Abstract
Sickle cell disease (SCD), which occurs primarily in individuals of African descent, has been identified as a preexisting health condition for COVID-19 with higher rates of hospitalization, intensive care unit admissions, and death. National data indicate Black individuals have higher rates of vaccine hesitancy and lower COVID-19 vaccination rates. Understanding the key predictors of intention to receive a COVID-19 vaccine is essential as intention is strongly associated with vaccination behavior. This multisite study examined attitudes, beliefs, intentions to receive COVID-19 vaccines, and educational preferences among adolescents, young adults, and caregivers of children living with SCD. Participants completed an online survey between July 2021 and March 2022. Multivariate logistic regression was used to examine the association between participant age and COVID-19 vaccine attitudes, beliefs, and vaccine intentions. Of the 200 participants, 65.1% of adolescents, 62.5% of young adults, and 48.4% of caregivers intended to receive a COVID-19 vaccine for themselves or their child. Perception that the vaccine was safe was statistically significant and associated with patient and caregiver intention to receive the COVID-19 vaccine for themselves or their child. Participant age was also statistically significant and associated with the intent to get a booster for patients. Study findings highlight key concerns and influencers identified by patients with SCD and their caregivers that are essential for framing COVID-19 vaccine education during clinical encounters. Study results can also inform the design of messaging campaigns for the broader pediatric SCD population and targeted interventions for SCD subpopulations (eg, adolescents, caregivers).
Key Words: sickle cell disease, immunizations, COVID-19
Adults and children with sickle cell disease (SCD) are at increased risk of developing SCD-related complications in response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection1 including acute chest syndrome, severe pain crises (vaso-occlusive episode), strokes, and changes in kidney function.1 Immunizations have been developed for SARS-CoV-2 and found to significantly reduce disease severity and mortality2,3 and COVID-19 vaccines have now been approved and recommended for those aged 6 months and older . Despite this, a significant portion of children and adolescents remain unvaccinated, with even lower rates for booster shots. As of April 2023, it is estimated that ∼40% of adolescents (12 to 17 y old) and 34% of young adults (18 to 24 y old) have not completed the COVID-19 vaccine 2-dose series (CDC).4 The rates for COVID-19 booster for this population are significantly lower: 7.7% of 12- to 17-year olds and 7.3% of 18- to 24-year olds who are fully vaccinated have received a booster.4 This trend is concerning, particularly in light of potential annual booster recommendations by the FDA and CDC.5
To address suboptimal vaccination rates, understanding the reasons behind vaccine hesitancy among adolescents, young adults, and parents/caregivers is crucial. For minoritized populations, such as in the case of SCD where the majority of individuals are Black individuals, contextual factors such as disease context (eg, treatments), prior vaccination experiences, and socio/cultural influences such as dissemination must be considered.6 These factors can inform targeted educational and communication strategies.
Frameworks like the Health Belief Model and Theory of Planned Behavior7–10 offer insights into how beliefs about vaccine side effects and other factors influence intentions and behaviors. For example, a person’s belief that a vaccine has serious side effects increases their perception of risk, which in turn, can decrease their intention to receive a vaccine. Tailored interventions can provide accurate risk-benefit information in accessible language, addressing concerns about vaccine safety and necessity.
The limited research available on COVID-19 vaccine attitudes among pediatric SCD patients and their families reveals high levels of hesitancy driven by mistrust and perceived lack of benefit.11 Similar trends are found in the broader literature on child and adolescent vaccine hesitancy, including fears of side effects and doubts about necessity.12–15 Parental hesitancy often stems from safety concerns and personal experiences with COVID-19.
Our study sought to fill this gap by assessing attitudes, beliefs, and intentions regarding COVID-19 vaccination among pediatric SCD patients and their caregivers, using established theoretical frameworks. Specifically, our multisite study aimed to: (1) describe adolescents, young adults, and caregivers of children with SCD’s attitudes, beliefs, and intentions regarding COVID-19 vaccination; (2) examine associations between participant characteristics (ie, age, discrimination experience), COVID-19 vaccine attitudes and beliefs, and the intention to receive a COVID-19 vaccine; and (3) assess preferred sources of information and educational methods. Based on the literature,11,16–19 we hypothesized that beliefs about vaccine side effects/safety, participant characteristics and experiences of discrimination would be associated with COVID-19 intention to receive a COVID-19 vaccine or booster. By understanding these factors and preferences for information sources and educational methods, we can develop targeted interventions to improve COVID-19 vaccination rates in the pediatric SCD population.
METHODS
Population
Approximately 1147 participants were eligible across 3 pediatric SCD clinics. Participants were eligible for participation if they were an adolescent (12 to 17 y old) living with SCD, an adult (18 y old and up) living with SCD, or a parent/primary caregiver of children living with SCD (0 to 17 y old). Eligibility criteria also included the ability to read and answer questions without assistance. The study was approved by the primary children’s hospital Institutional Review Board (IRB) and the IRBs of 2 of the clinical sites. With respect to the informed consent process, this study was approved for a waiver of documentation of consent. Therefore, all eligible adolescents, adults, and caregivers who selected to begin the survey provided implicit consent.
