Abstract
Background and Aim:
Irradiation is one of the most effective microbial decontamination treatments for eliminating foodborne pathogens and enhancing chicken meat safety. The effect of gamma irradiation on the overall quality of chicken meat and its products must be observed to provide a comprehensive explanation to the public. This meta-analysis examined the effects of gamma irradiation on the oxidation parameters, microbial activity, physicochemical characteristics, sensory parameters, and nutrient quality of chicken meat and meat products.
Materials and Methods:
We conducted a literature search using various search engines (Scopus®, PubMed®, and Google Scholar®) with “irradiation,” “gamma,” “chicken,” and “meat” as keywords. Gamma irradiation treatment was set as a fixed effect, and the difference between experiments was set as a random effect. This study used a mixed-model methodology. After evaluation, we selected 43 articles (86 studies) for inclusion in the database.
Results:
Gamma irradiation significantly increased (p < 0.01) thiobarbituric acid-reactive substance levels on days 0, 7, and 14 of storage. Gamma irradiation reduced total aerobic bacteria, coliforms, Salmonella, yeast, and mold activity (p < 0.01). According to our meta-analysis, 21.75 kGy was the best dose for reducing total aerobic bacteria. On day 0, gamma irradiation did not affect the color parameters (L*, a*, b*). However, a significant difference (p < 0.01) was noted for a* and b* parameters between the control and irradiation treatments at 7 and 14 days. Although irradiation treatment was less consistent in sensory parameters, overall acceptability decreased on days 0, 7, and 14 after storage (p < 0.05). Regarding nutrient composition, gamma irradiation reduced moisture content and free fatty acid (FFA) content (p < 0.05). Although irradiation significantly reduces the microbial population, it increases the oxidation of chicken meat and its products. Irradiation decreases FFA content and overall acceptability, but it does not affect flavor, tenderness, juiciness, or cooking loss.
Conclusion:
Gamma irradiation positively reduces the microbial activity in chicken meat and its products but increases the oxidation parameters. Although gamma irradiation does not alter the flavor, tenderness, juiciness, or cooking loss, gamma irradiation can reduce the FFA content and overall acceptability.
Keywords: chicken, gamma irradiation, meat, meta-analysis, product
Introduction
In developing countries, poultry meat is an important source of protein due to its low price compared with red meat. In addition, poultry meat is also popular in developed countries because of its health benefits. Globally, chicken meat as the main source of poultry products is projected to account for 41% of total protein derived from meat sources by 2030, an increase of 2% over the baseline period [1]. Poultry and beef meat are expected to represent the largest imports of extra meat into Asia and Africa, where consumption growth will exceed domestic production [1]. Producing and disseminating meat with the longest possible shelf life is a primary challenge for traders [2]. Therefore, the food industry utilizes various food preservation techniques, such as cooling, freezing, evaporation, fermentation, and adding chemical preservatives [3]. Moreover, irradiation has been recognized as an effective method for preventing the spread of diseases and parasites and extending the shelf life of products [4].
In 2003, the FAO recommended the irradiation technique in the Codex Alimentarius, and it has been widely adopted in 50 nations, including the United States, Egypt, China, and most Latin American nations [5]. In the food processing industry, irradiation is commonly used to preserve food. Irradiation is one of the most effective microbial decontamination treatments for eliminating foodborne pathogens and enhancing meat safety [6]. Gamma irradiation can be applied to raw materials because of its high level of effectiveness in reducing the number of germs and its capacity to produce little changes to the natural characteristics of the product [7]. Gamma irradiation is more effective than electron beam irradiation against foodborne pathogens [8]. Irradiation treatment is usually combined with packaging [9–11], cooking [12–14], or the addition of antioxidants [15–17] to minimize the effects of free radical reactions that affect meat quality.
Apart from commercial purposes, gamma irradiation research focuses on developing foods for specialized applications, such as space programs, military operations, and care of geriatric and immunocompromised people [18]. However, consumers’ perception and acceptance of irradiated meat are among the most crucial aspects of adopting irradiation technology in meat production [6]. The effect of gamma irradiation on the overall quality of chicken meat and its products must be observed to provide a comprehensive explanation to the public. Previously, Dimov [2] published a meta-analysis on the impact of gamma irradiation on the lipid oxidative processes exhibited by peroxide value (POV) and thiobarbituric acid-reactive substances (TBARS) in raw chicken meat. A meta-analysis on the impact of gamma irradiation on raw chicken meat quality (color and microbiology) [19] has also been published. Fallah et al. [20] reviewed the combined effects of ionizing radiation and bio-based active packaging on the quality of muscle food. However, there is no comprehensive study on the effect of gamma irradiation on the quality of raw chicken meat and its products.
Therefore, this meta-analysis aimed to investigate the effects of gamma irradiation on the oxidation parameters, microbial activity, physicochemical characteristics, sensory parameters, and nutrient quality of chicken meat and meat products.
Materials and Methods
Ethical approval
Ethical clearance was not required for the present meta-analysis study. This study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Protocol.
Study period and location
The study was conducted from August 2, 2023, to October 31, 2023 at the Research Center for Food Technology and Processing, National Research and Innovation Agency of Indonesia and Faculty of Animal Science, Universitas Gadjah Mada, Indonesia.
Search strategy
The literature search was performed using Harzing’s Publish or Perish version 8 (Windows GUI Edition). Keywords used were irradiation, gamma radiation, chicken, and meat. The outputs were 1129 articles published between 1998 and 2022 from Scopus®, PubMed®, and Google Scholar®. Only scientific articles were included in the selected literature.
Selection criteria
The article selection procedure followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses protocol [21]. Inclusion criteria were as follows: (1) The article was published in a scientific journal; (2) the article was an experimental research-based study; (3) the irradiation treatment only uses gamma rays (not electron beams); (4) the irradiation dosage level was reported; and (5) chicken meat or meat products were used as the subjects of this study. Figure-1 shows a diagram of the literature selection. After full-text evaluation, 43 articles (86 experiments) were entered into the database (Table-1) [7, 9–18, 22–53].
Figure-1.
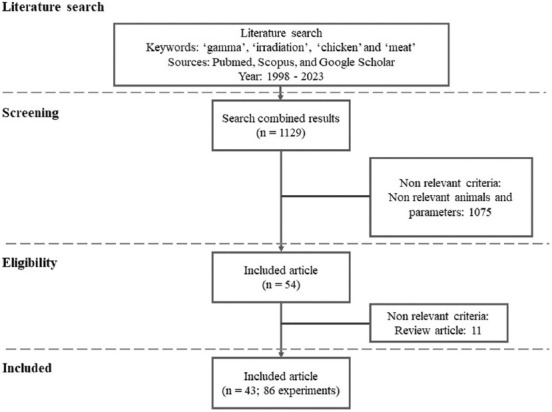
Flow chart for literature selection included in meta-analysis.
Table 1.
List of eligible studies for meta-analysis of gamma irradiation effects on chicken meat and meat products.
| No. | References | Meat sample | Dosage (kGy) | Dosage rate (kGy/h) | Sample treatment |
|---|---|---|---|---|---|
| 1 | Brito et al. [7] | Deboned chicken meat | 0; and 3 | 0.32 and 4.04 | Untreated |
| 2 | Yoon et al. [9] | Marinated diced chicken meat | 0; 5; 10; 15; 20; 25; and 30 | 10 | Vacuum-packaged |
| 3 | Mantilla et al. [10] | Chicken breast filets | 0; 2; and 3 | No information | Vacuum-packaged |
| 4 | Nisar et al. [11] | Chicken meat | 0; 1.5; and 3 | No information | Vacuum-packaged and moringa leaf powder addition |
| 5 | Jayathilakan et al. [12] | Hurdle-processed chicken meat | 0; 1; and 2 | No information | Cooked with Masala and lactic acid treatment |
| 6 | Muhammad et al. [13] | Chicken meat | 0; 5; and 7.5 | No information | Boiled; and fried |
| 7 | Chen et al. [14] | Chicken dices meat | 0; 10; 20 | 2.1 | Stir-fried with dry chili |
| 8 | Abdeldaiem [15] | Minced chicken meat | 0; 2; 4; and 6 | No information | Coating with ethanolic extract of papaya leaves |
| 9 | Hwang et al. [16] | Chicken sausage | 0; 2.5; and 5 | 5 | Untreated; emulsified with mugwort extract; and mugwort extract+ascorbic acid |
| 10 | Arshad et al. [17] | Chicken meat | 0; 1; and 2 | No information | Turmeric powder; aerobic packaging; and vacuum packaging |
