Abstract
The E2 protein of papillomaviruses is a site-specific DNA binding nuclear protein. It functions as the primary replication origin recognition protein and assists in the assembly of the preinitiation complex. It also helps regulate transcription from the native viral promoter. The E2 protein consists of an amino-terminal (N) trans-acting domain, a central hinge (H) domain, and a carboxyl-terminal (C) protein dimerization and DNA binding domain. The hinge is highly divergent among papillomaviruses, and little is known about its functions. We fused the enhanced green fluorescent protein (GFP) with the full-length human papillomavirus type 11 (HPV-11) E2 protein and showed that the resultant fusion, called gfpE2, maintained transcription and replication functions of the wild-type protein and formed similar subnuclear foci. Using a series of GFP fusion proteins, we showed that the hinge conferred strong nuclear localization, whereas the N or C domain was present in both cytoplasm and nucleus. Biochemical fractionation demonstrated that the N domain and hinge, but not the C domain, independently associated with the nuclear matrix. Mutational analyses showed that a cluster of basic amino acid residues, which is conserved among many mucosotropic papillomaviruses, was required for efficient nuclear localization and nuclear matrix association. This mutation no longer repressed the HPV-11 upstream regulatory region-controlled reporter expression. However, a very small fraction of this mutant colocalized with E1 in the nucleus, perhaps by a piggyback mechanism, and was able to support transient replication. We propose that the hinge is critical for the diverse regulatory functions of the HPV-11 E2 protein during mRNA transcription and viral DNA replication.
The papillomavirus E2 protein is a multifunctional nuclear phosphoprotein with a molecular mass of approximately 40 kDa. With high affinity and specificity, it functions as a dimer, binding to multiple copies of a consensus E2 binding site (E2BS), ACCN6GGT, located in the upstream regulatory region (URR) and regulating both viral DNA replication and mRNA transcription from the viral E6 promoter located immediately upstream of the E6 gene. The E2 protein is the primary origin recognition protein and helps recruit the viral replication initiator E1 protein (8, 30, 40, 55, 64, 66, 70). It also plays a role in the assembly of the preinitiation complex (39; K.-Y. Lee, T. R. Broker, and L. T. Chow, unpublished data). In vitro, E2 is able to exclude nucleosome formation on the origin, which would otherwise inhibit the initiation of DNA replication (36). Furthermore, E2 modulates the native human papillomavirus (HPV) URR-E6 promoter or a surrogate promoter linked to the URR or to one or more copies of synthetic E2BS (16–18, 26, 59, 62, 63). The bovine papillomavirus type 1 (BPV-1) E2 protein tethers the BPV-1 DNA to mitotic chromosomes, ensuring plasmid segregation during mitosis (28, 35, 58). Moreover, the BPV-1 E2 has been postulated to play a role in virion morphogenesis (14).
The full-length E2 protein consists of three distinct domains, the amino-terminal (N) trans-acting domain, a central, apparently unstructured hinge (also called the H domain), and the carboxyl-terminal (C) protein dimerization and DNA binding domain. The structure and function of the N and C domains are relatively conserved among human and animal papillomaviruses, whereas the H domain is highly variable in sequence and length. Little is known about its function, and it is generally considered a flexible linker between the two functional domains (19). Alternative promoter usage and mRNA splicing generate additional forms of E2 polypeptides. In these E2-related proteins, the N domain is either truncated or substituted, but all contain the H and C domains. Each is a nuclear protein and inhibits replication or transcription by competing for binding to the E2BS (6, 7, 9, 10, 27, 32, 37, 39, 53). Since only the full-length E2 protein can activate a promoter and support replication, these observations indicate that the N domain plays a critical role in transcription activation and replication by interacting with other proteins.
Upon synthesis in the cytoplasm, proteins may enter the nucleus by passive diffusion or by energy-dependent active transport. All protein transport into and out of the nucleus occurs through the nuclear pore complexes in the nuclear envelope. Proteins larger than 45 to 60 kDa require active transportation via nuclear localization signals (NLSs) encoded within the protein or by association with another protein that is actively transported into the nucleus via a piggyback mechanism (reviewed in reference 29). Although there are no highly conserved consensus sequences for the NLS, peptide tracts rich in basic amino acid residues, exemplified by those in the simian virus 40 T antigen, can function as an NLS (56). In addition, phosphorylation can also play a role in nuclear localization (29). Two NLSs have been identified in the BPV-1 E2 protein: residues 107 to 115 (KRCFKKGAR) in the N domain and residues 339 to 352 (KCYRFRVKKNHRHR) in the C domain (57). Partially homologous sequences exist in the N and C domains of E2 proteins of many HPV types, including HPV type 11 (HPV-11). Whether they also function as NLSs has not been tested.
In a recent study using indirect immunofluorescence staining, we showed that the full-length HPV-11 E2 protein is located exclusively in the nucleus (61). Most prominent are the distinct subnuclear foci. Furthermore, the virus-encoded E1 helicase (reviewed in references 11 and 60), which is required for both initiation and elongation (39), invariably colocalizes with the E2 protein in these subnuclear foci. The E2 foci are observed in the presence or absence of E1 or HPV origin-containing DNA. In contrast, E1, which is also a nuclear phosphoprotein, only infrequently exhibits a similar punctate pattern when expressed alone. Thus, E2 appears to play a role in recruiting E1 protein and the origin-containing DNA to certain subnuclear compartments. When E1 and E2 expression vectors were cotransfected with an HPV origin-containing plasmid, some of these subnuclear foci became larger or merged. These large foci are identified to be replication compartments by their colocalization with the single-stranded DNA binding protein RPA and with bromodeoxyuridine incorporated during pulse-labeling. They also colocalize with HPV origin-containing DNA, which is below the level of detection elsewhere in the nucleus or in cells negative for E1 or E2 protein. We hypothesize that these subnuclear compartments are attributable to association with the nuclear matrix, but this hypothesis has not been examined.
Operationally, the eucaryotic nucleus is comprised of nucleoplasm, a soluble fraction of the nucleus, and two insoluble fractions: chromatin, which is composed of DNA and histones, and a fibrogranular structure called the nuclear matrix (NM) or nuclear scaffold, which remains after the nucleus has been treated with DNase and high salt or lithium-3,5-diiodosalicylate (1, 51). NM is composed of lamin polymers, core filaments, and associated proteins and is known to organize chromosomal DNA into looped domains via matrix attachment regions. It is this scaffold on which RNA transcription and processing as well as DNA replication take place (1, 2, 15).
In this study, we used the enhanced green fluorescent protein (GFP) as a tracer to delineate the domains of HPV-11 E2 responsible for its nuclear localization and to investigate the nature of the subnuclear E2 compartmentation. Our results show that, unexpectedly, the H domain contains a strong NLS and is responsible for nuclear localization of the protein. We also demonstrate that the E2 foci are indeed attributable to association with NM. Moreover, both the N and H domains, but not the C domain, contain sequences that bind to NM. Mutational analyses identified in the hinge a cluster of basic amino acids critical to NLS and for NM association. The implications of these findings in terms of HPV E2 protein functions will be discussed.
MATERIALS AND METHODS
Plasmids.
