Abstract
The vaccinia virus B5R type I integral membrane protein accumulates in the Golgi network, from where it becomes incorporated into the envelope of extracellular virions. Our objective was to determine the domains of B5R responsible for Golgi membrane targeting in the absence of other viral components. Fusion of an enhanced green fluorescent protein to the C terminus of B5R allowed imaging of the chimeric protein without altering intracellular trafficking and Golgi network localization in transfected cells. Deletion or swapping of B5R domains with corresponding regions of the vesicular stomatitis virus G protein, which is targeted to the plasma membrane, indicated that (i) the N-terminal extracellular domain of B5R had no specific role in Golgi apparatus localization, (ii) the transmembrane domain of B5R was sufficient for exiting the endoplasmic reticulum, and (iii) removal of the cytoplasmic tail impaired Golgi network localization and increased the accumulation of B5R in the plasma membrane. Further experiments demonstrated that the cytoplasmic tail mediated internalization of B5R from the plasma membrane, suggesting a retrieval mechanism. Mutagenesis revealed residues required for Golgi membrane localization and efficient plasma membrane retrieval of the B5R protein: a tyrosine at residue 310 and two adjacent leucines at residues 315 and 316.
Vaccinia virus replicates in the cytoplasm and produces two related infectious forms: intracellular mature virions (IMV) and extracellular enveloped virions (EEV) (32). EEV arise from IMV that have been wrapped by a trans-Golgi network (TGN) or endosomal cisternae (18, 21, 31, 44, 47). While IMV comprise the majority of progeny virions, they are released only following cell lysis. Cell surface adherent and detached EEV are believed to be largely responsible for cell-to-cell spread and long-range transmission of vaccinia virus (1, 4, 7, 37). Six proteins, encoded by the A33R (42), A34R (12), A36R (36), A56R (38, 45), B5R (13, 22), and F13L (19) open reading frames (ORFs), are known to be EEV specific. When expression of A33R, A34R, A36R, B5R, or F13L was prevented, the size of virus plaques was drastically reduced because of inefficient virus spread (3, 30, 36, 41, 51). F13L and B5R play crucial roles in morphogenesis, as deletion of either inhibited the wrapping of IMV causing decreased EEV production (3, 14, 51). In contrast, deletion or repression of A33R, A34R, or A36R had relatively little effect on EEV formation but decreased the number of actin tails associated with intracellular enveloped virions (IEV) (41, 42a, 52, 53).
While there is considerable information regarding the consequences of EEV gene deletions on morphogenesis and virus spread, little is known about how EEV proteins are targeted to the viral membrane. The 42-kDa type I integral membrane glycoprotein encoded by the B5R ORF (13, 22) was found in the Golgi network when it was expressed in the absence of other viral proteins (24), suggesting that it contains the requisite transport and localization signals. Several studies with infected cells showed that removal or replacement of either the lumenal domain or the cytoplasmic tail had no effect on the incorporation of B5R into EEV (17, 24, 26, 29). Taken together, these results implied that the transmembrane domain of B5R was necessary and sufficient for Golgi membrane localization and EEV targeting. However, no mutant with a deletion of both the lumenal domain and cytoplasmic tail of B5R was examined, leaving open the possibility that redundant targeting signals are present in the lumenal domain and cytoplasmic tail and absent from the transmembrane domain. Furthermore, since all studies with mutated proteins were carried out in the context of permissive virus infections, other viral proteins may have served as chaperones. For these reasons, we thought it important to test the roles of the transmembrane domain and cytoplasmic tail individually and in the absence of other vaccinia virus proteins.
In the present study, we modified and interchanged domains of the B5R and vesicular stomatitis virus glycoprotein (VSVG) and demonstrated that the transmembrane domain of B5R allowed endoplasmic reticulum (ER)-to-Golgi membrane transport and that specific sequences in the cytoplasmic tail of B5R were responsible for its accumulation in the Golgi network and retrieval from the plasma membrane.
MATERIALS AND METHODS
Construction of chimeric proteins.
The following primers, with restriction endonuclease cleavage sites in italics, overlapping nucleotides (nt) for two-stage PCR underlined, and mutations in the B5R coding sequence in bold letters, were used in this study: B5RSacF, CGGAGCTCTGCAACTTATCATATAATC; B5R3′NcoR, GCTTACACCATGGGTAGCAATTTATGGAACT; VSVGctF, TGTTCCTGTCGAGTTGGTATTTATC; B5RtmdR, AAATACCAACTCGACAGGAACAAACTAAT; B5R5′D3F, GAAGCTTCATAAATAAAAATGAAAACG; B5RΔctR, ACCATGGTACAGGAACAAACTAATAC; B5RctF, TTGGTTCTCTGTTCCTGTGACAAAAAT; VSVGtmdR, GGAACAGAGAACCAAGAATAGTCC; B5R310AF, GACCAAGCTAAGTTCCATAAATTGCTA; B5R310AR, GAACTTAGCTTGGTCATTATTTTTGTC; B5RSacR, AGAGCTCTCTAACGATTCTATTTCTTGT; B5RΔLLR, CCATGGGTTTATGGAACTTATATTGGTCATT; VSVGΔctR, ATCCATGGCAATACCAACTCGGAGAACCAA; B5R310A/ΔLLR, ACCATGGGTTTATGGAACTTAGCTTGG.
