Abstract
Herpes simplex virus type 1 (HSV-1) mutants that are attenuated for neurovirulence are being used for the treatment of cancer. We have examined the safety of G207, a multimutated replication-competent HSV-1 vector, in mice. BALB/c mice inoculated intracerebrally or intracerebroventricularly with 107 PFU of G207 survived for over 20 weeks with no apparent symptoms of disease. In contrast, over 80% of animals inoculated intracerebrally with 1.5 × 103 PFU of HSV-1 wild-type strain KOS and 50% of animals inoculated intracerebroventricularly with 104 PFU of wild-type strain F died within 10 days. Similarly, after intrahepatic inoculation of G207 (3 × 107 PFU) all animals survived for over 10 weeks, whereas no animals survived for even 1 week after inoculation with 106 PFU of KOS. After intracerebroventricular inoculation, LacZ expression was initially observed in the cells lining the ventricles and subarachnoid space; expression decreased until almost absent within 5 days postinfection, with no apparent loss of ependymal cells. G207 DNA could be detected by PCR in the brains of mice 8 weeks after intracerebral inoculation; however, no infectious virus could be detected after 2 days. As a model for latent HSV in the brain, we used survivors of an intracerebral inoculation of HSV-1 KOS at the 50% lethal dose. Inoculation of a high dose of G207 at the same stereotactic coordinates did not result in reactivation of detectable infectious virus or symptoms of disease. We conclude that G207 is safe at or above doses that were efficacious in mouse tumor studies.
Herpes simplex virus type 1 (HSV-1) is a neurotropic DNA virus which infects a wide range of cell types in different animals. Natural infections either follow a lytic, replicative cycle or establish latency. Latency is characterized by the long-term persistence of viral DNA in latently infected neurons, the lack of infectious or replicating virus, the lack of viral gene expression except for the latency-associated transcripts (LATs), and in some situations episodic reactivation of infectious virus (74). In humans, the natural host, HSV-1 causes a number of diseases, including gingivostomatitis and pharyngitis after primary oral-facial infection, recurrent herpes labialis, genital herpes, keratitis after eye infection, disseminated visceral infections in immunocompromised patients, hepatitis, and encephalitis after spread to the central nervous system (CNS) (13).
HSV encephalitis is the most common CNS viral infection, occurring in two to three persons per million (13). Brain pathology is usually localized to the temporal lobe and limbic system, with asymmetric necrotizing encephalitis, inflammation, and hemorrhage (19, 33). The majority of cases of encephalitis occur in patients with recurrent HSV who are seropositive at onset of disease. Even with acyclovir therapy, mortality is about 20%, and about 20% of survivors have long-term morbidity, including cognitive abnormalities (23, 51).
A number of animal models of HSV encephalitis involving spread from peripheral infection (3, 80) or direct inoculation in the CNS (11, 39, 50) have been developed. Direct inoculation into the olfactory bulb in rabbits and rats leads to encephalitis, with virus isolatable from the temporal cortex (69) and surviving animals exhibiting learning deficits (3, 53). In addition, encephalitis has been induced in rabbits by reactivation of latent HSV established by intranasal inoculation of a neurovirulent strain (75). About a quarter of the reactivated rabbits developed behavioral changes (lethargy and unresponsiveness), and a similar proportion had gross necrotic lesions in the cortex within 1 to 2 weeks postreactivation (75).
Considerable progress has been made in understanding latency in the sensory ganglia of the peripheral nervous system (PNS). Unfortunately, latency in the CNS is much less characterized. The role of CNS latency in pathogenesis has become an important issue with the demonstration of HSV DNA in human brain tissue from patients without encephalitis. HSV DNA was detected in brains from 14 of 40 HSV-seropositive patients, mostly in the olfactory bulb, pons, and medulla, (2), and from 6 of 22 patients with nonneurologic disease and 5 of 22 patients with Alzheimer's disease (68). PCR primers in these studies could not differentiate HSV-1 from HSV-2. In a study of 109 human corpses at forensic postmortem, 16% were positive for HSV-1 DNA in the olfactory bulbs, whereas 72% of the trigeminal ganglia were positive (43). In animal models, HSV DNA can often be detected in the CNS of animals surviving HSV-induced encephalitis (17, 67).
Attenuated replication-competent mutants of HSV-1 have been shown to be efficacious in the treatment of a variety of experimental tumor models (1, 5, 6, 30, 49, 56, 63, 65, 88). We have created a second-generation, multimutated HSV-1, G207, that is currently in clinical trial for the treatment of recurrent gliomas. G207 has deletions of both copies of the γ34.5 gene (RL1) and an Escherichia coli lacZ insertion that inactivates the ICP6 gene (UL39) (57). Mutations in γ34.5 causes a large decrease in neurovirulence, so that after intracerebral (i.c.) inoculation in mice, the 50% lethal dose (LD50) is >107 PFU within the standard 3-week observation period (10, 47). In prior studies, we were unable to detect an LD50 for G207 after i.c. inoculation in mice or Aotus nancymae primates (25, 57). However, as part of the preclinical evaluation of these vectors for use in tumor therapy, we expanded on these studies and examined other potential pathological consequences of G207 inoculation. Other safety concerns include inoculation into the ventricles of the brain, leakage of virus into the bloodstream, or infection of susceptible peripheral organs. Another issue to consider is whether i.c. inoculation of G207 could lead to reactivation of, or complementation or recombination with, a patient's latent virus to cause disease. It has been shown in mice that mixed infections with two nonlethal nonneuroinvasive HSV-1 strains can result in a lethal infection and spread to the brain (29). To address this, we used a mouse model involving i.c. inoculation of wild-type HSV-1 strain KOS and subsequent challenge of the survivors with a high dose of G207 at the same i.c. location. The present studies found that G207 caused no detectable disease in mice at titers many logs above the lethal dose of the parental virus, HSV-1 strain F.
MATERIALS AND METHODS
Viruses.
HSV-1 G207 (57), parental wild-type strain F (obtained from B. Roizman, University of Chicago, Chicago, Ill.), wild-type strain 17syn+ (obtained from J. Subak-Sharpe, Institute of Virology, Glasgow, United Kingdom), and wild-type strain KOS1.1 (obtained from D. Knipe, Harvard Medical School, Boston, Mass.) were propagated on mycoplasma-free Vero (African green monkey kidney) cells (American Type Culture Collection). Virus stocks were prepared by infecting subconfluent monolayers of Vero cells cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% heat-inactivated fetal calf serum (IFCS) (HyClone, Logan, Utah) at a multiplicity of infection of 0.01. Infected cells were incubated at 34 or 37°C and harvested when total cytopathic effect was observed. After a freeze-thaw/sonication regimen, cell debris was removed by low-speed centrifugation (2,000 × g at 4°C for 10 min) and virus (F and G207) was concentrated by high-speed centrifugation (45,000 × g at 4°C for 60 min). The viral pellet was resuspended in virus buffer (VB; 150 mM NaCl, 20 mM Tris [pH 7.5]) and titered by plaque assay on Vero cells. Viral stocks were stored at −80°C and thawed rapidly for use. Mock extracts were prepared identically, except that VB was used in place of virus during the infection step.
Intracerebral inoculation of HSV-1.
