Abstract
Background A widely accepted set of imaging criteria or classification has not yet been adopted to evaluate response to treatment by percutaneous sclerotherapy for aneurysmal bone cyst (ABC). In this article, we described and illustrated the Royal Orthopaedic Hospital (ROH) scoring system which is a new, reproducible, and objective tool to evaluate the radiological response. We also reported our institutional experience in the efficacy of computed tomography (CT)-guided sclerotherapy for treating such lesions.
Patients and Methods A retrospective analysis was conducted for 19 patients who underwent CT-guided sclerotherapy with doxycycline and albumin to treat ABC. Follow-up magnetic resonance imaging, at a minimum of 12 months, was assessed according to the four ROH scoring system parameters: cystic component, fluid–fluid level, presence of consolidation, and cortical integrity. The cumulative score was used to grade response as either: excellent, good, equivocal, or poor.
Results Out of 19 patients with a mean age of 17.8 years, 11 cases occurred in the long bones, 5 cases in the pelvis, and 1 in each of the C3 vertebral body, scapula, and talus. The mean parameter of response score for cystic component was 2, fluid–fluid level was 1.3, consolidation was 2, and cortical integrity was 2.1. Four cases showed excellent response, 12 cases showed good response, 2 cases showed equivocal response, and 1 case showed poor response. Interrater reliability was excellent (κ = 0.9).
Conclusion The ROH scoring system provides the radiologist and surgeon with an objective method to score imaging parameters of response independently and achieve a grade based on the cumulative score.
Keywords: sclerotherapy, aneurysmal bone cyst, MRI, scoring system
Introduction
Aneurysmal bone cyst (ABC) is a benign but locally aggressive bone neoplasm of uncertain etiology. It has an annual reported incidence of 0.14 per 100,000 and constitutes 1% of all bone tumors. 1 2 Primary ABC represents approximately 70% of all such lesions and most commonly occurs in the first or second decade but can occasionally be encountered in older patients. 3 Secondary ABC (“ABC-like changes”) according to the latest World Health Organization Classification of Tumours of Bone) 4 occur in the remaining 30% of cases at the site of a preexisting bone lesion such as giant cell tumor (most common), chondroblastoma, osteoblastoma, or rarely osteosarcoma. 4 5
Traditional methods of treatment by en bloc resection or curettage have been complemented or replaced by newer treatments such as percutaneous sclerotherapy where sclerosing agents are injected directly into the lesion. 6 7 8 These minimally invasive methods demonstrate a lower postprocedure morbidity and complication rate, are often more acceptable to patients and parents, but may require multiple treatments to achieve a satisfactory therapeutic outcome. 9 10 11 12 13 Given that ABCs occur on a spectrum of morphology, location, and aggressive features, the treatment is tailored according to radiological and pathological features, institutional experience, and patient preference. 1
Radiological evaluation of sclerotherapy treatments typically starts after 3 months of treatment and may be assessed by radiographs, computed tomography (CT) or magnetic resonance imaging (MRI). A widely accepted set of imaging criteria or classification to assess treatment response has not yet been adopted, although some authors have proposed reproducible scoring systems. 14 15 16 17 Given that a comprehensive radiological assessment involves the assessment of multiple parameters to evaluate overall response, an ideal imaging scoring system should interrogate each one individually with a subsequent cumulative score.
In this article, we describe and illustrate a new, reproducible, and objective radiological scoring system which was used to evaluate response to multisession CT-guided sclerotherapy, performed with doxycycline and albumin, in patients with primary ABC. We also evaluate the efficacy of this treatment according to our institutional experience in a cohort of 19 patients.
Patients and Methods
Study Design
Following local hospital committee ethical approval, a retrospective evaluation of our Radiology Information System, Picture Archiving and Communication System, Clinical Record Interactive Search system computer system database over a 3-year period was undertaken to identify patients who had CT-guided sclerotherapy for the treatment of primary ABC. Two fellowship-trained and experienced musculoskeletal radiologists performed a retrospective analysis evaluating radiological consolidation, as a measure of treatment response, using the Royal Orthopaedic Hospital (ROH) scoring system.
Inclusion and Exclusion Criteria
Adult and pediatric patients with a primary ABC who had (1) at least three CT-guided sclerotherapy sessions with 200 mg of doxycycline and 5 mL 20% human albumin, (2) at least one preprocedure MRI scan, and (3) at least one follow-up MRI following this treatment regimen at a minimum interval of 1 year were included. Out of 27 patients, 8 patients did not meet these criteria and were excluded; 5 patients did not receive at least three treatment sessions, of which 1 went on to have surgery, and 3 patients were lost to follow-up.
Image Protocol and Analysis Using Royal Orthopaedic Hospital Scoring System
The standard MRI protocol for bone lesions at our institution comprises a T1-weighted coronal, short tau inversion recovery (STIR) coronal, T1-weighted axial, and T2-weighted fat-saturated (T2FS) axial sequences. For patients who had an MRI at another institution, a combination of fluid- and nonfluid-sensitive sequences were obtained.
