Abstract
Diabetes may impact physical and psychosocial well-being; the diabetes incidence has seen a drastic increase globally. There is also a rise in poor mental health and well-being in children with and without chronic illness; problems are being seen at a younger age. The objective of this review was to understand the determinants of these problems in a family context. We conducted a systematic review to investigate what lifestyle and psychological factors influence children with Type 1 diabetes and their parents. A focused literature search was performed using a combination of keywords that covered the relevant terminology for diabetes, target population, and associated emotional distress, using electronic bibliographic databases containing publications until May 2022. Methodological quality was assessed using the Quality Assessment Tools for Quantitative Studies. Twenty articles met the inclusion criteria. Quality scores were weak because of a lack of comparison groups, information about the type of therapy, or adequate sample sizes. Many of the studies included a wide age range in their sample. The majority of the studies reported that parents and their children showed depression symptoms, fear of hypoglycaemia, and higher parenting stress. We conclude that sufficiently powered studies employing appropriate control groups and measures are needed to elucidate the psychological variables associated with Type1 diabetes in children and the effects on parents, especially considering primary-age children who are increasingly reported to suffer from poor mental health, and its implications. This should help to introduce better targeted interventions and improve behavioural outcomes.
Keywords: Type1 diabetes, systematic review, children and parents, anxiety, depression
Introduction
Diabetes mellitus Type 1, commonly known as Type 1 diabetes, is caused by the destruction of islet ß cells in the pancreas, usually leading to absolute insulin deficiency (Donath et al., 2003). Over the years, diabetes has become a major public health concern globally, affecting not only people with diagnosis, but also their families and caregivers. According to the available literature, this disease is becoming more common in children and their parents (Saraswathi et al., 2019). Anxiety, anger, and depression are common emotions experienced by children and their families upon receiving the diagnosis (Diabetes and Emotions, 2017). In one longitudinal study, primary school-age children with diabetes reported anxiety and mild depression, which resolved 6 months after diagnosis; while depression symptoms increased after one to 2 years, anxiety decreased only for boys, while it increased for girls over the first 6 years (Silverstein et al., 2005). In this situation, children with diabetes may perceive that they are different from their peers and may be at risk for difficulties in social competence. Type 1 diabetes in children can be intense and may lead to behaviour-related disease management problems such as anxiety, depression, social anxiety, and lower self-esteem. Diagnosis often leads to worry and stress-related responses regarding the complex care plan that needs to be adhered to by the patient and delivered by the caregivers (Silverstein et al., 2005). For example, a child with diabetes may potentially be anxious about how their condition will develop in the future, be fearful of leaving their house or communicating with others, and be prone to avoid social interactions with others (Diabetes and Anxiety, 2017). It may also affect the household in numerous ways; financially, socially, and emotionally (McCarthy & Kushner, 2007). Therefore, it is imperative for families to learn management and coping with diabetes, and the effects that the disease might have on their children’s life-span development (including normal peer relationships) as early as possible. Yet there are few published studies regarding this situation, especially among younger-aged children (e.g., 8–11 years), who are increasingly likely to report poor mental health, even in the absence of chronic illness (Silverstein et al., 2005).
Coping with behavioural changes as a result of the disease can be challenging for both children and families (Calentine & Porter, 2012). Considering the effects of diabetes diagnosis and illness, it is expected that children’s behaviour will have an effect on their diet, education, and lifestyle. If families are not aware of the risks, the situation may become more difficult to manage and control in the future for both parents and children.
The area of diabetes and depression in children and adolescents has not been researched extensively. Children with diabetes have a two-fold higher prevalence of depression, and adolescents have a three-fold higher prevalence than their non-diabetic peers (Grey et al., 2002). The combination of diabetes and depression is influenced by many variables, including gender, family behaviours, and poorer metabolic control. Diabetes and depression co-morbidity is a significant issue in children and adolescents, affecting an estimated 20% of diabetic individuals, compared to less than 7% of youth without diabetes (Grey et al., 2002). This presents the risk of disability, and negative long-term consequences. Therefore, health practitioners need to pay attention to the emotional functioning and family functioning of children with diabetes, as diabetes can cause a significant impact on families and caregivers in terms of providing support and promoting a healthy family environment (Hood et al., 2006).
Lowes et al. (2015) conducted a qualitative study aimed to explore the experience of attending paediatric diabetes services and living with and managing Type 1 diabetes. They recruited children aged 7–15 years old and their parents. Most parents reported that attending the clinic was a source of anxiety. For example, one carer said, “I often feel stressed up to about a week before I go to clinic. I worry about what my son’s HbA1c results will be.” Children also worried about attending the diabetes clinic and reported that their experience is often represented negatively. Some parents reported feeling exhausted as a result of the responsibility of caring for their child. Children expressed their feelings of fear, unhappiness, anger or distress about the presence of Type 1 diabetes in their lives. For example, one child told the researchers, “I don’t like having diabetes. I don’t like injections (insulin). I don’t like going out with other people for the day cos they don’t understand diabetes. My friends sometimes say they don’t like me because I have diabetes so I feel sad.” The findings of this study also showed that paediatric diabetes nurses interacted with patients and families in a more compassionate manner than medical professionals. A mother said, “Feeling confident when going to clinic and speaking to members of the team is crucial in the learning process and enables you to ask questions as often as you need to! Parents with diabetic children have lots of questions and fears!” (Lowes et al., 2015). Similarly, Hawthorne et al. (2011) found that children with Type 1 diabetes and their parents or careers believe that doctors struggle to link the demands of diabetes with daily life, such as school and social activities, in their consultations, and to consider the emotional impact of living with Type 1 diabetes. However, it is well documented that most parents are likely to experience significant distress (e.g., anxiety and depression symptoms) after their child is diagnosed with Type 1 diabetes (Kokkonen et al., 1997). Kovacs et al. (1997) and Jaser et al. (2008) reported that increases in parental distress have been linked to higher levels of child distress; maternal depressive symptoms are one of the most powerful risk factors for depressive symptoms and a lower quality of life in children. Ongoing parental involvement in treatment management is linked to improved health and psychosocial outcomes in children with Type 1 diabetes (Anderson et al., 2002). Furthermore, observational research may shed light on specific aspects of parent-child interactions that influence diabetes adaptation. One study found that higher levels of observed emotional support, acceptance, and conflict resolution in children and their parents, as well as lower levels of observed parent anger and sadness, were related to better glycaemic control during a diabetes-related task (Martin et al., 1998). Another study discovered that higher levels of observed hostility by mothers, as well as lower levels of child-centered behaviour and positive reinforcement, were associated with poorer psychosocial adjustment and glycaemic control in adolescents (Jaser and Grey, 2010). A higher frequency of negative parent-child interactions has also been linked to a lower quality of life (Weissberg-Benchell et al., 2009). This intense level of responsibility is likely to increase family stress and conflict, especially as children reach adolescence, which is unique to Type 1 diabetes.