Three-week-old BALB/c female mice, obtained from the National Cancer Institute (Frederick, Md.), were anesthetized with a 250-μl intraperitoneal (i.p.) injection of sodium pentobarbital solution (84% bacteriostatic saline, 10% sodium pentobarbital [50 mg/ml; Abbott Laboratories, Chicago, Ill.], 6% ethyl alcohol) and placed in a KOPF stereotactic frame. A small burr hole was made 1 mm rostral of the bregma and 2 mm right of midline, and a beveled 29-gauge Hamilton needle was used to inoculate 2 to 5 μl of virus at a depth of 2 mm from the skull surface over a period of 10 min. After another 3 min, the needle was slowly withdrawn (26). Animals were monitored for 6 weeks to 1 year postinfection (p.i.). All procedures involving animals were approved by the Georgetown University Animal Care and Use Committee.
Three-week-old BALB/c female mice were inoculated i.c. with increasing amounts of KOS virus diluted in VB and monitored for 21 days. The PFU/LD50 ratio was calculated (84). At 56 days p.i., a subset of surviving animals was sacrificed and the presence of HSV DNA in the brain was determined by PCR. Sixty days after KOS inoculation, the surviving mice (5 × 102 to 20 × 102 PFU) were randomly divided into groups and inoculated i.c. at the same stereotactic coordinates with 5 μl of G207 (107 PFU) in VB or VB alone.
Intracerebroventricular (i.c.v.) inoculation of HSV-1.
Ten- to twelve-week-old BALB/c female mice were anesthetized by i.p. injection of 250 μl of sodium pentobarbital solution and placed in a KOPF stereotactic frame. A 2-mm burr hole was made 0.8 mm anterior and 1 mm off midline. A Hamilton microliter syringe containing G207 virus (107 PFU) or mock extract in 10 μl was introduced to a 2-mm depth, and virus was injected slowly over 10 min. To confirm that an i.c.v. injection occurred, two mice in each group were injected with Evans blue (Sigma) dye (2% in saline) and sacrificed at the end of surgery.
For 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry, mice were anesthetized with 500 μl of sodium pentobarbital solution and perfused transcardially with cold 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed, postfixed for 2 h in 4% paraformaldehyde, cut in half coronally, and stained with X-Gal substrate solution (56). Brain fragments were washed in PBS, cryoprotected overnight in 30% sucrose, sectioned on a cryostat at 10 or 40-μm thickness, mounted on gelatin-coated slides, and counterstained with carmalum or hematoxylin and eosin.
Intravenous (i.v.) inoculation of G207.
Three-week-old BALB/c female mice were anaesthetized by i.p. injection of 250 μl of sodium pentobarbital solution. Virus (100 μl) was administered by percutaneous puncture of the lateral tail vein.
Intrahepatic (i.h.) inoculation of G207.
Four- to five-week-old BALB/c mice were inoculated with 30 μl of virus or PBS into the right hepatic lobe as described elsewhere (58).
Titration of virus from the brain.
Mice were sacrificed at different times following G207 challenge (1, 2, 4, 7, 14, and 30 days; two mice per time point) by lethal injection (500 μl of sodium pentobarbital solution). Brains were removed and quick-frozen. Brain homogenates were made in DMEM–10% FCS at a 10% wt/vol) ratio, using a Thomas pestle tissue grinder (Thomas Scientific, Swedesboro, N.J.). After centrifugation at 700 × g at 4°C for 10 min, the supernatants were serially diluted in PBS–1% IFCS, and viral titer on Vero cells was determined.
PCR amplification.
Brain tissue (∼25 mg) was isolated from the right frontal cortex (inoculation site), and DNA was purified using a QIAamp tissue kit (QIAGEN, Chatsworth, Calif.) as described by the manufacturer. Amplification reactions were performed in 50-μl volumes containing DNA, 2.0 mM MgCl2, 200 μM each deoxyribonucleoside triphosphate (Perkin-Elmer), 1 μM each primer (Gibco BRL, Gaithersburg, Md.), and 2.5 U of Taq polymerase (Perkin-Elmer) with PCR buffer II (Perkin-Elmer). Primer sequences and their expected product sizes are shown in Table 1. The conditions for PCR amplification were 94°C (denaturation) for 90 s, annealing at 55°C for 60 s, and extension at 72°C for 120 s (35 cycles) on a PTC-100 thermal controller (MJ Research Inc., Watertown, Mass.). Amplification products were separated by electrophoresis on a 2.5% NuSieve agarose (FMC) gel in Tris-acetate-EDTA (TAE) buffer and visualized by ethidium bromide fluorescence. To determine the sensitivity of the PCR assay, known amounts of virus was mixed with uninfected mouse brain, and DNA was purified and amplified as described above.
TABLE 1.
Primer pairs used in this study
| Namea | Gene | Primer sequenceb | PCR product size (bp) | Reference |
|---|---|---|---|---|
| RR (ICP6) | UL39 | 5′ GACAG CCATA TCCTG AGC 3′ | 221 | 15 |
| 3′ GCCAG CAGTT GCTAG ACACT CA 5′ | ||||
| TK | UL23 | 5′ CTGCA GATAC CGCTC CGTAT T 3′ | 273 | 46 |
| 3′ TACCC TACCG CCAGC TTCTA C 5′ | ||||
| gE | US8 | 5′ GAGATGCGAATATACGAAT 3′ | 320 | 18 |
| 3′ TAAAC CGGCT CGGGT GGGTG 5′ | ||||
| LacZ | 5′ TTGCT GATTC GAGGG GTTAA CCGTC ACGAG 3′ | 300 | 64 | |
| 3′ TAGCA TTAGT GGCGT CACAC TAGTAG ACCA 5′ | ||||
| Fx | 5′ AGACT CTTAC CTTTG GGGAT A 3′ | 127 | 40 | |
| 3′ TCGAT TACTT CGTGA CCAGT G 5′ |
Fx is a single-copy mouse gene, RR, TK, and gE amplify HSV-1 sequences, and LacZ is unique to G207.
Sequences listed 5′ to 3′ are upstream primers; 3′-to-5′ sequences are downstream primers.
RESULTS
Safety of G207 in BALB/c mice.
The safety of G207 inoculation in young BALB/c mice was determined after four different routes of inoculation: i.c., i.c.v., i.v., and i.h. The first three are likely sites of viral spread after inoculation of brain tumors in situ. Intracerebral inoculation of 107 PFU of G207 or mock extract resulted in no symptomatic evidence of disease during the 1-year observation period (Table 2). In an earlier set of studies from this laboratory (57), we found that 50% of mice died after an i.c. inoculation of 103 PFU of strain F, similar to the reported LD50 (10). The dose for i.c. injection of G207 was the maximum that could be administered in the small volumes required. No HSV-related mortality or morbidity was noted. Ten-week-old BALB/c mice were injected i.v. with G207 (107 PFU) or mock extract and followed for 1 year with no evidence of disease (Table 2). HSV infection of the liver can cause a lethal necrotic hepatitis in both mice and humans (22, 32, 81). Direct injection of G207 into the liver at a very high dose (3 × 107 PFU) caused no disease, whereas injection of 106 PFU of KOS was uniformly lethal.
TABLE 2.
