Abstract
Radish exhibits significant variation in color, particularly in sprouts, leaves, petals, fleshy roots, and other tissues, displaying a range of hues such as green, white, red, purple, and black. Although extensive research has been conducted on the color variation of radish, the underlying mechanism behind the variation in radish flower color remains unclear. To date, there is a lack of comprehensive research investigating the variation mechanism of radish sprouts, leaves, fleshy roots, and flower organs. This study aims to address this gap by utilizing transcriptome sequencing to acquire transcriptome data for white and purple radish flowers. Additionally, the published transcriptome data of sprouts, leaves, and fleshy roots were incorporated to conduct a systematic analysis of the regulatory mechanisms underlying anthocyanin biosynthesis in these four radish tissues. The comparative transcriptome analysis revealed differential expression of the anthocyanin biosynthetic pathway genes DFR, UGT78D2, TT12 and CPC in the four radish tissues. Additionally, the WGCNA results identified RsDFR.9c and RsUGT78D2.2c as hub genes responsible for regulating anthocyanin biosynthesis. By integrating the findings from the comparative transcriptome analysis, WGCNA, and anthocyanin biosynthetic pathway-related gene expression patterns, it is hypothesized that genes RsDFR.9c and RsUGT78D2.2c may serve as pivotal regulators of anthocyanins in the four radish tissues. Furthermore, the tissue-specific expression of the four copies of RsPAP1 is deemed crucial in governing anthocyanin synthesis and accumulation. Our results provide new insights into the molecular mechanism of anthocyanin biosynthesis and accumulation in different tissues of radish.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10519-4.
Keywords: Radish, Anthocyanin, Transcriptome, DFR, PAP1
Introduction
Anthocyanins, which are flavonoid secondary metabolites, play a pivotal role as natural pigments in plants. They impart red, purple, or blue colors to different plant tissues, including leaves, stems, roots, flowers, and fruits, thereby contributing to plant growth and development [19, 29]. Moreover, these compounds serve as attractants for pollinators and aid in seed dispersal [10]. Furthermore, anthocyanins have been extensively studied for their involvement in plant responses to both biotic and abiotic stresses, encompassing UV protection, pigmentation, and defense mechanisms [30, 38]. The antioxidant properties and associated health benefits of anthocyanins have attracted considerable attention. These compounds, when included in the diet, not only act as safe and non-toxic natural food colorants but also exhibit potent free radical scavenging activity [1, 27]. This activity has the potential to reduce the risk of certain cancers, cardiovascular diseases, and other chronic ailments [16, 38]. Consequently, the cultivation of vibrantly colored vegetable varieties enriched with anthocyanins holds substantial commercial value. This endeavor aligns closely with the goals of breeders, as it addresses both consumer preferences and the pursuit of healthier dietary choices.
Since its discovery in 1986, the C1 gene has been identified as a key regulator in the biosynthesis of anthocyanin pigments in the aleurone layer of maize endosperm [6]. Subsequently, numerous studies have elucidated the biosynthetic and transcriptional regulation mechanisms of anthocyanins in various plant species [13, 15, 21, 26, 29, 41, 42]. Genes related to the anthocyanin biosynthetic pathway mainly include early biosynthetic genes (EBGs) phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate CoA ligase 4 (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3’-hydroxylase (F3’H), and late biosynthetic genes (LBGs) dihydroflavonol reductase (DFR), anthocyanin synthetase/ leucoanthocyanin dioxygenase (ANS /LDOX), and UDP-flavonoid glucosyltransferase (UFGT). Concurrently, extensive research has demonstrated that the expression of anthocyanin biosynthetic genes is controlled at the transcriptional level, involving transcription factors from the MYB, bHLH, and WD40 protein families [9, 29, 32]. In the phenylpropanoid biosynthetic pathway, the regulation of PAL, C4H, and 4CL expression is mediated by MYB transcription factors MYB3, MYB4, MYB7, and MYB32 [7, 12, 22]. Similarly, in the flavonoid biosynthetic pathway, the expression of CHS, CHI, and F3'H is controlled by MYB transcription factors MYB11, MYB12, and MYB111 [24, 28, 31]. Lastly, in the anthocyanin biosynthetic pathway, the regulation of DFR, ANS (LDOX), and UFGT expression is orchestrated by the MBW transcriptional regulatory complex, which is composed of MYB transcription factors MYB75, MYB90, MYB113, MYB114, and bHLH transcription factors TT8, GL3, EGL3, and WAD40 proteins [9, 14, 32, 36].
Radish (Raphanus sativus L., RR, 2n = 18) is a highly nutritious vegetable, abundant in phenols, vitamin C, chlorophyll, carotenoids, and glucosinolates, which confer potential health benefits to individuals. Consequently, it is extensively cultivated and consumed globally [43]. The roots, leaves, and flowers of radish exhibit considerable diversity in terms of color, shape, and size. Notably, the wide range of color variations has emerged as a prominent characteristic that appeals to consumers. Previous studies have investigated the color variation in various tissues of radish, including sprouts, leaves, and roots. Muleke et al. [17] utilized four inbred lines of radish to conduct a comparative transcriptome analysis at three different developmental stages (10DAS, 30DAS, 50DAS), there findings indicate that the primary regulatory factor in radish anthocyanin biosynthesis is RsUFGT. Furthermore, they observed a close association between the transcript levels of RsUFGT, RsF3H, RsANS, RsCHS3, and RsF3'H1 and the overall concentration of anthocyanins [17]. Upon subjecting radish sprouts to UV-A treatment, there was a significant increase in anthocyanin content, through comparative transcriptome sequencing analysis, the transcript levels of transcriptional regulators related to the MYB-bHLH-WD40 complex were consistent with the observed concentration of anthocyanins [40]. In the study conducted by Pu et al. [23], it was observed that the expression levels of the structural genes PAL4, 4CL4, CHS, F3H, DFR, ANS, OMT1, as well as the transcription factors TT8, CPC, and MYB114, were significantly elevated in purple leaves. Similarly, Gao et al. [8] found that in radish fleshy roots, the transcription factors RsMYB_9, RsERF070, and the anthocyanin synthesis-related genes RsCHS, RsCHI, RsANS, RsMT2-4, RsUF3GT, RsUFGT78D2-like, and RsUDGT-75C1-like play crucial roles in anthocyanin biosynthesis in radish varieties. In the context of root-skin color variants, the CHS gene exhibits specific expression in red skin, suggesting its potential role as a crucial regulator in the accumulation of red pigment in red-skinned radish. Furthermore, the expression levels of transcription factors MYB1/2/75, bHLH (TT8), WD40, and the structural genes involved in the anthocyanin biosynthetic pathway are significantly higher in red skin compared to white skin [35]. In a recent study by Zhang et al. [37, 39], a comparative transcriptome analysis was conducted using six radish materials with varying skin and flesh colors, aiming to identify key genes responsible for anthocyanin biosynthesis. The findings of this study demonstrated that RsTT4, RsC4H, RsTT7, RsCCOAMT, RsDFR, and RsLDOX were significantly upregulated in the red- and purple-colored accessions. The metabolic and transcriptome analysis findings of the dark red taproot of radish indicate that RsCHS and RsDFR exhibit the highest up-regulation in this particular taproot. Additionally, the heightened co-expression of RsMYB1 and RsMYB2 potentially plays a role in the development of the dark red coloration [11]. However, the molecular underpinnings of radish flower color variation remain unexplored, and there exists a dearth of comprehensive investigations into the mechanisms governing color variation in radish hypocotyl, leaves, fleshy roots, and petals. In this study, the most recent published radish reference genome information (Xinlimei) was utilized as a reference. Sequencing and transcriptome data were gathered from radish hypocotyl, leaves, fleshy roots, and petals to perform a comparative analysis. The findings revealed that 10 genes exhibited significant differential expression across all tissues. The results of GO enrichment and KEGG annotation indicated that these differentially expressed genes were enriched in the pathways associated with flavonoid metabolism and anthocyanin metabolism. Specifically, among the 10 genes that were differentially expressed, four exhibited a close association with anthocyanin biosynthesis. Following this, we employed the known anthocyanin-related genes of Arabidopsis thaliana to perform a comprehensive search for genes associated with the anthocyanin biosynthetic pathway in radish at a genome-wide level. A total of 103 genes were successfully identified, with several genes displaying tandem duplications. Additionally, we conducted weighted correlation network analysis (WGCNA) utilizing transcriptome data from four distinct tissues, encompassing 24 sets of gene expression data related to anthocyanin synthesis. The findings indicate that RsDFR.9c and RsUGT78D2.2c may serve as hub genes in the biosynthesis of anthocyanins. Moreover, by combining comparative transcriptome data and WGCNA results, we have constructed potential regulatory patterns for anthocyanin production in the four tissues of radish. The distinct synthesis and accumulation of anthocyanins in various radish tissues may be intricately linked to the tissue-specific expression of distinct copies of RsPAP1. Consequently, our investigation contributes to the advancement of knowledge regarding the genetic and molecular mechanisms that govern the tissue-specific biosynthesis and accumulation of anthocyanins in radish.
