Abstract
Background
Variceal and nonvariceal upper gastrointestinal bleeding (VUGIB and NVUGIB, respectively) require prompt intervention. Existing studies offer limited insight into the impact of interhospital transfers on patients with VUGIB and NVUGIB.
Methods
We conducted a retrospective study using the US National Inpatient Sample database from 2017 to 2020. The outcomes included in-hospital mortality, incidence of complications, procedural performance, and resource utilization.
Results
A total of 28,275 VUGIB and 781,370 NVUGIB adult patients were included. Transferred VUGIB and NVUGIB patients, when compared to nontransferred ones, demonstrated higher inpatient mortality (adjusted odds ratio [AOR] 1.49 and 1.86, P < 0.05). Patients with VUGIB and NVUGIB had a higher likelihood of acute kidney injury requiring dialysis (AOR 3.79 and 1.76, respectively, P = 0.01), vasopressor requirement (AOR 2.13 and 2.37, respectively, P < 0.01), need for mechanical ventilation (AOR 1.73 and 2.02, respectively, P < 0.01), and intensive care unit admission (AOR 1.76 and 2.01, respectively, P < 0.01). Compared to their nontransferred counterparts, transferred VUGIB patients had a higher rate of undergoing transjugular intrahepatic portosystemic shunt (AOR 3.26, 95% CI 1.92–5.54, P < 0.01), while transferred NVUGIB patients had a higher rate of interventional radiology-guided embolization (AOR 2.01, 95% CI 1.73–2.34, P < 0.01) and endoscopic hemostasis (AOR 1.10, 95% CI 1.05–1.15, P < 0.01).
Conclusion
Interhospital transfer is associated with worse clinical outcomes and higher resource utilization for VUGIB and NVUGIB patients.
Keywords: Inpatient mortality, interhospital transfer, National Inpatient Sample, resource utilization, upper gastrointestinal bleeding
Variceal upper gastrointestinal bleeding (VUGIB) and nonvariceal upper gastrointestinal bleeding (NVUGIB) require immediate attention, given their association with heightened morbidity and mortality rates.1,2 Despite continuous advances in pharmacotherapy, endoscopic techniques, intensive care management, and surgical interventions, the mortality attributed to acute upper gastrointestinal bleeding has remained consistently between 5% and 10% since 1945.3 In the United States, annual estimates indicate about 350 hospital admissions per 100,000 individuals for various gastrointestinal bleeding presentations, culminating in over a million hospitalizations annually.4–6
Timely and appropriate management is critical; however, multidisciplinary modalities may not be available at all healthcare centers.7 This often necessitates the transfer of patients to tertiary care facilities capable of providing advanced treatments such as endoscopic procedures, interventional radiology procedures, and intensive care.8 However, the literature on the impact of such interhospital transfers (IHT) on clinical outcomes in patients with VUGIB and NVUGIB is scarce at the population level. Studies evaluating the impact of IHT on percutaneous coronary intervention and stroke outcomes have shown that patients transferred between hospitals generally experience worse clinical outcomes, including higher mortality rates and increased resource utilization, such as longer hospital stays and higher hospitalization costs.9–16 Various factors contribute to these outcomes, including delays in diagnosis and treatment initiation, complexities in the transition of care, and logistical challenges associated with IHT.17,18
Given the significant clinical implications and challenges associated with VUGIB and NVUGIB, it is imperative to investigate the impact of IHT on clinical outcomes and resource utilization within these patient cohorts. In this study, we aimed to determine whether VUGIB and NVUGIB patients transferred between hospitals experience elevated in-hospital mortality, an increased incidence of complications, and greater consumption of hospital resources compared to directly admitted patients.
METHODS
Database information
We performed a retrospective cohort study utilizing data for the years 2017 to 2020 from the US National Inpatient Sample (NIS) database.19 NIS is the largest publicly available all-payer inpatient healthcare database, covering 48 states and more than 98% of the US population. It is sampled from the State Inpatient Databases and contains data on roughly 7 million hospitalizations each year. A 20% stratified sample is gathered from all US community hospitals, excluding rehabilitation and long-term care hospitals. These data contain information on all hospital stays, regardless of the expected payer for the hospital stay. Each discharge from the resultant data is then weighted (where the weight equals the total number of discharges from all acute care hospitals in the United States divided by the number of discharges included in the 20% sample) to make the NIS nationally representative. When weighted, the NIS data estimates around 35 million hospitalizations across the US. The NIS aims to make regional and national estimates of healthcare utilization, cost, quality, and outcomes. It contains deidentified clinical and demographic elements for each hospital stay at the hospital and patient level. Given the deidentified data in the NIS, the institutional review board deemed this study exempt.
