Abstract
Background
Mammary adenocarcinomas are one of the most common tumour diseases in bitches. The relationship between oxidative stress and the degree of malignancy of the tumour has not been sufficiently researched in veterinary medicine.
Objectives
The main objective was to investigate the potential role of MDA as a practice‐relevant biomarker for the assessment of systemic oxidative stress and to determine whether this parameter can indicate the malignancy grade of a mammary adenocarcinoma.
Methods
In the present pilot study, MDA plasma concentrations were analysed in 55 bitches with (n = 28) and without (n027) malignant adenocarcinomas of the mammary gland using two different measurement methods and the relationship to tumour size was investigated.
Results
The mean MDA concentration measured by enzyme‐linked immunosorbent assay (ELISA) was 289 ng/mL (range 365–634 ng/mL) in dogs with grade 1 adenocarcinoma (n = 13), 288.5 ng/mL (range 85–752 ng/mL) in dogs with grade 2 adenocarcinoma (n = 10), 332 ng/mL (range 239–947 ng/mL) in dogs with grade 3 (n = 5) adenocarcinoma and 293 ng/mL (range 175–549 ng/mL) in dogs without a mammary tumour (n = 27). When MDA was measured by HPLC, the average MDA concentration in the study group (n = 11) was 0.24 µmol/L (range 0.16–0.37) and that of the control group (n = 15) was 0.27 µmol/L (range 0.16–1.62). Thus, there were no significant differences between the study group with malignant adenocarcinomas and the control group in both examination methods (p > 0.05). Furthermore, there was no correlation between the MDA concentrations and the approximate volume of the mammary tumour.
Conclusion
The results highlight the challenges of providing a prognosis for the malignancy of a mammary adenocarcinoma based on MDA concentrations in plasma using ELISA or HPLC. As a result, histopathological examination remains the gold standard for diagnosing and differentiating adenocarcinomas of the mammary gland.
Keywords: dog, ELISA, HPLC, malondialdehyde, mammary tumour, oxidative stress, plasma
In the present pilot study, malondialdehyde (MDA), a parameter for oxidative stress, was measured in plasma of bitches with and without malignant mammary adenocarcinomas. The results showed no correlation between MDA concentrations in the blood and malignancy or approximative volume of the mammary adenocarcinomas. As a result, histopathological examination remains the gold standard for diagnosing and differentiating adenocarcinomas of the mammary gland.

1. INTRODUCTION
Mammary tumours are the most common type of tumour in intact bitches (Sorenmo, 2003). According to previous literature, at least 40–50% of these tumours appear to be malignant (Sorenmo, 2003). There is a growing interest in the investigation of pathogenesis and prognostic biomarkers for the assessment of malignancy (Burrai et al., 2022; Estaller et al., 2022; Michishita et al., 2011; Montalli et al., 2017; Rasotto et al., 2017; Santos et al., 2023). The aetiopathogenesis of tumours of the mammary gland in dogs is not yet fully understood, although genetic, nutritional and hormonal factors are discussed (Sarli et al., 2002; Sorenmo, 2003). Therapy, such as surgical resection and, if necessary, adjuvant treatment, depends on the type of tumour, hormone and receptor status as well as cell proliferation (Funakoshi et al., 2000; Rasotto et al., 2017; Vinothini et al., 2009). Based on the World Health Organization (WHO) International Histological Classification of Mammary Tumours, the malignancy of the tumour is first concluded based on the morphology and histology. Goldschmidt et al. published a revised grading for the determination of malignancy in 2011. The authors focus primarily on the tubular formation, the nuclear pleomorphism and the mitosis number/10 HPF (high‐power field). On the basis of this scoring, a distinction can be made between low (well differentiated; grade 1), moderate (moderately differentiated; grade 2) and high‐grade (poorly differentiated; grade 3) malignancy of the tumours. The prognosis depends on the histogenesis and the staging (Funakoshi et al., 2000; Nieto et al., 2003; Sarli et al., 2002; Sorenmo, 2003; Vinothini et al., 2009).