Materials and methods
Plant materials
The purple-flowered and white-flowered radish inbred lines used in this study were grown at the Wuhan Academy of Agriculture Science and Technology experimental base. Under the same cultivation conditions, white-flowered and purple-flowered radish were planted in the experimental plot, and flower buds (about 3 mm in length) to be opened were selected for sampling during the flowering period. Three biological replicates were taken from each material. All samples were then frozen in liquid nitrogen and stored at 80 °C for RNA extraction. The RNA-seq data of hypocotyl and fleshy roots were collected from National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/) with the following biological projects PRJNA388018 (hypocotyl), PRJNA810281 (fleshy roots), PRJNA810914 (fleshy roots). The RNA-seq data of leaves were collected from National Genomics Data Center (https://ngdc.cncb.ac.cn/gsa/browse/CRA008485) under accession ID CRA008485.
Transcriptome analysis of the purple and green/white radish tissues
The raw data obtained from the NCBI was initially converted into fastq format using the SRA toolkit. Then, all raw data processed with default parameters using trimmatic (v0.39, [2]). Subsequently, the RNA-seq clean reads were aligned to the reference genome of radish (Xinlimei, http://brassicadb.cn/#/) using the HISAT2 software (v2.1.0, [20]). The obtained millions of fragments per thousand bases (FPKM) values were then calculated using StringTie (v2.1.1, [20]). The expression histogram was generated using Excel, while the heat map was generated using TBtools software (v1.133, [4]). In addition, Gene Ontology (GO) and KEGG pathway functional enrichment analyses of DEGs were significantly enriched in GO terms and metabolic pathways at Bonferroni-corrected P-value ≤ 0.05 compared with the whole-transcriptome background. GO functional enrichment and KEGG pathway analysis were carried out by Goatools (https://github.com/tanghaibao/Goatools) and KOBAS (http://kobas.cbi.pku.edu.cn/home.do). The 25 enrichment items with the highest correlation (Q-value) were used to draw the GO enrichment map, and the map was generated using TBtools software (v1.133, [4]).
Genome-wide identification of genes related to anthocyanin biosynthesis in radish
Here, all the protein sequences and CDS sequences radish (Xinlimei) were obtained from the Brassicaceae Database http://www.brassicadb.cn/#/ [37, 39]. The genes related to anthocyanin biosynthesis from Arabidopsis was selected as the reference for identifying homologous genes in the radish. To ensure accurate identification homologous genes of anthocyanin biosynthesis related genes in radish, the following steps were primarily employed. Firstly, initial searches were conducted using local BLASTP and local BLASTN algorithms, with a significance threshold of E < 1e—20. Secondly, the screening process involved identifying candidate genes that exhibited a consistency rate exceeding 65% and a coverage rate surpassing 60%.
Construction of co-expression network modules using association analysis modules and phenotypes
To explore the metabolite fluxes of the anthocyanin biosynthetic pathway and identification of hub genes regulating anthocyanin biosynthesis in radish, we employed the weighted gene co-expression network analysis (WGCNA) to establish a gene co-expression network for the transcriptional regulation of anthocyanin in four tissues of radish. The construction of co-expression networks was carried out using the WGCNA software package, specifically version R-4.3.1. Subsequently, correlation analysis was performed to determine the correlation between each co-expression module and the collected tissue data of radish, enabling the identification of hub genes associated with anthocyanin. All 24 sets of transcriptome expression data of anthocyanin biosynthesis-related genes in four radish tissues were then utilized to construct a co-expression network module. For the detailed method, refer to the articles of Chen et al. [4]. Finally, the gene regulatory network data analyzed by WGCNA was imported into Cytoscape (v 3.80, [25]) for visual display.
RNA extraction, and qRT-PCR analysis
The green and purple hypocotyls, leaves, roots, and the white and purple flowers with three biological replicates were collected, all samples were collected and immediately frozen in liquid nitrogen for RNA extraction and qRT-PCR analysis. eight genes (RsDFR.9c, RsUGT78D2.2c, RsCPC.1c, RsTT8.9c, RsPAP1.5c, RsPAP1.7c1, and RsPAP1.7c2) have been used for qRT-PCR analysis, and the Actin3 gene as an internal reference gene control. The primers information was list in Supplementary Table S6. For the detailed methods, please refer to Chen et al. [4]. All materials of this research were collected from Gannan Normal University research base.