Overall, the relationship between psychological variables and lifestyle in children with Type 1 diabetes and their parents at primary age has not been reviewed in the existing literature. We conducted a systematic review of quantitative studies to investigate what lifestyle and psychological variables influence children with Type 1 diabetes at primary age and their parents.
Method
Search process
A literature search was carried out in five databases using a web browser: ProQuest; Science Direct, Web of Science, Google Scholar, via Bangor University Library. This search reviewed scientific and electronic literature without employing a specific set of years, until May 2022. The report follows PRISMA guidelines for systematic review. The terms used in the search as keywords or phrases to describe the target population were in the English language: (childhood diabetes), (children with diabetes), (diabetes mellitus in children), (children with Type 1 diabetes), and (parenting and Type 1 diabetes in children). Psychological variables were also used in the search: (psychological status of children with Type 1 diabetes), (psychological problems in children with diabetes), (depression in children with diabetes), and (psychological problems in parents of children with Type 1). Due to the number of different constructs investigated across the papers, a meta-analysis of studies was not conducted.
Literature search selection
Two reviewers independently screened titles, abstracts, and full texts to determine article eligibility. Any disagreements were settled through discussion. The titles and abstracts were read to choose studies to be included in the present review. We considered cohort studies, randomised and non-randomised controlled trials, cross-sectional studies, and case-control (observational) studies including the following criteria: (1) they reported children with Type 1 diabetes and their parents; (2) included in the sample primary school age range; (3) included parents of children with diabetes; (4) included psychological variables or examined lifestyle and physical activity. Studies were eliminated if: (1) the sample were over 11 years of age; (2) results were neuropsychology based; (3) interventions were used without targeting both children and their parents; (4) results solely reported memory problems and cognitive behaviour; (5) sample did not include children with Type 1 diabetes and their parents. The papers published in the past 2 years on the effects of the COVID pandemic mostly fell in the last category; some relevant results have been reported for children, or for parents, but not for both.
Data extraction and quality assessment tool for quantitative studies
We extracted the following data; first author, year of publication, participants’ characteristics, measurements used in the study, analysis, main finding, therapy type (pump or daily injection), and HbA1c.
The Effective Public Health Practice Project (EPHPP) generic tool was used to assess all selected studies in this review (Quality assessment tool for quantitative studies, 2003). The EPHPP tool was chosen for its inclusiveness of a variety of research study designs, not restricting to only Randomised Controlled Trials (RCTs), but also considering nonrandomised studies. This tool has been shown to have good content and construct validity (Quality assessment tool for quantitative studies, 2003). The components of the study methodology were assessed across six key domains: selection bias, study design, confounding variables, blinding, data collection methods, and withdrawals and dropouts. Following the guidelines for the quality assessment tool, each domain was rated as either strong, moderate, or weak, and scores were collated to provide the total score (Quality assessment tool for quantitative studies, 2003; Thomas et al., 2004). Based on the final scores, those with no weak ratings and at least four strong ratings were considered strong; less than four strong ratings and one weak rating were considered moderate, and those with two or more weak ratings are considered weak (Thomas et al., 2004).
Results
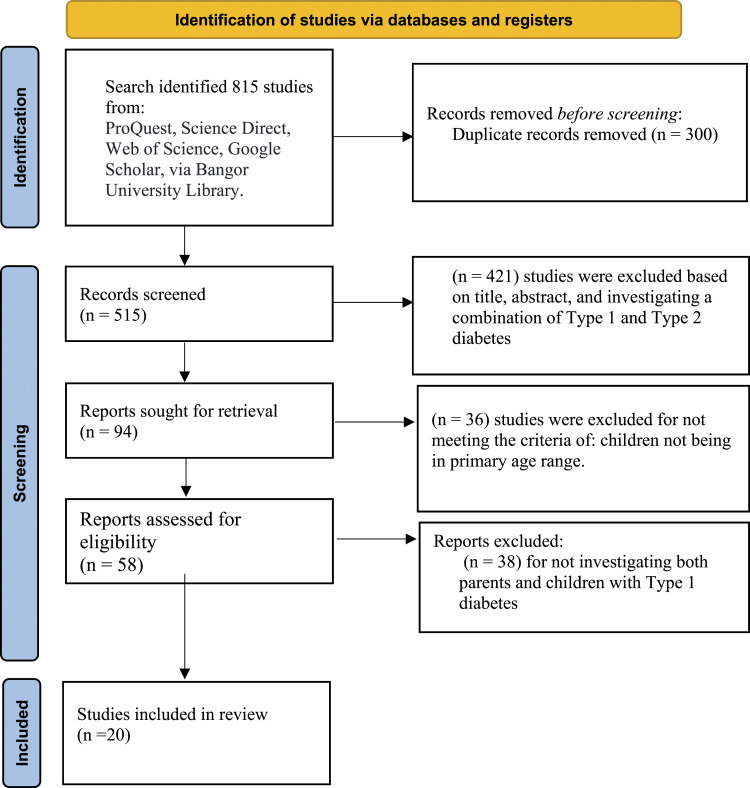
Out of the 815 studies gathered by the search, 300 were removed due to duplicate records, and 421 were excluded based on the title, abstract, and combination of Type 1 and Type 2 diabetes. Out of the remaining 94 studies, 36 were excluded because children were not in the primary age range; 38 studies out of 58 were excluded for not investigating both parents and children with Type 1 diabetes. Therefore, the total number of studies included in the final review was 20. Figure 1 summarises the selection procedure.