Mortality and morbidity of HSV in BALB/c mice after i.c., i.c.v., i.v., or i.h. injectiona
| Virus | Dose (PFU) | Injection site | Survival (survived/injected) | Time to death (days p.i.) | Observation (wk) |
|---|---|---|---|---|---|
| VB | i.c. | 12/12 | 8 | ||
| Mock extract | i.c. | 8/8 | 52 | ||
| Fb | 1 × 103 | i.c. | 4/8 | 3, 3, 5, 14 | 3 |
| KOS | 1.5 × 103 | i.c. | 0/8 | 4, 4, 5, 5, 5, 5, 6, 7 | |
| 17syn+ | 1 × 102 | i.c. | 2/4 | 4, 5 | 3 |
| G207 | 1 × 107 | i.c. | 7/8 | 140c | 52 |
| Mock | i.c.v. | 8/8 | 28 | ||
| F | 1 × 104 | i.c.v. | 4/8 | 7, 7, 7, 10 | 52 |
| G207 | 1 × 107 | i.c.v. | 10/11 | 168d | 28 |
| VB | i.v. | 4/4 | 52 | ||
| G207 | 1 × 107 | i.v. | 4/4 | 52 | |
| PBSe | i.h. | 3/3 | 11 | ||
| KOSe | 1 × 106 | i.h. | 0/4 | 2, 2, 6, 6 | <1 |
| G207 | 3 × 107 | i.h. | 6/6 | 11 |
Intracerebroventricular injection of G207.
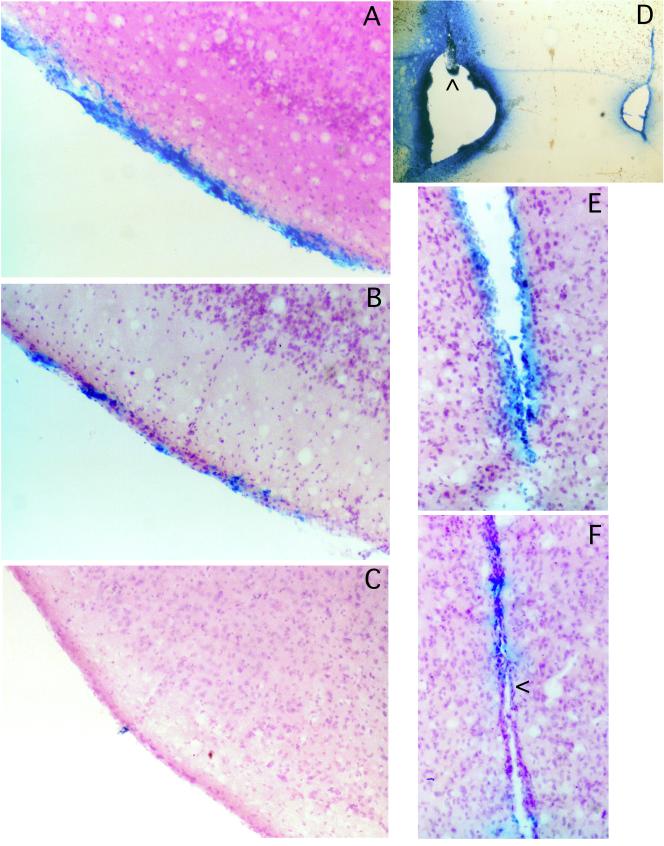
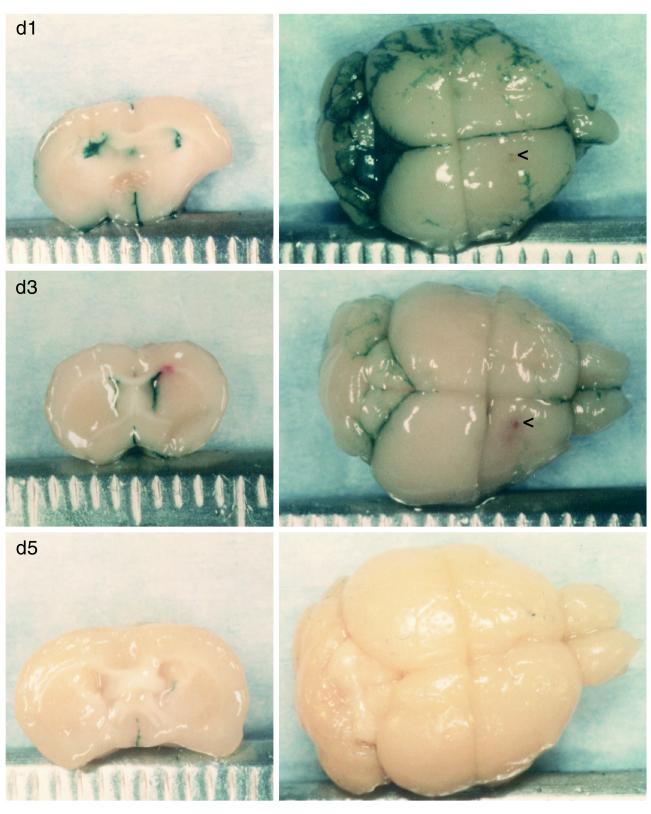
Brain tumors are very often located near ventricles, and therefore it was important to determine the toxicity of G207 inoculation into the ventricles, where the virus could spread throughout the CNS. The accuracy of the i.c.v. injections was confirmed by injecting the first and last animal in a group with Evans blue dye, sacrificing the animal, and visualizing bilateral ventricular staining (see Fig. 2D). No symptoms of disease were noted in any of the animals inoculated with G207 (107 PFU) or mock extract, whereas 50% of the strain F (104 PFU)-inoculated animals died within 10 days (Table 2). The spread of G207 in the ventricular system was examined by sacrificing G207-injected mice on days 1, 3, and 5 p.i. and histochemically staining for LacZ expression (Fig. 1 and 2). In G207, the lacZ transgene is driven by the HSV-1 ICP6 promoter. The most intense X-Gal staining was seen 1 day p.i., in the bilateral ventricles and subarachnoid spaces (Fig. 1, d1; Fig. 2A). By day 3, the contralateral, left ventricle (Fig. 1, d3, left) is much less intensely stained, as are the subarachnoid spaces (Fig. 1, d3, right). There is almost no staining visible by day 5 (Fig. 1, d5; Fig. 2C) and no apparent loss of ependymal cells (Fig. 2F). Sectioning of these brains further demonstrated that LacZ expression was limited to cells in contact with cerebrospinal fluid (CSF) (Fig. 2). No X-Gal-stained cells of obvious neuronal morphology were detected. We have found that X-Gal staining is more sensitive than immunohistochemical staining for β-galactosidase (60).
FIG. 2.
Histology of i.c.v.-injected brains. Mouse brains were isolated 1 (A), 3 (B and E), or 5 (C and F) days after i.c.v. injection of G207 (107 PFU) as in Fig. 1, sectioned, and counterstained. X-Gal staining of meningeal cells is apparent in the subarachnoid space adjacent to the piriform cortex (A to C) and ependymal cells of the third ventricle (E and F). There is no evidence of meningitis or loss of ependymal cells (<, ependymal single-cell layer). The accuracy of the i.c.v. injections was confirmed in separate animals by injection of Evans blue dye (D). Injection site in the ventricle is indicated by an arrowhead; Evans blue dye is apparent in the injected and noninjected, contralateral ventricle.
FIG. 1.
Intracerebroventricular injection of G207. Brains were isolated 1, 3, and 5 days after i.c.v. injection of G207 (107 PFU) and stained for LacZ expression by X-Gal histochemistry. On the left are brains cut coronally through the ventricles; on the right are the upper surface of the brain, with the olfactory bulb to the right and cerebellum to the left. A small red spot is visible at the site of injection (<) in the day 3 (d3) brain below the midline, as well as above the ventricle in the coronal section (left).
Persistent HSV-1 KOS in the brain.