Interest in the investigation of adequate biomarkers for canine mammary tumours is steadily growing (Kaszak et al., 2018). Kaszak et al. (2018) have already investigated tumour‐associated genes such as the breast cancer 1 gene (BRCA1), the breast cancer 2 gene (BRCA2), antigens (Ki‐67), the endothelial growth factor receptor (HER‐2), the progesterone receptor and cyclooxygenase 1 in the affected tissue and in serum.
Nevertheless, the detection of these biomarkers is not yet established in current veterinary practice to indicate the need for further therapy, such as surgical removal of the tumour or subsequent chemotherapy.
With regard to the pathogenesis of adenocarcinomas of the mammary gland, studies have shown that oxidative stress plays a decisive role in humans with regard to the invasion and progression of a tumour (Kangari et al., 2018). Free radicals are produced by lipid peroxidation, which seems to be related to tumour growth (Kangari et al., 2018). Malondialdehyde (MDA) has so far been established in human medicine as a biomarker for the detection of oxidative stress. MDA is a degradation product of lipid peroxidation (Canakci et al., 2009; Cherian et al., 2019). The clinical relevance of this biomarker has been shown in human medicine studies regarding the diagnosis of dental diseases and mammary tumours, among others (Canakci et al., 2009; Cherian et al., 2019; Kangari et al., 2018; Taba et al., 2005). For example, Kangari et al. (2018) studied 38 women diagnosed with early‐stage breast cancer. The aim was to examine whether the MDA concentrations in the blood were increased in these patients and whether enzymatic antioxidants such as glutathione peroxidase (GPX) and superoxide dismutase (SOD) were also systemically increased or decreased. Patients were included who did not have any other cancer, liver or kidney disease or diabetes. Compared to the control group, the MDA concentrations in the serum of the breast cancer patients were significantly higher than in the control group. Interestingly, SOD levels were also significantly increased, while GPX levels were decreased. The results of the SOD and GPX concentrations suggest an individual compensatory capacity of the enzymes in response to oxidative stress (Kangari et al., 2018).
With regard to small animal medicine, Jayasri et al. (2016) determined the increased presence of reactive oxygen species, including fructose‐1, hexokinase and glucose‐6‐phosphate, in the diseased tissue in a pathohistological study using six tissue samples from canine mammary tumours and six healthy tissue samples. These results indicate increased oxidative stress in tumour tissue (Jayasri et al., 2016).
Karamagouruparan et al. (2005) were also able to demonstrate increased reactive oxygen species in canine mammary tumour tissue as a result of lipid peroxidation and a resulting upregulation of antioxidants such as glutathione, SOD and vitamin E. In another clinical study, an increase in serum SOD was detected in 28 dogs with mammary tumours, including 15 bitches with malignant tumours, 13 dogs with benign tumours and 10 healthy bitches (Szczubial et al., 2004). In addition to oxidative stress, another study with 33 bitches suffering from a mammary tumour (log grade of malignancy: n = 26; high grade: n = 7) compared to healthy bitches (n = 10) also demonstrated increased inflammatory reactions based on elevated cytokines (TNF‐α, INF‐γ, IL‐1 and IL‐6) (Machado et al., 2015).
A correlation between MDA concentrations in the blood and malignant tumour disease has also already been demonstrated in dogs (Macotpet et al., 2013). Dogs with mast cell tumours (n = 15), osteosarcomas (n = 8), mammary carcinomas (n = 27) and other malignant tumours were included. However, in this study, the tumours were not differentiated more precisely with regard to the grading scheme. Since there are still no differentiated studies in the accessible literature with regard to grading, the aim of the present study was to check to what extent plasma MDA in dogs with adenocarcinomas of the mammary gland provides indications of the malignancy of the tumour.