The cosmid pWR133-168 (46) was digested with ScaI-NsiI and a 2.15-kbp fragment containing B5R was cloned into pGEM-7Zf (Promega) that had been digested with SmaI-NsiI. The resulting plasmid, pBMW-4, contained B5R and approximately 500 bp of flanking sequence on each side. Primers B5R5′D3F and B5R3′NcoR were used to amplify the entire B5R sequence and add a HindIII site 17 bp upstream from the translational start site and an NcoI site immediately in front of the termination codon. PCR products were separated on a 1% low-melting-point agarose (GIBCO) gel, and the appropriately sized fragment was excised, purified by using Wizard PCR preps (Promega), cloned into pGEM-T (Promega), and sequenced. Subsequently, a 970-bp HindIII-NcoI fragment containing the coding sequence for B5R was ligated together with a 734-bp NcoI-XbaI fragment from pEGFP-N1 (Clontech) containing the enhanced green fluorescent protein (GFP) ORF and the expression vector pCDM8 (Invitrogen), which had been linearized with HindIII-XbaI to yield pB5R-GFP. To simplify the nomenclature of the chimeras, each was given a three-letter designation, divided by slashes, corresponding to the three domains of the protein (lumenal/transmembrane/cytoplasmic) and the protein from which it was derived, G for VSVG and B for B5R (Fig. 1). VSVG-GFP (39) was a generous gift from Jennifer Lippincott-Schwartz. Primers B5RSacF and B5R3′NcoR were used to amplify a fragment of B5R containing the transmembrane domain and cytoplasmic tail and add a SacI site at nt 825 and an NcoI site immediately in front of the termination codon. The amplified fragment was cloned into pGEM-T as described above to yield pBMW1-T. To construct G/B/B-GFP, a fragment containing the transmembrane domain and cytoplasmic tail was excised from pBMW1-T by using SacI and NcoI. This fragment was ligated with a 734-bp NcoI-XbaI fragment containing GFP from pEGFP-N1 and VSVG-GFP that had been linearized with XbaI and partially digested with SacI to cleave only the SacI site at nt 1389 of VSVG to yield G/B/B-GFP. To make templates for the construction of other chimeras, the plasmids containing VSVG-GFP and G/B/B-GFP were each digested with PstI-XbaI, and the resulting fragments were cloned into pUC19 that had been digested the same way to yield pBMW-10 and pBMW-3, respectively. Standard two-stage PCR was used with the following primers and template pairs: primer pair B5RctF and M13 forward (Promega) with template pBMW-3 and primer pair VSVGtmdR and M13 reverse (Promega) with template pBMW-10 to construct G/G/B-GFP. After amplification, fragments were separated as before and joined by a third PCR. The resulting amplified fragment was cloned as described before to yield pBMW-5T. A 730-bp PstI-NcoI fragment from G/B/B-GFP was replaced with a 720-bp PstI-NcoI fragment from pBMW-5T to yield G/G/B-GFP. Construct G/B/G-GFP was made in a similar way by using the following primer pairs and templates: primer pair B5RtmdR and M13 reverse (Promega) with template pBMW-3 and primer pair VSVGctF and M13 forward and template pBMW-10 for the initial amplification.
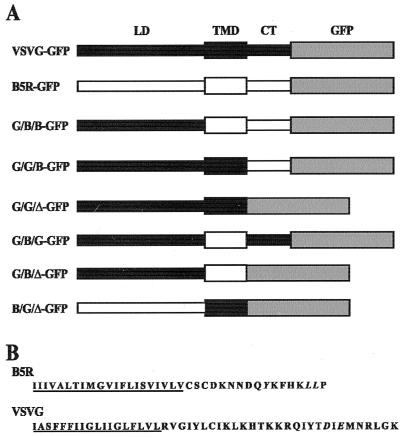
FIG. 1.
VSVG, B5R, and GFP chimeras. (A) Schematic representation of chimeras. VSVG-GFP and B5R-GFP are VSVG and B5R proteins with GFP appended to the cytoplasmic tail, respectively. Chimeras are constructed from the lumenal domain (LD), transmembrane domain (TMD), and cytoplasmic tail (CT) of VSVG (■) or B5R (□), and GFP ( ). ▵, deleted cytoplasmic domain. (B) Amino acid sequences of predicted transmembrane domains (underlined) and cytoplasmic tails of B5R and VSVG. Tyrosine 310 and leucines 315 and 316 of B5R and the diacidic signal of VSVG are shown in italics.
). ▵, deleted cytoplasmic domain. (B) Amino acid sequences of predicted transmembrane domains (underlined) and cytoplasmic tails of B5R and VSVG. Tyrosine 310 and leucines 315 and 316 of B5R and the diacidic signal of VSVG are shown in italics.
To construct G/B/Δ-GFP, primers B5RΔctR and M13 reverse were used to amplify a 703-bp fragment from pBMW-4 that contained an NcoI site at nt 911 of B5R that is in frame with the 5′ NcoI site in pEGFP-N1. Similarly, to construct G/G/Δ-GFP, primers VSVGΔctR and M13 reverse were used to amplify a 686-bp fragment from pBMW-10 that contained an NcoI site at nt 1461 of VSVG. Amplified fragments were initially cloned into pGEM-T for sequencing and then inserted into VSVG-GFP as described above. To construct B/G/Δ-GFP, primers T7 and B5RSacR were used to amplify a fragment from pBMW-10 that contained a SacI site at nt 825 of B5R. The resulting fragment was cloned into pGEM-T as described above to yield pBMW-24T. A 550-bp EcoRI-SacI fragment from pBMW-24T was ligated with an 813-bp SacI-XbaI fragment from construct G/G/Δ-GFP and B5R-GFP that had been linearized with EcoRI-XbaI to yield B/G/Δ-GFP.
Construct G/B/BY310A-GFP, in which the nucleotide sequence was changed to encode an alanine at residue 310 instead of a tyrosine, was made by two-stage PCR using pBMW-3 as the template and primer pairs B5R310AF-M13 forward and B5R310AR-M13 for the initial amplification. The second stage and subsequent cloning were carried out as described earlier. Primers B5RΔLLR and M13 forward were used to amplify a 750-bp fragment from pBMW-3 that placed an NcoI site at nt 349 of B5R and removed the sequence that coded for the two leucines at residues 315 and 316 of B5R. A fragment containing both mutations was amplified with primers B5R310A/ΔLLR and T7 with G/B/BΔ315/316-GFP as the template. Both fragments were cloned into pGEM-T for sequencing and inserted into G/B/B-GFP as previously described.
Cells and transfections.
HeLa and COS-7 cell monolayers were grown in Dulbecco's modified Eagle's medium (Quality Biologicals, Gaithersburg, Md.) supplemented with 10% fetal calf serum. For transfection, cells were grown on coverslips in six-well plates to approximately 80% confluency and then transfected with Lipofectamine reagent (Gibco BRL) as recommended by the manufacturer. DNA for transfection was prepared with the Wizard Maxipreps DNA Purification System according to the directions of Promega.
Fluorescence microscopy.
At 36 to 48 h after transfection, cells expressing GFP chimeras were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C. To stain the Golgi network, fixed cells were permeabilized with 0.05% saponin in PBS, quenched for 10 min in 0.1 M glycine, and incubated with anti-58K antibody (Sigma) followed by CY5-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories) that had been diluted 1:100 in PBS.
Hybridoma cells expressing the anti-VSVG monoclonal antibody (MAb) I1 (10, 25) were a generous gift of Jonathan Yewdell. For live staining, cells expressing GFP chimeras were washed twice with ice-cold PBS and incubated for 1 h on ice with hybridoma I1 supernatant diluted 1:100 in PBS. Following incubation, cells were washed twice with ice-cold PBS, fixed for 1 h, and stained with secondary antibody as described above. For internalization of antibody complexes, live cells expressing chimeras were incubated with MAb I1 as described above. After incubation, cells were washed twice with ice-cold PBS and incubated in Opti-MEM medium (Gibco BRL) for 2 h at 31°C. After incubation, cells were washed, fixed, permeabilized with PBS containing 0.5% saponin for 1 h, and quenched. Antibody staining was detected as described above.