Results
Identification of differentially expressed genes (DGEs) in different tissues of radish
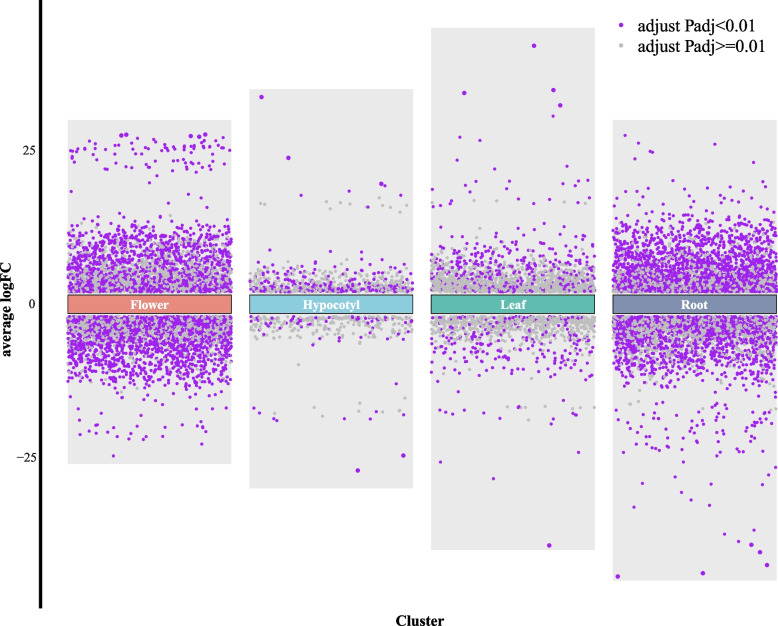
The radish plant exhibits a diverse range of organ and color variations, rendering it a valuable specimen for investigating the mechanisms underlying color formation and regulation. In order to elucidate the genome-wide gene expression patterns associated with purple pigmentation in different radish tissues, we performed a comparative transcriptome analysis encompassing hypocotyls, leaves, radish taproots, and petals. The application of principal component analysis (PCA) yielded distinct separation among these various radish tissues (Supplementary Fig. 1). Significantly, the three biological replicates of the same tissue, albeit with distinct colors, were successfully clustered together, thereby emphasizing the reproducibility and consistency of the RNA-seq data. Additionally, we conducted a differential expression analysis using DESeq2, whereby genes were deemed significantly differentially expressed if they displayed |log2FC|≥ 2 and a P-value ≤ 0.01. Our expression analysis unveiled that, out of the 44109 annotated genes in radish (reference genome RS00, Xin-li-mei), 25152, 27607, 28209, and 27607 genes exhibited differential expression in the hypocotyl, leaf, radish taproot and flower, respectively (Table 1, Fig. 1).
Table 1.
Number of differentially expressed genes in different tissues
| Tissue | Significant | Not-significant | Total | ||
|---|---|---|---|---|---|
| Up-regulated | Down-regulated | Total | |||
| Hypocotyl | 248 | 78 | 326 | 24826 | 25152 |
| Leaf | 453 | 267 | 720 | 26887 | 27607 |
| Root | 2110 | 1756 | 3866 | 24343 | 28209 |
| Flower | 1534 | 1863 | 3397 | 24210 | 27607 |
Fig. 1.
Volcano plot of differentially expressed genes in four radish tissues
Specifically, the number of significantly differentially expressed genes (DEGs) in purple tissues compared to green/white tissues varied across different plant organs. In the hypocotyl, leaf, radish taproot, and flower, there were 326, 720, 3866, and 3397 DEGs, respectively (Table 1, Fig. 1, Supplementary Table S1). Further analysis revealed that there were 114 genes specifically expressed in the hypocotyl, 331 genes specifically expressed in the leaf, 2636 genes specifically expressed in the radish taproot, and 2213 genes specifically expressed in the flower (Supplementary Fig. 2). Notably, there were 10 genes that exhibited differential expression in all four plant organs, namely the hypocotyl, leaf, radish taproot, and flower (Supplementary Fig. 2, Supplementary Table S2).
GO and KEGG enrichment analysis of DGEs
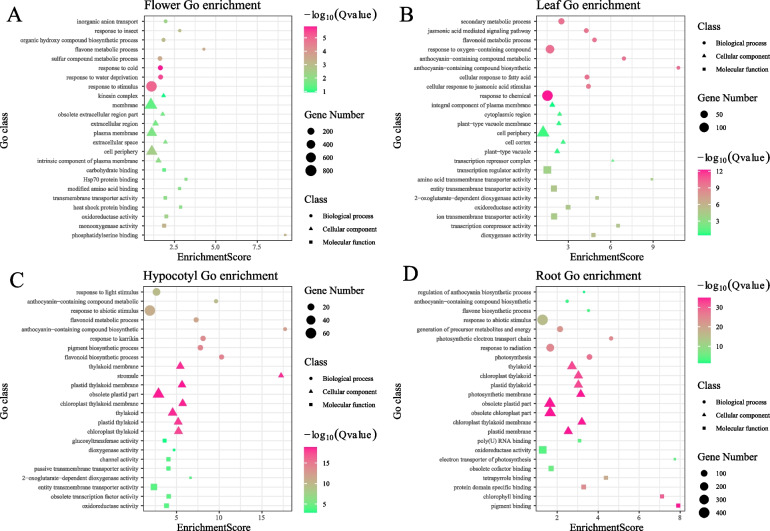
To further assess the biological functionalities of differentially expressed genes (DEGs) in four distinct tissues of radish, we conducted GO and KEGG enrichment analyses on the DEGs identified in these tissues and obtained 25 enrichment items with the highest Q-value (Fig. 2, Supplementary Fig. 3). Notably, in petals, DEGs exhibited the most significant enrichment score in pathways related to phosphatidylserine binding and flavone metabolic processes (Fig. 2A). Conversely, in leaves, DEGs displayed the highest enrichment score in pathways associated with anthocyanin-containing compound biosynthesis, amino acid transmembrane transporter activity, and anthocyanin-containing compound metabolism (Fig. 2B). In the hypocotyl, differentially expressed genes (DEGs) are primarily involved in the biosynthesis of anthocyanin-containing compounds, with the stromule pathway exhibiting the highest enrichment score (Fig. 2C). In the root, DEGs are predominantly enriched in pathways related to pigment binding, biosynthesis of anthocyanin-containing compounds, and flavone biosynthesis (Fig. 2D). KEGG annotation analysis reveals that DEGs in the hypocotyl, leaf, radish taproot, and flower are enriched in metabolic pathways associated with flavonoid biosynthesis, phenylpropanoid biosynthesis, and isoflavonoid biosynthesis (Supplementary Fig. 3).The results of the GO functional enrichment and KEGG pathway annotation analysis indicate that the different color tissues of radish hypocotyl, leaf, radish taproot, and flower exhibit significant enrichment in the flavonoid/anthocyanin metabolism pathway. This finding suggests that the biosynthesis and accumulation of flavonoids/anthocyanins may play a crucial role in the observed color variation in these tissues. Furthermore, the GO functional enrichment and KEGG pathway annotation analysis of the 10 differentially expressed genes (DEGs) shared by hypocotyl, leaf, radish taproot, and flower tissues revealed significant enrichment of Rs0 × 7c033250, Rs0 × 9c039896, Rs0 × 2c007983 and Rs0 × 1c000114 in the flavonoid/anthocyanin biosynthesis pathway (Supplementary Table S2).
Fig. 2.