| 11 | Baptista et al. [18] | ready-to-eat broiler breast filets | 0; and 48 | 1 | Frozen storage |
| 12 | Bhoir et al. [22] | Minced chicken meat | 0; and 0.5 | 2 | Untreated and mixed with Chitosan (0.1% w/w) |
| 13 | De Azevedo Gomes et al. [23] | Refrigerated mechanically deboned chicken | 0; 3; and 4 | 7.32 | Untreated |
| 14 | Khalid et al. [24] | Chicken meat | 0; and 3 | No information | Untreated; and treated with 1% and 2% kale leaf powder |
| 15 | Javanmard et al. [25] | Chicken meat | 0; 0.75; 3; and 5 | 1.98 | Frozen storage |
| 16 | Rima et al. [26] | Fresh chicken meat | 0; 1; 2; and 3.5 | 4.29 | Untreated |
| 17 | Al-Bachir and Othman [27] | Chicken sausage | 0; 2; 4; and 6 | 8.49 | Untreated |
| 18 | Hassanzadeh et al. [28] | Chicken breast meat | 0; and 2.5 | No information | Untreated; immersed in chitosan solution; and chitosan solution containing 0.1% grape seed extract |
| 19 | Fallah et al. [29] | Ready-to-cook Iranian barbecued chicken | 0; 1.5; 3; and 4.5 | 3.06 | Untreated |
| 20 | Yun et al. [30] | Ready-to-eat chicken breast | 0; 5; and 40 | No information | Heated |
| 21 | Mahrour et al. [31] | Chicken leg meat | 0; and 5 | No information | Packed in air and marinated |
| 22 | Kim et al.[32] | Chicken breast meat | 0; 2.5; 5; 7.5; and 10 | 7 | Vacuum-packaged; stored at 5°C and 30°C |
| 23 | Zahran [33] | Minced chicken meat | 0; 1; and 2 | 3.49 | Untreated; 100; and 200 micro g/g nisin |
| 24 | Shankar et al. [34] | Boneless chicken thighs | 0; and 1 | 9.57 | Untreated and mixed with Essential oils (Oregano+Thyme+Cannelle 2% w/w) |
| 25 | Aly and El-Aragi [35] | Cold-sliced chicken meat | 0; 2; 4; and 6 | 1.37 | Untreated |
| 26 | Mrityunjoy et al. [36] | Chicken meat | 0; 6; and 8 | No information | Untreated |
| 27 | Chouliara et al. [37] | Chicken breast meat | 0; 2; and 4 | 1 | Packed-in air and modified atmosphere packaging |
| 28 | Spoto et al. [38] | Chicken breast meat | 0; 2; and 4 | No information | Untreated |
| 29 | Irmanita et al. [39] | Chicken breast and legs meat | 0; 3; and 5 | No information | Untreated |
| 30 | Al-Bachir et al. [40] | Chicken kabab | 0; 2; 4; and 6 | 0.73 | Untreated |
| 31 | Millar et al. [41] | Chicken breast and chicken leg meat | 0; and 5 | No information | Untreated |
| 32 | De Toledo et al. [42] | Chicken breast meat | 0; 2; 4; 6; and 8 | 1.2 | Untreated; and frozen storage |
| 33 | Kang et al. [43] | Minced chicken meat | 0; 3; 5; 7; and 10 | No information | Non-thermal pasteurization |
| 34 | Pelicia et al. [44] | Chicken breast meat | 0; 2; 4; and 8 | No information | Vacuum and no-vacuum packaging |
| 35 | Kumar et al. [45] | Chicken pulav | 0; 2; 3; 4; and 5 | 0.6 | Untreated |
| 36 | Min et al. [46] | Minced chicken meat | 0; 1; 3; 5;10; and 15 | 10 | Untreated |
| 37 | Kanatt et al. [47] | Chicken chilly meat | 0; 1; 2; and 3 | 3 | Frozen storage |
| 38 | Yusof et al. [48] | Chicken sausage and burger | 0; 3.5; 5.5; and 10 | No information | Untreated |
| 39 | Chong-Nam et al. [49] | Chicken breast meat | 0; 0.5; 1; and 1.5 | No information | Cold storage |
| 40 | Balamatsia et al. [50] | Chicken breast-filet | 0; 0.5; 1; and 2 | 1 | Stored Aerobically at 4°C |
| 41 | Kanatt et al. [51] | Chicken chunks, chicken mince, and chicken leg meat | 0; and 2.5 | 3 | Untreated |
| 42 | Yoon [52] | Chicken breast meat | 0; and 2.55 | 0.92 | Untreated |
| 43 | Kanatt et al. [53] | Chicken meat | 0; and 2.5 | 2.7 | Untreated; treated with citric acid; ascorbic; tocopherol; butylated hydroxytoluene; nitrite; and sodium tripolyphosphate |
Inclusion data
As shown in Table-1, the dosage of gamma irradiation ranged from 0 to 48 kGy and the dosage rate ranged from 0 to 10 kGy/h in this meta-analysis. Meat samples included fresh chicken meat, boneless chicken thighs, minced meat, diced meat, breast filets, marinated meat, chicken sausage, chicken kabab, chicken burger, chicken pulav, chicken chilly meat, and ready-to-cook barbecue chicken. Oxidation parameters were TBARS, total volatile base nitrogen (TVBN), and POV. Myoglobin (Mb), metmyoglobin (MMb), and oxymyoglobin (MbO2) were the hemoglobin parameters. Total aerobic bacteria, coliforms, lactic acid bacteria, enterobacteria, Salmonella, Pseudomonas, staphylococci, yeast, and mold were the microbial load parameters. The pH, lightness (L*), redness (a*), and yellowness (b*) were included physicochemical parameters. The sensory parameters assessed were appearance, texture, taste, color, odor, flavor, tenderness, juiciness, cooking loss, and overall acceptability. Moisture, protein, fat, ash, free fatty acid (FFA) profiles, C14:0, C16:0, C16:1, C18:0, C18:1, C18:2, C18:3, C20:3n6, C20:4, C22:0, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids were the nutrient composition.
Statistical analysis
The database had compatible measurement units and statistical meta-analysis based on mixed-model methodology [54, 55] was performed. Various studies were classified as random effects, and gamma irradiation dose was classified as fixed effects. All statistical analyses were conducted using R software version 4.1.2 by the R Core Team (http://www.r-project.org/index.html) [56] equipped with lme4 library version 1.1-28 (https://cran.r-project.org/web/packages/lme4/index.html). The root-mean-square error (RMSE) and the determination coefficient of Nakagawa or RGLMM(c)2 were used to validate the model [56–58]. Statistical significance was stated at p < 0.05. When the p-value ranged between 0.05 and 0.10, there was a tendency for the result to be significant.
Results
The effects of gamma irradiation on the oxidation parameters of chicken meat and its products are summarized in Table-2. Irradiation treatment increased TBARS and POV after 0, 7, and 14 days of storage (p < 0.01). However, gamma irradiation treatment reduced TVBN after 7 and 14 days of storage (p < 0.05). Furthermore, gamma irradiation increased TBARS after 14 days of storage (p < 0.05). In the present meta-analysis, a dose of 3.24 kGy was sufficient to increase the TBARS value. With regard to hemopigment, irradiation treatment decreased Mb levels after 0, 7, and 14 days of storage (p < 0.01). In contrast, MMb levels increased after gamma irradiation (p < 0.05). With the exception of 14 days of storage, gamma irradiation did not affect the MbO2 level. Table-3 shows the effects of gamma irradiation on the microbial activity of poultry meat and meat products (log colony forming unit/g). In general, gamma irradiation treatment reduced microbial activity in meat and meat products (i.e., total aerobic bacteria, coliforms, lactic acid bacteria, enterobacteria, Salmonella, Pseudomonas, Staphylococcus, yeast, and mold; p < 0.05).
Table 2.
Meta-analysis results of gamma irradiation effects on oxidation parameters and haem pigment of chicken meat and meat products.
| Response parameter | Unit | n | Intercept | SE Intercept | Slope | SE slope | p-value | RMSE | R2 |
|---|---|---|---|---|---|---|---|---|---|
| TBARS (d) | mg MDA/kg | ||||||||
| 0 | 106 | 0.62 | 0.09 | 0.04 | 0.01 | 0.001 | 0.31 | 0.72 | |
| 7 | 76 | 0.72 | 0.18 | 0.17 | 0.05 | 0.003 | 0.56 | 0.58 | |
| 14 | 84 | 0.78 | 0.15 | 0.10 | 0.02 | 0.023 | 0.23 | 0.88 | |
| 21 | 6 | 0.43 | 0.23 | 0.49 | 0.13 | 0.129 | 0.32 | 0.00 | |
| TVBN (d) | mg/100 mL | ||||||||
| 0 | 49 | 7.02 | 1.75 | 0.18 | 0.06 | 0.006 | 1.82 | 0.90 | |
| 7 | 30 | 9.42 | 2.42 | −0.77 | 0.27 | 0.011 | 1.47 | 0.94 | |
| 14 | 26 | 11.8 | 3.58 | −1.62 | 0.67 | 0.028 | 3.58 | 0.84 | |
| POV (d) | meq peroxide/kg | ||||||||
| 0 | 38 | 0.43 | 0.07 | 0.08 | 0.01 | 0.001 | 0.08 | 0.89 | |
| 7 | 30 | 0.52 | 0.09 | 0.06 | 0.01 | 0.001 | 0.03 | 0.99 | |
| 14 | 30 | 0.60 | 0.09 | 0.06 | 0.01 | 0.001 | 0.03 | 0.99 | |
| Mb (d) | % | ||||||||
| 0 | 18 | 36.5 | 1.44 | −0.90 | 0.22 | 0.002 | 0.76 | 0.93 | |
| 7 | 18 | 22.5 | 1.13 | −0.85 | 0.20 | 0.002 | 0.71 | 0.91 | |
| 14 | 18 | 11.9 | 1.85 | −0.68 | 1.19 | 0.005 | 0.66 | 0.97 | |
| MMb (d) | % | ||||||||
| 0 | 24 | 46.7 | 3.55 | 0.51 | 0.18 | 0.013 | 0.72 | 0.99 | |
| 7 | 18 | 49.6 | 0.98 | 0.71 | 0.17 | 0.002 | 0.61 | 0.91 | |
| 14 | 18 | 58.2 | 1.12 | 1.07 | 0.19 | 0.001 | 0.69 | 0.92 | |
| MbO2 (d) | % | ||||||||
| 0 | 12 | 12.3 | 0.62 | 0.37 | 0.19 | 0.108 | 0.44 | 0.82 | |
| 7 | 12 | 15.4 | 0.63 | 0.33 | 0.19 | 0.130 | 0.43 | 0.82 | |
| 14 | 12 | 20.4 | 0.65 | 0.33 | 0.13 | 0.036 | 0.28 | 0.93 |
TBARS=Thiobarbituric acid-reactive substances, TVBN=Total volatile base nitrogen, POV=Peroxide value, Mb=Myoglobin, MMb: Metmyoglobin, MbO2=Oxymyoglobin, SE=Standard error, RMSE=Root mean square error, R2=The proportion of a dependent variable’s variation that can be explained by an independent variable (bigger is better)
Table 3.
