Abstract
Background:
Proton-pump inhibitors (PPIs) and potassium-competitive acid blockers (P-CABs) are recommended for erosive esophagitis (EE), with good safety and tolerance. However, it is unclear which is the best treatment option for EE.
Objectives:
This study aimed to evaluate the comparative efficacy of P-CABs and PPIs for healing EE patients, seeking an appropriate treatment choice in the 4- or 8-week treatment and standard or double dose.
Design:
A systematic review and network meta-analysis.
Data sources and methods:
Relevant databases were searched to collect randomized controlled trials of PPIs and P-CABs in the treatment of EE up to 31 May 2023. Studies on standard or double-dose PPIs or P-CABs which were published in English and assessed 4- or 8-week healing effects in EE were included. A network meta-analysis was performed to evaluate the efficacy of the treatments under the frequentist framework. Sensitivity and subgroup analyses of patients with different baseline EE were also conducted.
Results:
In all, 34 studies involving 25,054 patients and 9 PPIs, 6 P-CABs, or placebo treatment interventions were included. The pooled 4-week healing rate was significantly statistically lower than the pooled 8-week healing rate for most treatments. Besides, the higher healing rate of double-dose treatment than standard-dose treatment was not observed in the initial treatment of most drugs. The main analysis only included studies conducted for both patients with and without severe EE at baseline, and the proportion of severe EE included in the study was >10%, Keverprazan 20 mg qd ranked best with a surface under the cumulative ranking curve (SUCRA) value of 84.7, followed by Ilaprazole 10 mg qd with a SUCRA value of 82.0, for the healing rate at 8 weeks. Sensitivity analysis showed that the results were robust. Subgroup analysis showed that most P-CABs had higher healing rates than PPIs, particularly for patients with severe EE. And the healing rate of Keverprazan 20 mg qd at 8 weeks ranked best in the subgroup without or with severe EE at baseline.
Conclusion:
This study showed that an 8-week treatment seemed more effective than the 4-week treatment for healing EE patients. The healing effect of Keverprazan (20 mg qd) ranked best in 8-week treatment, for both severe and non-severe EE patients.
Trial registration:
The study protocol was registered with INPLASY (registration number INPLASY2023120053).
Keywords: erosive esophagitis, network meta-analysis, potassium-competitive acid blockers, proton-pump inhibitors
Plain language summary
A review and network meta-analysis of different medications for treating erosive esophagitis: potassium-competitive acid blockers and proton-pump inhibitors
Why was the study done? Proton-pump inhibitors (PPIs) and potassium-competitive acid blockers (P-CABs) are commonly used to treat Erosive esophagitis (EE) due to their good safety and tolerance. This study aimed to compare the effectiveness of P-CABs and PPIs in healing EE patients. We wanted to determine the best treatment choice in terms of the duration of treatment (4 or 8 weeks) and the dosage (standard or double-dose). What did the researchers do? The researchers searched relevant databases for randomized controlled trials that studied the use of PPIs and P-CABs in treating EE up until May 31, 2023. They included studies that evaluated the healing effects of standard or double-dose PPIs or P-CABs over a period of 4 or 8 weeks. A network meta-analysis was performed to compare the effectiveness of these treatments. They also conducted sensitivity analysis and subgroup analysis to examine the effects on patients with different levels of EE. What did the researchers find? The results showed that the healing rate after 4 weeks of treatment was significantly lower than the healing rate after 8 weeks for most treatments. Additionally, the higher healing rate observed with double-dose treatment compared to standard-dose treatment was not seen in the initial treatment of most drugs. In the main analysis, which included studies with patients both with and without severe EE at the beginning, Keverprazan 20mg qd was ranked as the most effective treatment with a healing rate of 84.7, followed by Ilaprazole 10mg qd with a healing rate of 82.0 at 8 weeks. The results were robust in sensitivity analysis. Subgroup analysis showed that most P-CABs had higher healing rates than PPIs, especially for patients with severe EE. What do the findings mean? Treating EE patients for 8 weeks was more effective than treating them for 4 weeks. Keverprazan (20mg once a day) with the 8-week treatment is the optimal method.
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastroenterological diseases, with a prevalence of 13.3% worldwide, covering all age groups and both genders. 1 GERD results from the reflux of gastric contents into the esophagus, often accompanied by symptoms such as heartburn, acid regurgitation, and dysphagia. 2 The symptomatic nature and high prevalence of GERD not only impact patients’ quality of life and well-being 3 but also bring a huge economic burden on social medical systems. 4
Erosive esophagitis (EE) is a severe condition of GERD, with an estimated proportion of 25–50% occurring in patients with GERD.5,6 And EE is graded by the severity of mucosal breaks using the Hetzel–Dent, 7 Savary–Miller, 8 or Los Angeles scale. 9 Without effective treatment, EE may develop into esophageal stricture, esophageal bleeding, or Barrett’s esophagus, the risk factor for esophageal adenocarcinoma.10,11 The principal aim of treatment for patients with EE is gastric acid suppression. Currently, proton-pump inhibitors (PPIs) are the first-line drug for treating EE. 12 However, the efficacy of PPIs depends on the polymorphism of CYP2C19, 13 and three to five dosages are needed to maximize the efficacy. 14 Furthermore, PPIs need to be activated by gastric acid before they can bind to the proton pumps, and thus their onset is gradual and needs to be taken before meals.14,15
Potassium-competitive acid blockers (P-CABs) are recent alternatives to PPIs for EE. In contrast to PPIs, P-CABs inhibit H+/K+-ATPase in a reversible and K+-competitive manner without any conversion.16,17 The inhibitory effect of P-CABs is impacted less by the CYP2C19 enzyme, enabling gastric acid suppression faster and more potent. 18 In addition, P-CABs are more stable in an acidic environment, water soluble, and capable of combining with both activated and inactivated proton pumps.19,20 Several types of P-CABs, such as Vonoprazan developed by Takeda Pharmaceutical Company Ltd, in Japan, 21 Tegoprazan developed by CJ Healthcare Corp. in South Korea, 22 Keverprazan developed by Jiangsu Carephar Pharmaceutical Co. Ltd in China, 23 and Fexuprazan developed by Daewoong Pharmaceutical Co. Ltd in South Korea, 24 have been launched for EE treatment. In addition, PPIs or P-CABs at standard dose or double dose, if ineffective with the standard dose, are recommended in GERD guidelines or consensuses for treating EE. 25 However, as many types of PPIs and P-CABs are available in clinical practice, it is unclear which is the best treatment option for EE. Three meta-analyses have compared Vonoprazan and PPIs in healing GERD, but they did not include other P-CABs.26–28
The confirmed safety and tolerance of PPIs and P-CABs have been demonstrated in clinical practice. For example, previous meta-analyses showed no significant difference in the incidence of adverse events among all PPIs, Vonoprazan, and placebo.27,28 Some head-to-head randomized controlled trials (RCTs) have also shown that other P-CABs such as Tegoprazan, Keverprazan, and Fexuprazan had similar safety outcomes to those of some PPIs.23,29–31 Therefore, we conducted a systematic review and network meta-analysis to evaluate the comparative efficacy of P-CABs and PPIs for healing EE patients. A subgroup analysis of patients with different baseline erosive grades would be also conducted, given that P-CABs could be more effective in patients with severe EE who could not benefit from PPIs. 19 We ranked the efficacy on the 4- and 8-week healing rate of each treatment to help establish evidence-based hierarchies. In addition, the pooled 4- and 8-week healing rates were compared, to determine the optimal main outcome as well as the appropriate treatment course.
Methods
This study was conducted using the recommended method by the Cochrane Handbook for Systematic Reviews of Interventions and reported according to the PRISMA statement and the PRISMA extension for network meta-analysis. 32 The study protocol was registered with INPLASY (registration number INPLASY2023120053). We have already registered it with PROSPERO, and it is under review.
Data sources and searches
PubMed, Embase, Web of Science, Cochrane Library, and Medline were searched for all years up to 31 May 2023, to identify RCTs of PPIs and P-CABs in the treatment of EE. The search terms and meshes were mainly ‘Esophagitis’, ‘Esomeprazole’, ‘Fexuprazan’, ‘Ilaprazole’, ‘Lansoprazole’, ‘Omeprazole’, ‘Pantoprazole’, ‘Rabeprazole’, ‘Tegoprazan’, ‘Vonoprazan’, and ‘Keverprazan’. More detailed terms are listed in Supplemental Material S1.