Study population
We included adult patients (aged ≥18 years) admitted with principal diagnoses of VUGIB and NVUGIB, utilizing International Classification of Diseases, Tenth Revision, Clinical Modifications (ICD-10-CM) codes. The patients were further classified into two groups based on their transfer status. The transfer status is defined in the NIS by using the variable TRAN_IN. In our study, we included patients who were either admitted directly or transferred from another acute care hospital. Patients transferred from nonacute long-term facilities were excluded. We reviewed previously published literature on NIS to identify our patient population. The ICD-10-CM diagnosis and procedure codes used in our study are listed in Supplementary Table 1.
Study variables and outcomes
The primary outcomes assessed included all-cause mortality, acute kidney injury (AKI) requiring dialysis, blood transfusion, septic shock, admission to the intensive care unit (ICU), and resource utilization with total hospitalization charges and length of stay (LOS). We also compared procedure performance in both groups (i.e., esophagogastroduodenoscopy [EGD] without intervention, endoscopic hemostasis [EH], interventional radiology [IR]-guided embolization, and transjugular intrahepatic portosystemic shunt [TIPS]).
Statistical analysis
STATA version 14.2 (StataCorp, College Station, Texas, USA) was used for the analyses. Multivariate linear and logistic regression analyses were performed using confounders with a P value ≤ 0.2 on univariate regression analysis.20 In addition, we incorporated variables that were considered clinically significant to the outcome, as indicated by previous research, regardless of their statistical significance to the outcome in the univariate analysis. We also included comorbidities and variables included in Rockall and AIMS score in the regression model (including ischemic heart disease, renal failure, liver failure, metastatic disease, presence of hypovolemic shock, and coagulopathy). The variables included in the final regression analysis for NVUGIB were age (in years), gender, race, Charlson’s comorbidity index (CCI), hypovolemic shock, insurance status (Medicare, Medicaid, private, and uninsured), hospital bed size (small, medium, and large), hospital location (urban and rural), end-stage renal disease, thrombocytopenia, obesity, and use of anticoagulants or underlying coagulopathy. Since most transfers were to teaching hospitals, we excluded teaching status from the regression model to avoid collinearity.
Regression analysis for VUGIB included age, gender, race, CCI, hypovolemic shock, insurance status, hospital bed size, hospital region, thrombocytopenia, end-stage renal disease, and obesity. Furthermore, as we were unable to use MELD-Na and Child-Pugh to adjust for decompensated liver disease in patients with VUGIB, we devised a surrogate severity scale. It included the following clinical parameters: presence of ascites, international normalized ratio abnormalities, hepatic encephalopathy, hepatorenal syndrome, hyponatremia, and hypoalbuminemia.
Normality was checked by plotting histograms for the individual variables before applying descriptive statistics. Continuous variables (i.e., LOS and total hospitalization charges) had a Poisson distribution, whereas the binary outcomes had a normal distribution. Poisson regression analysis was used to calculate continuous outcomes, whereas logistic regression analysis was used to assess binary outcomes. All P values were two sided, and the threshold for statistical significance was set at P < 0.05.
