Abstract
Cancer is a major global health issue. Effective therapeutic strategies can prolong patients' survival and reduce the costs of treatment. Drug repurposing, which identifies new therapeutic uses for approved drugs, is a promising approach with the advantages of reducing research costs, shortening development time, and increasing efficiency and safety. Disulfiram (DSF), a Food and Drug Administration (FDA)-approved drug used to treat chronic alcoholism, has a great potential as an anticancer drug by targeting diverse human malignancies. Several studies show the antitumor effects of DSF, particularly the combination of DSF and copper (DSF/Cu), on a wide range of cancers such as glioblastoma (GBM), breast cancer, liver cancer, pancreatic cancer, and melanoma. In this review, we summarize the antitumor mechanisms of DSF/Cu, including induction of intracellular reactive oxygen species (ROS) and various cell death signaling pathways, and inhibition of proteasome activity, as well as inhibition of nuclear factor-kappa B (NF-κB) signaling. Furthermore, we highlight the ability of DSF/Cu to target cancer stem cells (CSCs), which provides a new approach to prevent tumor recurrence and metastasis. Strikingly, DSF/Cu inhibits several molecular targets associated with drug resistance, and therefore it is becoming a novel option to increase the sensitivity of chemo-resistant and radio-resistant patients. Studies of DSF/Cu may shed light on its improved application to clinical tumor treatment.
Keywords: Disulfiram, Aldehyde dehydrogenase, Reactive oxygen species, Proteasome activity, Cancer stem cells, Drug resistance
Introduction
Cancer is becoming one of the most common causes of death, and its prevalence is expected to increase worldwide.[1] Developing effective new pharmacotherapies improves survival and reduces mortality of patients with cancer. Currently, in addition to radical surgery, radiotherapy, and immunotherapy, chemotherapy that employs broad-spectrum cytotoxic drugs remains one of the most effective cancer treatments, despite having significant side effects.[2] Thus, discovering new anticancer drugs is of great importance for fulfilling a highly unmet medical need. However, developing new anticancer drugs is challenging because of high cost and being time consuming. To overcome these challenges, drug repurposing is a practical alternative strategy for using approved drugs with known toxicological and pharmacokinetic characteristics for new indications, which saves research costs and reduces the time to find new ways to treat various diseases.[3,4]
Disulfiram (DSF), the Food and Drug Administration (FDA)-approved drug to treat chronic alcoholism, has been used since 1951 and is well tolerated with minimal side effects.[5] DSF irreversibly inhibits the activity of aldehyde dehydrogenase (ALDH), leading to excessive accumulation of acetaldehyde in the body, thereby establishing the alcohol aversion reflex.[6] Recently, growing evidence shows the potential of repurposing DSF to treat various pathologies such as inflammation, Lyme disease, metabolic disorders, and cancer.[7–10] Numerous mechanistic studies reveal that DSF exhibits excellent anticancer effects such as triggering oxidative stress,[11] inhibiting proteasomes activity,[12] reducing angiogenesis,[13] arresting the cell cycle,[11,14] reducing the stemness of cancer cells,[15] reversing drug resistance,[16,17] constraining tumor metastasis,[18,19] and regulating the immune microenvironment.[20,21]
Currently, the studies on effects of DSF are progressing through several clinical trials designed to treat malignant tumors, including glioblastoma (GBM), metastatic breast cancer, and recurrent pancreatic carcinoma. Furthermore, the trace metal copper (Cu) plays a key role in potentiating the antitumor effect of disulfiram.[22] In this review, we summarize the molecular mechanism of DSF and its metabolites in the treatment of cancers, and we evaluate the contribution of DSF/Cu to enhancing drug sensitivity or reversing drug resistance, which will contribute comprehensive data for repurposing DSF in the future.
Anticancer Mechanisms of DSF/Cu
Numerous studies reveal that DSF serves as an antitumor drug. Although the anticancer mechanism of DSF is unclear, there is no doubt that the combination of DSF and Cu2+ achieves a better antitumor effect than DSF alone.[23–25] Strikingly, Cu2+ is essential for human cells as it participates in numerous processes such as mitochondrial respiration, reactive oxygen species (ROS) generation, and antioxidant/detoxification processes.[26,27] Furthermore, mounting evidence indicates that patients with malignancies have significantly higher levels of serum Cu and intracellular Cu compared with those of healthy controls.[28] Cu plays a prominent role in oncogenesis, cancer progression and severity, because Cu accumulation promotes cell proliferation, angiogenesis, and metastasis.[27,29] Elevated Cu levels in tumor cells serve as a specific target for DSF, which binds tumor cellular copper and impairs the activities of Cu-dependent enzymes, leading to inhibition of cuproplasia (Cu-dependent cellular proliferation).[30] On the other hand, a high concentration of Cu in cancer cells causes cytotoxicity through oxidative stress or by inhibiting enzyme activity to induce specific copper-dependent cell death, called cuproptosis.[31] The Cu ionophore DSF facilitates increased Cu uptake into cancer cells, enabling DSF to specifically target cancer cells while sparing normal cells.[32] Numerous studies show that the administration of DSF with Cu significantly increases anticancer activity.[33,34] Recent mechanistic studies demonstrate that Cu(DDC)2 (bis-diethyldithiocarbamate-copper, also known as CuET), which is a major metabolite of DSF combined with Cu2+, is the active form responsible for its tumor suppressing effects [Figure 1].[35,36] Because Cu(DDC)2 is a potent anticancer agent, we focused on several targets of Cu(DDC)2, including alteration of ROS levels, activation of the mitogen-activated protein kinase (MAPK) pathway, and inhibition of ubiquitin proteasome activity, as well as suppression of NF-κB signaling. Strikingly, apart from Cu(DDC)2, the Zn(DDC)2 complex formed by DDC binding to Zn2+ also represents an important antitumor activity, confirming that DSF-based tumor therapy is metal ion-dependent.[37]
Figure 1.
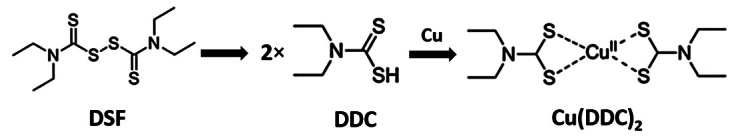
Chemical structure of DSF, DDC, and Cu(DDC)2. Cu(DDC)2: Bis-diethyldithiocarbamate-copper; DDC: Diethyldithiocarbamate; DSF: Disulfiram.
Effects of DSF on ROS
Oxidative stress occurs when the accumulation of ROS exceeds the body's antioxidant capacity. Increased ROS levels are toxic by destroying cellular structures and damaging vital organs, leading to cell death.[38] DSF-mediated cytotoxicity is partially caused by increased ROS production. Evidence indicates that excessive ROS exposure will exhaust cellular antioxidant capacity and selectively induce cancer cell apoptosis.[38] Accumulation of DSF, DDC, and its copper complex Cu(DDC)2 in cancer cells can promote ROS generation, which eventually triggers apoptosis of cancer cells.[39,40] DSF/Cu-induced metallothionein expression results in oxidative stress and inhibits DNA replication in prostate cancer cells.[41,42] Furthermore, the reaction between DDC and Cu2+ reduces Cu2+ to Cu+,[43] a more toxic form of copper ion, which further reacts with O2 and Fe2+ to produce highly cytotoxic ·OH through a Fenton-like reaction.[43] Moreover, the DSF/Cu complex promotes the transport of copper into inflammatory breast cancer (IBC) cells.[32] Cu accumulation causes the intercellular generation of ROS, which alters membrane permeability and further promotes copper uptake, and therefore induces oxidative stress-mediated apoptosis in multiple IBC cellular models.[32] DSF specifically transports Cu ions into tumor tissues, thus preventing Cu from interacting with non-specifically binding proteins.