2. MATERIALS AND METHODS
The aim of the present study was to determine the plasma concentrations of MDA in dogs presenting to the Clinif of Small Animal Surgery and Reproduction, Ludwig‐Maximilians‐Universität Munich, Germany, for surgical removal of a mammary tumour. Blood samples were taken as part of the routine preanaesthetic examination. The study was approved by the Ethics Committee of the Veterinary Faculty, Ludwig‐Maximilians‐ Universität Munich, Germany (AZ 285‐30‐09‐2021).
A prerequisite for inclusion in the study was that one or more adenocarcinomas were diagnosed in the pathohistological examination of the tumour(s). In the case of other benign or malignant tumours, such as fibrosarcoma or squamous cell carcinoma, these patients were subsequently excluded. If not all tumours could be removed in one session, these patients were also excluded. Dogs in which the mammary tumour presented as a benign adenoma served as a control group. In addition, young, healthy bitches ≤2 years of age that presented for spaying or cycle stage determination and did not have mammary tumours were included in the control group. These dogs also had blood routinely drawn for preanaesthesia or cycle stage determination. A prerequisite for inclusion in the study was also the absence of other tumourous or inflammatory diseases, a normal general examination, a normal laboratory examination (red blood count, white blood count and clinical chemistry with liver, kidney values and total protein). In case of spaying, the pathohistological examination of the ovaries had to be without abnormal findings.
The MDA determination in plasma was done by ELISA; if sufficient plasma was left, a determination by high‐pressure liquid chromatography (HPLC) was also initiated.
MDA concentrations in plasma were analysed in an in‐house laboratory using a commercial ELISA kit (MDA ELISA Kit, Nordic Biosite AB; Sweden). For this purpose, the ELISA plate was first washed twice, the samples diluted with an appropriate dilution buffer solution and then pipetted onto the ELISA plate. Then the BDA working solution (biotin‐detection‐antibody) was pipetted and the plate was incubated covered at 37°C for 45 min. In the next step, the ELISA plate was washed three times and then the horseradish peroxidase (HRP)‐streptavidin conjugate (SABC) solution was pipetted and the plate was incubated again at 37°C for 30 min. After washing the plate five times, the tetramethylbenzidine (TMB) substrate, which was also prewarmed at 37°C, was pipetted onto the plate and the plate was covered again and incubated at 37°C for 15 min. Finally, a stop solution was pipetted onto the plate and the plate was immediately measured (ELISA Reader Infinite® F50, Tecan Deutschland GmbH, Crailsheim, Germany). For MDA measurement by high‐performance liquid chromatography (HPLC), the plasma samples were shipped frozen to the SYNLAB MVZ Laboratory Munich Centre, Germany, where they were analysed accordingly. The histopathological examination was carried out at the Institute of Animal Pathology, Faculty of Veterinary Medicine, xx. Based on the findings, tumours were classified according to Goldschmidt et al. (2011) into low‐grade (grade 1) (Figure 1), intermediate‐grade (grade 2) (Figure 2) and high‐grade malignant (grade 3) (Figure 3). If a patient had multiple mammary tumours, the highest grade was scored. When estimating the volumes (length × width × height), the values of several tumours were added together.
FIGURE 1.
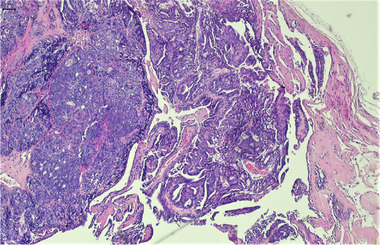
Mammary tumour grade: 1, multilobular, epithelial proliferates, accompanied by fibrovascular stroma rich in collagen fibres, partly with formation of papillary structures. Two mitoses 10 per high‐power field (HPF), HE staining, 40× magnification.
FIGURE 2.

Mammary tumour grade: 2, focal multinodular, well circumscribed, capsule‐like epithelial proliferation, predominantly tubulopapillary to partly cystic growth, multifocal with myxoid ground substance. HE staining, 40× magnification.
FIGURE 3.

