Abstract
Patients with decompensated end-stage liver disease (ESLD) are at increased risk for mortality, and only liver transplantation (LT) offers meaningful hope for survival. These patients are at risk for kidney dysfunction through the continuum of care for ESLD including LT. We discuss the role of accurate estimation and measurement of baseline glomerular filtration rate in assessment of kidney dysfunction among those with ESLD. Optimizing kidney function is a vital goal in the management of these patients before LT. In this review, we summarize salient aspects of assessing and optimizing kidney function in this patient population. Precipitating factors and different causes of acute kidney injury are discussed, including hepatorenal syndrome. We further review treatment options for acute kidney injury including volume management. The role of vasopressor therapy, renal replacement therapy, and transjugular intrahepatic portosystemic shunting are discussed.
INTRODUCTION
Optimizing kidney function is vital in patients with end-stage liver disease (ESLD). Although commonly described as “replaceable” with renal replacement therapies (ie, dialysis), the kidneys remain central to homeostasis, doing much more than what dialysis can do. There remains a considerable difference in survival among patients with kidney dysfunction or those requiring dialysis compared with those without kidney dysfunction.1,2 This remains true in a number of chronic medical conditions, including those with ESLD, where it correlates closely with morbidity and mortality.1 This is epitomized using the Model for End-stage Liver Disease (MELD), which includes serum creatinine as a key variable, for prioritizing organ allocation in liver transplantation (LT) in the United States since 2002.
Patients with ESLD have significant nonliver medical comorbidities, such as esophageal varices, refractory ascites, hepatic encephalopathy, and so on. Kidney dysfunction is an important comorbid condition that can arise because of ESLD, for example, hepatorenal syndrome, or because of unrelated mechanisms, for example, intrinsic renal diseases. Here, we review the commonly encountered challenges in these complex patients and focus on ways to optimize and preserve kidney health in candidates for LT.
MEASUREMENT OF GFR IN ESLD
The first and most crucial step in evaluation of kidney dysfunction is the assessment of accuracy for measuring “normal” kidney function in patients with ESLD. By default, serum creatinine is used to estimate glomerular filtration rate (GFR) in patients with ESLD. Creatinine is a component of MELD score and, hence, directly impacts clinical decision-making regarding candidacy for either LT versus simultaneous liver kidney transplant (SLKT). Additionally, changes in serum creatinine lag the onset of acute kidney injury (AKI), significantly limiting its utility in reflecting a change in measured GFR. Failure to detect a true drop in GFR can lead to a failure to identify AKI early, leading to subclinical progression of chronic kidney disease (CKD). Conversely, improvement in serum creatinine is taken as “resolution of kidney dysfunction”; however, studies have shown that it can be inaccurate.3 Thus, accurate GFR measurement has wide-ranging implications from measuring incidence, resolution, and treatment of AKI to subsequent candidacy for LT alone versus SLKT.
Endogenous and Exogenous Molecules for GFR Measurement Among Those With ESLD
Creatine is produced in the liver and is metabolized to creatinine in skeletal muscle. Kidney handling of creatinine includes free filtration in the glomerulus with no reabsorption or metabolism, although approximately 10%–40% can be secreted by proximal convoluted tubule.4 Cirrhosis leads to reduced creatine production, and sarcopenia leads to decreased metabolism leading to reduced serum creatinine. This can further be diluted because of increased extracellular volume in a volume expanded state such as ESLD. All these factors can lead to an overestimation of eGFR.
Urinary creatinine and urine output along with serum creatinine have been utilized to estimate eGFR with 24-h CrCl. This can be advantageous over SCr-based estimations, as it takes into account urine output. However, suboptimal urine collection both in duration and quantity can impact its reliability and can be cumbersome for those with ESLD already burdened by a variety of symptoms, such as loose stools because of laxatives for hepatic encephalopathy prevention. Proulx et al5 reviewed several studies and a meta-analysis concluded that 24-h CrCl overestimates mean GFR significantly among those with GFR of <30 mL/min by up to 49% (13 mL/min).
Cystatin C is another endogenous molecule produced by all nucleated cells at a constant rate that has been studied extensively to estimate GFR in ESLD. Serum cystatin C levels are inversely related to eGFR, as it is completely filtered and reabsorbed/metabolized by proximal convoluted tubule. Cystatin C levels promise to be a great molecule for eGFR, as it is independent of gender, ethnicity, muscle mass, elevated bilirubin levels, and ESLD. However, it is impacted by non-ESLD and nonkidney-related medical conditions resulting in under and over estimation of eGFR among those with ESLD.
Exogenous Molecules for Measurement of GFR
Inulin is a naturally occurring fructon carbohydrate that it is freely filtered and not reabsorbed or secreted. It is considered the gold standard against which other methods of GFR estimation are validated. Other exogenous molecules include iohexol, sinistrin, and nuclear radiolabeled isotopes, such as 51Cr-EDTA, 51Chromium-ethylenediamine tetraacetic acid, 99mTc-DTPA, 99mTc-diethylene triamine penta-acetic acid, 125I-IOT, 125I-iothalamate, and so on. Advantages and disadvantages of exogenous molecular GFR measurement are discussed in Table 1.