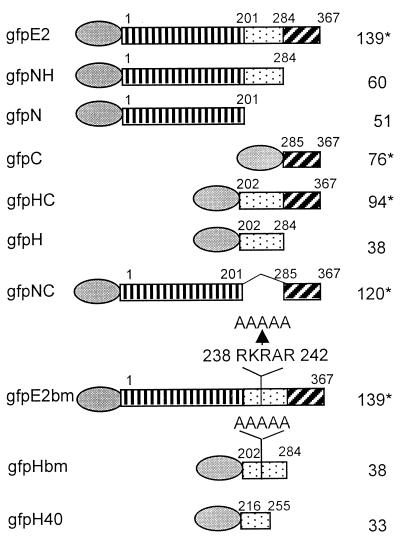
The enhanced GFP expression plasmid pEGFP-C1 (Clontech, Inc., Palo Alto, Calif.) was used to construct the GFP fusion protein expression plasmids. DNA fragments spanning the full-length HPV-11 E2, or a portion of it, such as the N domain, the intact or truncated hinge region, and the C domain, alone or in combinations (Fig. 1), were amplified by PCR using DNA polymerase Pfu (Stratagene, La Jolla, Calif.). These fragments were each fused in frame to the carboxyl terminus of the GFP coding gene via the BglII and SmaI cloning sites in plasmid pEGFP-C1. GFP fusion proteins containing mutations in the cluster of basic amino acids in the hinge (RKRAR to AAAAA) (HPV-11 genomic nucleotides 3434 to 3458, corresponding to amino acid residues 238 to 242) were constructed by PCR-mediated site-directed mutagenesis. Fusion junctions and mutations were confirmed by DNA sequence analyses of up to 400 bases around the site of interest. All constructs expressed proteins of the anticipated molecular masses in a Western blot.
FIG. 1.
Schematic representations of the GFP fusion proteins. The full-length HPV-11 E2 protein consists of the N, H, and C domains, each denoted by a different boxed symbol. The amino acid residues of the E2 protein expressed in each fusion protein are indicated. Mutations of the H domain are also illustrated. The GFP moiety is placed at the amino terminus of each fusion protein and is represented by an oval. The calculated molecular masses (kilodaltons) are given at the right; ∗ signifies the dimeric form of the protein when the C domain is present.
Cell cultures and transient transfections.
The simian virus 40-transformed monkey kidney epithelial cell line COS-7, adenovirus-transformed human kidney epithelial cell line 293, and human cervical carcinoma cell line C33A were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Cells were grown at 37°C under 5% CO2. Plasmids were electroporated into 5 × 106 human 293 cells at 960 μF and 180 V or into COS-7 and C33A cells at 960 μF and 170 V (8). After electroporation, the cells were pelleted through 10 ml of medium, resuspended in fresh medium, and plated on 22- by 22-mm glass coverslips at a concentration of 2 × 105 per coverslip or on 60-mm plates at 5 × 105 per plate and grown at 37°C for 24 or 48 h as specified for each experiment.
Transient HPV DNA replication and transcription regulation assays.
Transient replication assays were performed as described elsewhere (8). Briefly, 0.5 μg of HPV-11 origin-containing plasmid p7730-99 (HPV-11 nucleotides 7730 to 7933 and 1 to 99), 5 μg of an HPV-11 E1 protein expression vector (pMT2-11E1*) (70), and 5 μg of an HPV-11 E2 protein expression vector (pMT2-11E2) (8), or alternatively 10 μg of pEGFP-E2, pEGFP-E2bm, or pEGFP that expressed the GFP-E2 fusion protein (here called gfpE2), gfpE2 with mutations in the basic region of the hinge, or GFP, were cotransfected by electroporation into 5 × 106 293 cells, and half of the cells were cultured on a 100-mm-diameter plate for 48 h. Low-molecular-weight DNA was harvested via Hirt lysis as described elsewhere (8). Half of the DNA was subjected to HindIII digestion, which linearizes pMT2-11E1*, pMT2-11E2, and p7730-99 but does not cut the pEGFP plasmids. The other half was digested with HindIII and DpnI, which cuts the unreplicated input plasmid DNA into small pieces. The digestion mixtures were resolved by electrophoresis through 0.8% agarose gels, blotted onto nylon membranes, probed with [32P]dCTP-labeled origin-containing plasmid, and documented by autoradiography. To perform the transcription regulation assay, 1 μg of pURR-LacZ, a plasmid which contains the bacterial lacZ gene driven by the HPV-11 URR-E6 promoter (50), was cotransfected into 293 cells with 10 μg of vector, pMT2-11 E2, pEGFP-E2, or pEGFP-E2bm. After 24 h, β-galactosidase activity was determined with an assay kit (Promega, Madison, Wis.).
Immunofluorescence detection of E1 and E2 proteins.
Indirect immunofluorescence to detect the HPV-11 E1 protein was conducted as described previously (61), with slight modifications (14). Briefly, the transfected cells were grown on glass coverslips for 24 h, fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS), washed three times with PBS containing 200 mM glycine, and then incubated with monoclonal antibody against the EE epitope (21) at a 1:500 dilution at room temperature for 2 h. Goat anti-mouse antibody conjugated with Texas red fluorophor (Southern Biotechnology Associates, Birmingham, Ala.) was used as the secondary antibody at a 1:500 dilution. Incubation was conducted at room temperature overnight, followed by extensive washing with PBS. Coverslips were mounted on slides with DAPI (4′,6-diamidino-2-phenylindole)-containing mounting medium (Vector Laboratories, Inc., Burlingame, Calif.). E1 protein was revealed by Texas red, and E2 was visualized via GFP by using an Olympus IX70 fluorescence microscope, appropriate narrow-band-pass filters, and a SenSys digital imaging camera.
Subcellular fractionation.
In situ fractionation was modified from the protocol described by McNeil et al. (46). Briefly, monolayer COS-7 cells transfected with expression plasmid were cultured on petri plates or coverslips for 24 h. Cells were then washed with PBS and extracted successively to generate various subcellular fractions as follows. The cytoplasmic fraction was obtained after incubation for 60 s on ice in CSK buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8), 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA] containing 0.1% Triton X-100. The remaining material was washed once with cold PBS. An extraction solution containing 0.5% Triton X-100 in CSK buffer was added and incubated on ice for 10 to 20 min. This solution was collected as the nucleoplasm fraction. After washing with PBS, the residual nuclear structures, still attached to the substratum, were incubated with DNase I at 100 μg/ml in CSK buffer at room temperature or 37°C for 15 min and then were extracted in the same solution adjusted to 0.25 M (NH4)2SO4 for 10 min on ice or at room temperature. This solution was then collected as the chromatin fraction. After final washing, residues left on the substrate were NMs. To visualize directly the distribution of GFP fusion proteins after each extraction, the coverslips were fixed with 4% paraformaldehyde for 15 min and mounted on slides with DAPI-containing mounting medium.
For Western blots, the cytosol, nucleoplasm, and chromatin fractions were collected as described above. The whole cell extract and NM fraction on the substratum were solubilized in TES buffer (1% sodium dodecyl sulfate [SDS], 2 mM EDTA, 20 mM Tris-HCl [pH 7.4]). Immunoblot analyses were performed after polyacrylamide gel electrophoresis (PAGE) through SDS–10% polyacrylamide gels. GFP and gfpE2 fusion proteins were detected with a monoclonal antibody against GFP at a 1:1,000 dilution (Clontech, Inc., Palo Alto, Calif.). Histone H1 and lamin A and C antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) were used at a 1:1,000 dilution.
RESULTS
GFP-tagged full-length E2 protein functions similarly to the native E2.
We previously demonstrated that the HPV-18 glutathione S-transferase–E2 fusion protein can support cell-free HPV origin-specific replication in the presence of HPV-18 E1 protein (34), indicating that it is possible for the E2 protein to accommodate a relatively large addition at its amino terminus without losing functions. To track the localization of HPV-11 E2 protein and to facilitate its subsequent genetic dissection into functional domains, we created gfpE2, a recombinant expression clone that encodes the full-length HPV-11 E2 protein fused in frame to the carboxyl terminus of an enhanced GFP (Fig. 1). The GFP fusion proteins would bypass the dependence on our polyclonal E2 antibody, which requires permeabilization of the cell membrane and might recognize various E2 domains with different efficiencies, thus confounding the analyses.