Procedures
Recruitment occurred from July 2021 through March 2022. Adolescent, young adult, and caregiver participants were recruited from one of the 3 SCD clinics via email, mail (study flyer), phone call, or at a clinic visit. All participants began the study by clicking on a link directing them to an eligibility survey. After completing the eligibility survey and receiving a study information sheet, interested adolescents, adults, and caregivers completed an anonymous online survey (see survey, Supplemental Digital Content 1, http://links.lww.com/JPHO/A685) via REDCap.20 The survey took ∼10 to 15 minutes to complete and participants were mailed a $10 gift card after completion to the address they provided on a separate survey.
The online survey assessed constructs from health behavior models used to examine vaccine intention and uptake, specifically the Health Belief Model and Theory of Planned Behavior.7–10 Items assessing knowledge, perceptions, normative beliefs, and intention to receive a COVID-19 vaccine or booster were adapted from validated instruments.9,21–27
Measures
Demographics
Participants completed a sociodemographic survey, providing information about age, sex, race, ethnicity, insurance type, and zip code. As a part of this survey, they also indicated whether they received the 2020/2021 influenza vaccine. These data were used as potential predictors of intent to obtain the COVID-19 vaccine. Age was included as a predictor in all regression analyzes.
Zip code was used as a measure of community resources. Each zip code was recoded using the Distressed Communities Index which assigns a score of 0 (not distressed) to 100 (severely distressed) by zip code based on several socioeconomic (SES) factors (eg, poverty rate, housing vacancy rate, high school diploma rate, etc.),28 zip codes were divided into Prosperous, Comfortable, Mid-tier, At Risk, or Distressed. The percentage of the population living in zip codes with scores in the Prosperous, Comfortable, and Mid-tier ranges were coded as “High Resourced” communities, while zip codes with scores in the At Risk or Distressed range were coded as “Low-Resourced communities.”
Perceived Discrimination
Perceived discrimination was measured by asking participants to indicate whether they have, “ever experienced discrimination, been prevented from doing something, or been hassled or made to feel inferior in any of the following situations because of [their] race, ethnicity or color,” at: school, getting hired or getting a job, at work, getting housing, on the street or in a public setting.”22 Responses to the dichotomous item were used as a predictor in all analyzes.
Perceptions
Perceptions about vaccine safety, side effects, and risk for a SCD pain crisis were measured using items from different scales measuring vaccine perceptions, vaccine hesitancy,27 and attitudes and beliefs about vaccines, respectively. Each of these items was rated on a 5-point scale anchored by 1 (strongly disagree) and 5 (strongly agree). To measure perceptions of safety, participants were asked, “It would be safe for me to get a COVID-19 vaccine.” To measure perceptions of side effects, participants were asked, “A COVID-19 vaccine might have serious side effects,” or “I am concerned about vaccine side effects (eg, pain, fever, headache) in my child with sickle cell.” To measure perceptions of risk for a SCD pain crisis, participants were asked, “I am concerned the COVID-19 vaccine may cause my child/me to have a sickle cell pain crisis.” These items were used in all regression analyzes individually as predictors.
Normative Beliefs
Normative beliefs were measured using 2 items: “My healthcare provider would want me to get the COVID-19 vaccine,” and “Most people I know would think the COVID-19 vaccine is good for my health.” Participants responded using a 5-point scale with responses ranging from 1 (strongly disagree) to 5 (strongly agree). Similar items were used to assess parents’ normative beliefs regarding the COVID-19 vaccine for their child. For analysis, the items were included in the models as a scale and included in all regression analyzes. Cronbach ⺠values for the scale included 0.66 for adolescent participants, 0.66 for young adult participants, 0.66 for parent participants, and 0.64 for parent participants reporting their vaccine intention for their child.
Intention to Receive the Vaccine
For adolescents and young adults, intention to receive the COVID-19 vaccine for oneself was measured by the item, “Based on what you know RIGHT NOW about the COVID-19 vaccine, how likely are you to accept the vaccine if it was offered to you today?” Caregiver participants received an item assessing their intention to vaccinate their child: “Based on what you know RIGHT NOW about the COVID-19 vaccine, how likely are you to accept the vaccine for your child with sickle cell if it was offered to your child today?”21 Participants responded using a 5-point scale anchored by 1 (strongly disagree) and 5 (strongly agree). The same question was asked for intention to receive and COVID-19 booster. For vaccine and booster intention, “strongly disagree,” “disagree,” and “neutral” were categorized into “no intention to vaccinate/booster” and “strongly agree,” and “agree” were categorized into “intention to vaccinate/booster.”
Information and Education
Sources of information about COVID-19 were assessed by asking participants to indicate which of the following sources applied to them: self-guided research on the internet; doctor; federal government/agency; co-worker, friend or family; local public health officials; newspapers and news on television or internet; and social media. Participants then ranked their top 3 educational preferences for information sources from the following: videos from SCD leaders and experts; videos from others living with SCD; email updates, websites for self-learning, townhall meetings with questions and answers, and podcasts. These data were used descriptively.