Fab fragments of the MAb I1 were generated by using the ImmunoPure Fab preparation Kit (Pierce) according to directions of the manufacturer. Cells expressing chimeras were washed twice in Opti-MEM followed by incubation overnight at 31°C with the Fab fragments that had been diluted 1:10 in Opti-MEM.
Nuclei were visualized by staining with 6 μg of Hoechst 33258 (Pierce) per ml in PBS for 10 min, followed by three 5-min washes with PBS. Coverslips were mounted in PBS and sealed with rubber cement. Fluorescence was detected on a Leica TCS NT inverted confocal microscope with a UV laser (Coherent, Santa Clara, Calif.) attached. Images were overlaid by using Adobe PhotoShop version 5.0.2.
Flow cytometry.
HeLa cells were harvested with PBS containing 2% EDTA at 48 h after transfection. Harvested cells were incubated for 1 h on ice with I1 hybridoma supernatant, diluted 6:100 in PBS, followed by CY5-conjugated goat anti-mouse secondary antibody. Stained cells were analyzed for GFP and CY5 staining on a FACSCalibur instrument (Becton Dickinson). At least 1,400 GFP-positive cells were analyzed.
RESULTS
Localization of a B5R-GFP fusion protein in the Golgi network.
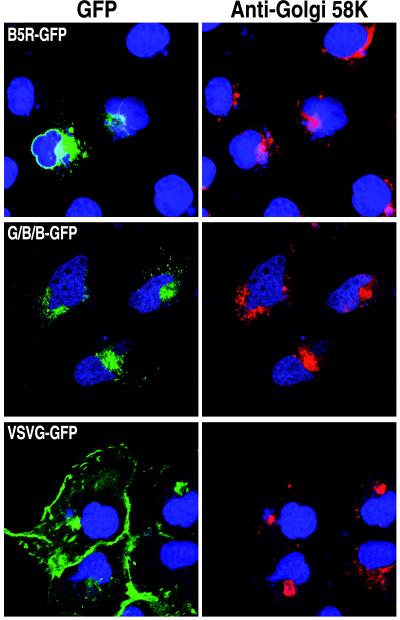
The B5R ORF encodes a type I integral membrane protein that localizes to the Golgi network independently of other viral proteins (24). In a previous study, Presley et al. (39) had shown that fusion of GFP to the C terminus of VSVG, another type I membrane glycoprotein, did not affect the trafficking of VSVG from the ER to the plasma membrane as detected by fluorescence microscopy. Based on this result, we decided to add GFP to the C terminus of the B5R protein (Fig. 1). A plasmid containing the B5R-GFP ORF regulated by a cytomegalovirus promoter was transfected into HeLa cells. As shown in Fig. 2, an intense green fluorescence was detected in perinuclear and juxtanuclear structures characteristic of the ER and Golgi complex, respectively. The GFP fluorescence colocalized with antibody to the Golgi 58K protein (Fig. 2) which has been shown to be associated with the Golgi apparatus (5, 11). As will be shown later, constructs with an intact B5R cytoplasmic tail are retained in the Golgi network even after a prolonged chase in the presence of cycloheximide, an inhibitor of protein synthesis. Whereas cells transfected with B5R-GFP exhibited very low fluorescence in the plasma membrane, strong Golgi and plasma membrane fluorescence was noted in HeLa cells transfected with a plasmid expressing VSVG-GFP (Fig. 2). (The VSVG protein expressed in this study has a reversible temperature-sensitive mutation in the lumenal domain that inhibits proper folding and subsequent exit from the ER at the nonpermissive temperature of 40°C but refolds at the permissive temperature of 31°C. Unless specifically mentioned, all cited studies were carried out at 31°C.) Thus, the addition of GFP to the C terminus of B5R did not interfere with its normal ability to traffic to the Golgi network and accumulate there.
FIG. 2.
Golgi network localization of B5R-GFP and G/B/B-GFP. HeLa cells were transfected with plasmids containing B5R-GFP, G/B/B-GFP, or VSVG-GFP (Fig. 1). Fixed, permeabilized cells were stained with anti-Golgi 58K antibody, followed by CY5-conjugated goat anti-mouse antibody to show location of the Golgi apparatus and with Hoechst dye to show location of nuclei, and then examined by confocal microscopy. Colors: green, GFP; red, 58K antibody; blue, Hoechst dye. Note that green fluorescence only occurred in transfected cells, whereas the Hoechst dye and antibody stained all cells.
The transmembrane domain and cytoplasmic tail of B5R are sufficient for ER to Golgi network transport.
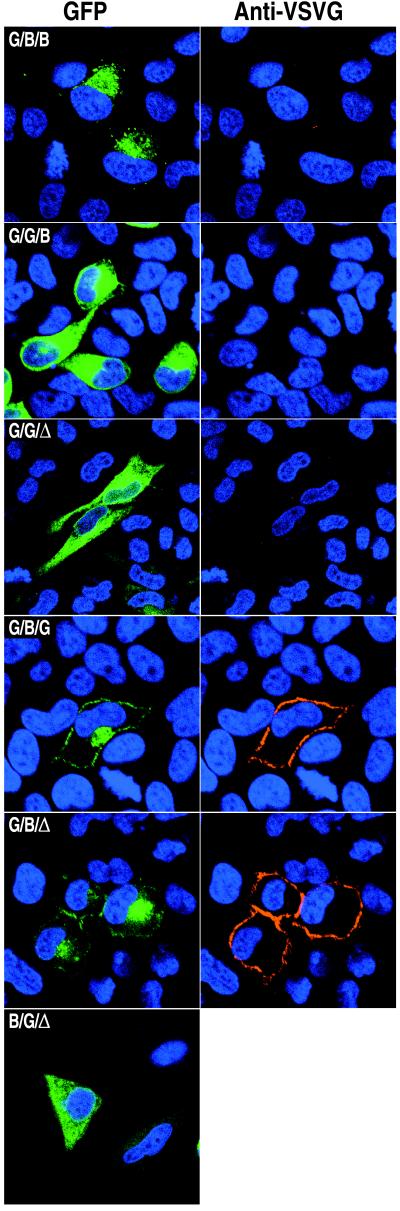
A segment containing most of the lumenal domain of B5R (residues 1 to 275) was replaced with residues 1 to 464 of VSVG to form a protein with the lumenal domain of VSVG, the transmembrane domain of B5R, and the cytoplasmic tail of B5R fused to GFP (G/B/B-GFP; Fig. 1). Expression of G/B/B-GFP at the permissive temperature resulted in green fluorescence in a juxtanuclear region that colocalized with the Golgi 58K marker (Fig. 2) indicating that the B5R lumenal domain was not needed for this localization. As with B5R-GFP, low fluorescence was detected on the plasma membrane. When G/B/B-GFP or VSVG-GFP was expressed at the nonpermissive temperature, widespread cytoplasmic staining characteristic of the ER was observed (not shown), indicating that the VSVG lumenal domain was temperature sensitive even with a heterologous transmembrane domain and cytoplasmic tail.