Study selection
The inclusion criteria for eligible studies were (1) Patients: patients with EE. Exclude refractory EE or resistance to previous PPI treatment. (2) Interventions and comparisons: drugs included either PPIs or P-CABs administered with the standard or double dose and placebo, excluding medications that are not yet licensed in the market. The included drugs and doses were described as follows: Esomeprazole 40 and 80 mg qd; Ilaprazole 10 and 20 mg qd; Lansoprazole 30 and 60 mg qd; Omeprazole 20 and 40 mg qd; Pantoprazole 40 and 80 mg qd; Rabeprazole 10 and 20 mg qd; Vonoprazan 20 and 40 mg qd; Tegoprazan 50 and 100 mg qd; Keverprazan 20 and 40 mg qd; and Fexuprazan 20 and 40 mg qd. (3) Outcomes: 4- or 8-week healing rate. (4) Study design: only RCTs published in English. Studies were excluded if informally published literature such as conference abstracts and academic papers, or patients received combined therapy for EE, such as two types of PPIs.
Data extraction and quality assessment
Two authors independently screened the title and abstract, reviewed the full texts, performed data extraction, and assessed the risk of bias. Disagreement was solved via discussion or consultation with the senior authors.
Study characteristics (ID, authors, year of publication), population (sample size, patient demographics, grade of EE at baseline, proportion of patients with severe EE at baseline), description of interventions (drug class, name, dose), and outcomes were extracted from each treatment group from each study. Intention-to-treat data were collected for all outcomes if available; otherwise, completer-only data were collected. The risk of bias was assessed using the Cochrane Risk of Bias Tool for randomized clinical trials. 32
Data synthesis and analysis
The network meta-analyses were performed under the frequentist framework using Stata 13 software (StataCorp, College Station, TX, USA).
Inconsistency was assessed by global Wald χ2, with a p value >0.05 defined as no inconsistency, and the fixed-effects model was used; otherwise, a random-effects model with restricted maximum likelihood variance estimation was used. 33 Pairwise odds ratios (ORs) and the 95% confidence interval (95% CI) were calculated to compare the efficacy of treatments. The surface under the cumulative ranking curve (SUCRA) was used to rank the efficacy of the treatments, and the larger SUCRA indicated the better efficacy of the treatment regimen. 34 The funnel plot and Egger’s test of the intercept were employed to assess indications of publication bias. 34
To control the impact of the proportion of severe EE at baseline on the outcomes, studies were included in the main analysis if (1) they were originally conducted for both patients with and without severe EE at baseline and (2) the proportion of severe EE at baseline included in the study was >10%. Sensitivity analysis was also conducted to examine the validity and robustness of the main analysis using all studies that were originally conducted for both patients with and without severe EE at baseline. Subgroup analysis was conducted on the data of patients with or without a severe EE baseline grade, which was defined as grade 3 or higher on the Hetzel–Dent or Savary–Miller scales, or grade C or D on the Los Angeles scale.35–39 If the study was originally conducted only for patients with or without severe EE at baseline, the data were only used in the subgroup analysis. The pooled 4- and 8-week healing rates were compared based on each treatment arm, via calculating OR and 95% CI.
Results
Study selection and characteristics
Figure 1 shows the number of included and excluded studies at each stage of the process. A total of 11,420 studies were identified from the databases, of these, 34 were eligible for analyses and included a total of 25,054 patients.23,29–31,40–69
Figure 1.
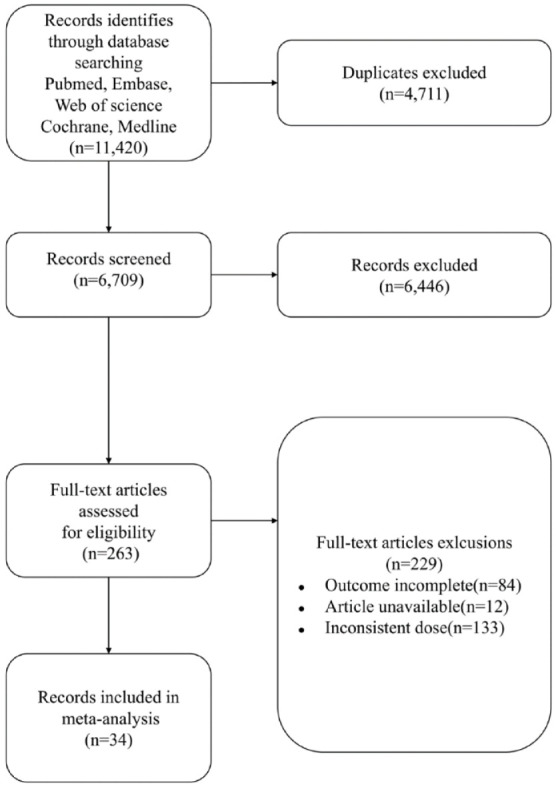
Study flow diagram. Inconsistent dose: PPIs and P-CABs were not administered with the standard or double dose.
P-CAB, potassium-competitive acid blockers; PPI, proton-pump inhibitors.
Characteristics of the eligible studies are shown in Table 1. After literature screening, the dose of Rabeprazole 10 mg bid was identified in one article. Given that Rabeprazole 10 mg bid was similar to the double dose of Rabeprazole (20 mg qd), we supplemented this dose to this study. A total of 16 treatment interventions were included, including nine PPIs (Esomeprazole 40 mg qd, Ilaprazole 10 mg qd, Lansoprazole 30 mg qd, Lansoprazole 60 mg qd, Omeprazole 40 mg qd, Omeprazole 20 mg qd, Pantoprazole 40 mg qd, Rabeprazole 10 mg bid, and Rabeprazole 20 mg qd), six P-CABs (Tegoprazan 100 mg qd, Tegoprazan 50 mg qd, Vonoprazan 20 mg qd, Vonoprazan 40 mg qd, Keverprazan 20 mg qd, and Fexuprazan 40 mg qd), and placebo. Data on the healing rate at 4 weeks were reported in 31 studies. Of these, 28 studies were originally conducted for both patients with and without severe EE at baseline but 3 studies included a proportion of less than 10% of severe EE at baseline, 2 studies only included patients without severe EE at baseline, and 1 study only included patients with severe EE at baseline.
Table 1.
Characteristics of the eligible studies.
| Study ID | Study | Sample size | Treatment | Age | Male (%) | Diagnosis level | Proportion of patients with severe EE at baseline (%) | 4-Week healing rate | 8-Week healing rate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Total | Event | Total | ||||||||
| 1 | Sontag et al., 199240 | 91 | Omeprazole 40 mg qd | NR | NR | HD II–IV | About 50 | 40 | 91 | 66 | 91 |
| 1 | Sontag et al., 199240 | 93 | Omeprazole 20 mg qd | NR | NR | HD II–IV | 36 | 93 | 68 | 93 | |
| 1 | Sontag et al., 199240 | 46 | Placebo | NR | NR | HD II–IV | 3 | 46 | 7 | 46 | |
| 2 | Hatlebakk et al., 199341 | 116 | Lansoprazole 30 mg qd | 54.3 | 66.4 | 1–2 a | 0 | 71 | 113 | 95 | 112 |