Data Analysis
All analyzes were completed using SPSS 26 and Stata 18. Descriptive analyzes (frequencies, percentages, means, ranges, and SD) were conducted to examine participant characteristics, COVID and COVID-19 vaccine perceptions, normative beliefs, and intention to receive a COVID-19 vaccine or booster.
Next, logistic regressions were conducted using a robust variance estimator to assess variables associated with our 2 outcomes: intention to receive a COVID-19 vaccine (0=no, 1=yes) and intention to receive a COVID-19 booster (0=no, 1=yes). For these analyzes, adolescents and young adults were combined to serve as one independent sample (sample 1) and caregivers served as a second (sample 2), and each sample was analyzed separately. Predictors included in all models were: age, perceived discrimination, perceptions of vaccine safety, perceptions of side effects, perceptions of risk of SCD crisis, and normative beliefs. Data utilized to examine the aims are available from the corresponding author upon reasonable request.
RESULTS
Participant Characteristics
Of the potential participants (n=1147), 774 emails were sent, 690 did not respond and 69 completed at least eligibility screening. We called or left voicemails for 43 patients. One hundred eighty-two patients were approached in the clinic, with 16 not consenting to participate. In total, 51 individuals started the screening/consenting process and ultimately did not complete the survey (primary children’s hospital=18; second children’s hospital=20; third children’s hospital=11; 2 from other states). Fourteen participants were removed from analyzes due to being duplicates (n=9) or ineligible based on screening criteria (n=5). In total, 43 adolescents (M age=15, SD=4.74), 64 young adults (M age=21.9, SD=5.73), and 93 caregivers (M age=37.7, SD=7.87) completed the survey and were included in the analyzes, resulting in a total sample size of N=107 in sample 1 and N=93 in sample 2, and an overall sample size of N=200 (Fig. 1).
FIGURE 1.
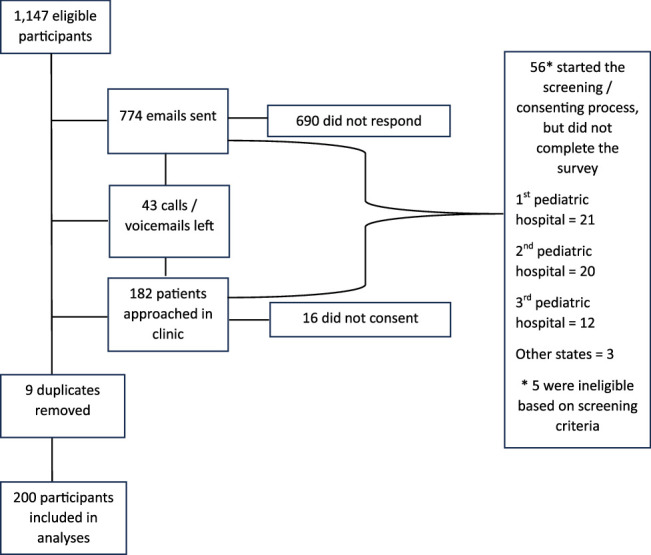
Consort diagram of study sample recruitment and enrollment.
Patients and caregivers primarily self-identified as Black or African American (n=177, 88.5%) and female (n=146, 73%). Most patients received the flu vaccine for the 2020-2021 season (n child=72, 77.4%; n teen=35, 81.4%; n young adult=48, 75.0%). Overall, 23% (n=45) of participants reported experiencing discrimination. Zip code data revealed that 55.6% (n=105; 11 missing) of participants reside in “Low-Resourced” communities. Table 1 displays participant demographic and clinical characteristics. There were no differences between those who completed the survey and did not complete the survey with respect to age group or SCD clinic where the participant/child receives care.
TABLE 1.
Participant Characteristics by Age Group
| n (%) | |||
|---|---|---|---|
| Adolescents (N=43) | Young adults (N=64) | Parents/caregivers (N=93) | |
| Age, mean (SD) (y) | 15.1 (4.74) | 21.9 (5.73) | 37.7 (7.87) |
| Range | 12-17 | 5-47 | 25-60 |
| Sex | |||
| Female | 21 (49) | 46 (72) | 79 (85) |
| Male | 22 (51) | 18 (28) | 14 (15) |
| Race | |||
| Black or African American | 39 (91) | 55 (86) | 83 (89) |
| Multiracial | 3 (7) | 5 (8) | 4 (4) |
| White | 1 (2) | — | 3 (3) |
| Asian/Pacific Islander | — | 1 (2) | 1 (1) |
| Other | — | 3 (5) | 2 (2) |
| Ethnicity | |||
| Hispanic | 3 (7) | 5 (92) | 7 (7) |
| Non-Hispanic | 40 (93) | 59 (8) | 86 (93) |
| SCD Genotype | n=13 | ||
| HbSS | 25 (58) | 40 (63) | 3 (23) |
| HbSC | 11 (26) | 16 (25) | 8 (62) |
| HbSβ + thalassemia | 4 (9) | 4 (6) | — |
| HbSβ 0 thalassemia | 1 (2) | 2 (3) | 1 (8) |
| Other | — | 2 (3) | — |
| Do not know | 2 (5) | — | 1 (8) |
| SCD treatments | |||
| Transfusions | 8 (19) | 26 (41) | 18 (19) |
| Hydroxyurea | 20 (47) | 34 (53) | 57 (61) |
| Crizilizumab | — | 3 (5) | 18 (19) |
| Voxelotor | 3 (7) | 3 (5) | 2 (2) |
| l-Glutamine | — | 1 (2) | 2 (2) |
| Deferasirox | 1 (2) | 3 (5) | 2 (2) |
| Flu shot in 2020-2021 | 35 (81) | 48 (75) | 49 (53) |
| Child flu shot in 2020-2021 | — | — | 72 (77) |
| Experienced discrimination | 2 (5) | 20 (31) | 23 (25) |
Sample demographics were consistent with data from the overall sample (N=200).