RESULTS
Patient characteristics
VUGIB
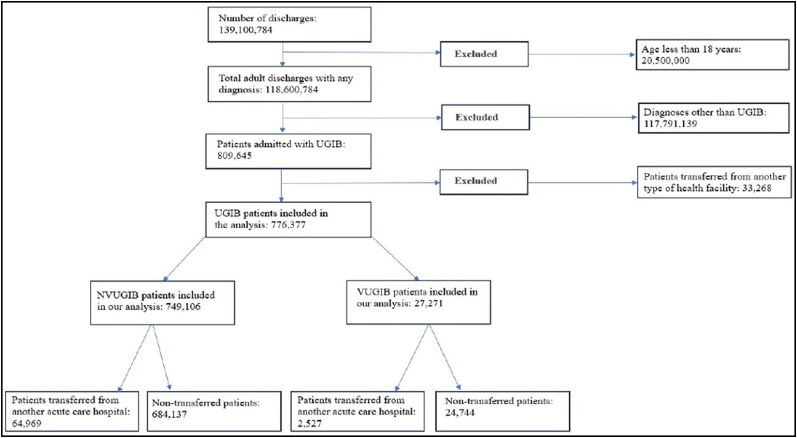
We included a total of 28,275 adult patients admitted with VUGIB. Of these, 2527 (9.37%) were transferred from other acute care hospitals, while 24,744 (87.51%) were admitted directly (Figure 1). Patients admitted from long-term nonacute care facilities were excluded from the study. Table 1 shows the characteristics of the patients and hospitals in which they were treated. We found no statistically significant differences in mean age, gender, or comorbid status between the two groups. Regarding racial differences, transferred patients were more likely to be White (81.66% vs 67.99%, P < 0.01). From a hospital standpoint, both groups had a similar distribution.
Figure 1.
Flow diagram depicting the inclusion and exclusion of patients with variceal and nonvariceal upper gastrointestinal bleed.
Table 1.
Baseline characteristics of transferred and nontransferred adult patients with variceal and nonvariceal upper gastrointestinal bleeding
| Baseline characteristics | VUGIB = 28,275 |
NVUGIB = 781,369 |
||||
|---|---|---|---|---|---|---|
| Nontransferred (n = 24,744) | Transferred (n = 2887) | P value | Nontransferred (n = 684,136) | Transferred (64,969) | P value | |
| Age: mean (95% CI) (years) | 57.59 (57.23–57.95) |
56.63 (55.53–57.73) |
0.07 | 67.07 (66.97–67.17) |
66.66 (66.38–66.94) |
<0.01 |
| Female gender, % | 35.73 | 32.7 | 0.36 | 45.63 | 42.99 | <0.01 |
| Race, % | < 0.01 | < 0.01 | ||||
| White | 67.99 | 81.66 | 70.63 | 81.74 | ||
| Black | 7.93 | 3.55 | 15.38 | 10.12 | ||
| Hispanic | 20.38 | 13.41 | 10.03 | 6.13 | ||
| Asians | 3.70 | 1.38 | 3.96 | 2.01 | ||
| Charlson’s comorbidity index, % | 0.64 | < 0.01 | ||||
| 1 | 2.34 | 2.61 | 26.34 | 22.17 | ||
| 2 | 1.55 | 0.87 | 20.04 | 19.33 | ||
| ≥3 | 96.11 | 96.52 | 53.62 | 58.5 | ||
| Median household income in the patient’s zip code (quartile), % | < 0.01 | < 0.01 | ||||
| 1st (0–25th) | 31.08 | 38.05 | 29.55 | 38.22 | ||
| 2nd (26th–50th) | 27.08 | 31.68 | 26.18 | 33.19 | ||
| 3rd (51st–75th) | 24.32 | 20.18 | 24.29 | 18.48 | ||
| 4th (76th–100th) | 17.53 | 10.09 | 19.98 | 10.11 | ||
| Insurance status, % | 0.01 | < 0.01 | ||||
| Medicare | 35.91 | 39.82 | 64.51 | 66.51 | ||
| Medicaid | 26.79 | 26.79 | 12.41 | 12 | ||
| Private | 28.29 | 25.69 | 18.45 | 17.35 | ||
| Uninsured | 9.03 | 7.71 | 4.64 | 4.14 | ||
| Hospital region, % | < 0.01 | < 0.01 | ||||
| Northeast | 15.32 | 14.43 | 16.77 | 11.76 | ||
| Midwest | 19.4 | 31.13 | 21.02 | 31.97 | ||
| South | 40.15 | 34.78 | 40.34 | 36.59 | ||
| West | 25.13 | 19.65 | 21.87 | 19.68 | ||
| Hospital bed size, % | < 0.01 | < 0.01 | ||||
| Small | 19.77 | 10.26 | 21.53 | 12.99 | ||
| Medium | 32.31 | 26.26 | 31.32 | 25.19 | ||
| Large | 47.92 | 63.48 | 47.16 | 61.82 | ||
| Hospital location, % | < 0.01 | < 0.01 | ||||
| Rural | 6.62 | 6.26 | 7.78 | 5.74 | ||
| Urban | 93.38 | 93.74 | 92.22 | 94.26 | ||
| Thrombocytopenia, % | 38.68 | 42.43 | 0.2 | 8.6 | 11.15 | <0.01 |
| Anticoagulant use, % | 4.08 | 5.39 | 0.05 | 14.55 | 14.16 | <0.01 |
| End-stage renal disease, % | 2.42 | 2.09 | 0.8 | 6.25 | 6.72 | <0.01 |
| Obesity, % | 12.87 | 15.3 | 0.1 | 14.12 | 15.95 | <0.01 |
| Coagulopathy, % | 2.54 | 3.13 | 0.13 | 8.43 | 10.86 | <0.01 |
NVUGIB indicates nonvariceal upper GI bleed; VUGIB, nonvariceal upper GI bleed.