Neither the cytoplasmic tail nor the lumenal domain of B5R was needed for export from the ER.
A diacidic signal in the cytoplasmic tail of VSVG is required for net export from the ER (34). Accordingly, a construct with the VSVG lumenal and transmembrane domains but lacking the VSVG cytoplasmic tail (G/G/Δ-GFP; Fig. 1) largely remained in the ER (Fig. 3). In addition, unpermeabilized cells that expressed G/G/Δ-GFP could not be stained with MAb I1 to the VSVG extracellular domain (Fig. 3), whereas cells expressing VSVG-GFP were readily labeled on the cell surface by this procedure (not shown). Similarly, little surface staining of G/B/B-GFP was detected by MAb I1 in unpermeabilized cells (Fig. 3). The above results allowed us to investigate whether an ER export signal capable of replacing that of VSVG occurs in the cytoplasmic domain of B5R. Transfection experiments indicated that a construct consisting of the lumenal and transmembrane domains of VSVG attached to the cytoplasmic domain of B5R (G/G/B-GFP) also remained in the ER and was not transported to the Golgi or plasma membrane (Fig. 3). Permeabilized cells expressing either G/G/Δ-GFP or G/G/B-GFP were readily stained with MAb I1 (not shown), which is conformation specific (10, 25), indicating that the lumenal domain of these proteins was properly folded and that misfolding was not the reason for their inability to leave the ER. Thus, there was no evidence of an ER-exiting signal in the cytoplasmic tail of B5R.
FIG. 3.
Intracellular and surface expression of chimeric proteins. HeLa cells were transfected with plasmids containing the indicated GFP chimeras, and the live, unpermeabilized cells were stained with VSVG extracellular domain MAb I1, followed by CY5-conjugated goat anti-mouse antibody to detect surface expression and Hoechst dye to show location of nuclei by confocal microscopy. Colors: green, GFP; red, MAb I1; blue, Hoechst dye.
Next, we exchanged the lumenal domain of VSVG with that of B5R to test its ability to facilitate ER export. Cells expressing the lumenal domain of B5R and the transmembrane domain of VSVG linked to GFP (B/G/Δ-GFP; Fig. 1) showed widespread fluorescence of the cytoplasm similar to that seen for G/G/Δ-GFP (Fig. 3), indicating that the lumenal domain of B5R could not restore efficient export from the ER. Taken together, these data suggested that neither the cytoplasmic domain nor the lumenal domain of B5R contains a strong ER-to-Golgi transport signal which could substitute for the one in VSVG.
The transmembrane domain of B5R facilitated ER to Golgi transport.
When the transmembrane domain of B5R and the lumenal domain and cytoplasmic tail of VSVG were expressed as part of the same construct (G/B/G-GFP; Fig. 1), the protein was present in Golgi and plasma membranes as determined by green fluorescence and staining of live cells with VSVG MAb I1 (Fig. 3). With this construct, however, transport out of the ER could have been mediated by either the transmembrane domain of B5R or the diacidic signal in the cytoplasmic tail of VSVG. To distinguish between these two possibilities, the cytoplasmic tail of G/B/G-GFP was removed and GFP was placed directly after the transmembrane domain of B5R (G/B/Δ-GFP; Fig. 1). As shown in Fig. 3, expression of G/B/Δ-GFP resulted in green fluorescence of both the Golgi and plasma membranes; fluorescence of the plasma membrane also occurred when the live cells were stained with MAb I1. Considering that G/G/Δ-GFP was unable to exit the ER efficiently, the ability of G/B/Δ-GFP to traffic to the Golgi network indicated that the transmembrane domain of B5R allowed efficient protein export from the ER.
Signals in the cytoplasmic tail are required to maintain B5R in the Golgi network and prevent accumulation on the plasma membrane.
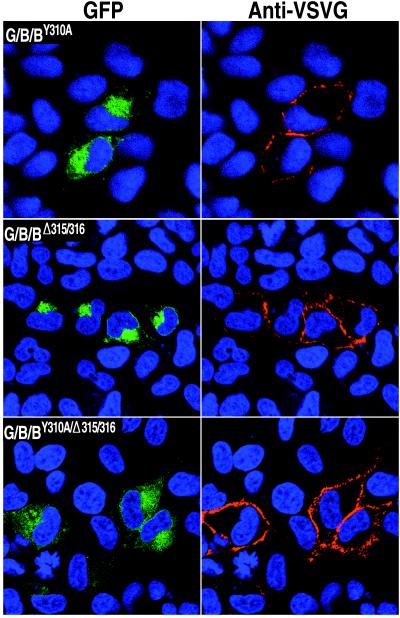
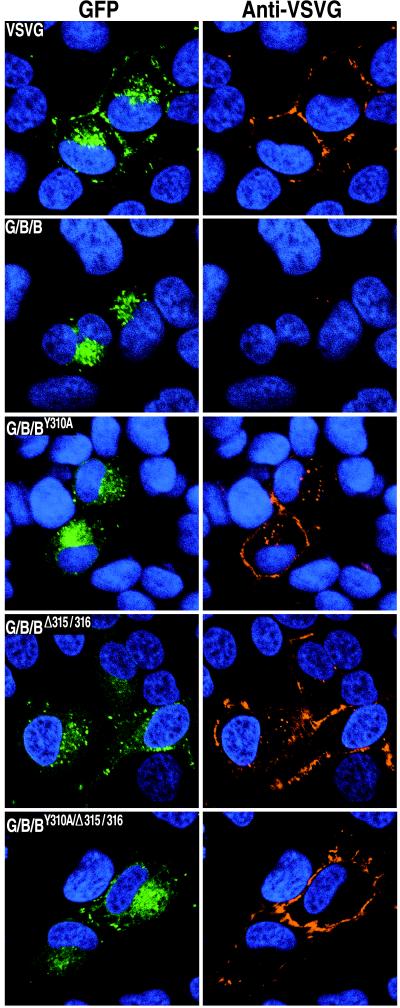
The striking difference in the localization of G/B/B-GFP and G/B/Δ-GFP (Fig. 3) suggested that the cytoplasmic tail of B5R prevented the accumulation of this protein in the plasma membrane. The cytoplasmic tail could mediate Golgi membrane localization either by acting as a Golgi membrane anchor or as a retrieval signal from the plasma membrane via the endocytic pathway (33). Inspection of the sequence constituting the short cytoplasmic tail of B5R revealed a tyrosine at residue 310 and a two adjacent leucines at residues 315 and 316 (Fig. 1B) which could form parts of motifs for selective inclusion in clathrin-coated vesicles. Such a receptor-mediated retrieval pathway has been shown to function for both furin and the Golgi 58K protein (6, 20, 33, 43). To determine if tyrosine or leucine mutations affect the localization of B5R, three new constructs were made. In one, the tyrosine at position 310 was changed to alanine (G/B/BY310A-GFP); in another, the two leucines at positions 315 and 316 were deleted (G/B/BΔ315/316-GFP); and in the third, both mutations were made (G/B/BY310A/Δ315/316-GFP). Each construct was tested independently and shown to express the GFP-fusion protein (Fig. 4). Live, unpermeabilized cells that had been transfected with plasmids capable of expressing these mutated proteins were incubated at 0°C with VSVG MAb I1. As shown in Fig. 4, the proteins with the mutated tyrosine or deleted leucines were more highly expressed on the cell surface than was G/B/B-GFP (Fig. 3), suggesting that these amino acids are part of a signal that normally plays a role in preventing plasma membrane accumulation.