Figure 1.
Flow diagram of literature search and study selection.
Results of quality assessment tool for quantitative studies of included studies
The overall quality scores, shown in Table 1 indicated that 16 studies were rated as weak, 3 studies were rated as moderate, and 1 study was rated as strong. Regarding the selection bias, 12 studies were rated as moderate, because participants were referred from a clinic and the participating percentages were 60%–79%. Only six studies were rated weak because the participation was less than 60% of the total number. In accordance with the QATQS criteria on study design, 18 studies were rated as weak because they used cross-sectional designs, and only the two randomised control trial studies were rated as strong (Armstrong et al., 2011; Sweenie et al., 2014). Eighteen studies were rated as strong in the confounders domain because there was no difference between groups, whiles 2 studies were rated as weak. With respect to their data collection methods, 17 studies were rated as strong due to the clarity, validity, and reliability of their data collection tools, and the remaining 3 studies were rated as weak. Regarding blinding, 14 studies were rated as weak due to the participant’s knowledge of the research question, while 6 studies were rated as strong. 15 studies were rated as weak in the withdraw and drop-out domain, while 3 studies were moderate and only 2 studies were rated as strong, due to retention greater than 80%.
Table 1.
Quality assessment according to EPHPP assessment tool for quantitative studies.
| Authors in alphabetical order | Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and dropouts | Overall quality score |
|---|---|---|---|---|---|---|---|
| Armstrong et al. (2011) | M | S | S | W | S | M | M |
| Barzel & Reid (2011) | W | W | S | W | S | W | W |
| Cohen et al. (2004) | W | W | W | S | W | W | W |
| Feeley et al., (2019) | M | W | S | S | S | S | M |
| Gruhn et al. (2016) | M | W | S | W | S | W | W |
| Jaser et al. (2008) | W | W | S | W | S | W | W |
| Jaser and Grey (2010) | M | W | S | W | S | W | W |
| Jaser et al. (2014) | W | W | S | W | S | W | W |
| Jabbour et al., (2016) | M | W | S | S | S | W | W |
| Jaser et al. (2017) | M | W | S | W | S | W | W |
| Michaud et al. (2017) | M | W | W | W | S | M | W |
| Moreira et al. (2014) | M | W | S | S | S | S | M |
| Mullins et al. (2004) | W | W | S | W | S | W | W |
| Patton et al. (2011) | W | W | S | W | S | W | W |
| Sweenie et al. (2014) | M | S | S | S | S | M | S |
| Troncone et al. (2017) | W | W | S | W | W | W | W |
| Viaene et al. (2017) | M | W | S | W | W | W | W |
| Van Gampelaere et al. (2020) | M | W | S | W | S | W | W |
| Whittemore et al. (2003) | W | W | S | W | S | W | W |
| Wilson et al. (2009) | M | W | S | S | S | W | W |
Note. Studies were rated as strong (S), moderate (M), or weak (W) on each specified dimension.
Study characteristics
Table 2 shows the summary of the finding and the characteristics of the studies included in the present review. These clinical trials were performed in several countries, including the United States of America (USA), Italy, Canada, Portugal, and Belgium. Most of the studies were conducted without a comparison group; only one study had a comparison group. Seven studies did not specify the therapy type of the participants. Regarding the haemoglobin (HbA1C) measurements, seven studies specified the mean of the HbA1C, nine studies reported the range of HbA1C, and four studies did not report this measure. All the studies included in this systematic review used self-report questionnaires for both children and their parents. Their data analysis was conducted using IBM SPSS Statistics, and most of the studies were correlational in design.
Table 2.
Characteristics of the 20 studies included in the present review and their main results.
