Abstract
Although the assembly of herpesviruses has remained an active area of investigation, considerable controversy continues to surround the cellular location of tegument and envelope acquisition. This controversy is particularly evident when the proposed pathways for α- and β-herpesvirus assembly are compared. We have approached this aspect of human cytomegalovirus (HCMV) assembly, specifically, envelopment, by investigating the intracellular trafficking of viral tegument proteins which localize in the cytoplasms of infected cells. In this study we have demonstrated that the virion tegument protein pp28 (UL99), a true late protein, was membrane associated as a result of myristoylation. A mutation in this protein which prevented incorporation of [3H]myristic acid also altered the detergent solubility and intracellular distribution of the protein when it was expressed in transfected cells. Using a panel of markers for intracellular compartments, we could localize the expression of wild-type pp28 to an intracellular compartment which colocalized with the endoplasmic reticulum-Golgi-intermediate compartment (ERGIC), a dynamic compartment of the secretory pathway which interfaces with both the ER and Golgi apparatus. The localization of this viral tegument protein within an early secretory compartment of the cell provided further evidence that the assembly of the HCMV tegument likely includes a cytoplasmic phase. Because pp28 has been shown to be localized to a cytoplasmic assembly compartment in HCMV-infected cells, our findings also suggested that viral tegument protein interactions within the secretory pathway may have an important role in the assembly of the virion.
The assembly of human herpesviruses remains an active area of investigation. Several different models of virion assembly have been proposed (12, 16, 21, 30, 34, 38, 47, 48). Although all models include steps of capsid formation and tegumentation within the nucleus of an infected cell, the intracellular locations of virion tegumentation and envelopment remain contentious. Early models of envelopment suggested that tegumented particles budded through the inner leaflet of the nuclear envelope and entered the secretory pathway through the endoplasmic reticulum (ER) (9, 34, 38, 39). Evidence supporting this model has come from electron microscopic analysis of herpes simplex virus (HSV)- and human cytomegalovirus (HCMV)-infected cells, studies using inhibitors of glycoprotein processing, and characterizations of viruses with mutations in envelope glycoprotein genes (10, 18, 19, 34, 38, 40). More recently, several laboratories have provided evidence that the final envelopment of α-herpesviruses occurs in the cytoplasm following transient envelopment and de-envelopment at the nuclear membrane (7, 16, 47–49). These studies have led to an alternative model of envelopment which appears to be more consistent with recently published findings, yet the intracellular compartment in which the final envelopment of herpesviruses takes place remains incompletely defined and may differ for different members of this family of viruses. The final envelopment of varicella-zoster virus appears to take place in the Golgi apparatus and trans-Golgi network (TGN), based on studies of the trafficking of the major glycoprotein of varicella-zoster virus, gE (12, 16, 21, 31, 51, 52). Similarly, results of early studies were consistent with a similar site of envelopment for pseudorabies virus, and although more recent studies have suggested that the site of virion envelopment might include the TGN, additional compartments within the infected cell may also serve as assembly compartments (15, 44, 47, 48). Our study of the assembly of HCMV was consistent with envelopment occurring in the TGN or an intracellular site contiguous with the TGN (36). This proposed site of HCMV virion assembly was also consistent with several previous studies which indicated that the major glycoprotein of the envelope of HCMV, gB, was cleaved into its mature form by the cellular enzyme furin (28, 45, 46). Thus, evidence from several sources has suggested that the final envelopment of β-herpesviruses likely takes place in the cytoplasm, perhaps in the TGN or in a compartment contiguous to this intracellular compartment.
We have studied the envelopment of HCMV using a different approach. It was our hypothesis that understanding envelopment and assembly could be accomplished by studying the intracellular trafficking of HCMV virion tegument proteins, as these proteins are major constituents of the extracellular particle. Furthermore, a large number of different studies of assembly of several different enveloped RNA viruses have indicated that budding of the subviral particle involves passage of a capsid through a viral glycoprotein anchored within a biological membrane (11). In many cases this step was facilitated by interaction of the capsid and/or envelope glycoprotein with a viral matrix protein (11). Thus, if HCMV was enveloped in the cytoplasm as existing evidence suggested, then it was possible that one or more virion tegument proteins were participating in this intracellular budding event. Interestingly, early ultrastructural studies of HSV and HCMV consistently noted that nonenveloped cytoplasmic particles in HCMV-infected cells were coated with a thick tegument layer but that nonenveloped HSV particles often had the appearance of naked capsids (38). Whether these HSV cytoplasmic capsids were actually devoid of tegument or coated with a layer of unrecognized tegument was not addressed in this study. In the case of HCMV, at least four different tegument or matrix proteins have been shown to localize within the cytoplasms of infected cells in the late phases of the replicative cycle, a time of maximal production of progeny virions (3, 24, 36). We have shown that three of these tegument proteins, pp150 (UL32), pp65 (UL83), and pp28 (UL99), accumulated in a stable juxtanuclear, membranous cytoplasmic structure together with three envelope glycoproteins (36). Thus, any of these three tegument proteins or an as yet unstudied tegument protein may facilitate the cytoplasmic budding and envelopment of the subviral particle of HCMV.