Mammary tumour grade: 3, nodular, partly infiltrative, partly pseudo‐encapsulated proliferation of epithelial and myoepithelial cells. The epithelial component is partly papillary in growth. Eleven mitoses per 10 HPF, HE staining, 40× magnification.
The results were then statistically analysed. First, the Shapiro–Wilk test was used to check whether the data were normally distributed. To assess a correlation between MDA concentration and degree of malignancy or size of the adenocarcinoma, the Kruskal–Wallis test was used. To check whether there was a difference between the examination and control groups, the Mann–Whitney U test was used. Results with a p‐value ≤ 0.05 were considered statistically significant.
3. RESULTS
Fifty bitches were included in the study, among them 28 bitches with a malignant mammary tumour (study group) and 27 bitches without a malignant mammary tumour (control group). The average age of the study group was 9 years (4–14) and the average age of the control group was 3 years (1–13). The average weight of the study group was 17 kg (range 3–31) and that of the control group 12 kg (range 5–40). Overall, in the study group of dogs with adenocarcinoma, 13 patients were diagnosed with grade 1, 10 with grade 2 and 5 with grade 3 tumours.
The mean MDA concentration by ELISA was 289 ng/mL (range 117–634 ng/mL) in dogs with grade 1 adenocarcinoma, 288.5 ng/mL (85–752 ng/mL) in those with grade 2, 332 ng/mL (range 239–947 ng/mL) in those with grade 3 and 293 ng/mL (range 175–594 ng/mL) in those without malignant adenocarcinoma (n = 27) (Figure 4). Among the animals in the control group were two animals with benign adenomas and one animal with a benign mixed mammary tumour. The average MDA concentration of these three animals was 290 ng/mL and thus did not deviate significantly from the overall average of the control group. The remaining dogs in the control group had no mammary tumours.
FIGURE 4.

MDA concentrations (ng/mL) in plasma (ELISA method) of bitches with and without adenocarcinoma of the mammary groin depending on the degree of malignancy of the adenocarcinoma.
The measurement of MDA by HPLC also showed no significant differences (p < 0.05) between the study group (n = 11) and the healthy control group (n = 15) (Figure 5). The average MDA concentration in the study group was 0.24 µmol/L (range 0.16–0.37), in the control group 0.27 µmol/L (range 0.16–1.62).
FIGURE 5.

MDA concentrations (µmol/L) in plasma (HPLC method) of the study group with adenocarcinoma compared to the control group.
There was also no correlation between the MDA concentrations and the estimated volumes of the tumours (p = 0.9). Thus, the approximate volume of bitches with grade 1 tumours was 12.4 cm3 (range 0.25–40.3), with grade 2 tumours 14.9 cm3 (range 0.3–31.4) and with grade 3 tumours 73.3 cm3 (range 1.4–216) in the study group and that of bitches with benign mammary tumours in the control group 1.47 cm3 (range 0.1–4). On the basis of MDA concentrations in the blood alone, it was not possible to distinguish between bitches without and bitches with malignant mammary tumours.
4. DISCUSSION
In the present study, MDA concentrations in plasma of dogs with malignant adenocarcinoma of the mammary gland were determined by ELISA and HPLC and compared with MDA concentrations of healthy dogs without adenocarcinoma. The aim was to check whether a blood test can be used to determine whether a dog with a mammary tumour has malignant adenocarcinoma. There are currently no concrete studies on this in the accessible literature, which also include the dependence on the degree of malignancy of the tumour. Overall, there were no differences between the examination and control groups, regardless of the malignancy or the size of the tumour. These results illustrate the challenges of providing a prognosis for the malignancy of mammary adenocarcinoma based on MDA concentrations in plasma.
This is in contrast to a previous study in which MDA concentrations in the serum of dogs with tumours were higher than in dogs without tumours (Macotpet et al., 2013). That study included various tumours, including mast cell tumours, osteosarcomas, mammary carcinomas and others, although the degree of malignancy was not considered for the mammary tumours. The control group consisted of dogs older than 2 years that had no specific findings in the clinical general examination and no parasites; the average age of subjects was 5 years (range 3–7) (Macotpet et al., 2013). Here, the MDA concentrations of the bitches with mammary tumours were higher than those of the control group.