Meta-analysis results of gamma irradiation effects on microbial activity of chicken meat and meat products (log colony forming unit/g).
| Response parameter | n | Intercept | SE Intercept | Slope | SE slope | p-value | RMSE | R2 |
|---|---|---|---|---|---|---|---|---|
| Total aerobic bacteria (d) | ||||||||
| 0 | 167 | 4.70 | 0.44 | −0.29 | 0.07 | 0.001 | 4.36 | 0.14 |
| 7 | 69 | 5.37 | 0.28 | −0.52 | 0.07 | 0.001 | 1.85 | 0.45 |
| 14 | 69 | 7.51 | 0.28 | −0.99 | 0.09 | 0.001 | 1.49 | 0.69 |
| Coliforms (d) | ||||||||
| 0 | 106 | 3.86 | 0.34 | −0.58 | 0.07 | 0.001 | 1.55 | 0.60 |
| 7 | 43 | 3.09 | 0.41 | −0.49 | 0.10 | 0.001 | 1.29 | 0.56 |
| 14 | 37 | 3.74 | 0.43 | −0.63 | 0.10 | 0.001 | 1.31 | 0.64 |
| Lactic acid bacteria (d) | ||||||||
| 0 | 16 | 3.87 | 0.77 | −0.60 | 0.24 | 0.026 | 1.34 | 0.51 |
| 7 | 21 | 4.63 | 0.51 | −0.99 | 0.15 | 0.001 | 0.79 | 0.80 |
| 14 | 20 | 6.30 | 0.67 | −0.95 | 0.20 | 0.001 | 1.32 | 0.64 |
| Enterobacteriaceae (d) | ||||||||
| 0 | 12 | 2.07 | 0.55 | −0.51 | 0.19 | 0.024 | 1.10 | 0.42 |
| 7 | 17 | 3.98 | 0.60 | −1.19 | 0.25 | 0.001 | 1.54 | 0.01 |
| 14 | 18 | 5.02 | 0.66 | −1.29 | 0.24 | 0.001 | 1.75 | 0.01 |
| Yeast and mold (d) | ||||||||
| 0 | 47 | 4.32 | 0.77 | −0.54 | 0.06 | 0.001 | 0.75 | 0.93 |
| 7 | 30 | 3.83 | 0.50 | −0.78 | 0.14 | 0.001 | 1.42 | 0.62 |
| 14 | 23 | 4.45 | 0.41 | −0.76 | 0.13 | 0.001 | 1.39 | 0.01 |
| Salmonella (0 d) | 17 | 5.89 | 0.82 | −0.40 | 0.09 | 0.001 | 0.99 | 0.74 |
| Pseudomonas (d) | ||||||||
| 0 | 12 | 7.11 | 0.22 | −0.41 | 0.03 | 0.001 | 0.29 | 0.95 |
| 7 | 9 | 4.50 | 0.80 | −1.35 | 0.31 | 0.003 | 1.34 | 0.01 |
| 14 | 6 | 6.15 | 1.03 | −1.74 | 0.39 | 0.012 | 1.30 | 0.01 |
| Staphylococcal (d) | ||||||||
| 0 | 37 | 5.94 | 0.75 | −0.67 | 0.09 | 0.001 | 0.99 | 0.87 |
| 7 | 7 | 5.22 | 1.39 | −1.66 | 0.19 | 0.003 | 0.37 | 0.97 |
| 14 | 5 | 5.83 | 1.53 | −1.59 | 0.23 | 0.018 | 0.43 | 0.96 |
SE=Standard error, RMSE: Root mean square error, R2=The proportion of a dependent variable’s variation that can be explained by an independent variable (bigger is better)
Table-4 shows the effects of gamma irradiation on the physicochemical and sensory parameters of meat and products. In terms of physicochemical parameters, gamma irradiation did not have any significant effect on the pH value at 0, 7, and 14 days after storage. Furthermore, on day 0, irradiation did not affect L*, a*, and b* values in meat. In contrast, a* and b* values were elevated after irradiation treatment at 7 and 14 days of storage (p < 0.01). Irradiation treatment decreased the appearance, texture, taste, and odor on storage day 0 (p = 0.01). Overall acceptability at 0, 7, and 14 days of storage decreased with increasing irradiation dose (p < 0.05). A dose of 12.26 kGy was sufficient to decrease the overall acceptability score. Gamma irradiation did not affect flavor, tenderness, juiciness, and cooking loss of meat and its products. Gamma irradiation treatment did not affect the appearance, texture, taste, color, and odor after 7 days of storage. Gamma irradiation did not affect the overall sensory parameters of meat and its product after 14 days of storage, except for taste and acceptability values, which were negatively affected (p < 0.05).
Table 4.
Meta-analysis results of gamma irradiation effects on physicochemical and sensory parameters of chicken meat and meat products.
| Response parameter | n | Intercept | SE intercept | Slope | SE slope | p-value | RMSE | R2 |
|---|---|---|---|---|---|---|---|---|
| pH (d) | ||||||||
| 0 | 59 | 5.89 | 0.08 | 0.01 | 0.01 | 0.514 | 0.07 | 0.94 |
| 7 | 20 | 5.92 | 0.07 | 0.01 | 0.01 | 0.596 | 0.14 | 0.37 |
| 14 | 8 | 5.76 | 0.05 | 0.01 | 0.01 | 0.789 | 0.06 | 0.16 |
| Lightness (L*) (d) | ||||||||
| 0 | 38 | 53.3 | 1.59 | −0.14 | 0.11 | 0.221 | 0.97 | 0.96 |
| 7 | 33 | 52.7 | 1.38 | 0.01 | 0.13 | 0.999 | 0.77 | 0.96 |
| 14 | 27 | 51.8 | 1.34 | −0.36 | 0.17 | 0.048 | 0.89 | 0.93 |
| Redness (a*) (d) | ||||||||
| 0 | 38 | 11.9 | 1.38 | 0.11 | 0.07 | 0.125 | 0.58 | 0.98 |
| 7 | 33 | 10.3 | 1.35 | 0.29 | 0.07 | 0.001 | 0.43 | 0.99 |
| 14 | 27 | 10.5 | 1.30 | 0.36 | 0.07 | 0.001 | 0.38 | 0.99 |
| Yellowness (b*) (d) | ||||||||
| 0 | 38 | 11.6 | 1.23 | 0.001 | 0.06 | 0.897 | 0.53 | 0.98 |
| 7 | 33 | 10.4 | 1.02 | 0.23 | 0.06 | 0.001 | 0.39 | 0.98 |
| 14 | 27 | 10.8 | 0.76 | 0.41 | 0.08 | 0.001 | 0.46 | 0.94 |
| Appearance (d) | ||||||||
| 0 | 61 | 7.83 | 0.24 | −0.04 | 0.09 | 0.001 | 0.29 | 0.89 |
| 7 | 30 | 7.64 | 0.26 | −0.04 | 0.02 | 0.113 | 0.14 | 0.96 |
| 14 | 30 | 7.34 | 0.30 | −0.02 | 0.04 | 0.714 | 0.27 | 0.88 |
| Texture (d) | ||||||||
| 0 | 67 | 7.28 | 0.23 | −0.03 | 0.01 | 0.002 | 0.37 | 0.83 |
| 7 | 29 | 6.85 | 0.37 | −0.01 | 0.02 | 0.520 | 0.20 | 0.95 |
| 14 | 29 | 6.75 | 0.37 | −0.01 | 0.016 | 0.591 | 0.19 | 0.96 |
| Taste (d) | ||||||||
| 0 | 60 | 7.09 | 0.21 | −0.03 | 0.01 | 0.001 | 0.29 | 0.86 |
| 7 | 35 | 6.02 | 0.44 | −0.01 | 0.03 | 0.689 | 0.20 | 0.97 |
| 14 | 26 | 6.66 | 0.41 | −0.07 | 0.02 | 0.001 | 0.89 | 0.99 |
| Color (d) | ||||||||
| 0 | 63 | 7.27 | 0.31 | −0.01 | 0.01 | 0.552 | 0.37 | 0.91 |
| 7 | 26 | 7.84 | 0.57 | 0.01 | 0.01 | 0.729 | 0.15 | 0.99 |
| 14 | 26 | 7.79 | 0.56 | 0.01 | 0.01 | 0.491 | 0.18 | 0.98 |
| Odor (d) | ||||||||
| 0 | 52 | 7.76 | 0.23 | −0.05 | 0.01 | 0.001 | 0.41 | 0.79 |
| 7 | 44 | 6.94 | 0.39 | 0.01 | 0.07 | 0.890 | 0.58 | 0.79 |
| 14 | 44 | 6.34 | 0.56 | 0.09 | 0.08 | 0.230 | 0.63 | 0.87 |
| Flavor (0 d) | 39 | 7.06 | 0.49 | −0.01 | 0.02 | 0.751 | 0.50 | 0.87 |
| Tenderness (0 d) | 14 | 5.10 | 0.26 | −0.02 | 0.03 | 0.565 | 0.28 | 0.60 |
| Juiciness (0 d) | 14 | 5.89 | 0.23 | −0.01 | 0.04 | 0.828 | 0.35 | 0.29 |
| Cooking loss (0 d) | 16 | 23.5 | 1.04 | −0.02 | 0.13 | 0.862 | 1.28 | 0.57 |
| Overall acceptability (d) | ||||||||
| 0 | 86 | 7.41 | 0.19 | −0.03 | 0.01 | 0.004 | 0.50 | 0.71 |
| 7 | 40 | 7.57 | 0.20 | −0.02 | 0.01 | 0.025 | 0.13 | 0.95 |
| 14 | 40 | 7.54 | 0.21 | −0.02 | 0.01 | 0.026 | 0.14 | 0.95 |
SE=Standard error, RMSE=Root mean square error, R2=The proportion of a dependent variable’s variation that can be explained by an independent variable (bigger is better)
The effects of gamma irradiation on the nutrient and fatty acid characteristics of chicken meat and meat products are summarized in Table-5. Gamma irradiation decreased the moisture and FFA content (7 and 14 days of storage) of chicken meat and its product (p < 0.05). Interestingly, gamma irradiation enhanced crude protein (CP) content (p < 0.01). Meanwhile, irradiation did not significantly affect the fat content, ash, and FFA (0-day storage). Except for C20:3n6 and C22:0, gamma irradiation decreased the fatty acid profile of meat and its products (p < 0.01). Figure-2 shows an illustrative outline of the effect of gamma irradiation on the quality of chicken meat and its products.