NVUGIB
We identified a total of 781,370 adult hospitalizations for NVUGIB, of which 64,969 (8.32%) were transferred from other hospitals, while 684,137 (87.57%) were nontransferred admissions (excluding admissions from nonacute long-term facilities) (Figure 1). We found that transferred patients, when compared to nontransferred ones, were younger (66.66% vs 67.07%, P < 0.01), more likely to be males (57.01% vs 54.37%, P < 0.01), and had higher CCI scores (58.50% vs 53.62%, P < 0.01). From the hospital standpoint, transferred patients were more often admitted to large hospitals (61.82% vs 47.16%, P < 0.01) located in urban areas (94.26% vs 92.22%, P < 0.01).
Mortality and morbidity
VUGIB
VUGIB patients who were transferred from other hospitals had higher odds of inpatient mortality (adjusted odds ratio [AOR] 1.49, 95% CI 4.82–8.19, P = 0.04). We also found that transferred patients had higher adjusted odds of AKI requiring dialysis (AOR 3.79, 95% CI 1.35–10.63, P = 0.01), vasopressor requirement (AOR 2.13, 95% CI 1.26–3.62, P < 0.01), mechanical ventilation (AOR 1.73, 95% CI 1.35–2.21, P < 0.01), and ICU admission (AOR 1.76, 95% CI 1.38–2.24, P < 0.01). Additionally, transferred VUGIB patients had increased mean LOS by 0.45 days (P = 0.03) when compared to nontransferred patients (Table 2).
Table 2.
Outcomes analysis by transfer status for adults with variceal and nonvariceal upper gastrointestinal bleeding
| Outcomes | VUGIB = 28,275 |
NVUGIB = 781,369 |
||||
|---|---|---|---|---|---|---|
| Nontransferred (n = 24,744) | Transferred (n = 2887) | AOR (95% CI; P value) | Nontransferred (n = 684,136) | Transferred (64,969) | AOR (95% CI; P value) | |
| Mortality, % | 4.82 | 8.19 | 1.49 (1.02–2.18; 0.04) | 1.60 | 3.40 | 1.86 (1.65–2.09; <0.01) |
| Vasopressor requirement, % | 1.62 | 3.83 | 2.13 (1.26–3.62; <0.01) | 0.87 | 2.46 | 2.37 (2.02–2.78; <0.01) |
| Mechanical ventilation, % | 12.48 | 22.26 | 1.73 (1.35–2.21; <0.01) | 3.61 | 7.99 | 2.02 (1.86–2.20; <0.01) |
| Requiring ICU admission, % | 12.97 | 23.13 | 1.76 (1.38–2.24; <0.01) | 4.08 | 8.92 | 2.01 (1.86–2.18; <0.01) |
| Septic shock, % | 0.77 | 1.91 | 1.87 (0.87–4.05; 0.11) | 0.60 | 1.32 | 2.03 (1.69–2.44; <0.01) |
| AKI requiring dialysis, % | 0.39 | 1.57 | 3.79 (1.35–10.63; 0.01) | 0.53 | 1.02 | 1.76 (1.43–2.17; <0.01) |
| Adjusted mean difference | ||||||
| Mean LOS, days (95% CI) | 4.50 (4.39–4.61) |
5.25 (4.83–5.67) |
0.45 (0.04–0.85; 0.03) |
4.42 (4.40–4.45) |
5.5 (5.42–5.65) |
0.95 (0.82–1.07; <0.01) |
| THC, mean, $US | 61,354 (59,457–63,250) |
68,613 (61,064–76,161) |
5677 (−2240–13,595; 0.16) |
54,839 (54,158–55,520) |
61,755 (59,431–64,079) |
3754 (1,556–5,953; <0.01) |
AKI indicates acute kidney injury; AOR, adjusted odds ratio; CI, confidence interval; ICU, intensive care unit; LOS, length of stay; NVUGIB, nonvariceal upper GI bleed; THC, total hospitalization charges; VUGIB, variceal upper GI bleed.