Gene Ontology (GO) enrichment analysis of the DEGs between four tissues of radish. A GO enrichment analysis of flower DEGs; B GO enrichment analysis of llower DEGs; C GO enrichment analysis Hypocotyl DEGs; D GO enrichment analysis of root DEGs
Identification of anthocyanin biosynthesis-related genes in radish
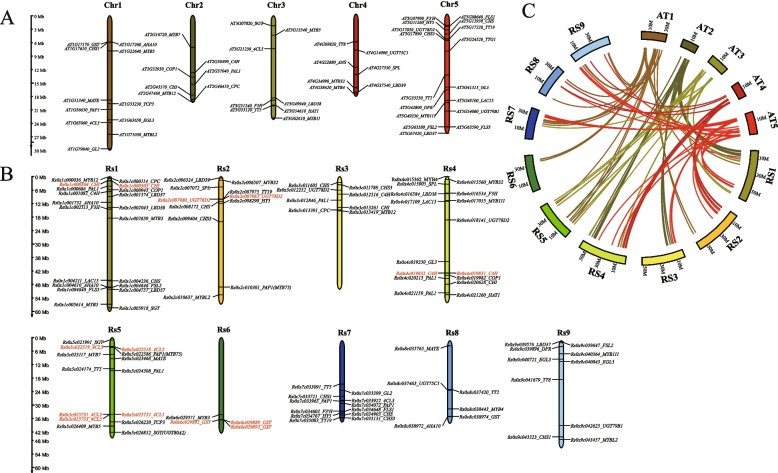
Both Radish and Arabidopsis thaliana belong to the Brassicaceae family, sharing a common ancestor and exhibiting a close relationship [18]. The structural genes and transcriptional regulators associated with the anthocyanin biosynthetic pathway in Arabidopsis have been extensively studied and characterized [9, 33]. By employing a method that combines homologous gene identification with collinearity analysis, we successfully identified a total of 103 genes related to anthocyanin biosynthesis in radish (Fig. 3, Supplementary Table S3). Among the identified genes, 48 were determined to possess homology with the 50 known anthocyanin biosynthesis-related genes in Arabidopsis (Fig. 3A, Supplementary Table S3). Notably, PAL1 and 4CL5 exhibited the most significant degree of homology, with each gene having up to five homologous counterparts. In contrast, no homologous genes for TTG1 and MYB11 were detected. The total count of genes associated with anthocyanin biosynthesis in radish amounts to 103, distributed across nine chromosomes. The number of these genes varies on each chromosome, with 20 on Rs1, 12 on Rs2, eight on Rs3, 17 on Rs4, 14 on Rs5, four on Rs6, 12 on Rs7, six on Rs8, and ten on Rs9 (Fig. 3B, Supplementary Table S3). Significantly, all five homologous genes of 4CL5 are situated on chromosome Rs5. Specifically, Rs0 × 5c022518 and Rs0 × 5c022519 are arranged in tandem on one arm of chromosome Rs5, while Rs0 × 5c025731, Rs0 × 5c025732, and Rs0 × 5c025733 are arranged in tandem on the opposite arm of chromosome Rs5 (Fig. 3B, C, Supplementary Table S4). A comparable pattern is observed for C4H (Rs0 × 4c019831, Rs0 × 4c019832), CHI (Rs0 × 1c000304, Rs0 × 1c000305), and UGT78D2 (Rs0 × 2c007980, Rs0 × 2c007983) genes, as well as GST (Rs0 × 6c029889, Rs0 × 6c029892, Rs0 × 6c029893) genes (Fig. 3B, C, Supplementary Table S4). It is noteworthy to mention that Brassica species have experienced whole-genome tripling events throughout their evolutionary trajectory in comparison to Arabidopsis [5]. The observed tandem duplications in radish may potentially arise as a consequence of the aforementioned whole-genome tripling event.
Fig. 3.
Distribution of anthocyanin biosynthesis-related genes in Arabidopsis and radish and their collinear relationship. A Distribution of anthocyanin biosynthesis-related genes on Arabidopsis chromosomes; B Distribution of anthocyanin biosynthesis-related genes on radish chromosomes; C Collinearity of anthocyanin-related genes in Arabidopsis and radish. Bars represent chromosomes, and the relative positions of anthocyanin biosynthesis-related genes are marked on the chromosomes, and the scale on the left shows the physical distance of the chromosomes
Analysis of anthocyanin related gene expression patterns in different tissues and colors of radish
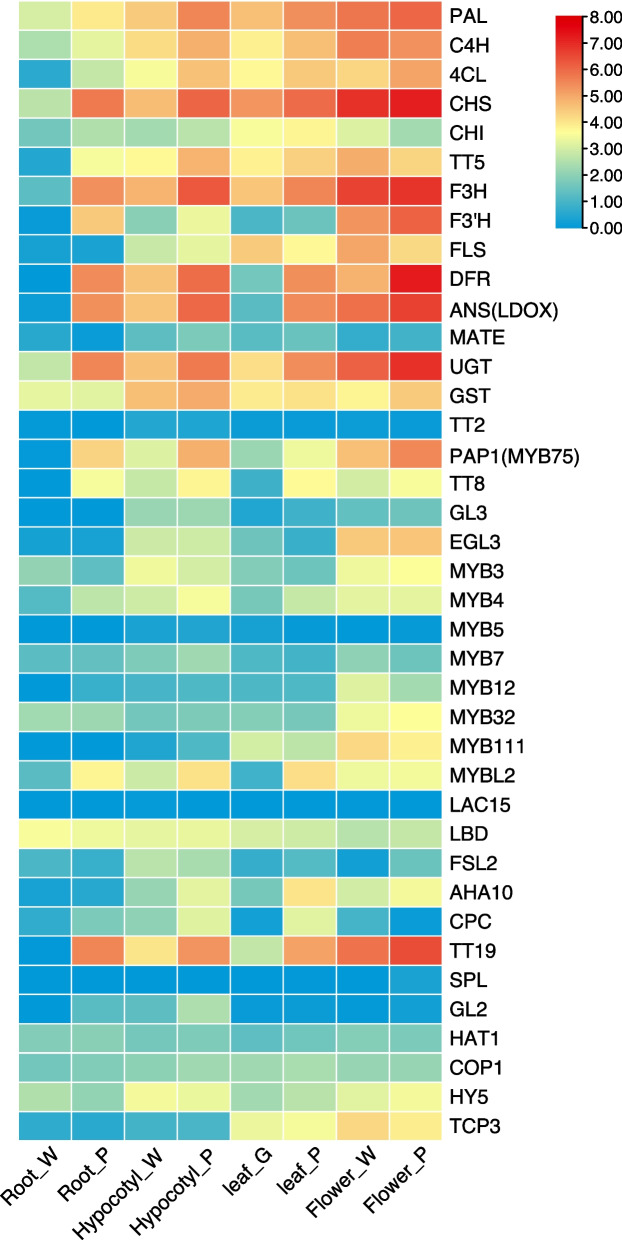
To further investigate the expression patterns of genes associated with the anthocyanin biosynthesis pathway in various tissues of radish, including hypocotyl, leaf, taproot, and flower, we utilized comparative transcriptome data from these tissues. This data was used to construct a heat map illustrating the expression levels of genes involved in the anthocyanin metabolism pathway (Fig. 4). Notably, early anthocyanin biosynthetic genes (EBGs) such as PAL, C4H, 4CL, CHS, CHI, and F3'H exhibit a certain level of expression in both white/green and purple tissues. This can be attributed to the fact that anthocyanins are also involved in flavonol biosynthesis (Fig. 4, Supplementary Table S5). In this pathway, the elevated expression of flavonol synthase (FLS), a crucial catalytic enzyme for flavonol synthesis, in white flowers and green leaves provides strong evidence for this assertion (Fig. 4). Conversely, the late anthocyanin biosynthetic genes (LBGs) DFR, ANS (LDOX), and UGT exhibited significantly higher expression levels in purple tissues (Fig. 4). Among the transcriptional regulators, the expression pattern of MYB3, MYB4, MYB7, MYB12, MYB32, and MYB111, which are genes regulated by EBGs, closely resembles that of EBGs and is observed to a certain degree in both white/green and purple tissues. The regulatory genes PAP1 and TT8 of LBGs exhibited a significantly higher expression in purple tissues, aligning with the expression pattern of LBGs. Furthermore, we observed a significant up-regulation of glutathione transferase TT19 in purple tissues, which displayed a similar expression trend to that of LBGs (Fig. 4).