Table 5.
Meta-analysis results of gamma irradiation effects on nutrient and fatty acid characteristics of chicken meat and meat products.
| Response parameter | Unit | n | Intercept | SE Intercept | Slope | SE slope | p-value | RMSE | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Moisture | % | 18 | 71.8 | 1.84 | −0.05 | 0.02 | 0.039 | 0.62 | 0.97 |
| Protein | % | 17 | 22.6 | 1.67 | 0.06 | 0.02 | 0.008 | 0.58 | 0.97 |
| Fat | % | 18 | 4.46 | 1.20 | 0.01 | 0.01 | 0.945 | 0.37 | 0.97 |
| Ash | % | 18 | 2.65 | 0.75 | −0.01 | 0.01 | 0.642 | 0.14 | 0.99 |
| Free fatty acids | mg/g meat | ||||||||
| 0 | 18 | 0.47 | 0.09 | −0.05 | 0.04 | 0.293 | 0.24 | 0.01 | |
| 7 | 14 | 1.33 | 0.21 | −0.27 | 0.11 | 0.049 | 0.45 | 0.38 | |
| 14 | 14 | 2.39 | 0.43 | −0.54 | 0.24 | 0.047 | 1.06 | 0.01 | |
| Fatty acids | % Total FA | ||||||||
| C14:0 | 12 | 10.6 | 0.23 | −0.08 | 0.03 | 0.022 | 0.09 | 0.94 | |
| C16:0 | 15 | 52.8 | 7.81 | −1.15 | 0.20 | 0.001 | 0.69 | 1.00 | |
| C16:1 | 12 | 15.3 | 0.55 | −0.30 | 0.05 | 0.001 | 0.17 | 0.97 | |
| C18:0 | 15 | 19.8 | 1.84 | −1.11 | 0.17 | 0.001 | 0.59 | 0.97 | |
| C18:1 | 15 | 26.9 | 2.31 | −0.87 | 0.20 | 0.001 | 0.69 | 0.97 | |
| C18:2 | 15 | 13.1 | 1.36 | −0.35 | 0.08 | 0.002 | 0.27 | 0.99 | |
| C18:3 | 15 | 2.25 | 0.34 | −0.17 | 0.04 | 0.003 | 0.15 | 0.94 | |
| C20:3n6 | 12 | 0.66 | 0.12 | −0.01 | 0.01 | 0.701 | 0.05 | 0.93 | |
| C20:4 | 15 | 2.61 | 0.54 | −0.11 | 0.03 | 0.006 | 0.11 | 0.99 | |
| C22:0 | 12 | 3.61 | 2.73 | −0.13 | 0.11 | 0.273 | 0.37 | 0.99 | |
| SFA | 12 | 0.44 | 0.16 | −0.27 | 0.04 | 0.001 | 0.13 | 0.87 | |
| MUFA | 12 | 27.4 | 0.21 | −0.78 | 0.09 | 0.001 | 0.32 | 0.88 | |
| PUFA | 12 | 15.7 | 0.12 | −0.38 | 0.04 | 0.001 | 0.15 | 0.89 |
FA=Fatty acids, SFA=Saturated fatty acids, MUFA=Monounsaturated fatty acids, PUFA=Polyunsaturated fatty acids, SE=Standard error, RMSE=Root mean square error, R2=The proportion of a dependent variable’s variation that can be explained by an independent variable (bigger is better)
Figure-2.

Gamma irradiation influences the oxidation parameters, heme pigment, microbial load, and fatty acid profiles of chicken meat and meat products.
Discussion
Influence of gamma irradiation on oxidation parameters of chicken meat and its products
TBARS is one of the oxidation parameters in meat in units of malondialdehyde (MDA). Lipid peroxidation generates MDA as a byproduct. This MDA interacts with thiobarbituric acid to produce pink chromogen TBARS [59]. We measured lipid peroxidation and its secondary products in terms of TBARS [22]. Because sensory evaluation of oxidized odor levels in meat correlates well with TBARS results, the TBARS variable is particularly essential [23, 60]. In the present meta-analysis, irradiation increased TBARS values at 0, 7, and 14 days of storage (p < 0.05). Although this study integrated data from the addition of antioxidants, packaging, and cooking, statistical analysis revealed an increase in the TBARS value. In a previous study, Lee and Ahn [61] demonstrated that irradiation only enhanced TBARS in raw and cooked meat under aerobic packaging conditions, whereas lipid oxidation in irradiated meat did not occur in the absence of oxygen. According to our findings, there is an increase in TBARS in irradiated meat or meat products after additional treatment. Increased gamma-ray dose promoted the production of hydroperoxide. On the other hand, package type contributed to the variability and overall effect of TBARS generation [2]. According to Arshad et al. [17], irradiation can increase TBARS in both cooked and raw beef only when packaging is aerobically performed. As the storage time progressed, TBARS levels increased, and the rate of lipid oxidation was higher in irradiated samples than in non-irradiated samples [16]. Khalid et al. [24] reported that increasing the irradiation dose enhances the TBARS value. In this meta-analysis, a dose of 3.24 kGy was sufficient to increase the TBARS level. According to Dimov [2], 4 kGy irradiation has no negative effect on raw chicken meat, maintains low oxidation levels, and may be recommended for use in practice.
TVBN may be used as a quality index for chicken meat because its increase is associated with the activity of spoilage bacteria and endogenous enzymes [15]. Non-protein nitrogenous substances and protein breakdown produce TVBN values [24]. Intrinsic factors, such as the amount and types of spoilage microflora and pH, may influence TVBN levels during storage [20], depending on the type of muscle food. In our findings, TVBN increased after radiation treatment at the 0-day storage point. Khalid et al. [24] demonstrated that ionization enhances the number of volatile compounds in ready-to-eat chicken breast. TVBN decreased after radiation treatment at the 7- and 14-day storage points. This result is similar to that reported by Arshad et al. [17], who observed that TVBN increased on day 0 without radiation treatment but decreased during the 40-day storage interval for irradiated samples compared with non-irradiated samples. This reduction indicates a decrease in spoilage inside the sample, which is a positive indication. Increasing the dose decreases the rate of TVBN synthesis during storage by decreasing the initial concentrations of the most common spoilage bacteria [25].
In addition to TBARS, POV is a crucial quality indicator in irradiated meat samples because it reflects the extent of lipid damage induced by irradiation [11]. Irradiation enhances the lipid oxidation process, which is particularly significant in products with high fat and unsaturated fatty acid content because it generates numerous free radicals [26]. Similar to TBARS values, POV increased with increasing irradiation dose and storage period [17]. Increased gamma-ray dose promoted the production of hydroperoxide [2]. At the end of storage, POV production increased, and the highest POV value was detected at the highest dose of irradiation [24]. Because free radicals created during food irradiation processing are catalysts of lipidic auto-oxidation, a significant increase in POV is consistent with the predictions [11]. In previous studies by Nisar et al. [11], Khalid et al. [24], the addition of antioxidants prevented lipid oxidation. However, according to our findings, an increase in POV still occurs in post-irradiated samples.
The decrease in Mb level and the increase in MMb and MbO2 levels demonstrate that oxidation increases while the gamma irradiation dose increases. Different mechanisms are responsible for the pro-oxidant capacity of Mb. One of these mechanisms is the ability to decompose hydroperoxide, and another is the conversion to ferryl/perferryl form, which allows them to serve as free radicals. Both of these mechanisms are responsible for the pro-oxidant capacity of Mb [17]. With the passage of time and an increase in dose through an intermediary MbO2 phase, Mb is oxidized into MMb [62]. MMb-containing meat subjected to irradiation resulted in MbO2 regeneration. MMb reacts with free radicals during MbO2 regeneration. A small amount of MbO2 was produced when purified MMb was irradiated in an aqueous solution [63]. The red color of the meat will change with an increase in both MbO2 and MMb. MMb reacts with hydroxyl radicals to generate MbO2, resulting in irradiated light meat with a bright red color [6]. However, the change in the Mb level after irradiation also varied according to the meat type. Zhou et al. [5] showed that poultry muscles are not drastically discolored because they contain less Mb.
Influence of gamma irradiation on chicken meat microbial activity and its products
In recent years, there has been a growth in the consumption of foods derived from animals; however, little is known about their contamination with the most prevalent non-spore-producing pathogenic bacteria in foods [8]. The production of chicken-based products at the factory may have been contaminated with a high population of microorganisms, as well as the chicken meat and ingredients used to produce products, which may have contained significant amounts of bacteria [27]. Therefore, gamma irradiation plays an important role in preventing the growth of pathogens in meat. However, the type and quantity of microorganisms present in food affect the effectiveness of the irradiation [28]. In conclusion, gamma irradiation treatment significantly reduced all microorganism activity in chicken meat and meat products. The microbial populations decreased significantly as the gamma irradiation dose increased [17]. Furthermore, based on our meta-analysis, the optimum dose of gamma irradiation was 21.74, 6.84, 3.76, 3.93, 7.82, 7.52, and 8.87 kGy, which removed total aerobic bacteria, coliforms, lactic acid bacteria, Enterobacteriaceae, yeast and mold, Salmonella, and staphylococcal activity, respectively.