HbSβ+thalassemia indicates hemoglobin genotype sickle beta thalassemia; HbSC, hemoglobin genotype SC; HbSS, hemoglobin genotype SS; SCD, sickle cell disease.
Perceptions and Normative Beliefs
Approximately, ∼30% of adolescents and young adults reported having COVID-19 and 27% were concerned they were likely to get COVID-19. Among caregivers, 23% reported having COVID-19 and 18% thought their child was likely to get COVID-19. About 50% of adolescents, young adults, and caregivers reported perceiving that COVID-19 might make them or their child seriously ill.
With respect to COVID-19 vaccines, ∼50% of adolescents and young adults worried that COVID-19 vaccines might make their SCD worse, or cause pain or SCD complications. Fewer caregivers reported these concerns about SCD complications (30% to 42%). Of the adolescents and young adults, 35% and 39%, respectively, reported concerns about COVID-19 vaccine side effects. Approximately 60% and 67% of adolescents and young adults, respectively, perceived it would be safe to get the COVID-19 vaccine. Overall, participants felt strongly that their doctors would want them to get a COVID-19 vaccine and held positive beliefs about vaccines in general (Table 2).
TABLE 2.
Attitudes, Beliefs, and Intentions About COVID-19 and Vaccines by Adolescents, Young Adults, and Caregiver
| n (%) | |||
|---|---|---|---|
| Adolescents (N=43) | Young adults (N=64) | Caregiver (N=93) | |
| N (%) | |||
| COVID-19 attitudes and beliefs | |||
| Risk perception for COVID-19 | |||
| Ever have COVID-19 | 13 (30.2) | 21 (32.8) | 22 (23.7) |
| Susceptibility for COVID-19 | |||
| Likely to get COVID-19 | 10 (27)* | 16 (27.1)† | 17 (18.3) |
| Severity of COVID-19 | |||
| No worse than cold | 7 (6.5) | 3 (7.3)‡ | 7 (6.5) |
| Worse than cold but not serious | 24 (22.4) | 6 (14.6) | 24 (22.4) |
| Seriously Ill | 54 (50.5) | 20 (48.8) | 54 (50.5) |
| Not sure | 22 (20.6) | 12 (29.3) | 22 (20.6) |
| Perceived stigma related to COVID-19 | |||
| Discriminate against me | 4 (9.3) | 9 (14.1) | — |
| Vaccine-related attitudes, beliefs, intentions | |||
| SCD and COVID-19 vaccine | |||
| Make SCD worse | 22 (51.2) | 33 (53.2)§ | 60 (30) |
| Cause a pain crises | 11 (25.6) | 20 (31.3) | 39 (42.9)∥ |
| Perceived barriers to COVID-19 vaccine | |||
| Side effects | 17 (39.5) | 23 (35.9) | — |
| Not been tested on people like me | 11(25.6) | 14 (22.6)§ | — |
| Perceived benefits of COVID-19 Vaccine | |||
| Safe to get the vaccine | 29 (67.4) | 38 (59.3) | 40 (44)§ |
| Normative beliefs about vaccines | |||
| My doctor would want me to get vaccine | 34 (79) | 57 (89) | 75 (80.6) |
| General vaccine beliefs | |||
| Vaccines are effective | 26 (60.4) | 48 (75) | 72 (77.4) |
| Vaccines are important for health | 28 (65.1) | 51 (79.7) | 62 (66.7) |
| Do what doctor tells about vaccines | 25 (58.1) | 39 (60.9) | 60 (64.5) |
| COVID-19 vaccine intentions | |||
| Intention to receive COVID-19 vaccines | 28 (65.1) | 40 (62.5) | 45 (48.4) |
| Intention to receive COVID-19 booster | 12 (27.9)¶ | 14 (21.9)# | 16 (17.2)** |
Data from 6 participants missing.
Data from 5 participants missing.
Data from 23 participants missing.
Data from 2 participants missing.
Data from 1 participant missing.
Data from 17 participants missing.
Data from 26 participants missing.
Data from 49 participants missing.
SCD indicates sickle cell disease.