TABLE 1.
Estimation and measurement of GFR techniques and formulae in ESLD
| Estimation of GFR | Variables | Pros | Cons |
|---|---|---|---|
| CG equation5,6 | Age, weight, SCr, TBW, sex | Still used for drug dosing, because of availability of pharmacokinetic data from drug dosing studies | Does not take into account body surface area Creatinine laboratory values were not standardized Did not include ESLD patients in derivation |
| MDRD-4 and MDRD-67 | Age, sex, ethnicity, serum creatinine, urea, and albumin | MDRD-6 has relatively better correlation with mGFR especially among those with mGFR of <30 mL/min/m2 Accounts for BSA Albumin was included |
Derived from cohort of healthy individuals, ESLD not included Lack of pharmacokinetic data for drug dosing Race was included as a variable Overestimates eGFR |
| CKD-EPI: SCr8 | Age, sex, creatinine, race | Standardized creatinine Better accuracy at eGFR >60 mL/min/1.73 m2 |
Race as a variable led to over estimation of eGFR in AA. Less accuracy of <60 mL/min/m2 ESLD population not included Overestimation SCr secretion increases with reduction of GFR Impacted by muscle mass and diet Hyperbilirubinemia may interfere with SCr measurement |
| CKD-EPI: CysC | Cystatin C | Independent of muscle mass, gender, and diet | Underestimates eGFR >60 mL/min Affected by nonkidney nonliver medical conditions Costly and not widely available ESLD population not included Lack of standardized testing |
| CKD-EPI SCr-CysC | Better accuracy for eGFR of <60 mL/min compared with CKD-EPI | ESLD population not included | |
| GRAIL9 | Creatinine, blood urea nitrogen, age, gender, race, and albumin Temporal testing CKD stage |
Prognostic ability to predict CKD post-LT Superior accuracy in estimating eGFR of <30 mL/min, that is, group requiring decision for LT alone vs SLKT Specifically modeled for those with ESLD |
Inclusion of race as a variable More studies needed to validate |
| CKD-EPI NMR10 | Age, sex, and creatinine | Removes as a variable can account for sarcopenia | Needs more studies |
| Measurement of GFR | |||
| Inulin | Urinary clearance of inulin | Gold standard, completely filtered, no reabsorption or secretion | Costly Time consuming, limiting serial assessments Invasive |
| Iohexol, 51Cr-EDTA, 99mTc-DTPA, 99mTc- 125I-IOT, 125I |
Exogenous markers | Less expensive and more available than inulin Less technically challenging |
Costly and time consuming limiting Serial measurements Anaphylactic risk with iohexol Radiation exposure Overestimation of GFR in hypervolemia/ascites in ESLD |
| Carboxymethylated dextran3,10 | Exogenous dextran | Point of care, rapid testing Ease of testing and serial measurements Validated in other hypervolemic state, that is, CHF |
Needs validation studies in ESLD |
AA, African Americans; BFA, body surface area; CG, Cockcroft Gault; CHF, congestive heart failure; CKD-EPI, chronic kidney disease epidemiology collaboration; CKD NMR, chronic kidney disease nuclear magnetic resonance; 51Cr-EDTA, 51Chromium-ethylenediamine tetraacetic acid; Cys, cystatin C; ESLD, end-stage liver disease; GFR, glomerular filtration rate; 125I-IOT, 125I-iothalamate; MDRD, modification of diet in renal disease; 99mTc-DTPA, 99mTc-diethylene triamine penta-acetic acid; SCr, serum creatinine; TBW, total body water.
Refining of Various eGFR Equations for Better Accuracy
To account for various factors impacting estimation of GFR various formulae and its modifications have been developed and studied to improve its accuracy including Cockcroft Gault, MDRD, and CKD-EPI equations. Their limitations among those with ESLD are summarized in Table 1. To further improve its accuracy, several variations of the MDRD formulae were developed though none of the cohorts studied in the derivation of these formulae included patients with ESLD. Despite this, these formulae are utilized for the vital aforementioned clinical purposes. In the past decade, cystatin C–based estimation of kidney function has been studied in patients with ESLD.6 A recent meta-analysis demonstrated that in general serum creatinine–based equations overestimate GFR and cystatin C–based equations underestimated GFR in ESLD.11 Notably, a CKD-EPI-based model, using both creatinine and cystatin C, had the highest accuracy and precision in those with preserved GFR and the least bias (ie, difference between estimated GFR and measured GFR). The same meta-analysis showed that in patients with eGFR of <60 or ascites, neither molecule was accurate in matching measured GFR. Francoz et al7 compared the relative accuracy of MDRD-4,12 MDRD-6,13 and CKD-EPI against standardized iohexol measurement. They showed that MDRD-4 (bias +15 mL/min/1.73 m2) and CKD-EPI (+9 mL/min/1.73 m2) overestimated GFR, whereas MDRD-6 underestimated (−9 mL/min/1.73 m2) compared with iohexol GFR. Overall, for those patients with ESLD who are in the eGFR range to be potentially considered for SLKT, that is, eGFR of <60 mL/min/1.73 m2, all 3 equations overestimated GFR, with MDRD still performing the best (6 mL/min/1.73 m2) for accuracy.