Furthermore, DSF inhibits the scavenging of ROS. DSF was recently reported to downregulate glutathione peroxidase 4 (GPX4) expression to prevent ROS clearance and induce ferroptosis in GBM, which is rescued by the ferroptosis inhibitor ferrostatin-1.[44] Moreover, DSF/Cu treatment also leads to hepatocellular carcinoma (HCC)-cell death via induction of ferroptosis, associated with a compensatory activation of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2),[45] which plays a key role in counteracting oxidative stress via regulating the expression of antioxidant genes.[46] In particular, DSF, as a specific inhibitor of ALDH, prevents ROS scavenging and detoxification mediated by ALDH isozymes.[6,15] ROS are an inevitable side-product of redox reactions. Increased ROS levels and inhibition of ROS scavenging subject cells to subsequent oxidative stress and this further causes damage to DNA, lipids, and proteins, which triggers cell death.[47]
Mounting studies confirm that DSF enhances oxidative stress and induces ROS production, which is necessary for DSF to exert cytotoxicity in various malignancies, including nasopharyngeal cancer (NPC),[48] gastric cancer,[49] prostate cancer,[50] acute myeloid leukemia (AML),[11] lymphoid malignancies,[51] osteosarcoma,[52] head and neck squamous cell carcinoma (HNSC),[53] lung cancer,[54] breast cancer,[55] thyroid cancer,[56] and HCC.[57] Among them, DSF/Cu is cytotoxic for NPC[48] and HCC[57] through ROS/MAPK promoted apoptosis. Moreover, DSF/Cu is highly toxic to AML cell lines because of the alteration of the ROS balance, cell cycle arrest, and apoptosis, as manifested by an increased rate of apoptosis of approximately 70%.[11] The c-Jun N-terminal kinase (JNK), a critical member of the MAPK family, plays a key role in apoptosis. DSF/Cu activates the ROS-JNK proapoptotic pathway in osteosarcoma cells[52] and HNSC[53] and simultaneously inhibits antiapoptotic pathways such as those mediated by NF-κB and NRF2 signaling in malignant lymphoid cell lines and AML stem cells.[40,51] Furthermore, Xie et al[56] demonstrated that DSF/Cu kills thyroid cancer cells with a lower IC50 (half-maximal inhibitory concentration, 62.88 ± 0.01 nmol/L) in IHH4 cell line via inhibiting the activities of the MAPK/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/serine/threonine kinase 1 (AKT) pathways in a ROS-dependent manner. Notably, Lu et al[39] compared the anticancer effects of DSF and DSF/Cu, and they found that DSF/Cu exhibits a significantly greater effect than DSF alone in A549 cells. They further revealed that DSF is metabolized to form Cu(DDC)2, which accumulates in cancer cell and initiates apoptosis with overproduction of ROS and induces cell cycle arrest. To confirm the effect of ROS, several studies attempted to reverse the cytotoxicity of DSF using the ROS scavenger N-acetylcysteine (NAC).[51,58,59] These studies show a significant reversing effect of NAC on ROS induction, and the toxic effects of DSF are obviously blocked with NAC treatment. It is noteworthy that NAC contains the reactive cysteine structure, which inactivates DSF. Thus, alternatives to ROS inhibitors such as cynarin should be used to confirm ROS levels in the future. Together, the evidence shows that the anticancer effect of DSF is related to its induction of ROS and subsequent cell death [Figure 2], although further in-depth mechanistic research on the cytotoxicity of DSF should be performed.
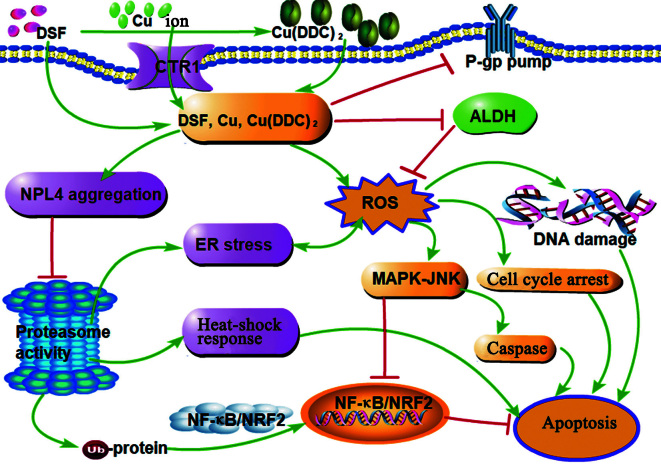
Figure 2.
Anticancer mechanisms of DSF. DSF combined with Cu or Cu(DDC)2 inhibits proteasome activity via NPL4 aggregation, leading to reduced NF-κB and NRF2 activity and consequently apoptosis. Furthermore, DSF, DSF/Cu, and Cu(DDC)2 induce ROS production and inhibit ALDH activity to inhibit ROS scavenging, then initiating DNA damage, cell cycle arrest, and caspase pathway-mediated apoptosis. ALDH: Aldehyde dehydrogenase; Cu(DDC)2: Bis-diethyldithiocarbamate-copper; CTR1: Copper-transport-related protein; DDC: Diethyldithiocarbamate; DSF: Disulfiram; ER: Edoplasmic reticulum; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; NF-κB: Nuclear factor-kappa B; NPL4: Nuclear protein localization protein 4; NRF2: Nuclear factor erythroid 2-related factor 2; P-gp: P-glycoprotein; ROS: Reactive oxygen species; Ub: Ubiquitination.
Effects of DSF on proteasome inhibition
The proteasome complex, which comprises a catalytic 20S core and a 19S regulator, selectively regulates and degrades ubiquitinated proteins.[60] The ubiquitin proteasome system (UPS) is critical for maintaining the balance of protein degradation as well as the physiological functions of cells. Cancer cells depend on UPS more than normal cells, indicating that UPS may serve as an attractive pharmacological target for cancer therapy.[61] DSF/Cu or Cu(DDC)2 blocks the upstream p97 pathway of the proteasome, which induces the accumulation of polyubiquitinated proteins, ultimately leading to cell death, and Cu(DDC)2 induces higher cytotoxicity for diverse types of cancer cells compared with DSF or DDC.[33] Mechanistically, Skrott et al[33] demonstrated that Cu(DDC)2, with high affinity for thiol-containing proteins, induces aggregation and dysfunction of the nuclear protein localization protein 4 (NPL4), an adaptor of the p97 segregase essential for proteasome activity, which consequently blocks p97-NPL4-dependent processes, leading to accumulation of misfolded or even toxic proteins. Furthermore, inactivated p97 segregase induces endoplasmic reticulum (ER) stress and the heat-shock response (HSR), as indicated by detected biomarkers of ER stress and HSP70 after Cu(DDC)2 treatment of U-2OS cells.[33,62] In a follow-up study, Majera et al[63] further demonstrated that disabling the vital NPL4-p97 pathway by DSF/Cu(DDC)2 interferes DNA replication and causes DNA damage, enhancing replication stress. Strikingly, Cu(DDC)2 treatment also impairs the replication protein A (RPA)–ataxia telangiectasia and Rad3 related-interacting protein (ATRIP)–ATR–checkpoint kinase (CHK1) signaling cascade that is critical for prosurvival responses to replication stress, thus collectively provoking a toxic scenario in cancer cells.[63] NF-κB is well known for its antiapoptotic role, and aberrant NF-κB activation is involved in the pathogenesis of many malignant tumors.[64] The activity of proteasomes is critical for activating the NF-κB pathway, because proteasomes cleave the inhibitor-κB molecule (IκB), thereby releasing the NF-κB p50/p65 heterodimer from the inhibitory complex to translocate into the nucleus, leading to gene transcription.[64] Thus, proteasome inhibition leads to the inhibition of NF-κB signaling and cancer cell death. Recently, growing evidence shows that DSF inhibits cancer cell proliferation and promotes apoptosis in vitro and in vivo by increasing NPL4 aggregation, confirming that the p97/NPL4 pathway is a promising therapeutic target of DSF in oncology.[65–69] Notably, the DSF/Cu complex potently inhibits the proteasomal activity of cancer cells, but not that of normal or immortalized cells in in vitro and in vivo experiments.[68] As mentioned above, selective induction of apoptosis of tumor cells is associated with elevated copper levels and these are more dependent on proteasome activity for their survival [Figure 2], further suggesting DSF as an ideal antitumor drug.