Vaccine/Booster Intentions
Of the 200 participants, 65.1% of adolescents, 62.5% of young adults, and 48.4% of caregivers intended to receive a COVID-19 vaccine for their child and 46.2% of adolescent and 36.8% of young adults intended to receive a booster; 36.4% of caregivers intended to receive a booster for their child (n=16). No caregivers intended to receive a booster for themselves (Table 2).
Sources of Information
Most participants reported that the doctor was their primary source of information (n=127, 60.8%), followed by self-guided research on the internet (n=46, 22%), co-workers, friends, or family (n=12, 5.7%), and local public health officials (n=11, 5.3%).
Educational Preferences
Participants preferred the following ways to learn about COVID-19 vaccines: videos from sickle cell leaders and experts (ranked first), videos from others living with sickle cell (second), email updates or websites for self-learning and (tied for third), townhall meetings with questions and answers (fourth) and podcasts (fifth).
Factors Associated with Patients’ Intention to Receive a COVID-19 Vaccine/Booster
In the logistic regression analysis for sample 1 (adolescents and adults), we observed one statistically significant predictor of patients’ intention to receive a COVID-19 vaccine (Table 3): perception that the COVID-19 vaccine is safe (odds ratio [OR]=4.29, P=0.005, 95% CI=1.55-11.86), such that participants are over 4 times more likely to intend to receive the COVID-19 vaccine with every 1-point increase an safety perception scores. Normative beliefs, SCD pain crisis, side effects, discrimination, and age were not statistically significant predictors of intention to receive the vaccine. For patients’ intention to receive a COVID-19 booster, participant age was a statistically significant predictor, (Table 3) OR=0.86, P<0.001, 95% CI=0.81-0.92), such that participants were ∼14% less likely to intend to receive a COVID-19 booster with each one-year increase in age. Perceived discrimination, safety perception, side effects, normative beliefs, and SCD pain crisis were not statistically significant predictors of intention to receive a booster.
TABLE 3.
Multivariable Logistic Regression Analyzes for Assessing Associations With Vaccine Intentions by Patient and Caregiver
| Adolescents and young adults | Caregivers | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| COVID-19 vaccine intention | ||||
| Participant characteristics | ||||
| Age | 1.02 (0.92-1.14) | 0.63 | 0.96 (0.86-1.07) | 0.43 |
| Experienced discrimination | 1.11 (0.31-3.94) | 0.16 | 1.14 (0.16-7.92) | 0.90 |
| Attitudes and beliefs | ||||
| Vaccine will cause SCD pain crisis | 0.99 (0.60-1.65) | 0.98 | 0.62 (0.33-1.15) | 0.13 |
| Vaccine will cause side effects | 0.63 (0.34-1.14) | 0.12 | 0.89 (0.43-1.85) | 0.76 |
| Safe to get COVID-19 vaccine | 4.29 (1.55-11.86) | 0.01* | 10.29 (3.28-32.2) | 0.00* |
| SCD doctor recommend vaccine | 1.02 (0.61-1.73) | 0.91 | 0.93 (0.64-1.36) | 0.72 |
| COVID-19 vaccine booster | ||||
| Participant characteristics | ||||
| Age | 0.86 (0.81-0.92) | 0.00* | 1.01 (0.89-1.14) | 0.81 |
| Experienced discrimination | 0.85 (0.25-2.94) | 0.80 | 0.46 (0.07-3.10) | 0.43 |
| Attitudes and beliefs | ||||
| Vaccine will cause SCD pain crisis | 1.16 (0.68-1.98) | 0.58 | 0.76 (0.38-1.50) | 0.43 |
| Vaccine will cause side effects | 0.80 (0.45-1.43) | 0.45 | 0.90 (0.45-1.82) | 0.78 |
| Safe to get COVID-19 vaccine | 1.43 (0.71-2.87) | 0.32 | 3.30 (1.21-9.00) | 0.02* |
| SCD doctor recommend vaccine | 1.33 (0.88-2.00) | 0.17 | 1.07 (0.63-1.83) | 0.81 |
OR Indicates odds ratio; SCD, sickle cell disease.
Statistically significant, P<0.05.
Factors Associated With Caregivers’ Intention to Receive a COVID-19 Vaccine/Booster
For sample 2 (caregivers), perception that the COVID-19 vaccine is safe was also the only factor associated with caregivers’ intention (OR=10.29, P<0.001, 95% CI=3.28-32.23), such that caregivers are over 10 times more likely to intend to accept the vaccine for their child with every one-point increase in safety perception scores. Similarly, perception that the COVID-19 vaccine is safe was a statistically significant predictor of caregivers’ intention (OR=3.30, P=0.02, 95% CI=1.21-9.00; Table 3), such that caregivers were 3.3 times more likely to intend to accept the COVID-19 booster for their child with every one-point increase in safety perception scores. Normative beliefs, SCD pain crisis, side effects, discrimination, and age were not statistically significant predictors of caregivers’ intention to receive the vaccine or the booster for their child.