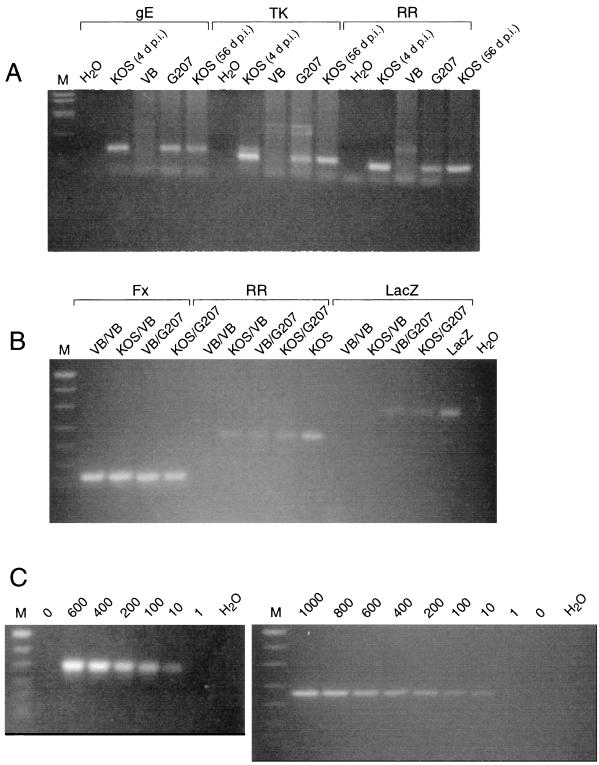
Groups of eight 3-week-old female BALB/c mice were inoculated i.c. with doses of HSV-1 KOS ranging from 2.5 × 102 to 6 × 103 PFU per mouse (Table 3). Most of the mice exhibited signs of CNS disease or encephalitis, such as ruffled fur, hunched posture, hind limb paralysis, and lethargy. Clinical symptoms in mice usually resolved within 48 h of onset, or death occurred. At the highest inoculation doses (3 × 103 and 6 × 103 PFU), HSV-1 infection was fatal in all of the mice. The LD50 of i.c.-inoculated KOS was approximately 1.5 × 103 PFU (Table 3). It should be noted that in a separate experiment, 100% of animals died after i.c. inoculation of 1.5 × 103 PFU KOS (Table 2). At death, infectious virus could be recovered from brain tissue homogenates. The titer of infectious KOS virus recovered after i.c. inoculation was about 3 logs greater than the inoculated dose (3 × 106 PFU after 3 × 103-PFU inoculation and 5 × 106 PFU after 6 × 103-PFU inoculation on day 3 or 4 p.i.). The persistence of viral DNA in brain tissue was detected by PCR amplification, using primers from ICP6 (ribonucleotide reductase [RR]), thymidine kinase (TK), and glycoprotein E (gE), which amplify sequences of 221, 273, and 320 bp, respectively (Table 1). In all brains from KOS-inoculated survivors analyzed at 56 and 112 days p.i., viral DNA was detectable (Fig. 3A; Table 4).
TABLE 3.
Incidence of mortality after i.c. inoculationa
| Inoculation | Viral dose (PFU, 103) | Time of death (days p.i.) | Mortality (%) |
|---|---|---|---|
| KOS | 0.25 | 0 | |
| 0.5 | 4, 5, 5 | 37 | |
| 0.75 | 10, 11 | 25 | |
| 1.5 | 3, 3, 3, 4, 7 | 58 | |
| 2.0 | 5, 5, 6, 7 | 50 | |
| 3.0 | 2, 3, 3, 3, 3, 4, 4, 7 | 100 | |
| 6.0 | 2, 3, 3, 3, 3, 3, 5, 6 | 100 | |
| VB | 0 |
Animals were observed from 60 to 240 days p.i. There were 8 animals at each dose of HSV-1 KOS and 12 animals in the control VB group. These animals were different from those described in Table 2. Surviving animals after inoculations of 5 × 102 to 20 × 102 PFU of KOS were used in the G207 challenge studies (Table 4).
FIG. 3.
(A) Persistence of viral DNA in the mouse brain after i.c. inoculation of HSV-1. DNA was isolated from the brains of the following animals: KOS (6 × 103 PFU) at time of death, 4 days p.i. (d p.i.) (lanes 2, 7, and 12); VB at 56 days postinoculation (lanes 3, 8, and 13); G207 (107 PFU) at 56 days p.i. (lanes 4, 9, and 14) and KOS (103 PFU) survivor at 56 days p.i. [KOS (56 d p.i.)] (lanes 5, 10, and 15). Isolated DNA was amplified with the gE (lanes 1 to 5), TK (lanes 6 to 10), and RR (lanes 11 to 15) primer pairs (Table 1), separated by agarose gel (2.5% NuSieve) electrophoresis, and visualized with ethidium bromide. The controls (lanes 1, 6, and 11) contained water in place of DNA. DNA size markers (M) are as in panel B. (B) Detection of HSV DNA sequences in the brain by PCR. DNA was isolated from the brains of animals listed in Table 4, after injections (first/second) as indicated above the lanes. Isolated DNA was amplified with the Fx (lanes 1 to 4), RR (lanes 5 to 9), and LacZ (lanes 10 to 14) primer pairs (Table 1), separated by agarose gel (2.5% NuSieve) electrophoresis, and visualized with ethidium bromide. The DNA size markers (M) are MW VIII from Boehringer Mannheim (501/489, 404, 320, 242, 190, 147, and 127 bp), the positive control for KOS (lane 9) is 100 PFU equivalents of KOS mixed with mouse brain, the positive control for LacZ (lane 14) is 10 ng of pHCL (31), and the negative control (lane 15) is water in place of DNA. The LacZ primer pair uniquely detects G207 DNA. (C) Detection sensitivity of PCR assay. HSV-1 G207 (left) or KOS (right) was mixed with mouse brain, and DNA was isolated. DNA size markers (M) are as in panel B. G207 DNA (0, 600, 400, 200, 100, 10, and 1 PFU equivalents), and water in place of DNA in lane 8, was amplified with the LacZ primer pair (Table 1) (left); KOS DNA (1,000, 800, 600, 400, 200, 100, 10, 1, and 0 PFU equivalents), and water in place of DNA in lane 10, was amplified with the RR primer pair (Table 1) (right).
TABLE 4.
Detection of viral sequences in the brain by PCRa
| Injection
|
Primer
|
No. of animals
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | RR | TK | gE | LacZ | Fx | PCR | Alive |
| KOS | VB | + | + | + | − | + | 1 | 3 |
| G207 | + | + | + | + | + | 3 | 5 | |
| VB | VB | − | − | − | − | + | 2 | 3 |
| G207 | + | + | + | + | + | 2 | 3 | |
Three-week-old BALB/c female mice were i.c. inoculated with ≤2 × 103 PFU of KOS or VB (first injection); after viral latency had been established (8 to 9 weeks), G207 (107 PFU) or VB was inoculated at the same stereotactic site (second injection). Animals were sacrificed 8 weeks after the second injection, except in the KOS/G207 group, where an animal was sacrificed at 8, 16, and 24 weeks (PCR); DNA was extracted from brain tissue and subjected to PCR with the indicated primers (Table 1). The remainder of the animals were alive and well at 1.7 years.
Attempts to reactivate KOS by superinfection with G207.