Modeling of variables specific to those with ESLD have also been studied to better characterize eGFR. Asrani et al9 did one of the largest studies among those with ESLD to develop the GRAIL (GFR assessment in liver disease) model comparing estimated GFR. Although the variables used are similar to MDRD-6, timing of eGFR measurement and renal dysfunction were incorporated in the model relative to LT improving the predictability of accurate eGFR of <30 mL/min/1.73 m2. Overall, the GRAIL model correctly classified 75% as having mGFR of <30 mL/min/1.73 m2 versus 36.1% (CKD-EPI), 36.1% (MDRD-4), and 52.8% (MDRD-6) (P < 0.01).
More recently, race as a variable has been removed from the CKD-EPI equation.14 Stammler et al10 showed an equation for eGFR based on serum myo-inositol, valine, and creatinine quantified by nuclear magnetic resonance spectroscopy in combination with cystatin C, age, and sex, labeled (GFRNMR). They showed that among ESLD patients with a MELD score of >15 or higher, eGFRNMR performed better with lower median bias (1 mL/min/1.73 m2 compared with CKD-EPI [Cr] with race excluded [16. 5 mL/min/1.73 m2] versus CKD-EPI eGFR Cr-cystatin C with race excluded [2 mL/min/1.73 m2]). More data are needed on GFRNMR among those with higher MELD scores.
Direct Measurement of GFR
Recently, a new technique using fluorescent-labeled intravenous dextran has been shown to accurately and quickly measure kidney function in patients with heart failure. Use of a large carboxymethylated dextran allows the calculation of total plasma volume, whereas smaller molecular dextran is freely filtered matching that of inulin.3,15 This technique allows measurement of GFR in real time, among those with normal or deranged kidney function. This study did not include any ESLD participants, but clinically, those with ESLD and heart failure have extracellular volume expansion with third spacing. Direct measurement of kidney function has the potential to benefit those who are at a disadvantage in the MELD scoring system (ie, frail, sarcopenic individuals, women who tend to have a lower serum creatinine). Direct measurement of GFR needs validation studies in those with ESLD, as it provides for an opportunity to perform serial assessment of kidney function at bedside in future clinical practice.
The MELD scoring system predicts 90-d mortality in LT candidates, and therefore, identifying and utilizing a more accurate index of GFR other than serum creatinine has the potential to make it an even more useful tool in this regard.
KIDNEY HEALTH IN ESLD
Patients with ESLD are vulnerable for kidney dysfunction in the form of AKI. Appreciation of kidney health in this period is important, as this has an overall impact on patient management and early and long-term prognosis. The main risk factors for kidney dysfunction and potential strategies to protect and preserve kidney function in this population are discussed below. Recognition and management of CKD in this population is equally important; however, it is beyond the scope of this review.
AKI
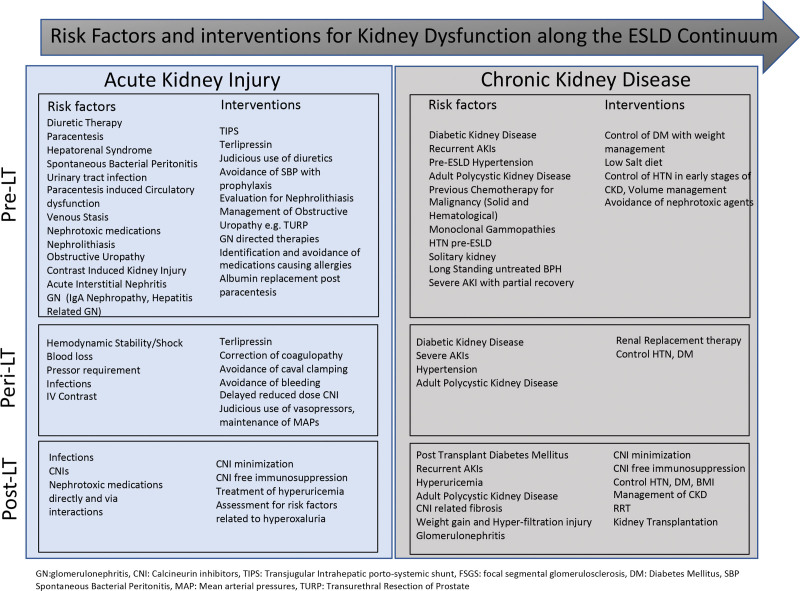
AKI among those with ESLD can be broadly classified as structural or functional (Figure 1). Structural AKI can be related to damage to any of the 4 histological components of the kidney, that is, glomeruli, tubules, interstitium, and/or vasculature. Functional AKI is related to altered systemic and intrarenal hemodynamics leading to low flow state, that is, hypoperfusion to the kidneys, reduction in venous outflow, and obstruction of urine flow. Usually, functional AKI is a result of pathology outside of the kidney, and quick diagnosis is important for potential reversibility. However, if severe or persistent, this can lead to structural AKI, causing damage to the kidney components leading to organ dysfunction. Structural AKI is more common in patients with ESLD because of repeated AKI episodes and limited capacity of the kidneys to respond to stressors.16 We know from living kidney donors that this capacity can account for up to 50% of GFR reserve, and patients with ESLD lacking this reserve come to light as AKI. The definition of AKI in ESLD has evolved over time, and 3 of the most common criteria used are summarized in Table 2.