DSF targets cancer stem cells (CSCs)
CSCs, which comprise a small population of quiescent cancer cells capable of self-renewal and differentiation, play a critical role in tumor initiation, progression, relapse, metastasis, and resistance to therapy.[70] As a result, targeting CSCs may serve as a promising strategy to improve cancer therapeutics in the future.[70,71] ALDH, as a typical marker of CSC as well as an enzyme required for the stemness of CSCs during oncogenesis, is irreversibly inhibited by DSF.[71] Data from recent studies show that DSF potently inhibits CSCs in various cancers, including AML, breast cancer, and ovarian cancer (OC) owing to the inhibitory effect of DSF on ALDH through diverse mechanisms.[15,40,55,72] For example, DSF/Cu targets aldehyde dehydrogenase isoform-1A1 (ALDH1A1) to inhibit non-small cell lung cancer (NSCLC) growth and recurrence[34] and to overcome cisplatin resistance in breast cancer[72] via inhibition of stemness-related transcription factor expression in ALDH-positive CSCs. Notably, a high-throughput drug screen (HTS) identified DSF as one of the most potent anti-OC compounds. Under CSC-enriching conditions, DSF treatment efficiently inhibits ALDH activity and represses sphere formation, suggesting DSF is able to inhibit CSCs formation in OC cells. Moreover, DSF decreases CSCs populations and reduces relapse in an in vitro model, and DSF also shows efficacy in an in vivo model of postsurgery, postchemotherapy OC relapse, demonstrating that targeting CSCs prevents OC recurrence.[73] Likewise, using HTS, researchers tested the sensitivity of glioma stem cells (GSCs) to 2000 compounds, among which DSF significantly inhibits the proliferation of GSCs. Notably, DSF toxicity for cancers is enhanced by Cu, which significantly increases CSC death via inactivation of the ubiquitin-proteasome pathway.[74]
However, recent evidence seems to challenge the notion that DSF-induced CSC toxicity is attributed to ALDH inhibition. A recent study demonstrates that repurposing DSF modulates the cell cycle distribution and significantly decreases clonogenic survival of GBM stem cells independent of ALDH3 expression.[75] Skrott et al[66] found that inhibition of ALDH is only secondary to membrane damage and cell death, rather than the preferential cytotoxicity of DSF/Cu. Wang et al[76] revealed that DSF/Cu complexes block the formation of breast cancer CSCs by downregulating the NF-κB-stemness gene pathway. Correspondingly, in vivo, combined treatment of radiotherapy and DSF significantly inhibited mammary primary tumor growth and spontaneous lung metastasis compared with olive oil-treated mice (vehicle control).[76] As expected, DSF increased DNA damage, and induced apoptosis and autophagy as well as cell cycle arrest in irradiated CSCs of an atypical teratoid /rhabdoid tumor.[77] Concurrently, DSF combined with radiotherapy significantly potentiates the anticancer effects of radiotherapy manifested by inhibited tumor growth and prolonged survival in atypical teratoid /rhabdoid tumor mouse models.[77] More recently, Sun et al[59] revealed that DSF/Cu induces robust antitumor immune responses, triggering a higher level of immunogenic cell death (ICD) of breast cancer CSCs, partly caused by ROS generation and ER stress. Notably, DDC binding to Zn2+ also suppresses the stem cell properties of lung cancer cells.[78] Cui et al[79] found that DSF/Zn nanoparticles significantly inhibit the growth of CSCs and tumors without damaging non-involved organs during oral cancer therapy. Collectively, studies in the past five years demonstrate that DSF exerts strong cytotoxicity upon various CSCs through diverse mechanisms. The research on DSF targeting CSCs is listed in Table 1.[40,54,59,69,72,73,75,79–87]
Table 1.
Disulfiram plays a critical role in inhibiting various CSCs in vitro and in vivo.
| Type of cancer | Cell line | In vitro | Mechanisms | In vivo | Administration/dose | Efficacy | References |
|---|---|---|---|---|---|---|---|
| Glioblastoma | LK17 | DSF/Cu (0.1/0.1 μmol/L) | DSF/Cu inhibits clonogenic survival of glioblastoma CSCs, independent of ALDH1A3 expression. | NA | NA | NA | [75] |
| Glioblastoma | U87MG, U251MG, U373MG | DSF/Cu (1/10 μmol/L) | DSF inhibits hypoxia-induced GSC and EMT phenotypes by inhibiting NF-κB-p65 protein expression. | BALB/c Nu/Nu mice xenografts | DSF-PLGA (10 mg/kg) CuGlu (6 mg/kg), 3 days/week for 4 weeks | DSF-PLGA/Cu significantly reduced the intracranial and subcutaneous tumor size and tumor weight in mice. | [80] |
| Breast cancer | MCF-7, SKB-R3, MDA-MB-435S | DSF (1 μmol/L) | DSF inhibits ALDH activity and inhibits Sox, Nanog, and Oct expression in CSCs, and modulates ROS generation. | NA | NA | NA | [72] |
| Breast cancer | MDA-MB-231, UACC-812 | DSF/Cu (0.15/1 μmol/L) | DSF/Cu induces ICD in breast CSCs partially by ROS generation and IRE1α/XBP1 pathway activation. | NA | NA | NA | [59] |
| Breast cancer | MCF-7, BT549, MDA-MB-231 | DSF (0.5–15 μmol/L) | DSF suppresses EMT and CSC by inhibiting SOX4, which is induced by upregulating miR-30a expression. | NA | NA | NA | [81] |
| NSCLC | H292 | DSF/Cu (0.05–0.15/15 μmol/L) | DSF/Cu complex induces oxidative stress, including superoxide, peroxide, lipid peroxidation, and mitochondrial damage. | Athymic nude mice xenografts | DSF 100 mg·kg–1·day–1 by oral gavage for 25 days. | DSF decreased xenograft tumor growth and exerted chemo- and radio-therapy-sensitizing effects. | [54] |
| AML | KG1α, Kasumi-1 | DSF/Cu (0.5/1 μmol/L) | DSF/Cu induces ROS-JNK pathway and inhibites pro-survival NRF2 and NF-κB pathways to kill CSCs. | NOD/SCID xenograft models | DSF (3 mg·20 g–1·day–1) Cu (0.03 mg·20g–1·day–1) for 2 weeks | DSF/Cu also significantly inhibited tumor growth and reduced tumor burden. | [40] |
| AML | THP1, UT7 | DSF (0.9 μmol/L) | DSF in combination with Ara-c suppresses P65 expression and increases intracellular γ-H2AX formation in CSCs. | NOD/SCID mice xenograft model |
3 mg·20g–1·day–1 DSF for 4 consecutive days |
DSF eliminated the ALDH high leukemia cells and enhanced sensitivity to Ara-c in transplanted mice. | [82] |
| DTCs | K1, WRO | DSF/Cu (0.1/1 μmol/L) | DSF/Cu targets CSCs in DTCs by inhibiting c-Myc- or E2F1-mediated BMI1 expression. | NA | NA | NA | [83] |
| Cervical cancer | SiHa, HeLa | DSF/Cu (0.1/0.01 μmol/L) | DSF/Cu inhibites the expression of stemness markers (ALDH, CD49f) and reduces the LGR5+CSCs. | BALB/c-nude mice xenograft models | DSF (30 mg/kg), CuCl2 (1.5 mg/kg) twice per week for the experiment | DSF/Cu complex inhibited tumor growth and had the greater antitumor efficacy on cervical cancer than cisplatin in vivo. | [84] |
| Medulloblastoma | D425med, D341 | DSF/Cu (0.1/0.01 μmol/L) | DSF/Cu reduces ALDH activity and CD133 expression. | Athymic Nu/Nu mice xenografts | DSF (150 mg·kg–1·day–1), Cu2+ (2 mg·kg–1·day–1) of 5 days/week for 3 weeks | DSF/Cu inhibited tumor growth and prolonged survival in vivo. | [69] |
| Ovarian cancer | IGROV1, SKOV3 SKOV3IP1 | DSF/Cu (1/1 μmol/L) | DSF/Cu increases intracellular ROS levels triggering apoptosis of ovarian CSC. | NA | NA | NA | [85] |
| Ovarian cancer | OV90, OVCAR8 | DSF (0.25 μmol/L, OV90), DSF (0.5 μmol/L, OVCAR8) | DSF promotes ROS generation and enhances oxidative stress in CSCs, thus increasing cell death. | Athymic Nu/Nu mice xenografts mice | DSF (10 mg/kg) three times per week for 3 weeks | DSF was effective in a post-surgery, post-chemotherapy ovarian cancer relapse model in vivo. | [73] |
| Chondrosarcoma | SW1353, CS-1 | DSF/Cu (0.05/1 μmol/L) | DSF/Cu decreases NF-κB-stemness pathway in CSCs. | Xenograft nude or NSG mouse | DSF (50 mg·kg–1·day–1), Cu (0.03 mg·kg–1·day–1) for 7 days | DSF/Cu inhibited tumor growth and prolonged survival in vivo. | [86] |
| Multiple myeloma | NCI-H929 | DSF/Cu (0.1/1 μmol/L) | DSF/Cu can inhibit the ALDH+ stem cells through suppressing ALDH1A1 and Hedgehog pathway. | NOD/SCID xenograft mouse model | DSF (150 mg·kg–1·day–1), Cu (2 mg·kg–1·day–1) for 3 weeks | DSF/Cu reduced the tumor growth and inhibited stemness of multiple myeloma in xenograft model. | [87] |
| Oral carcinoma | Cal27 | DSF (25 mg/g IRMOF3), IRMOF3 (100 μg/mL), Zn (100 μmol/L) | Folic acid-modified DSF/Zn-IRMOF3 nanoparticles could inhibit ALDH1+ CSCs by downregulating the expressions of ALDH1A1, Nanog, OCT4, and SOX2. | BALB/c mouse xenograft model | IRMOF3-DSF-FA every 3 days for 30 days | IRMOF3-DSF-FA could inhibit tumor growth and had a good tumor-targeting ability in vivo. | [79] |
ALDH: Aldehyde dehydrogenase; ALDH1A3: Aldehyde dehydrogenase isoform-1A3; AML: Acute myeloid leukemia; Ara-C: Arabinocytidine; BCSCs: Breast cancer stem cells; BMI1: B lymphoma Mo-MLV insertion region 1 homolog; c-Myc: cellular-myelocytomatosis viral oncogene; CSCs: Cancer stem cells; CuGlu: Copper gluconate; DSF: Disulfiram; DSF/Cu: Combination of DSF and copper; DTCs: Differentiated thyroid carcinomas; E2F 1: Early 2 factor transcription factor 1; EMT: Epithelial mesenchymal transition; GSC: Glioma stem cell; ICD: Immunogenic cell death; IRE1α: Inositol-requiring enzyme 1α; JNK: c-Jun N-terminal kinase; LGR5: Leucine-rich repeat-containing G-protein coupled receptor 5; MiR: Micro RNA; NA: Not available; NF-κB: Nuclear factor-kappa B; NOD: Non obese diabetes; NRF2: Nuclear factor erythroid 2-related factor 2; NSCLC: Non-small cell lung cancer; PLGA: Poly lactic-co-glycolic acid; ROS: Reactive oxygen species; SCID: Severe combined immune deficiency; XBP1: X-box binding protein 1; γH2AX: γ-H2A histone family member X.