| 2 | Hatlebakk et al., 199341 | 113 | Omeprazole 20 mg qd | 55.4 | 65.5 | 1–2 a | 73 | 112 | 96 | 111 | |
| 3 | Corinaldesi et al., 199542 | 121 | Omeprazole 20 mg qd | 52 | 62 | SM II–III | 18.3 | 83 | 105 | 96 | 105 |
| 3 | Corinaldesi et al., 199542 | 120 | Pantoprazole 40 mg qd | 50 | 65 | SM II–III | 81 | 103 | 97 | 103 | |
| 4 | Mössner et al., 199543 | 95 | Omeprazole 20 mg qd | 55 | 69 | SM II–III | 20.3 | 67 | 86 | 81 | 86 |
| 4 | Mössner et al., 199543 | 191 | Pantoprazole 40 mg qd | 53 | 70 | SM II–III | 126 | 170 | 153 | 170 | |
| 5 | Castell et al., 199644 | 422 | Lansoprazole 30 mg qd | 48.6 | 68.4 | II–IV a | 34.8 | 335 | 421 | 367 | 421 |
| 5 | Castell et al., 199644 | 431 | Omeprazole 20 mg qd | 47.5 | 60.3 | II–IV a | 343 | 431 | 375 | 431 | |
| 5 | Castell et al., 199644 | 213 | Placebo | 47.6 | 66.7 | II–IV a | 62 | 213 | 68 | 213 | |
| 6 | Mee and Rowley, 199645 | 300 | Lansoprazole 30 mg qd | 53.4 | 66.0 | SM 1–4 | 16.6 | 186 | 300 | 226 | 300 |
| 6 | Mee and Rowley, 199645 | 304 | Omeprazole 20 mg qd | 52.4 | 67.1 | SM 1–4 | 172 | 304 | 216 | 304 | |
| 7 | Earnest et al., 199846 | 71 | Lansoprazole 30 mg qd | NR | NR | II–IV a | NR | 52 | 71 | 62 | 71 |
| 7 | Earnest et al., 199846 | 79 | Lansoprazole 60 mg qd | NR | NR | II–IV a | 60 | 79 | 70 | 79 | |
| 7 | Earnest et al., 199846 | 66 | Placebo | NR | NR | II–IV a | 20 | 66 | 31 | 66 | |
| 8 | Dekkers et al., 199947 | 102 | Omeprazole 20 mg qd | 52 ± 15.56 | 71.6 | HD II–IV | 56.9 | 83 | 102 | 96 | 102 |
| 8 | Dekkers et al., 199947 | 100 | Rabeprazole 20 mg qd | 54 ± 15.70 | 53 | HD II–IV | 81 | 100 | 92 | 100 | |
| 9 | Vcev et al., 199948 | 60 | Omeprazole 20 mg qd | NR | NR | SM 1–2 | 0 | 43 | 60 | 56 | 60 |
| 9 | Vcev et al., 199948 | 60 | Pantoprazole 40 mg qd | NR | NR | SM 1–2 | 46 | 60 | 57 | 60 | |
| 10 | Delchier et al., 200049 | 103 | Omeprazole 20 mg qd | 53 ± 15.1 | 39 | HD II–IV | Median grade: 3.0; mean grade: 2.6 | 94 | 103 | 97 | 103 |
| 10 | Delchier et al., 200049 | 103 | Rabeprazole 10 mg bid | 52 ± 14.3 | 30 | HD II–IV | 88 | 103 | 94 | 103 | |
| 10 | Delchier et al., 200049 | 104 | Rabeprazole 20 mg qd | 55 ± 15.7 | 45 | HD II–IV | 92 | 104 | 95 | 104 | |
| 11 | Kahrilas et al., 200050 | 654 | Esomeprazole 40 mg qd | 44.8 ± 13.0 | 58.7 | LA A–D | 26.7 | 496 | 654 | 615 | 654 |
| 11 | Kahrilas et al., 200050 | 650 | Omeprazole 20 mg qd | 46.5 ± 13.5 | 61.4 | LA A–D | 421 | 650 | 565 | 650 | |
| 12 | Richter and Bochenek, 200051 | 173 | Pantoprazole 40 mg qd | 49.3 ± 13.6 | 69.9 | HD II–IV | 44.5 | 125 | 173 | 152 | 173 |
| 12 | Richter and Bochenek, 200051 | 82 | Placebo | 48.3 ± 14.0 | 64.6 | HD II–IV | 11 | 82 | 27 | 82 | |
| 13 | Dupas and Houcke, 200152 | 235 | Lansoprazole 30 mg qd | 55.0 ± 14.7 | 75 | SM II–III | 17 | 189 | 235 | 201 | 235 |
| 13 | Dupas and Houcke, 200152 | 226 | Pantoprazole 40 mg qd | 53.0 ± 14.5 | 73 | SM II–III | 184 | 226 | 203 | 226 | |
| 14 | Richter et al., 200153 | 1216 | Esomeprazole 40 mg qd | NR | 59.4 | LA I–IV | 26.4 | 993 | 1216 | 1139 | 1216 |
| 14 | Richter et al., 200153 | 1209 | Omeprazole 20 mg qd | NR | 62.9 | LA I–IV | 831 | 1209 | 1018 | 1209 | |
| 15 | Castell et al., 200254 | 2624 | Esomeprazole 40 mg qd | 47.0 ± 13.0 | 57.4 | LA A–D | 24.6 | 2083 | 2624 | 2430 | 2624 |
| 15 | Castell et al., 200254 | 2617 | Lansoprazole 30 mg qd | 47.4 ± 13.1 | 57.3 | LA A–D | 1965 | 2617 | 2324 | 2617 | |
| 16 | Howden et al., 200255 | 141 | Esomeprazole 40 mg qd | 46 ± 13 | 76 | II–IV a | 39.4 | 108 | 138 | 123 | 138 |
| 16 | Howden et al., 200255 | 143 | Lansoprazole 30 mg qd | 47 ± 12 | 82 | II–IV a | 107 | 139 | 127 | 139 | |
| 17 | Gillessen et al., 200456 | 114 | Esomeprazole 40 mg qd | 54 ± 14 | 50 | LA B–C | 16.5 | 68 | 103 | 92 | 103 |
| 17 | Gillessen et al., 200456 | 113 | Pantoprazole 40 mg qd | 53 ± 15 | 57 | LA B–C | 55 | 94 | 94 | 94 | |
| 18 | Fennerty et al., 200557 | 498 | Esomeprazole 40 mg qd | 47.3 ± 13.2 | 65.7 | LA C–D | 100 | 292 | 498 | 410 | 498 |
| 18 | Fennerty et al., 200557 | 501 | Lansoprazole 30 mg qd | 47.1 ± 12.9 | 66.5 | LA C–D | 247 | 501 | 388 | 501 | |
| 19 | Labenz et al., 200558 | 1562 | Esomeprazole 40 mg qd | 50.6 ± 14.0 | 62 | LA I–IV | 24.4 | 1265 | 1562 | 1492 | 1562 |
| 19 | Labenz et al., 200558 | 1589 | Pantoprazole 40 mg qd | 50.5 ± 13.8 | 63.7 | LA I–IV | 1184 | 1589 | 1462 | 1589 | |
| 20 | Pace et al., 200559 | 277 | Omeprazole 20 mg qd | 47.1 ± 14.9 | 67.7 | SM I–III | 29.7 | 213 | 237 | 231 | 237 |
| 20 | Pace et al., 200559 | 283 | Rabeprazole 20 mg qd | 47.7 ± 14.2 | 68.6 | SM I–III | 212 | 233 | 228 | 233 | |
| 21 | Schmitt et al., 200661 | 576 | Esomeprazole 40 mg qd | 47.1 ± 13.3 | 60.1 | LA A–D | 26.6 | 393 | 576 | 501 | 576 |
| 21 | Schmitt et al., 200661 | 572 | Omeprazole 20 mg qd | 46.2 ± 13.6 | 58.6 | LA A–D | 379 | 572 | 491 | 572 | |
| 22 | Vcev et al., 200662 | 90 | Esomeprazole 40 mg qd | 51.2 ± 14.5 | 63.3 | LA I–III | 31.4 | 70 | 90 | 83 | 90 |
| 22 | Vcev et al., 200662 | 90 | Pantoprazole 40 mg qd | 49.4 ± 13.9 | 65.6 | LA I–III | 65 | 90 | 82 | 90 | |
| 23 | Bardhan et al., 200763 | 293 | Esomeprazole 40 mg qd | 54 ± 14 | 53 | LA A–D | 34.6 | 202 | 293 | 243 | 293 |
| 23 | Bardhan et al., 200763 | 288 | Pantoprazole 40 mg qd | 53 ± 14 | 49 | LA A–D | 199 | 288 | 248 | 288 | |
| 24 | Zheng, 200964 | 68 | Esomeprazole 40 mg qd | 57.4 ± 12.8 | 48.5 | LA A–D | 32.1 | NR | NR | 65 | 68 |
| 24 | Zheng, 200964 | 69 | Lansoprazole 30 mg qd | 58.1 ± 13.0 | 50.7 | LA A–D | NR | NR | 62 | 69 | |
| 24 | Zheng, 200964 | 68 | Omeprazole 20 mg qd | 57.9 ± 14.1 | 48.5 | LA A–D | NR | NR | 60 | 68 | |
| 24 | Zheng, 200964 | 69 | Pantoprazole 40 mg qd | 57.8 ± 13.2 | 49.3 | LA A–D | NR | NR | 63 | 69 | |
| 25 | Ashida et al., 201530 | 140 | Lansoprazole 30 mg qd | 55.8 ± 13.92 | 70.7 | LA A–D | 33.6 | 123 | 132 | 126 | 132 |
| 25 | Ashida et al., 201530 | 154 | Vonoprazan 20 mg qd | 58.3 ± 13.86 | 74.7 | LA A–D | 136 | 144 | 139 | 144 | |