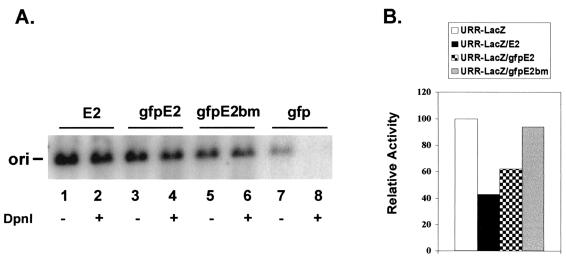
To ascertain that the GFP moiety does not interfere with E2 protein functions, we tested the ability of gfpE2 to support HPV-11 origin-dependent replication in the presence of HPV-11 E1 protein in transiently transfected 293 cells. gfpE2 yielded a level of replication comparable to that of the wild-type E2 protein (Fig. 2A, compare lanes 3 and 4 with lanes 1 and 2). In the presence of E1 and GFP, no replication was observed (Fig. 2A, lanes 7 and 8). In transfected COS-7 or C33A cells, gfpE2 colocalized with E1 protein to subnuclear foci in patterns identical to the previously reported replication complexes (61) (Fig. 3D and data not shown). Furthermore, the fusion protein was able to repress the HPV-11 enhancer-E6 promoter activity to an extent similar to that imposed by the wild-type E2 (Fig. 2B). Thus, fusion to GFP does not significantly affect subnuclear localization or the replication and transcription functions of the E2 protein.
FIG. 2.
Regulatory functions of gfpE2 and gfpE2bm. (A) Transient replication of HPV-11 origin (ori) plasmid p7730-99 in the presence of HPV-11 E1 plus E2 (lanes 1 and 2), gfpE2 (lanes 3 and 4), gfpE2bm (lane 5 and 6), or GFP (lanes 7 and 8) expression vectors. An autoradiogram of one of two experiments reveals HindIII-linearized, total origin-containing DNA prior to DpnI digestion (lanes 1, 3, 5, and 7) and replicated, origin-containing DNA after DpnI digestion (lanes 2, 4, 6, and 8). (B) Repression of the HPV-11 URR-E6 promoter-driven lacZ reporter by E2, gfpE2, or gfpE2bm in transfected cells. The results shown were averages of two separate experiments, each performed in duplicate. Both functional assays were conducted in 293 cells as described in Materials and Methods.
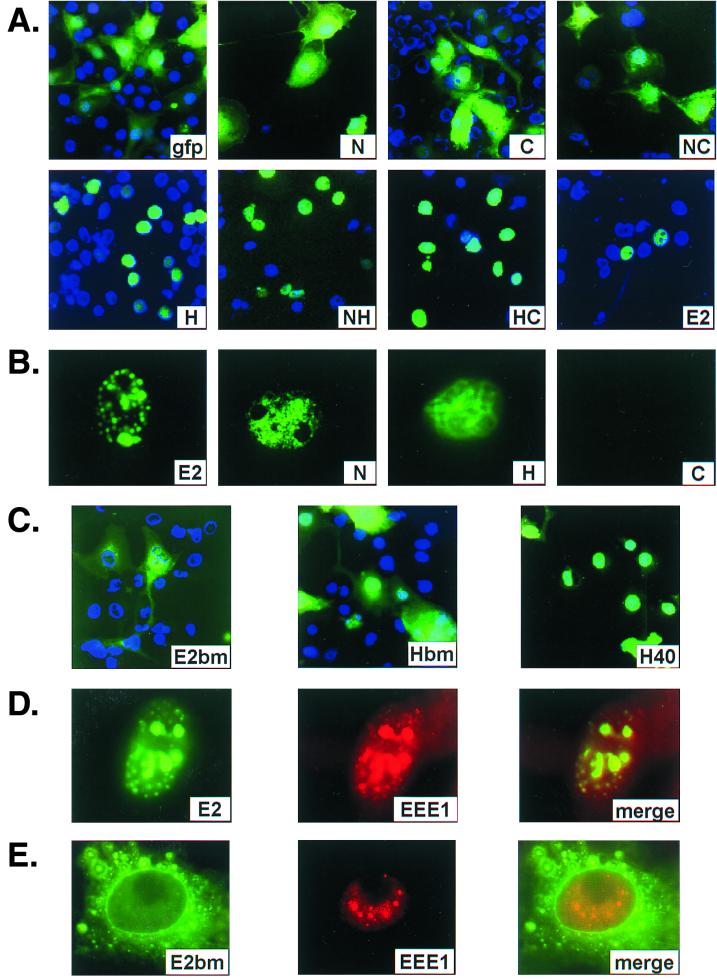
FIG. 3.
Localization of GFP and GFP fusion proteins as visualized microscopically via GFP fluorescence. (A and C) Subcellular distribution of GFP and GFP fusion proteins in whole cells as indicated in each panel. COS cells were transfected with various expression vectors; 24 h posttransfection, cells were fixed with paraformaldehyde and treated with a mounting medium containing DAPI to reveal their nuclei. (B) Typical patterns of GFP fluorescence which remained in transfected COS-7 cells after in situ fractionation as described in Materials and Methods. (D and E) Typical COS-7 cells cotransfected with an origin-containing plasmid and plasmids expressing EE-E1 and gfpE2 (D) or gfpE2bm (E). E2 was directly visualized via GFP (left). The EE-E1 protein was revealed by the Texas red through indirect immunofluorescence after staining with a monoclonal antibody against the EE epitope (center). Merged images reveal colocalization (right). The images were acquired through a 40× (A and C) or 100× (B, D, and E) objective lens of a monochromatic camera, pseudo-colored, and digitally processed. Relative to panels A and C, images in panels B, D, and E have been enlarged to reveal detailed subnuclear structures.
The H region is required for exclusive nuclear localization.
To determine the E2 domain responsible for its nuclear localization, we constructed a panel of GFP fusion proteins with one or two of the three functional domains, each fused to the C terminus of GFP and designated gfpN, gfpH, gfpC, gfpNH, gfpHC, or gfpNC (Fig. 1). The calculated molecular mass or that of a dimer for fusion protein containing the C domain is also presented in Fig. 1. The boundaries of the domains are based on a sequence alignment of the E2 proteins of a large number of papillomavirus types as well as the coding region of the E4 protein, which coincides with the E2 H domain in a different reading frame (12, 48, 69).
COS-7 cells were transiently transfected with the expression plasmid for each fusion protein and, after 24 h of incubation, were fixed with paraformaldehyde. The cellular localization of each fusion protein was visualized directly via GFP with a fluorescence microscope, while nuclei were revealed by the fluorescence of DAPI contained in the mounting medium (Fig. 3A). The same subcellular distribution was observed in 293 and C33A cells. The examples shown are for COS-7 cells because, of the three cells lines tested, COS-7 is the most suitable for in situ subcellular fractionation (described below); 293 cells do not attach to substrates securely enough to allow fractionation, while C33A cells have a low transfection efficiency such that the percentage of GFP-positive cells and the signal intensities were low and not optimal for subcellular fractionation.
The results are presented in Fig. 3A. Of all the fusion proteins, the signals from gfpE2, as well as the fraction of positive cells, were consistently the lowest. This low expression might suggest that E2 is unstable or overexpression is toxic to the cells. gfpE2 was detected virtually exclusively in the nucleus in a punctate pattern when visualized at a high magnification as shown previously (61) (data not shown, but see Fig. 3B and D). In contrast, GFP alone was diffused throughout the cells. Unexpectedly, gfpN and gfpC fusion proteins were present in both the cytoplasm and the nucleus, with a higher concentration in the nucleus, indicative of an inward flux of the fusion proteins to the nucleus greater than the outward flux. Since the calculated molecular masses of gfpN and dimerized gfpC are about 51 and 76 kDa, respectively, near or above the approximately 45- to 60-kDa limit for passive diffusion through the nuclear pore complex, a weak NLS in the N and C domains or nuclear retention signal may explain their biased subcellular distribution.