DSF Reverses Drug Resistance
Drug resistance, either intrinsic or acquired, is a serious problem associated with the treatment of malignant tumors, which is mainly caused by factors such as hypoxia, preexisting CSC populations, enhanced drug efflux pumps, and activation of NF-κB.[16,54,88,89] Hypoxic cells and CSCs represent two key factors contributing to failure of treatment for NSCLC. DSF/Cu lengthens survival and decreases the progression of NSCLC.[54] Mechanistically, DSF induces superoxide production and mitochondrial stress, which significantly decrease the viability of hypoxic cells, mitigating resistance to radiation and chemotherapy in vitro and in vivo.[54] As mentioned above, the presence of CSCs has profound implications for drug resistance. DSF-Cu complex reverses the Taxol-resistant (A549/Taxol) cells and vincristine-resistant cells (KB/VCR cells) via decreasing the expression of ALDH2 and stem cell transcription factors.[88] On the other hand, cancer cells exert multidrug resistance (MDR) by accelerating efflux or blocking the influx of drugs through various membrane transporters such as P-glycoprotein (P-gp), multidrug resistance-associated protein 1 (MRP1), and ATPase copper transporting proteins alpha and beta (ATP7A and ATP7B). DSF inhibits ATP7A expression, which increases the levels of platinum–DNA adducts as well as apoptosis of human urothelial carcinoma (UC) cells, indicating that DSF confers UC cells higher sensitivity to chemotherapy.[16] Furthermore, chemoresistance is closely related to the activation of NF-κB in cancer cells.[89] Liu et al[90] found that administration of liposome-encapsulated DSF reverses the chemoresistance of breast cancer cells by targeting the NF-κB pathway in vitro and in vivo. Collectively, these data support the conclusion that DSF serves as an adjuvant strategy for overcoming drug resistance. Moreover, DSF significantly increases the sensitivity to ionizing radiation of pancreatic cancer cells and xenografted nude mice by aggravating DNA damage as well as inducing cell cycle arrest and apoptosis.[14] Similarly, DSF improves T-cell-mediated antitumor immunity via directly activating T-cell antigen receptor (TCR) signaling. Furthermore, DSF-induced antitumor immunity against colon cancer and melanoma in mouse models is further enhanced when combined with anti-programed cell death-1 (PD-1).[91] However, Zirjacks et al[75] demonstrated that DSF/Cu does not improve the treatment response to radiotherapy and temozolomide in mesenchymal GBM cells. And the further mechanism research found that temozolomide can reduce the effect of DSF on clonogenic survival, likely caused by pharmacological interactions between DSF and temozolomide. Here, the effects of DSF/Cu-based therapy on improving radio/chemo-sensitivity are summarized in Table 2,[48,54,59,69,72,88,92–99] which will hopefully provide insights and inspirations for researchers in this field.
Table 2.
DSF/Cu-enhanced drug sensitivity by targeting specific molecules.
| Type of cancer | Compound (dose) | Mechanisms | In vivo | Administration/dose | Efficacy | References |
|---|---|---|---|---|---|---|
| NPC | DSF/Cu + cisplatin | DSF/Cu induced NPC apoptosis by ROS/MAPK and inactive CAFs by inhibiting α-SMA. | BALB/c nude mouse 5-8F xenograft model | 150 mg·kg–1·day–1 DSF, 2 mg·kg–1·day–1 Cu, 5 mg/kg CDDP per 3 days for 15 days. | Combined with CDDP, DSF/Cu significantly inhibited tumor growth of NPC tissues in vivo. | [48] |
| TGCTs | DSF + cisplatin | DSF sensitized resistant NTERA-2 CisR cells by decreasing ALDH activity and alteration of stemness-associated genes expression. | Balb/c-nu/nu xenograft model | 50 mg·kg–1·day–1 DSF i.p., 3 mg·kg–1·day–1 cisplatin i.p. for 28 days. | DSF in combination with cisplatin inhibited tumor growth of NTERA-2 CisR xenografts. | [92] |
| ESCC | DSF/Cu + cisplatin + radiation | DSF/Cu sensitized chemo/radio-resistant ALDH1-positive ESCC cells by inhibiting ALDH1 and downregulating the PI3K/Akt pathway. | BALB/c nude mice | 50 mg/kg DSF (i.p.), 0.15 mg/kg Cu (orally), radiotherapy (4 Gy) | DSF/Cu complex enhances the radiosensitivity in ESCC via inhibition of ALDH1 in tumor-initiating cells. | [93] |
| Breast cancer | DSF/Cu + cisplatin | DSF overcame cisplatin resistance by targeting ALDH, inhibiting the expression of Sox, Nanog, and Oct, and modulating ROS generation. | NA | NA | NA | [72] |
| Breast cancer | DSF + DOX | Lipo-DSF-DOX effectively overcame DOX resistance by inhibiting P-gp activity and its ubiquitination. | NA | NA | NA | [94] |
| Breast cancer | DSF/Cu + radiation | DSF/Cu induced ICD and improved the sensitivity in IR-resistant CSCs partially by ROS generation and IRE1α/XBP1 pathway. | NA | NA | NA | [59] |
| Breast cancer | DSF + DTX | DSF inhibited P-gp expression and increased ROS production and apoptosis. | Balb/c mice orthotopic breast cancer | 73 mg/kg DSF (i.p.), 20 mg/kg DTX (i.v.), 0.085 ppm Cu in drinking water | DSF/Cu enhanced anti-tumor efficacy and prevented lung metastasis in vivo. | [95] |
| NSCLC | DSF/Cu + Taxol/VCR | DSF/Cu downregulated the expression of ALDH2 and reduced the levels of P-gp and stem cell transcription factors in vitro | Balb/c nude mice xenograft model | 60 mg/kg DSF, 1.92 mg/kg Cu, 10 mg/kg Taxol; 30 mg/kg or 60 mg/kg DSF, 9.6 mg/kg Cu, 1 mg/kg VCR | DSF/Cu significantly inhibited tumor growth and reversed microtubule inhibitor resistance in vivo. | [88] |
| HNSCC | DSF/Cu + cisplatin + irradiation | DSF/Cu inhibited cisplatin-/IR-induced G2/M phase arrest. Triple treatment of DSF/Cu + cisplatin + IR significantly increased the cytotoxicity by enhancing the ROS accumulation. | NMRI nu/nu mice PDX model | Disulfiram (60 mg/kg, s.c.) three times a week, cisplatin (8 mg/kg, i.v.) once a week and irradiation (10 Gy) | DSF inhibited tumor growth in three different HNSCC-derived PDX models, supporting DSF as a strong radio-chemosensitizer. | [96] |
| NSCLC | DSF/Cu + cisplatin + irradiation | DSF/Cu enhanced radiation and chemotherapy toxicity in tumor cells dependent on ROS overproduction and Cu retention. | Athymic nude mice xenograft model | Radiation (3 × 6 Gy)+carboplatin (2 × 15 mg/kg)+DSF(100 mg/kg) | DSF decreased xenograft tumor growth when combined with radiation and carboplatin in vivo. | [54] |