| 25 | Ashida et al., 201530 | 146 | Vonoprazan 40 mg qd | 57.6 ± 12.83 | 78.1 | LA A–D | 130 | 134 | 130 | 134 | |
| 26 | Ashida et al., 201629 | 202 | Lansoprazole 30 mg qd | 57.4 ± 13.2 | 76.2 | LA A–D | 36.2 | 184 | 199 | 190 | 199 |
| 26 | Ashida et al., 201629 | 207 | Vonoprazan 20 mg qd | 58.3 ± 13.8 | 66.2 | LA A–D | 198 | 205 | 203 | 205 | |
| 27 | Xue et al., 201665 | 105 | Esomeprazole 40 mg qd | 47.8 ± 11.65 | 68.6 | LA A–D | 10.8 | 75 | 105 | 89 | 105 |
| 27 | Xue et al., 201665 | 107 | Ilaprazole 10 mg qd | 48.9 ± 12.63 | 70.1 | LA A–D | 87 | 107 | 95 | 107 | |
| 28 | Xue et al., 201866 | 215 | Esomeprazole 40 mg qd | 47.5 ± 12.32 | 70.7 | LA A–D | 6.1 | 167 | 215 | 178 | 215 |
| 28 | Xue et al., 201866 | 322 | Ilaprazole 10 mg qd | 48.2 ± 11.96 | 72.0 | LA A–D | 245 | 322 | 269 | 322 | |
| 29 | Xiao et al., 202067 | 237 | Lansoprazole 30 mg qd | 53.8 ± 12.53 | 75.5 | LA A–D | 29.9 | 192 | 230 | 210 | 230 |
| 29 | Xiao et al., 202067 | 244 | Vonoprazan 20 mg qd | 54.1 ± 13.16 | 72.1 | LA A–D | 203 | 238 | 220 | 238 | |
| 30 | Lee et al., 201931 | 99 | Esomeprazole 40 mg qd | 50.4 (21.0–75.0) | 53.5 | LA A–D | 4.3 | 87 | 99 | 92 | 99 |
| 30 | Lee et al., 201931 | 102 | Tegoprazan 100 mg qd | 52.8 (20.0–74.0) | 64.7 | LA A–D | 92 | 102 | 97 | 102 | |
| 30 | Lee et al., 201931 | 99 | Tegoprazan 50 mg qd | 52.7 (21.0–74.0) | 62.6 | LA A–D | 87 | 99 | 95 | 99 | |
| 31 | Chen et al., 202223 | 119 | Keverprazan 20 mg qd | 49.7 ± 12.1 | 83.2 | LA A–D | 20.6 | 98 | 119 | 114 | 119 |
| 31 | Chen et al., 202223 | 119 | Lansoprazole 30 mg qd | 48.8 ± 12.3 | 76.5 | LA A–D | 97 | 119 | 107 | 119 | |
| 32 | Laine et al., 202368 | 510 | Lansoprazole 30 mg qd | 51.7 ± 14.1 | 45.7 | LA A–D | 34.3 | NR | NR | 431 | 510 |
| 32 | Laine et al., 202368 | 514 | Vonoprazan 20 mg qd | 51.0 ± 13.4 | 50.2 | LA A–D | NR | NR | 478 | 514 | |
| 33 | Lee et al., 202269 | 115 | Esomeprazole 40 mg qd | 55.05 ± 12.89 | 64.3 | LA A–D | 6.9 | 92 | 104 | 110 | 111 |
| 33 | Lee et al., 202269 | 116 | Fexuprazan 40 mg qd | 53.70 ± 12.44 | 67.2 | LA A–D | 93 | 103 | 106 | 107 | |
| 34 | Lightdale et al., 200660 | 588 | Omeprazole 20 mg qd | 45.3 ± 13.0 | 63.9 | LA A–D | 26.2 | NR | NR | 484 | 588 |
These articles do not specify the endoscopic grading scale.
HD, Hetzel–Dent; LA, Los Angeles; SM, Savary–Miller; NR, not report.
Data on the healing rate at 8 weeks were reported in 34 studies. Of these, 31 studies were originally conducted for both patients with and without severe EE at baseline but 3 studies included a proportion of severe EE at a baseline of >0–10%, 2 studies only included patients without severe EE at baseline, and 1 study only included patients with severe EE at baseline.
For the subgroup analysis, 11 studies reported a 4-week healing rate for patients without severe EE at baseline, 11 reported a 4-week healing rate for patients with severe EE at baseline, 14 studies reported an 8-week healing rate for patients without severe EE at baseline, and 14 studies reported an 8-week healing rate for patients with severe EE at baseline.
Quality assessment of the included studies
As shown in Supplemental Figure S1, 18 studies had a low risk of bias in random sequence generation; 28 used appropriate allocation concealment; and 32 studies had a low risk of blinding participants, personnel, and outcome assessment. All 34 studies had complete data and none selectively reported the findings, and it was unclear whether other sources of bias existed.
Pooled 4- versus 8-week healing rates
As shown in Supplemental Table S9, the pooled 4-week healing rate was significantly statistically lower than the pooled 8-week healing rate for most drugs, except for Tegoprazan 100 qd (OR: 0.474, 95% CI: 0.156–1.440), Vonoprazan 40 mg qd (OR: 1.000, 95% CI: 0.245–4.084), and Rabeprazole 10 mg bid (OR: 0.562, 95% CI: 0.234–1.349).
4-Week healing rate
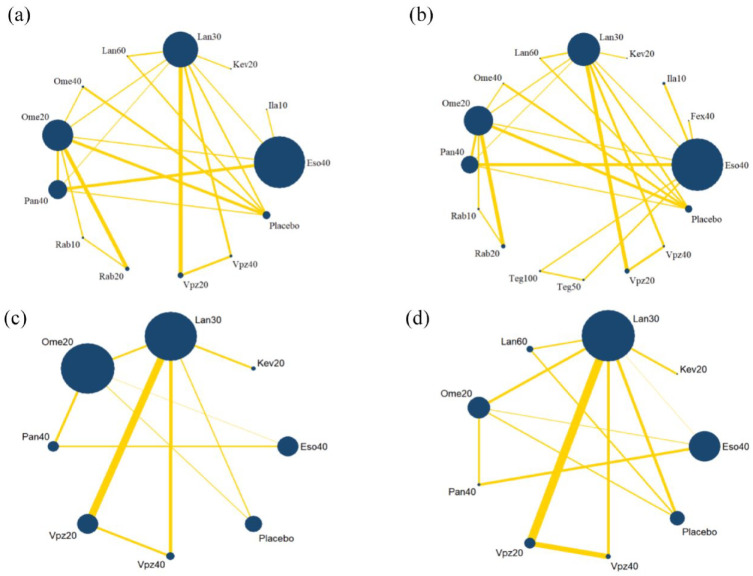
The network plot is shown in Figure 2, the area of nodes indicates the number of studies included in the corresponding nodes, and the width of the lines connecting nodes suggests the number of relevant data. No inconsistency was detected in the main, sensitivity, or subgroup analysis (Table 2). The funnel plot is shown in Supplemental Figure S2, indicating that publication bias was acceptable.
Figure 2.
Network plots for 4-week healing rate. (a) Main analysis. (b) Sensitivity analysis. (c) Subgroup analysis on patients without severe baseline grade. (d) Subgroup analysis on patients with severe baseline grade.
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 20 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
Table 2.
Results of inconsistency testing.
| Outcomes | Wald χ2 | p Value |
|---|---|---|
| 4-Week healing rate | ||
| Main analysis | 4.63 | 0.865 |
| Sensitivity analysis | 0.65 | 0.741 |
| Subgroup analysis | ||
| Patients without severe baseline grade | 1.03 | 0.470 |
| Patients with a severe baseline grade | 1.07 | 0.499 |
| 8-Week healing rate | ||
| Main analysis | 0.49 | 0.893 |
| Sensitivity analysis | 0.51 | 0.886 |
| Subgroup analysis | ||
| Patients without severe baseline grade | 0.81 | 0.552 |
| Patients with a severe baseline grade | 1.33 | 0.382 |
In the main analysis, 25 studies were included with the proportions of severe EE at a baseline of >10%. For all relative treatment pairwise comparisons, all PPIs and P-CABs showed a significantly better 4-week healing rate than the placebo (Supplemental Table S1). Ilaprazole 10 mg qd and Esomeprazole 40 mg qd showed significantly higher rates than Rabeprazole 10 mg bid, Pantoprazole 40 mg qd, Omeprazole 20 mg qd, and Lansoprazole 30 mg qd. Ilaprazole 10 mg qd was also significantly superior to Rabeprazole 20 mg qd on a 4-week healing rate. Vonoprazan 40 mg qd showed significantly higher rates than Rabeprazole 10 mg bid. Ilaprazole 10 mg qd was ranked as the best treatment with a SUCRA value of 89.3, followed by Vonoprazan 40 mg qd with a SUCRA value of 86.7 (Table 3).
Table 3.