FIGURE 1.
Risk factors and interventions for kidney dysfunction along the ESLD continuum. AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; CNI, calcineurin inhibitor; DM, diabetes mellitus; ESLD, end-stage liver disease; GN, glomerulonephritis; HTN, hypertension; IgA, immunoglobulin A; IV, intravenous; LT, liver transplant; MAP, mean arterial pressure; RRT, renal replacement therapy; TIPS, transjugular intrahepatic portosystemic shunt.
TABLE 2.
Definitions of AKI: 3 common staging systems to define and stage AKI
| System | Stage I | Stage II | Stage III |
|---|---|---|---|
| AKIN17 | SCr increase by 1.5- to 1.9-fold from baseline; SCr increase of ≥0.3 mg/dL; or urine output of <0.5 mL/kg/h >6 h | SCr increase 2.0–2.9 times baseline or urine output of <0. 5 mL/kg/h for >12 h | SCr increase 3.0 times baseline; SCr increase of ≥4.0 mg/dL with an acute increase of at least 0.5 mg/dL; or urine output of <0. 3 mL/kg/h >24 h or anuric for >12 h |
| KDIGO18 | SCr increase 1.5–1.9 times baseline; or Cr increase of ≥0.3 mg/dL or urine output of <0.5 mL/kg/h for 6–12 h | SCr increase 2.0–2.9 times baseline; or urine output of <0.5 mL/kg/h for >12 h | SCr increase 3.0 times baseline; SCr increase of ≥4.0 mg/dL; initiation of renal replacement therapy; or urine output of <0.3 mL/kg/h for 24 h or anuric for >12 h |
| IAC19 | SCr increase (0.3 mg/dL) or increase in SCr of ≥1.5–2.0 times from baseline | SCr increase >2.0–3.0 times from baseline | SCr increase >3.0 times from baseline; SCr of ≥4.0 mg/dL with an acute rise of 0.3 mg/dL; or initiation of renal replacement therapy |
AKIN, Acute Kidney Injury Network; IAC, International Ascites Club; KDIGO, Kidney Disease Improving Global Outcomes; SCr, serum creatinine.
RISK FACTORS FOR AKI
Understanding the cause of AKI in ESLD is important. In patients who develop AKI, an urgent diagnostic work up should be performed to identify a cause of the AKI for therapeutic and prognostic purposes. The most common mechanisms of AKI in ESLD are summarized in Table 3.20,21 The most common cause of AKI in patients with ESLD is infection.22 This is followed by volume depletion (prerenal azotemia) followed by hepatorenal syndrome–related AKI (HRS-AKI). Furthermore, ascites requiring LVP, refractory ascites, and over diuresis are important additional risk factors. It is important to know that often cause for AKI is multifactorial, and several primary and secondary causes may occur at the same time.
TABLE 3.
Etiologies of AKI and clinical scenarios
| Cause | Examples |
|---|---|
| “Prerenal” absolute or functional hypovolemia | Infection, gastrointestinal bleeding, gastrointestinal losses for example, diarrhea, hypovolemia |
| ATN | Nephrotoxins, progression of prerenal injury |
| GN | Hepatitis B and C related |
| IgA nephropathy | Most common finding, however, mostly with minimal clinical and biopsy findings |
| AIN | Antibiotics or PPI related |
| Drug-induced AKI | Inadequately adjusted doses for antimicrobials |
AIN, acute interstitial nephritis; AKI, acute kidney injury; ATN, acute tubular necrosis; GN, glomerulonephritis; IgA, immunoglobulin A; PPI, proton pump inhibitor.
Infections
Spontaneous bacterial peritonitis (SBP) and urinary tract infections23 remain the 2 most common infections among patients with ESLD.24 SBP accounts for up to 30%–50%25 of infections among hospitalized patients with ESLD. Increased propensity for infections results from immune system dysfunction, bacterial overgrowth, and increase intestinal permeability with passage of bacteria into the ascitic fluid. Urinary tract infections are the second most common cause of infections among patients with ESLD and are associated with increased 90-d mortality.23
Prerenal Azotemia
The kidneys receive about 25% of the cardiac output. Any condition that results in decreased effective blood volume with or without total body volume depletion can cause AKI (gastrointestinal bleeding, gastrointestinal losses, poor oral intake, and over diuresis).