Postprocedural MRI was compared with the pre-procedure MRI using the ROH scoring system. Postprocedure images were assessed using five parameters: (1) size and volume, (2) cystic component, (3) fluid–fluid levels, (4) presence of consolidation (fibrous or fatty), and (5) cortical integrity.
The cystic component and fluid–fluid levels were evaluated using the fluid-sensitive STIR coronal and T2FS sequences. A good response was indicated by a reduction in size of the hyperintense signal, and reduction in size of the juxtaposed-dependent hyper- and hypointense signals, respectively. Tumor consolidation was assessed on T1-weighted and fluid-sensitive coronal and axial sequences. A good response was identified as fatty change indicating the formation of normal bone, typically starting at the periphery of the lesion. Cortical integrity was predominantly assessed on T1-weighted sequences; restoration of a normal cortical thickness indicated a good response. Size and volume were assessed using a combination of T1- and T2FS sequences and incorporate cystic, solid, and consolidated contents within defined tumor borders.
Each parameter was given a score from 1 to 4 according to the percentage reduction compared with the preprocedure MRI: score 1 = > 75% response, score 2 = 51 to 75% response, score 3 = 26 to 50% response, and score 4 = < 25% response ( Table 1 ). For the preprocedure score, the maximum score of 4 was applied to all parameters in all lesions; this provided a universal and standard baseline to allow comparison of the postprocedure score. The grade of response was assessed by calculating the total score of the individual parameters as follows: poor (total score ≥13), equivocal (total score 9–12), good (total score 5–8), excellent (total score ≤4) ( Table 2 ). The better the grade (and lower the overall score), the better the radiological treatment response.
Table 1. Royal Orthopaedic Hospital scoring system.
| Response (reduction compared to preprocedure scan) for each parameter | Parameter score |
|---|---|
| <25% | 4 |
| 26–50% | 3 |
| 51–75% | 2 |
| >75% | 1 |
Note: Parameter scores. A response score from 1 to 4 (1 is the best score, and 4 is the worst score) was given to the following parameters: (1) size in volume, (2) cystic component, (3) fluid–fluid level, (4) presence of consolidation (fibrous or fatty), and (5) cortical integrity.
Table 2. Royal Orthopaedic Hospital scoring system.
| Grade of response | Total response score of the lesion |
|---|---|
| 1 (Poor) | ≥13 |
| 2 (Equivocal) | 9–12 |
| 3 (Good) | 5–8 |
| 4 (Excellent) | ≤4 |
Note: Grade of response. The total of each parameter response score provides the grade of response (minimum = 4, maximum = 20); 1 is the worst grade, and 4 is the best grade.
The interrater reliability between the two observers was calculated using Cohen's kappa coefficient (κ). In cases of discrepancy, scoring was performed by consensus discussion.
Protocol CT-Guided Sclerotherapy with Doxycycline and Albumin
Patients were initially discussed at our multidisciplinary team (MDT) meeting to confirm imaging features of ABC where a decision was made on whether to proceed with sclerotherapy. The treatment was performed as a day case in the CT suite under general anesthesia.
Percutaneous access of ABC with 14 to 25G needle was performed under CT guidance. Omnipaque or Visipaque contrast was injected to confirm and identify any potential large vessels; 200 mg doxycycline was mixed with 5 mL of 20% albumin and then agitated with 10 mL of air. It was delivered as 10 mg/mL doxycycline foam injection into the ABC after aspiration of blood. Multilocular ABC required two or more punctures. Postprocedural pain was managed by oral nonsteroidal anti-inflammatory drugs or oral narcotics. The procedure was repeated after 6 weeks for a minimum of three sessions in the included patients.
Data Collection
The patient's age at presentation (in years) and gender were recorded. The lesion location, size (in cm 3 ), number of sessions of CT-guided sclerotherapy, postprocedure parameter scores, and duration of follow-up were recorded.
Results
A total of 19 patients (male = 11 and female = 8) with a mean age of 17.8 years (range: 6–33 years) who had a histological or MDT consensus diagnosis of ABC and had three completed sessions of CT-guided sclerotherapy with 200 mg of doxycycline and 5 mL 20% human albumin were evaluated. Ten patients were followed up to 12 to 24 months and 11 patients for more than 24 months. A total of 11 cases occurred in long bones (femur = 5, metatarsals = 2, humerus = 1, ulna = 1, fibula = 1, clavicle = 1), 5 cases in the pelvis (all confined to the pubic bone), 1 case in the C3 vertebral body (neural arch), 1 case in the scapula, and 1 case in the talus ( Table 3 ).