In this study we have characterized the membrane association and intracellular trafficking of pp28 (UL99), one of the first HCMV virion tegument proteins which was shown to be expressed exclusively in the cytoplasms of infected cells (24). This viral protein is an example of a true late protein and is expressed only very late in the infectious cycle during the time of maximal virus production (22, 24). It is a phosphorylated protein component of the virion which can be demonstrated in vacuole-like structures in HCMV-infected human fibroblasts, suggesting that it is membrane associated (24, 36). Interestingly, the HSV protein homolog of HCMV UL99, UL11, has been suggested to be essential for replication of HSV in vitro (1, 26). A UL11 null mutant virus exhibited impaired growth, and in one study it was noted that the UL11 null mutant exhibited deficits in the nuclear egress of the capsid, suggesting that this tegument protein may have a role in virion envelopment (1, 26, 39).
In the present investigation, we studied the intracellular localization of HCMV pp28 (UL99). We have demonstrated that pp28 was a myristoylated protein in infected cells and that this modification accounted for its membrane association in infected cells and in virions. In the absence of other viral proteins, pp28 was not associated with the ER, Golgi apparatus, TGN, or lysosomal compartments but was restricted to the ER-Golgi-intermediate compartment (ERGIC). Additional evidence of the location of pp28 within the ERGIC was provided by colocalization of pp28 with ERGIC 53, a protein which cycles within the ERGIC, when cells were incubated at 15°C or treated with nocadazole. This cellular localization was dependent on myristoylation of pp28. Together, these results demonstrated that this major virion tegument protein was membrane associated and localized to an early compartment of the cellular secretory pathway when it was expressed in the absence of other viral proteins. Because this abundant virion tegument protein is located beneath the virion envelope, our findings provided additional evidence that budding of the subviral particle likely takes place in a nonnuclear cellular membrane derived from the secretory pathway. Furthermore, the localization of pp28 to the ERGIC in the absence of other viral proteins suggested that a viral function was required for localization of pp28 to the cytoplasmic assembly compartment observed in HCMV-infected HF cells (36). Thus, it appears that the assembly program of HCMV has a very complex cytoplasmic phase which likely involves the interaction between tegument proteins and envelope proteins localized to the cellular secretory pathway.
MATERIALS AND METHODS
Cells, recombinant vaccinia viruses, plasmids, and antibodies.
Cos7 and baby hamster kidney (BHK) cells were obtained from Eric Hunter (University of Alabama at Birmingham, Birmingham, Ala.) and propagated as described previously (35). 293T cells were also propagated as described previously (36). Plasmid DNA was introduced into cells by using a modification of the calcium chloride-mediated transfection process (35). Recombinant vaccinia viruses were generated as we have described previously (6). The pp28 (UL99) open reading frame was cloned into the expression plasmid pcDNA3 (Invitrogen, Carlsbad, Calif.). The G2A mutant pp28 was generated by PCR using a mutagenic oligonucleotide which altered the second codon from glycine to alanine (G2A). All PCR products were subjected to nucleotide sequencing, and their sequences were compared to the published sequence of UL99 prior to use.
Monoclonal antibodies (MAbs) reactive with pp28 (UL99) included MAbs 41-18 and 21-21 (36). A rabbit anti-pp28 antiserum which was produced by immunization of rabbits with Escherichia coli-derived pp28 fusion protein was kindly provided by Michael Mach (University of Erlangen, Erlangen, Germany) (27). Antibodies reactive with cellular markers were as follows: (i) RAP, a resident ER protein (29); (ii) ERGIC 53, a recycling ERGIC protein (Peter Hauri, University of Basel, Basel, Switzerland); (iii) GM130, a Golgi protein (29); (iv) LAMP-1, a lysosomal membrane protein (M. Fukuda, La Jolla Cancer Research Center, La Jolla, Calif.); and (v) polyclonal rabbit anti-calreticulin (Affinity BioReagents, Golden, Colo.). Texas red-conjugated wheat germ agglutinin (WGA) was purchased from Molecular Probes, Eugene, Oreg. Fluorochrome-conjugated secondary antibodies were purchased from Southern Biotechnology Associates, Birmingham, Ala.