NVUGIB
Transferred NVUGIB patients, compared with their nontransferred counterparts, demonstrated increased odds of inpatient mortality (AOR 1.86, 95% CI 1.65–2.09, P < 0.01). Furthermore, transferred patients had higher odds of AKI requiring dialysis (AOR 1.76, 95% CI 1.43–2.17, P < 0.01), septic shock (AOR 2.03, 95% CI 1.69–2.44, P < 0.01), vasopressor requirement (AOR 2.37, 95% CI 2.02–2.78, P < 0.01), need for mechanical ventilation (AOR 2.02, 95% CI 1.86–2.20, P < 0.01), and ICU admission (AOR 2.01, 95% CI 1.69–2.44, P < 0.01). From a resource utilization viewpoint, transferred patients had an increased mean LOS by 0.95 days (P < 0.01) and higher total hospitalization charges ($3754, 95% CI 1556–5953, P < 0.01).
Procedural performance
VUGIB
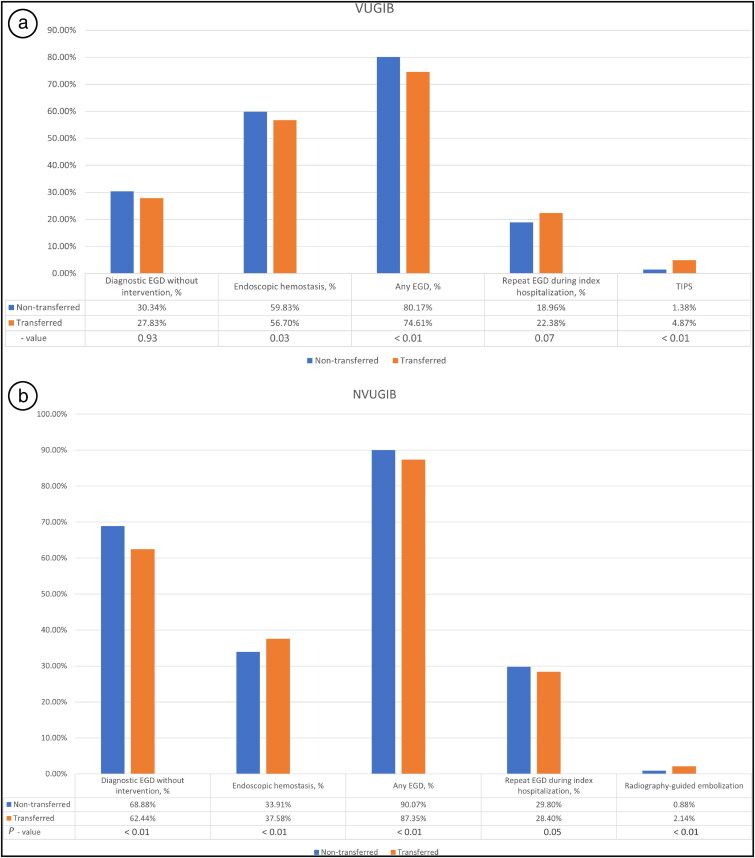
Compared to nontransferred patients, transferred VUGIB patients had significantly higher rates of undergoing TIPS (AOR 3.26, 95% CI 1.92–5.54, P < 0.01) and lower rates of EH (AOR 0.81, 95% CI 0.66–0.98, P = 0.03), overall EGD (AOR 0.72, 95% CI 0.57–0.90, P < 0.01), and repeat EGD (AOR 1.29, 95% CI 0.98–1.68, P = 0.07) (Figure 2a). This implies that transferred patients are more likely to undergo specialized procedures, such as TIPS, possibly indicating a higher severity of illness or complications that require advanced intervention.
Figure 2.
Procedural performance for (a) variceal upper gastrointestinal bleed and (b) nonvariceal upper gastrointestinal bleed.