We attempted to reactivate i.c. KOS virus or generate neurovirulent recombinants by superinfection with a high dose of G207 (107 PFU). At 2 months after KOS i.c. inoculation, a time when HSV-1 DNA was detectable in the brain by PCR, surviving mice were inoculated with G207 at the same stereotactic coordinates as the prior KOS injection. Some of these animals were followed for over 1 year with no sign of disease, suggesting that neurovirulent recombinants were not generated. The persistence of viral genomes after G207 superinfection was determined by PCR (Table 4). The LacZ primer (Table 1) was used to differentially identify G207 sequences, with the RR, TK, and gE primers common to both G207 and KOS DNA (Fig. 3B). We have been unable to generate useful PCR primers that would uniquely identify KOS DNA and not G207. Amplification of the cellular fatty acid binding protein gene (Fx) served as internal control and to normalize the amount of tissue DNA in all experiments. G207 and likely KOS DNA sequences were detected from 8 to 24 weeks after the second injection with G207 (Table 4). To determine the sensitivity of the PCR assay, we mixed known amounts of KOS or G207 virus with normal mouse brain tissue and extracted the DNA as was done with the inoculated brain samples. We could detect approximately 10 PFU equivalents of KOS and G207 with the ICP6 and LacZ primers, respectively (Fig. 3C).
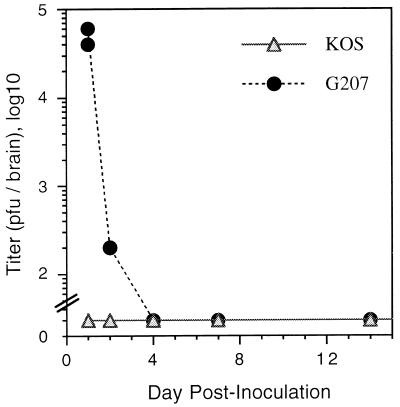
It was possible that KOS reactivated but was unable to cause detectable disease. Therefore, we assayed brain homogenates for infectious virus on days 1, 2, 4, 7, 14, and 30 after G207 challenge by plaque assay (Fig. 4). G207 plaques could be distinguished from KOS plaques because of the expression of β-galactosidase which is histochemically stained with X-Gal. Infectious G207 could be detected 1 day after injection (4 × 105 PFU/brain), with titers declining by day 2 and no plaques detectable at day 4 or later (Fig. 4). We were unable to detect any KOS plaques (white after X-Gal staining) at any of these time points, in contrast to the results obtained after the initial i.c. inoculation of KOS.
FIG. 4.
Recovery of infectious HSV from G207-challenged brains. Whole brain homogenates, from surviving KOS i.c.-inoculated BALB/c mice that were rechallenged with G207 (107 PFU) on day 0, were titered on Vero cells, and the PFU per brain was determined. Plaques that stained with X-Gal were considered G207, and those that did not were considered KOS. No plaques were detected from day 4 onward; no white plaques were found at any time point. The minimal detectable titer was >30 PFU per brain. Two animals were sacrificed at each time point.
DISCUSSION
G207 is a second-generation, attenuated replication-competent HSV vector that was constructed for the original purpose of treating brain tumors. We have shown that G207 is efficacious in the treatment of brain tumors, inhibiting the growth and/or prolonging survival in animal models of human glioma (57), meningioma (88), and breast cancer metastatic to the brain (79). G207 is also effective in treating a variety of solid peripheral human tumors, such as head and neck squamous cell carcinoma, breast adenocarcinoma, prostate adenocarcinoma, and colorectal cancer, after intraneoplastic injection (9, 37, 79, 82). Hepatic portal vein injection of G207 was shown to reduce the number of liver metastases in a syngeneic hepatoma model (37). Recently, we found that i.v. injection of G207 in nude mice was able to induce tumor regression in distant subcutaneous LNCaP and DU-145 human prostate adenocarcinomas (82). In humans, HSV infection of the CNS often causes a rapidly debilitating or fatal encephalitis (13). Blood-borne virus concentrates in the liver and HSV infections of the liver can cause a fulminant hepatitis (22). It is therefore important to demonstrate that inoculation with G207 in the brain and possible leakage to CSF or blood will not pose a significant risk to any patient undergoing therapy.
G207 is derived from HSV-1 strain F, one of the most attenuated laboratory strains (16), and contains engineered mutations in two viral genes affecting neurovirulence, γ34.5 (10, 77, 78) and ICP6 (8, 28, 87). These features were incorporated into G207 in order to maximally decrease neurovirulence and yet maintain the ability of the virus to replicate in tumor cells. We found no morbidity associated with injection of 107 PFU of G207 into the cerebral cortex, ventricles, liver, or tail vein of BALB/c mice. There was no or minimal G207 replication after i.c. inoculation, so that the titer of recoverable infectious virus decreased from the input of 107 PFU to 2 × 102 PFU on day 2 p.i. to undetectable on day 4. This recovered G207 could be remaining input virus or newly synthesized. Intracerebral injection of 106 PFU R3613, the strain F γ34.5Δ parent of G207, was found to cause no deaths, and only 102 PFU/g of brain tissue could be recovered (10).
In contrast, other attenuated, replication-competent HSV vectors being used for cancer therapy are derived from the strain 17+ background, and γ34.5 mutations in this background are more neurovirulent than those in the strain F background (35, 47, 52, 54). Intracerebral injection of 106 PFU of 1716, γ34.5Δ in strain 17+, in both AO rats and BALB/c mice, caused a severe inflammatory response in the brain that was accompanied by excessive weight loss and clinical illness (54). Interestingly, C3H/He mice remained healthy after similar injections (54), even though C3H/He mice are similar to BALB/c mice in sensitivity to wild-type HSV infection (44). After i.c. inoculation of R3616 or 1716, virus is widely distributed via retrograde transport in neurons, and there is some expression of viral antigens and possible viral replication within the first week, which then ceases (48, 55). In studies using G207 as a helper virus for defective HSV vectors, we found that inoculation in the rat substantia nigra resulted in LacZ expression only in cells surrounding the site of inoculation and not at retrograde sites that were identified by expression of the alkaline phosphatase reporter gene on the defective vector (59).
A feature common to both R3616 and 1716 is a predilection to replicate in and destroy ependymal cells in the ventricles. When R3616 was injected into the striatum of mice, virus spread to the ventricles, presumably through leakage into the CSF, so that within 7 days the ependymal cells in the lateral ventricles had disappeared (48). Direct inoculation of 1716 into the ventricles of BALB/c mice also led to a loss of ependymal cells and hydrocephalus but no mortality (34). When 1716 was injected into the ventricles of nude mice, it caused high mortality even at low doses (103 PFU) (41). Intrathecal injection of hrR3 (KOS backbone with same ICP6− mutation as G207) into rats caused significant neurologic morbidity and some mortality (38). In contrast, animals receiving i.c.v. inoculations of G207 exhibited no symptoms of disease. The virus spread throughout the ventricular system, with large numbers of cells bilaterally expressing LacZ within the first day. LacZ expression then rapidly decreased, so that by day 5 postinoculation very few positive cells were detected, with no apparent loss of ependymal cells. LacZ expression is a marker for active viral transcription. In G207, LacZ is driven by the ICP6 promoter, a leaky early (E) or ß promoter, that is activated by VP16 (αTIF) and ICP0 but not ICP4 (14, 24, 76).
Mutations in a number of nonessential viral genes, including the γ34.5 (61, 66, 71, 86), ICP6 (27, 28), TK (12), uracil DNA glycosylase (UL2) (62), and ICP0 (42) genes, affect the ability of HSV to establish latency in the PNS and to be reactivated. In many cases the degree of reactivation and/or establishment of latency is dependent on the in vivo model, animal species, route of infection (e.g., corneal infection/latent trigeminal ganglia, footpad infection/latent dorsal root ganglia, or vaginal infection/latent sacral ganglia) (71), and means of inducing reactivation (e.g., explant cocultivation or in vivo stimuli) (20, 62). Based on the ability of HSV to establish latency, replication-defective mutants of HSV have been used for gene delivery to the CNS, usually by injection into the hippocampal region (4, 21, 64). Unfortunately, in most cases the delivered gene is only transiently expressed. However, viral DNA transcripts and LATs can be detected for long periods of time in the region of injection in the brain (4, 21, 64), suggesting that a latent infection has been established. Intracerebral inoculation of strain 1716 was similarly found to lead to the establishment of a latent infection in large numbers of neurons, as detected by LAT expression (35). We found that G207 DNA was present in the brains of mice 6 months after i.c. inoculation.