Table 3. Table of patients.
| Patient | Age at presentation (y) | Gender | Location | Size of lesion at presentation (cm 2 ) | Final score (4–20) a | Final grade (1–4) a | Duration of follow-up (mo) |
|---|---|---|---|---|---|---|---|
| 1 | 32 | Male | Proximal femur | 15 | 12 | 2 | >24 |
| 2 | 18 | Male | Proximal femur | 22.6 | 8 | 3 | >24 |
| 3 | 21 | Male | Pubic bone | 9.8 | 4 | 4 | >24 |
| 4 | 9 | Male | Pubic bone | 9.5 | 11 | 2 | >24 |
| 5 | 7 | Male | Scapula | 32.8 | 7 | 3 | >24 |
| 6 | 12 | Female | Ulna | 5 | 7 | 3 | >24 |
| 7 | 13 | Female | Fourth metatarsal | 14.6 | 7 | 3 | >24 |
| 8 | 13 | Female | Pubic bone | 598.6 | 4 | 4 | 12-24 |
| 9 | 8 | Male | Humerus | 29 | 7 | 3 | >24 |
| 10 | 14 | Female | Clavicle | 14.3 | 16 | 1 | 12–24 |
| 11 | 23 | Female | Second metatarsal | 1.4 | 7 | 3 | 12–24 |
| 12 | 16 | Female | Pubic bone | 13.6 | 7 | 3 | 12–24 |
| 13 | 21 | Male | Pubic bone | 27 | 4 | 4 | >24 |
| 14 | 27 | Female | Proximal femur | 13.6 | 7 | 3 | 12–24 |
| 15 | 26 | Female | Proximal femur | 49.5 | 7 | 3 | 12–24 |
| 16 | 16 | Male | C3 posterior elements | 1.05 | 4 | 4 | 12–24 |
| 17 | 33 | Male | Fibular head | 50.4 | 7 | 3 | 12–24 |
| 18 | 20 | Male | Talus | 18.5 | 8 | 3 | 12–24 |
| 19 | 26 | Male | Proximal femur | 23 | 7 | 3 | 12–24 |
Note: All patients received three sessions of computed tomography-guided sclerotherapy with doxycycline and albumin.
Royal Orthopaedic Hospital score or grade at the time of final follow-up.
The mean parameter of response score from the preprocedure MRI to the final follow-up MRI (12–24 or >24 months) for cystic component was 2, fluid–fluid level was 1.3, consolidation was 2, and cortical integrity was 2.1 ( Fig. 1 ). The mean total score of response score was 7.4 (range: 4–16, median = 7). The mean reduction in volume was 30.5%, although this did not always correlate to the overall response to treatment; a persistent consolidated (sclerotic) area or expanded area was observed in many patients despite a good radiological response. Consequently, this was not incorporated into the scoring system as the relative change in size and volume of lesion was thought to skew the score. The preprocedure volume (cm 2 ) for each lesion has been provided ( Table 3 ).
Fig. 1.
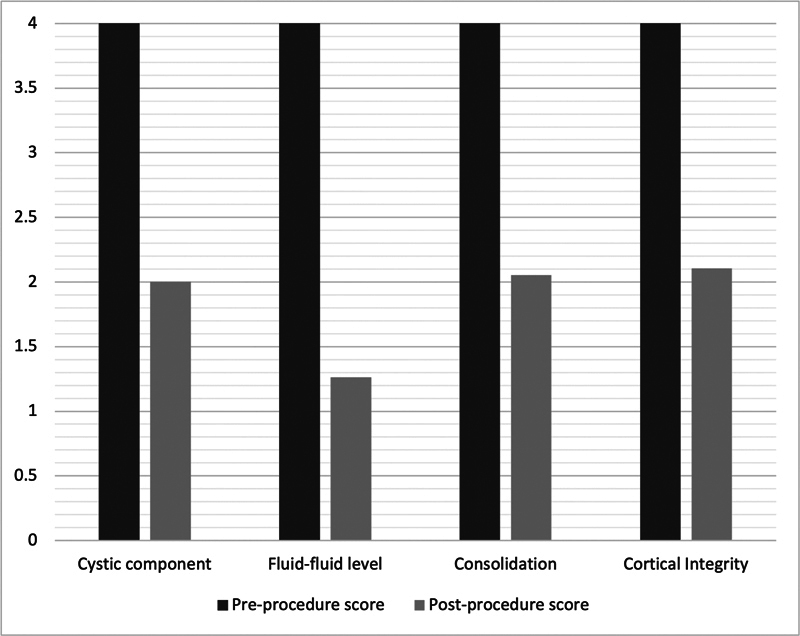
Chart showing the average pre- and postprocedure parameter results, for aneurysmal bone cysts following computed tomography-guided sclerotherapy with doxycycline and albumin, according to the Royal Orthopaedic Hospital scoring system.
A total of 4 cases showed excellent response ( Figs. 2 , 3 and 4 ), 12 cases showed good response ( Figs. 5 , 6 and 7 ), 2 cases showed equivocal response ( Fig. 8 ), and 1 case showed poor response. Representative images, with breakdown of scoring, have been provided in the respective figures and legends ( Fig. 9 ). There was excellent interrater reliability between the two radiologists (κ = 0.9). Some cases required consensus discussion.
Fig. 2.