NVUGIB
Transferred NVUGIB patients, compared to nontransferred counterparts, had a significantly higher rate of IR-guided embolization (AOR 2.01, 95% CI 1.73–2.34, P < 0.01) and EH (AOR 1.10, 95% CI 1.05–1.15, P < 0.01). However, EGD without intervention and overall EGD were performed less frequently in the transferred patients (AOR 0.77 and 0.70, respectively, P < 0.01) (Figure 2b). This implies that transferred NVUGIB patients are more likely to undergo specialized intervention-focused procedures such as IR-guided embolization.
DISCUSSION
Our study analyzed the VUGIB and NVUGIB outcomes in 28,275 and 781,369 adult patients, respectively. Among these patients, 2887 were transferred for VUGIB and 64,969 for NVUGIB. The data highlight the differences in outcomes between transferred and nontransferred patients, further elucidating the complexities of gastrointestinal bleeding management across care settings.21,22 In line with current literature,10 transferred VUGIB or NVUGIB patients, when compared with nontransferred patients, had significantly higher odds of mortality and complications, including AKI, vasopressor use, mechanical ventilation, and ICU admission, along with longer hospital stays and increased costs.
A broad study spanning 2008 to 2014 identified 58,362 cases of esophageal variceal bleeding from the NIS database in the United States and found that patients transferred from another acute care hospital faced a heightened risk of mortality.23 The evident increase in mortality among these transferred patients underscores the importance of refining transfer protocols and implementing immediate care interventions tailored to esophageal variceal bleeding. In our study cohort, individuals with VUGIB who underwent IHT demonstrated a significantly higher mortality rate than directly admitted patients. This outcome was statistically significant despite adjustments for severity of gastrointestinal bleed, decompensated liver disease, and other underlying comorbidities, highlighting the potential influence of transfer protocols on the outcomes.
On the other hand, the NVUGIB group displayed a markedly elevated mortality risk among the transferred individuals. Prior studies have indicated that age, the presence and characterization of comorbid illnesses, healthcare access and standards, and the timing of admissions are potential causes for such an increase in mortality among transferred NVUGIB patients.21,24 However, there is a scarcity of data regarding the impact of IHT on NVUGIB outcomes on a national scale. It is crucial to acknowledge that despite no statistically significant difference in transferred VUGIB patients, mortality rates in NVUGIB cases during IHT remain elevated. This emphasizes the need for continued enhancements in transfer protocols.
The transferred VUGIB and NVUGIB patients, compared to nontransferred patients, had increased mean LOS by 0.45 and 0.95 days, respectively. These extended hospital stays correlate with higher total hospitalization charges, implying a significant economic burden. Moreover, our findings are consistent with those of a prior study that compared the outcomes of variceal esophageal bleeding in teaching and nonteaching hospitals.23 While our study did not specifically focus on teaching versus nonteaching hospitals, these data suggest that higher costs and longer stays are a concern across various healthcare settings and may be exacerbated in transferred patients.25,26
Transferred patients in both the VUGIB and NVUGIB categories had significantly higher odds of experiencing acute complications, such as vasopressor requirement, mechanical ventilation, ICU admission, and AKI requiring dialysis. In our study, transferred VUGIB patients were more likely to undergo TIPS, while demonstrating lower rates of EGD without intervention and EH compared to their nontransferred counterparts. One plausible explanation for this trend is the limitation of smaller hospitals in providing advanced procedures, such as TIPS. It is conceivable that patients with VUGIB are transferred primarily for specialized interventions like TIPS, which are often not available at smaller facilities. In the NVUGIB cohort, we observed that transferred patients had higher odds of receiving specialized, intervention-focused procedures like IR-guided embolization. This trend, mirroring the trend in the VUGIB group, suggests that these NVUGIB patients likely presented with heightened severity or complications, warranting advanced interventions. The reduced likelihood of these patients undergoing standard EGD indicates prioritization of specialized care following transfer.
Our study adds to the growing evidence that transferred patients are likely to experience worse outcomes and require more aggressive initial management. It would be valuable to validate our findings in future studies to determine the effectiveness of different immediate care strategies on the outcomes of transferred patients. Moreover, the increased costs associated with the transfer highlight the need for an economic analysis to understand whether increased resource utilization translates into improved outcomes or if cost-saving measures can be implemented without compromising care. Better triaging scores need to be devised for gastrointestinal bleeding to improve access to bigger centers for patients who may be anticipated to require transfer later.