Fig. 4.
The expression patterns of anthocyanin-related genes in four tissues of radish were analyzed using a heat map that represents the FPKM values of these genes. The colors on the heat map, ranging from red to pink and white, indicate the expression levels from high to low
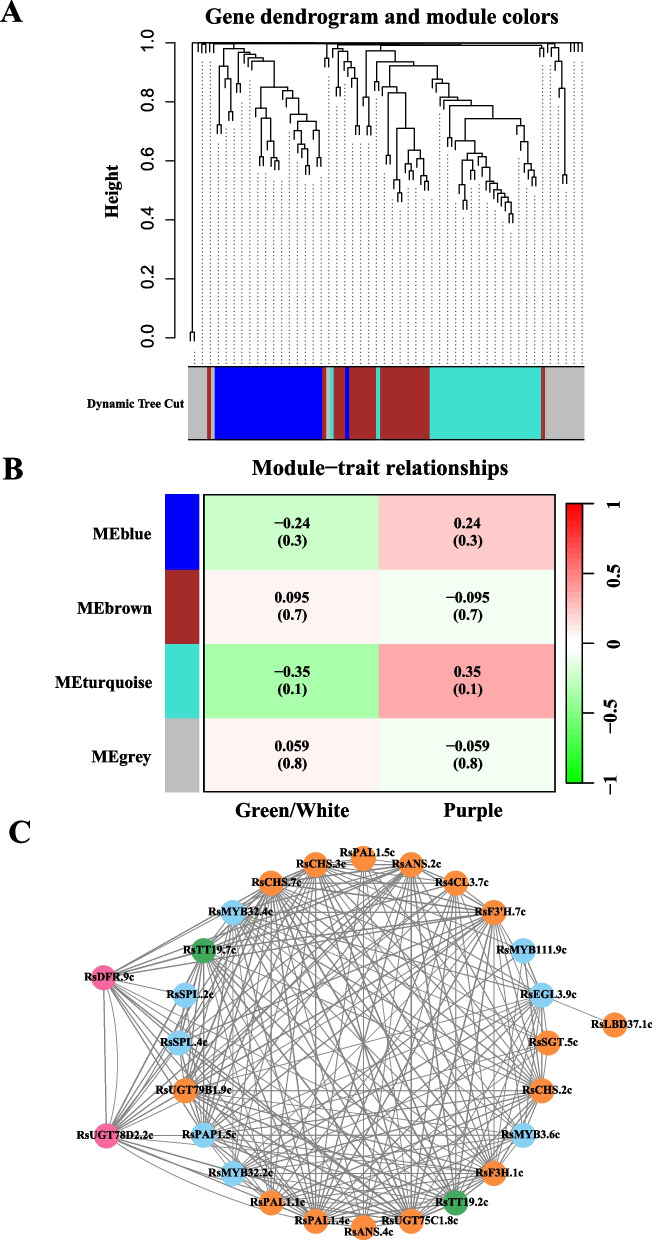
Co‑expression network analysis and identification of key regulatory genes
Based on the expression trend of genes associated with the anthocyanin biosynthetic pathway, all genes were categorized into four modules using the Weighted Gene Co-expression Network Analysis (WGCNA) approach, one of which was an unclustered gray module (Fig. 5A). Notably, the 'MEturquoise' module exhibited the strongest positive correlation with the purple phenotype (r = 0.35, p = 0.1), as well as the strongest negative correlation with the green/white phenotype (r = -0.35, p = 0.1), suggesting a potential close association between the genes within this module and anthocyanin biosynthesis (Fig. 5B). The ‘MEturquoise’ module exhibits the most significant positive correlation in the purple phenotype (r = 0.35, p = 0.1) and the most significant negative correlation in the green/white phenotype (r = -0.35, p = 0.1). This suggests that the genes within this module may have a close association with anthocyanin biosynthesis (Fig. 5B). To further investigate the connection between the genes within the module and the screening hub genes (genes with high connectivity), a correlation network was constructed for the ‘MEturquoise’ module. In the 'MEturquoise' module, a total of 27 genes associated with the synthesis of anthocyanin biopathways exhibited a significant level of expression correlation. These genes include PAL1, 4CL3, CHS, F3H, DFR, ANS, UGT, SGT and LBD37 (structural genes), SPL, MYB3, MYB32, MYB111, PAP1 and EGL3 (transcription factors), and TT19 (transporter). Notably, the comparative transcriptome analysis of four tissues revealed differential expression of RsUGT78D2.2c and RsDFR.9c between purple and green/white samples. This finding suggests that these two genes may play a crucial role in regulating anthocyanin biosynthesis and accumulation in various tissues of radish.
Fig. 5.
Co-expression analysis of genes related to anthocyanin biosynthesis was conducted in four tissues of radish. A Modular hierarchical clustering: The co-expression modules are depicted in different colors, while gray modules indicate no correlation between genes. B Module-to-sample correlation heatmap: Correlation analysis was performed between the co-expression modules of various genes associated with anthocyanin biosynthesis in different tissues. The numbers above the heat map indicate the Pearson correlation coefficient (r) values. C Cytoscape representation of co-expression network of the hub gene in the ‘MEturquoise’ module
Transcriptional regulation pattern of anthocyanin biosynthesis in four radish tissues
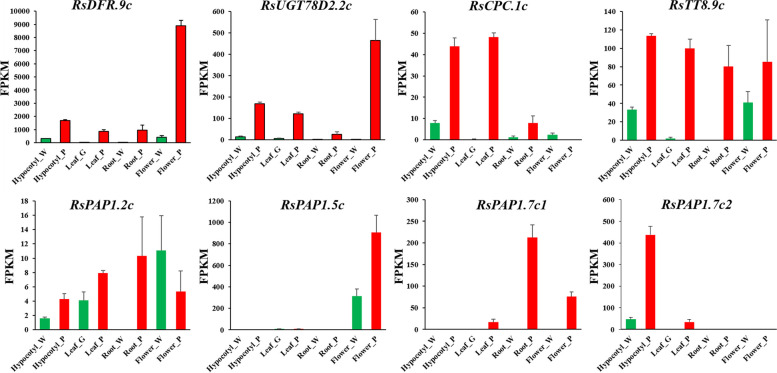
To investigate the expression pattern of hub genes identified through comparative transcriptome and WGCNA analysis, we conducted expression calculations for RsDFR.9c, RsUGT78D2.2c, RsCPC.1c, RsTT8.9c, RsPAP1.2c, RsPAP1.5c, RsPAP1.7c1, and RsPAP1.7c2 using transcriptome sequencing data from four different radish tissues (Fig. 6). The findings revealed a significant up-regulation of RsDFR.9c, RsUGT78D2.2c, RsCPC.1c, RsTT8.9c, RsPAP1.5c, RsPAP1.7c1, and RsPAP1.7c2 in purple tissues, whereas RsPAP1.2c exhibited expression primarily in white petals of flowers, with a higher expression level compared to purple tissues. Among the four homologous copies of RsPAP1, it is noteworthy that the expression levels of RsPAP1.5c, RsPAP1.7c1, and RsPAP1.7c2 exhibit distinct tissue expression specificity, in addition to RsPAP1.2c. Specifically, RsPAP1.5c demonstrates high expression in floral organs, while RsPAP1.7c1 displays high expression in fleshy roots and also exhibits differential expression in flowers and leaves. Furthermore, RsPAP1.7c2 exhibits high expression in the hypocotyl (Fig. 6).