The average age of the bitches in the control group in the present study was 3 years. Diseases such as kidney and liver diseases as well as acute and chronic inflammations were exclusion criteria for the study, which could be excluded as far as possible in the blood tests. It cannot be ruled out with certainty whether tumour diseases may nevertheless have been overlooked in the study group without a malignant mammary tumour.
In the present study, the ELISA and HPLC techniques were used as detection methods for the determination of MDA in plasma, while Macotpet et al. (2013) used a TBARS (thiobarbituric acid reactive substances) assay in serum. This is based on a reaction between thiobarbituric acid (TBA) and the products of lipid peroxidation. This method was also successfully used in other studies on oxidative stress in canine mammary tumours (Kangari et al., 2018; Kumaraguruparan et al., 2005), including a clinical study in bitches with benign (n = 13) and malignant (n = 15) mammary tumours, in which parameters such as GPX and SOD were examined (Szczubial et al., 2004). The use of a TBARS assay thus appears to be a promising procedure, although ELISA and HPLC methods for the determination of MDA are also generally considered to have accurate sensitivity and specificity (Bevan et al., 2003; Breusing et al., 2010; Mylnikov et al., 2022; Verlaet et al., 2019). In other studies with dogs, oxidative stress was successfully determined using MDA from plasma by means of HPLC (Kapun et al., 2012). The reasons why the results of this study did not show significant differences between the study and control groups, in contrast to other studies, could not be clarified conclusively, though differences in methodology between these studies cannot be excluded as a contributing factor.
The grading of malignancy is a complex issue and is handled differently in previous studies on oxidative stress in bitches with mammary tumours. For example, Macotpet et al. (2013) included mammary carcinomas in their study on oxidative stress in various canine tumour diseases, but did not further address the malignancy grading of these carcinomas (Macotpet et al., 2013). Kumaraguruparan et al. (2005) included both histopathological differentiation (adenocarcinoma, malignant mixed tumour and squamous cell carcinoma) and staging in their study on oxidative stress in bitches with mammary tumours (Kumaraguruparan et al., 2005). Goldschmidt et al. (2011) developed a simplified grading scheme based on different classifications (Pena et al., 2013), which was also applied in the present study. Follow‐up studies with a larger patient population per grade of malignancy of an adenocarcinoma would be useful to further investigate the relationship between grade of malignancy and an adequate biomarker, although in the present study it was not possible to distinguish between bitches with malignant and non‐malignant tumours regardless of the grade of malignancy of the tumour.
The rate of malignant tumours in the present study was significantly higher than described in the literature, with 3 adenomas in the study group and 28 adenocarcinomas in the control group. Demographic factors, which may be associated with a different breed distribution in different countries, may explain such differences, but the study population in the present study was too small to investigate this in more detail.
In order to assess the relationship between tumour size and oxidative stress, only the approximate volume was recorded in the pathological examination in the present study. A direct volume determination of the tumour after surgical resection would be much more accurate. In the other studies already mentioned on oxidative stress in dogs with mammary tumours, the size of the tumours was not taken into account at all (Kumaraguruparan et al., 2005; Macotpet et al., 2013; Szczubial et al., 2004), although they could see differences between dogs with mammary tumours and healthy dogs. A study of prognostic factors in canine mammary adenocarcinoma showed that tumour diameter correlated with survival time and metastasis rate (Rasotto et al., 2017).
These results highlight the challenges of predicting the malignancy of mammary adenocarcinomas based on plasma MDA concentrations using ELISA or HPLC. As a result, pathohistological examination remains the gold standard for diagnosing and differentiating mammary adenocarcinoma. There are already clinical studies that have successfully investigated other oxidative stress parameters besides MDA, including GPX and SOD, and have thus been able to differentiate between dogs with malignant tumours and healthy dogs. It therefore appears to useful to measure several oxidative stress parameters to investigate differences in groups. Follow‐up studies with a larger patient population per degree of malignancy of adenocarcinoma would be useful to possibly establish concrete threshold values of such parameters that are also used in everyday practice to possibly obtain an indication of the prognosis for the patient.