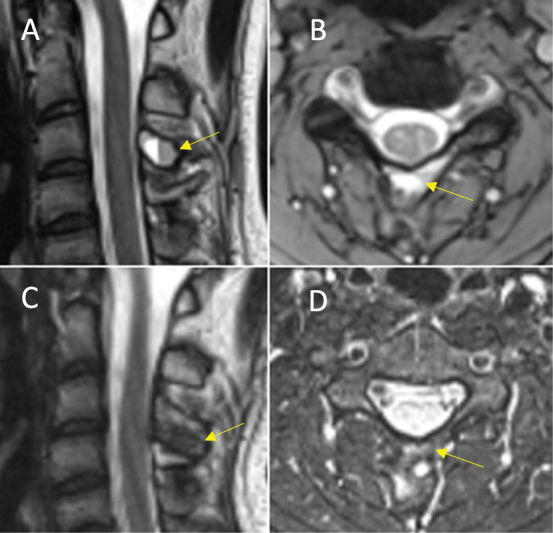
Excellent response (total score = 4) in an aneurysmal bone cyst of the C3 vertebral body neural arch (yellow arrow). ( A ) Sagittal T2 and ( B ) axial short tau inversion recovery (STIR) magnetic resonance imaging (MRI) preprocedure. ( C ) Sagittal T1 and ( D ) axial STIR MRI postprocedure. Royal Orthopaedic Hospital scoring system parameter scores: (1) cystic component = 1, (2) fluid–fluid level = 1, (3) consolidation = 1, and (4) cortical integrity = 1.
Fig. 3.
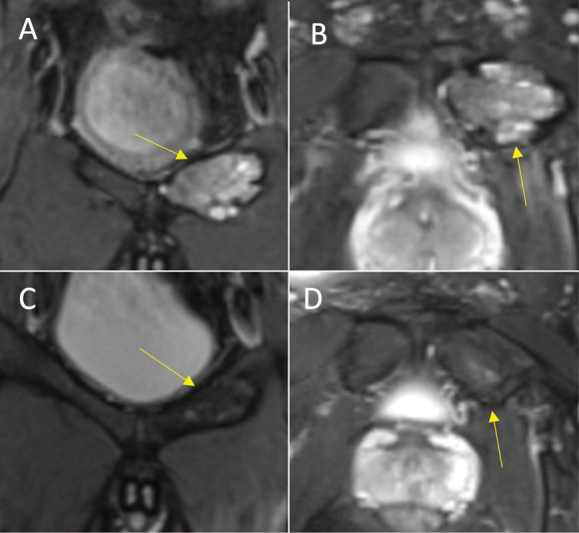
Excellent response (total score = 4) in an aneurysmal bone cyst of the left superior pubic ramus (yellow arrow). (A) Coronal T2 and (B) axial proton density-weighted fat-supressed (PDFS) magnetic resonance imaging (MRI) preprocedure. (C) Coronal and (D) axial PDFS MRI postprocedure. Royal Orthopaedic Hospital scoring system parameter scores: (1) cystic component = 1, (2) fluid–fluid level = 1, (3) consolidation = 1, and (4) cortical integrity = 1.
Fig. 4.
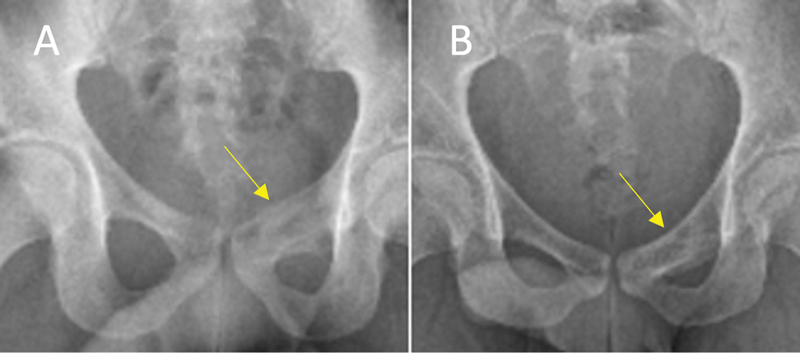
Excellent response (total score = 4) in an aneurysmal bone cyst of the left superior pubic ramus (yellow arrow). Anteroposterior radiograph correlate to Fig. 3. ( A ) Preprocedure. ( B ) Postprocedure demonstrates interval reduction in cystic component, bone sclerosis, and restoration of cortical integrity.
Fig. 5.
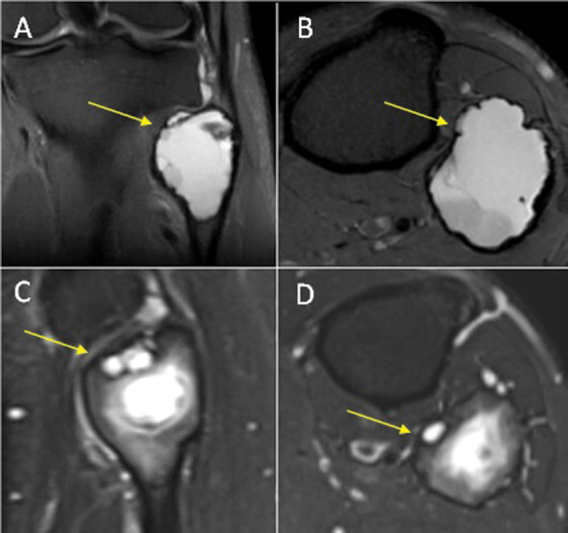
Good response (total score = 7) in an aneurysmal bone cyst of the fibular head (yellow arrow). ( A ) Coronal and ( B ) axial T2-weighted fat-saturated (T2FS) magnetic resonance imaging (MRI) preprocedure. ( C ) Sagittal and ( D ) axial T2FS MRI postprocedure. Royal Orthopaedic Hospital scoring system parameter scores: (1) cystic component = 2, (2) fluid–fluid level = 1, (3) consolidation = 2, and (4) cortical integrity = 2.