Much to our surprise, virtually all of the 38-kDa gfpH was detected in the nucleus, with little or no cytoplasmic signals (Fig. 3A), indicating that the H, rather than N or C, domain contains a strong NLS or nuclear retention signal. To substantiate this interpretation, we expressed gfpNC, which has a molecular mass of 120 kDa when dimerized. Highly efficient nuclear localization such as that demonstrated by gfpE2 was abolished. Cytoplasmic distribution of this protein ranged from very high to a level similar to that exhibited by gfpN or gfpC. In contrast, when the hinge was linked to the N (gfpNH) or C (gfpHC) domain, strong and efficient nuclear localization was restored (Fig. 3A). The former has a molecular mass of 60 kDa, whereas the latter is 94 kDa when dimerized, above the limit of passive diffusion into the nucleus. Collectively, these results demonstrate that the hinge indeed confers highly effective nuclear localization to each of the fusion proteins.
E2 nuclear foci are formed by association with NM.
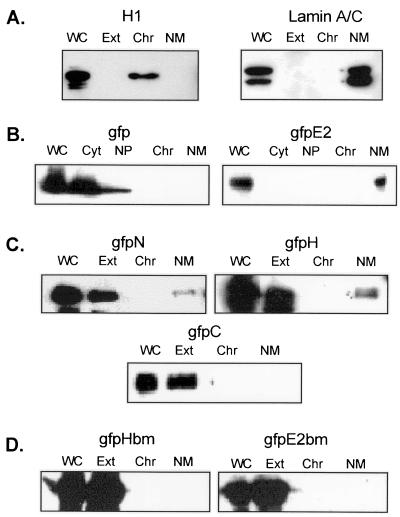
To ascertain whether the subnuclear foci observed with E2 and gfpE2 fusion proteins represent their association with NM, we examined the partition of gfpE2 expressed in COS-7 cells. The cells were fractionated while attached to the glass substrates by using an in situ protocol (46). Treatment with 0.5% Triton X-100 removed the cytoplasm, nuclear membrane, and soluble nucleoplasm, whereas chromatin and NM remained on the substrate as the insoluble fraction. DNase I treatment and subsequent 0.25 M (NH4)2SO4 extraction removed chromatin, and only NM remained on the substrate. This fractionation protocol was confirmed by Western blotting of the various fractions of cells expressing GFP alone for the distribution of GFP, histone H1, and lamins A and C that represent the various subcellular fractions (reference 1 and references therein). GFP was detected only in the cytoplasm and nucleoplasm fractions, not in the chromatin or NM fraction (Fig. 4B, left panel). Histone H1 was found only in the chromatin fraction, whereas lamins A and C were restricted to the NM fraction (Fig. 4A).
FIG. 4.
Subcellular distribution of GFP and GFP fusion proteins as detected by immunoblotting. COS-7 cells were fractionated by an in situ protocol. The whole cell extracts (WC) as well as the sequentially collected cytoplasm (Cyt) and nucleoplasm (NP) or, alternatively, pooled cytoplasm and nucleoplasm (Ext), chromatin (Chr), and nuclear matrix (NM) fractions were solubilized in PAGE loading buffer, separated by SDS-PAGE, and Western blotted with a monoclonal antibody against H1 (A, left), lamins A and C (A, right), or GFP (B to D).
The partitioning of various GFP fusion proteins to the NM was then directly visualized with a fluorescence microscope after in situ extraction (Fig. 3B). gfpE2 typically exhibited a punctate pattern in association with NM (Fig. 3B). The shape of the nucleus was well preserved. The distribution of the gfpE2 fusion protein into each subcellular fraction was further analyzed by SDS-PAGE and Western blotting with an antibody against GFP. In this and subsequent Western blots, all of the antibody-reactive proteins migrated at the appropriate molecular masses of monomeric proteins. Virtually all of the gfpE2 was found in the NM fraction, while the cytoplasm, nucleoplasm, and chromatin fractions contained no detectable signal (Fig. 4B, right panel). Similar subcellular fractionation patterns were observed after Western blotting with a polyclonal antibody against E2 (data not shown). Collectively, these observations indicate that NM provides the basic structural scaffold for the E2 protein subnuclear compartmentation and also validate that direct visual inspection of the NM fraction is an accurate means to assess the ability of GFP fusion proteins to associate with NM.
gfpN and gfpH associate with the NM.
To determine the E2 domain or domains required for the association with NM, the subcellular partitioning of GFP fusion proteins containing the individual E2 domains was examined following in situ fractionation as described above. Green fluorescence signal was removed from all of the gfpC-transfected cells (Fig. 3B), and the result was confirmed by Western blotting (Fig. 4C). Only trace nonspecific green flluorescence signal was occasionally observed to associate with cell debris following in situ extraction. Similar background was also observed in cells expressing GFP alone or other GFP fusion proteins, and it did not take on the shape of a nucleus, as observed in cells expressing gfpE2, gfpN, or gfpH. About 90% of the gfpN- or gfpH-positive cells lost most if not all of the green fluorescence after detergent extraction. These cells generally exhibited weaker signals before extraction. However, in the remaining 10% of GFP-positive cells that had relatively strong signals before extraction, the fusion proteins were retained on the NM (Fig. 3B). Consistent with the direct visualization with a fluorescence microscope, Western blot analysis revealed that although a majority of gfpN and gfpH fusion proteins were present in the pooled cytoplasm and nucleoplasm fractions (Fig. 4C, lanes labeled Ext), a small proportion was detected in the NM fraction. These results indicate that N and H domains can independently associate with NM, but with a much reduced affinity relative to the full-length E2 protein.
A cluster of basic amino acids in the hinge region is critical for nuclear localization and NM association.
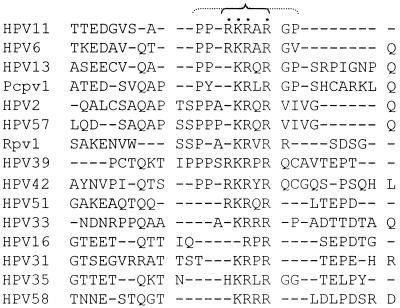
To localize the sequence in the hinge region important for its nuclear localization and NM association, we used alignment of the E2 hinge amino acid sequences of a large number of papillomavirus genotypes (69). Although no extensive homology was found, the central portion of the E2 hinge of a number of mucosotropic HPVs contains three or four basic amino acids in close proximity, generally flanked by proline and glycine residues (Fig. 5). In HPV-11, this sequence is PPRKRARPG (amino acid residues 236 to 244). To test whether this region might be important for nuclear localization, we converted the basic amino acid residues to alanine (RKRAR to AAAAA) by site-directed mutagenesis in the context of gfpH as well as gfpE2 (designated gfpHbm and gfpE2bm) (Fig. 1).
FIG. 5.
Alignment of a portion of the E2 H domain of 15 mucosotropic human and animal papillomaviruses spanning a cluster of basic amino acids residues. Pcpv, pigmy chimp papillomavirus; Rpv, rhesus monkey papillomavirus. Dashes represent gaps introduced for maximal alignment. The cluster of basic amino acids is marked by dots. The inner bracket denotes the pentapeptide mutated to alanine in the HPV-11 E2 fusion proteins. Note that the region is flanked by helix-breaking glycine and proline residues in many of the viruses (outer bracket).