FIG. 4.
Effects of point mutations in the cytoplasmic tail of B5R on intracellular trafficking of chimeric proteins. HeLa cells were transfected with GFP chimeras containing the VSVG lumenal domain, the B5R transmembrane domain, and the B5R cytoplasmic domain with mutations in the putative retrieval sequences. Live, unpermeabilized cells were stained with VSVG MAb I1, followed by CY5-conjugated goat anti-mouse antibody to detect surface expression and Hoechst dye to show the locations of nuclei by confocal microscopy. Colors: green, GFP; red, MAb I1; blue, Hoechst dye.
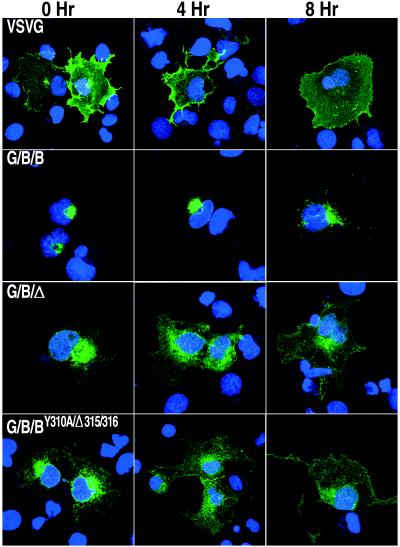
Although mutations of the tyrosine and leucine residues led to the presence of G/B/BY310A-GFP, G/B/BΔ315/316-GFP, and G/B/BY310A/Δ315/316-GFP in the plasma membrane, there were still appreciable amounts of the mutated proteins associated with the Golgi network (Fig. 4). We suspected that this protein was still in transit to the plasma membrane. To test this theory, we incubated cells expressing VSVG-GFP, G/B/B-GFP, G/B/Δ-GFP, or G/B/BY310A/Δ-315/316-GFP in the presence of cycloheximide to stop further protein synthesis and to provide additional time for the existing proteins in the ER and Golgi network to be transported to their final cellular destination. In the case of VSVG-GFP, which lacks Golgi retention or retrieval signals, there were considerable amounts of the protein in the plasma membrane at the start of cycloheximide treatment and most had moved out of the Golgi network after 4 h (Fig. 5). In contrast, G/B/B-GFP with an unmutated B5R cytoplasmic tail remained in the Golgi network at 8 h after the addition of cycloheximide (Fig. 5) and even after 20 h (not shown). With cells expressing constructs G/B/Δ-GFP or G/B/BY310A/Δ315/316-GFP, missing either the entire B5R cytoplasmic domain or the tyrosine and dileucine residues, respectively, the fluorescence was decreased in the Golgi network and increased at the cell surface at 4 and 8 h after cycloheximide addition (Fig. 5). Therefore, Golgi accumulation was dependent on signals in the cytoplasmic tail of the B5R protein.
FIG. 5.
Localization of chimeric proteins following a chase in the presence of cycloheximide. COS-7 cells expressing the indicated GFP chimeric proteins were incubated in medium containing 100 μg of cycloheximide per ml at 31°C. Coverslips were removed at 0, 4, and 8 h after addition of cycloheximide and stained with Hoechst dye. Colors: green, GFP; blue, Hoechst dye.
Flow cytometry was used to confirm differences in surface expression of proteins with intact or mutated B5R cytoplasmic domains. Live, unpermeabilized cells expressing our various GFP chimeras were incubated with VSVG MAb I1 followed by a CY5-conjugated secondary antibody. Cells expressing VSVG, which localizes to the plasma membrane, served as a positive control; cells expressing B5R served as a negative control as the epitope for VSVG MAb I1 is absent. Other negative controls consisted of cells expressing VSVG but not incubated with MAb or secondary antibody. Histograms showing representative data of the CY5 fluorescence of GFP-positive cells are shown in Fig. 6A. The percentages of CY5-positive cells are enumerated in Table 1 and confirm the higher surface expression when the B5R cytoplasmic domain was mutated or deleted as compared to that of G/B/B-GFP. To control for different levels of expression of the various proteins in the transfected cells, we divided the CY5 mean channel fluorescence (MCF) by the GFP MCF; the resulting ratios are shown in Table 1 and represented graphically in Fig. 6B. The highest MCF ratios were obtained for VSVG-GFP and G/B/G-GFP, whereas expression of G/G/B-GFP and G/G/Δ-GFP yielded very low MCF ratios due to their inability to exit the ER. The MCF ratios of cells transfected with G/B/B-GFP were higher than the negative controls, indicating that significant amounts of B5R were present on the cell surface, although this had been difficult to detect by confocal microscopy. Importantly, higher MCF ratios were obtained for cells transfected with either G/G/GY310A-GFP, G/B/BΔ315/316-GFP, G/B/BY310A/Δ315/316-GFP, or G/B/Δ-GFP than with G/B/B-GFP, indicating increased surface expression when signals in the cytoplasmic domain of B5R were removed.
FIG. 6.
Surface expression of chimeric proteins. Transfected HeLa cells were placed on ice and stained with VSVG MAb I1, followed by CY5-conjugated goat anti-mouse antibody except as indicated. (A) Shaded histograms of CY5 fluorescence of GFP-positive cells that were transfected with plasmids expressing VSVG-GFP, G/B/B-GFP, B/B/Δ-GFP, or G/B/BY310A/Δ315/316-GFP. Unshaded peak in each panel represents a negative control in which cells expressing VSVG were stained with the CY5-conjugated second antibody only. (B) Graphical representation of the CY5 MCF-to-GFP ratios in Table 1.