Fig. 6.
Expression analysis of eight hub genes in radish four different tissues. The FPKM (Fragments Per Kilobase Million) value obtained from transcriptome sequencing data analysis was used to represent the expression pattern of the hub genes in different tissues, both four different tissues with three biological replicates
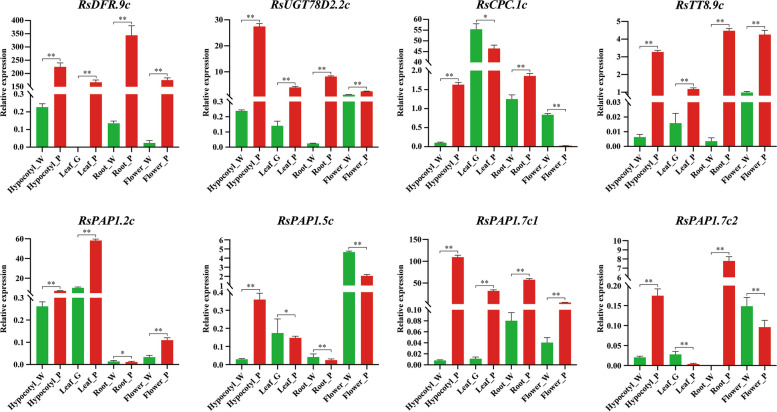
To further elucidate the transcriptional regulation pattern of anthocyanin biosynthesis in the four tissues of radish, we employed comparative transcriptome data and WGCNA analysis to construct a regulatory pattern map of key genes involved in anthocyanin biosynthesis in these tissues (Fig. 7). In both radish sprouts and leaves, the transcriptional regulation mode of anthocyanins is identical. Specifically, RsPAP1.7c2 and RsTT8.9c exhibit high expression exclusively in purple tissues, thereby governing the specific upregulation of the target gene DFR and facilitating anthocyanin biosynthesis (Fig. 7A-B). In fleshy roots, the transcriptional regulatory gene PAP1, responsible for regulating anthocyanin biosynthesis, has undergone a change. Specifically, RsPAP1.7c1 exhibits high expression exclusively in purple fleshy roots. In conjunction with RsTT8.9c, it governs the specific high expression of the target gene DFR, thereby driving anthocyanin biosynthesis (Fig. 7C). Interestingly, in petals, the transcriptional regulatory gene PAP1, responsible for regulating anthocyanin biosynthesis, has undergone another alteration. RsPAP1.5c is specifically highly expressed in purple petals and, in conjunction with RsTT8.9c, regulates the specific high expression of the target gene DFR, thereby driving anthocyanin biosynthesis (Fig. 7D). The findings of this study suggest that the elevated expression of the late structural genes DFR and UGT in the anthocyanin biosynthesis pathway serves as the foundation for the biosynthesis and accumulation of anthocyanins in purple tissues. Additionally, the distinct expression patterns of various copies of the PAP1 homologous gene in the four tissues may play a crucial role in the observed color variation among different radish tissues. Further investigation and validation are necessary to fully comprehend and confirm this phenomenon.
Fig. 7.
qRT-PCR validation of eight hub genes related to anthocyanin synthesis regulation in hypocotyl, leaves, fleshy roots, and petals. For qRT-PCR analysis, both white/green or purple sprouts, leaves, fleshy roots and flowers samples are with three biological replicates, the point represents the mean value of three technical replicates in a representative biological experiment, the error bars indicate s.d, student’s t-test, **P < 0.01, *P < 0.05
To enhance understanding of the transcriptional regulation of anthocyanin biosynthesis in various tissues of radish, including hypocotyls, leaves, fleshy roots, and petals, quantitative real-time polymerase chain reaction (qRT-PCR) was employed to validate the central genes identified through comparative transcriptome and WGCNA. The findings indicate that the expression profiles of the identified eight regulatory hub genes involved in anthocyanin biosynthesis closely align with the patterns observed in RNA sequencing data (Fig. 7). Notably, the expression levels of the key anthocyanin biosynthesis genes RsDFR.9c and RsUGT78D2.2c were significantly elevated in purple tissues, corroborating the results obtained from RNA-seq analysis. The expression levels of the negative regulatory transcription factor RsCPC.1c varied among different tissues in radish, with higher levels observed in purple hypocotyls and fleshy roots compared to green/white tissues, while the opposite trend was observed in leaves and petals. In contrast, RsTT8.9c, a key member of the MBW transcriptional regulatory complex, exhibited significantly higher expression levels in all four purple tissues. Notably, PAP1, another major member of the MBW complex, has four homologous copies in radish, and its expression pattern differs across hypocotyls, leaves, fleshy roots, and petals. The expression patterns of RsPAP1.2c, RsPAP1.5c, RsPAP1.7c1, and RsPAP1.2c in various tissues were analyzed. RsPAP1.2c exhibited significantly higher expression in purple hypocotyls, leaves, and petals compared to green/white tissues. Conversely, RsPAP1.5c showed low or negligible expression levels in hypocotyls, leaves, and roots, with white petals displaying significantly higher expression than purple petals. RsPAP1.7c1 demonstrated significantly higher expression in purple tissues compared to green tissues. RsPAP1.7c2 exhibited significantly higher expression in purple hypocotyls and roots compared to green/white tissues, while the opposite trend was observed in leaves. These results further prove that the four homologous copies of PAP1 play different functions in the regulation of anthocyanin synthesis in different tissues or organs of radish, and the relevant results need to be further verified.
Discussion
The radish exhibits a broad range of planting capabilities and diverse phenotypic characteristics, rendering it suitable for consumption as sprouts, leaf vegetables, and root vegetables. Notably, certain varieties of radish, particularly those with red and purple pigmentation, possess a high nutritional value due to their abundant anthocyanin content, thereby augmenting their desirability as a food source. The availability of comprehensive genomic data pertaining to high-quality radish cultivars has facilitated the identification and documentation of numerous biological traits by researchers.
Regulatory mechanism of color variation in different tissues of radish
In this study, a comprehensive analysis was conducted on various types of radishes, including those classified based on the color of their roots (white skin and white flesh, red skin and white flesh, red skin and red flesh, green skin and white flesh, green skin and green flesh, green skin and red flesh), as well as other variations such as yellow skin, purple skin, and black skin types. Additionally, the study also examined the variant types of radish sprouts and leaves, including those with green and purple colors. Comparative transcriptome and WGCNA analysis were performed using transcriptome sequencing data of purple-flowered and white-flowered radish, in conjunction with previously published transcriptome data of radish buds, leaves and fleshy roots. The findings of this study indicate that the late structural genes of the anthocyanin biosynthetic pathway, namely RsUGT78D2.2c and RsDFR.9c, exhibited differential expression in the purple and green/white tissues of the four radish samples. Additionally, the identification of the regulatory hub gene through WGCNA analysis revealed that UGT78D2.2c and RsDFR.9c serve as the hub gene regulating anthocyanin biosynthesis. This aligns with the previous research conducted by Muleke et al. [17], which demonstrated that RsUFGT plays a crucial role in regulating anthocyanin synthesis during various developmental stages of radish. DFR has been identified as the pivotal regulator of anthocyanin synthesis in purple leaves [23]. Extensive research has been conducted by scientific and technological experts on radish skin and radish meat in fleshy roots. The CHS gene has been recognized as the principal gene responsible for regulating red skin [35], whereas RsDFR and RsF3H play a crucial role in governing flavonoid synthesis in purple-skinned radish [37, 39]. In the taproot flesh, RsDFR and RsLDOX are considered indispensable for anthocyanin biosynthesis [37, 39]. In purple-skinned and purple-fleshed radish, the genes RsCHS and RsDFR have been identified as crucial regulators of anthocyanin synthesis [11]. Additionally, the high expression of RsANS and RsUF3GT has been implicated as pivotal in anthocyanin biosynthesis [8]. Overall, our findings indicate that the catalytic enzymes CHS, F3H, DFR, ANS (LDOX), and UGT, which are involved in the anthocyanin biosynthetic pathway, have been detected in various color variations across multiple tissues in radish. These genes may play a significant role in the diverse coloration observed in radish.