| Author | Country | Sample | Measurement | Analysis | Main results | Therapy type | HbA1c | ||
|---|---|---|---|---|---|---|---|---|---|
| Armstrong et al. (2011) | USA | Children with diabetes (N = 84) and their parents. Age 9–11 years. | - Diabetes family behaviour checklist | Correlation. | Children who reported critical parenting behaviours tended to have lower self-efficacy and more depressive symptoms. | Pump-daily injection | 8.1% | ||
| - Self-efficacy for diabetes questionnaire | |||||||||
| - Child depression inventory | |||||||||
| - Child version of the self-care inventory | |||||||||
| Barzel & Reid (2011) | Canada | Children with diabetes (N = 61) and their parents. Age 8–12 years. | - 14-Item Co-parenting questionnaire | Correlation | Co-parenting conflicts were observed whenever children internalised or externalised their problems. | - | 8.1% | ||
| - 14 items on the diabetes-specific Co- parenting questionnaire | |||||||||
| - Child behaviour checklist | |||||||||
| - Self-care inventory | |||||||||
| - Pediatric quality of life inventory diabetes module | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Cohen et al. (2004) | USA | Children with diabetes (N = 116) and their parents. Age 6–17 years | - Child behaviour checklist | Correlations, ancova and multivariate Analysis | High levels of family cohesion predicted good adherence and better control of glycaemia. | - | 4.8–17.9% | ||
| - Family adaptability and cohesion | |||||||||
| Evaluation scales | |||||||||
| - Adherence measures from medical chart | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Feeley et al. (2019) | USA | Children with diabetes (N = 18) and their parents. Age 6–12 years | -Pediatric fatigue short form | Correlations | Parents showed poor sleep quality in pittsburgh sleep quality. There was a significant correlation between children sleep and parent as measured by actigraphy | - | 7.52 ± 0.75 | ||
| -PROMIS pediatric anxiety short form | |||||||||
| - Pittsburgh sleep quality index | |||||||||
| - PROMIS sleep disturbance short form | |||||||||
| - Perceived stress scale | |||||||||
| - Center for epidemiological studies depression scale | |||||||||
| - Sleep diary | |||||||||
| - Actigraph sleep measure | |||||||||
| Gruhn et al. (2016) | USA | Children with diabetes (N = 93) and their mother. Age 10–16 years. | - Iowa family interaction rating scales | t-test, correlations, and regression Analyses. | Lower HbA1c counts were related to higher levels of collaborative parenting. Significantly greater child depressive symptoms after 1 year were linked to higher levels of observed overinvolved parenting. | Pump-daily injection | 5.4–12.9% | ||
| - Child depression inventory | |||||||||
| - Centre for epidemiologic studies of depression scale | |||||||||
| - Responses to stress questionnaire | |||||||||
| - State trait anxiety inventory | |||||||||
| - Glycaemia and blood measurement HbA1c. | |||||||||
| Jaser et al. (2008) | USA | Children with diabetes (N = 108) and their mother. Age 8–12 years. | - Children’s depression inventory | Linear Regression analyses and correlations. | There was a correlation between the maternal and depressive symptoms of the children | Pump-daily injection | 7.0% | ||
| - Issues in coping with IDDM—child scale | |||||||||
| - Diabetes quality of life scale for youth | |||||||||
| - Diabetes family behaviour scale | |||||||||
| - Center for epidemiologic depression scale. | |||||||||
| - Diabetes | |||||||||
| - Responsibility and conflict scale | |||||||||
| - Family adaptability and cohesion scale | |||||||||
| - Glycaemia and blood measurement HbA1c. | |||||||||
| Jaser and Grey (2010) | USA | Children with diabetes (N = 30) and their mother. Age 10–16 years. | - Responsibility and conflict scale | Correlation | Mothers’ symptoms of depression and anxiety were linked to a low level of child-centred parenting. | Pump-daily injection | 5.5–13.4% | ||
| - Iowa family interaction rating scales | |||||||||
| - Center for epidemiologic studies of depression scale | |||||||||
| - State trait anxiety inventory | |||||||||
| - Child depression inventory | |||||||||
| - Paediatric quality of life inventory | |||||||||
| - Glycaemia and blood measurement HbA1c. | |||||||||
| Jaser et al. (2014) | USA | Children with diabetes (N = 118) and their parents. Age 10–16 years. | - Responsibility and conflict scale | Anova and linear regression analyses. | - Secondary control coping interposed in the relationship between depression, maternal symptoms of anxiety, and diabetes related stress. There was no significant association between children’s outcomes and maternal coping. | Pump-daily injection | 5.4–12.9% | ||
| - Centre for epidemiologic studies depression scale | |||||||||
| - State trait anxiety inventory | |||||||||
| - Paediatric quality of life inventory | |||||||||
| - Child depression inventory | |||||||||
| - Haemoglobin HbA1c | |||||||||
| Jabbour et al., (2016) | Canada | Children with diabetes (N = 201) and their parents. Age two groups; younger than 12 years, 12 years of age or older | - Barriers to physical activity in type 1 diabetes scale | Correlations and 2-way analysis of variance. | - Fear of hypoglycaemia, external temperature, work schedule, and loss of control of diabetes had the highest barrier scores among children younger than 12 years. The lower barrier scores were associated with greater parental support for both younger and older children. | - | - | ||
| Jaser et al. (2017) | USA | Children with diabetes (N = 515) and their parents. Age 2 – 12 years. | - Child sleep habits questionnaire | Separate multivariable linear Regression and separate multivariable logistic regression. | Poor glycaemic control was related to the child’s sleep quality. Poorer sleep quality in children was associated with parental well-being; fear of hypoglycaemia; and poorer parental sleep quality. | Pump-daily injection | 7.8 ± 0.9% | ||
| - Pittsburgh sleep quality index | |||||||||
| - Self-reported HbA1c values | |||||||||
| Michaud et al. (2017) | Canada | Children with diabetes (N = 188) and their parents. Age 6–17 years | - Barriers to physical activity in type 1 diabetes scale | Chi-square, Mann–Whitney-Wilcoxon tests, and linear Regression. | The relations between the components were not significant. The hypoglycaemia phobia was the only barrier to physical activity. | Pump-daily injection | - | ||
| - Health measures survey | |||||||||
| - World health organization norms on metabolic equivalent task | |||||||||
| - Parents own PA habits | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Moreira et al. (2014) | Portugal | Children with diabetes (N = 88) and their parents. Children without diabetes (N= 121) and their parents. Age 8–18 years. | - Paediatric health-related | Anova and two-way manova. | Higher levels of cohesion were linked to higher HRQOL ratings in children with diabetes and lower scores of parental stress. Parents of children with diabetes felt more stress, anxiety, and perceived less cohesion compared to parents of healthy children. | - | 7.9% | ||