All types of ionizing radiation inactivate microorganisms through two major mechanisms: Direct interaction with cell components and indirect action from radiolytic products such as H+, OH-, and e-25 [64]. The direct target of ionizing radiation is chromosomal DNA, which loses its function when exposed to radiation. Ionizing water molecules generate reactive hydroxyl radicals that can damage the DNA of microbes, cause base alteration, break the DNA strand, and ultimately result in the death of microbial cells [20]. Arshad et al. [17] demonstrated that the total aerobic bacteria and coliform population was decontaminated after irradiation at a dose of 2 kGy in both aerobic and vacuum packaging samples. Irradiation dosages of 1.5 and 3 kGy reduced the initial population of anaerobic mesophilic bacteria by 2 and 3.4 log units, respectively, whereas 4.5 kGy dropped the population below the technique limit of detection during storage [29]. The total aerobic bacteria population decreased due to irradiation treatment has also been observed in red meat, ostrich, and seafood samples [20, 24]. Due to the observed microbial development in the samples irradiated at 5 kGy, specific-purpose foods must be irradiated at a high dose (40kGy) [30]. A combination of gamma irradiation with natural plant extracts [31], antioxidant treatment [24], vacuum packaging [17, 32], bioactive packaging [20], bacteriocin treatment [33], and essential oil treatment [34] is effective in reducing the total aerobic bacteria activity.
Coliform bacteria, which are responsible for spoilage of meat, are present in meat and its products [24]. Coliforms are microorganisms that include both pathogenic and nonpathogenic bacteria [22]. Li et al. [65] discovered that total coliforms were more sensitive to irradiation and that treatment with 2 kGy inhibited coliform growth. Increased irradiation intensity has a greater impact on the inactivation of microorganisms in food. In addition, irradiation was used to preserve meat for several days [59]. A previous meta-analysis by Dimov and Popova [19] also showed that irradiated treatment had a reduction effect on the coliform population. Irradiation at 2 kGy reduced coliform counts by approximately 4 log cycles [27]. In addition, coliforms were not detected in samples irradiated at 4 and 6 kGy throughout storage. Irradiation at 4 kGy significantly decontaminated and improved the hygienic quality of chicken-fermented products [66]. Ahn et al. [6] reported that the populations of the most common enteric pathogens such as Escherichia coli, Staphylococcus, and Salmonella can be significantly eliminated by a low dose (<3 kGy). As the gamma irradiation dose increases, the E. coli viable count decreases [8]. Intrinsic and extrinsic factors affect the intensity of radiation-induced damage, the amount and type of damage, and the radiosensitivity of microbes [20]. Irradiation and frozen storage are more effective in reducing coliforms than either method alone [19]. Irradiation and vacuum packaging are also more effective in reducing coliforms [17]. Reduction in the quantity of coliforms due to the combination treatment (irradiation and other treatments) is a crucial factor contributing to food safety [22]. The radiosensitivity of coliforms changes depending on the isolation source, strain, temperature, irradiation, oxygen content, and food matrix [8].
Among the microbial flora present in chicken meat products, lactic acid bacteria are the most resistant to irradiation treatment [29]. However, Abdeldaiem [15] reported that irradiated samples at doses of 0, 2, 4, and 6 kGy inhibited the development of lactic acid bacteria after 9, 18, 24, and 30 days of storage, respectively. Furthermore, gamma irradiation decreased lactic acid bacteria from day 0 of storage without a combination of treatments [22]. Although lactic acid bacteria are the most ionizing radiation-resistant bacteria, Enterobacteriaceae are the most sensitive bacteria [20]. Another study reported that compared to Enterobacteriaceae and Pseudomonas, lactic acid bacteria and Brochothrix thermosphacta are more resistant to irradiation [5, 29]. A previous meat study by Chouliara et al. [66] showed that irradiating meat at 2–5 kGy is more effective than eradicating lactic acid bacteria in eliminating Enterobacteriaceae and staphylococci.
Yeast and mold counts have been employed as sanitation indicators, and their high levels increase spoilage [33]. Yeasts are the most resistant microbes, followed by lactic acid bacteria, and their decrease in clippings is dose-dependent [66]. Because of their complex genetic structure, yeasts and molds are sensitive to irradiation [29]. However, irradiation should be combined with other treatments to maximize the efficacy of lowering yeast and mold activity. Abdeldaiem [15] reported reduced yeast and mold counts in samples of minced chicken thighs coated with an edible coating containing 2% ethanolic extract of papaya leaves. However, a single irradiation treatment is sufficient to remove yeast and mold. As reported by Aly and Aragi [35], gamma irradiation at doses of 2, 4, and 6 kGy reduced the initial counts of total bacteria, psychrophilic bacteria, spore-forming bacteria, total molds, and yeasts. However, only small volumes of bacterial and fungal growth were detected at 6 and 8 kGy [36]. Jayathilakan et al. [12] reported that a 2 log reduction in yeasts and molds can be achieved by applying a dosage of 2 kGy. The application of irradiation to certain chicken products requires further consideration. Meat yeasts, primarily Debaryomyces spp. and Micrococcaceae, play a secondary positive role in fermented sausages and are widely employed as starter cultures [66].
E. coli, Salmonella spp., and Staphylococcus spp. were highly prevalent in meat samples, which could degrade meat quality and increase the risk of foodborne infections [36]. Pseudomonas spp. is Gram-negative microbes regarded as one of the primary meat-spoiling microbes [37]. Mrityunjoy et al. [36] reported that Salmonella spp., Shigella spp., and Listeria spp. were completely eradicated (100%) from raw broiler meat following 6 kGy irradiation. Spoto et al. [38] reported that a minimum dose of 3.0 kGy is required to protect consumers against foodborne diseases associated with Staphylococcus aureus. When every living organism absorbs ionizing radiation during the sterilization process of S. aureus, there is a chance that the radiation will strike directly on the DNA, resulting in cell death [39]. Similar to the summary of our findings, irradiation is commonly used in the food processing industry to preserve food goods. It is effective against E. coli, Staphylococcus, Salmonella, and other harmful microorganisms [59].
Influence of gamma irradiation on the physicochemical and sensory parameters of chicken meat
In our study, gamma irradiation did not significantly affect pH. Similarly, studies by Mantilla et al. [10], Yun et al. [30], Al-Bachir and Othman [27], Hwang et al. [16], and Hassanzadeh et al. [28] showed that gamma irradiation had no effect on the pH values of chicken meat and its products during storage. This result may be due to undetected bacterial growth, which may prevent the synthesis of alkaline chemicals and maintain steady pH levels during storage [18]. However, irradiation may change the pH value under special conditions and treatments. Hassanzadeh et al. [28] showed that a combination of irradiation and chitosan treatment can decrease pH. Yun et al. [30] also reported that the pH of ready-to-eat meat samples irradiated at 5 kGy was not significantly different from that of meat samples irradiated at 40 kGy but was statistically significant. Bachir et al. [40] investigated the effect of irradiation on the pH of chicken kebabs and found an increase in the pH following irradiation. This may be due to changes in the chemical properties of the herbs used in the irradiated chicken product. However, further studies are required. In general, pH of aqueous system can influence the irradiation results. An acidic medium (excess H+) encourages the loss of aqueous electrons (), whereas an alkaline medium encourages their production [30]. In an extracellular environment in which microorganisms are suspended, pH significantly affects their survival after irradiation [6].
Irradiation alters meat flavor, color, and oxidative alterations, which significantly impact customer acceptance [6]. In this meta-analysis, we summarized color, expressed as total color difference (E), and hue angle (H°, 90° = yellow, 180° = green, and 0° = red) values [16]. On day 0, irradiation did not affect the values of L*, a*, and b* in meat. However, a* and b* values increased after irradiation treatment after 7 and 14 days of storage (p = 0.01). Brito et al. [7] also demonstrated a statistically significant difference (p = 0.05) in color parameters between the irradiated and control samples on certain days of refrigerated storage. Variable b* (yellowness) is primarily responsible for the color variation because chicken meat is predominantly yellow [24]. Persistent red pigments or brown pigments that turn red over time appear to result from the binding of irradiation-generated reactive oxygen species (O2) or gasses (CO) that form complexes bound by iron under modified reducing conditions [67]. Because of the sensitivity of the Mb molecule, specifically iron, to chemical transformations and changes in the energy input that eventually alter its structure, the irradiated meat changed color during refrigerated storage [7]. When the central iron atom in the heme group of Mb is oxidized, the ferrous heme iron changes to its ferric form, resulting in the transformation from Mb to MMb, which is responsible for meat discoloration [6, 19]. Color changes in irradiated fresh meat are caused by the inherent susceptibility of Mb molecules to energy input and changes in the chemical environment; heme iron is particularly vulnerable [5]. However, hemoglobin levels are higher than Mb levels in the muscles of low-pigmented meats such as chicken meat [41]. Several variables, such as irradiation dose, animal species, muscle type, additives, and packaging type, affect the color variations of irradiated meat [6]. With regard to meat type (i.e., pork meat, chicken meat, or products) and irradiation dose, packaging of meat (oxygen availability) may affect color changes of meat and products following gamma irradiation [68].
