMR signal assignments of 1–3 and computational methods for ECD calculations (PDF)
Accession Codes
CCDC 1556354 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: +44 1223 336033.
The authors declare no competing financial interest.
REFERENCES
- 1.Sarin S; Kumar M; Lau G; Abbas Z; Chan H; Chen C; Chen D; Chen H; Chen P; Chien R; et al. Hepatol. Int 2016, 10, 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cyong J-C; Kim S-M; Iijima K; Kobayashi T; Furuya M Am. J. Chin. Med 2000, 28, 351. [DOI] [PubMed] [Google Scholar]
- 3.Chiu HF; Chen TY; Tzeng YT; Wang CK Phytother. Res 2013, 27, 368. [DOI] [PubMed] [Google Scholar]
- 4.Wang T; Jin Y; Zhao R; Wu Y; Zhang Y; Wu D; Kong D; Jin X; Zhang F Int. J. Infect. Dis 2014, 20, 37. [DOI] [PubMed] [Google Scholar]
- 5.Cheeseman JR; Trucks GW; Keith TA; Frisch MJ J. Chem. Phys 1996, 104, 5497. [Google Scholar]
- 6.McWeeny R Phys. Rev 1962, 126, 1028. [Google Scholar]
- 7.Wolinski K; Hinton JF; Pulay PJ Am. Chem. Soc 1990, 112, 8251. [Google Scholar]
- 8.Grimblat N; Zanardi MM; Sarotti AM J. Org. Chem 2015, 80, 12526. [DOI] [PubMed] [Google Scholar]
- 9.Wang X; Liu J; Pandey P; Chen J; Fronczek FR; Parnham S; Qi X; Doerksen RJ; Ferreira D; Sun H; Li S; Hamann MT Biochim. Biophys. Acta, Gen. Subj 2017, 1861, 3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bifulco G; Riccio R; Martin GE; Buevich AV; Williamson RT Org. Lett 2013, 15, 654. [DOI] [PubMed] [Google Scholar]
- 11.Dalisay DS; Kim KW; Lee C; Yang H; Rübel O; Bowen BP; Davin LB; Lewis NG J. Nat. Prod 2015, 78, 1231. [DOI] [PubMed] [Google Scholar]
- 12.Li C-J; Ma J; Sun H; Zhang D; Zhang D-M Org. Lett 2016, 18, 168. [DOI] [PubMed] [Google Scholar]
- 13.Valeriote FA; Tenney K; Pietraszkiewicz H; Edelstein M; Johnson TA; Amagata T; Crews PJ Exp. Ther. Oncol 2012, 10, 119. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