SDS-PAGE, immunoblotting, immunoprecipitation, and radiolabeling of infected cells.
Electrophoresis under reducing conditions and immunoblotting were carried out as previously described (35). Immunoprecipitations using MAb and formalin-fixed staphylococcal bacteria to collect antigen-antibody complexes were done as described previously (4). Human fibroblasts infected with HCMV strain AD169 were radiolabeled for 16 h with 100 μCi of [3H]myristic acid (New England Nuclear, Boston, Mass.) per ml in medium supplemented with 1% delipidated calf serum (Sigma Chemical Co., St. Louis, Mo.). Following solubilization and preclearing with a nonreactive antibody, the radiolabeled proteins were precipitated overnight. The antigen-antibody complexes were collected, washed, and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following fixation, radiolabeled proteins were detected by fluorography using Biomax film (Kodak, Rochester, N.Y.).
TX114 solubilization.
We modified a phase-separation method originally described by Bordier (5). Infected cells were washed initially in PBS (phosphate-buffered saline, 0.15 M NaCl [pH 7.4]) and then with Tris-buffered saline (TBS) (0.05 M Tris, 0.15 M NaCl, 0.001 M EDTA [pH 7.4]). The cell pellet was resuspended in TBS containing 1.0% Triton X-114 (TX114; Sigma Chemical Co.) and incubated at 4°C for 1 h. Solubilized proteins were then subjected to phase separation by layering the supernatant over a cushion of 7% sucrose in TBS which contained 0.1% TX114. The tube was incubated for 10 min at 30°C and then centrifuged at 400 × g for 4 min. The detergent phase (cloudy phase) pelleted as a droplet on the bottom of the tube. The aqueous phase was carefully removed and extracted a second time with TX114 to a final concentration of 1.0%. The detergent-phase droplet from the second extraction was discarded. The detergent-phase pellet following the first extraction and the pooled aqueous phases were then analyzed by SDS-PAGE followed by immunoblotting.
Immunofluorescence microscopy.
Cos7 cells were grown on 12-mm-diameter coverslips and transfected 20 h after being seeded by a calcium chloride protocol for introduction of DNA (35). The cells on the coverslips were harvested 48 h later and fixed in 2% paraformaldehyde in PBS. The cells were permeabilized in PBS containing 0.05% NP-40 for 10 min at 4°C and then blocked with 20% normal goat serum for 30 min. Primary antibody and secondary antibody development was carried out as described previously (35). The coverslips were then postfixed in 2% paraformaldehyde, and images were captured and digitalized as described previously (36). In some cases transfected cells were incubated in 2 μM nocadazole (Sigma) or 2 μg of Brefeldin A (Sigma) per ml for 1 to 2 h prior to fixation.
RESULTS
pp28 (UL99) is myristoylated and membrane associated in infected cells.
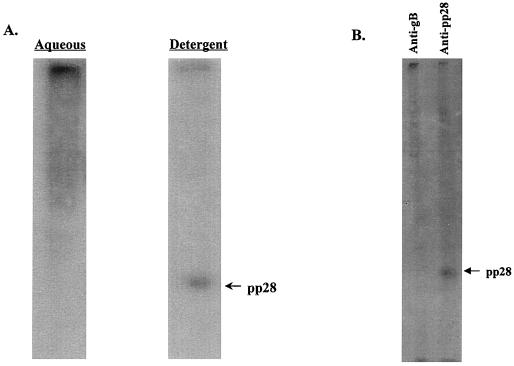
Previous studies have demonstrated that the late virion structural protein pp28 (UL99) remained localized to cytoplasmic vacuoles and membrane structures during productive infection of human fibroblasts (24, 36). Because these earlier findings were based primarily on electron and immunofluorescence microscopy, we investigated the biochemical properties of pp28 within infected cells using detergent solubility in TX114 to provide further evidence for pp28's association with cellular membranes (5). HCMV-infected fibroblasts were harvested 6 days postinfection and solubilized in TX114 at 4°C. The clarified detergent phase (membranes) was then warmed to 30°C and partitioned into a detergent and aqueous phase by low-speed centrifugation. An aliquot from each phase was analyzed by immunoblotting. The majority of pp28 partitioned into the detergent phase, suggesting that within infected cells this viral protein is membrane associated (Fig. 1A). In addition, similar treatment of gradient-purified extracellular virions indicated that the majority of virion pp28 also was associated with the viral envelope (data not shown). Together, these findings were consistent with those of previous studies in which immunofluorescence microscopy suggested that pp28 was membrane associated in HCMV-infected cells (36).