Even if there is a benefit to stability, consumers must once again rely on the esthetic appearance of the product in purchasing decisions for most processed poultry offered today. Moreover, consumers are interested in meat characteristics such as texture, flavor, juiciness, and appearance [69]. According to our findings, gamma irradiation affects the majority of sensory parameters (appearance, texture, taste, and odor) only on day 0 of storage or immediately after irradiation. In contrast, no significant effect was observed in the 7th and 14th storages. This pattern is similar to that reported in a previous study by Yoon et al. [9], Khalid et al. [24]. This shows that the effect of gamma irradiation on sensory parameters is not particularly noticeable after a long storage period. However, further research is required. Interestingly, the overall acceptability at 0, 7, and 14 days of storage decreased after irradiation. This result may be related to the effect of lipid oxidation on chicken meat and products after irradiation. Fats begin to oxidize on their own, resulting in rancid off-flavors when exposed to radiation [70]. Regarding overall quality and aroma, lipid oxidation was not the only major issue because the panelists did not recognize it [71]. Sulfur compounds appear to be the main volatile elements responsible for the odor of irradiated meat [32]. The volatile components responsible for these off-odors appear to result from the effects of electromagnetic energy (gamma radiation, accelerated electrons) on the production of high-energy species that destroy proteins and lipid molecules [72]. Sensorial analysis showed that irradiated samples had a significantly stronger rancid flavor than the control [17, 73]. It has been shown that the use of irradiation can result in some undesired changes in food if treated at high irradiation doses, mostly observed in food such as meat whose color and lipids are the significant indication elements, and a minor change in color and lipids may lead to rejection by consumers [59, 70]. On the other hand, Arvanitoyannis and Stratakos [71] recommended a maximum dose of 10 kGy for food irradiation without the need for toxicological or nutritional tests. These concentrations do not affect the flavor of the food. Variations in the effect of gamma irradiation on sensory parameters can be explained by various factors. Variations in free radicals, color changes, lipid oxidation, and off-odors depend on the irradiation dose and the type of meat [70, 74]. Accordingly, this study can be used as a reference for industries to anticipate changes in the sensory parameters of meat products following irradiation treatment.
In the current study, gamma irradiation did not significantly change tenderness, juiciness, and cooking loss of meat and its products. Therefore, our findings indicate that gamma irradiation does not affect the quality of meat for subsequent processing. The juiciness and tenderness of the irradiated, cooked chicken were only minimally affected and the sensory panel considered it satisfactory [75]. No differences in tenderness and juiciness evaluations were observed between treatments for beef patties irradiated at 0, 3, and 4.5 kGy [71]. Conversely, De Toledo et al. [42] reported that irradiation changed the tenderness and juiciness of fresh meat because of liquid loss after irradiation treatment. Rima et al. [26] have also demonstrated that the breakdown of myofibrillar and structural proteins after irradiation could reduce the cooking loss of irradiated meat samples. This is important because the disruption of muscle fibers is typically associated with a tenderizing effect [75]. Rababah et al. [76] reported that irradiation reduces tenderness in consumers and instrumental evaluations. In addition, infusion of plant extracts enhanced tenderness. These differences may be related to variations in the moisture content of the samples. The lack of variation in shear force and collagen solubility in the meat samples was another factor contributing to these inconsistent results. In addition, we assume that variations in the protein composition of the samples influence the irradiation effect. Proteolysis and protein oxidation play a significant role in the development of tenderness in irradiated meat during storage [77]. Kanatt et al. [78] also demonstrated a direct correlation between decreased shear force and increased collagen solubility in buffalo meat samples. In general, the perception of juiciness is increased by increasing moisture and/or fat content [69].
Influence of gamma irradiation on nutrient and fatty acid characteristics of chicken meat and its products
In conclusion, gamma irradiation decreases the moisture content of chicken meat and its products. This may be due to gamma-ray-induced degradation of meat protein fraction hydration capacity [18]. At higher irradiation dosages, the water content is generally released as a drip and remains on the surface of foods, reducing the moisture content of the food [79]. Chouliara et al. [66] observed that irradiation may have changed the functioning of meat proteins in such a way that the water-binding ability decreased, resulting in increased water loss. Moisture content can also be attributed to irradiation-induced protein denaturation [80]. Moisture reduction has a positive impact on the reduction of microbial activity. A lower moisture content helped to extend the shelf life of meat, which may be due to the lower availability of water for microorganisms [81]. However, it also changes the value of juiciness [69].
Based on the results of this study, gamma irradiation alters the protein content of chicken meat and its products. Higher doses of irradiation slightly enhanced the proportion of protein in the irradiated samples [26, 27]. The solubility of collagen and protein in irradiated meat is enhanced [78]. We assume that this change is generated by two aspects: (1) The oxidation of structural proteins in the meat and/or (2) the impact of lipid oxidation, which generates free radicals that interact with the protein. However, this method is also dependent on the presence of other ingredients in the sample, in particular chicken ready-to-eat products. Reactive oxygen species produced by lipid oxidation can change many intracellular and membrane proteins in the muscle [82]. Interactions between free radicals and other dietary components, such as amino acids, lipids, and proteins, are the main source of changes in product quality [68, 70]. Fallah et al. [20] demonstrated that radiation processing of muscle meals significantly increased the initial protein carbonyl content by 55.7% (R* = 1.557). In addition, irradiation significantly increased the protein carbonyl content of muscle meals by 17.1% during storage. Irradiation generates alkanes and alkenes that appear to originate from unsaturated fatty acids and amino acids [72]. Changes in food characteristics such as pH, ionic strength, dissolved gas level, viscosity, oxidation-reduction potential, and surface tension can also lead to alterations in enzymatic activity and protein denaturation [5].
In general, gamma irradiation decreases the fatty acid profile of meat and its products. Fatty acid composition plays an important role in determining the nutritional value, flavor, and textural characteristics of meat [62]. Fatty acid profile is typically used to predict lipid degradation in raw meat after irradiation [81]. A decrease in fatty acid content is induced by lipid oxidation. Unsaturated fatty acids are the principal resource for lipid oxidation [72, 83]. Ionizing radiation destroys biological components such as DNA, pigments, fatty acids, and membrane lipids [6]. Unsaturated fatty acids and carbonyl groups (fatty acids and amino acids) have electron-poor carbon-carbon double bonds that are extremely vulnerable to damage caused by free radical [72]. Lipid oxidation not only lowers the nutritional content of muscle meals through the decomposition of essential fatty acids and vitamins but also affects the sensory quality of the products due to the creation of chemicals that generate rancid odor and flavor [20]. Chicken meat is very sensitive to deterioration from oxidation processes because of the presence of relatively large levels of unsaturated fatty acids [81].
Conclusion
This study presents an in-depth overview of the influence of gamma irradiation on preservation technology in the chicken meat industry. Our results show that gamma irradiation positively reduces the microbial activity in chicken meat and its products but increases the oxidation parameters. Gamma irradiation can reduce the FFA content and overall acceptability but does not alter the flavor, tenderness, juiciness, or cooking loss. Gamma irradiation remains an important method for preserving chicken meat and its products. However, there is a need for a strategy to reduce the effect of oxidation, which affects reducing several sensory parameters in post-irradiated meats.
Authors’ Contributions
TW, MMS, ED, and ET: Conceptualization. RRA, TW, MMS, ED, DNA, and SA: Investigation. RRA, TW, MMS, TU, and WW: Methodology and formal analysis. RRA, ROS, ED, MA, and SA: Validation. RRA, TW, TU, MFK, and ROS: Data curation and writing of the original draft. RRA, SKW, DNA, MA, ET, and ROS: Resources and writing review and editing. RRA, TW, WW, MFK, SKW, and ET: Revised and edited the manuscript. TW, ET, and ROS: Supervision. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
The authors are highly thankful to the Research Organization for Agriculture and Food, the National Research and Innovation Agency of Indonesia, and Faculty of Animal Science, Universitas Gadjah Mada, for providing the collaboration platform for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Food and Agriculture Organization of United Nations (FAO) OECD-FAO Agricultural Outlook 2021-2030. 2023. [Retrieved on 23-01-2023]. Available from: https://www.fao.org/3/cb5332en/Meat.pdf .