DISCUSSION
The current study examined attitudes, beliefs and intentions about COVID-19 and COVID-19 vaccines in adolescents with SCD and caregivers of children with SCD across 3 pediatric SCD clinics. Approximately, 30% of adolescents reported having COVID-19 and about 23% of caregivers reported that their child with SCD had COVID-19. Despite SCD being considered an at-risk illness by the CDC, only about 50% of participants felt that COVID-19 would lead to serious illness. There are many possible reasons for this finding (eg, some participants experienced mild COVID-19 disease, some may not have had COVID-19, etc.). However, given the documented increased risk in SCD, patients may benefit from specific educational interventions that ensure they have an accurate understanding of the risk of COVID-19.
It is promising that most participants in our sample reported that they or their child received the 2020/2021 influenza vaccine; our rates were higher than previous reports.29,30 Studies have shown those who receive one recommended vaccine are more likely to receive other recommended vaccines. 31,32 However, given that there has been an overall decline in influenza vaccine rates,33 interventions to address the combination of the influenza and COVID-19 vaccines may be needed.
It is encouraging that more than half of adolescents and young adults intended to receive a COVID-19 vaccine for themselves but concerning that only about 48% of caregivers intended to receive a COVID-19 vaccine for their child with SCD. A recent study found self-reported vaccination rates for adolescents and caregivers with SCD ranged from 49% to 52%.11 Caregiver concerns about the efficacy and safety of the COVID-19 vaccine may impact their decision to vaccinate their child with SCD. It is also possible that this finding reflects the mistrust in health care that has been documented across studies including within pediatric SCD.11,34 To address this, mass communication campaigns using lay language could be implemented in building pro-COVID-19 vaccination normative beliefs, while targeted communication interventions by clinicians, could be used to address individual-level, caregiver and patient vaccine hesitancy as these types of interventions also help to build trust.
A major finding for all groups—adolescents, young adults, caregivers—was perceiving the COVID-19 vaccines as safe was associated with intention to receive the vaccine which is consistent with other studies.12–14,16,35 Half of adolescent and young adults reported a belief that the COVID-19 vaccine would make SCD worse and 25% to 43% of participants reported a belief that the COVID-19 vaccine would cause a pain crisis. Adolescent and young adults that reported a belief the COVID-19 vaccine would cause side effects were 20% less likely to intend to get the COVID-19 vaccine. Approaches to address concerns about COVID-19 vaccine safety and SCD-related complications and side effects can include: caregiver-centered motivational interviewing, focused responses to specific concerns, and provider statements endorsing the vaccine.36,37 However, clinicians have reported being underprepared and needing training on such strategies to address vaccine hesitancy.38,39 Therefore, developing and evaluating trainings on such strategies—for all vaccines and specifically for COVID-19 vaccines—is critical to increasing vaccination rates.38,39
A trusted source of COVID-19 vaccine information are doctors or the clinical team.16,35 Strong vaccine recommendations from clinicians is a well-established determinant of vaccination uptake.40 However, as previously noted, clinicians may lack specific training on addressing COVID-19 vaccine hesitancy. To fully leverage providers’ position as trusted sources of information and increase the likelihood of COVID-19 vaccine uptake, interventions should consider using effective modalities, such as interactive and immersive virtual reality curricula,41,42 to train providers in communication skills when encountering vaccine hesitancy.
Other studies have demonstrated that caregivers’ vaccine decision-making is influenced by their social networks, including family members and co-workers.43,44 Our sample ranked this group as less influential as a source of information than their medical team; yet, hearing from others in the SCD community about the vaccine was ranked as a preferred educational strategy. Thus, it will be important to develop educational campaigns specific to the SCD community using preferred channels and messaging. Based on current study findings and the literature, these campaigns could include nudges that focus on the need for COVID-19 vaccines, their safety, and the relative risk and duration of SCD specific side effects.11,45
Limitations
Our study had several limitations. First, we recruited a convenience sample primarily at 3 institutions, which may reduce the generalizability of the findings. Second, this was a cross-sectional study with data collection occurring over a 9-month period in 2021-2022. Perceptions of the vaccine may have changed over time as the vaccines became more widely available and more booster shots have been recommended. Third, these data were collected using self-reported measures which have the potential for recall biases and socially desirable responses. In addition, several predictors were single-item measures, thus we were unable to test validity and reliability. Fourth, though intention is correlated with behavior, the current study did not follow participants to ascertain if a COVID-19 vaccination was received. Finally, we did not measure other important factors that may impact vaccine hesitancy and intention such as educational attainment, health literacy, and financial hardship.11,33
CONCLUSIONS
Our study found moderate to low rates of COVID-19 vaccine intention among adolescents and young adults with SCD and their caregivers. Perceptions about COVID-19 vaccine safety and age were statistically significant factors impacting vaccine intention and may impact subsequent behavior (ie, receipt of the vaccine). Strengths of this study include its multisite design, theory-based examination, and goal of levering the data to guide the development of educational and communication interventions. Developing interventions to enhance positive COVID-19 vaccine attitudes and beliefs that increase exposure to reputable medical sources of information (eg, videos from sickle cell and vaccine experts) have potential to increase vaccine intention. Next steps include focus groups to better understand key messages for subpopulations (adolescents, caregivers) and an iterative process that includes collaboration with patients and caregiver to design educational media and scripts/support tools for clinicians and patients and caregivers during vaccine discussions. In conclusion, our study provides valuable and insightful data regarding salient concerns impacting vaccination intention for pediatric patients with SCD and is timely given that the FDA and CDC are considering annual COVID-19 boosters.5
Footnotes
B.L.R., L.M.S., and L.C., designed the research; C.A.M., E.O., and F.J. performed the research and collected data; C.A.M., C.M., B.M., and L.C. analyzed the data; L.S. and L.C. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Supported by research funding from the ASH Research Collaborative to A.A.T., L.M.S., and L.C. This study was also supported by funding from the Health Resource and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) as part of the Sickle Cell Treatment Demonstration Project Sickle Cell Treatment and Outcomes Research in the Midwest (STORM) to L.M.S.