Fig. 6.
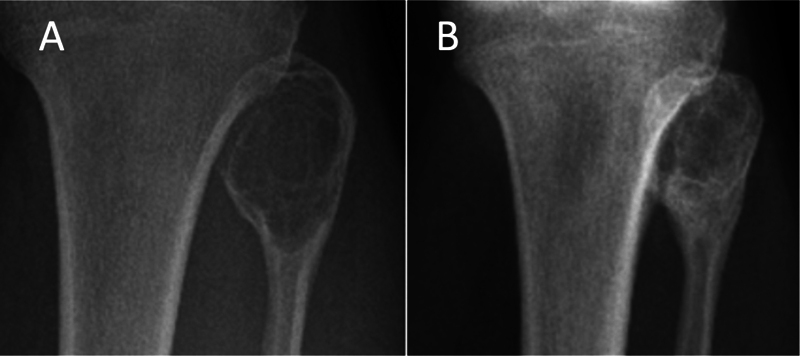
Good response (total Royal Orthopaedic Hospital score = 8) in an aneurysmal bone cyst of the fibular head. Anteroposterior radiograph correlate to Fig. 5. ( A ) Preprocedure. ( B ) Postprocedure demonstrates interval reduction in cystic component, bone sclerosis, and restoration of cortical integrity.
Fig. 7.
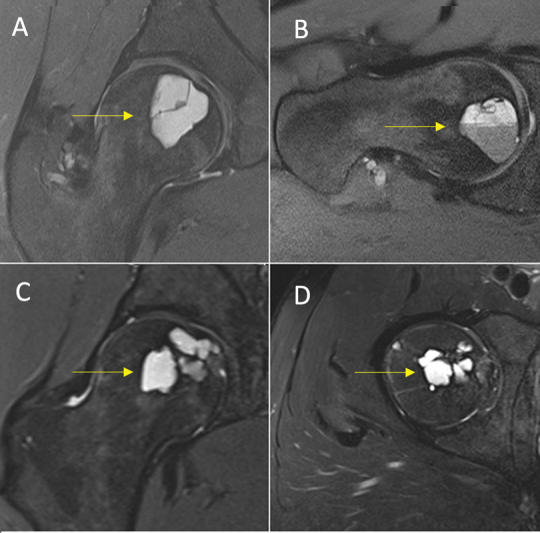
Good response (total score = 7) in an aneurysmal bone cyst of the femoral head (yellow arrow). ( A ) Sagittal and ( B ) Axial-oblique proton density-weighted fat-saturated (PDFS) magnetic resonance imaging (MRI) preprocedure. ( C ) Coronal and ( D ) axial PDFS MRI postprocedure. Royal Orthopaedic Hospital scoring system parameter scores: (1) cystic component = 2, (2) fluid–fluid level = 1, (3) consolidation = 2, and (4) cortical integrity = 2.
Fig. 8.
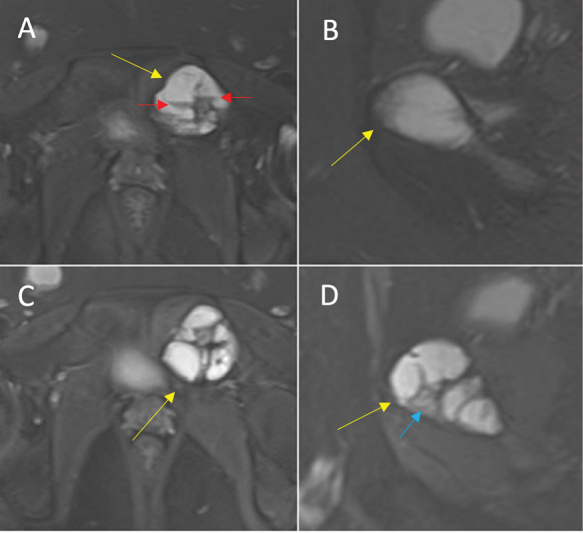
Equivocal response (total score = 11) in an aneurysmal bone cyst of the left superior pubic ramus (yellow arrow). ( A ) Axial and ( B ) sagittal proton density-weighted fat-saturated (PDFS) magnetic resonance imaging (MRI) preprocedure, note the fluid–fluid levels (red arrows). ( C ) Axial and ( D ) sagittal PDFS MRI postprocedure. Royal Orthopaedic Hospital scoring system parameter scores: (1) cystic component = 3, (2) fluid-–fluid level = 2, (3) consolidation = 3 (see blue arrow), and (4) cortical integrity = 3.
Fig. 9.