Limitations and strengths
There are a few limitations of our study. The retrospective nature of the study restricted the complete randomization of the cohorts. Despite adjusting for potential confounders using multivariate regression analysis, there is still the possibility of residual confounding. We used the NIS, a claims-based database that has inherent limitations of incomplete or missing data.27 Reliance on diagnosis codes instead of clinical parameters can lead to the misclassification of diagnoses. Nevertheless, we used ICD-10 codes for data retrieval, which are more specific than ICD-9 codes.3,28
Our study has several strengths. To our knowledge, since 2015, this is the first study that evaluated the impact of IHT status on the outcomes of NVUGIB and VUGIB at the national level. We employed the NIS database, which incorporates data from a wide range of hospitals across nearly all states in the United States. This confers enhanced external validity and generalizability to our study and helps mitigate biases associated with practice patterns observed in single-center or multicenter studies.29 Additionally, we were able to account for different socioeconomic and hospital factors, including household income estimates and hospitalization costs, which are not feasible in institution-based studies. Furthermore, although severity scales such as MELD-Na and Child-Pugh could not be calculated owing to the unavailability of laboratory values, we devised a surrogate severity scale to adjust for decompensated liver disease in our VUGIB patient population.
CONCLUSION
Our study provides valuable insights into the complexities of managing gastrointestinal bleeding and reveals distinct outcomes between transferred and nontransferred adult patients with VUGIB and NVUGIB. Transferred VUGIB and NVUGIB patients manifested a statistically significant increase in mortality compared to their nontransferred counterparts. The significant difference in outcomes in transferred VUGIB and NVUGIB patients highlights the urgent need to revisit and enhance IHT protocols and immediate care interventions. Moreover, the noticeable extension in hospital stays for transferred patients has both health and financial repercussions, underlining the broader economic impact of managing gastrointestinal bleeding. While our study, being retrospective and dependent on the NIS database, has limitations, it stands out as one of the first to probe the effects of transfers on VUGIB and NVUGIB outcomes nationally. Future studies should aim to further validate our findings and explore cost-efficient strategies without jeopardizing the quality of patient care.
Supplementary Material
Disclosure statement/Funding
The authors report no funding or conflicts of interest.
References
- 1.Farooq U, Tarar Z, Malik A, et al. . How does cirrhosis impact mortality, morbidity, and resource utilization in non-variceal upper gastrointestinal bleeding? A nationwide analysis. Gastroenterol Rev Gastroenterol. 18(2):204–215. doi: 10.5114/pg.2022.115232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esrailian E, Gralnek IM.. Nonvariceal upper gastrointestinal bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):589–605. doi: 10.1016/j.gtc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright DJ. ICD-9-CM to ICD-10-CM codes: what? why? how? Adv Wound Care (New Rochelle). 2013;2(10):588–592. doi: 10.1089/wound.2013.0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peura DA, Lanza FL, Gostout CJ, et al. . The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92(6):924–928. [PubMed] [Google Scholar]
- 5.Gilbert DA, Silverstein FE, Tedesco FJ.. National ASGE survey on upper gastrointestinal bleeding: complications of endoscopy. Dig Dis Sci. 1981;26(7 Suppl):55S–59S. doi: 10.1007/BF01300808. [DOI] [PubMed] [Google Scholar]
- 6.Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. . Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633–1641. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 7.Amin SK, Antunes C. Lower gastrointestinal bleeding. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 8.Kulshrestha A, Singh J.. Inter-hospital and intra-hospital patient transfer: Recent concepts. Indian J Anaesth. 2016;60(7):451–457. doi: 10.4103/0019-5049.186012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton H, Chapman P, Church N, et al. . P241 Clinical outcomes of inter-hospital transfers for upper GI bleeding and the utility of the Glasgow Blatchford Score. Gut. 2023;72:A177. doi: 10.1136/gutjnl-2023-BSG.309. [DOI] [Google Scholar]