FIG. 1.
pp28 is membrane associated and is myristoylated in AD169-infected HF cells. (A) TX114 partitioning of pp28 in AD169-infected HF cells. AD169-infected cells were solubilized in TX114 and then partitioned into aqueous and detergent phases as described in Materials and Methods. An aliquot from each phase was then subjected to SDS-PAGE, followed by immunoblotting using a pp28 specific MAb to develop the membrane. The migration of pp28 is shown in the right margin. (B) Myristoylation of pp28 in AD169-infected cells. AD169-infected HF cells were radiolabeled with [3H]myristic acid as described in Materials and Methods. The infected cell proteins were then precipitated with a pp28- or gB-specific MAb, and the precipitated proteins were separated by SDS-PAGE. The migration of pp28 is shown in the right margin.
Computer-aided analysis of the predicted primary amino acid sequence of pp28 (UL99) failed to identify regions of the molecule which may serve as hydrophobic membrane-spanning domains. Because of the membrane association of pp28, we next investigated the possibility that other posttranslational modifications, such as myristoylation or palmitoylation, could account for this characteristic of pp28. The presence of a glycine residue following the translation initiation methionine raised the possibility that pp28 was myristoylated. To examine this possibility, HCMV-infected cells were labeled with [3H]myristic acid for 16 h and infected cell proteins were solubilized in detergent-containing buffer. The infected cell proteins were then precipitated with a MAb specific for pp28 or, as a control, a MAb reactive with gB (UL55), and the precipitated proteins were analyzed by SDS-PAGE. After prolonged exposure of the fluorogram, we detected incorporation of the [3H]myristic acid into pp28 but not into gB (Fig. 1B). Both gB and pp28 could be precipitated by the respective MAbs from duplicate virus-infected cell cultures radiolabeled with [35S]methionine (data not shown).
The myristoylation of pp28 is required for its membrane localization within virus-infected cells.
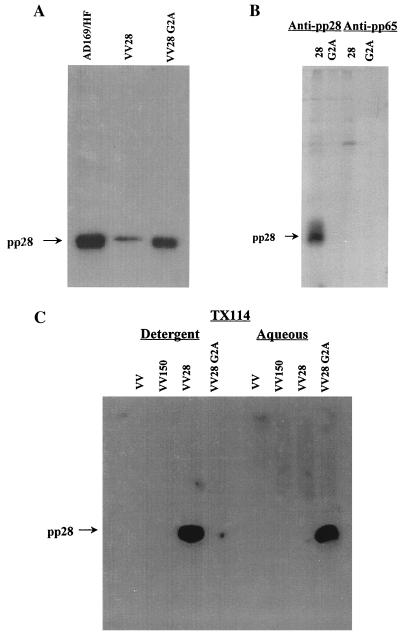
To directly determine whether myristoylation accounted for pp28's membrane association, we compared the solubilities of pp28 in the detergent TX114 and a mutant form of the protein which lacked a glycine residue at position 2. Wild-type pp28 and a mutant pp28 which had a glycine-to-alanine substitution at amino acid position 2 were generated as PCR products and then used to construct the respective recombinant vaccinia viruses. BHK cells infected with either the wild-type-pp28 or the G2A mutant recombinant vaccinia virus expressed a protein which comigrated with pp28 from HCMV-infected fibroblasts as determined by immunoblotting with a MAb specific for pp28 (Fig. 2A). Infected cell proteins from recombinant vaccinia virus-infected cells were radiolabeled with [3H]myristic acid, solubilized, and immunoprecipitated with a MAb specific for pp28 or a control MAb. Substituting an alanine for glycine at position 2 prevented the incorporation of [3H]myristic acid into the protein (Fig. 2B). To determine the effect of myristoylation on the membrane association of pp28, we next examined the partitioning of wild-type pp28 and the G2A mutant protein into the detergent and aqueous phases following TX114 solubilization. As controls for nonspecific reactivity, we included two different vaccinia viruses, a nonrecombinant vaccinia virus and a recombinant vaccinia virus encoding pp150 (UL32). The G→A substitution at amino acid position 2 resulted in the partitioning of the mutant pp28 into the aqueous phase (Fig. 2C). Together, these results demonstrated that pp28 was a myristoylated protein within HCMV-infected cells and that this modification accounted for its localization to intracellular membranes.
FIG. 2.