These two fusion proteins were each transiently expressed in COS-7 cells. Both mutated proteins were efficiently expressed. gfpHbm was distributed in both the nucleus and cytoplasm (Fig. 3C). In contrast, in the majority of the cells, gfpE2bm was detected in the cytoplasm, although a small amount may have been located in the nucleus. We next examined by in situ fractionation whether this mutation affected NM association. Neither gfpHbm nor gfpE2bm was associated with NM when probed by Western blotting (Fig. 4D) or viewed with a fluorescence microscope (data not shown). Collectively, these observations indicate that the cluster of basic amino acids in the hinge is critical for nuclear localization and contributes to the binding to the NM.
A truncated hinge retains the NLS but no longer associates with NM.
To delineate further the hinge region which contains the strong NLS and sequences that associate with NM, we expressed gfpH40. gfpH40 has a molecular mass of 33 kDa and contains amino acids (216 to 255) from the hinge region, including the basic peptide and the flanking proline and glycine residues (Fig. 1). In the majority of the transfected COS-7 cells, gfpH40 was primarily observed in the nucleus in a diffuse pattern with a much reduced cytoplasmic signal compared with gfpN or gfpC. However, significant cytoplasmic distribution was observed in some cells that overexpressed the gfpH40 fusion protein (Fig. 3C). Upon in situ fractionation, all GFP signal was lost from the NM fraction when viewed with a fluorescence microscope (data not shown), similar to gfpC (Fig. 3B). We thus conclude that this 40-amino-acid peptide still contains a strong NLS. However, an association with NM with an affinity high enough to be partially resistant to in situ fractionation requires additional hinge region sequences. This loss of NM association could have contributed to their increased cytoplasmic distribution relative to gfpH.
Functional assays of gfpE2bm.
We then tested whether gfpE2bm might retain any of the regulatory functions. When cotransfected with HPV-11 URR-lacZ, this mutation no longer repressed the URR promoter (Fig. 2B). This is not surprising considering that little or no gfpE2bm was transported into the nucleus (Fig. 3C). Unexpectedly, when cotransfected with E1 expression vector in a transient replication assay, a very small fraction of the mutated E2 colocalized with E1 in the nucleus. Despite a more abundant total E2 protein, the amount of nuclear gfpE2bm was significantly reduced relative to gfpE2 (compare Fig. 3D to E and 4B to D). Interestingly, this amount was sufficient to support transient replication (Fig. 2A).
DISCUSSION
The extensive amino acid sequence homology and functional conservation exhibited by E2 proteins encoded by different papillomavirus types are largely limited to the N and C domains. Other than being considered a flexible linker, the highly variable central hinge region has not been shown to serve any critical function (19). While high-resolution structures of the separated N and C domains have been determined by X-ray crystallography (5, 23–25), the H domain or the full-length E2 has not, presumably due to an unstructured nature of the hinge. Nonetheless, the BPV-1 E2 hinge region contains a number of regulatory phosphorylation sites, and mutations in these sites affect the association of E2 with mitotic chromosomes and extrachromosomal copy number (35, 45).
In this study, we prepared a panel of GFP fusion proteins containing the full-length HPV-11 E2 or one or two of the domains as a means to delineate those responsible for the nuclear localization and subnuclear focus formation exhibited by the full-length E2 protein. Our results demonstrate, unexpectedly, that the hinge region of HPV-11 E2 protein has a previously unrecognized critical function in conferring nuclear localization. In addition, the H and N domains each independently associate with apparently different components of NM. These functions of the hinge region may partly explain why all forms of E2 proteins naturally truncated by the use of alternative promoters or mRNA splice sites retain the hinge region, even though the C domain alone has the same E2BS binding property as the full-length E2 protein, critical for their inhibitory functions.
We showed that only the GFP fusion proteins containing the hinge domain are localized exclusively to the nucleus, whereas significant amounts of fusion proteins that do not have the hinge are found in the cytoplasm (Fig. 3A). These results indicate that the hinge contains a strong NLS and that the putative NLS in the N and C domains, as inferred by sequence homology to the NLS of BPV-1 E2, are insufficient for efficient nuclear localization. In fact, less gfpNC than either gfpN or gfpC was targeted to the nucleus, and in some cells it was virtually excluded from the nucleus. One possible explanation is that because of its large dimeric size (120 kDa), gfpNC is not efficiently transported into the nucleus in the absence of a strong NLS. Alternatively, there may be a mutual interference of the weak NLSs in the N and C domains when they are brought into close proximity. Masking of the NLS in the C domain has also been proposed to explain the cytoplasmic distribution of BPV-1 E2 in which the N-terminal NLS is mutated or deleted (57). However, this masking effect cannot account for the primarily cytoplasmic distribution of gfpE2bm in which the spacing between the N and C domains remains unchanged (Fig. 3C).
Mutagenic studies of the H domain revealed that the cluster of basic amino acids conserved among many of the mucosotropic HPVs (Fig. 5) is a critical element of the strong NLS of the HPV-11 E2 protein. Further truncation of the H domain localizes the strong NLS to a 40-amino-acid peptide spanning these basic amino acids, as this small fusion protein was transported to the nucleus almost as efficiently as the intact H domain (compare Fig. 3C and A).
Biochemical fractionation followed by in situ visualization or by Western blot analysis demonstrated that the great majority of the gfpE2 protein is associated with NM (Fig. 3B and 4). Therefore, we suggest that the specific subnuclear foci observed with HPV-11 E2 are at least in part due to its association with NM. A fraction of the BPV-1 E2 protein has also been found to partition into the NM fraction (27). Similar analyses of GFP fusions with the various HPV-11 E2 domains showed that the N and H domains each contain sequences that associate with NM, whereas C does not (Fig. 3B and 4C). Relative to the full-length E2, the affinity for NM was dramatically reduced when either the N or H domain was absent, suggesting that N and H domains may function synergistically rather than additively.
The cluster of basic amino acids in the hinge region is not only a critical element of the NLS but is also important for NM association, as gfpHbm failed to associate with NM upon extraction (Fig. 4D). However, these basic residues are not sufficient for NM association. Additional sequences in the hinge contribute to this affinity. This interpretation is suggested by the analysis of gfpH40, which retains the cluster of basic residues. Although primarily localized to the nucleus, gfpH40 was no longer associated with NM. Why was gfpE2bm unable to associate with NM, despite the ability of the N domain alone to bind to NM (Fig. 3B, 4C, and 4D)? This is likely because the NLS is largely incapacitated by the mutations in the hinge. When, at best, only a small amount of the mutated protein was transported into the nucleus, it was no longer possible to detect the weakened association with NM. Collectively, these data suggest that at least two activities are required to account for the subnuclear compartmentation exhibited by the full-length E2 protein. The first is a strong NLS which resides in the H domain primarily within a 40-amino-acid peptide spanning the cluster of basic residues. The second is a high affinity for NM, which requires both the N and H domains.
gfpE2bm no longer functioned as a transcription repressor or activator because little of this protein was able to find its way into the nucleus (Figs. 2B, 3C, and 4D). Unexpectedly, however, a small fraction of gfpE2bm was clearly colocalized with E1 in the nucleus (compare Fig. 3E and D). We suggest that this mutation is able to piggyback on the coexpressed E1 protein which is actively transported into the nucleus (61), as has been reported for other NLS-defective proteins (29). However, relative to gfpE2, only a fraction of the gfpE2bm formed distinct foci. Much of it exhibited a diffuse pattern (compare Fig. 3D and E), consistent with the abolished or diminished ability of gfpHbm and the N domain alone to associate with NM. Nevertheless, some of the mutated protein did associate with focal NM proteins via the N domain or via interaction with E1 (Fig. 3E) and supported transient replication of the HPV-11 origin-containing plasmid (Fig. 2A). We suggest that only a small amount of the E2 protein is sufficient to support DNA replication. Consistent with this interpretation, in a titration experiment, transfection of reduced amounts of gfpE2 or gfpE2bm expression vector in the presence of a constant amount of E1 expression vector increased rather than decreased transient replication (data not shown). However, it is not possible to compare the specific replication activity of the mutation relative to the wild-type E2 protein. This is because the fraction of cells that expressed these two proteins and the amounts of proteins expressed or transported into the nucleus were not comparable (compare Fig. 3A to C, 3D to E, and 4B to D). We speculate that in vivo, when E1 and E2 proteins were expressed at reduced levels from their native promoter compared to transfected cells, the probability of nuclear transport of E2 by piggybacking on E1 would be significantly reduced and this mutated virus might be defective.