Hepatorenal Syndrome
Hepatorenal syndrome (HRS) is a unique entity with reduced effective kidney perfusion leading to kidney dysfunction seen among those with ESLD and ascites. Historically HRS was divided into type 1 (acute) and type II (subacute). More recently, HRS is classified by the International Ascites Club as HRS-AKI and HRS-non-AKI (CKD) (HRS-CKD eGFR of <60 for >3 mo).26 HRS-AKI is defined as a stage II or higher stage AKI, with no response to diuretic withdrawal and/or volume expansion for at least 48 h, in the absence of shock, nephrotoxic medications, and lack of macroscopic signs of structural kidney damage, such as RBCs, absence of macroalbuminuria, or abnormalities on kidney imaging in the setting of cirrhosis with ascites.27 HRS-AKI is characterized by increased arteriolar vasodilation in the splanchnic circulation because of portal hypertension.28 Because of a resulting decrease in systemic vascular resistance, cardiac output rises, leading to a hyperdynamic circulation. Splanchnic vasodilation is thought to be a result of endothelial-derived vasoactive substances like nitric oxide29 and bacterial translocation30 into mesenteric lymph nodes. Net result of these hemodynamic changes result in intense vasoconstriction because of RAS activation and sympathetic nervous system of the renal arteries, as it senses a low flow state.31,32
Main risk factors include a higher baseline serum creatinine, lower mean arterial pressures, and cardiac dysfunction including diastolic dysfunction associated with persistent hyperdynamic circulation of decompensated cirrhosis.33,34 There are several precipitants of HRS, most notably LVP and infections, such as SBP and UTIs.
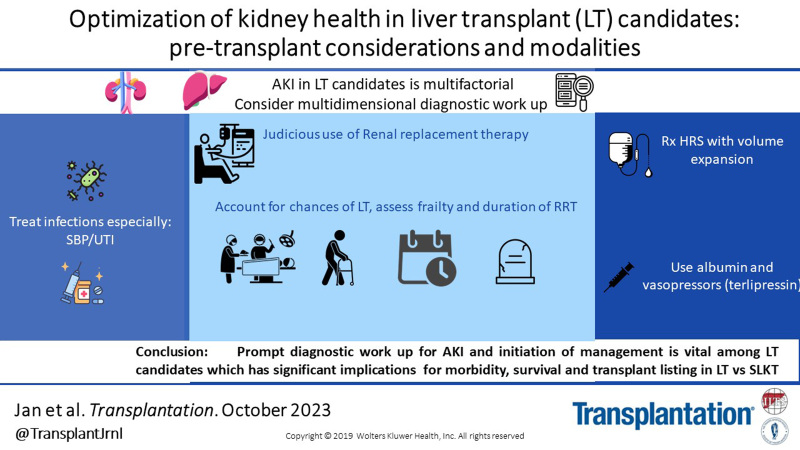
INTERVENTIONS/STRATEGIES TO PREVENT AND MANAGE AKI IN LIVER TRANSPLANT CANDIDATES
Management of HRS
Management of HRS focuses on reversal of the above hemodynamic changes. The goal is to reduce vasodilation and increase effective circulatory volume. When HRS is suspected, volume expansion with albumin at 1 g/kg/24 h for 48 h should be initiated early in the course, even if other causes for AKI are being simultaneously investigated. Other early interventions include discontinuation of nephrotoxic medications and avoidance of over diuresis. Any significant volume losses, for example, gastrointestinal losses because of ammonia-lowering therapies should be replaced appropriately. Therapies to increase the mean arterial pressures are key to reversing HRS-AKI. Additionally, a search for and treatment of any precipitating insults, such as infections or gastrointestinal bleeding, is vital. Initial studies35 of midodrine and octreotide showed improvement and reversal in HRS; however, more recent data are equivocal. Terlipressin, the vasoconstrictor of choice recommended by international guidelines, has been approved for HRS-AKI in Europe since 2010 with strong supporting evidence of efficacy.36–38 It recently received Food and Drug Administration approval for use in HRS-AKI in the United States based on the findings from the COMFIRM Trial.39 Terlipressin, a lysine vasopressin, has vasoconstrictor activity in the splanchnic circulation, leading to increase in venous return and resulting in decreasing renal artery vasoconstriction. Other studies done previously as summarized by Wang et al40 in a meta-analysis showed a reversal of HRS-AKI in up to 42% of those treated with terlipressin, whereas the CONFIRM trial showed a reversal of 32% as compared with 17% in the placebo arm.39 Interestingly, terlipressin infusion therapy was more effective and safer than bolus therapy and marks a new therapeutic opportunity for HRS-AKI.41 Caution is advised with the use of terlipressin in patients with HRS-AKI and grade-3 acute on chronic liver failure because of a higher incidence of pulmonary complications.42
Management of Ascites and Judicious Use of Diuretics
Ascites is a common and significant clinical complication of ESLD leading to significant symptom burden including abdominal discomfort, decreased oral intake, and severe cases intraabdominal hypertension.