The results of SUCRA in the main analysis.
| Treatment | 4-Week healing rate | 8-Week healing rate | ||||
|---|---|---|---|---|---|---|
| SUCRA | PrBest | MeanBank | SUCRA | PrBest | MeanBank | |
| Kev20 | 54.6 | 2.6 | 6.5 | 84.7 | 41.0 | 2.8 |
| Ila10 | 89.3 | 35.8 | 2.3 | 82 | 28.4 | 3.2 |
| Vpz20 | 73.9 | 4.9 | 4.1 | 80.4 | 7.2 | 3.3 |
| Eso40 | 68.6 | 0.3 | 4.8 | 72.5 | 0.7 | 4.3 |
| Vpz40 | 86.7 | 49.8 | 2.6 | 72.7 | 20.7 | 4.3 |
| Pan40 | 45.9 | 0.0 | 7.5 | 56.3 | 0.1 | 6.2 |
| Lan30 | 49.9 | 0.0 | 7.0 | 42 | 0.0 | 8.0 |
| Ome40 | 49.4 | 1.5 | 7.1 | 39.9 | 0.6 | 8.2 |
| Lan60 | 60.0 | 4.7 | 5.8 | 34 | 0.6 | 8.9 |
| Ome20 | 30.9 | 0.0 | 7.5 | 33.3 | 0.0 | 9.0 |
| Rab20 | 25.1 | 0.3 | 10.0 | 26.5 | 0.1 | 9.8 |
| Rab10 | 15.4 | 0.1 | 11.2 | 25.8 | 0.5 | 9.9 |
| Placebo | 0.3 | 0.0 | 13.0 | 0.0 | 0.0 | 13.0 |
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 40 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; SUCRA, surface under the cumulative ranking curve; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
In the sensitivity analysis, 28 studies were included with the proportions of both severe and non-severe EE at a baseline of >0%. The results showed that all PPIs and P-CABs showed a significantly better 4-week healing rate than the placebo. The superiority was observed in respective groups: Ilaprazole 10 mg qd versus Rabeprazole 10 mg bid and Omeprazole 20 mg qd; Esomeprazole 40 mg qd versus Pantoprazole 40 mg, Omeprazole 20 mg qd and Lansoprazole 30 mg qd; Vonoprazan 40 mg qd versus Rabeprazole 10 mg bid (Supplemental Table S2). Vonoprazan 40 mg qd was ranked as the best treatment with a SUCRA value of 87.3, followed by Ilaprazole 10 mg qd with a SUCRA value of 75.8 (Table 4).
Table 4.
The results of SUCRA in the sensitivity analysis.
| Treatment | 4-Week healing rate | 8-Week healing rate | ||||
|---|---|---|---|---|---|---|
| SUCRA | PrBest | MeanBank | SUCRA | PrBest | MeanBank | |
| Teg50 | 61.4 | 5.7 | 6.8 | 81.3 | 26.5 | 3.8 |
| Kev20 | 47.2 | 2 | 8.9 | 79.3 | 21.3 | 4.1 |
| Teg100 | 75 | 17.1 | 4.7 | 74.9 | 14.6 | 4.8 |
| Vpz20 | 66.9 | 1.6 | 6 | 73.7 | 2.2 | 4.9 |
| Ila10 | 75.8 | 4.1 | 4.6 | 71.7 | 2.5 | 5.3 |
| Vpz40 | 87.3 | 51.4 | 2.9 | 67.4 | 9.9 | 5.9 |
| Eso40 | 68.3 | 0.1 | 5.8 | 64.9 | 0.1 | 6.3 |
| Fex40 | 72.6 | 15.8 | 5.1 | 55.6 | 22.6 | 7.7 |
| Pan40 | 43.1 | 0 | 9.5 | 50.0 | 0 | 8.5 |
| Lan30 | 40.3 | 0 | 9.9 | 37.2 | 0 | 10.4 |
| Ome40 | 50.3 | 1.5 | 8.4 | 36.4 | 0.1 | 10.5 |
| Lan60 | 36.5 | 0.5 | 10.5 | 30.3 | 0.1 | 11.5 |
| Ome20 | 28.3 | 0 | 11.8 | 29.8 | 0 | 11.5 |
| Rab20 | 31.4 | 0.1 | 11.3 | 24.0 | 0 | 12.4 |
| Rab10 | 15.7 | 0.1 | 13.6 | 23.4 | 0.2 | 12.5 |
| Placebo | 0 | 0 | 16 | 0.2 | 0 | 16 |
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 40 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; SUCRA, surface under the cumulative ranking curve; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
In the subgroup analysis on patients without severe EE at baseline, all PPIs and P-CABs showed a significantly better 4-week healing rate than the placebo, and no significant differences were observed between any other two groups (Supplemental Table S3). Vonoprazan 40 mg qd was ranked as the best treatment with a SUCRA value of 90.7, followed by Lansoprazole 30 mg qd with a SUCRA value of 74.2, and Keverprazan 20 mg qd with a SUCRA value of 73.7 (Table 5).
Table 5.
The results of SUCRA in the subgroup analysis on patients without severe baseline grade.
| Treatment | 4-Week healing rate | 8-Week healing rate | ||||
|---|---|---|---|---|---|---|
| SUCRA | PrBest | MeanBank | SUCRA | PrBest | MeanBank | |
| Kev20 | 73.7 | 20.5 | 2.8 | 91.3 | 70.2 | 1.7 |
| Vpz40 | 90.7 | 73 | 1.7 | 76.2 | 14 | 2.9 |
| Lan30 | 74.2 | 4.5 | 2.8 | 66.6 | 0.7 | 3.7 |
| Rab20 | – | – | – | 63.7 | 14.5 | 3.9 |
| Vpz20 | 50 | 1.1 | 4.5 | 57.1 | 0.6 | 4.4 |
| Ome20 | 45.4 | 0.2 | 4.8 | 45.0 | 0.0 | 5.4 |
| Eso40 | 36.5 | 0.5 | 5.4 | 34.4 | 0.0 | 6.2 |
| Pan40 | 29.6 | 0.3 | 5.9 | 15.6 | 0.0 | 7.7 |
| Placebo | 0.0 | 0.0 | 8.0 | 0.1 | 0.0 | 9.0 |
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 40 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; SUCRA, surface under the cumulative ranking curve; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
In the subgroup analysis on patients with severe EE at baseline, all PPIs and P-CABs showed a significantly better 4-week healing rate than the placebo, and Vonoprazan 20 mg qd and Esomeprazole 40 mg qd showed a significantly higher rate than Lansoprazole 30 mg qd and Omeprazole 20 mg qd (Supplemental Table S4). Vonoprazan 20 mg qd was ranked as the best treatment with a SUCRA value of 85.1, followed by Vonoprazan 40 mg qd with a SUCRA value of 84.1 (Table 6).
Table 6.
The results of SUCRA in the subgroup analysis on patients with severe baseline grade.
| Treatment | 4-Week healing rate | 8-Week healing rate | ||||
|---|---|---|---|---|---|---|
| SUCRA | PrBest | MeanBank | SUCRA | PrBest | MeanBank | |
| Kev20 | 57.1 | 11.9 | 4.4 | 89.6 | 71.0 | 1.9 |
| Vpz20 | 85.1 | 27.2 | 2.2 | 75.8 | 11.0 | 3.2 |
| Eso40 | 70.8 | 2.1 | 3.3 | 74.4 | 3.8 | 3.3 |
| Vpz40 | 84.1 | 55.3 | 2.3 | 55.0 | 8.6 | 5.0 |
| Pan40 | 47.9 | 3.2 | 5.2 | 48.8 | 0.7 | 5.6 |
| Rab20 | – | – | – | 44.4 | 4.1 | 6.0 |
| Lan30 | 41.2 | 0.0 | 5.7 | 41.8 | 0.0 | 6.2 |
| Ome20 | 33.8 | 0.0 | 6.3 | 40.1 | 0.0 | 6.4 |
| Lan60 | 30.1 | 0.3 | 6.6 | 30.0 | 0.7 | 7.3 |
| Placebo | 0.0 | 0.0 | 9.0 | 0.0 | 0.0 | 10.0 |
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 40 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; SUCRA, surface under the cumulative ranking curve; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
8-Week healing rate
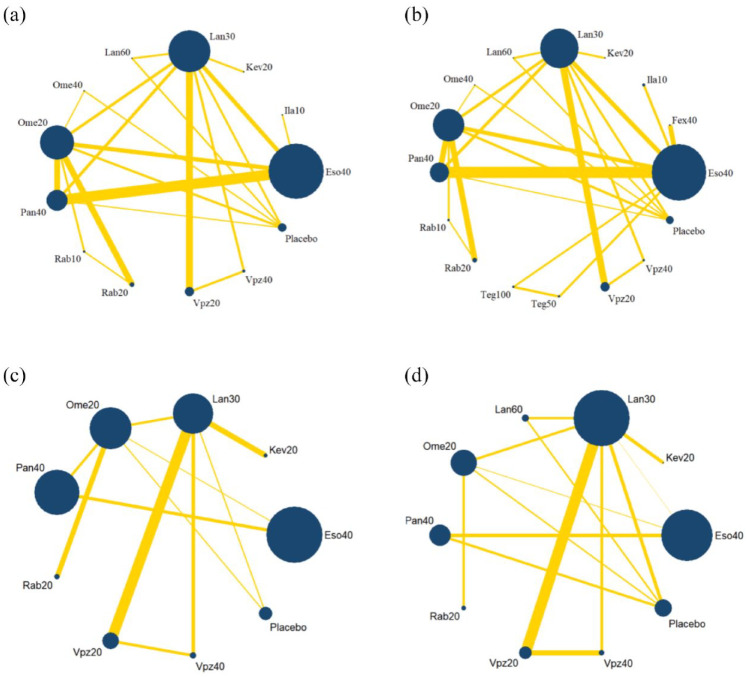
The network plot is shown in Figure 3. No inconsistency was detected in the main, sensitivity, or subgroup analysis (Table 2). The funnel plot is shown in Supplemental Figure S3, indicating that publication bias was acceptable.