- 2.Dimov K. Effect of gamma irradiation on the primary and secondary products of lipid oxidation in raw chicken meat, stored under different temperatures and packaging –a meta-analysis. Arch. Zootech. 2022;25(1):130–141. [Google Scholar]
- 3.Xavier M.M.B.B.S, Franco R.M, Souza M.C.L, Duque S.S, Esteves W.T.C. Implications of the use of irradiation in the processing of animal origin foods:Review. J. Bioenergy Food Sci. 2018;5(4):131–144. [Google Scholar]
- 4.Kwon J.H, Akram K, Nam K.C, Lee E.J, Ahn D.U. Evaluation of radiation-induced compounds in irradiated raw or cooked chicken meat during storage. Poult. Sci. 2011;90(11):2578–2583. doi: 10.3382/ps.2010-01237. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G.H, Xu X.L, Liu Y. Preservation technologies for fresh meat - A review. Meat Sci. 2010;86(1):119–128. doi: 10.1016/j.meatsci.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Ahn D.U, Kim I.S, Lee E.J. Irradiation and additive combinations on the pathogen reduction and quality of poultry meat. Poult. Sci. 2013;92(2):534–545. doi: 10.3382/ps.2012-02722. [DOI] [PubMed] [Google Scholar]
- 7.Brito P.P, Azevedo H, Cipolli K.M.V.A.B, Fukuma H.T, Mourao G.B, Roque C.V, Miya N.T. Effect of the gamma radiation dose rate on psychrotrophic bacteria, thiobarbituric acid reactive substances, and sensory characteristics of mechanically deboned chicken meat. J. Food Sci. 2011;76(2):S133–S138. doi: 10.1111/j.1750-3841.2010.02004.x. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahim H.M, El-Nour S.A, Hammad A.A.I, Abou Zeid M.A, Abdou D.A.M. Comparative effect of gamma and electron beam irradiation on some food borne pathogenic bacteria contaminating meat products. Egypt. J. Pure Appl. Sci. 2022;60(1):62–72. [Google Scholar]
- 9.Yoon Y, Cho W.J, Park J, Park J, Song B, Kim J, Byun M, Kim C, Sharma A.K, and Lee J.Y. Effect of Gamma irradiation on shelf-life extension and sensory characteristics of dak-galbi (marinated diced chicken) during accelerated storage. Korean J. Food Sci. Anim. Resour. 2009;29(5):573–578. [Google Scholar]
- 10.Mantilla S.P.S, Santos É.B, Vital H.D.C, Mano S.B, de Freitas M.Q, Franco R.M. Microbiology, sensory evaluation and shelf life of irradiated chicken breast fillets stored in air or vacuum. Braz. Arch. Biol. Technol. 2011;54(3):569–576. [Google Scholar]
- 11.Nisar M.F, Arshad M.S, Yasin M, Arshad M.U, Nadeem M.T. Influence of irradiation and moringa leaf powder on the amino acid and fatty acid profiles of chicken meat stored under various packaging materials. J. Food Process. Preserv. 2019;43(9):e14166. [Google Scholar]
- 12.Jayathilakan K, Sultana K, Radhakrishna K, Bawa A.S. Effect of lactic acid and irradiation on the shelf stability characteristics of hurdle processed chicken legs. Int. J. Poult. Sci. 2009;8(7):665–670. [Google Scholar]
- 13.Muhammad J, Bacha U, Anjum A.A, Sheikh A.A, Ahmad A, Hussain F, and Salman M. Assessment of different preservative methods for microbial quality and shelf life of chicken meat. J. Infect. Mol. Biol. 2013;1(3):38–40. [Google Scholar]
- 14.Chen Q, Cao M, Chen H, Gao P, Fu Y, Liu M, Wang Y, Huang M. Effects of gamma irradiation on microbial safety and quality of stir fry chicken dices with hot chili during storage. Radiat. Phys. Chem. 2016;127:122–126. [Google Scholar]
- 15.Abdeldaiem M.H. Using of combined treatment between edible coatings containing ethanolic extract of papaya (Carica papaya L.) Leaves and gamma irradiation for extending shelf-life of minced chicken meat. Am. J. Food Sci. Technol. 2014;2(1):6–16. [Google Scholar]
- 16.Hwang K.E, Kim H.W, Song D.H, Kim Y.J, Ham Y.K, Lee J.W, Choi Y.S, Kim C.J. Effects of antioxidant combinations on shelf stability of irradiated chicken sausage during storage. Radiat. Phys. Chem. 2015;106:315–319. [Google Scholar]
- 17.Arshad M.S, Amjad Z, Yasin M, Saeed F, Imran A, Sohaib M, Anjum F.M, Hussain S. Quality and stability evaluation of chicken meat treated with gamma irradiation and turmeric powder. Int. J. Food Prop. 2019;22(1):153–171. [Google Scholar]
- 18.Baptista R.F, Teixeira C.E, Lemos M, Monteiro M.L.G, Vital H.C, Mársico E.T, Júnior C.A.C, Mano S.B. Effect of high-dose irradiation on quality characteristics of ready-to-eat broiler breast fillets stored at room temperature. Poult. Sci. 2014;93(10):2651–2656. doi: 10.3382/ps.2014-03980. [DOI] [PubMed] [Google Scholar]
- 19.Dimov K, Popova T. A meta-analysis of the effect of gamma irradiation on chicken meat quality:Microbiology and colour. Food Sci. Appl. Biotechnol. 2022;5(2):160–172. [Google Scholar]
- 20.Fallah A.A, Sarmast E, Ghasemi M, Jafari T, Mousavi Khaneghah A, Lacroix M. Combination of ionizing radiation and bio-based active packaging for muscle foods:A global systematic review and meta-analysis. Food Chem. 2022;405:134960. doi: 10.1016/j.foodchem.2022.134960. [DOI] [PubMed] [Google Scholar]
- 21.Page M.J, McKenzie J.E, Bossuyt P.M, Boutron I, Hoffmann T.C, Mulrow C.D, Shamseer L, Tetzlaff J.M, Akl E.A, Brennan S.E, Chou R, Glanville J, Grimshaw J.M, Hróbjartsson A, Lalu M.M, Li T, Loder E.W, Mayo-Wilson E, McDonald S, McGuinness L.A, Stewart L.A, Thomas J, Tricco A.C, Welch V.A, Whiting P, Moher D. The PRISMA 2020 statement:An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhoir S.A, Jhaveri M, Chawla S.P. Evaluation and predictive modeling of the effect of chitosan and gamma irradiation on quality of stored chilled chicken meat. J. Food Process Eng. 2019;42(6):e13254. [Google Scholar]
- 23.De Azevedo Gomes H.D.A, da Silva E.N, Cardello H.M.A.B, Cipolli K.M.V.A.B. Effect of gamma radiation on refrigerated mechanically deboned chicken meat quality. Meat Sci. 2003;65(2):919–926. doi: 10.1016/S0309-1740(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 24.Khalid W, Arshad M.S, Yasin M, Imran A, Ahmad M.H. Quality characteristics of gamma irradiation and kale leaf powder treated ostrich and chicken meat during storage. Int. J. Food Prop. 2021;24(1):1335–1348. [Google Scholar]
- 25.Javanmard M, Rokni N, Bokaie S, Shahhosseini G. Effects of gamma irradiation and frozen storage on microbial, chemical and sensory quality of chicken meat in Iran. Food Control. 2006;17(6):469–473. [Google Scholar]
- 26.Rima F. J, Sadakuzzaman M, Hossain M.A, Ali M.S, Hashem M.A. Effect of gamma irradiation on shelf life and quality of broiler meat. SAARC J. Agric. 2019;17(1):149–159. [Google Scholar]
- 27.Al-Bachir M, Othman Y. Use of irradiation to control microorganisms and extend the refrigerated market life of chicken sausage. Innov. Rom. Food Biotechnol. 2013;13:63–70. [Google Scholar]
- 28.Hassanzadeh P, Tajik H, Mehdi S, Rohani R, Moradi M, Hashemi M, Aliakbarlu J. Effect of functional chitosan coating and gamma irradiation on the shelf-life of chicken meat during refrigerated storage. Radiat. Phys. Chem. 2017;141:103–109. [Google Scholar]
- 29.Fallah A.A, Saei-dehkordi S.S, Rahnama M. Enhancement of microbial quality and inactivation of pathogenic bacteria by gamma irradiation of ready-to-cook Iranian barbecued chicken. Radiat. Phys. Chem. 2010;79(10):1073–1078. [Google Scholar]
- 30.Yun H, Lee K.H, Lee H.J, Lee J.W, Ahn D.U, Jo C. Effect of high-dose irradiation on quality characteristics of ready-to-eat chicken breast. Radiat. Phys. Chem. 2012;81(8):1107–1110. [Google Scholar]
- 31.Mahrour A, Caillet S, Nketsa-Tabiri J, Lacroix M. Microbial and sensory quality of marinated and irradiated chicken. J. Food Prot. 2003;66(11):2156–2159. doi: 10.4315/0362-028x-66.11.2156. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.H, Jeon J.Y, Ryu S.R, Lee J.W, Kim J.H, Oh S.H, Seo J.H, Byun M.W. Quality ımprovement of chicken breast meat in a group-meal service by gamma ırradiation. Korean J. Food Preserv. 2005;12(1):28–35. [Google Scholar]
- 33.Zahran D.A. Combinations of nisin and γ irradiation to improve microbiological quality of minced chicken during refrigerated storage. Life Sci. J. 2015;12(2):147–152. [Google Scholar]
- 34.Shankar S, Karboune S, Salmieri S, Lacroix M. Development of antimicrobial formulation based on essential oils and gamma irradiation to increase the shelf life of boneless chicken thighs. Radiat. Phys. Chem. 2022;192(10):109893. [Google Scholar]
- 35.Aly A.A, El-Aragi G.M. Comparison between gamma irradiation and plasma technology to improve the safety of cold sliced chicken. Afr. J. Food Sci. 2013;7(12):461–467. [Google Scholar]
- 36.Mrityunjoy A, Israt I, Rashed N. Effects of gamma irradiation on the propagation of microbial growth in commonly available meat in Bangladesh. Int. Food Res. J. 2019;26(4):1211–1218. [Google Scholar]
- 37.Chouliara E, Badeka A, Savvaidis I, Kontominas M.G. Combined effect of irradiation and modified atmosphere packaging on shelf-life extension of chicken breast meat:microbiological, chemical and sensory changes. Eur. Food Res. Technol. 2008;226(4):877–888. [Google Scholar]
- 38.Spoto M.H.F, Gallo C.R, Alcarde A.R, Gurgel M.S.A, Blumer L, Walder J.M.M, Domarco R.E. Gamma irradiation in the control of pathogenic bacteria in refrigerated ground chicken meat. Sci. Agric. 2000;57(3):389–394. [Google Scholar]
- 39.Irmanita V, Wardani A.K, Harsojo H. The influence of gamma ırradiation against levels of protein and microbiologists broiler chicken meat modern market and traditional market South Jakarta. J. Pangan Agroindustri. 2016;4(1):428–434. [Google Scholar]
- 40.Al-Bachir M, Farah S, Othman Y. Influence of gamma irradiation and storage on the microbial load, chemical and sensory quality of chicken kabab. Radiat. Phys. Chem. 2010;79(8):900–905. [Google Scholar]