| Quality of life measurement | |||||||||
| - Self-report version of the DISABKIDS chronic | |||||||||
| Generic module | |||||||||
| - Family environment scale | |||||||||
| - Hospital anxiety and depression scale | |||||||||
| - Portuguese version of the | |||||||||
| Parenting stress index—short form | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Mullins et al. (2004) | USA | Children with diabetes (N = 43) and their parents. Age 8–12 years. | - Single 7- point likert scale | Correlations and multiple Regression. | High levels of depressive symptoms were related to two factors, child vulnerability and parenting stress. | - | 5–14% | ||
| - Parent protection scale | |||||||||
| - Child vulnerability scale | |||||||||
| - Parenting stress scale | |||||||||
| - Child depression inventory | |||||||||
| Patton et al. (2011) | USA | Children with diabetes (N = 39) and their parents. Age 0–7 years | - Behavioural paediatric feeding assessment scale | Correlations, and linear regression analyses. | Higher parenting stress was associated with a higher stress frequency, higher depressive symptoms, fear of hypoglycaemia, and greater mealtime issues. | Pump-daily injection | 8.6 ± 1.3% | ||
| - Paediatric inventory for parents | |||||||||
| - Hypoglycaemia fear survey-parents of young children | |||||||||
| - Beck depression inventory-second edition | |||||||||
| Sweenie et al. (2014) | USA | Children with diabetes (N = 86) and their parents. Age 9–11 years. | - The eyberg child behaviour inventory | Hierarchical linear regressions. | Parents, who reported their child’s psychological behaviour as more problematic, also stated more difficulty with paediatric parenting stress. | Pump-daily injection | 8.1% | ||
| - Diabetes family behaviour checklist | |||||||||
| - Paediatric inventory for parents | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Troncone et al. (2017) | Italy | Children with diabetes (N = 25) and their parents. Age 1–18 years. | - Patient’s health-related quality of life 3.0 DM | Anova. | - The patients were found to have lower global diabetes-specific problems and better experience. | Daily injection | 5.7–9.7% | ||
| - Parent’s perceived burden | - Parents described the treatment to be linked to treatment satisfaction. | ||||||||
| Viaene et al. (2017) | Belgium | Children with diabetes (N = 63) and their parents. Age 2–18 years. | - The Nijmegen parenting stress index-short form | Correlation, and mancova. | Results showed an indirect association between HbA1c values and parental FoH through parenting stress. | Pump-daily injection | 8.2% | ||
| - Parent’s fear of hypoglycaemia scale | |||||||||
| - Children’s fear of hypoglycaemia scale | |||||||||
| - Glycaemia and blood measurement HbA1c | |||||||||
| Van Gampelaere et al. (2020) | Belgium | Children with diabetes (N = 105) and their parents. Age 2–12 years Children without diabetes (N = 414) and their parents. | - Child quality of life/Quality of life Inventory-4.0 | Ancova | Children with type 1 diabetes (8–12 years) had higher quality of life compared with children without diabetes. Mothers of children with type 1 diabetes showed more anxiety and depressive stress than their counterparts with children without diabetes. | Pump-daily injection | <7.5%(78) >7.5%(27) | ||
| - Strengths and difficulties questionnaire | |||||||||
| - Perceived stress scale | |||||||||
| - The patient-reported outcomes measurement information system (PROMIS) for anxiety and depression] | |||||||||
| - Parental overprotection measure | |||||||||
| - The autonomy support scale | |||||||||
| Whittemore et al. (2003) | USA | Children with diabetes (N = 56) and their parents. Age 8–12 years. | - Diabetes quality of life youth | Correlations, and regression Analyses. | Families who found coping with diabetes less upsetting had children who reported a better quality of life. Children with diabetes type 1 who experienced a better quality of life reported fewer depression symptoms. | Pump-daily injection | 7.4% | ||
| - Child depression inventory | |||||||||
| - Issue in coping with IDDM- child version scale | |||||||||
| - The diabetes family behaviour scale | |||||||||
| - Centre for epidemiologic studies of depression scale | |||||||||
| - Haemoglobin A1c. | |||||||||
| Wilson et al. (2009) | USA | Children with diabetes (N = 46) and their parents. Age 5–12 years. | - The eyberg child behaviour inventory | Correlations and multiple regression. | Over-reactive parental discipline was associated with common child mealtime misbehaviour, and it was also linked to reports of less time spent managing child’s illness. | - | - | ||
| - Parenting scale | |||||||||
Study findings
Children with diabetes often showed symptoms of depression (Jaser et al., 2008; Whittemore et al., 2003; Mullins et al., 2004; Armstrong et al., 2011). In one of the studies, these depression symptoms related to children’s levels of metabolic control and adherence to medication (Cohen et al., 2004). Researchers reported that anxiety, aggressive behaviour, and attention showed a high relationship to depression (Jaser et al., 2014; Jaser and Grey, 2010; Gruhn et al., 2016). Researchers also reported that physical activity, poor glycaemia, higher stress, worrying, and children’s age were related to fear of hypoglycaemia (Michaud et al., 2017; Jabbour et al., 2016; Patton et al., 2011). Two studies reported that children with diabetes and their parents often showed symptoms of poor sleep quality (Jaser et al., 2017; Feeley et al., 2019). In one of the studies, children with diabetes had lower self-efficacy scores (Armstrong et al., 2011).
Parents of children with diabetes also showed an increased incidence of depression symptoms according to several studies (Patton et al., 2011; Gruhn et al., 2016; Whittemore et al., 2003; Van Gampelaere et al., 2020). Parents of children with diabetes often showed higher parenting stress (Mullins et al., 2004; Moreira et al., 2014; Viaene et al., 2017; Patton et al., 2011; Sweenie et al., 2014). In one of the studies, over-reactive discipline for the parents was linked to reports of less time spent managing the child’s illness (Wilson et al., 2009). In one study, according to parental reports, children with Type 1 diabetes had higher quality of life scores compared with the control group (Van Gampelaere et al., 2020). Children with diabetes and their parents have been shown to suffer from fear of hypoglycaemia (Michaud et al., 2017; Jabbour et al., 2016; Patton et al., 2011; Viaene et al., 2017; Jaser et al., 2017).