Dietary sodium restriction and diuretic therapy are the cornerstones of ascites management. In the outpatient setting, initiation of spironolactone followed by the addition of a loop diuretic is the recommended strategy. Spironolactone has been used as a first-line diuretic and has well-established efficacy in nonazotemic advanced liver disease.43,44 More recently, finerinone, a nonsteroidal mineralocorticoid receptor blocker, has been shown to reduce albuminuria and slow the progression of CKD in patients without ESLD and is an attractive agent for future study in ESLD.45 It is important to start diuretic therapy at the smallest doses and gradually titrate up to avoid large shifts in intravascular volume. validated in large cohortskg/d without edema and 1 kg/d with edema is the recommended daily weight-loss goal.46,47 Close follow-up is critical with specific instruction and counseling on holding diuretics if other complications develop such as bleeding, diarrhea, vomiting, or infection. Continuation of diuretics in such cases have the potential to cause AKI. In diuretic-resistant and intractable ascites, LVP is necessary. LVP has been associated with paracentesis-induced circulatory dysfunction, which can cause AKI. The use of albumin infusion post-LVP has been shown to prevent paracentesis-induced circulatory dysfunction48 and hyponatremia. Hyponatremia is a risk factor HRS morbidity and mortality among those with ESLD.49–51
Transjugular Intrahepatic Portosystemic Shunt
The transjugular intrahepatic portosystemic shunt (TIPS) aims to increase renal perfusion. An improvement in GFR has been shown in studies after TIPS placement, most notably in patients with an eGFR of <60,52 although most LT candidates may not qualify. TIPS lowers portal hypertension, diverting blood flow away from the cirrhotic liver, which can further compromise liver function. Factors determining candidacy for TIPS include the risk of hepatic encephalopathy, predicted time to transplant, serum bilirubin, and platelet count. In general, ideal candidates for TIPS are younger individuals with MELD scores of <19 and those with MELD score driven by serum creatinine.53
Timely and Judicious Use of Renal Replacement Therapy
Use of renal replacement therapy (RRT) plays an important role in management of ESLD patients with AKI with or without volume overload. Judicious use of RRT includes consideration of factors such as timeframe and cause of AKI, and the probability of kidney recovery. Although some studies have described poor short- and long-term survival (>1 y) among patients with ESLD needing RRT,1,2 in potential LT candidates, RRT offers a critical life-sustaining therapy. It can provide time needed to complete LT evaluation and/or to clarify goals of care. Additionally, duration of RRT in AKI is one of the medical eligibility criteria for SLKT candidacy.
Liver Allocation, Eligibility Criteria for SLKT, and Safety Net
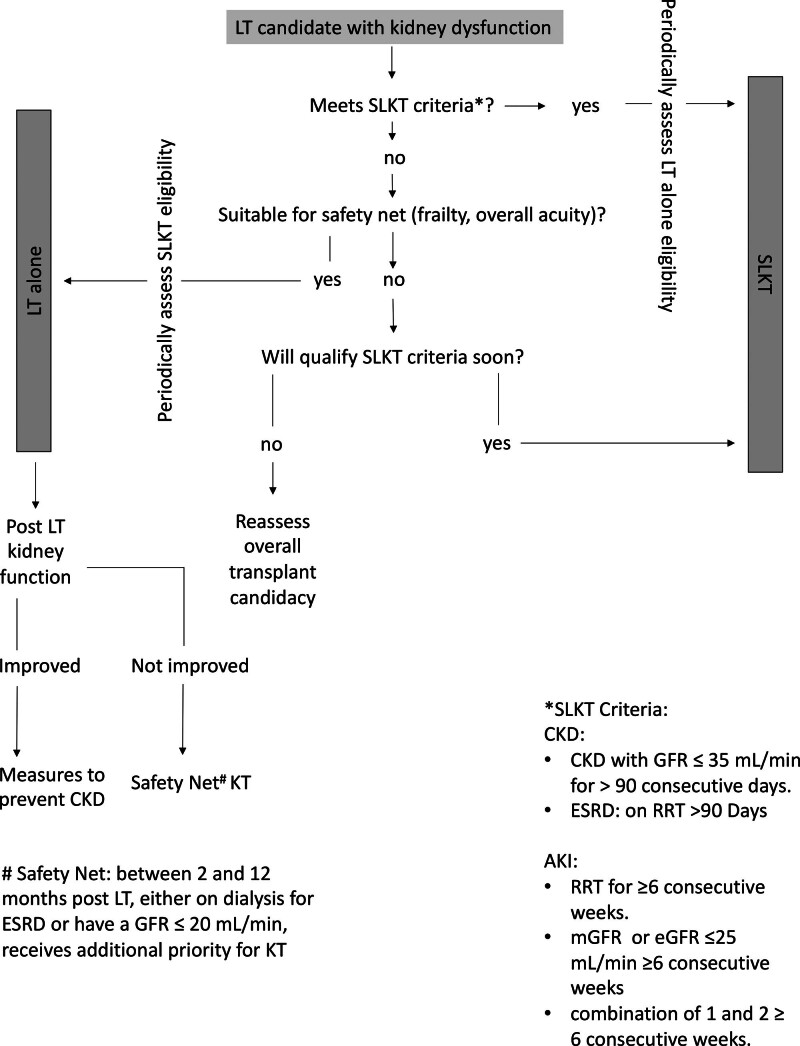
Understanding of organ allocation policies plays an important role in decision making and management of patients with ESLD with kidney dysfunction (Figure 2). Implemented in 2020, the acuity circles liver allocation policy has given even more importance to MELD score than before. Consequently, there is an increase in LT procedures in patients with kidney dysfunction. Previously, to address the steady rise in SLKT in the United States, the Organ Procurement and Transplant Network introduced a policy in August 2017,54 with a goal to establish clear rules for SLKT allocation and enhance equity in access to transplants. The eligibility for SLKT requires 6 wks of sustained AKI or 3 mo of CKD status. A key feature of the policy was the “safety net” provision, which permits LT recipients who did not meet SLKT criteria but with continued dialysis dependence or GFR of <20 mL/min within 2–12 mo after LT to receive priority for kidney transplantation (KT). It is crucial to periodically assess waitlisted patients’ candidacy for SLKT or LT alone as with time some patients would qualify for SLKT, and some patients listed for SLKT may show improvement in kidney function and may only need LT. It should be noted that some patients with persistent kidney dysfunction after LT may not be suitable candidates for KT because of frailty and post-LT complications. When recovery of kidney function is expected after LT, efforts must be made to support metabolic needs and volume status.
FIGURE 2.
Management algorithm for LT recipient with kidney dysfunction. AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; GFR, glomerular filtration rate; KT, kidney transplant; LT, liver transplant; mGFR, measured glomerular filtration rate; RRT, renal replacement therapy; SLKT, simultaneous liver kidney transplant.