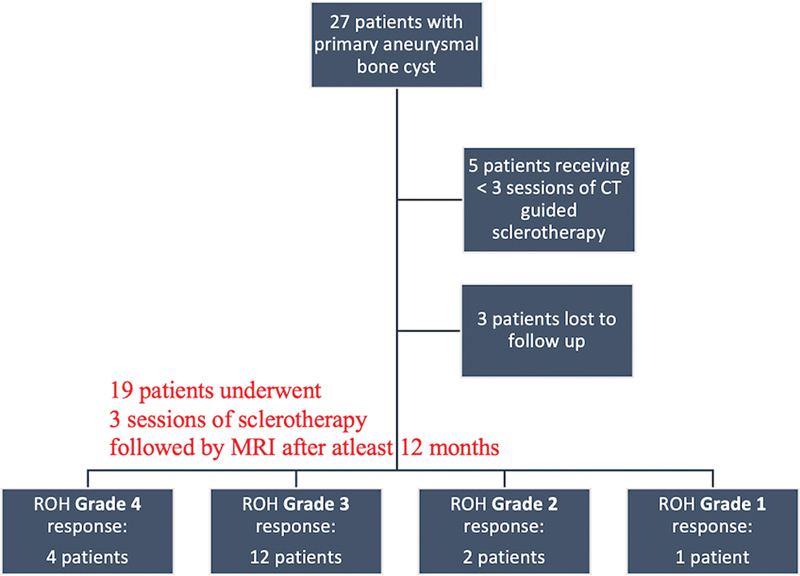
Flow chart showing the number of patients per grade of response according to the Royal Orthopaedic Hospital (ROH) scoring system. CT, computed tomography; MRI, magnetic resonance imaging.
Clinical outcome details were available for 7 out of 16 patients with excellent or good response, 1 out of 2 patients with equivocal response, and no information for the patient with poor response. Of the patients with excellent or good response, all were able to resume activities of daily living and two reported significant improvement in symptoms with mild residual tenderness at the lesion site. No patients had a physical deformity, limb length discrepancy, or further fracture. The patient with equivocal response (within the proximal femur) had persistent symptoms and went on to have further treatment with curettage and cement.
Discussion
This article provides an independent parameter-based MRI scoring system to evaluate ABC response to percutaneous sclerotherapy treatment. The study incorporates four established radiological parameters: (1) cystic component, (2) fluid–fluid level, (3) presence of consolidation (fibrous or fatty), and (4) cortical integrity. Given that ABCs occur on a spectrum of morphology, location, and aggressive features, imaging features after treatment may also be variable. The ROH scoring system provides the reader with an objective set of criteria which, independently and cumulatively, provide an overall grade of response. It is a reproducible model evidenced by excellent interreader reliability. The ROH scoring system may also guide how aggressive subsequent therapies, if required, should be. With larger data sets, individual parameters may be used as independent prognostic markers. Although the grading system was implemented in sclerotherapy with doxycycline and albumin in the treatment of ABC, it can be extrapolated to assess other cystic bone lesions (such as giant cell tumor) which often use a similar, or same, set of imaging features to evaluate treatment response. Our study was limited by a small population size and relatively short follow-up; 9 patients were followed up to 12 to 24 months and 10 patients to more than 24 months. The time frame for recurrence may vary but, in the majority of patients, has been reported to occur within 24 months, with a further 5% occurring after this. 13 Follow-up to 5 years may better evaluate long-term recurrence in future studies.
ABC is most commonly found at the metaphysis of long bones, followed by the spine and pelvis, but may occur in other parts of the skeleton. 1 18 Patients may present insidiously with pain and swelling or acutely as a consequence of pathological fracture. Radiographically, they classically appear as an expansile eccentrically placed metaphyseal lesion with multiple internal septations and thinning of the cortex. Imaging appearances may vary depending on the morphology, site, and phase of growth. 19 On MRI, lesions demonstrate fluid–fluid levels, representing blood products of differing age and postcontrast enhancement of the multiple internal septations. Lesions that demonstrate solid or other concerning features may indicate ABC-like changes within a preexisting primary lesion. 20 Most of the cases presented in this report conform to the aforementioned skeletal distribution and radiological appearances. However, a radiological diagnosis of ABC can prove difficult due to overlapping features with other benign and malignant entities which may feature bone expansion and fluid–fluid levels. At our institution, cases are discussed in a multidisciplinary meeting prior to performing a carefully planned biopsy to confirm the diagnosis.