L.C. has contributed to a Novartis speaker series for which she received an honorarium, has served on advisory boards for Novo Nordisk (Forma Therapeutics), and receives payment from Professional Resource Exchange for a book she co-authored. L.C. has served on advisory boards for Novo Nordisk, Sanofi Genzyme, Sobi, and Takeda. A.A.T. received research support from Alexion Pharmaceuticals and provided consulting work for Apellis Pharmaceuticals. L.C.'s institution has received research support on her behalf from Takeda. The remaining authors declare no competing financial interests.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.jpho-online.com.
Contributor Information
Lisa M. Shook, Email: lisa.shook@cchmc.org.
Brittany L. Rosen, Email: Brittany.Rosen@cchmc.org.
Constance A. Mara, Email: constance.mara@cchmc.org.
Cami Mosley, Email: cami.mosley@cchmc.org.
Alexis A. Thompson, Email: thompsona7@chop.edu.
Kim Smith-Whitley, Email: whitleyk@email.chop.edu.
Lisa Schwartz, Email: schwartzl@chop.edu.
Christina Barriteau, Email: CBarriteau@luriechildrens.org.
Allison King, Email: king_a@wustl.edu.
Eniola Oke, Email: EOke@luriechildrens.org.
Fatoumatou Jallow, Email: jallowf@chop.edu.
Bridget Murphy, Email: bridget.murphy@cchmc.org.
Lori Crosby, Email: lori.crosby@cchmc.org.
REFERENCES
- 1. Mucalo L, Brandow AM, Dasgupta M, et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv. 2021;5:2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliver SE, Wallace M, Link-Gelles R. COVID-19 vaccines: safe and effective in children aged 5 to 11 years. Pediatrics. 2022;150:e2022057314. [DOI] [PubMed] [Google Scholar]
- 3. Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). COVID Data Tracker; 2023.
- 5. Rubin R. Questions remain about what SARS-CoV-2 variants should go into the annual COVID-19 vaccines proposed by the FDA. JAMA. 2023;329:697–700. [DOI] [PubMed] [Google Scholar]
- 6. Kiviniemi MT, Ellis EM, Hall MG, et al. Mediation, moderation, and context: understanding complex relations among cognition, affect, and health behaviour. Psychol Health. 2018;33:98–116. [DOI] [PubMed] [Google Scholar]
- 7. Strecher VJ, Rosenstock IM. Andrew Baum, Stanton Newman, John Weinman, Robert West. The health belief model. Cambridge Handbook of Psychology, Pitt Building, Trumpington Street, Cambridge CB2 IRP, United Kingdom. Health and Medicine 1997: 117. [Google Scholar]
- 8. Glanz K, Rimer BK, Viswanath K. Health Behavior: Theory, Research, and Practice. John Wiley & Sons; 2015. [Google Scholar]
- 9. Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med. 2012;166:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu A, Gupta V, Unni EJ. Utilizing the theory of planned behavior to determine the intentions to receive the influenza vaccine during COVID-19: a cross-sectional survey of US adults. Prev Med Rep. 2021;23:101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Persaud Y, Mandrell BN, Sharma A, et al. Attitudes toward COVID-19 vaccine among pediatric patients with sickle cell disease and their caregivers. Pediatr Blood Cancer. 2023;70:e30274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams SH, Schaub JP, Nagata JM, et al. Young adult perspectives on COVID-19 vaccinations. J Adolesc Health. 2021;69:511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers AA, Cook RE, Button JA. Parent and peer norms are unique correlates of COVID-19 vaccine intentions in a diverse sample of US adolescents. J Adolesc Health. 2021;69:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brandt EJ, Rosenberg J, Waselewski ME, et al. National study of youth opinions on vaccination for COVID-19 in the US. J Adolesc Health. 2021;68:869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budhwani H, Maycock T, Murrell W, et al. COVID-19 vaccine sentiments among African American or Black adolescents in rural Alabama. J Adolesc Health. 2021;69:1041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher CB, Gray A, Sheck I. COVID-19 pediatric vaccine hesitancy among racially diverse parents in the United States. Vaccines. 2021;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher C, Bragard E, Madhivanan P. COVID-19 vaccine hesitancy among economically marginalized hispanic parents of children under five years in the United States. Vaccines. 2023;11:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourque SL, Weikel BW, Palmer C, et al. Prevalence and predictors of pediatric COVID-19 vaccine acceptance. Am J Perinatol. 2023;40:106–114. [DOI] [PubMed] [Google Scholar]
- 19. Rosen BL, Meisman A, Sun Q, et al. Factors associated with racially and ethnically diverse sample of adolescents, young adults, and parents’ intention to receive a COVID-19 vaccine. Am J Health Promot. 2024. [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Regional Office for Europe. Survey tool and guidance: rapid, simple, flexible behavioural insights on COVID-19: Copenhagen, Denmark; 2020.