Figure 3.
Network plots for 8-week healing rate. (a) Main analysis. (b) Sensitivity analysis. (c) Subgroup analysis on patients without severe baseline grade. (d) Subgroup analysis on patients with severe baseline grade.
Eso40, Esomeprazole 40 mg qd; Fex40, Fexuprazan 40 mg qd; Ila10, Ilaprazole 10 mg qd; Kev20, Keverprazan 20 mg qd; Lan30, Lansoprazole 30 mg qd; Lan60, Lansoprazole 60 mg qd; Ome 40, Omeprazole 40 mg qd; Ome 20, Omeprazole 20 mg qd; Pan40, Pantoprazole 40 mg qd; Rab10, Rabeprazole 10 mg bid; Rab20, Rabeprazole 20 mg qd; Teg100, Tegoprazan 100 mg qd; Teg50, Tegoprazan 50 mg qd; Vpz20, Vonoprazan 20 mg qd; Vpz40, Vonoprazan 40 mg qd.
In the main analysis, 28 studies were included with the proportions of severe EE at a baseline of >10%. All PPIs and P-CABs showed a significantly better 8-week healing rate than the placebo; Vonoprazan 20 mg qd (OR: 2.11, 95% CI: 1.16–3.85) and Esomeprazole 40 mg qd (OR: 1.73, 95% CI: 1.27–2.36) showed significantly higher rates than Omeprazole 20 mg qd; Vonoprazan 20 mg qd (OR: 1.88, 95% CI: 1.15–3.07) and Esomeprazole 40 mg qd (OR: 1.54, 95% CI: 1.09–2.18) also showed significantly higher rates than Lansoprazole 30 mg qd (Supplemental Table S5). Vonoprazan 20 mg qd (OR: 2.60, 95% CI: 1.01–6.68) also showed significantly higher rates than Rabeprazole 20 mg qd; Keverprazan 20 mg qd ranked best with a SUCRA value of 84.7, followed by Ilaprazole 10 mg qd with a SUCRA value of 82.0 (Table 3).
In the sensitivity analysis, 31 studies were included with the proportions of both severe and non-severe EE at baseline of >0%. All PPIs and P-CABs showed a significantly better 8-week healing rate than the placebo; Vonoprazan 20 mg qd (OR: 2.12, 95% CI: 1.17–3.82), Ilaprazole 10 mg qd (OR: 2.04, 95% CI: 1.06–3.94), and Esomeprazole 40 mg qd (OR: 1.74, 95% CI: 1.28–2.36) showed significantly higher rates than Omeprazole 20 mg qd; Vonoprazan 20 mg qd (OR: 1.88, 95% CI: 1.16–3.05) and Esomeprazole 40 mg qd (OR: 1.54, 95% CI: 1.10–2.17) also showed significantly higher rates than Lansoprazole 30 mg qd; Vonoprazan 20 mg qd showed a significantly better healing rate than Rabeprazole 20 mg qd (Supplemental Table S6). Tegoprazan 50 mg qd ranked best with a SUCRA value of 81.3, followed by Keverprazan 20 mg qd with a SUCRA value of 79.3 (Table 4).
In the subgroup analysis on patients without severe EE at baseline, all PPIs and P-CABs showed a significantly better 8-week healing rate than the placebo; Keverprazan 20 mg qd (OR: 23.82, 95% CI: 1.11–508.71), Lansoprazole 30 mg qd (OR: 3.18, 95% CI: 1.56–6.47), Omeprazole 20 mg qd (OR: 2.06, 95% CI: 1.19–3.57), and Esomeprazole 40 mg qd (OR: 1.69, 95% CI: 1.14–2.50) showed significantly higher rates than Pantoprazole 40 mg qd; Lansoprazole 30 mg qd (OR: 1.88, 95% CI: 1.02–3.47) also showed significantly higher rates than Esomeprazole 40 mg qd (Supplemental Table S7). Keverprazan 20 mg qd ranked best with a SUCRA value of 91.3, followed by Vonoprazan 40 mg qd with a SUCRA value of 76.2 (Table 5).
In the subgroup analysis on patients with severe EE at baseline, all PPIs and P-CABs showed a significantly better 8-week healing rate than the placebo, and no significant differences were observed between any other two groups (Supplemental Table S8). Keverprazan 20 mg qd ranked best with a SUCRA value of 89.6, followed by Vonoprazan 20 mg qd with a SUCRA value of 75.8 (Table 6).
Discussion
The results of this network meta-analysis demonstrated the efficacy of all kinds of P-CABs and PPIs in treating EE. All P-CABs and PPIs were more effective than placebo. Keverprazan 20 mg qd was found to have the highest healing rate in 8-week treatment, for both severe and non-severe EE patients, and Vonoprazan 40 mg had a relatively higher healing rate in 4-week treatment. To our knowledge, this is the first network meta-analysis including all types and the usual and double dosage of P-CABs and PPIs in treating EE. This analysis may therefore provide evidence for clinicians, enabling them to offer better treatment choices to patients with EE.
This network meta-analysis showed a higher efficacy for most P-CABs than PPIs, particularly in patients with severe EE. This finding may support the hypothesis that patients with severe EE benefit more from P-CABs than those with mild-to-moderate EE. 70 P-CABs may have an advantage over PPIs because of their special mechanism of action, but the evidence is insufficient. In the current guideline or consensus, PPIs are still recommended as the first-choice treatment; P-CABs or the optimization of PPI therapy are suggested for patients with PPI resistance.71,72 Further studies were needed, to help inform the appropriate treatment of patients with complaints of differing severity.
In terms of healing rates at 4- and 8-week, the results showed that the pooled 4-week healing rate was significantly statistically lower than the pooled 8-week healing rate for most drugs. Thus, an 8-week treatment may be preferable for treating EE. Under this condition, Keverprazan 20 mg qd is recommended to be the best choice, despite that the sensitivity analysis revealed Keverprazan 20 mg qd was slightly less effective than Tegoprazan 50 mg qd. Only one study included Tegoprazan 50 mg qd, and the proportion of patients with severe EE was much lower (4.3%) than that in the study including Keverprazan 20 mg qd (20.6%). This may be why Tegoprazan 50 mg qd ranked better than Keverprazan 20 mg qd in the 8-week sensitivity analysis. Keverprazan, a new oral P-CAB, was launched in China in February 2023. It was designed based on the structure of Vonoprazan and has a high distribution in the stomach, providing better control of stomach acid. 73 A published clinical trial (ChiCTR2100050136) indicated that the percentages of time of intragastric pH greater than 4 [pH >4 holding-time ratio (HTR)] in Placebo, 20 mg Vonoprazan, and 20 mg Keverprazan groups were 5.6 ± 2.4%, 82.2 ± 12.6%, and 85.0 ± 3.0% on day 1, respectively, and the corresponding night-time HTR values were 3.9 ± 4.7%, 87.9 ± 15.7%, and 99.9 ± 0.0%, respectively. 67 Another phase I study showed that starting 4 h after administration, the pH levels in the 20–60 mg dose groups of Keverprazan were consistently higher than those in the 30 mg Lansoprazole group. Specifically, pH levels were maintained above 6 in the Keverprazan groups after 16 h of administration, which was remarkably higher when compared to Lansoprazole. This head-to-head study therefore demonstrated that the acid suppression effect of Keverprazan at a dose of 20 mg was more potent and stable than 30 mg of Lansoprazole. 74 Tegoprazan was also a novel P-CAB. The pharmacodynamic data showed that the 50, 100, and 200 mg doses of Tegoprazan demonstrated longer HTRs above pH >4 up to 12 h after evening dosing than the Dexlansoprazole group. 75 A randomized, open-label, three-period, six-sequence crossover study showed that night-time intragastric pH greater than four HTRs in 50 mg Tegoprazan, 20 mg Vonoprazan, and 40 mg Esomeprazole groups were 66.0 ± 15.7%, 60.5 ± 13.5%, and 36.1 ± 14.7%, respectively. 76 Further RCT studies, particularly head-to-head studies, are needed to compare Keverprazan and Tegoprazan, to inform the better choice for patients with EE.
In the main analysis, although some drugs, such as Omeprazole showed higher healing rates using double dose compared to standard dose after 4- and 8-week treatment, some drugs such as Vonoprazan and Lansoprazole demonstrated healing rate results unrelated to the dosage after 8-week treatment. Thus, the healing rate of a double dose may not necessarily be higher than that of a standard dose in the initial treatment. In some clinical practice guidelines, a standard dose is often recommended as an initial treatment choice, and a double dose is recommended as a therapeutic strategy for PPI-resistant GERD.71,72 The EE population included in this network meta-analysis only received initial treatment; therefore, the therapeutic advantage of a double dose might not have been observed. Further research is needed to confirm the optimal dosage at different treatment stages.