Several treatment options have been proposed and implemented in the treatment of ABCs. Curettage with or without bone grafting, or cement, is one of the traditional methods used to treat ABC. Recurrence rates following this treatment vary considerably but are relatively high between 10 and 30%. 6 7 8 Adjuvant therapy with radiation therapy, 21 22 cryotherapy, 8 and high-speed burr 23 has been utilized to reduce recurrence rates. En bloc resection is a more invasive method of treatment and can be utilized for eccentric lesions of expendable bones. 24 Although it may result in a lower rate of recurrence, it carries a higher postoperative morbidity, recovery time, and long-term complications such as limb length discrepancy. 25 Reddy et al reported a novel treatment in which spontaneous resolution was observed following biopsy. 26 The term “curopsy” was coined to describe a limited percutaneous curettage, from various quadrants of the cyst, obtained at the time of diagnostic biopsy. No additional treatment was required in 81% of patients within the curopsy group as compared with 90% in traditional curettage. Patients in the curopsy group had shorter recovery time compared with the curettage group. Spontaneous resolution of ABC in the absence of any treatment has been reported albeit rarely. It may be a consideration for very small lesions with a low risk of fracture in selected cases. 27
In recent decades, percutaneous sclerotherapy with agents such as doxycycline, polidocanol, ethanol, and sodium tetradecyl sulphate has emerged as a minimally invasive method of treatment. First performed in 1993 by Adamsbaum et al, two pediatric patients with ABC were treated with sclerotherapy and demonstrated satisfactory healing of the lesion evidenced by radiographic consolidation. 10 The foreign injectate acts as an irritant within bone or, in the case of doxycycline, an inhibitor to angiogenesis and osteoclastic function, ultimately inducing fibrosis and sclerosis. 10 28 Reported side effects include mild localized inflammation, vomiting, and fever with few reports of serious complications such as pulmonary embolus and skin necrosis. 12 Ethibloc, an earlier agent, has been discontinued due to its major side effect profile. 29 In 2010, Varshney et al compared (1) percutaneous sclerotherapy with polidocanol against (2) curettage with high-speed bur treatment. 9 There were no significant difference in recurrence rates between each group (94.3 and 84.8%, respectively), but the prior treatment was considered safer and resulted in fewer complications. Multiple reports have found similar positive results with recurrence rates between 5 and 20%. 11 12 13 30 31 32 As a result, percutaneous sclerotherapy has been adopted as a first-line treatment in many centers. In our study, 16 out of 19 patients (84%) demonstrated treatment response, reflecting existing outcomes in the literature.
The aim of any treatment is to induce healing of cystic cavities and thereby resolve pain, reduce deformity, and minimize risk of future pathological fractures. Radiographically, this may be observed by a decrease in size, reduction in bone expansion, ossification of cystic spaces, and restoration of cortical thickness. These findings may be evaluated in greater detail by utilizing MRI with the additional benefit of assessing resolution of fluid–fluid levels. The first follow-up imaging should ideally be performed after a minimum of 3 months to allow for sufficient consolidation to have occurred. 13
Several authors have utilized such parameters to evaluate response to treatment. 14 33 Ramkumar et al proposed a radiological scale for response following sclerotherapy with doxycycline and looked at features on both postprocedure MRI and CT, with each grade of response incorporating multiple imaging parameters. 14 The scale showed low interreader reliability in imaging interpretation between interventional radiologists and orthopaedic surgeons. The scale does not, however, allow each parameter of response (decrease in size, bone expansion, etc.) to be individually scored; a variation in radiological response among different parameters may be difficult to incorporate into one grade alone. Other authors have utilized a modified Neer et al's classification 15 which comprises parameters of sclerosis, persistent radiolucent areas, and cortical thickness but does not include fluid–fluid levels. 16 17 The scoring system is clear and reproducible but again does not allow for the independent assessment of each parameter. Other studies have used a variety of individual follow-up criteria on radiographs, MRI and CT. 9 11 13 23 32 33 A direct comparison of postprocedure radiological features reported in the literature can prove difficult owing to the variety and nuances of different classification systems.
Conclusion
Several studies have evaluated ABC treatment response on imaging, but radiological parameters, though valid, can vary between investigators. Adopting one technique may allow comparability between future studies. Utilizing MRI can provide a highly detailed and anatomic depiction of treatment response. The ROH scoring system provides the radiologist and surgeon with an objective and reproducible method to score imaging parameters independently and achieve a grade based on the cumulative score.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Cottalorda J, Kohler R, Sales de Gauzy J et al. Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B. 2004;13(06):389–394. doi: 10.1097/01202412-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Leithner A, Windhager R, Lang S, Haas O A, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;(363):176–179. [PubMed] [Google Scholar]
- 3.Bonakdarpour A, Levy W M, Aegerter E. Primary and secondary aneurysmal bone cyst: a radiological study of 75 cases. Radiology. 1978;126(01):75–83. doi: 10.1148/126.1.75. [DOI] [PubMed] [Google Scholar]