TABLE 1.
Flow cytometry analysis of surface expression of chimeric proteins
| Sample no. | Construct | % CY5 positive | CY5 MCF | GFP MCF | MCF ratioa |
|---|---|---|---|---|---|
| 1 | VSVGb | 0.0 | 1.97 | 224.45 | 0.01 |
| 2 | VSVGc | 0.0 | 2.91 | 231.46 | 0.01 |
| 3 | B5R | 0.1 | 3.21 | 140.10 | 0.02 |
| 4 | VSVG | 79.6 | 170.84 | 218.94 | 0.78 |
| 5 | G/B/B | 14.9 | 34.49 | 359.10 | 0.10 |
| 6 | G/B/G | 38.3 | 89.83 | 234.71 | 0.38 |
| 7 | G/G/B | 5.7 | 16.29 | 389.95 | 0.04 |
| 8 | G/G/Δ | 7.7 | 17.26 | 293.56 | 0.06 |
| 9 | G/B/Δ | 40.3 | 104.99 | 399.24 | 0.26 |
| 10 | G/B/BY310A | 47.2 | 109.84 | 533.14 | 0.21 |
| 11 | G/B/BΔ315/316 | 29.8 | 60.21 | 328.19 | 0.18 |
| 12 | G/B/BY310A/Δ315/316 | 43.4 | 100.48 | 473.17 | 0.21 |
CY5 MCF/GFP MCF.
Neither MAb I1 nor CY5-conjugated secondary antibody was added to the cells.
Only CY5-conjugated secondary antibody was added to the cells.
Retrieval of the B5R protein from the plasma membrane.
Thus far, we have not specifically examined the roles of retention and retrieval in the accumulation of the B5R protein in the Golgi network, although the latter was suggested. To investigate these mechanisms, we needed to determine whether the B5R protein cycles between the Golgi network and the plasma membrane and if mutation of either the tyrosine or leucines could inhibit this cycling. Our plan was to use extracellular antibody to bind proteins on the cell surface and then see if the complexes are internalized and returned to the Golgi network. Live, unpermeabilized cells expressing VSVG-GFP, G/B/B-GFP, G/B/BY310A-GFP, G/B/BΔ315/316-GFP, or G/B/BY310A/Δ315/316-GFP were incubated on ice for 1 h with the MAb I1. Afterwards, the cells were washed to remove unbound antibody and prevent its fluid phase endocytosis and then incubated at 31°C for 2 h to allow time for the internalization of antibody-protein complexes. After incubation, cells were washed, fixed, and processed for immunofluorescence, and also examined for green fluorescence. Only cells expressing GFP showed antibody staining, demonstrating the specificity of MAb I1. As shown in Fig. 7, cells expressing VSVG-GFP exhibited green fluorescence in the Golgi and plasma membranes but antibody labeling was restricted to the cell surface, indicating that no detectable antibody complexes had been internalized. This was an important control to ensure that internalization of antibody does not occur because of cross-linking or fluid phase endocytosis. Cells expressing G/B/B-GFP showed green fluorescence in the Golgi network but almost no antibody labeling, presumably because there are small amounts of protein with an intact cytoplasmic tail on the cell surface (Fig. 7). As before, cells expressing either G/B/BY310A- GFP or G/B/BΔ315/316-GFP exhibited intense plasma membrane labeling with antibody. However, after the 2-h incubation, antibody could also be detected in the juxtanuclear region of these cells, suggesting that some complexes had been internalized from the cell surface (Fig. 7). Cells expressing G/B/BY310A/Δ315/316-GFP showed antibody labeling on the plasma membrane but not in the juxtanuclear region (Fig. 7). Thus, mutation of the tyrosine or leucines impaired retrograde transport, whereas mutation of both sites abrogated it.
FIG. 7.
Surface staining and internalization of chimeric proteins complexed with VSVG MAb I1. HeLa cells expressing the indicated GFP chimeric proteins were labeled with MAb I1 on ice and then incubated at 31°C to allow internalization of protein-MAb complexes. Cells were stained with CY5-conjugated goat anti-mouse antibody and Hoechst dye. Colors: green, GFP; red, MAb I1; blue, Hoechst dye.
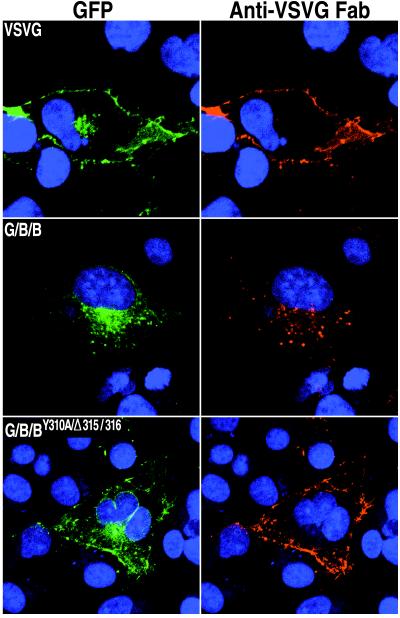
In the previous experiments, antibody binding and internalization were carried out for a relatively short time to minimize the possibility that antibody-mediated cross-linking contributed to endocytosis. Under those conditions, however, there was sufficient protein on the cell surface for antibody staining only when the tyrosine was mutated or the leucines deleted. Presumably, because of the efficiency of internalization mediated by the unmutated cytoplasmic tail, the steady-state amount of G/B/B-GFP on the surface was too small for antibody staining under these conditions. To allow longer continuous labeling without the potential of antibody cross-linking, monovalent Fab fragments were made from MAb I1. Transfected cells were incubated overnight at 31°C with the Fab fragments and then washed, fixed, and further processed to detect both GFP fluorescence and Fab staining. As shown in Fig. 8, Fab fragments that had bound to antigen on the cell surface were mostly detected in overlapping regions of green fluorescence in the juxtanuclear region of cells expressing G/B/B-GFP, suggesting that this chimera recycles between the plasma and Golgi membranes. It is possible, however, that some internalized Fab-protein complexes dissociated or were directed toward the lysosome for degradation as confocal microscopy is not quantitative. In contrast, VSVG-GFP and G/B/BY310A/Δ315/316-GFP showed intense Fab staining of the plasma membrane but little, if any, Fab staining in the juxtanuclear area (Fig. 8), indicating that the mutations effectively blocked retrieval from the plasma membrane even after prolonged incubation. The observation that there was little internal Fab staining of cells transfected with VSVG-GFP or G/B/BY310A/Δ315/316-GFP or of cells that were not expressing chimeras indicated that intracellular Fab staining of G/B/B-GFP was specific for internalized protein-Fab complexes and was not due to fluid phase endocytosis of the Fab fragments.