AUTHOR CONTRIBUTIONS
Maike Schroers: Conceptualisation; formal analysis; investigation; methodology; project administration; writing – original draft; Beate Walter: Data curation; supervision; writing – review and editing; Stine Fischer: Data curation; writing – review and editing; Jessica Cremer: Data curation; writing – review and editing; Eva‐Maria Bauer: Data curation; Yury Zablotzki: Formal analysis; Monir Majzoub‐Altweck: Formal analysis; writing – review and editing; Andrea Meyer‐Lindenberg: Conceptualisation; Supervision; Writing – review & editing; All authors contributed to both performing of the study and writing of the manuscript
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. The study was approved by the Ethics Committee of the Veterinary Faculty, Ludwig‐Maximilians‐Universität München, Munich, Germany (AZ 285‐30‐09‐2021).
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/vms3.1496.
Schroers, M. , Walter, B. , Fischer, S. , Cremer, J. , Bauer, E.‐M. , Zablotzki, Y. , Majzoub‐Altweck, M. , & Meyer‐Lindenberg, A. (2024). Studies on the association of malondialdehyde as a biomarker for oxidative stress and degree of malignancy in dogs with mammary adenocarcinomas. Veterinary Medicine and Science, 10, e1496. 10.1002/vms3.1496
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Bevan, R. J. , Durand, M. F. , Hickenbotham, P. T. , Kitas, G. D. , Patel, P. R. , Podmore, I. D. , Griffiths, H. R. , Waller, H. L. , & Lunec, J. (2003). Validation of a novel ELISA for measurement of MDA‐LDL in human plasma. Free Radical Biology and Medicine, 35(5), 517–527. 10.1016/s0891-5849(03)00359-9 [DOI] [PubMed] [Google Scholar]
- Breusing, N. , Grune, T. , Andrisic, L. , Atalay, M. , Bartosz, G. , Biasi, F. , Borovic, S. , Bravo, L. , Casals, I. , Casillas, R. , Dinischiotu, A. , Drzewinska, J. , Faber, H. , Fauzi, N. M. , Gajewska, A. , Gambini, J. , Gradinaru, D. , Kokkola, T. , Lojek, A. , … Spickett, C. M. (2010). An inter‐laboratory validation of methods of lipid peroxidation measurement in UVA‐treated human plasma samples. Free Radical Research, 44(10), 1203–1215. 10.3109/10715762.2010.499907 [DOI] [PubMed] [Google Scholar]
- Burrai, G. P. , Baldassarre, V. , Brunetti, B. , Iussich, S. , Maniscalco, L. , Mariotti, F. , Facteria, A. , Cocumelli, C. , Grieco, V. , Millanta, F. , Paciello, O. , Papparella, S. , Rasotto, R. , Romanucci, M. , & Zappulli, V. (2022). Canine and feline in situ mammary carcinoma: A comparative review. Veterinary Pathology, 59(6), 894–902. 10.1177/03009858221105060 [DOI] [PubMed] [Google Scholar]
- Canakci, C. F. , Cicek, Y. , Yildirim, A. , Sezer, U. , & Canakci, V. (2009). Increased levels of 8‐hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. European Journal of Dentistry, 3(2), 100–106. https://www.ncbi.nlm.nih.gov/pubmed/19421389 [PMC free article] [PubMed] [Google Scholar]
- Cherian, D. A. , Peter, T. , Narayanan, A. , Madhavan, S. S. , Achammada, S. , & Vynat, G. P. (2019). Malondialdehyde as a marker of oxidative stress in periodontitis patients. Journal of Pharmacy and Bioallied Sciences, 11(Suppl 2), 297–300. 10.4103/JPBS.JPBS_17_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaller, A. , Kessler, M. , Wehrend, A. , Hirschberger, J. , & Neumann, S. (2022). Utility of serum Ki‐67 as a marker for malignancy in dogs. Animals (Basel), 12(10), 1263. 10.3390/ani12101263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi, Y. , Nakayama, H. , Uetsuka, K. , Nishimura, R. , Sasaki, N. , & Doi, K. (2000). Cellular proliferative and telomerase activity in canine mammary gland tumors. Veterinary Pathology, 37(2), 177–183. 10.1354/vp.37-2-177 [DOI] [PubMed] [Google Scholar]