| GBM | DSF/Cu + temozolomide | DSF/Cu impaird DNA repair and enhanced the effects of DNA alkylating agents to augment temozolomide activity. | SCID mice orthotopic transplantation | 100 mg·kg–1·day–1 DSF, 2 mg·kg–1·day–1 Cu, 50 mg·kg–1·mouse–1·day–1 temozolomide | DSF/Cu prolonged in vivo survival in patient-derived BTIC models established from both newly diagnosed and recurrent tumors. | [97] |
| GBM | DSF + galunisertib | DSF inhibited ALDH activity and decreased TGF-β signaling. | SCID mice orthotopic xenograft model | 50 mg/kg DSF, 75 mg/kg galunisertib | DSF and galunisertib suppressed therapeutic-resistant GBM growth in vivo. | [98] |
| PDAC | DSF/Cu + IR + 5-FU/FOLFIRINOX | DSF/Cu targeted PCSCs and inhibited the NF-κB-stemness gene pathway. | C57BL/6 xenograft model | 50 mg/kg DSF, 8 Gy IR,10 mg/kg 5-FU, FOLFIRINOX (mixture: 4.75 mg/kg irinotecan, 10.5 mg/kg leucovorin, 2.25 mg/kg oxaliplatin, 20 mg/kg 5-FU) | DSF/Cu combined with IR and 5-FU was more effective than either IR + 5-FU or IR + FOLFIRINOX therapy in inhibiting tumor growth of the mouse. | [99] |
α-SMA: α-Smooth muscle actin; A549/Taxol cells: Taxol-resistant A549 cells; ALDH: Aldehyde dehydrogenase; BTIC: Brain tumor-initiating cells; CAFs: Cancer-associated fibroblasts; CDDP: Cisplatin; CisR: Chemoresistant; CSCs: Cancer stem cells; DOX: Doxorubicin; DSF: Disulfiram; DSF/Cu: Combination of DSF and copper; DTX: Docetaxel; ESCC: Esophageal squamous cell carcinoma; 5-FU: 5-Fluorouracil; FOLFIRINOX: Mix of 4 drugs: Irinotecan, Leucovorin, Oxaliplatin, and 5-Fluorouracil; GBM: Glioblastoma; HNSCC: Head and neck squamous cell carcinoma; ICD: Immunogenic cell death; i.p.: Intraperitoneal injection; IR: Ionizing radiation; IRE1α: Inositol-requiring enzyme 1α; i.v.: Intravenous injection; KB/VCR cells: Vincristine-resistant KB cells; MAPK: Mitogen-activated protein kinase; NA: Not applicable; NF-κB: Nuclear factor-kappa B;NPC: Nasopharyngeal cancer; NSCLC: Non-small cell lung cancer; PCSCs: Pancreatic cancer stem cells; PDAC: Pancreatic ductal adenocarcinoma; PDX: Patient-derived tumor xenograft; P-gp: P-glycoprotein; PI3K: Phosphoinositide 3-kinase; ROS: Reactive oxygen species; s.c.: Subcutaneous injection; SCID: Server combined immune-deficiency; TGCTs: Testicular germ cell tumors; TGF-β: Transforming growth factor β; VCR: Vincristine; XBP1: X-box binding protein 1.
Improved Drug Delivery System for DSF
Although DSF/Cu exhibits high toxicity in various cancer cells, clinical studies of DSF/Cu in cancer patients are not satisfactory. These inconsistent outcomes may be attributed to the rapid degradation of DSF or the unwanted modification of its metabolite DDC in the liver. Consequently, DDC loses its functional sulfhydryl groups, resulting in reduced chelation between DDC and Cu, which deceases the levels of the active Cu(DDC)2 complex.[58] To overcome its instability and to realize the maximal therapeutic efficacy of DSF, various combination therapies and drug delivery systems (DDSs) have been extensively explored. For example, the use of nano-encapsulation technologies such as liposomes, polymers, polymeric micelles, or protein (albumin) particles encapsulating the DSF/DDC to protect the functional thiol groups of DDC has been widely applied to the treatment of various cancers.[90,94,100–103] Furthermore, nanoparticles are easily captured by tumor cells, increasing drug concentrations at the lesion site, which alleviates cytotoxicity.[104] In contrast, the codelivery systems for DSF and other chemotherapeutics may efficiently overcome drug resistance, promoting a synergistic effect.[105–107] Collectively, recent DSF-based treatment strategies have undergone dramatic development with huge potential for the treatment of cancer, which are well summarized in a recent review by Lu et al.[108]
Challenges and Perspectives
DSF is a first-line anti-alcoholism drug with good safety profiles that has been used in clinics for over 70 years. Recently, drug repurposing research has shown its great potential for developing an antitumor agent. However, the clinical trials of DSF encountered some challengesthat must be addressed. First, given that DSF-induced cytotoxicity depends on Cu, supplementation with copper should be applied to patients with Cu deficiency. Notably, DSF/Cu manifested more serious and uncontrollable toxicity against cancer cells than DSF alone. Thus, considering its safety profile, balancing between DSF with slight efficacy and DSF/Cu with strong cytotoxicity should be fully explored to allow the clinical application of DSF for comprehensive cancer treatment. Second, DSF/Cu regulates the immune microenvironment and induces the death of immunogenic cells that attack cancer cells. However, further research is needed to explore how to prevent excessive cytokine release and inflammatory response during antitumor treatment. Furthermore, when DSF is used as an adjuvant therapy for cancers, the pharmacological interactions with comedications should be carefully investigated in advanced for the success of future clinical trials. Finally, DSF can also be combined with another metal, such as Zn ion which also shows anticancer activities, hence the application of DSF in vivo in complicated and should be further investigated in the future. Despited these challenges, we firmly believe that the prospect of DSF in serving as a treatment for cancer is promising. Further studies should focus on the in-depth mechanism of DSF as an anticancer reagent, and trials designed to bridge the gap between the laboratory and the clinic are required.
Conclusions
DSF, especially DSF/Cu, shows high anti-tumor effects on diverse cancers, which is associated with induction of the intracellular ROS, inhibition of proteasome activity, as well as inhibition of nuclear factor-kappa B (NF-κB) signaling. In addition, DSF or DSF/Cu targets CSCs and improves radio/chemo-sensitivity, which will provide a novel avenue for cancer treatment in the future.
Funding
This study was supported by grants from the Undergraduate Research and Innovation Project of University of South China (Nos. X202110555528, S202210555245, and X202210555136).
Conflicts of interest
None.
Footnotes
Min Zeng, Baibei Wu, and Wenjie Wei contributed equally to this work.