Discussion
In this systematic review, 20 research studies reported that children living with Type 1 diabetes suffered from psychological issues such as anxiety, depression, sleep disturbance, and lifestyle adjustment. At present, there is not much empirical evidence to draw inferences on the cause of these psychological issues. However, several studies pointed to the interactions and associations between child and parental variables. For example, it has been shown that both children who have diabetes and their parents suffer from a fear of hypoglycemia (Michaud et al., 2017; Jabbour et al., 2016), an increased incidence of depression (Patton et al., 2011; Gruhn et al., 2016), and often show symptoms of poor sleep quality (Michaud et al., 2017; Jabbour et al., 2016). (Jaser et al., 2017). This would be expected because childhood chronic illness affects parents’ mental health and life quality, while familial variables have been shown to affect child outcomes (Vonneilich et al., 2016). Furthermore, given the consequences of diabetes diagnosis and illness, it is reasonable to expect that children’s behaviour will influence their diet, education, and lifestyle. If families are not aware of the risks, the situation for both parents and children may become more difficult to manage and control in the future.
With regards to the methodology, the authors of these 20 studies typically did not choose an adequate sample size prior to the examination, thus resulting in low statistical power. Further, 11 studies did not specify the age range of the target population. The examiners combined young children with older youth that can be considered adults in one category (e.g., 6–18 years old). These choices, probably driven by pragmatic concerns, cannot be justified in terms of child development: while older children may have developed their coping skills with the illness, the younger children were possibly at the beginning of developing their coping skills with the diagnosis and illness (Compas et al., 2012). The opposite could also be true, as older children may show a greater appreciation of the long-term problems likely to be caused by their illness and show an increase in anxiety and depression symptoms; for these children, elevated symptoms are more likely to occur (Beardslee et al., 1996). In addition, 18 studies did not have a comparison group, which is an important factor when focusing on the change variable necessary for making meaningful comparisons between the target cohort and the general population (Mingle, 2018). In four studies, the authors did not provide sufficient medical data regarding the type of therapy used by the patients; whether the children used the pump or daily injection, which plays a significant role in understanding their quality of life (Birkebaek et al., 2014).
The weakness and limitations identified in the present review should be considered in future research. More detailed medical and technical data are required when investigating the children’s and their parents' behavioural problems. Getting a better understanding of the correlates of different behavioural outcomes for children diagnosed with Type 1 diabetes is crucial in helping them and their parents minimise the behavioural impacts of the illness. Future research should incorporate lifestyle variables with mental health outcomes because we know that having a healthy lifestyle in early age can reduce the burden of mental health in the future (Loewen et al., 2019). The researchers should consider that young children respond to traumatic events in ways that are different from older children and adults, especially in primary school age (Early Childhood Mental Health, n.d). Establishing determinants of poor outcomes in younger, primary-aged children is essential for our understanding of the aetiology of psychological issues, and for introducing timely and targeted intervention to address the areas of need (e.g., parenting programmes, healthy lifestyle interventions). Increasing the involvement of diabetes educators, utilizing integrated behavioural health care models that consider costs and benefits, and extending the follow-up period are additional recommendations for future research.
Finally, we consider that stressors brought about by the enforced proximity, social isolation, medical care restrictions, and anxiety of the COVID pandemic, may have heightened or altered some of the relationships identified in pre-pandemic research. Quantitative, and qualitative familial studies in this age group will be needed to update our understanding and aid in the implementation of more targeted interventions to improve behavioural outcomes.
Conclusion
The present review has investigated the psychological and lifestyle variables that may impact the health and well-being of primary-age children and their families. Despite the procedural limitations such as the lack of control groups for most of the studies, combining young children with older youth that can be considered adults in one category, and insufficient medical data, we can conclude that children with Type 1 diabetes and their parents are at risk of experiencing a multitude of psychological problems. Lifestyle changes contributing to this may include poorer sleep quality and reduced physical activity.
Author biographies
Afrah Alazmi, PhD School of Human and Behavioural Sciences, Bangor University, United Kingdom.
Masha Boi Bashiru, MsC,Department of Obstetrics and Gynaecology, Korle Bu Teaching Hospital, Ghana.
Simon Viktor, PhD School of Human and Behavioural Sciences, Bangor University, United Kingdom.
Mihela Erjavec, PhD School of Human and Behavioural Sciences, Bangor University, United Kingdom.
Footnotes
Authors’ contributions: AA conducted the review, completed Quality Assessment Tool for Quantitative Studies assessment, processed and analysed data, and drafted the manuscript. MBB assisted with methodology and editing. SV provided methodological and academic guidance. ME supervised the research and co-wrote the final manuscript. All authors have read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ministry of health (MOH) in Kuwait supported research project, in collaboration with Bangor University. None of these funding bodies had a role in the design of this systematic review or in writing the manuscript.