This study has several limitations. First, literature-based network meta-analysis included heterogeneity and bias based on each study. We integrated RCTs using variable grading scales to identify the severity for sensitivity and subgroup analyses. Thus, the analyses might be biased by the individual grading criteria adopted in each grading scale. Second, the majority of included studies did not report the outcomes according to severity grading under endoscopy so we could not assess the efficacy of all PPIs and P-CABs with different severity of EE. Finally, as the P-CABs are novel, there are few head-to-head trials comparing their efficacy. For example, only one RCT study assessed the efficacy of Keverprazan, Tegoprazan, and Fexuprazan, respectively, which meant a statistically significant difference in the efficacy of different drugs could not be determined. Therefore, further high-quality RCTs of P-CABs are required to confirm the efficacy in treating EE.
Conclusion
Our network meta-analysis suggests that the healing effect of Keverprazan (20 mg qd) ranked best in 8-week treatment, for both severe and non-severe EE patients. Most P-CABs showed a higher healing rate than PPIs, particularly for patients with severe EE. As current evidence comparing PPIs and P-CABs for EE is insufficient, our results may help inform future directions of treatment for EE patients. Furthermore, high-quality RCTs of P-CABs are required to confirm the healing effect in patients with EE.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_17562848241251567 for Potassium-competitive acid blockers and proton-pump inhibitors for healing of erosive esophagitis: a systematic review and network meta-analysis by Yin Liu, Zhifeng Gao and XiaoHua Hou in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_17562848241251567 for Potassium-competitive acid blockers and proton-pump inhibitors for healing of erosive esophagitis: a systematic review and network meta-analysis by Yin Liu, Zhifeng Gao and XiaoHua Hou in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Yin Liu  https://orcid.org/0000-0003-0961-2276
https://orcid.org/0000-0003-0961-2276
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yin Liu, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China.
Zhifeng Gao, Department of Gastroenterology, The First People’s Hospital of Xuzhou, Xuzhou, China.
XiaoHua Hou, Division of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430022 Wuhan, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Yin Liu: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft.
Zhifeng Gao: Data curation; Methodology; Validation; Writing – review & editing.
XiaoHua Hou: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018; 67: 430–440. [DOI] [PubMed] [Google Scholar]
- 2. Locke GR, 3rd. Natural history of nonerosive reflux disease. Is all gastroesophageal reflux disease the same? What is the evidence? Gastroenterol Clin North Am 2002; 31(Suppl. 4): S59–S66. [DOI] [PubMed] [Google Scholar]
- 3. Prasad M, Rentz AM, Revicki DA. The impact of treatment for gastro-oesophageal reflux disease on health-related quality of life: a literature review. Pharmacoeconomics 2003; 21: 769–790. [DOI] [PubMed] [Google Scholar]
- 4. Joish VN, Donaldson G, Stockdale W, et al. The economic impact of GERD and PUD: examination of direct and indirect costs using a large integrated employer claims database. Curr Med Res Opin 2005; 21: 535–544. [DOI] [PubMed] [Google Scholar]
- 5. Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 2005; 40: 275–285. [DOI] [PubMed] [Google Scholar]
- 6. Thomson AB, Chiba N, Armstrong D, et al. The Second Canadian Gastroesophageal Reflux Disease Consensus: moving forward to new concepts. Can J Gastroenterol 1998; 12: 551–556. [DOI] [PubMed] [Google Scholar]
- 7. Hetzel DJ, Dent J, Reed WD, et al. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology 1988; 95: 903–912. [DOI] [PubMed] [Google Scholar]
- 8. Sontag SJ, Kogut DG, Fleischmann R, et al. Lansoprazole prevents recurrence of erosive reflux esophagitis previously resistant to H2-RA therapy. The Lansoprazole Maintenance Study Group. Am J Gastroenterol 1996; 91: 1758–1765. [PubMed] [Google Scholar]
- 9. Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996; 111: 85–92. [DOI] [PubMed] [Google Scholar]
- 10. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017; 152: 706–715. [DOI] [PubMed] [Google Scholar]
- 11. Modiano N, Gerson LB. Risk factors for the detection of Barrett’s esophagus in patients with erosive esophagitis. Gastrointest Endosc 2009; 69: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 12. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013; 108: 308–328; quiz 329. [DOI] [PubMed] [Google Scholar]
- 13. Furuta T, Shirai N, Sugimoto M, et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet 2005; 20: 153–167. [DOI] [PubMed] [Google Scholar]
- 14. Horn JR, Howden CW. Review article: similarities and differences among delayed-release proton-pump inhibitor formulations. Aliment Pharmacol Ther 2005; 22(Suppl. 3): 20–24. [DOI] [PubMed] [Google Scholar]
- 15. Fock KM, Ang TL, Bee LC, et al. Proton pump inhibitors: do differences in pharmacokinetics translate into differences in clinical outcomes? Clin Pharmacokinet 2008; 47: 1–6. [DOI] [PubMed] [Google Scholar]
- 16. Laine L, Sharma P, Mulford DJ, et al. Pharmacodynamics and pharmacokinetics of the potassium-competitive acid blocker vonoprazan and the proton pump inhibitor lansoprazole in US subjects. Am J Gastroenterol 2022; 117: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 17. Abdel-Aziz Y, Metz DC, Howden CW. Review article: potassium-competitive acid blockers for the treatment of acid-related disorders. Aliment Pharmacol Ther 2021; 53: 794–809. [DOI] [PubMed] [Google Scholar]
- 18. Andersson K, Carlsson E. Potassium-competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacol Ther 2005; 108: 294–307. [DOI] [PubMed] [Google Scholar]
- 19. Hori Y, Matsukawa J, Takeuchi T, et al. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- 20. Matsukawa J, Hori Y, Nishida H, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 21. Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther 2011; 339: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi N, Take Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther 2018; 364: 275–286. [DOI] [PubMed] [Google Scholar]
- 23. Chen S, Liu D, Chen H, et al. The efficacy and safety of keverprazan, a novel potassium-competitive acid blocker, in treating erosive oesophagitis: a phase III, randomised, double-blind multicentre study. Aliment Pharmacol Ther 2022; 55: 1524–1533. [DOI] [PubMed] [Google Scholar]
- 24. Sunwoo J, Oh J, Moon SJ, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2018; 48: 206–218. [DOI] [PubMed] [Google Scholar]
- 25. Xiao YL, Zhou LY, Hou XH, et al. Chinese expert consensus on gastroesophageal reflux disease in 2020. J Dig Dis 2021; 22: 376–389. [DOI] [PubMed] [Google Scholar]
- 26. Miyazaki H, Igarashi A, Takeuchi T, et al. Vonoprazan versus proton-pump inhibitors for healing gastroesophageal reflux disease: a systematic review. J Gastroenterol Hepatol 2019; 34: 1316–1328. [DOI] [PubMed] [Google Scholar]
- 27. Yang S, Deng W, Xie Z, et al. Efficacy and safety of proton pump inhibitors versus vonoprazan in treatment of erosive esophagitis: a PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 2022; 101: e31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng Y, Liu J, Tan X, et al. Direct comparison of the efficacy and safety of vonoprazan versus proton-pump inhibitors for gastroesophageal reflux disease: a systematic review and meta-analysis. Dig Dis Sci 2021; 66: 19–28. [DOI] [PubMed] [Google Scholar]
- 29. Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016; 43: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015; 42: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther 2019; 49: 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jørgensen L, Paludan-Müller AS, Laursen DR, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev 2016; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012; 3: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wo JM, Mendez C, Harrell S, et al. Clinical impact of upper endoscopy in the management of patients with gastroesophageal reflux disease. Am J Gastroenterol 2004; 99: 2311–2316. [DOI] [PubMed] [Google Scholar]
- 36. Wu JC, Sung JJ, Chan FK, et al. Helicobacter pylori infection is associated with milder gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2000; 14: 427–432. [DOI] [PubMed] [Google Scholar]
- 37. Shiwaku H, Sato H, Shimamura Y, et al. Risk factors and long-term course of gastroesophageal reflux disease after peroral endoscopic myotomy: a large-scale multicenter cohort study in Japan. Endoscopy 2022; 54: 839–847. [DOI] [PubMed] [Google Scholar]