With the recent findings of HSV DNA in the CNS of patients dying without encephalitis (2, 43, 68), it became important to determine whether i.c. injection of G207 might lead to reactivation of or recombination with latent virus, leading to a neurovirulent phenotype. Mixed infections in the periphery with two nonneuroinvasive HSV-1 strains can lead to a lethal infection that spreads to the brain at doses logs less than for each of the viruses alone (29). This is due to both complementation and generation of neuroinvasive recombinants (70) and occurs with mixtures of HSV-1 KOS plus F (parental strain of G207) (73). There have been only a few reports of attempts to establish HSV-1 latency models in the CNS, most unable to demonstrate reactivation (35, 45, 72, 75, 85). For example, using a variety of explant protocols with mouse brainstem, Steiner et al. were unable to demonstrate any evidence of reactivation in 44 samples where reactivation from trigeminal ganglia was readily apparent (72). In an HSV-1 latency model established in motor neurons of the spinal cord, explanting spinal cord did not lead to viral reactivation, in contrast to 100% reactivation from latently infected dorsal root ganglia (85). In the two studies where latent HSV-1 could be reactivated from the mouse CNS, virus was identified in explants of 1 of 20 brain hemispheres and none of 20 brainstems, whereas it could be isolated from 38 of 40 trigeminal ganglia (7), and in 4 of 53 explanted spinal cords, whereas 36 of 53 spinal ganglia were positive (36). The difficulty in demonstrating HSV reactivation from the CNS as opposed to the PNS may be due to the ability to explant and maintain viable ganglia from the adult PNS, whereas it is not readily possible to maintain viable tissue from the adult brain in vitro.
To establish a mouse model with persistent HSV DNA in the brain, we i.c. inoculated mice with approximately 1 LD50 of wild-type HSV-1 KOS 1.1, which was about 103.2 PFU. This can be compared to previously determined LD50s of 102.2 PFU for KOS 321 in DBA/2 mice (11) and 101.7 PFU for KOS-63 in BALB/c mice (16). Large amounts of infectious virus (>106 PFU) could be isolated from the brains of animals that did not survive KOS i.c. injection. It seems likely that in those animals that survived, significant numbers of cells were infected. Superinfection with a high dose of G207 (107 PFU), at the same stereotactic coordinates as the initial inoculation with HSV-1 KOS, did not lead to detectable reactivation of infectious virus, nor did it result in disease through the generation of neurovirulent recombinants. While this work was in progress, Wang et al. (83) similarly used an i.c. inoculation of a sublethal dose of HSV-1 KOS to establish latency in the brains of rats. They were unable to detect ICP6 transcripts from this virus, as a marker of reactivation, after i.c. inoculation of hrR3 (ICP6−) at the same stereotactic coordinates (83).
These studies provide strong support for the safety of G207 when inoculated into the brain, liver, or systemic venous circulation of mice, even at high doses. In light of the serious neurologic complications that HSV infection causes, it is rather remarkable that mutations in two viral genes can have such a dramatic effect on the pathogenesis of the virus. It will be important to determine what aspects of viral replication or virus-host interactions are blocked in the CNS or peripheral organs that permit G207 replication and destruction of brain and other solid tumors but not normal tissue.
ACKNOWLEDGMENTS
We thank Bernard Roizman, John Subak-Sharpe, and David Knipe for providing viruses, Anu Iyer for generating HSV stocks, Masahiro Toda and Tomoki Todo for technical assistance, Joseph T. Newsome and the Georgetown University Research Resource Facility staff for assistance with the animals, and Herbert J. Manz for assistance with pathology.
This study was supported in part by grants from the National Institutes of Health (NS32677) and NeuroVir, Inc. Samuel D. Rabkin and Robert L. Martuza are consultants to NeuroVir, Inc., which has a license from Georgetown University to commercialize G207.
REFERENCES
- 1.Andreansky S S, He B, Gillespie G Y, Soroceanu L, Markert J, Chou J, Roizman B, Whitley R J. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baringer J R, Pisani P. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol. 1994;36:823–829. doi: 10.1002/ana.410360605. [DOI] [PubMed] [Google Scholar]
- 3.Beers D R, Henkel J S, Kesner R P, Stroop W G. Spatial recognition memory deficits without notable CNS pathology in rats following herpes simplex encephalitis. J Neurol Sci. 1995;131:119–127. doi: 10.1016/0022-510x(95)00099-n. [DOI] [PubMed] [Google Scholar]
- 4.Bloom D C, Maidment N T, Tan A, Dissette V B, Feldman L T, Stevens J G. Long-term expression of a reporter gene from latent herpes simplex virus in the rat hippocampus. Mol Brain Res. 1995;31:48–60. doi: 10.1016/0169-328x(95)00031-m. [DOI] [PubMed] [Google Scholar]
- 5.Boviatsis E J, Scharf J M, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 6.Brandt C R, Imesch P D, Robinson N L, Syed N A, Untawale S, Darjatmoko S R, Chappell R J, Heinzelman P, Albert D M. Treatment of spontaneously arising retinoblastoma tumors in transgenic mice with an attenuated herpes simplex virus mutant. Virology. 1997;229:283–291. doi: 10.1006/viro.1996.8414. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera C V, Wohlenberg C, Openshaw H, Rey-Mendez M, Puga A, Notkins A L. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature. 1980;288:288–290. doi: 10.1038/288288a0. [DOI] [PubMed] [Google Scholar]
- 8.Cameron J M, McDougall I, Marsden H S, Preston V G, Ryan D M, Subak-Sharpe J H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988;69:2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- 9.Chahlavi A, Todo T, Martuza R L, Rabkin S D. Replication-competent herpes simplex virus vector G207 and cisplatin combination therapy for head and neck squamous cell carcinoma. Neoplasia. 1999;1:162–169. doi: 10.1038/sj.neo.7900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou J, Kern E R, Whitley R J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 11.Chrisp C E, Sunstrum J C, Averill D R J, Levine M, Glorioso J C. Characterization of encephalitis in adult mice induced by intracerebral inoculation of herpes simplex virus type 1 (KOS) and comparison with mutants showing decreased virulence. Lab Investig. 1989;60:822–830. [PubMed] [Google Scholar]
- 12.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey L, Spear P G. Infections with herpes simplex viruses. N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 14.Desai P, Ramakrishnan R, Lin Z W, Osak B, Glorioso J C, Levine M. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J Virol. 1993;67:6125–6135. doi: 10.1128/jvi.67.10.6125-6135.1993. . (Erratum, 68:1264, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dix R D, McKendall R R, Baringer J R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983;40:103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond C W, Eglin R P, Esiri M M. Herpes simplex virus encephalitis in a mouse model: PCR evidence for CNS latency following acute infection. J Neurol Sci. 1994;127:159–163. doi: 10.1016/0022-510x(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 18.Dubin G, Basu S, Mallory D L, Basu M, Tal-Singer R, Friedman H M. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J Virol. 1994;68:2478–2485. doi: 10.1128/jvi.68.4.2478-2485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esiri M M. Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen with the brain. J Neurol Sci. 1982;54:209–226. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- 20.Fawl R L, Roizman B. Induction of reactivation of herpes simplex virus in murine sensory ganglia in vivo by cadmium. J Virol. 1993;67:7025–7031. doi: 10.1128/jvi.67.12.7025-7031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink D J, Sternberg L R, Weber P C, Mata M, Goins W F, Glorioso J C. In vivo expression of β-galactosidase in hippocampal neurons by HSV-mediated gene transfer. Hum Gene Ther. 1992;3:11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- 22.Goodman Z D, Ishak K G, Sesterhenn I A. Herpes simplex hepatitis in apparently immunocompetent adults. Am J Clin Pathol. 1986;85:694–699. doi: 10.1093/ajcp/85.6.694. [DOI] [PubMed] [Google Scholar]