Different copies of RsPAP1 specifically regulate anthocyanin synthesis in different tissues of radish
In this study, a whole-genome identification approach was employed to identify four homologous genes (RsPAP1.2c, RsPAP1.5c, RsPAP1.7c1, and RsPAP1.7c2) belonging to the PAP1(MYB75)/PAP2(MYB90)/MYB113/MYB114 family from the radish genome (Xinlimei). Subsequently, an analysis of the expression patterns revealed an intriguing phenomenon. Specifically, the expression patterns of the four copies of RsPAP1 exhibited variations across the four different tissues of radish, with no single copy showing significant and specific up-regulation across all four tissues. Previous studies have reported the involvement of MYB114 (PAP1 homologous gene) in the development of purple leaves in radish, while MYB75 (PAP1) is responsible for regulating anthocyanins in the red skin of radish [23, 35]. Notably, our research has revealed, for the first time, that RsPAP1.7c2 exhibits specific and high expression levels in radish sprouts and leaves, RsPAP1.7c1 is specifically highly expressed in purple fleshy roots, and RsPAP1.5c is specifically expressed in purple radish petals. In Brassica napus, a relative of radish, a similar phenomenon has been observed. Specifically, BnaPAP2.A7 has been identified as a crucial regulator of anthocyanin biosynthesis in leaves [4]. Furthermore, the genes BnaA07.PAP2In−184–317 [34] and BnaPAP2.A7a [3] play a pivotal role in determining the flower color of orange-red rapeseed. Additionally, the specific upregulation of BnaPAP2.C6a in the purple stems of purple leaf rapeseed is essential for the synthesis and accumulation of anthocyanins in the stems [3]. In conclusion, our research findings offer novel perspectives for the subsequent investigation of the anthocyanin transcriptional regulation mechanism across various radish tissues. The tissue-specific expression of RsPAP1, which consequently influences the expression of DFR genes, could potentially account for the extensive diversity in radish coloration. Therefore, it is imperative to conduct further exploration into the regulatory mechanism underlying this phenomenon.
Conclusion
In this study, a total of 103 genes associated with the anthocyanin biosynthetic pathway were identified from the entire radish genome. Through comparative transcriptome analysis of radish hypocotyls, leaves, fleshy roots, and petals, along with the utilization of weighted gene co-expression network analysis (WGCNA), it was determined that RsUGT78D2.2c and RsDFR.9c potentially serve as central genes involved in anthocyanin synthesis across different radish tissues. Furthermore, our analysis revealed that the tissue-specific expression of RsPAP1, a transcription factor responsible for regulating the expression of DFR genes, likely contributes significantly to the observed variations in radish coloration. These findings contribute novel insights into the understanding of anthocyanin synthesis in radish, thereby expanding our knowledge in this field.
Supplementary Information
Acknowledgements
Thanks for the sequencing service was provided by Bioyi Biotechnology Co., Ltd. Wuhan, China, and thanks to Dr Shubei Wan for his help in the data analysis process of the thesis.
Statement on plant guidelines and permission
All materials in this study comply with relevant institutional, national, and international guidelines, legislation, and sub-section ethical approval and consent to participate.
Gannan Normal University agreed to the sampling of various experimental materials in this study.
Conflicts of interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be envisaged and/or construed as a conflict of interest.
Authors’ contributions
YL, XZ and DC conceived and designed the experiments. YL, CW, HC, GD, QC, WS, CT, LG, LC, CG and BZ analyzed the data. YL, XZ and CT wrote the manuscript. All authors approved the manuscript and consent to publication this manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangxi Province, grant number: 20224BAB215016, and 20212BAB215003; Wuhan knowledge innovation special project, grant number: 2022020801010413; the Science and Technology Foundation of Jiangxi Education Department, grant number: GJJ211447; the Key Research and Development Program of Jiangxi Province, grant number: 20223BBF61002.
Availability of data and materials
All RNA-seq data in this study were downloaded from the NCBI (https://www.ncbi.nlm.nih.gov/), with biological projects PRJNA388018 (hypocotyl), PRJNA810281 (fleshy roots), PRJNA810914 (fleshy roots). The RNA-seq data of leaves were collected from National Genomics Data Center (https://ngdc.cncb.ac.cn/gsa/browse/CRA008485) under accession ID CRA008485.
Declarations
Ethics approval and consent to participate
This study including the collection on plants material complies with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueli Zhang, Email: zywweirui2020@163.com.
Chen Tan, Email: tanchen2020@gnnu.edu.cn.