FIG. 8.
Surface staining and internalization of chimeric proteins complexed with Fab fragments. COS-7 cells expressing the indicated GFP chimeric proteins were incubated overnight at 31°C with Fab fragments prepared from VSVG MAb I1. Cells were stained with CY5-conjugated goat anti-mouse antibody and Hoechst dye. Colors: green, GFP; red, Fab fragments.
DISCUSSION
The outer membrane of extracellular vaccinia virus particles is derived from the TGN or endosomal cisternae that have been modified by the insertion of specific viral proteins. The type I integral membrane protein encoded by the B5R ORF is crucial as deletion of it prevents the wrapping of intracellular particles by the modified cellular membranes (14, 51). Thus far, the B5R protein is the only one encoded by vaccinia virus that has been shown to localize within the Golgi network when expressed in the absence of other viral proteins (24). The purpose of the present study was to identify the B5R domains responsible for intracellular trafficking and localization. We adapted an approach, successfully used for other integral membrane proteins, involving the construction of chimeras between proteins that localize in Golgi and plasma membranes (33). The intracellular locations of the chimeras were visualized by appending GFP to the C terminus of the cytoplasmic domain of the B5R protein. GFP is particularly suitable as a reporter because it has a compact, independently folding structure and its stable fluorescence is easily monitored by confocal microscopy (48). Moreover, Presley et al. (39) had reported that GFP did not perturb the plasma membrane localization of VSVG and we found that it also did not alter the normal Golgi membrane localization of the B5R protein. By swapping domains between VSVG and B5R, we showed that the transmembrane domain of B5R was responsible for export from the ER and the cytoplasmic tail was needed for accumulation of chimera in the Golgi network. Further investigations provided evidence for cycling of B5R between the Golgi and plasma membranes mediated by internalization sequences within the cytoplasmic tail.
Integral membrane proteins exit the ER in COPII-coated vesicles. Accumulation of a protein in the ER can occur for different reasons, including improper folding, absence of exit signals, or recycling. Signals that allow movement of proteins from the ER are generally located in the transmembrane or cytoplasmic domains. A well-studied example, in the cytoplasmic tail of VSVG and some other type 1 integral membrane proteins, is a diacidic motif which interacts with COPII machinery (2, 35). Another signal, present in the cytoplasmic tail of the p24 family of type I integral membrane proteins which move both anterograde and retrograde in the secretory pathway, includes a diphenylalanine motif that interacts with COPII component Sec23Ap (9, 23). A conserved glutamine in the transmembrane domain acts in conjunction with the diphenylalanine for efficient ER export (15). However, neither a diacidic nor a diphenylalanine motif is present in the cytoplasmic tail of B5R. Moreover, the inability of the cytoplasmic tail of B5R to functionally replace the corresponding part of VSVG suggested that it lacks an independent ER-exiting signal. Instead, ER exiting was determined by the transmembrane domain of B5R. Thus, a chimera consisting of the B5R transmembrane domain fused to either the lumenal domain of B5R or VSVG efficiently exited the ER. Whether constructs containing the B5R transmembrane domain exit by a default pathway or through specific signals cannot be distinguished from this study. In the absence of any recognizable motif in the B5R transmembrane domain, extensive mutagenesis would be required to correlate structure with function.
Having exited the ER, membrane proteins may accumulate in the Golgi network because of retention or retrieval mechanisms. Retention can be mediated by the transmembrane domain and has been attributed to formation of large oligomeric complexes or to lipid sorting based on the length of the transmembrane domain (reviewed in reference 33). Although a chimeric protein containing the VSVG lumenal and B5R transmembrane domains was exported out of the ER, it did not accumulate in the Golgi network but continued on to the plasma membrane. Addition of the B5R cytoplasmic domain, however, allowed Golgi network accumulation. Furthermore, internalization of B5R from the plasma membrane was indicated by the internalization of extracellular antibody as well as Fab fragments that targeted the extracellular domain of B5R chimera. Although antibody cross-linking and fluid phase endocytosis were ruled out, we cannot be absolutely certain that the B5R chimera and antibody or Fab fragments remained complexed in the cytoplasm after internalization. However, it was likely that the complexes did remain intact, because the internalized Fab fragment staining overlapped with GFP and because the antibody and Fab fragments were found in juxtanuclear regions where the GFP chimera accumulated. Therefore, the cytoplasmic tail of B5R appeared to mediate retrograde transport from the plasma membrane to the Golgi apparatus. It is possible that along with their role in internalization, signals in the cytoplasmic tail are responsible for retaining B5R in the Golgi network. In that case, mutation of these residues would prevent retention, and this could also lead to increased levels of B5R on the cell surface. Further analysis will be required to determine if these signals can mediate Golgi retention as well as plasma membrane internalization.
Endosomal targeting signals have been found in the cytoplasmic domain of some TGN resident proteins. The best characterized of these are two tyrosine-containing motifs, YXXØ (where Y is tyrosine, X is any amino acid, and Ø is an amino acid with a bulky hydrophobic side group) and NPXY (where N and P are asparagine and proline, respectively) (reviewed in reference 28). Studies have shown that internalized TGN38 containing the YXXØ motif was delivered to the TGN via an endocytic recycling compartment, whereas a chimera containing the NPXY motif bypassed this compartment en route to the TGN (16, 27). Overexpression studies further suggested that these two signals are internalized by distinct saturable mechanisms (50). The YXXØ signal interacts with the μ2 subunit of the clathrin adapter protein complex AP-2, accounting for its selective recruitment into coated pits (40). Another motif, consisting of two adjacent leucine residues, has been found in the cytoplasmic tail of several proteins and shown to direct endocytosis (reviewed in reference 28). This dileucine motif also interacts with the μ2 subunit of the clathrin adapter protein complex AP-2 (8). Inspection of the B5R cytoplasmic tail sequence revealed a tyrosine at residue 310, although the neighboring amino acids did not conform exactly to either of the known tyrosine motifs, as well as adjacent leucine residues at positions 315 and 316. Mutation of either the tyrosine or the dileucine motif increased surface expression of the B5R protein and impaired its internalization from the plasma membrane, whereas mutation of both abrogated internalization. It is possible that the tyrosine and two leucines constitute parts of a single retrieval motif that has reduced efficiency when either the tyrosine or leucines are missing. Alternatively, the tyrosine and leucines could be parts of independent internalization signals that act coordinately. Construction and analysis of additional mutations would be needed to discriminate between these models and to determine the roles of adjacent and intervening amino acid residues.