- 23.Gordon B, Selnes O A, Hart J, Jr, Hanley D F, Whitley R J. Long-term cognitive sequelae of acyclovir-treated herpes simplex encephalitis. Arch Neurol. 1990;47:646–647. doi: 10.1001/archneur.1990.00530060054017. [DOI] [PubMed] [Google Scholar]
- 24.Holland L E, Anderson K P, Stringer J R, Wagner E K. Isolation and localization of herpes simplex virus type 1 mRNA abundant before viral DNA synthesis. J Virol. 1979;31:447–462. doi: 10.1128/jvi.31.2.447-462.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter W D, Martuza R L, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome J T, Platenberg R C, Manz H J, Rabkin S D. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter W D, Yazaki T, Rabkin S D, Martuza R L. Replication-competent viral therapy of tumors in the central nervous system. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. Chichester, England: John Wiley & Sons; 1996. pp. 305–317. [Google Scholar]
- 27.Idowu A D, Fraser-Smith E B, Poffenberger K L, Herman R C. Deletion of the herpes simplex virus type 1 ribonucleotide reductase gene alters virulence and latency in vivo. Antiviral Res. 1992;17:145–156. doi: 10.1016/0166-3542(92)90048-a. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson J G, Leib D A, Goldstein D J, Bogard C L, Schaffer P A, Weller S K, Coen D M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 29.Javier R T, Sedarati F, Stevens J G. Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science. 1986;234:746–748. doi: 10.1126/science.3022376. [DOI] [PubMed] [Google Scholar]
- 30.Jia W W, McDermott M, Goldie J, Cyander M, Tan J, Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type 1. J Natl Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- 31.Kaplitt M G, Pfaus J G, Kleopoulos S P, Hanlon B A, Rabkin S D, Pfaff D W. Expression of a functional foreign gene in adult mammalian brain following in vivo transfer via a herpes simplex virus type 1 defective viral vector. Mol Cell Neurosci. 1991;2:320–330. doi: 10.1016/1044-7431(91)90062-s. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman B, Gandhi S A, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24:334–338. doi: 10.1093/clinids/24.3.334. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy P G, Adams J H, Graham D I, Clements G B. A clinico-pathological study of herpes simplex encephalitis. Neuropathol Appl Neurobiol. 1988;14:395–415. doi: 10.1111/j.1365-2990.1988.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 34.Kesari S, Lasner T M, Balsara K R, Randazzo B P, Lee V M, Trojanowski J Q, Fraser N W. A neuroattenuated ICP34.5-deficient herpes simplex virus type 1 replicates in ependymal cells of the murine central nervous system. J Gen Virol. 1998;79:525–536. doi: 10.1099/0022-1317-79-3-525. [DOI] [PubMed] [Google Scholar]
- 35.Kesari S, Lee V M, Brown S M, Trojanowski J Q, Fraser N W. Selective vulnerability of mouse CNS neurons to latent infection with a neuroattenuated herpes simplex virus-1. J Neurosci. 1996;16:5644–5653. doi: 10.1523/JNEUROSCI.16-18-05644.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knotts F B, Cook M L, Stevens J G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973;138:740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooby D A, Carew J F, Halterman M W, Mack J E, Bertino J R, Blumgart L H, Federoff H J, Fong Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999;13:1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 38.Kramm C M, Rainov N G, Sena-Esteves M, Barnett F H, Chase M, Herrlinger U, Pechan P A, Chiocca E A, Breakefield X O. Long-term survival in a rodent model of disseminated brain tumors by combined intrathecal delivery of herpes vectors and ganciclovir treatment. Hum Gene Ther. 1996;7:1989–1994. doi: 10.1089/hum.1996.7.16-1989. [DOI] [PubMed] [Google Scholar]
- 39.Kristensson K. Experimental herpes simplex virus infection in the immature mouse brain. Acta Neuropathol (Berlin) 1976;35:343–351. [PubMed] [Google Scholar]
- 40.Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 41.Lasner T M, Tal-Singer R, Kesari S, Lee V M, Trojanowski J Q, Fraser N W. Toxicity and neuronal infection of a HSV-1 ICP34.5 mutant in nude mice. J Neurovirol. 1998;4:100–105. doi: 10.3109/13550289809113487. [DOI] [PubMed] [Google Scholar]
- 42.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liedtke W, Opalka B, Zimmermann C W, Lignitz E. Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci. 1993;116:6–11. doi: 10.1016/0022-510x(93)90082-a. [DOI] [PubMed] [Google Scholar]
- 44.Lopez C. Genetics of natural resistance to herpesvirus infection in mice. Nature. 1975;258:152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 45.Lynas C, Hill T J, Maitland N J, Love S. Latent infection with the MS strain of herpes simplex virus type 2 in the mouse following intracerebral inoculation. J Neurol Sci. 1993;120:107–114. doi: 10.1016/0022-510x(93)90033-u. [DOI] [PubMed] [Google Scholar]
- 46.Lynas C, Laycock K A, Cook S D, Hill T J, Blyth W A, Maitland N J. Detection of herpes simplex virus type 1 gene expression in latently and productively infected mouse ganglia using the polymerase chain reaction. J Gen Virol. 1989;70:2345–2355. doi: 10.1099/0022-1317-70-9-2345. [DOI] [PubMed] [Google Scholar]
- 47.MacLean A R, ul-Fareed M, Robertson L, Harland J, Brown S M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 48.Markovitz N S, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the γ134.5-mutant of herpes simplex virus 1. J Virol. 1997;71:5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 50.McFarland D J, Sikora E, Hotchin J. The production of focal herpes encephalitis in mice by stereotaxic inoculation of virus. Anatomical and behavioral effects. J Neurol Sci. 1986;72:307–318. doi: 10.1016/0022-510x(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 51.McGrath N, Anderson N E, Croxson M C, Powell K F. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKie E A, Brown S M, MacLean A R, Graham D I. Histopathological responses in the CNS following inoculation with a non-neurovirulent mutant (1716) of herpes simplex virus type 1 (HSV 1): relevance for gene and cancer therapy. Neuropathol Appl Neurobiol. 1998;24:367–372. doi: 10.1046/j.1365-2990.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 53.McLean J H, Shipley M T, Bernstein D I, Corbett D. Selective lesions of neural pathways following viral inoculation of the olfactory bulb. Exp Neurol. 1993;122:209–222. doi: 10.1006/exnr.1993.1121. [DOI] [PubMed] [Google Scholar]