How to cite this article: Zeng M, Wu BB, Wei WJ, Jiang ZH, Li PQ, Quan YT, Hu XB. Disulfiram: A novel repurposed drug for cancer therapy. Chin Med J 2024;137:1389–1398. doi: 10.1097/CM9.0000000000002909
References
- 1.Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71: 209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020;395: 1078–1088. doi: 10.1016/S0140-6736(20)30164-1. [DOI] [PubMed] [Google Scholar]
- 3.Pushpakom S Iorio F Eyers RA Escott KJ Hopper S Wells A, et al. Drug repurposing: Progress, challenges and recommendations. Nat Rev Drug Discov 2019;18: 41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 4.Turanli B Altay O Borén J Turkez H Nielsen J Uhlen M, et al. Systems biology based drug repositioning for development of cancer therapy. Semin Cancer Biol 2021;68: 47–58. doi: 10.1016/j.semcancer.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Fuller RK, Williford WO, Lee KK, Derman R. Veterans administration cooperative study of disulfiram in the treatment of alcoholism: Study design and methodological considerations. Control Clin Trials 1984;5: 263–273. doi: 10.1016/0197-2456(84)90030-8. [DOI] [PubMed] [Google Scholar]
- 6.Kleczkowska P, Sulejczak D, Zaremba M. Advantages and disadvantages of disulfiram coadministered with popular addictive substances. Eur J Pharmacol 2021; 904: 174143. doi: 10.1016/j.ejphar.2021.174143. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Chen S, Li C, Xu J, Wang L. Application of disulfiram and its metabolites in treatment of inflammatory disorders. Front Pharmacol 2022; 12: 795078. doi: 10.3389/fphar.2021.795078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omran Z, Sheikh R, Baothman OA, Zamzami MA, Alarjah M. Repurposing disulfiram as an anti-obesity drug: Treating and preventing obesity in high-fat-fed rats. Diabetes Metab Syndr Obes 2020;13: 1473–1480. doi: 10.2147/DMSO.S254267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liegner KB. Disulfiram (tetraethylthiuram disulfide) in the treatment of lyme disease and babesiosis: Report of experience in three cases. Antibiotics (Basel) 2019; 8: 72. doi: 10.3390/antibiotics8020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Wang JY, Wu CF, Wang LH, Chen ZS, Cui W. The combination of disulfiram and copper for cancer treatment. Drug Discov Today 2020;25: 1099–1108. doi: 10.1016/j.drudis.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Hassani S Ghaffari P Chahardouli B Alimoghaddam K Ghavamzadeh A Alizadeh S, et al. Disulfiram/copper causes ROS levels alteration, cell cycle inhibition, and apoptosis in acute myeloid leukaemia cell lines with modulation in the expression of related genes. Biomed Pharmacother 2018;99: 561–569. doi: 10.1016/j.biopha.2018.01.109. [DOI] [PubMed] [Google Scholar]
- 12.Chen C Nie D Huang Y Yu X Chen Z Zhong M, et al. Anticancer effects of disulfiram in T-cell malignancies through NPL4-mediated ubiquitin-proteasome pathway. J Leukoc Biol 2022;112: 919–929. doi: 10.1002/JLB.5MA1121-644R. [DOI] [PubMed] [Google Scholar]
- 13.Roy B, Palaniyandi SS. Aldehyde dehydrogenase 2 inhibition potentiates 4-hydroxy-2-nonenal induced decrease in angiogenesis of coronary endothelial cells. Cell Biochem Funct 2020;38: 290–299. doi: 10.1002/cbf.3468. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y Lu L Luo J Wang L Zhang Q Cao J, et al. Disulfiram alone functions as a radiosensitizer for pancreatic cancer both in vitro and in vivo. Front Oncol 2021; 11: 683695. doi: 10.3389/fonc.2021.683695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo F, Yang Z, Sehouli J, Kaufmann AM. Blockade of ALDH in cisplatin-resistant ovarian cancer stem cells in vitro synergistically enhances chemotherapy-induced cell death. Curr Oncol 2022;29: 2808–2822. doi: 10.3390/curroncol29040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita Y Hamada A Saito R Teramoto Y Tanaka R Takano K, et al. Systematic chemical screening identifies disulfiram as a repurposed drug that enhances sensitivity to cisplatin in bladder cancer: A summary of preclinical studies. Br J Cancer 2019;121: 1027–1038. doi: 10.1038/s41416-019-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammad IS, Teng C, Chaurasiya B, Yin L, Wu C, He W. Drug-delivering-drug approach-based codelivery of paclitaxel and disulfiram for treating multidrug-resistant cancer. Int J Pharm 2019;557: 304–313. doi: 10.1016/j.ijpharm.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 18.Bu W Wang Z Meng L Li X Liu X Chen Y, et al. Disulfiram inhibits epithelial-mesenchymal transition through TGFβ-ERK-Snail pathway independently of Smad4 to decrease oral squamous cell carcinoma metastasis. Cancer Manag Res 2019;11: 3887–3898. doi: 10.2147/CMAR.S199912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y Wang LH Zhang HT Wang YT Liu S Zhou WL, et al. Disulfiram combined with copper inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma through the NF-κB and TGF-β pathways. J Cell Mol Med 2018;22: 439–451. doi: 10.1111/jcmm.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu JJ Liu X Xia S Zhang Z Zhang Y Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 2020;21: 736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao P Wang Y Kang X Wu A Yin W Tang Y, et al. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci 2018;9: 2674–2689. doi: 10.1039/c7sc04853j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveri V. Selective targeting of cancer cells by copper ionophores: An overview. Front Mol Biosci 2022; 9: 841814. doi: 10.3389/fmolb.2022.841814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu C, Li X, Ren Y, Zhang X. Disulfiram: A novel repurposed drug for cancer therapy. Cancer Chemother Pharmacol 2021;87: 159–172. doi: 10.1007/s00280-020-04216-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L Yang L Yang C Liu Y Chen Q Pan W, et al. Membrane loaded copper oleate PEGylated liposome combined with disulfiram for improving synergistic antitumor effect in vivo. Pharm Res 2018; 35: 147. doi: 10.1007/s11095-018-2414-5. [DOI] [PubMed] [Google Scholar]
- 25.McMahon A, Chen W, Li F. Old wine in new bottles: Advanced drug delivery systems for disulfiram-based cancer therapy. J Control Release 2020;319: 352–359. doi: 10.1016/j.jconrel.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz LM, Libedinsky A, Elorza AA. Role of copper on mitochondrial function and metabolism. Front Mol Biosci 2021; 8: 711227. doi: 10.3389/fmolb.2021.711227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michniewicz F Saletta F Rouaen JRC Hewavisenti RV Mercatelli D Cirillo G, et al. Copper: An intracellular achilles' heel allowing the targeting of epigenetics, kinase pathways, and cell metabolism in cancer therapeutics. ChemMedChem 2021;16: 2315–2329. doi: 10.1002/cmdc.202100172. [DOI] [PubMed] [Google Scholar]
- 28.Chen F Wang J Chen J Yan L Hu Z Wu J, et al. Serum copper and zinc levels and the risk of oral cancer: A new insight based on large-scale case-control study. Oral Dis 2019;25: 80–86. doi: 10.1111/odi.12957. [DOI] [PubMed] [Google Scholar]
- 29.Aubert L Nandagopal N Steinhart Z Lavoie G Nourreddine S Berman J, et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat Commun 2020; 11: 3701. doi: 10.1038/s41467-020-17549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock JL Kalimutho M Straube J Lim M Gresshoff I Saunus JM, et al. COMMD3 loss drives invasive breast cancer growth by modulating copper homeostasis. J Exp Clin Cancer Res 2023; 42: 90. doi: 10.1186/s13046-023-02663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsvetkov P Coy S Petrova B Dreishpoon M Verma A Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022;375: 1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allensworth JL Evans MK Bertucci F Aldrich AJ Festa RA Finetti P, et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol Oncol 2015;9: 1155–1168. doi: 10.1016/j.molonc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skrott Z Mistrik M Andersen KK Friis S Majera D Gursky J, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017;552: 194–199. doi: 10.1038/nature25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X Xue X Wang L Wang W Han J Sun X, et al. Suppressing autophagy enhances disulfiram/copper-induced apoptosis in non-small cell lung cancer. Eur J Pharmacol 2018;827: 1–12. doi: 10.1016/j.ejphar.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Xu L Xu J Zhu J Yao Z Yu N Deng W, et al. Universal anticancer Cu(DTC)2 discriminates between thiols and zinc(II) thiolates oxidatively. Angew Chem Int Ed Engl 2019;58: 6070–6073. doi: 10.1002/anie.201814519. [DOI] [PubMed] [Google Scholar]
- 36.Kannappan V Ali M Small B Rajendran G Elzhenni S Taj H, et al. Recent advances in repurposing disulfiram and disulfiram derivatives as copper-dependent anticancer agents. Front Mol Biosci 2021; 8: 741316. doi: 10.3389/fmolb.2021.741316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao YZ Lin MT Lan QH Zhai YY Xu HL Xiao J, et al. Silk fibroin-modified disulfiram/zinc oxide nanocomposites for pH triggered release of Zn2+ and synergistic antitumor efficacy. Mol Pharm 2020; 17: 3857–3869. doi: 10.1021/acs.molpharmaceut.0c00604. [DOI] [PubMed] [Google Scholar]