The membrane association of pp28 is dependent on myristoylation. (A) The G2A mutant pp28 is expressed in recombinant vaccinia virus-infected cells. BHK cells were infected with a recombinant vaccinia virus encoding wild-type pp28 (VV28) or the G2A mutant pp28 (VV28 G2A), and infected cell proteins were analyzed by immunoblotting using a pp28-specific MAb to develop the membranes. As a control, infected cell proteins from AD169-infected cells were also analyzed in an identical manner. The migration of pp28 is shown in the left margin. (B) The G2A pp28 mutation prevents myristoylation of pp28. BHK cells were infected with the recombinant vaccinia viruses encoding either pp28 or the G2A pp28 mutant and radiolabeled with [3H]myristic acid as described in the legend to Fig. 1. Infected cell proteins were then immunoprecipitated with either a pp28- or pp65 (UL83)-specific MAb and analyzed by SDS-PAGE. The migration of pp28 is shown in the left margin. (C) Myristoylation of pp28 is required for membrane association. BHK cells infected with wild-type (VV) or recombinant vaccinia viruses encoding pp150 (UL32, VV150), pp28, or the G2A mutant pp28 were partitioned by TX114 into a aqueous or detergent fraction as described in the legend to Fig. 1. The different fractions were then analyzed by immunoblotting, and the membranes were developed with a pp28-specific MAb. The migration of pp28 is shown in the left margin.
pp28 is localized to the ERGIC when it is expressed in the absence of other viral proteins.
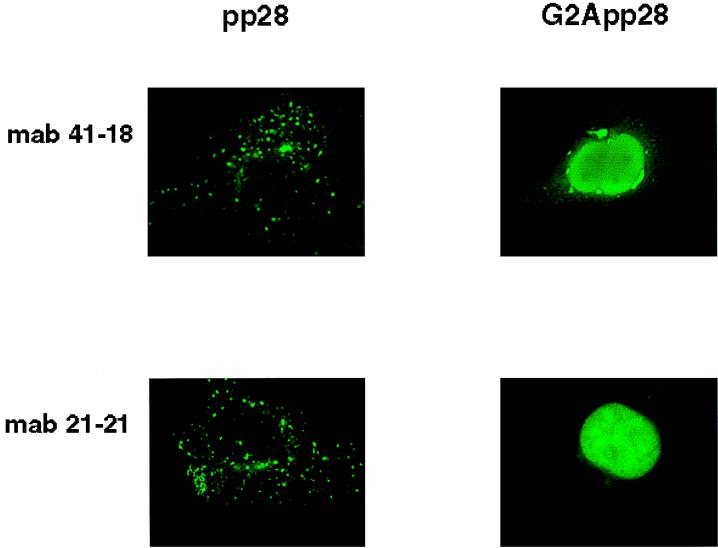
The association of pp28 with cellular membranes was consistent with its localization in cytoplasmic vacuoles and in a recently described membranous compartment which formed in HCMV-infected human fibroblast cells late in infection (36). We next examined the intracellular trafficking of wild-type pp28 and the G2A mutant protein in Cos7 cells transfected with expression plasmids bearing the respective genes. The intracellular distribution of the transiently expressed protein was determined by immunofluorescence microscopy. Wild-type pp28 was expressed in distinct, similarly sized cytoplasmic vacuoles which often surrounded the nuclei (Fig. 3). Although the signal was subtle, the nuclear envelope also appeared to be demarcated by a bead-like distribution of pp28 (Fig. 3, panels with MAb 21-21). The signal from pp28 was also noted to be concentrated asymmetrically in a perinuclear or juxtanuclear distribution (Fig. 3). These observations were confirmed using a second, independently derived MAb (Fig. 3). In contrast, the G2A mutant pp28 was expressed throughout the cell and in this experiment exhibited a prominent nuclear localization (Fig. 3). This localization was in marked contrast to what occurred with wild-type pp28, which was not expressed within nuclei, regardless of the system used to express the recombinantly derived protein, or in the HCMV-infected human fibroblast cells. This finding was also consistent with our results using detergent partitioning and provided further evidence that the myristoylation of pp28 at amino acid position 2 accounted for its intracellular association with membrane-containing vacuoles.
FIG. 3.
Intracellular localization of pp28 is dependent on its myristoylation. Cos7 cells were transfected with an expression plasmid bearing either wild-type pp28 (pp28) or the G2A mutant pp28 (G2App28) and imaged with pp28-specific MAbs and then fluorescein isothiocyanate anti-murine immunoglobulin G antibodies. Note the prominent nuclear distribution of the G2A pp28 protein compared to that of wild-type pp28.