- 41.Millar S.J, Moss B.W, Stevenson M.H. The effect of ionising radiation on the colour of leg and breast of poultry meat. Meat Sci. 2000;55(3):361–370. doi: 10.1016/s0309-1740(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 42.De Toledo T.C.F, Canniatti-Brazaca S.G, Spoto M.H.F, Arthur V. Sensory evaluation of chicken breast under gamma irradiation at commercial doses. J. Food Sci. 2005;70(1):S8–S12. [Google Scholar]
- 43.Kang S.W, Hwang J.H, Jung A.H, Park E, Park S, Yoon Y, Park S.H. Effect of non-thermal pasteurization on minced chicken meat based pet food and its quality attributes through gamma ray and electron beam irradiation. Food Eng. Prog. 2021;25(2):139–146. [Google Scholar]
- 44.Pelicia K, Garcia E.A, Molino A.B, Santos G.C, Vieira Filho J.A, Santos T.A, Berto D.A. Chicken meat submitted to gamma radiation and packed with or without oxygen. Braz. J. Poult. Sci. 2015;17(2):255–262. [Google Scholar]
- 45.Kumar R, Johnsy G, Rajamanickam R, Lakshmana J.H, Kathiravan T, Nataraju S, Nadanasabapathi S. Effect of gamma irradiation and retort processing on microbial, chemical and sensory quality of ready-to-eat (RTE) chicken pulav. Int. Food Res. J. 2013;20(4):1579–1584. [Google Scholar]
- 46.Min Y.M, Park J.H, Lee J.H, Park J.N, Park J.K, Sung N.Y, Song B.S, Kim J.H, Yoon Y, Gao M, Yook H.S, Lee J.W. Effects of gamma-irradiation before and after cooking on bacterial population and sensory quality of Dakgalbi. Radiat. Phys. Chem. 2012;81(8):1121–1124. [Google Scholar]
- 47.Kanatt S.R, Rao M.S, Chawla S.P, Sharma A. Shelf-life extension of convenience meat products sold in Indian supermarkets by radiation processing. Radiat. Phys. Chem. 2010;79(12):1259–1263. [Google Scholar]
- 48.Yusof S.C.M, Juri M.L, Ali F, Majid S.A, Deraman M, Ramli R.A.A, Ahmad R, Harun Z, Babji A.S. The acceptance of gamma irradiated pre-cooked processed chicken meat products. J. Sains Nukl. Malaysia. 2009;21(1):64–71. [Google Scholar]
- 49.Chong-Nam A, Hyun-Seok C, Young-Mo Y, Hyo-Soon Y, Jun-Sang H, Seok-Geun J, Kwang-Yup K, Aera J. Effect of gamma irradiation on meat quality in chicken breast during cold storage. Korean J. Food Sci. Anim. Resour. 2008;28(3):289–294. [Google Scholar]
- 50.Balamatsia C.C, Rogga K, Badeka A, Kontominas M.G, Savvaidis I.N. Effect of low-dose radiation on microbiological, chemical, and sensory characteristics of chicken meat stored aerobically at 4o C. J. Food Prot. 2006;69(5):1126–1133. doi: 10.4315/0362-028x-69.5.1126. [DOI] [PubMed] [Google Scholar]
- 51.Kanatt S.R, Chander R, Sharma A. Effect of radiation processing on the quality of chilled meat products. Meat Sci. 2005;69(2):269–275. doi: 10.1016/j.meatsci.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Yoon K.S. Effect of gamma irradiation on the texture and microstructure of chicken breast meat. Meat Sci. 2003;63(2):273–277. doi: 10.1016/s0309-1740(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 53.Kanatt S.R, Paul P, Souza S.F.D, Thomas P. Lipid peroxidation in chicken meat during chilled storage as affected by antioxidants combined with low-dose gamma irradiation. J. Food Sci. 1998;63(2):198–200. [Google Scholar]
- 54.St-Pierre N.R. Invited review:Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001;84(4):741–755. doi: 10.3168/jds.S0022-0302(01)74530-4. [DOI] [PubMed] [Google Scholar]
- 55.Sauvant D, Schmidely P, Daudin J.J, St-Pierre N.R. Meta-analyses of experimental data in animal nutrition. Animal. 2008;2(8):1203–1214. doi: 10.1017/S1751731108002280. [DOI] [PubMed] [Google Scholar]
- 56.R Core Team. R Foundation for Statistical Computing. Vienna: 2022. [Retrieved on 25-05-2023]. A language and environment for statistical computing. Available from: http://www.r-project.org/index.html . [Google Scholar]
- 57.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4(2):133–142. [Google Scholar]
- 58.Nakagawa S, Johnson P.C.D, Schielzeth H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface. 2017;14(134):20170213. doi: 10.1098/rsif.2017.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jadhav H.B, Annapure U.S, Deshmukh R.R. Non-thermal technologies for food processing. Front. Nutr. 2021;8:657090. doi: 10.3389/fnut.2021.657090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes H.A, Da Silva E.N. Effects of ionizing radiation on mechanically deboned chicken meat during frozen storage. J. Radioanal. Nucl. Chem. 2006;270(1):225–229. [Google Scholar]
- 61.Lee E.J, Ahn D.U. The Use of Irradiation in Processed Meat Products. Cambridge: Woodhead Publishing Limited; 2011. [Google Scholar]
- 62.Reddy K.J, Jayathilakan K, Pandey M.C. Effect of ionizing radiation on the protein and lipid quality characteristics of mutton kheema treated with rice bran oil and sunflower oil. Radiat. Phys. Chem. 2015;117(4):217–224. [Google Scholar]
- 63.Tappel A.L. Regeneration and stability of oxymyoglobin in some gamma irradiated meats. Food Res. 1956;21:650–656. [Google Scholar]
- 64.Dinçer A.H, Baysal T. Decontamination techniques of pathogen bacteria in meat and poultry. Crit. Rev. Microbiol. 2004;30(3):197–204. doi: 10.1080/10408410490468803. [DOI] [PubMed] [Google Scholar]
- 65.Li G, Lin P, Li Y, He Y, Liu Z. Quality and stability evaluation of Guizhou spicy chicken treated with gamma irradiation during the storage period. Food Sci. Nutr. 2023;11(4):1982–1993. doi: 10.1002/fsn3.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chouliara I, Samelis J, Kakouri A, Badeka A, Savvaidis I.N, Riganakos K, Kontominas M.G. Effect of irradiation of frozen meat/fat trimmings on microbiological and physicochemical quality attributes of dry fermented sausages. Meat Sci. 2006;74(2):303–311. doi: 10.1016/j.meatsci.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Brewer S. Irradiation effects on meat color - A review. Meat Sci. 2004;68(1):1–17. doi: 10.1016/j.meatsci.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Ferdousi R, Ghasemzadeh-Mohammadi V, Eskandari S, Mostashari P, Abedi A.S, Mahmoudzadeh M. Effects of gamma irradiation on physicochemical and sensory properties of cooked beef sausages. Philipp. J. Sci. 2022;151(6):2447–2457. [Google Scholar]
- 69.Barbut S, Leishman E.M. Quality and processability of modern poultry meat. Animals (Basel) 2022;12(20):2766. doi: 10.3390/ani12202766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khalid W, Maggiolino A, Kour J, Arshad M.S, Aslam N, Afzal M.F, Meghwar P, Zafar K.W, De Palo P, Korma S.A. Dynamic alterations in protein, sensory, chemical, and oxidative properties occurring in meat during thermal and non-thermal processing techniques:A comprehensive review. Front. Nutr. 2023;9:1057457. doi: 10.3389/fnut.2022.1057457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arvanitoyannis I.S, Stratakos A.C. Applications of Irradiation on Meat and Meat Products. 1st ed. Amsterdam: Elsevier; 2010. [Google Scholar]
- 72.Brewer M.S. Irradiation effects on meat flavor:A review. Meat Sci. 2009;81(1):1–14. doi: 10.1016/j.meatsci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim H.M. Prediction of meat and meat products by gamma rays, electron beams and X-ray irradiations-A Review. Int. J. Agric. Sci. 2013;3(5):521–534. [Google Scholar]
- 74.Kaur R, Kaur L, Gupta T.B, Singh J, Bronlund J. Multitarget preservation technologies for chemical-free sustainable meat processing. J. Food Sci. 2022;87(10):4312–4328. doi: 10.1111/1750-3841.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Bryan C.A, Crandall P.G, Ricke S.C, Olson D.G. Impact of irradiation on the safety and quality of poultry and meat products:A review. Crit. Rev. Food Sci. Nutr. 2008;48(5):442–457. doi: 10.1080/10408390701425698. [DOI] [PubMed] [Google Scholar]
- 76.Rababah T, Hettiarachchy N.S, Eswaranandam S, Meullenet J.F, Davis B. Sensory evaluation of irradiated and nonirradiated poultry breast meat infused with plant extracts. J. Food Sci. 2005;70(3):S228–S235. [Google Scholar]
- 77.Zhang M, He L, Li C, Yang F, Zhao S, Liang Y, Jin G. Effects of gamma ray irradiation-induced protein hydrolysis and oxidation on tenderness change of fresh pork during storage. Meat Sci. 2020;163:108058. doi: 10.1016/j.meatsci.2020.108058. [DOI] [PubMed] [Google Scholar]
- 78.Kanatt S.R, Chawla S.P, Sharma A. Effect of radiation processing on meat tenderisation. Radiat. Phys. Chem. 2015;111:1–8. [Google Scholar]
- 79.Wang H, Yang R, Liu Y, Zhang W, Zhao W, Zhang Y, Hua X. Effects of low dose gamma irradiation on microbial inactivation and physicochemical properties of fried shrimp (Penaeus vannamei) Int. J. Food Sci. Technol. 2010;45(6):1088–1096. [Google Scholar]
- 80.Choi Y.S, Kim H.W, Hwang K.E, Song D.H, Jeong T.J, Seo K.W, Kim Y.B, Kim C.J. Effects of gamma irradiation on physicochemical properties of heat-induced gel prepared with chicken salt-soluble proteins. Radiat. Phys. Chem. 2015;106:16–20. [Google Scholar]
- 81.Zu X.Y, Li H.L, Xiong G.Q, Liao T, Yu Y.H, Qiu J.H. Gamma irradiation on moisture migration and lipid degradation of Micropterus salmoides meat. Radiat. Phys. Chem. 2022;192(368):109915. [Google Scholar]
- 82.Xiao S, Zhang W.G, Lee E.J, Ma C.W, Ahn D.U. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poult. Sci. 2011;90(6):1348–1357. doi: 10.3382/ps.2010-01244. [DOI] [PubMed] [Google Scholar]
- 83.Henry F.C. Irradiation effects on meat:A review. Rev. Ciênc. Agrár. 2009;32(2):255–262. [Google Scholar]