We propose that, as is the case with cellular nucleic acid metabolism, NM is where HPV DNA replication and mRNA transcription take place. Since E2 protein plays critical roles in both processes, the nuclear localization and proper subnuclear compartmentation conferred by the H and N domains are integral to these diverse functions. We note that the N domain alone typically exhibits a punctate pattern, similar to that of full-length E2, whereas the H domain usually assumes a more fibrous pattern (Fig. 3B). Collectively, these results suggest that the N and H domains interact with different components of the NM. We suggest that the N domain plays a major role in selecting focal NM-associated proteins whereas the H domain helps anchor the protein to the scaffold.
We have no information on the focal components to which the N domain associates, but there are a number of potential candidates. For instance, our recent immunofluorescence studies of HPV replication compartments in transfected cells demonstrated that PML (or promyelocytic oncogenic domains), a component of nuclear domains 10 (ND10), is partially redistributed and colocalizes with HPV-11 E1 and E2 proteins (61). This colocalization is independent of HPV DNA replication and is thus dictated by one or both viral proteins. A variety of other DNA viruses, such as adenoviruses, polyomaviruses, and herpesviruses, also target to or disrupt ND10. To date, the significance of these relationships is not understood (reviewed in reference 44). We also note that many of the transcription factors that bind to HPV URR (references 50, 67, and 68 and references therein) also associate with NM (65), as do at least some cellular replication proteins, such as RPA (47), DNA polymerase α/primase (reference 42 and references therein), and topoisomerase II (reviewed in reference 33). Interestingly, our recent data indicated that E2 protein binds to RPA via the 70-kDa subunit and enhances the single-stranded DNA binding activities of both (Lee et al., unpublished data). E1 also binds to the same RPA subunit (22) as well as to DNA polymerase α/primase (3, 13, 43, 49).
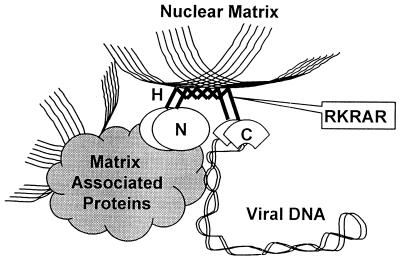
Based on the above observations, we propose a model for the mechanics of how the E2 protein of HPV-11 functions (Fig. 6); this hypothesis may extend to some of the other mucosotropic HPVs, in view of the conservation of the cluster of basic amino acids in the hinge region. The hinge region is involved in active transport of the E2 protein into the nucleus and helps anchor the protein to the nuclear scaffold. The relative lack of structure and apparent flexibility due to the high proline and glycine content of the H domain enable the E2 protein to adopt new conformations when the C domain secures viral DNA, while the N domain associates with focally located NM-associated proteins. Once anchored to the NM, the E2 protein and viral DNA can then interact with additional matrix-associated proteins or recruit additional proteins to the complex, resulting in the regulation of viral mRNA transcription and DNA replication.
FIG. 6.
A model for the structure and function of the dimeric HPV-11 E2 protein. The N domain (oval) binds to focal NM-associated proteins, while the flexible hinge (H; bold zig zag lines) helps anchor the protein by association with the fibrous matrix scaffold (thin lines). The C domain (fans) secures the supercoiled viral DNA (ribbon). The E2 protein and the viral DNA then interact with additional proteins on NM or recruit proteins to the matrix-bound DNA, leading to the regulation of viral mRNA transcription or DNA replication. The cluster of basic residues in the hinge is an important element for both nuclear localization and for NM association.
It is worth noting that papillomaviruses appear to use different NLS and NM attachment sequences to target E2 to the proper nuclear loci. For instance, the hinge of BPV-1 E2 can be approximately aligned with that of a number of cutaneous animal and human papillomavirus types but not with the mucosotropic HPVs (69). This lack of homology would perhaps explain the different NLS between HPV-11 and BPV-1 E2 proteins (this work and reference 57). Similarly, only the full-length BPV-1 E2, not the other N-terminus-truncated forms of E2, associates with NM (27). Our results are also different from those for the mucosotropic HPV-16. When expressed in insect cells, the HPV-16 E2 peptide truncated of the C domain was found in the cytoplasm, whereas the C domain peptide was found in both the nucleus and the cytoplasm (54). We note that among the mucosotropic HPVs, HPV-16 has the least homology in the corresponding hinge region, with only two basic residues (Fig. 5). This distinction may explain their different distribution in insect cells. Interestingly, the cutaneous HPV-5 E2 protein appears to have properties that are similar partially to those of HPV-11 and partially to those of BPV-1 E2 proteins. It colocalizes with an mRNA splicing coactivator in a speckled nuclear pattern (31), similar to the punctate nuclear pattern exhibited by HPV-11 E2 (reference 61 and this work). Since no common cellular antigens were used in their and our studies, it remains to be determined whether both E2 proteins indeed associate with the same cellular proteins. As with BPV-1 E2, the hinge of HPV-5 E2 had little homology to that of HPV-11 E2. However, an HPV-5 E2 peptide lacking the hinge region is observed in the nucleus, but in a diffuse pattern (31). Evidently, the N or C domain or both of HPV-5 E2 contain the NLS, but the H domain appears to be critical to its association with NM, as it is for HPV-11 E2.
It is intriguing that in all papillomaviruses, the hinge region overlaps and coincides with the coding region of the E4 protein in a different translation frame. The E4 protein is very abundant in productively infected tissues (4). It is directly or indirectly associated with cytokeratins, but its precise function has yet to be defined (52). The E2 hinge and the E4 proteins of many papillomaviruses have several features in common. Both are hypervariable. Both are rich in proline and glycine residues that are helix breakers, indicating that both are rather unstructured or flexible peptides. These properties apparently enable them to associate with their respective interacting protein partners in the nuclear or cytoplasmic skeletons (this work and reference 52). Both are also rich in serine and threonine residues, and some of these residues in HPV-1 E4 and BPV-1 E2 are substrates for kinases (20, 35, 45). HPV-11 E2 protein is a substrate of cyclin E-, A-, or B-dependent kinases (CDKs) in vitro in a complex which also contains the HPV-11 E1 protein (41). However, only cyclin E/cdk2 plays a unique role in initiation of replication that cannot be fulfilled by other CDKs (38). We note that several candidate CDK phosphorylation sites are located in the hinge region. Whether phosphorylation of these residues modulates the association with NM or its regulatory functions in the context of the full-length E2 will be of interest for future investigations.
ACKNOWLEDGMENTS
This research was supported by USPHS grants CA36200 and CA83679 and the Cystic Fibrosis Foundation. The Digital Imaging Microscopy Facility was established with grant funds provided in large measures by the UAB Health Services Foundation and by Oral Cancer Research Center grant DE/CA 11910.
The DNA sequence was determined by a core facility of the UAB Center for AIDS Research.