The B5R protein including the cytoplasmic tail is highly conserved among orthopoxviruses, and the latter is therefore likely to have an important role in virus replication or spread. One would expect the cytoplasmic tail to allow retrieval of B5R that transited through the Golgi network to the plasma membrane as well as B5R that was deposited in the plasma membrane during the membrane fusion events that occur during budding of EEV. The active internalization of B5R from the plasma membrane could be related to the finding of enhanced traffic between the plasma membrane and the TGN after vaccinia virus infection (44). The retrieved B5R could be recycled for IEV membrane formation. However, Lorenzo et al. (26) recently reported that vaccinia virus expressing a mutated form of B5R without the cytoplasmic tail formed similar amounts of EEV as wild-type virus in cultured cells. Either the transit through the TGN of B5R lacking a cytoplasmic tail is slow enough to permit wrapping of IMV or else other viral proteins help retain B5R in the TGN or retrieve it from the plasma membrane. Recovery of B5R from the plasma membrane for conservation purposes, however, is only one possible function of retrieval. Alternatively, there could be negative consequences of having large amounts of B5R on the infected cell surface in vivo. Still another possibility is that B5R associates with proteins on the cell surface and transports them to the TGN either to remove them from the plasma membrane or for their incorporation into EEV membranes. The extracellular portion of B5R could be the interacting domain, as it contains short consensus repeats that are homologous to those in complement regulatory proteins. These repeats, however, were not necessary for the incorporation of cellular complement regulatory proteins in the EEV membrane (49). Therefore, either the transmembrane domain of B5R is involved in the transport of the latter proteins or other mechanisms are involved.
In future experiments, we plan to study the recycling of native and mutated forms of the B5R protein between the plasma membrane and TGN in infected cells and investigate the role of this process on intracellular trafficking, EEV formation, and virulence in vivo.
ACKNOWLEDGMENTS
We thank Jonathan Yewdell for MAb I1 and the corresponding hybridoma cell line, Jennifer Lippincott-Schwartz for a plasmid containing VSVG-GFP, Elizabeth Wolffe for advice and comments on the manuscript, Chris Norbury and Jonathan Yewdell for help with the flow cytometry, and Norman Cooper for provision of tissue culture cells.
REFERENCES
- 1.Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- 2.Aridor M, Bannykh S I, Rowe T, Balch W E. Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J Biol Chem. 1999;274:4389–4399. doi: 10.1074/jbc.274.7.4389. [DOI] [PubMed] [Google Scholar]
- 3.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco R, Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom G S, Brashear T A. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- 6.Bos K, Wraight C, Stanley K K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulter E A, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- 8.Dietrich J, Kastrup J, Nielsen B L, Odum N, Geisler C. Regulation and function of the CD3gamma DxxxLL motif: a binding site for adaptor protein-1 and adaptor protein-2 in vitro. J Cell Biol. 1997;138:271–281. doi: 10.1083/jcb.138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud J P, Thomas D Y, Bergeron J J, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doms R W, Ruusala A, Machamer C, Helenius J, Helenius A, Rose J K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988;107:89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaldson J G, Lippincott-Schwartz J, Bloom G S, Kreis T E, Klausner R D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990;111:2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan S A, Smith G L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992;66:1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelstad M, Howard S T, Smith G L. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 14.Engelstad M, Smith G L. The vaccinia virus 42-kDa envelope protein is required for the envelopment and egress of extracellular virus and for virus virulence. Virology. 1993;194:627–637. doi: 10.1006/viro.1993.1302. [DOI] [PubMed] [Google Scholar]
- 15.Fiedler K, Rothman J E. Sorting determinants in the transmembrane domain of p24 proteins. J Biol Chem. 1997;272:24739–24742. doi: 10.1074/jbc.272.40.24739. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh R N, Mallet W G, Soe T T, McGraw T E, Maxfield F R. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera E, del Mar Lorenzo M, Blasco R, Isaacs S N. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72:294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirt P, Hiller G, Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986;58:757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey J S, Peters P J, Yuan L C, Bonifacino J S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichihashi Y, Matsumoto S, Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971;46:507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs S N, Wolffe E J, Payne L G, Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappeler F, Klopfenstein D R, Foguet M, Paccaud J P, Hauri H P. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- 24.Katz E, Wolffe E J, Moss B. The cytoplasmic and transmembrane domains of the vaccinia virus B5R protein target a chimeric human immunodeficiency virus type 1 glycoprotein to the outer envelope of nascent vaccinia virions. J Virol. 1997;71:3178–3187. doi: 10.1128/jvi.71.4.3178-3187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefrancios L, Lyles D S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982;121:157–167. [PubMed] [Google Scholar]
- 26.Lorenzo M D, Herrera E, Blasco R, Isaacs S N. Functional analysis of vaccinia virus B5R protein: role of the cytoplasmic tail. Virology. 1998;252:450–457. doi: 10.1006/viro.1998.9483. [DOI] [PubMed] [Google Scholar]
- 27.Mallet W G, Maxfield F R. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks M S, Woodruff L, Ohno H, Bonifacino J S. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew E, Sanderson C M, Hollinshead M, Smith G L. The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation. J Virol. 1998;72:2429–2438. doi: 10.1128/jvi.72.3.2429-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh A A G, Smith G L. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J Virol. 1996;70:272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976;73:43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- 32.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 33.Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura N, Balch W E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch W E. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274:15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- 36.Parkinson J E, Smith G L. Vaccinia virus gene A36R encodes a Mr 43-50 K protein on the surface of extracellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- 37.Payne L G. Significance of extracellular virus in the in vitro and in vivo dissemination of vaccinia virus. J Gen Virol. 1980;50:89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- 38.Payne L G, Norrby E. Presence of hemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976;32:63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 39.Presley J F, Cole N B, Schrer T A, Hirschberg K, Zaal K J M, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 40.Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley L C, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roper R, Wolffe E J, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J Virol. 1998;72:4192–4204. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roper R L, Payne L G, Moss B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.Sanderson C M, Frischknecht F, Way M, Hollinshead M, Smith G L. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J Gen Virol. 1998;79:1415–1425. doi: 10.1099/0022-1317-79-6-1415. [DOI] [PubMed] [Google Scholar]
- 43.Schafer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse M L, Kern H F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shida H. Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology. 1986;150:451–462. doi: 10.1016/0042-6822(86)90309-0. [DOI] [PubMed] [Google Scholar]
- 46.Thompson C L, Condit R C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986;150:10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- 47.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 48.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 49.Vanderplasschen A, Mathew E, Hollinshead M, Sim R B, Smith G L. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci USA. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren R A, Green F A, Stenberg P E, Enns C A. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J Biol Chem. 1998;273:17056–17063. doi: 10.1074/jbc.273.27.17056. [DOI] [PubMed] [Google Scholar]
- 51.Wolffe E J, Isaacs S N, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolffe E J, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolffe E J, Weisberg A S, Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;244:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]