- 10.Sedarous M, Alayo QA, Nwaiwu O, et al. . S0623 time trends and outcomes of inter-hospital transfer in patients with upper gastrointestinal bleeding: a nationwide analysis. Am J Gastroenterol. 2020;115(1):S311–S312. doi: 10.14309/01.ajg.0000704540.05350.55. [DOI] [Google Scholar]
- 11.Sakowitz S, Ng A, Williamson CG, et al. . Impact of inter-hospital transfer on outcomes of urgent cholecystectomy. Am J Surg. 2023;225(1):107–112. doi: 10.1016/j.amjsurg.2022.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Mueller SK, Fiskio J, Schnipper J.. Interhospital transfer: transfer processes and patient outcomes. J Hosp Med. 2019;14(8):486–491. doi: 10.12788/jhm.3192. [DOI] [PubMed] [Google Scholar]
- 13.Chen E, Longcoy J, McGowan SK, et al. . Interhospital transfer outcomes for critically ill patients with coronavirus disease 2019 requiring mechanical ventilation. Crit Care Explor. 2021;3(10):e0559. doi: 10.1097/CCE.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudlow P, Burns KEA, Adhikari NKJ, et al. . Inter-hospital transfers and outcomes of critically ill patients with severe acute kidney injury: a multicenter cohort study. Crit Care. 2014;18(5):513. doi: 10.1186/s13054-014-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baig SH, Gorth DJ, Yoo EJ.. Critical care utilization and outcomes of interhospital medical transfers at lower risk of death. J Intensive Care Med. 2022;37(5):679–685. doi: 10.1177/08850666211022613. [DOI] [PubMed] [Google Scholar]
- 16.Cini C, Neto AS, Burrell A, et al. . Inter‐hospital transfer and clinical outcomes for people with COVID‐19 admitted to intensive care units in Australia: an observational cohort study. Med J Aust. 2023;218(10):474–481. doi: 10.5694/mja2.51917. [DOI] [PubMed] [Google Scholar]
- 17.Myers V, Nolan B.. Delays to initiate interfacility transfer for patients transported by a critical care transport organization. Air Med J. 2021;40(6):436–440. doi: 10.1016/j.amj.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Luster J, Yanagawa FS, Bendas C, et al. . Interhospital transfers: Managing competing priorities while ensuring patient safety. In: Vignettes in Patient Safety-Volume 2. Rijeka, Croatia: IntechOpen; 2017. [Google Scholar]
- 19.NIS Overview . The Healthcare Cost and Utilization Project. 2023. https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 20.Talavera B, García-Azorín D, Martínez-Pías E, et al. . Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. 2020;419:117163. doi: 10.1016/j.jns.2020.117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandel MH, Kolkman JJ, Kuipers EJ, et al. . Nonvariceal upper gastrointestinal bleeding: differences in outcome for patients admitted to internal medicine and gastroenterological services. Am J Gastroenterol. 2000;95(9):2357–2362. doi: 10.1111/j.1572-0241.2000.02230.x. [DOI] [PubMed] [Google Scholar]
- 22.Haddad FG, El Imad T, Nassani N, et al. . In-hospital acute upper gastrointestinal bleeding: What is the scope of the problem? World J Gastrointest Endosc. 2019;11(12):561–572. doi: 10.4253/wjge.v11.i12.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel P, Rotundo L, Orosz E, et al. . Hospital teaching status on the outcomes of patients with esophageal variceal bleeding in the United States. World J Hepatol. 2020;12(6):288–297. doi: 10.4254/wjh.v12.i6.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcão D, Alves da Silva J, Pereira Guedes T, et al. . The current portrayal of non-variceal upper gastrointestinal bleeding in a Portuguese tertiary center. GE Port J Gastroenterol. 2021;28(6):392–397. doi: 10.1159/000516139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryer BL, Wilcox CM, Henk HJ, et al. . The economics of upper gastrointestinal bleeding in a US managed-care setting: a retrospective, claims-based analysis. J Med Econ. 2010;13(1):70–77. doi: 10.3111/13696990903526676. [DOI] [PubMed] [Google Scholar]
- 26.Campbell HE, Stokes EA, Bargo D, et al. . Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open. 2015;5(4):e007230–e007230. doi: 10.1136/bmjopen-2014-007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klabunde CN, Warren JL, Legler JM.. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Duncan Saunders L, et al. . Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade C. Internal, external, and ecological validity in research design, conduct, and evaluation. Indian J Psychol Med. 2018;40(5):498–499. doi: 10.4103/IJPSYM.IJPSYM_334_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.