REFERENCES
- 1.Berezney R, Wei X. The new paradigm: integrating genomic function and nuclear architecture. J Cell Biochem Suppl. 1998;30-31:238–242. [PubMed] [Google Scholar]
- 2.Blencowe B J, Nickerson J A, Issner R, Penman S, Sharp P A. Association of nuclear matrix antigens with exon-containing splicing complexes. J Cell Biol. 1994;127:593–607. doi: 10.1083/jcb.127.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne-Andrea C, Santucci S, Clertant P, Tillier F. Bovine papillomavirus E1 protein binds specifically DNA polymerase alpha but not replication protein A. J Virol. 1995;69:2341–2350. doi: 10.1128/jvi.69.4.2341-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown D R, Chin M T, Strike D G. Identification of human papillomavirus type 11 E4 gene products in human tissue implants from athymic mice. Virology. 1988;165:262–267. doi: 10.1016/0042-6822(88)90680-0. [DOI] [PubMed] [Google Scholar]
- 5.Bussiere D E, Kong X, Egan D A, Walter K, Holzman T F, Lindh F, Robins T, Giranda V L. Structure of the E2 DNA-binding domain from human papillomavirus serotype 31 at 2.4 Å. Acta Crystallogr Sect D. 1998;54:1367–1376. doi: 10.1107/s0907444998005587. [DOI] [PubMed] [Google Scholar]
- 6.Chiang C-M, Broker T R, Chow L T. An E1M∧E2C fusion protein encoded by human papillomavirus type 11 is a sequence-specific transcription repressor. J Virol. 1991;65:3317–3329. doi: 10.1128/jvi.65.6.3317-3329.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang C-M, Dong G, Broker T R, Chow L T. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J Virol. 1992;66:5224–5231. doi: 10.1128/jvi.66.9.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang C-M, Ustav M, Stenlund A, Ho T F, Broker T R, Chow L T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992;89:5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin M T, Hirochika R, Hirochika H, Broker T R, Chow L T. Regulation of human papillomavirus type 11 enhancer and E6 promoter by activating and repressing proteins from the E2 open reading frame: functional and biochemical studies. J Virol. 1988;62:2994–3002. doi: 10.1128/jvi.62.8.2994-3002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe J, Vaillancourt P, Stenlund A, Botchan M. Bovine papillomavirus type 1 encodes two forms of a transcriptional repressor: structural and functional analysis of new viral cDNAs. J Virol. 1989;63:1743–1755. doi: 10.1128/jvi.63.4.1743-1755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow L T, Broker T R. Papillomavirus DNA replication. Intervirology. 1994;37:150–158. doi: 10.1159/000150373. [DOI] [PubMed] [Google Scholar]
- 12.Chow L T, Nasseri M, Wolinsky S M, Broker T R. Human papillomavirus types 6 and 11 mRNAs from genital condylomata acuminata. J Virol. 1987;61:2581–2588. doi: 10.1128/jvi.61.8.2581-2588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conger K L, Liu J-S, Kuo S-R, Chow L T, Wang T S-F. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J Biol Chem. 1999;274:2696–2705. doi: 10.1074/jbc.274.5.2696. [DOI] [PubMed] [Google Scholar]
- 14.Day P M, Roden R B, Lowy D R, Schiller J T. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J Virol. 1998;72:142–150. doi: 10.1128/jvi.72.1.142-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong L, Grande M A, Mattern K A, Schul W, van Driel R. Nuclear domains involved in RNA synthesis, RNA processing, and replication. Crit Rev Eukaryotic Gene Expr. 1996;6:215–246. doi: 10.1615/critreveukargeneexpr.v6.i2-3.60. [DOI] [PubMed] [Google Scholar]
- 16.Demeret C, Desaintes C, Yaniv M, Thierry F. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J Virol. 1997;71:9343–9349. doi: 10.1128/jvi.71.12.9343-9349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong G, Broker T R, Chow L T. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J Virol. 1994;68:1115–1127. doi: 10.1128/jvi.68.2.1115-1127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dostatni N, Lambert P F, Sousa R, Ham J, Howley P M, Yaniv M. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 1991;5:1657–1671. doi: 10.1101/gad.5.9.1657. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier J M, Dillner J, Yaniv M. Structural analysis of the human papillomavirus type 16-E2 transactivator with antipeptide antibodies reveals a high mobility region linking the transactivation and the DNA-binding domains. Nucleic Acids Res. 1991;19:7073–7079. doi: 10.1093/nar/19.25.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grand R J, Doorbar J, Smith K J, Coneron I, Gallimore P H. Phosphorylation of the human papillomavirus type 1 E4 proteins in vivo and in vitro. Virology. 1989;170:201–213. doi: 10.1016/0042-6822(89)90367-x. [DOI] [PubMed] [Google Scholar]
- 21.Grüssenmeyer T, Scheidtmann K H, Hutchinson M A, Eckhart W, Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci USA. 1985;82:7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Loo Y M, Militello K T, Melendy T. Interactions of the papovavirus DNA replication initiator proteins, bovine papillomavirus type 1 E1 and simian virus 40 large T antigen, with human replication protein A. J Virol. 1999;73:4899–4907. doi: 10.1128/jvi.73.6.4899-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris S F, Botchan M R. Crystal structure of the human papillomavirus type 18 E2 activation domain. Science. 1999;284:1673–1677. doi: 10.1126/science.284.5420.1673. [DOI] [PubMed] [Google Scholar]
- 24.Hegde R S, Androphy E J. Crystal structure of the E2 DNA-binding domain from human papillomavirus type 16: implications for its DNA binding-site selection mechanism. J Mol Biol. 1998;284:1479–1489. doi: 10.1006/jmbi.1998.2260. [DOI] [PubMed] [Google Scholar]
- 25.Hegde R S, Wang A F, Kim S S, Schapira M. Subunit rearrangement accompanies sequence-specific DNA binding by the bovine papillomavirus-1 E2 protein. J Mol Biol. 1998;276:797–808. doi: 10.1006/jmbi.1997.1587. [DOI] [PubMed] [Google Scholar]
- 26.Hirochika H, Hirochika R, Broker T R, Chow L T. Functional mapping of the human papillomavirus type 11 transcriptional enhancer and its interaction with the trans-acting E2 proteins. Genes Dev. 1988;2:54–67. doi: 10.1101/gad.2.1.54. [DOI] [PubMed] [Google Scholar]
- 27.Hubbert N L, Schiller J T, Lowy D R, Androphy E J. Bovine papilloma virus-transformed cells contain multiple E2 proteins. Proc Natl Acad Sci USA. 1988;85:5864–5868. doi: 10.1073/pnas.85.16.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilves I, Kivi S, Ustav M. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which is mediated by the viral E2 protein and its binding sites. J Virol. 1999;73:4404–4412. doi: 10.1128/jvi.73.5.4404-4412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jans D A, Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- 30.Kuo S-R, Liu J-S, Broker T R, Chow L T. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J Biol Chem. 1994;269:24058–24065. [PubMed] [Google Scholar]
- 31.Lai M C, Teh B H, Tarn W Y. A human papillomavirus E2 transcriptional activator. The interactions with cellular splicing factors and potential function in pre-mRNA processing. J Biol Chem. 1999;274:11832–11841. doi: 10.1074/jbc.274.17.11832. [DOI] [PubMed] [Google Scholar]
- 32.Lambert P F, Hubbert N L, Howley P M, Schiller J T. Genetic assignment of multiple E2 gene products in bovine papillomavirus-transformed cells. J Virol. 1989;63:3151–3154. doi: 10.1128/jvi.63.7.3151-3154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen A K, Skladanowski A, Bojanowski K. The roles of DNA topoisomerase II during the cell cycle. Prog Cell Cycle Res. 1996;2:229–239. doi: 10.1007/978-1-4615-5873-6_22. [DOI] [PubMed] [Google Scholar]