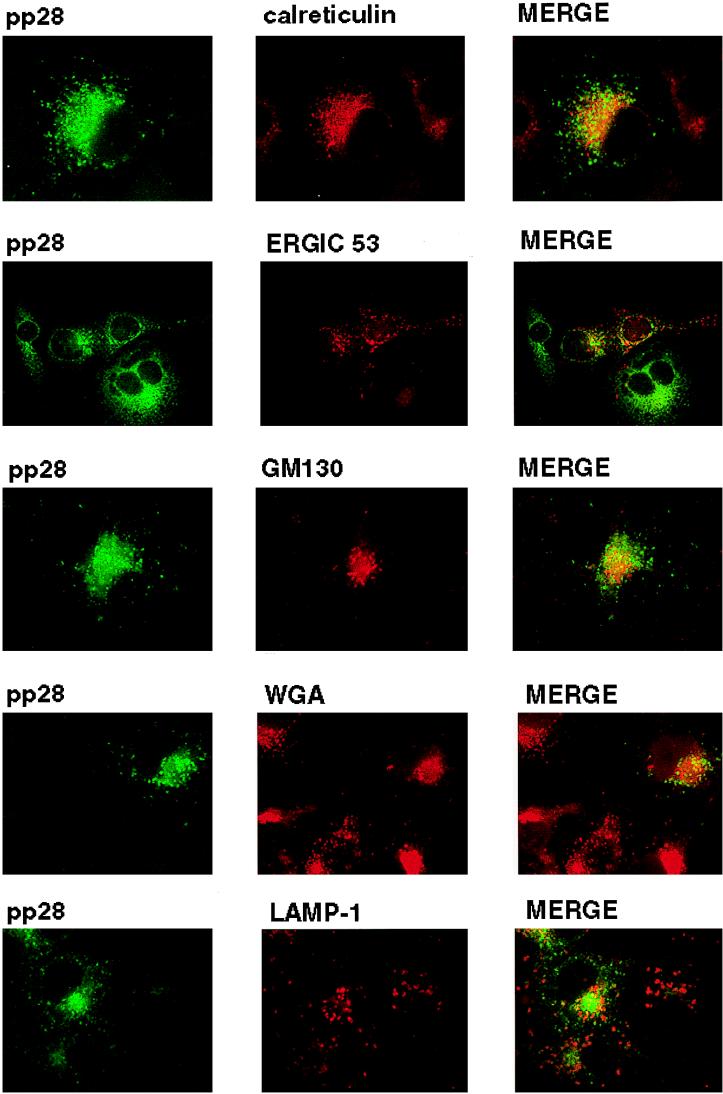
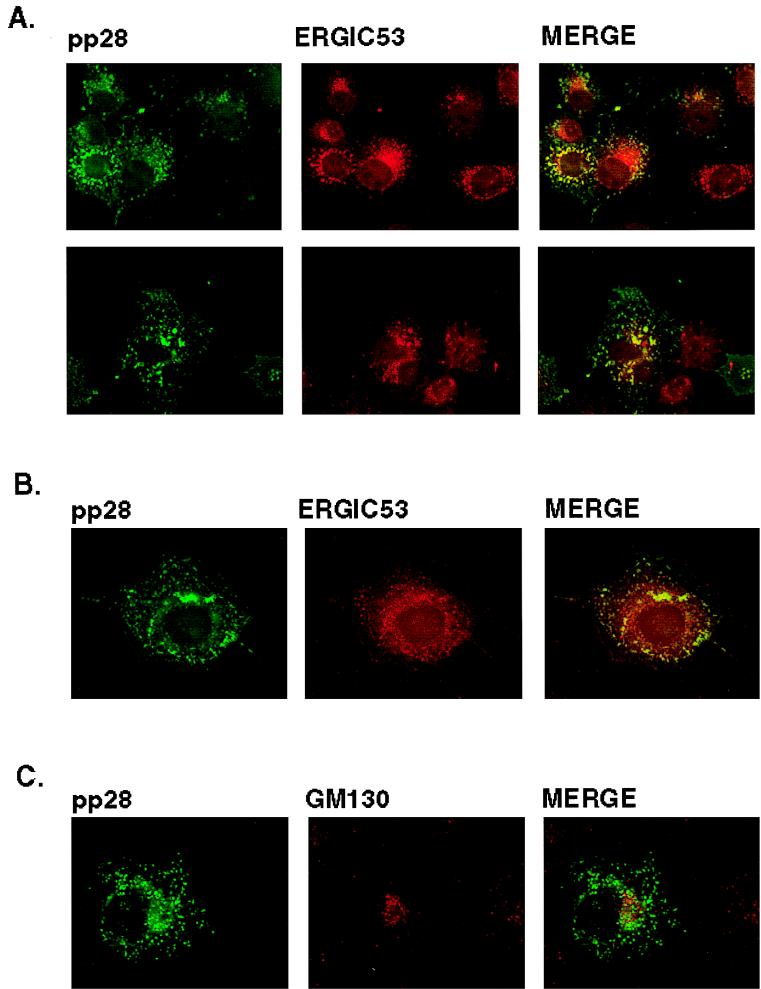
The intracellular distribution of pp28 was consistent with the protein being present in the ER and Golgi apparatus, yet the more peripheral vacuolar distribution was not consistent with localization in these intracellular compartments. To define the intracellular localization of pp28, we utilized transient expression of the protein in Cos7 cells followed by colocalization of the pp28 with known protein markers of intracellular compartments. pp28 failed to colocalize with calreticulin or GM130, resident proteins of the ER and the Golgi apparatus, respectively (Fig. 4). In addition, we failed to observe significant overlap between pp28 and WGA, a marker for the TGN (Fig. 4). Similarly, we failed to colocalize pp28 with LAMP-1, a marker for the lysosomal compartment (Fig. 4). Although some signal overlap was noted for all cell markers, we did not view this overlap as significant colocalization because distinct structures exhibiting signal overlap were not observed in merged images. In contrast, there was partial but less than complete colocalization with ERGIC 53, a protein found within the ERGIC (17). This overlap was most readily seen as limited but distinct colocalization signals from the pp28 and ERGIC 53 that surrounded the nucleus (Fig. 4).