ORCID iD
Afrah Alazmi https://orcid.org/0000-0002-2875-810X
References
- Anderson B. J., Vangsness L., Connell A., Butler D., Goebel‐Fabbri A., Laffel L. M. B. (2002). Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association, 19(8), 635–642. 10.1046/j.1464-5491.2002.00752.x [DOI] [PubMed] [Google Scholar]
- Armstrong B., Mackey E. R., Streisand R. (2011). Parenting behavior, child functioning, and health behaviors in preadolescents with type 1 diabetes. Journal of Pediatric Psychology, 36(9), 1052–1061. 10.1093/jpepsy/jsr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzel M., Reid G. J. (2011). Coparenting in relation to children’s psychosocial and diabetes- specific adjustment. Journal of Pediatric Psychology, 36(5), 618–629. 10.1093/jpepsy/jsr022 [DOI] [PubMed] [Google Scholar]
- Beardslee W. R., Keller M. B., Seifer R., Lavori P. W., Staley J., Podorefsky D., Shera D. (1996). Prediction of adolescent affective disorder: Effects of prior parental affective disorders and child psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry, 35(3), 279–288. 10.1097/00004583-199603000-00008 [DOI] [PubMed] [Google Scholar]
- Birkebaek N. H., Kristensen L. J., Mose A. H., Thastum M., Danish Society for Diabetes in Childhood and Adolescence . (2014). Quality of life in Danish children and adolescents with type 1 diabetes treated with continuous subcutaneous insulin infusion or multiple daily injections.Diabetes Research and Clinical Practice, 106(3), 474–480. 10.1016/j.diabres.2014.09.028 [DOI] [PubMed] [Google Scholar]
- Calentine L., Porter R. (2012). Kids First diabetes second: Tips for parenting a child with Type 1 diabetes. Spry Pub. LLC. [Google Scholar]
- Cohen D. M., Lumley M. A., Naar-King S., Partridge T., Cakan N. (2004). Child behavior problems and family functioning as predictors of adherence and glycemic control in economically disadvantaged children with type 1 diabetes: A prospective study. Journal of Pediatric Psychology, 29(3), 171–184. 10.1093/jpepsy/jsh019 [DOI] [PubMed] [Google Scholar]
- Compas B. E., Jaser S. S., Dunn M. J., Rodriguez E. M. (2012). Coping with chronic illness in childhood and adolescence. Annual Review of Clinical Psychology, 8, 455–480. 10.1146/annurev-clinpsy-032511-143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes.co.uk (2017). Diabetes and anxiety. Retrieved from https://www.diabetes.co.uk/emotions/diabetes-and-anxiety.html. [Google Scholar]
- Diabetes.co.uk (2017). Diabetes and emotions - coping with diabetes. Retrieved from https://www.diabetes.co.uk/emotions/ [Google Scholar]
- Donath M. Y., Størling J., Maedler K., Mandrup-Poulsen T. (2003). Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. Journal of Molecular Medicine, 81(8), 455–470. [DOI] [PubMed] [Google Scholar]
- Early Childhood mental health . (n.d.). Center on the developing child at Harvard university. Retrieved from https://developingchild.harvard.edu/science/deep-dives/mental-health/
- Feeley C. A., Sereika S. M., Chasens E. R., Siminerio L., Charron-Prochownik D., Muzumdar R. H., Viswanathan P. (2019). Sleep in parental caregivers and children with type 1 diabetes. The Journal of School Nursing, 37(4), 259–269. 10.1177/1059840519865942 [DOI] [PubMed] [Google Scholar]
- Grey M., Whittemore R., Tamborlane W. (2002). Depression in type 1 diabetes in children: Natural history and correlates. Journal of Psychosomatic Research, 53(4), 907–911. 10.1016/s0022-3999(02)00312-4 [DOI] [PubMed] [Google Scholar]
- Gruhn M. A., Lord J. H., Jaser S. S. (2016). Collaborative and overinvolved parenting differentially predict outcomes in adolescents with type 1 diabetes. Health Psychology, 35(7), 652–660. 10.1037/hea0000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne K., Bennert K., Lowes L., Channon S., Robling M., Gregory J. W., DEPICTED Study Team . (2011). The experiences of children and their parents in paediatric diabetes services should inform the development of communi- cation skills for healthcare staff (the DEPICTED Study). Diabetic Medicine: A Journal of the British Diabetic Association, 28(9), 1103–1108. 10.1111/j.1464-5491.2011.03292.x [DOI] [PubMed] [Google Scholar]
- Hood K. K., Huestis S., Maher A., Butler D., Volkening L., Laffel L. M. (2006). Depressive symptoms in children and adolescents with type 1 diabetes: Association with diabetes-specific characteristics. Diabetes Care, 29(6), 1389–1391. 10.2337/dc06-0087 [DOI] [PubMed] [Google Scholar]
- Jabbour G., Henderson M., Mathieu M. E. (2016). Barriers to active lifestyles in children with type 1 diabetes. Canadian Journal of Diabetes, 40(2), 170–172. 10.1016/j.jcjd.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Jaser S. S., Foster N. C., Nelson B. A., Kittelsrud J. M., DiMeglio L. A., Quinn M., Willi S. M., Simmons J. H., T1D Exchange Clinic Network . (2017). Sleep in children with type 1 diabetes and their parents in the T1D Exchange. Sleep Medicine, 39(39), 108–115. 10.1016/j.sleep.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S. S., Grey M. (2010). A pilot study of observed parenting and adjustment in adolescents with type 1 diabetes and their mothers. Journal of Pediatric Psychology, 35(7), 738–747. 10.1093/jpepsy/jsp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S. S., Linsky R., Grey M. (2014). Coping and psychological distress in mothers of adolescents with type 1 diabetes. Maternal and Child Health Journal, 18(1), 101–108. 10.1007/s10995-013-1239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S. S., Whittemore R., Ambrosino J. M., Lindemann E., Grey M. (2008). Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychology, 33(5), 509–519. 10.1093/jpepsy/jsm104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkonen J., Taanla A., Kokkonen E. R. (1997). Diabetes in adolescence: The effect of family and psychologic factors on metabolic control. Nordic Journal of Psychiatry, 51(3), 165–172. 10.3109/08039489709109091 [DOI] [Google Scholar]
- Kovacs M., Goldston D., Obrosky D. S., Bonar L. K. (1997). Psychiatric disorders in youths with IDDM: Rates and risk factors. Diabetes Care, 20(1), 36–44. 10.2337/diacare.20.1.36 [DOI] [PubMed] [Google Scholar]