References
- 1.Bendokas V, Skemiene K, Trumbeckaite S, Stanys V, Passamonti S, Borutaite V, Liobikas J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit Rev Food Sci Nutr. 2020;60(19):3352–3365. doi: 10.1080/10408398.2019. [DOI] [PubMed] [Google Scholar]
- 2.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed]
- 3.Chen D, Jin Q, Pan J, Liu Y, Tang Y, E Y, Xu L, Yang T, Qiu J, Chen X, Wang J, Gong D, Ge X, Li Z, Cui C. Fine mapping of genes controlling pigment accumulation in oilseed rape (Brassica napus L.). Mol Breed. 2023;43(3):19. 10.1007/s11032-023-01365-5. [DOI] [PMC free article] [PubMed]
- 4.Chen D, Liu Y, Yin S, Qiu J, Jin Q, King GJ, Wang J, Ge X, Li Z. Alternatively spliced BnaPAP2.A7 isoforms play opposing roles in anthocyanin biosynthesis of Brassica napus L. Front Plant Sci. 2020;11:983. doi: 10.3389/fpls.2020.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng F, Mandáková T, Wu J, Xie Q, Lysak MA, Wang X. iphering the diploid ancestral genome of the Mesohexaploid Brassica rapa. Plant Cell. 2013;25(5):1541–1554. doi: 10.1105/tpc.113.110486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cone KC, Burr FA, Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci U S A. 1986;83(24):9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008;55(6):940–953. doi: 10.1111/j.1365-313X.2008.03564.x. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Li WB, Liu HF, Chen FB. Identification of differential expression genes related to anthocyanin biosynthesis in carmine radish (Raphanus sativus L) fleshy roots using comparative RNA-Seq method. PLoS One. 2020;15(4):e0231729. doi: 10.1371/journal.pone.0231729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53(5):814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 10.Grotewold E. The genetics and biochemistry of floral pigments. Annu Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 11.Heng S, Gao C, Cui M, Fu J, Ren S, Xin K, He C, Wang A, Song L, Tang L, Wang B, Zhang X. Metabolic and transcriptome analysis of dark red taproot in radish (Raphanus sativus L) PLoS One. 2022;17(5):e0268295. doi: 10.1371/journal.pone.0268295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000;19(22):6150–6161. doi: 10.1093/emboj/19.22.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaFountain AM, Yuan YW. Repressors of anthocyanin biosynthesis. New Phytol. 2021;231(3):933–949. doi: 10.1111/nph.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Ma K, Qi Y, Lv G, Ren X, Liu Z, Ma F. Transcriptional regulation of anthocyanin synthesis by MYB-bHLH-WDR complexes in Kiwifruit (Actinidia chinensis) J Agric Food Chem. 2021;69(12):3677–3691. doi: 10.1021/acs.jafc.0c07037. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Tikunov Y, Schouten RE, Marcelis LFM, Visser RGF, Bovy A. Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: A review. Front Chem. 2018;6:52. doi: 10.3389/fchem.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25(17):3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muleke EM, Fan L, Wang Y, Xu L, Zhu X, Zhang W, Cao Y, Karanja BK, Liu L. Coordinated regulation of anthocyanin biosynthesis genes confers varied phenotypic and spatial-temporal anthocyanin accumulation in radish (Raphanus sativus L.) Front Plant Sci. 2017;8:1243. doi: 10.3389/fpls.2017.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolov LA, Shushkov P, Nevado B, Gan X, Al-Shehbaz IA, Filatov D, Bailey CD, Tsiantis M. Resolving the backbone of the Brassicaceae phylogeny for investigating trait diversity. New Phytol. 2019;222(3):1638–1651. doi: 10.1111/nph.15732. [DOI] [PubMed] [Google Scholar]
- 19.Passeri V, Koes R, Quattrocchio FM. New challenges for the design of high value plant products: stabilization of anthocyanins in plant vacuoles. Front Plant Sci. 2016;7:153. doi: 10.3389/fpls.2016.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11(9):1650–67. 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed]
- 21.Petroni K, Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011;181(3):219–229. doi: 10.1016/j.plantsci.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004;40(6):979–995. doi: 10.1111/j.1365-313X.2004.02280.x. [DOI] [PubMed] [Google Scholar]
- 23.Pu Q, He Z, Xiang C, Shi S, Zhang L, Yang P. Integration of metabolome and transcriptome analyses reveals the mechanism of anthocyanin accumulation in purple radish leaves. Physiol Mol Biol Plants. 2022;28(10):1799–1811. doi: 10.1007/s12298-022-01245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilbert HM, Glover BJ. Analysis of flavonol regulator evolution in the Brassicaceae reveals MYB12, MYB111 and MYB21 duplications and MYB11 and MYB24 gene loss. BMC Genomics. 2022;23(1):604. doi: 10.1186/s12864-022-08819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi MZ, Xie DY. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat Biotechnol. 2014;8(1):47–60. doi: 10.2174/1872208307666131218123538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health-a focus on oxidative stress, inflammation and disease. Antioxidants (Basel) 2020;9(5):366. doi: 10.3390/antiox9050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50(4):660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008;54(4):733–749. doi: 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- 30.Tohge T, de Souza LP, Fernie AR. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J Exp Bot. 2017;68(15):4013–4028. doi: 10.1093/jxb/erx177. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Xu H, Jiang S, Zhang Z, Lu N, Qiu H, Qu C, Wang Y, Wu S, Chen X. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana) Plant J. 2017;90(2):276–292. doi: 10.1111/tpj.13487. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015;20(3):176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Grain D, Bobet S, Le Gourrierec J, Thévenin J, Kelemen Z, Lepiniec L, Dubos C. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 2014;202(1):132–144. doi: 10.1111/nph.12620. [DOI] [PubMed] [Google Scholar]
- 34.Ye S, Hua S, Ma T, Ma X, Chen Y, Wu L, Zhao L, Yi B, Ma C, Tu J, Shen J, Fu T, Wen J. Genetic and multi-omics analyses reveal BnaA07.PAP2In-184–317 as the key gene conferring anthocyanin-based color in Brassica napus flowers. J Exp Bot. 2022;73(19):6630–6645. doi: 10.1093/jxb/erac312. [DOI] [PubMed] [Google Scholar]
- 35.Yu R, Du X, Li J, Liu L, Hu C, Yan X, Xia Y, Xu H. Identification and differential expression analysis of anthocyanin biosynthetic genes in root-skin color variants of radish (Raphanus sativus L.) Genes Genomics. 2020;42(4):413–424. doi: 10.1007/s13258-020-00915-x. [DOI] [PubMed] [Google Scholar]
- 36.Yue M, Jiang L, Zhang N, Zhang L, Liu Y, Lin Y, Zhang Y, Luo Y, Zhang Y, Wang Y, Li M, Wang X, Chen Q, Tang H. Regulation of flavonoids in strawberry fruits by FaMYB5/FaMYB10 dominated MYB-bHLH-WD40 ternary complexes. Front Plant Sci. 2023;14:1145670. doi: 10.3389/fpls.2023.1145670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Zhao J, Tan Q, Qiu X, Mei S. Comparative transcriptome analysis reveals key genes associated with pigmentation in radish (Raphanus sativus L.) skin and flesh. Sci Rep. 2021;11(1):11434. doi: 10.1038/s41598-021-90633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Jing P. Anthocyanins in Brassicaceae: composition, stability, bioavailability, and potential health benefits. Crit Rev Food Sci Nutr. 2022;62(8):2205–2220. doi: 10.1080/10408398.2020.1852170. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Liu T, Wang J, Wang P, Qiu Y, Zhao W, Pang S, Li X, Wang H, Song J, Zhang W, Yang W, Sun Y, Li X. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol Plant. 2021;14(12):2032–2055. doi: 10.1016/j.molp.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Su N, Jia L, Tian J, Li H, Huang L, Shen Z, Cui J. Transcriptome analysis of radish sprouts hypocotyls reveals the regulatory role of hydrogen-rich water in anthocyanin biosynthesis under UV-A. BMC Plant Biol. 2018;18(1):227. doi: 10.1186/s12870-018-1449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Butelli E, Martin C. Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol. 2014;19:81–90. doi: 10.1016/j.pbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Zhao X, Zhang Y, Long T, Wang S, Yang J. Regulation mechanism of plant pigments biosynthesis: anthocyanins, carotenoids, and betalains. Metabolites. 2022;12(9):871. doi: 10.3390/metabo12090871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou C, Zhu Y, Luo Y. Effects of sulfur fertilization on the accumulation of health-promoting phytochemicals in radish sprouts. J Agric Food Chem. 2013;61(31):7552–7559. doi: 10.1021/jf402174f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All RNA-seq data in this study were downloaded from the NCBI (https://www.ncbi.nlm.nih.gov/), with biological projects PRJNA388018 (hypocotyl), PRJNA810281 (fleshy roots), PRJNA810914 (fleshy roots). The RNA-seq data of leaves were collected from National Genomics Data Center (https://ngdc.cncb.ac.cn/gsa/browse/CRA008485) under accession ID CRA008485.