- 54.McMenamin M M, Byrnes A P, Charlton H M, Coffin R S, Latchman D S, Wood M J. A gamma34.5 mutant of herpes simplex 1 causes severe inflammation in the brain. Neuroscience. 1998;83:1225–1237. doi: 10.1016/s0306-4522(97)00513-7. [DOI] [PubMed] [Google Scholar]
- 55.McMenamin M M, Byrnes A P, Pike F G, Charlton H M, Coffin R S, Latchman D S, Wood M J A. Potential and limitations of a γ34.5 mutant of herpes simplex 1 as a gene therapy vector in the CNS. Gene Ther. 1998;5:594–604. doi: 10.1038/sj.gt.3300639. [DOI] [PubMed] [Google Scholar]
- 56.Mineta T, Rabkin S D, Martuza R L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 57.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 58.Miyatake S-I, Tani S, Feigenbaum F, Sundaresan P, Toda H, Narumi O, Kikuchi H, Hashimoto N, Hangai M, Martuza R L, Rabkin S D. Hepatoma-specific antitumor activity of an albumin enhancer/promoter regulated herpes simplex virus in vivo. Gene Ther. 1999;6:564–572. doi: 10.1038/sj.gt.3300861. [DOI] [PubMed] [Google Scholar]
- 59.New K C. Ph.D. thesis. Washington, D.C.: Georgetown University; 1996. [Google Scholar]
- 60.New K C, Gale K, Martuza R L, Rabkin S D. Novel synthesis and release of GABA in cerebellar granule cell cultures after infection with defective herpes simplex virus vectors expressing glutamic acid decarboxylase. Brain Res Mol Brain Res. 1998;61:121–135. doi: 10.1016/s0169-328x(98)00203-4. [DOI] [PubMed] [Google Scholar]
- 61.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. High-dose ocular infection with a herpes simplex virus type 1 ICP34.5 deletion mutant produces no corneal disease or neurovirulence yet results in wild-type levels of spontaneous reactivation. J Virol. 1996;70:2883–2893. doi: 10.1128/jvi.70.5.2883-2893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pyles R B, Thompson R L. Evidence that the herpes simplex virus type 1 uracil DNA glycosylase is required for efficient viral replication and latency in the murine nervous system. J Virol. 1994;68:4963–4972. doi: 10.1128/jvi.68.8.4963-4972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyles R B, Warnick R E, Chalk C L, Szanti B E, Parysek L M. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 64.Ramakrishnan R, Fink D J, Jiang G, Desai P, Glorioso J C, Levine M. Competitive quantitative PCR analysis of herpes simplex virus type 1 DNA and latency-associated transcript RNA in latently infected cells of the rat brain. J Virol. 1994;68:1864–1873. doi: 10.1128/jvi.68.3.1864-1873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Randazzo B P, Kesari S, Gesser R M, Alsop D, Ford J C, Brown S M, MacLean A, Fraser N W. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995;211:94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- 66.Robertson L M, MacLean A R, Brown S M. Peripheral replication and latency reactivation kinetics of the non-neurovirulent herpes simplex virus type 1 variant 1716. J Gen Virol. 1992;73:967–970. doi: 10.1099/0022-1317-73-4-967. [DOI] [PubMed] [Google Scholar]
- 67.Rock D L, Fraser N W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983;302:523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 68.Sanders V J, Waddell A E, Felisan S L, Li X M, Conrad A J, Tourtellotte W W. Herpes simplex virus in postmortem multiple sclerosis brain tissue. Arch Neurol. 1996;53:125–133. doi: 10.1001/archneur.1996.00550020029012. [DOI] [PubMed] [Google Scholar]
- 69.Schlitt M, Lakeman A D, Wilson E R, To A, Acoff R W, Harsh G R d, Whitley R J. A rabbit model of focal herpes simplex encephalitis. J Infect Dis. 1986;153:732–735. doi: 10.1093/infdis/153.4.732. [DOI] [PubMed] [Google Scholar]
- 70.Sedarati F, Javier R T, Stevens J G. Pathogenesis of a lethal mixed infection in mice with two nonneuroinvasive herpes simplex virus strains. J Virol. 1988;62:3037–3039. doi: 10.1128/jvi.62.8.3037-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spivack J G, Fareed M U, Valyi-Nagy T, Nash T C, O'Keefe J S, Gesser R M, McKie E A, MacLean A R, Fraser N W, Brown S M. Replication, establishment of latent infection, expression of the latency-associated transcripts and explant reactivation of herpes simplex virus type 1 gamma 34.5 mutants in a mouse eye model. J Gen Virol. 1995;76:321–332. doi: 10.1099/0022-1317-76-2-321. [DOI] [PubMed] [Google Scholar]
- 72.Steiner I, Mador N, Reibstein I, Spivack J G, Fraser N W. Herpes simplex virus type 1 gene expression and reactivation of latent infection in the central nervous system. Neuropathol Appl Neurobiol. 1994;20:253–260. doi: 10.1111/j.1365-2990.1994.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 73.Stevens J G. HSV-1 neuroinvasiveness. Intervirology. 1993;35:152–163. doi: 10.1159/000150306. [DOI] [PubMed] [Google Scholar]
- 74.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stroop W G, Schaefer D C. Production of encephalitis restricted to the temporal lobes by experimental reactivation of herpes simplex virus. J Infect Dis. 1986;153:721–731. doi: 10.1093/infdis/153.4.721. [DOI] [PubMed] [Google Scholar]
- 76.Sze P, Herman R C. The herpes simplex virus type 1 ICP6 gene is regulated by a ‘leaky’ early promoter. Virus Res. 1992;26:141–152. doi: 10.1016/0168-1702(92)90153-z. [DOI] [PubMed] [Google Scholar]
- 77.Taha M Y, Clements G B, Brown S M. A variant of herpes simplex virus type 2 strain HG52 with a 1.5 kb deletion in RL between 0 to 0.02 and 0.81 to 0.83 map units is non-neurovirulent for mice. J Gen Virol. 1989;70:705–716. doi: 10.1099/0022-1317-70-3-705. [DOI] [PubMed] [Google Scholar]
- 78.Thompson R L, Wagner E K, Stevens J G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983;131:180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- 79.Toda M, Rabkin S D, Martuza R L. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 80.Tomlinson A H, Esiri M M. Herpes simplex encephalitis. Immunohistological demonstration of spread of virus via olfactory pathways in mice. J Neurol Sci. 1983;60:473–484. doi: 10.1016/0022-510x(83)90158-2. [DOI] [PubMed] [Google Scholar]
- 81.Ulbricht A, Farber I, Wutzler P. Herpes simplex virus hepatitis in mice: effects of treatment with trisodium phosphonoformate. Acta Virol. 1985;29:493–498. [PubMed] [Google Scholar]
- 82.Walker J R, McGeagh K G, Sundaresan P, Jorgensen T J, Rabkin S D, Martuza R L. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther. 1999;10:2237–2243. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Guo J, Jia W. Intracerebral recombinant HSV-1 vector does not reactivate latent HSV-1. Gene Ther. 1997;4:1300–1304. doi: 10.1038/sj.gt.3300535. [DOI] [PubMed] [Google Scholar]
- 84.Weil C S. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instructions in their use. Biometrics. 1952;8:249–263. [Google Scholar]
- 85.Wharton S B, Meyers N L, Nash A A. Experimental herpes simplex virus type 1 (HSV-1) infection of the spinal cord and dorsal root ganglia. Neuropathol Appl Neurobiol. 1995;21:228–237. doi: 10.1111/j.1365-2990.1995.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 86.Whitley R J, Kern E R, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Investig. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada Y, Kimura H, Morishima T, Daikoku T, Maeno K, Nishiyama Y. The pathogenicity of ribonucleotide reductase-null mutants of herpes simplex virus type1 in mice. J Infect Dis. 1991;164:1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]
- 88.Yazaki T, Manz H J, Rabkin S D, Martuza R L. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]