SUMMARY AND CONCLUSIONS
Kidney health is paramount to waitlisted patients with ESLD. Timely diagnosis and management of kidney dysfunction are crucial for patient survival and LT.
Greater emphasis should be placed on accurate measurement and/or estimation of baseline GFR using multiple techniques or equations where available. This should be followed with serial measurements over time. Rapid bedside measurement of GFR using carobymethylated dextrans holds significant potential in this regard. For estimation of GFR, ESLD-specific formulae need to be developed and validated in large cohorts, especially among those with GFR of <30 mL/min.
Multidimentional diagnostic work up should be pursued in LT candidates with AKI. Treatment for common causes of AKI should be started concurrently. In general volume expansion with albumin, withholding diuretics, and treatment of potential infections should be started with antibiotics. Further therapies should be directed at specific causes.
Elevating MAPs with the help of vasopressor agents are important in reversing HRS pathology. Terlipressin, which has been recently approved for use in the United States, should be used early in suspected HRS, and protocols need to be developed in expanding its use.
RRT therapy should be used judiciously, taking into account chances of getting to LT, frailty, and overall potential duration of RRT. It can be used as an effective bridge to LT.
Footnotes
The authors declare no funding or conflicts of interest.
M.Y.J. reviewed the literature and wrote the article, tables, conclusions, and figures. K.P. reviewed the literature and wrote and reviewed the article. M.G. reviewed the article. C.A.K. wrote the article and revised the article, tables, and figures.
REFERENCES
- 1.Allegretti AS, Parada XV, Eneanya ND, et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol. 2018;13:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung TH, Tsai CC, Tseng KC, et al. High mortality of cirrhotic patients with end-stage renal disease. Medicine (Baltim). 2016;95:e3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swolinsky JS, Nerger NP, Leistner DM, et al. Serum creatinine and cystatin C-based estimates of glomerular filtration rate are misleading in acute heart failure. ESC Heart Fail. 2021;8:3070–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shemesh O, Golbetz H, Kriss JP, et al. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. [DOI] [PubMed] [Google Scholar]
- 5.Proulx NL, Akbari A, Garg AX, et al. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transplant. 2005;20:1617–1622. [DOI] [PubMed] [Google Scholar]
- 6.Torre A, Aguirre-Valadez JM, Arreola-Guerra JM, et al. Creatinine versus cystatin c for estimating GFR in patients with liver cirrhosis. Am J Kidney Dis. 2016;67:342–344. [DOI] [PubMed] [Google Scholar]
- 7.Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology. 2014;59:1514–1521. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asrani SK, Jennings LW, Trotter JF, et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology. 2019;69:1219–1230. [DOI] [PubMed] [Google Scholar]
- 10.Stämmler F, Derain-Dubourg L, Lemoine S, et al. Impact of race-independent equations on estimating glomerular filtration rate for the assessment of kidney dysfunction in liver disease. BMC Nephrol. 2023;24:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singapura P, Ma TW, Sarmast N, et al. Estimating glomerular filtration rate in cirrhosis using creatinine-based and cystatin C-based equations: systematic review and meta-analysis. Liver Transpl. 2021;27:1538–1552. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Eneanya ND, Coresh J, et al. ; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swolinsky JS, Tuvshinbat E, Leistner DM, et al. Discordance between estimated and measured changes in plasma volume among patients with acute heart failure. ESC Heart Fail. 2022;9:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armenta A, Madero M, Rodriguez-Iturbe B. Functional reserve of the kidney. Clin J Am Soc Nephrol. 2022;17:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angeli P, Garcia-Tsao G, Nadim MK, et al. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. [DOI] [PubMed] [Google Scholar]
- 20.du Cheyron D, Bouchet B, Parienti JJ, et al. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;31:1693–1699. [DOI] [PubMed] [Google Scholar]
- 21.Fang JT, Tsai MH, Tian YC, et al. Outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transplant. 2008;23:1961–1969. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Llahí M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–496.e4. [DOI] [PubMed] [Google Scholar]
