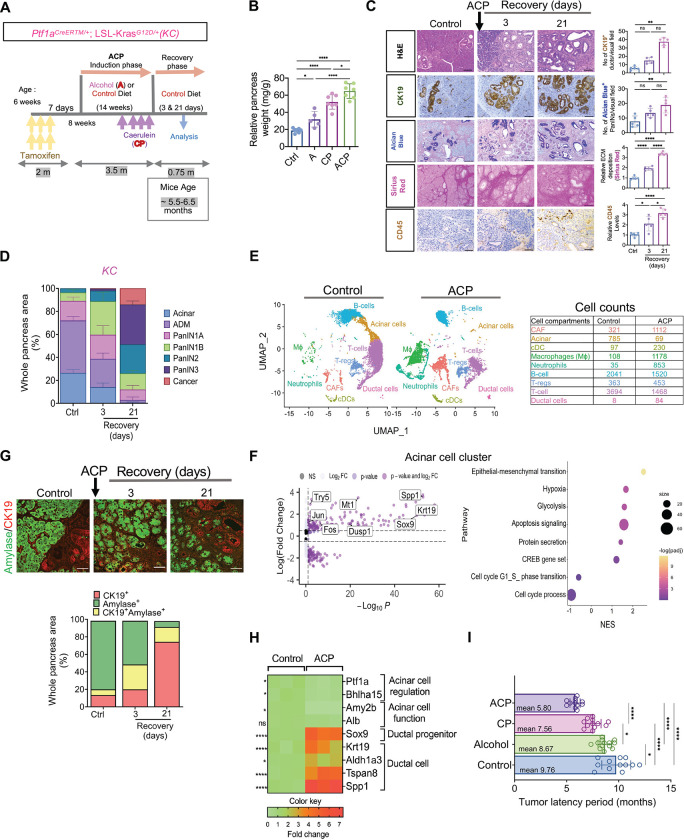
Figure 2.
ACP induction accelerates ADM reprogramming towards PanIN and pancreatic cancer in KrasG12D/+ mutant mice. (A) Schematic for ACP induction Ptf1aCreERTM/+; LSL-KrasG12D/+ (KC) mice exposed to alcohol enriched liquid diet (A) and repetitive caerulein administration (CP). Mice were euthanized after 3 and 21 days of recovery period. (B) Relative pancreas weight measurements in ctrl, A, CP, and ACP induced KC mice with n=5–7 mice per group. (C) Representative pancreatic images depicting H&E, of CK19+ ducts, PanINs (Alcian Blue), collagen (Sirius red) and immune cells (CD45+) in pancreata of control and ACP-induced KC mice with 3 and 21 days of recovery period (left) and their quantification (right) (n=5 mice per group). (D) Histopathological assessment using H&E staining was employed to quantify areas within the pancreas corresponding to acinar, ADMs, PanINs and cancer regions with n=3 mice per group. (E) UMAP projection displaying cell clusters (left) and cell count table (right) in the scRNA sequencing analysis of pancreata from control and ACP induction in KC mice. (F) Volcano plot (left) illustrating differentially expressed genes (DEG) and bubble plot (right) showing gene set enrichment analysis (GSEA) in the acinar cell subcluster of ACP vs. control in KC mice. (G) Representative pancreas images depicting amylase (green)/ CK19 (red) co-immunofluorescent labeling (top) and the corresponding quantification of CK19+ amylase+ cells (bottom) in ctrl and ACP-induced KC mice with 3 and 21 days of recovery period (n=3 mice per group). (H) Heat map illustrating the differential expression of genes associated with acinar or ductal phenotypes in the pancreas of control and ACP-induced KC mice with n=3 mice per group. (I) Measurement of tumor latency period in control, alcohol, CP, and ACP-induced KC mice (n=12–13 mice per group). Scale bar, 50μm. ns nonsignificant; *p<0.05; **p< 0.01; ****p<0.0001 by ANOVA or unpaired t test.