- 4.WHO . 5 ed. International Agency for Research on Cancer; Lyon, France: 2020. Soft Tissue and Bone Tumours, WHO Classification of Tumours. [Google Scholar]
- 5.Martinez V, Sissons H A. Aneurysmal bone cyst. A review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61(11):2291–2304. doi: 10.1002/1097-0142(19880601)61:11<2291::aid-cncr2820611125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Mankin H J, Hornicek F J, Ortiz-Cruz E, Villafuerte J, Gebhardt M C. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23(27):6756–6762. doi: 10.1200/JCO.2005.15.255. [DOI] [PubMed] [Google Scholar]
- 7.Ramírez A R, Stanton R P. Aneurysmal bone cyst in 29 children. J Pediatr Orthop. 2002;22(04):533–539. [PubMed] [Google Scholar]
- 8.Vergel De Dios A M, Bond J R, Shives T C, McLeod R A, Unni K K. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Varshney M K, Rastogi S, Khan S A, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res. 2010;468(06):1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamsbaum C, Kalifa G, Seringe R, Dubousset J. Direct Ethibloc injection in benign bone cysts: preliminary report on four patients. Skeletal Radiol. 1993;22(05):317–320. doi: 10.1007/BF00198389. [DOI] [PubMed] [Google Scholar]
- 11.Brosjö O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84(05):502–505. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rastogi S, Varshney M K, Trikha V, Khan S A, Choudhury B, Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J Bone Joint Surg Br. 2006;88(09):1212–1216. doi: 10.1302/0301-620X.88B9.17829. [DOI] [PubMed] [Google Scholar]
- 13.Shiels W E, II, Mayerson J L. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop Relat Res. 2013;471(08):2675–2683. doi: 10.1007/s11999-013-3043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramkumar D B, Ercolano L B, Allar B G, Miller P E, Padua H, Anderson M E. Sclerotherapy for aneurysmal bone cysts: scale for response. J Pediatr Orthop. 2021;41(07):444–449. doi: 10.1097/BPO.0000000000001864. [DOI] [PubMed] [Google Scholar]
- 15.Neer C S, II, Francis K C, Marcove R C, Terz J, Carbonara P N. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am. 1966;48(04):731–745. [PubMed] [Google Scholar]
- 16.Wong M N, Braswell L E, Murakami J W. Doxycycline sclerotherapy of cervical spine aneurysmal bone cysts: single-institution 13-year experience. Pediatr Radiol. 2022;52(08):1528–1538. doi: 10.1007/s00247-022-05328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiba H, Kobayashi M, Waguri-Nagaya Y et al. Treatment of simple bone cysts using endoscopic curettage: a case series analysis. J Orthop Surg Res. 2018;13(01):168. doi: 10.1186/s13018-018-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Gan Y, Shi D, Zhao J. Huge aneurysmal bone cyst secondary to giant cell tumor of the hand phalanx: a case report and related literature. BMC Cancer. 2020;20(01):233. doi: 10.1186/s12885-020-06746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F, Campanacci M. Aneurysmal cysts of long bones. Ital J Orthop Traumatol. 1985;11(04):409–417. [PubMed] [Google Scholar]
- 20.Rapp T B, Ward J P, Alaia M J. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20(04):233–241. doi: 10.5435/JAAOS-20-04-233. [DOI] [PubMed] [Google Scholar]
- 21.Marcove R C, Sheth D S, Takemoto S, Healey J H. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995;(311):157–163. [PubMed] [Google Scholar]
- 22.Delloye C, De Nayer P, Malghem J, Noel H.Induced healing of aneurysmal bone cysts by demineralized bone particles. A report of two cases Arch Orthop Trauma Surg 1996115(3-4):141–145. [DOI] [PubMed] [Google Scholar]
- 23.Dormans J P, Hanna B G, Johnston D R, Khurana J S. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;(421):205–211. doi: 10.1097/01.blo.0000126336.46604.e1. [DOI] [PubMed] [Google Scholar]
- 24.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;(204):25–36. [PubMed] [Google Scholar]
- 25.Flont P, Kolacinska-Flont M, Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J Surg Oncol. 2013;11:109. doi: 10.1186/1477-7819-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy K I, Sinnaeve F, Gaston C L, Grimer R J, Carter S R. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res. 2014;472(06):1901–1910. doi: 10.1007/s11999-014-3513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cottalorda J, Chotel F, Kohler R et al. Aneurysmal bone cysts of the pelvis in children: a multicenter study and literature review. J Pediatr Orthop. 2005;25(04):471–475. doi: 10.1097/01.bpo.0000158002.30800.8f. [DOI] [PubMed] [Google Scholar]
- 28.Fife R S, Rougraff B T, Proctor C, Sledge G W., Jr Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med. 1997;130(05):530–534. doi: 10.1016/s0022-2143(97)90130-x. [DOI] [PubMed] [Google Scholar]
- 29.Topouchian V, Mazda K, Hamze B, Laredo J D, Penneçot G F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232(02):522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 30.Woon J TK, Hoon D, Graydon A, Flint M, Doyle A J. Aneurysmal bone cyst treated with percutaneous doxycycline: is a single treatment sufficient? Skeletal Radiol. 2019;48(05):765–771. doi: 10.1007/s00256-019-03188-y. [DOI] [PubMed] [Google Scholar]
- 31.Ghanem I, Nicolas N, Rizkallah M, Slaba S. Sclerotherapy using Surgiflo and alcohol: a new alternative for the treatment of aneurysmal bone cysts. J Child Orthop. 2017;11(06):448–454. doi: 10.1302/1863-2548.11.170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marie-Hardy L, El Sayed L, Alves A et al. Percutaneous alcohol-based sclerotherapy in aneurysmal bone cyst in children and adolescents. Orthop Traumatol Surg Res. 2020;106(07):1313–1318. doi: 10.1016/j.otsr.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, Kumar S, Kumar D et al. Sclerotherapy for aneurysmal bone cyst: a single-center experience. Cureus. 2021;13(10):e18469. doi: 10.7759/cureus.18469. [DOI] [PMC free article] [PubMed] [Google Scholar]