- Loewen O. K., Maximova K., Ekwaru J. P., Faught E. L., Asbridge M., Ohinmaa A., Veugelers P. J. (2019). Lifestyle behavior and mental health in early adolescence. Pediatrics, 143(5), Article e20183307. 10.1542/peds.2018-3307 [DOI] [PubMed] [Google Scholar]
- Lowes L., Eddy D., Channon S., McNamara R., Robling M., Gregory J. W., DEPICTED Study Team . (2015). The experience of living with type 1 diabetes and attending clinic from the perception of children, adolescents and carers: Analysis of qualitative data from the DEPICTED study. Journal of Pediatric Nursing, 30(1), 54–62. 10.1016/j.pedn.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Martin M. T., Miller-Johnson S., Kitzmann K. M., Emery R. E. (1998). Parent–child relationships and insulin-dependent diabetes mellitus: Observational ratings of clinically relevant dimensions. Journal of Family Psychology, 12(1), 102–111. 10.1037/0893-3200.12.1.102 [DOI] [Google Scholar]
- McCarthy M., Kushner J. (2007). The everything parent's guide to children with juvenile diabetes: Reassuring advice for managing symptoms and raising a happy, healthy child. Simon and Schuster. [Google Scholar]
- Michaud I., Henderson M., Legault L., Mathieu M. E. (2017). Physical activity and sedentary behavior levels in children and adolescents with type 1 diabetes using insulin pump or injection therapy–the importance of parental activity profile. Journal of Diabetes and Its Complications, 31(2), 381–386. 10.1016/j.jdiacomp.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Mingle L. (2018). Evaluating a program’s “research base” part 1: Importance of a comparison group. Retrieved from https://ensemblelearning.org/evaluating-a-programs-research-base-part-1-importance-of-a-comparison-group/
- Moreira H., Frontini R., Bullinger M., Canavarro M. C. (2014). Family cohesion and health-related quality of life of children with type 1 diabetes: The mediating role of parental adjustment. Journal of Child and Family Studies, 23(2), 347–359. 10.1007/s10826-013-9758-6 [DOI] [Google Scholar]
- Mullins L. L., Fuemmeler B. F., Hoff A., Chaney J. M., Van Pelt J., Ewing C. A. (2004). The relationship of parental overprotection and perceived child vulnerability to depressive symptomotology in children with type 1 diabetes mellitus: The moderating influence of parenting stress. Children's Health Care, 33(1), 21–34. 10.1207/s15326888chc3301_2 [DOI] [Google Scholar]
- Patton S. R., Dolan L. M., Smith L. B., Thomas I. H., Powers S. W. (2011). Pediatric parenting stress and its relation to depressive symptoms and fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings, 18(4), 345–352. 10.1007/s10880-011-9256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quality assessment tool for quantitative studies . (2020). Effective public health practice project, Retrieved from: http://www.ephpp.ca/tools.html
- Saraswathi S., Al-Khawaga S., Elkum N., Hussain K. (2019). A systematic review of childhood diabetes research in the middle east region. Frontiers in Endocrinology, 10, 805. 10.3389/fendo.2019.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J., Klingensmith G., Copeland K., Plotnick L., Kaufman F., Laffel L., Deeb L., Grey M., Anderson B., Holzmeister L. A., Clark N. (2005). Care of children and adolescents with type 1 diabetes: A statement of the American diabetes association. Diabetes Care, 28(1), 186–212. 10.2337/diacare.28.1.186 [DOI] [PubMed] [Google Scholar]
- Sweenie R., Mackey E. R., Streisand R. (2014). Parent–child relationships in Type 1 diabetes: Associations among child behavior, parenting behavior, and pediatric parenting stress. Families, Systems & Health: The Journal of Collaborative Family Healthcare, 32(1), 31–42. 10.1037/fsh0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. H., Ciliska D., Dobbins M., Micucci S. (2004). A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews on Evidence‐Based Nursing, 1(3), 176–184. 10.1111/j.1524-475X.2004.04006.x [DOI] [PubMed] [Google Scholar]
- Troncone A., Cascella C., Zanfardino A., Chianese A., Confetto S., Giglio M., Villano P., Perrone L., Iafusco D. (2017). Psychological outcomes of injection port therapy in children and adolescents with type 1 diabetes and their primary caregivers. Acta Diabetologica, 54(10), 975–978. 10.1007/s00592-017-1014-x [DOI] [PubMed] [Google Scholar]
- Van Gampelaere C., Luyckx K., van der Straaten S., Laridaen J., Goethals E. R., Casteels K., Vanbesien J., den Brinker M., Depoorter S., Klink D., Cools M., Goubert L., Ghent University . (2020). Families with pediatric type 1 diabetes: A comparison with the general population on child well‐being, parental distress, and parenting behavior. Pediatric Diabetes, 21(2), 395–408. 10.1111/pedi.12942 [DOI] [PubMed] [Google Scholar]
- Viaene A. S., Van Daele T., Bleys D., Faust K., Massa G. G. (2017). Fear of hypoglycemia, parenting stress, and metabolic control for children with type 1 diabetes and their parents. Journal of Clinical Psychology in Medical Settings, 24(1), 74–81. 10.1007/s10880-017-9489-8 [DOI] [PubMed] [Google Scholar]
- Vonneilich N., Lüdecke D., Kofahl C. (2016). The impact of care on family and health-related quality of life of parents with chronically ill and disabled children. Disability and Rehabilitation, 38(8), 761–767. 10.3109/09638288.2015.1060267 [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Nansel T., Holmbeck G., Chen R., Anderson B., Wysocki T., Laffel L., Steering Committee of the Family Management of Diabetes Study . (2009). Generic and diabetes-specific parent–child behaviors and quality of life among youth with type 1 diabetes.Journal of Pediatric Psychology, 34(9), 977–988. 10.1093/jpepsy/jsp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R., Urban A. D., Tamborlane W. V., Grey M. (2003). Quality of life in school-aged children with type 1 diabetes on intensive treatment and their parents. The Diabetes Educator, 29(5), 847–854. 10.1177/014572170302900514 [DOI] [PubMed] [Google Scholar]
- Wilson A. C., DeCourcey W. M., Freeman K. A. (2009). The impact of managing school-aged children’s diabetes: The role of child behavior problems and parental discipline strategies. Journal of Clinical Psychology in Medical Settings, 16(3), 216–222. 10.1007/s10880-009-9163-x [DOI] [PubMed] [Google Scholar]