FIG. 4.
Intracellular localization of pp28. Cos7 cells were transfected with an expression plasmid bearing the gene encoding pp28 and then fixed 48 h later as described in Materials and Methods. The cells were reacted with either pp28-specific MAb or polyclonal rabbit anti-pp28 antibodies and the corresponding cell marker. We used the following markers for compartments in the secretory system: (i) calreticulin for the ER, (ii) ERGIC 53 for the ERGIC, (iii) GM130 for the Golgi apparatus, (iv) WGA for the TGN, and (v) LAMP-1 for lysosomes. Colocalization was considered significant when the merged signal revealed a yellow color associated with a distinct cellular site or structure and not in a more generalized location within the cell.
We consistently localized pp28 with ERGIC 53 but not with other cell markers when multiple fields from several experiments were examined. To further investigate the apparent colocalization of pp28 and ERGIC 53, we determined if the intracellular distribution of pp28 was sensitive to incubation of transferred cells at 15°C, an experimental condition which has been shown to decrease the rate of anterograde transport through the ERGIC (29, 43). Incubation of cells at this lower temperature has been shown to result in redistribution of protein components of the ERGIC into the periphery of the cell (29, 43). Incubation of transfected cells at 15°C followed by brief rewarming to 37°C resulted in the redistribution of pp28 from discrete vacuoles with an asymmetric perinuclear accumulation to a more peripheral, lacy pattern which was consistent with the accumulation of this protein in the vesiculotubular complexes of the ERGIC (Fig. 5A). A similar redistribution of ERGIC 53 in many of these structures was also noted, and overlap between the signals from pp28 and ERGIC 53 could be appreciated (Fig. 5A). To confirm the colocalization of pp28 with ERGIC 53, we incubated cells in nocadazole, an agent which depolymerizes cellular microtubules and causes redistribution of intracellular proteins dependent on microtubules for their compartmentalization. Nocadazole caused redistribution of pp28 and ERGIC 53 in Cos7 cells transfected with the pp28 expression plasmid (Fig. 5B). An overlap in the signals from ERGIC 53 and pp28 was also noted in nocadazole-treated cells (Fig. 5B). Finally, ERGIC is contiguous with the Golgi apparatus, and as a result, treatment of cells with the toxin Brefeldin A has been reported to result in partial redistribution or vesiculation of the ERGIC (2, 17, 43). When Cos7 cells transfected with the pp28 expression plasmid were incubated with Brefeldin A, vesiculation of the Golgi apparatus as well as some increased vesiculation of the compartment containing pp28 was seen (Fig. 5C). As noted previously, there was no colocalization between the Golgi marker GM130 and pp28 (Fig. 5C). Together, these findings argued that, when expressed in the absence of other viral functions and under steady-state conditions, pp28 was localized primarily to the ERGIC and not to the Golgi apparatus.
FIG. 5.
pp28 colocalizes with the ERGIC 53 protein. (A) Cos7 cells transfected with the expression plasmid bearing the gene encoding pp28 were incubated for 2 h at 15°C, rewarmed briefly to 37°C, and then fixed as described in Materials and Methods. The cells were then reacted with a rabbit anti-pp28 antiserum or with a MAb reactive with ERGIC 53. Colocalization can be appreciated by the yellow signal surrounding the nucleus. The two panels represent the results of two independent experiments. (B) Cos7 cells transiently expressing pp28 were incubated in medium containing 2 μM nocadazole for 2 h and then fixed and processed as described above. Colocalization can be observed in structures surrounding the nucleus. (C) pp28 does not colocalize with the Golgi apparatus after Brefeldin A treatment. Cos7 cells expressing pp28 were treated with 2 μg of Brefeldin A per ml for 2 to 3 h and then fixed as described in Materials and Methods. The fixed cells were then reacted with a pp28-specific MAb and a rabbit serum reactive with the Golgi marker, GM130, and developed with fluorochrome-conjugated secondary antibodies. Note the vesiculation of the signals from both pp28 and GM130 compared to that of the corresponding panels in Fig. 4, but colocalization was not observed.