- 38.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell 2020;38: 167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu XL, Lin B, Xu N, Huang H, Wang Y, Lin JM. Evaluation of the accumulation of disulfiram and its copper complex in A549 cells using mass spectrometry. Talanta 2020; 211: 120732. doi: 10.1016/j.talanta.2020.120732. [DOI] [PubMed] [Google Scholar]
- 40.Xu B Wang S Li R Chen K He L Deng M, et al. Disulfiram/copper selectively eradicates AML leukemia stem cells in vitro and in vivo by simultaneous induction of ROS-JNK and inhibition of NF-κB and Nrf2. Cell Death Dis 2017; 8: e2797. doi: 10.1038/cddis.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iljin K Ketola K Vainio P Halonen P Kohonen P Fey V, et al. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res 2009;15: 6070–6078. doi: 10.1158/1078-0432.CCR-09-1035. [DOI] [PubMed] [Google Scholar]
- 42.Ruttkay-Nedecky B Nejdl L Gumulec J Zitka O Masarik M Eckschlager T, et al. The role of metallothionein in oxidative stress. Int J Mol Sci 2013;14: 6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis DJ, Deshmukh P, Tedstone AA, Tuna F, O'Brien P. On the interaction of copper(II) with disulfiram. Chem Commun (Camb) 2014;50: 13334–13337. doi: 10.1039/c4cc04767b. [DOI] [PubMed] [Google Scholar]
- 44.Qiu C Zhang X Huang B Wang S Zhou W Li C, et al. Disulfiram, a ferroptosis inducer, triggers lysosomal membrane permeabilization by up-regulating ROS in glioblastoma. Onco Targets Ther 2020;13: 10631–10640. doi: 10.2147/OTT.S272312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren X Li Y Zhou Y Hu W Yang C Jing Q, et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol 2021; 46: 102122. doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang Q, Zhao Y, Lin J, Jiang S, Li W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp Mol Med 2022;54: 1067–1075. doi: 10.1038/s12276-022-00829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Y Lu Y Saredy J Wang X Drummer Iv C Shao Y, et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol 2020; 37: 101696. doi: 10.1016/j.redox.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y Chen F Chen J Chan S He Y Liu W, et al. Disulfiram/copper induces antitumor activity against both nasopharyngeal cancer cells and cancer-associated fibroblasts through ROS/MAPK and ferroptosis pathways. Cancers (Basel) 2020; 12: 138. doi: 10.3390/cancers12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y Guan X Wang M Wang N Chen Y Li B, et al. Disulfiram/copper induces antitumor activity against gastric cancer via the ROS/MAPK and NPL4 pathways. Bioengineered 2022;13: 6579–6589. doi: 10.1080/21655979.2022.2038434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safi R Nelson ER Chitneni SK Franz KJ George DJ Zalutsky MR, et al. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res 2014;74: 5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zha J Chen F Dong H Shi P Yao Y Zhang Y, et al. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J Transl Med 2014; 12: 163. doi: 10.1186/1479-5876-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo W Zhang X Lin L Wang H He E Wang G, et al. The disulfiram/copper complex induces apoptosis and inhibits tumour growth in human osteosarcoma by activating the ROS/JNK signalling pathway. J Biochem 2021;170: 275–287. doi: 10.1093/jb/mvab045. [DOI] [PubMed] [Google Scholar]
- 53.Park YM, Go YY, Shin SH, Cho JG, Woo JS, Song JJ. Anti-cancer effects of disulfiram in head and neck squamous cell carcinoma via autophagic cell death. PLoS One 2018; 13: e0203069. doi: 10.1371/journal.pone.0203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falls-Hubert KC Butler AL Gui K Anderson M Li M Stolwijk JM, et al. Disulfiram causes selective hypoxic cancer cell toxicity and radio-chemo-sensitization via redox cycling of copper. Free Radic Biol Med 2020;150: 1–11. doi: 10.1016/j.freeradbiomed.2020.01.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yip NC Fombon IS Liu P Brown S Kannappan V Armesilla AL, et al. Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer 2011;104: 1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie J Liu J Zhao M Li X Wang Y Zhao Y, et al. Disulfiram/Cu kills and sensitizes BRAF-mutant thyroid cancer cells to BRAF kinase inhibitor by ROS-dependently relieving feedback activation of MAPK/ERK and PI3K/AKT pathways. Int J Mol Sci 2023; 24: 3418. doi: 10.3390/ijms24043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiba T Suzuki E Yuki K Zen Y Oshima M Miyagi S, et al. Disulfiram eradicates tumor-initiating hepatocellular carcinoma cells in ROS-p38 MAPK pathway-dependent and -independent manners. PLoS One 2014; 9: e84807. doi: 10.1371/journal.pone.0084807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butcher K Kannappan V Kilari RS Morris MR McConville C Armesilla AL, et al. Investigation of the key chemical structures involved in the anticancer activity of disulfiram in A549 non-small cell lung cancer cell line. BMC Cancer 2018; 18: 753. doi: 10.1186/s12885-018-4617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun T Yang W Toprani SM Guo W He L DeLeo AB, et al. Induction of immunogenic cell death in radiation-resistant breast cancer stem cells by repurposing anti-alcoholism drug disulfiram. Cell Commun Signal 2020; 18: 36. doi: 10.1186/s12964-019-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enenkel C, Kang RW, Wilfling F, Ernst OP. Intracellular localization of the proteasome in response to stress conditions. J Biol Chem 2022; 298: 102083. doi: 10.1016/j.jbc.2022.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J, Cho J, Song EJ. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res 2020;43: 1144–1161. doi: 10.1007/s12272-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnaghi P D'Alessio R Valsasina B Avanzi N Rizzi S Asa D, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol 2013;9: 548–556. doi: 10.1038/nchembio.1313. [DOI] [PubMed] [Google Scholar]
- 63.Majera D, Skrott Z, Chroma K, Merchut-Maya JM, Mistrik M, Bartek J. Targeting the NPL4 adaptor of p97/VCP segregase by disulfiram as an emerging cancer vulnerability evokes replication stress and DNA damage while silencing the ATR pathway. Cells 2020; 9: 469. doi: 10.3390/cells9020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verzella D Pescatore A Capece D Vecchiotti D Ursini MV Franzoso G, et al. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis 2020; 11: 210. doi: 10.1038/s41419-020-2399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshino H Yamada Y Enokida H Osako Y Tsuruda M Kuroshima K, et al. Targeting NPL4 via drug repositioning using disulfiram for the treatment of clear cell renal cell carcinoma. PLoS One 2020; 15: e0236119. doi: 10.1371/journal.pone.0236119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skrott Z Majera D Gursky J Buchtova T Hajduch M Mistrik M, et al. Disulfiram's anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene 2019; 38: 6711–6722. doi: 10.1038/s41388-019-0915-2. [DOI] [PubMed] [Google Scholar]
- 67.Gao X Huang H Pan C Mei Z Yin S Zhou L, et al. Disulfiram/copper induces immunogenic cell death and enhances CD47 blockade in hepatocellular carcinoma. Cancers (Basel) 2022; 14: 4715. doi: 10.3390/cancers14194715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res 2006;66: 10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 69.Serra R Zhao T Huq S Gorelick NL Casaos J Cecia A, et al. Disulfiram and copper combination therapy targets NPL4, cancer stem cells and extends survival in a medulloblastoma model. PLoS One 2021; 16: e0251957. doi: 10.1371/journal.pone.0251957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Telang N. Stem cell models for cancer therapy. Int J Mol Sci 2022; 23: 7055. doi: 10.3390/ijms23137055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Triscott J, Rose Pambid M, Dunn SE. Concise review: bullseye: Targeting cancer stem cells to improve the treatment of gliomas by repurposing disulfiram. Stem Cells 2015;33: 1042–1046. doi: 10.1002/stem.1956. [DOI] [PubMed] [Google Scholar]
- 72.Yang Z, Guo F, Albers AE, Sehouli J, Kaufmann AM. Disulfiram modulates ROS accumulation and overcomes synergistically cisplatin resistance in breast cancer cell lines. Biomed Pharmacother 2019; 113: 108727. doi: 10.1016/j.biopha.2019.108727. [DOI] [PubMed] [Google Scholar]