- Goldschmidt, M. , Pena, L. , Rasotto, R. , & Zappulli, V. (2011). Classification and grading of canine mammary tumors. Veterinary Pathology, 48(1), 117–131. 10.1177/0300985810393258 [DOI] [PubMed] [Google Scholar]
- Jayasri, K. , Padmaja, K. , & Saibaba, M. (2016). Altered oxidative stress and carbohydrate metabolism in canine mammary tumors. Veterinary World, 9(12), 1489–1492. 10.14202/vetworld.2016.1489-1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangari, P. , Zarnoosheh Farahany, T. , Golchin, A. , Ebadollahzadeh, S. , Salmaninejad, A. , Mahboob, S. A. , & Nourazarian, A. (2018). Enzymatic antioxidant and lipid peroxidation evaluation in the newly diagnosed breast cancer patients in Iran. Asian Pacific Journal of Cancer Prevention, 19(12), 3511–3515. 10.31557/APJCP.2018.19.12.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapun, A. P. , Salobir, J. , Levart, A. , Kotnik, T. , & Svete, A. N. (2012). Oxidative stress markers in canine atopic dermatitis. Research in Veterinary Science, 92(3), 469–470. 10.1016/j.rvsc.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Kaszak, I. , Ruszczak, A. , Kanafa, S. , Kacprzak, K. , Krol, M. , & Jurka, P. (2018). Current biomarkers of canine mammary tumors. Acta Veterinaria Scandinavica, 60(1), 66. 10.1186/s13028-018-0417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraguruparan, R. , Balachandran, C. , Manohar, B. M. , & Nagini, S. (2005). Altered oxidant‐antioxidant profile in canine mammary tumours. Veterinary Research Communications, 29(4), 287–296. 10.1023/b:verc.0000048499.38049.4b [DOI] [PubMed] [Google Scholar]
- Machado, V. S. , Crivellenti, L. Z. , Bottari, N. B. , Tonin, A. A. , Pelinson, L. P. , Borin‐Crivellenti, S. , Santana, A. E. , Torbitz, V. D. , Moresco, R. N. , Duarte, T. , Duarte, M. M. , Schetinger, M. R. , Morsch, V. M. , Jaques, J. A. , Tinucci‐Costa, M. , & Da Silva, A. S. (2015). Oxidative stress and inflammatory response biomarkers in dogs with mammary carcinoma. Pathology – Research and Practice, 211(9), 677–681. 10.1016/j.prp.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Macotpet, A. , Suksawat, F. , Sukon, P. , Pimpakdee, K. , Pattarapanwichien, E. , Tangrassameeprasert, R. , & Boonsiri, P. (2013). Oxidative stress in cancer‐bearing dogs assessed by measuring serum malondialdehyde. BMC Veterinary Research, 9, 101. 10.1186/1746-6148-9-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita, M. , Akiyoshi, R. , Yoshimura, H. , Katsumoto, T. , Ichikawa, H. , Ohkusu‐Tsukada, K. , Nakagawa, T. , Sasaki, N. , & Takahashi, K. (2011). Characterization of spheres derived from canine mammary gland adenocarcinoma cell lines. Research in Veterinary Science, 91(2), 254–260. 10.1016/j.rvsc.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Montalli, V. A. , Passador‐Santos, F. , Martinez, E. F. , Furuse, C. , Aguiar, M. C. , Soares, F. A. , Brown, A. L. , de Araújo, N. S. , & de Araújo, V. C. (2017). Mammaglobin and DOG‐1 expression in polymorphous low‐grade adenocarcinoma: An appraisal of its origin and morphology. Journal of Oral Pathology & Medicine, 46(3), 182–187. 10.1111/jop.12491 [DOI] [PubMed] [Google Scholar]