- 34.Lee K-Y, Broker T R, Chow L T. Transcription factor YY1 represses cell-free replication from human papillomavirus origins. J Virol. 1998;72:4911–4917. doi: 10.1128/jvi.72.6.4911-4917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehman C W, Botchan M R. Segregation of viral plasmids depends on tethering to chromosomes and is regulated by phosphorylation. Proc Natl Acad Sci USA. 1998;95:4338–4343. doi: 10.1073/pnas.95.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Botchan M R. Acidic transcription factors alleviate nucleosome-mediated repression of DNA replication of bovine papillomavirus type 1. Proc Natl Acad Sci USA. 1994;91:7051–7055. doi: 10.1073/pnas.91.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim D A, Gossen M, Lehman C W, Botchan M R. Competition for DNA binding sites between the short and long forms of E2 dimers underlies repression in bovine papillomavirus type 1 DNA replication control. J Virol. 1998;72:1931–1940. doi: 10.1128/jvi.72.3.1931-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin, B. Y., T. Ma, J.-S. Liu, S.-R. Kuo, G. Jin, T. R. Broker, J. W. Harper, and L. T. Chow. HeLa cells are phenotypically limiting in cyclin E/cdk2 for efficient human papillomavirus DNA replication. J. Biol. Chem., in press. [DOI] [PubMed]
- 39.Liu J-S, Kuo S-R, Broker T R, Chow L T. The functions of human papillomavirus type 11 E1, E2, and E2C proteins in cell-free DNA replication. J Biol Chem. 1995;270:27283–27291. doi: 10.1074/jbc.270.45.27283. [DOI] [PubMed] [Google Scholar]
- 40.Lusky M, Hurwitz J, Seo Y-S. The bovine papillomavirus E2 protein modulates the assembly of but is not stably maintained in a replication-competent multimeric E1-replication origin complex. Proc Natl Acad Sci USA. 1994;91:8895–8899. doi: 10.1073/pnas.91.19.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma T, Zou N, Lin B-Y, Chow L T, Harper J W. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc Natl Acad Sci USA. 1999;96:382–387. doi: 10.1073/pnas.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martelli A M, Capitani S, Neri L M. Prereplicative increase of nuclear matrix-bound DNA polymerase-alpha and primase activities in HeLa S3 cells following dilution of long-term cultures. J Cell Biochem. 1998;71:11–20. doi: 10.1002/(sici)1097-4644(19981001)71:1<11::aid-jcb2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Masterson P J, Stanley M A, Lewis A P, Romanos M A. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J Virol. 1998;72:7407–7419. doi: 10.1128/jvi.72.9.7407-7419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maul G G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.McBride A A, Howley P M. Bovine papillomavirus with a mutation in the E2 serine 301 phosphorylation site replicates at a high copy number. J Virol. 1991;65:6528–6534. doi: 10.1128/jvi.65.12.6528-6534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeil S, Guo B, Stein J L, Lian J B, Bushmeyer S, Seto E, Atchison M L, Penman S, van Wijnen A J, Stein G S. Targeting of the YY1 transcription factor to the nucleolus and the nuclear matrix in situ: the C-terminus is a principal determinant for nuclear trafficking. J Cell Biochem. 1998;68:500–510. [PubMed] [Google Scholar]
- 47.Murti K G, He D C, Brinkley B R, Scott R, Lee S H. Dynamics of human replication protein A subunit distribution and partitioning in the cell cycle. Exp Cell Res. 1996;223:279–289. doi: 10.1006/excr.1996.0083. [DOI] [PubMed] [Google Scholar]
- 48.Nasseri M, Hirochika R, Broker T R, Chow L T. A human papilloma virus type 11 transcript encoding an E1∧E4 protein. Virology. 1987;159:433–439. doi: 10.1016/0042-6822(87)90482-x. [DOI] [PubMed] [Google Scholar]
- 49.Park P, Copeland W, Yang L, Wang T, Botchan M R, Mohr I J. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc Natl Acad Sci USA. 1994;91:8700–8704. doi: 10.1073/pnas.91.18.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker J N, Zhao W, Askins K J, Broker T R, Chow L T. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 1997;8:751–762. [PubMed] [Google Scholar]
- 51.Pederson T. Thinking about a nuclear matrix. J Mol Biol. 1998;277:147–159. doi: 10.1006/jmbi.1997.1618. [DOI] [PubMed] [Google Scholar]
- 52.Roberts S, Ashmole I, Rookes S M, Gallimore P H. Mutational analysis of the human papillomavirus type 16 E1-E4 protein shows that the C terminus is dispensable for keratin cytoskeleton association but is involved in inducing disruption of the keratin filaments. J Virol. 1997;71:3554–3562. doi: 10.1128/jvi.71.5.3554-3562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rotenberg M O, Chow L T, Broker T R. Characterization of rare human papillomavirus type 11 mRNAs coding for regulatory and structural proteins, using the polymerase chain reaction. Virology. 1989;172:489–497. doi: 10.1016/0042-6822(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 54.Sanders C M, Stern P L, Maitland N J. Characterization of human papillomavirus type 16 E2 protein and subdomains expressed in insect cells. Virology. 1995;211:418–433. doi: 10.1006/viro.1995.1424. [DOI] [PubMed] [Google Scholar]
- 55.Seo Y-S, Müller F, Lusky M, Gibb E, Kim H Y, Phillips B, Hurwitz J. Bovine papilloma virus (BPV)-encoded E2 protein enhances binding of E1 protein to the BPV replication origin. Proc Natl Acad Sci USA. 1993;90:2865–2869. doi: 10.1073/pnas.90.7.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silver P A. How proteins enter the nucleus. Cell. 1991;64:489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- 57.Skiadopoulos M H, McBride A A. The bovine papillomavirus type 1 E2 transactivator and repressor proteins use different nuclear localization signals. J Virol. 1996;70:1117–1124. doi: 10.1128/jvi.70.2.1117-1124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skiadopoulos M H, McBride A A. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J Virol. 1998;72:2079–2088. doi: 10.1128/jvi.72.3.2079-2088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spalholz B A, Yang Y C, Howley P M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985;42:183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- 60.Stenlund A. Papillomavirus DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 679–697. [Google Scholar]
- 61.Swindle C S, Zou N, Van Tine B A, Shaw G M, Engler J A, Chow L T. Human papillomavirus DNA replication compartments in a transient DNA replication system. J Virol. 1999;73:1001–1009. doi: 10.1128/jvi.73.2.1001-1009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan S H, Leong L E, Walker P A, Bernard H U. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J Virol. 1994;68:6411–6420. doi: 10.1128/jvi.68.10.6411-6420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thierry F, Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987;6:3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Wijnen A J, Bidwell J P, Fey E G, Penman S, Lian J B, Stein J L, Stein G S. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993;32:8397–8402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]
- 66.Yang L, Li R, Mohr I J, Clark R, Botchan M R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991;353:628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]
- 67.Zhao W, Broker T R, Chow L T. Transcriptional activities of human papillomavirus type 11 promoter-proximal elements in raft and submerged cultures of foreskin keratinocytes. J Virol. 1997;71:8832–8840. doi: 10.1128/jvi.71.11.8832-8840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao W, Chow L T, Broker T R. A distal element in the HPV-11 upstream regulatory region contributes to promoter repression in basal keratinocytes of squamous epithelium. Virology. 1999;253:219–229. doi: 10.1006/viro.1998.9478. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Q L, Smith T F, Lefkowitz E J, Chow L T, Broker T R. Nucleic acid and protein sequence alignments of human and animal papillomaviruses constrained by functional sites. University of Alabama at Birmingham; 1994. [Google Scholar]
- 70.Zou N, Liu J-S, Kuo S-R, Broker T R, Chow L T. The carboxyl-terminal region of the human papillomavirus type 16 E1 protein determines E2 protein specificity during DNA replication. J Virol. 1998;72:3436–3441. doi: 10.1128/jvi.72.4.3436-3441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]