- 73.Harrington BS Ozaki MK Caminear MW Hernandez LF Jordan E Kalinowski NJ, et al. Drugs targeting tumor-initiating cells prolong survival in a post-surgery, post-chemotherapy ovarian cancer relapse model. Cancers (Basel) 2020; 12: 1645. doi: 10.3390/cancers12061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hothi P Martins TJ Chen L Deleyrolle L Yoon JG Reynolds B, et al. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget 2012;3: 1124–1136. doi: 10.18632/oncotarget.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zirjacks L Stransky N Klumpp L Prause L Eckert F Zips D, et al. Repurposing disulfiram for targeting of glioblastoma stem cells: An in vitro study. Biomolecules 2021; 11: 1561. doi: 10.3390/biom11111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y Li W Patel SS Cong J Zhang N Sabbatino F, et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget 2014;5: 3743–3755. doi: 10.18632/oncotarget.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YE Choi SA Kwack PA Kim HJ Kim IH Wang KC, et al. Repositioning disulfiram as a radiosensitizer against atypical teratoid/rhabdoid tumor. Neuro Oncol 2017;19: 1079–1087. doi: 10.1093/neuonc/now300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ninsontia C, Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P. Zinc suppresses stem cell properties of lung cancer cells through protein kinase C-mediated β-catenin degradation. Am J Physiol Cell Physiol 2017;312: C487–C499. doi: 10.1152/ajpcell.00173.2016. [DOI] [PubMed] [Google Scholar]
- 79.Cui J Li W Bu W Liu J Chen X Li X, et al. Folic acid-modified disulfiram/Zn-IRMOF3 nanoparticles for oral cancer therapy by inhibiting ALDH1A1+ cancer stem cells. Biomater Adv 2022; 139: 213038. doi: 10.1016/j.bioadv.2022.213038. [DOI] [PubMed] [Google Scholar]
- 80.Kannappan V Liu Y Wang Z Azar K Kurusamy S Kilari RS, et al. PLGA-nano-encapsulated disulfiram inhibits hypoxia-induced NF-κB, cancer stem cells, and targets glioblastoma in vitro and in vivo. Mol Cancer Ther 2022;21: 1273–1284. doi: 10.1158/1535-7163.MCT-22-0066. [DOI] [PubMed] [Google Scholar]
- 81.Liu Z Mi M Zheng X Zhang C Zhu F Liu T, et al. miR-30a/SOX4 double negative feedback loop is modulated by disulfiram and regulates EMT and stem cell-like properties in breast cancer. J Cancer 2021;12: 5053–5065. doi: 10.7150/jca.57752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang W Xie J Hou R Chen X Xu Z Tan Y, et al. Disulfiram/cytarabine eradicates a subset of acute myeloid leukemia stem cells with high aldehyde dehydrogenase expression. Leuk Res 2020; 92: 106351. doi: 10.1016/j.leukres.2020.106351. [DOI] [PubMed] [Google Scholar]
- 83.Ni YL Chien PJ Hsieh HC Shen HT Lee HT Chen SM, et al. Disulfiram/copper suppresses cancer stem cell activity in differentiated thyroid cancer cells by inhibiting BMI1 expression. Int J Mol Sci 2022; 23: 13276. doi: 10.3390/ijms232113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao HZ, Yang WT, Zheng PS. Cytotoxic effect of disulfiram/copper on human cervical cancer cell lines and LGR5-positive cancer stem-like cells. BMC Cancer 2022; 22: 521. doi: 10.1186/s12885-022-09574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo F, Yang Z, Kulbe H, Albers AE, Sehouli J, Kaufmann AM. Inhibitory effect on ovarian cancer ALDH+ stem-like cells by disulfiram and copper treatment through ALDH and ROS modulation. Biomed Pharmacother 2019; 118: 109371. doi: 10.1016/j.biopha.2019.109371. [DOI] [PubMed] [Google Scholar]
- 86.Wang K Michelakos T Wang B Shang Z DeLeo AB Duan Z, et al. Targeting cancer stem cells by disulfiram and copper sensitizes radioresistant chondrosarcoma to radiation. Cancer Lett 2021;505: 37–48. doi: 10.1016/j.canlet.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin N, Zhu X, Cheng F, Zhang L. Disulfiram/copper targets stem cell-like ALDH+ population of multiple myeloma by inhibition of ALDH1A1 and hedgehog pathway. J Cell Biochem 2018;119: 6882–6893. doi: 10.1002/jcb.26885. [DOI] [PubMed] [Google Scholar]
- 88.Wang NN Wang LH Li Y Fu SY Xue X Jia LN, et al. Targeting ALDH2 with disulfiram/copper reverses the resistance of cancer cells to microtubule inhibitors. Exp Cell Res 2018;362: 72–82. doi: 10.1016/j.yexcr.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Cvek B, Dvorak Z. Targeting of nuclear factor-kappa B and proteasome by dithiocarbamate complexes with metals. Curr Pharm Des 2007;13: 3155–3167. doi: 10.2174/138161207782110390. [DOI] [PubMed] [Google Scholar]
- 90.Liu P Wang Z Brown S Kannappan V Tawari PE Jiang W, et al. Liposome encapsulated disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget 2014; 5: 7471–7485. doi: 10.18632/oncotarget.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Q Zhu T Miao N Qu Y Wang Z Chao Y, et al. Disulfiram bolsters T-cell anti-tumor immunity through direct activation of LCK-mediated TCR signaling. EMBO J 2022; 41: e110636. doi: 10.15252/embj.2022110636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidtova S Kalavska K Gercakova K Cierna Z Miklikova S Smolkova B, et al. Disulfiram overcomes cisplatin resistance in human embryonal carcinoma cells. Cancers (Basel) 2019; 11: 1224. doi: 10.3390/cancers11091224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qian L Murakami K Toyozumi T Matsumoto Y Otsuka R Sekino N, et al. An anti-alcoholism drug, disulfiram and copper complex improves radio-resistance of tumor-initiating cells in esophageal squamous cell carcinoma. Esophagus 2023;20: 134–142. doi: 10.1007/s10388-022-00948-z. [DOI] [PubMed] [Google Scholar]
- 94.Rolle F Bincoletto V Gazzano E Rolando B Lollo G Stella B, et al. Coencapsulation of disulfiram and doxorubicin in liposomes strongly reverses multidrug resistance in breast cancer cells. Int J Pharm 2020; 580: 119191. doi: 10.1016/j.ijpharm.2020.119191. [DOI] [PubMed] [Google Scholar]
- 95.Swetha KL, Paul M, Maravajjala KS, Kumbham S, Biswas S, Roy A. Overcoming drug resistance with a docetaxel and disulfiram loaded pH-sensitive nanoparticle. J Control Release 2023;356: 93–114. doi: 10.1016/j.jconrel.2023.02.023. [DOI] [PubMed] [Google Scholar]
- 96.Yao W Qian X Ochsenreither S Soldano F DeLeo AB Sudhoff H, et al. Disulfiram acts as a potent radio-chemo sensitizer in head and neck squamous cell carcinoma cell lines and transplanted xenografts. Cells 2021; 10: 517. doi: 10.3390/cells10030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lun X Wells JC Grinshtein N King JC Hao X Dang NH, et al. Disulfiram when combined with copper enhances the therapeutic effects of temozolomide for the treatment of glioblastoma. Clin Cancer Res 2016;22: 3860–3875. doi: 10.1158/1078-0432.CCR-15-1798. [DOI] [PubMed] [Google Scholar]
- 98.Liu CC, Wu CL, Lin MX, Sze CI, Gean PW. Disulfiram sensitizes a therapeutic-resistant glioblastoma to the TGF-β receptor inhibitor. Int J Mol Sci 2021; 22: 10496. doi: 10.3390/ijms221910496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cong J Wang Y Zhang X Zhang N Liu L Soukup K, et al. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett 2017;409: 9–19. doi: 10.1016/j.canlet.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: Progress, challenges and opportunities. Nat Rev Cancer 2017; 17: 20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fasehee H, Ghavamzadeh A, Alimoghaddam K, Ghaffari S, Faghihi S. A comparative cytotoxic evaluation of disulfiram encapsulated PLGA nanoparticles on MCF-7 Cells. Int J Hematol Oncol Stem Cell Res 2017; 11: 102–107. [PMC free article] [PubMed] [Google Scholar]
- 102.Zhuo X Lei T Miao L Chu W Li X Luo L, et al. Disulfiram-loaded mixed nanoparticles with high drug-loading and plasma stability by reducing the core crystallinity for intravenous delivery. J Colloid Interface Sci 2018;529: 34–43. doi: 10.1016/j.jcis.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 103.Banerjee P, Geng T, Mahanty A, Li T, Zong L, Wang B. Integrating the drug, disulfiram into the vitamin E-TPGS-modified PEGylated nanostructured lipid carriers to synergize its repurposing for anti-cancer therapy of solid tumors. Int J Pharm 2019; 557: 374–389. doi: 10.1016/j.ijpharm.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 104.Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A. Design of liposomal formulations for cell targeting. Colloids Surf B Biointerfaces 2015;136: 514–526. doi: 10.1016/j.colsurfb.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 105.Mohammad IS, He W, Yin L. A smart paclitaxel-disulfiram nanococrystals for efficient MDR reversal and enhanced apoptosis. Pharm Res 2018; 35: 77. doi: 10.1007/s11095-018-2370-0. [DOI] [PubMed] [Google Scholar]
- 106.Huo Q Zhu J Niu Y Shi H Gong Y Li Y, et al. pH-triggered surface charge-switchable polymer micelles for the co-delivery of paclitaxel/disulfiram and overcoming multidrug resistance in cancer. Int J Nanomedicine 2017;12: 8631–8647. doi: 10.2147/IJN.S144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu L Sun Y Li Y Sun J Guo Y Shen Q, et al. Disulfiram: A Food and Drug Administration-approved multifunctional role in synergistically drug delivery systems for tumor treatment. Int J Pharm 2022; 626: 122130. doi: 10.1016/j.ijpharm.2022.122130. [DOI] [PubMed] [Google Scholar]
- 108.Lu Y Pan Q Gao W Pu Y Luo K He B, et al. Leveraging disulfiram to treat cancer: mechanisms of action, delivery strategies, and treatment regimens. Biomaterials 2022; 281: 121335. doi: 10.1016/j.biomaterials.2021.121335. [DOI] [PubMed] [Google Scholar]