- 22. Krieger N, Smith K, Naishadham D, et al. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61:1576–1596. [DOI] [PubMed] [Google Scholar]
- 23. Kahn JA, Rosenthal SL, Jin Y, et al. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstet Gynecol. 2008;111:1103–1110. [DOI] [PubMed] [Google Scholar]
- 24. Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt). 2009;18:1679–1686. [DOI] [PubMed] [Google Scholar]
- 25. Schneider G, Rosenthal SL, Lan D, et al. 6: HPV-related stigma in female adolescents. J Adolesc Health. 2006;38:107. [Google Scholar]
- 26. Centers for Disease Control and Prevention (CDC). Quarantine and isolation; 2021.
- 27. Shapiro GK, Tatar O, Dube E, et al. The vaccine hesitancy scale: psychometric properties and validation. Vaccine. 2018;36:660–667. [DOI] [PubMed] [Google Scholar]
- 28. Ferrell B, Tharakan J, Nguyen D, et al. Utilization of distressed communities index to examine the impact of socioeconomic status on lung transplant outcomes. J Heart Lung Transplant. 2022;41:S145. [Google Scholar]
- 29. Beverung LM, Brousseau D, Hoffmann RG, et al. Ambulatory quality indicators to prevent infection in sickle cell disease. Am J Hematol. 2014;89:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Payne AB, Adamkiewicz TV, Grosse SD, et al. Influenza vaccination rates and hospitalizations among Medicaid enrollees with and without sickle cell disease, 2009–2015. Pediatr Blood Cancer. 2021;68:e29351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kessels SJ, Marshall HS, Watson M, et al. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30:3546–3556. [DOI] [PubMed] [Google Scholar]
- 32. Bish A, Yardley L, Nicoll A, et al. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29:6472–6484. [DOI] [PubMed] [Google Scholar]
- 33. Bonacina F, Boëlle P-Y, Colizza V, et al. Global patterns and drivers of influenza decline during the COVID-19 pandemic. Int J Infect Dis. 2023;128:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khairat S, Zou B, Adler-Milstein J. Factors and reasons associated with low COVID-19 vaccine uptake among highly hesitant communities in the US. Am J Infect Control. 2022;50:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szilagyi PG, Shah MD, Delgado JR, et al. Parents’ intentions and perceptions about COVID-19 vaccination for their children: results from a national survey. Pediatrics. 2021;148:e2021052335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edwards KM, Hackell JM, COmmittee on Infectious Diseases, The Committee on Practice and Ambulatory Medicine . Countering vaccine hesitancy. Pediatrics. 2016;138:e20162146. [DOI] [PubMed] [Google Scholar]
- 37. Braun C, O’Leary ST. Recent advances in addressing vaccine hesitancy. Curr Opin Pediatr. 2020;32:601–609. [DOI] [PubMed] [Google Scholar]
- 38. Mohanty S, Carroll-Scott A, Wheeler M, et al. Vaccine hesitancy in pediatric primary care practices. Qual Health Res. 2018;28:2071–2080. [DOI] [PubMed] [Google Scholar]
- 39. Gowda C, Schaffer SE, Dombkowski KJ, et al. Understanding attitudes toward adolescent vaccination and the decision-making dynamic among adolescents, parents and providers. BMC Public Health. 2012;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18:149–207. [DOI] [PubMed] [Google Scholar]
- 41. Real FJ, Ollberding NJ, Meisman AR, et al. Impact of a virtual reality curriculum on human papillomavirus vaccination: a pilot trial. Am J Prev Med. 2022;63:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Real FJ, DeBlasio D, Beck AF, et al. A virtual reality curriculum for pediatric residents decreases rates of influenza vaccine refusal. Acad Pediatr. 2017;17:431–435. [DOI] [PubMed] [Google Scholar]
- 43. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397–e1404. [DOI] [PubMed] [Google Scholar]
- 44. Stokes S, Ismail K. Uptake of the H1N1 vaccine by maternity staff at a university hospital in the UK. Int J Gynaecol Obstet. 2011;112:247. [DOI] [PubMed] [Google Scholar]
- 45. Navin MC. The Ethics of Vaccination Nudges in Pediatric Practice. HEC forum: Springer; 2017:43–57. [DOI] [PubMed] [Google Scholar]