- Mylnikov, P. Y. , Shchulkin, A. V. , Abalenikhina, Y. V. , & Yakusheva, E. N. (2022). Development and validation of a methodology for quantitative determination of malondialdehyde by HPLC‐MC/MS. Kliniceskaja Laboratornaja Diagnostika, 67(7), 369–373. 10.51620/0869-2084-2022-67-7-369-373 [DOI] [PubMed] [Google Scholar]
- Nieto, A. , Perez‐Alenza, M. D. , Del Castillo, N. , Tabanera, E. , Castano, M. , & Pena, L. (2003). BRCA1 expression in canine mammary dysplasias and tumours: Relationship with prognostic variables. Journal of Comparative Pathology, 128(4), 260–268. 10.1053/jcpa.2002.0631 [DOI] [PubMed] [Google Scholar]
- Pena, L. , De Andres, P. J. , Clemente, M. , Cuesta, P. , & Perez‐Alenza, M. D. (2013). Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two‐year follow‐up: Relationship with clinical and histological characteristics. Veterinary Pathology, 50(1), 94–105. 10.1177/0300985812447830 [DOI] [PubMed] [Google Scholar]
- Rasotto, R. , Berlato, D. , Goldschmidt, M. H. , & Zappulli, V. (2017). Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Veterinary Pathology, 54(4), 571–578. 10.1177/0300985817698208 [DOI] [PubMed] [Google Scholar]
- Santos, D. B. , Fernandez, G. J. , Pardini, L. M. C. , Pardini, M. , & Ferrasi, A. C. (2023). Transcriptomic profile of canine mammary ductal carcinoma. International Journal of Molecular Sciences, 24(6), 5212. 10.3390/ijms24065212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarli, G. , Preziosi, R. , Benazzi, C. , Castellani, G. , & Marcato, P. S. (2002). Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. Journal of Veterinary Diagnostic Investigation, 14(1), 25–34. 10.1177/104063870201400106 [DOI] [PubMed] [Google Scholar]
- Sorenmo, K. (2003). Canine mammary gland tumors. Veterinary Clinics: Small Animal Practice, 33(3), 573–596. 10.1016/s0195-5616(03)00020-2 [DOI] [PubMed] [Google Scholar]
- Szczubial, M. , Kankofer, M. , Lopuszynski, W. , Dabrowski, R. , & Lipko, J. (2004). Oxidative stress parameters in bitches with mammary gland tumours. Journal of Veterinary Medicine. A, Physiology, Pathology, Clinical Medicine, 51(7‐8), 336–340. 10.1111/j.1439-0442.2004.00647.x [DOI] [PubMed] [Google Scholar]
- Taba, M., Jr. , Kinney, J. , Kim, A. S. , & Giannobile, W. V. (2005). Diagnostic biomarkers for oral and periodontal diseases. Dental Clinics of North America, 49(3), 551–571. 10.1016/j.cden.2005.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaet, A. A. J. , Breynaert, A. , Ceulemans, B. , De Bruyne, T. , Fransen, E. , Pieters, L. , Savelkoul, H. F. J. , & Hermans, N. (2019). Oxidative stress and immune aberrancies in attention‐deficit/hyperactivity disorder (ADHD): A case‐control comparison. European Child & Adolescent Psychiatry, 28(5), 719–729. 10.1007/s00787-018-1239-4 [DOI] [PubMed] [Google Scholar]
- Vinothini, G. , Balachandran, C. , & Nagini, S. (2009). Evaluation of molecular markers in canine mammary tumors: Correlation with histological grading. Oncology Research, 18(5‐6), 193–201. 10.3727/096504009x12596189659042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