- 38. Genta RM, Spechler SJ, Kielhorn AF. The Los Angeles and Savary–Miller systems for grading esophagitis: utilization and correlation with histology. Dis Esophagus 2011; 24: 10–17. [DOI] [PubMed] [Google Scholar]
- 39. Pandolfino JE, Vakil NB, Kahrilas PJ. Comparison of inter- and intraobserver consistency for grading of esophagitis by expert and trainee endoscopists. Gastrointest Endosc 2002; 56: 639–643. [DOI] [PubMed] [Google Scholar]
- 40. Sontag SJ, Hirschowitz BI, Holt S, et al. Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: the U.S. Multicenter Study. Gastroenterology 1992; 102: 109–118. [DOI] [PubMed] [Google Scholar]
- 41. Hatlebakk JG, Berstad A, Carling L, et al. Lansoprazole versus omeprazole in short-term treatment of reflux oesophagitis. Results of a Scandinavian multicentre trial. Scand J Gastroenterol 1993; 28: 224–228. [DOI] [PubMed] [Google Scholar]
- 42. Corinaldesi R, Valentini M, Belaïche J, et al. Pantoprazole and omeprazole in the treatment of reflux oesophagitis: a European multicentre study. Aliment Pharmacol Ther 1995; 9: 667–671. [DOI] [PubMed] [Google Scholar]
- 43. Mössner J, Hölscher AH, Herz R, et al. A double-blind study of pantoprazole and omeprazole in the treatment of reflux oesophagitis: a multicentre trial. Aliment Pharmacol Ther 1995; 9: 321–326. [DOI] [PubMed] [Google Scholar]
- 44. Castell DO, Richter JE, Robinson M, et al. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. The Lansoprazole Group. Am J Gastroenterol 1996; 91: 1749–1757. [PubMed] [Google Scholar]
- 45. Mee AS, Rowley JL. Rapid symptom relief in reflux oesophagitis: a comparison of lansoprazole and omeprazole. Aliment Pharmacol Ther 1996; 10: 757–763. [DOI] [PubMed] [Google Scholar]
- 46. Earnest DL, Dorsch E, Jones J, et al. A placebo-controlled dose-ranging study of lansoprazole in the management of reflux esophagitis. Am J Gastroenterol 1998; 93: 238–243. [DOI] [PubMed] [Google Scholar]
- 47. Dekkers CP, Beker JA, Thjodleifsson B, et al. Double-blind comparison [correction of double-blind, placebo-controlled comparison] of rabeprazole 20 mg vs. omeprazole 20 mg in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. The European Rabeprazole Study Group. Aliment Pharmacol Ther 1999; 13: 49–57. [DOI] [PubMed] [Google Scholar]
- 48. Vcev A, Stimac D, Vceva A, et al. Pantoprazole versus omeprazole in the treatment of reflux esophagitis. Acta Med Croatica 1999; 53: 79–82. [PubMed] [Google Scholar]
- 49. Delchier JC, Cohen G, Humphries TJ. Rabeprazole, 20 mg once daily or 10 mg twice daily, is equivalent to omeprazole, 20 mg once daily, in the healing of erosive gastrooesophageal reflux disease. Scand J Gastroenterol 2000; 35: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 50. Kahrilas PJ, Falk GW, Johnson DA, et al. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther 2000; 14: 1249–1258. [DOI] [PubMed] [Google Scholar]
- 51. Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo-controlled, randomized clinical trial. Pantoprazole US GERD Study Group. Am J Gastroenterol 2000; 95: 3071–3080. [DOI] [PubMed] [Google Scholar]
- 52. Dupas JL, Houcke P, Samoyeau R. Pantoprazole versus lansoprazole in French patients with reflux esophagitis. Gastroenterol Clin Biol 2001; 25: 245–250. [PubMed] [Google Scholar]
- 53. Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 2001; 96: 656–665. [DOI] [PubMed] [Google Scholar]
- 54. Castell DO, Kahrilas PJ, Richter JE, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol 2002; 97: 575–583. [DOI] [PubMed] [Google Scholar]
- 55. Howden CW, Ballard ED, 2nd, Robieson W. Evidence for therapeutic equivalence of lansoprazole 30 mg and esomeprazole 40 mg in the treatment of erosive oesophagitis. Clin Drug Investig 2002; 22: 99–109. [DOI] [PubMed] [Google Scholar]
- 56. Gillessen A, Beil W, Modlin IM, et al. 40 mg pantoprazole and 40 mg esomeprazole are equivalent in the healing of esophageal lesions and relief from gastroesophageal reflux disease-related symptoms. J Clin Gastroenterol 2004; 38: 332–340. [DOI] [PubMed] [Google Scholar]
- 57. Fennerty MB, Johanson JF, Hwang C, et al. Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2005; 21: 455–463. [DOI] [PubMed] [Google Scholar]
- 58. Labenz J, Armstrong D, Lauritsen K, et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther 2005; 21: 739–746. [DOI] [PubMed] [Google Scholar]
- 59. Pace F, Annese V, Prada A, et al. Rabeprazole is equivalent to omeprazole in the treatment of erosive gastro-oesophageal reflux disease. A randomised, double-blind, comparative study of rabeprazole and omeprazole 20 mg in acute treatment of reflux oesophagitis, followed by a maintenance open-label, low-dose therapy with rabeprazole. Dig Liver Dis 2005; 37: 741–750. [DOI] [PubMed] [Google Scholar]
- 60. Lightdale CJ, Schmitt C, Hwang C, et al. A multicenter, randomized, double-blind, 8-week comparative trial of low-dose esomeprazole (20 mg) and standard-dose omeprazole (20 mg) in patients with erosive esophagitis. Dig Dis Sci 2006; 51: 852–857. [DOI] [PubMed] [Google Scholar]
- 61. Schmitt C, Lightdale CJ, Hwang C, et al. A multicenter, randomized, double-blind, 8-week comparative trial of standard doses of esomeprazole (40 mg) and omeprazole (20 mg) for the treatment of erosive esophagitis. Dig Dis Sci 2006; 51: 844–850. [DOI] [PubMed] [Google Scholar]
- 62. Vcev A, Begić I, Ostojić R, et al. Esomeprazole versus pantoprazole for healing erosive oesophagitis. Coll Antropol 2006; 30: 519–522. [PubMed] [Google Scholar]
- 63. Bardhan KD, Achim A, Riddermann T, et al. A clinical trial comparing pantoprazole and esomeprazole to explore the concept of achieving ‘complete remission’ in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007; 25: 1461–1469. [DOI] [PubMed] [Google Scholar]
- 64. Zheng RN. Comparative study of omeprazole, lansoprazole, pantoprazole and esomeprazole for symptom relief in patients with reflux esophagitis. World J Gastroenterol 2009; 15: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xue Y, Qin X, Zhou L, et al. A randomized, double-blind, active-controlled, multi-center study of Ilaprazole in the treatment of reflux esophagitis. Clin Drug Investig 2016; 36: 985–992. [DOI] [PubMed] [Google Scholar]
- 66. Xue Y, Qin X, Zhou L, et al. A randomized, double blind, controlled, multi center study of ilaparazole in the treatment of reflux esophagitis-phase III clinical trial. Contemp Clin Trials 2018; 68: 67–71. [DOI] [PubMed] [Google Scholar]
- 67. Xiao Y, Zhang S, Dai N, et al. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut 2020; 69: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Laine L, DeVault K, Katz P, et al. Vonoprazan versus lansoprazole for healing and maintenance of healing of erosive esophagitis: a randomized trial. Gastroenterology 2023; 164: 61–71. [DOI] [PubMed] [Google Scholar]
- 69. Lee KN, Lee OY, Chun HJ, et al. Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitis. World J Gastroenterol 2022; 28: 6294–6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 2010; 335: 231–238. [DOI] [PubMed] [Google Scholar]
- 71. Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022; 117: 27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Iwakiri K, Fujiwara Y, Manabe N, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2021. J Gastroenterol 2022; 57: 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou S, Xie L, Zhou C, et al. Keverprazan, a novel potassium-competitive acid blocker: multiple oral doses safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy subjects. Clin Transl Sci 2023; 16: 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou S, Xie L, Zhou C, et al. Keverprazan, a novel potassium-competitive acid blocker: single ascending dose safety, tolerability, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Eur J Pharm Sci 2023; 190: 106578. [DOI] [PubMed] [Google Scholar]
- 75. Han S, Choi HY, Kim YH, et al. Comparison of pharmacodynamics between tegoprazan and dexlansoprazole regarding nocturnal acid breakthrough: a randomized crossover study. Gut Liver 2023; 17: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang E, Kim S, Kim B, et al. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol 2022; 88: 3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_17562848241251567 for Potassium-competitive acid blockers and proton-pump inhibitors for healing of erosive esophagitis: a systematic review and network meta-analysis by Yin Liu, Zhifeng Gao and XiaoHua Hou in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_17562848241251567 for Potassium-competitive acid blockers and proton-pump inhibitors for healing of erosive esophagitis: a systematic review and network meta-analysis by Yin Liu, Zhifeng Gao and XiaoHua Hou in Therapeutic Advances in Gastroenterology