- 23.Reuken PA, Stallmach A, Bruns T. Mortality after urinary tract infections in patients with advanced cirrhosis—relevance of acute kidney injury and comorbidities. Liver Int. 2013;33:220–230. [DOI] [PubMed] [Google Scholar]
- 24.Ekpanyapong S, Reddy KR. Infections in cirrhosis. Curr Treat Options Gastroenterol. 2019;17:254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans LT, Kim WR, Poterucha JJ, et al. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897–901. [DOI] [PubMed] [Google Scholar]
- 26.Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–974. [DOI] [PubMed] [Google Scholar]
- 27.Angeli P, Gines P, Wong F, et al. ; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. [DOI] [PubMed] [Google Scholar]
- 28.Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. [DOI] [PubMed] [Google Scholar]
- 29.Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–541. [DOI] [PubMed] [Google Scholar]
- 30.Wiest R, Das S, Cadelina G, et al. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Seara J, Prieto J, Quiroga J, et al. Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology. 1989;97:1304–1312. [DOI] [PubMed] [Google Scholar]
- 32.Wadei HM, Mai ML, Ahsan N, et al. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–1079. [DOI] [PubMed] [Google Scholar]
- 33.Wong F, O’Leary J, Reddy K, et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcomes. Am J Gastroenterol. 2017;112:1103–1110. [DOI] [PubMed] [Google Scholar]
- 34.Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angeli P, Volpin R, Gerunda G, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–1697. [DOI] [PubMed] [Google Scholar]
- 36.Kalambokis GN, Christaki M, Tsiakas I, et al. Efficacy of treatment with terlipressin plus albumin in hepatorenal syndrome diagnosed with the new acute kidney injury versus the conventional criteria. Eur J Gastroenterol Hepatol. 2019;31:1292–1294. [DOI] [PubMed] [Google Scholar]
- 37.Boyer TD, Sanyal AJ, Wong F, et al. ; REVERSE Study Investigators. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology. 2016;150:1579–1589.e2. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal AJ, Boyer T, Garcia-Tsao G, et al. ; Terlipressin Study Group. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong F, Pappas SC, Curry MP, et al. ; CONFIRM Study Investigators. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–828. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Liu A, Bo W, et al. Terlipressin in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. Medicine (Baltim). 2018;97:e0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63:983–992. [DOI] [PubMed] [Google Scholar]
- 42.Wong F, Pappas SC, Reddy KR, et al. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment Pharmacol Ther. 2022;56:1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fogel MR, Sawhney VK, Neal EA, et al. Diuresis in the ascitic patient: a randomized controlled trial of three regimens. J Clin Gastroenterol. 1981;3(Suppl 1):73–80. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Ayuso RM, Arroyo V, Planas R, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84(5 Pt 1):961–968. [PubMed] [Google Scholar]
- 45.Bakris GL, Agarwal R, Anker SD, et al. ; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. [DOI] [PubMed] [Google Scholar]
- 46.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. [DOI] [PubMed] [Google Scholar]
- 47.Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaccherini G, Tufoni M, Bernardi M. Albumin administration is efficacious in the management of patients with cirrhosis: a systematic review of the literature. Hepat Med. 2020;12:153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angeli P, Wong F, Watson H, et al. ; CAPPS Investigators. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–1542. [DOI] [PubMed] [Google Scholar]
- 50.Sersté T, Gustot T, Rautou PE, et al. Severe hyponatremia is a better predictor of mortality than MELDNa in patients with cirrhosis and refractory ascites. J Hepatol. 2012;57:274–280. [DOI] [PubMed] [Google Scholar]
- 51.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allegretti AS, Ortiz G, Cui J, et al. Changes in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: a matched cohort analysis. Am J Kidney Dis. 2016;68:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. [DOI] [PubMed] [Google Scholar]
- 54.United Network of Organ Sharing. Simultaneous liver-kidney allocation (SLK) policy changes now in effect. Available at https://unos.org/news/simultaneous-liver-kidney-allocation-slk-policy-changes-now-in-effect/. Accessed October 2, 2023. [DOI] [PMC free article] [PubMed]