DISCUSSION
Previously we and others have shown that pp28 (UL99) remained within the cytoplasm throughout the infectious cycle of HCMV (36). Furthermore, in productively infected human fibroblasts, pp28 has been shown to accumulate in cytoplasmic vacuoles and in a large, membranous juxtanuclear structure which also contained additional tegument proteins and at least three envelope proteins (36). The localization of pp28 to these membranous intracellular compartments could not be explained by examination of its predicted primary sequence. However, the presence of a glycine residue following the translation initiation of methionine suggested that myristoylation of the protein could account for its membrane association. We were able to directly demonstrate that this posttranslational modification was necessary for its association with intracellular membranes. Myristoylation of proteins is thought to occur on free polyribosomes, suggesting that a myristoylated protein may associate nonspecifically with a number of different intracellular membranes (20). Our findings from image analysis did not support such nonspecific association between pp28 and intracellular membranes and indicated more restricted and specific membrane targeting. Consistent with the specific targeting of myristoylated viral matrix proteins to intracellular membranes have been the observations from studies of the myristoylated Gag proteins of type C retroviruses and lentiviruses (8, 14, 33, 37, 42). With Moloney murine leukemia virus (Mo-MuLV), the Gag protein has been shown to be associated with the cytoplasmic face of the plasma membrane (42). The mechanism which permits localization of this protein to the plasma membrane is not entirely understood but may result from the trafficking of the Gag protein with a cellular protein to the plasma membrane or possibly from the cotranslational insertion of Gag into the plasma membrane because of the proximity of the Gag mRNA to the plasma membrane during translation (13, 41, 42). Likewise, the Gag protein of human immunodeficiency virus (HIV) is also myristoylated and has been shown to be targeted to the plasma membrane (8, 14, 50). However, in contrast to the findings with some C-type retroviruses, it appeared that HIV Gag may require an interaction with the envelope glycoprotein for efficient targeting to the plasma membrane (32, 42). Interestingly, mutations in the Gag protein of HIV-1 which prevented myristoylation also prevented assembly of capsid structures and budding of virus, yet had no effect on the proteolytic processing of the Gag polyprotein (14). Thus, it appears that myristoylation of viral matrix protein provides at least one mechanism of membrane association; however, this modification alone is insufficient for targeting the viral matrix protein to a specific intracellular membrane. Although several sequences within the primary sequence of pp28 resemble known intracellular targeting sequences, our preliminary studies with pp28 deletion mutants have failed to identify a specific targeting sequence within this protein.
Previously, studies have shown that the HCMV pp28 homolog of HSV, UL11, was also myristoylated (25). The importance of myristoylation to the intracellular targeting of the HSV UL11 protein has recently been suggested by Bowzard et al. (J. B. Bowzard, R. J. Visalli, C. B. Wilson, E. M. Callahan, J. S. Loomis, R. J. Courtney, and J. W. Wills, Abstr. 24th Int. Herpesvirus Workshop, abstr. 7.021, 1999). Similar to the requirements for the targeting of Mo-MuLV to the plasma membrane, both myristoylation and a signal within the NH2 terminus of this protein have been postulated to be required for targeting to intracellular membranes, specifically the TGN (Bowzard et al., 24th Int. Herpesvirus Workshop). A cluster of basic amino acids in the NH2 terminus of the MoMuLV Gag protein has been suggested to be responsible for the interaction of MoMuLV Gag with the plasma membrane (42). Bowzard et al. noted that an acidic cluster in the amino terminus of HSV UL11 might have a similar role in the intracellular targeting of UL11 to the TGN (Bowzard et al., 24th Int. Herpesvirus Workshop). Those investigators further suggested that this cluster was conserved in UL11 homologs of several different herpesviruses and that it thus may represent a consensus signal for localization to the TGN. Although our results were consistent with specific intracellular targeting of pp28 and although pp28 contained an acidic cluster of amino acids in a location similar to that of the acidic cluster of HSV UL11, the HCMV pp28 (UL99) protein was not localized to the TGN when it was expressed in the absence of other viral proteins. Thus, this region in HCMV pp28 did not appear to have the same targeting function as the homologous region of HSV UL11, at least when UL99 was expressed in the absence of other viral proteins. Additional experiments will be directed towards determining the function of different domains of pp28 in its intracellular trafficking; however, some caution must be applied to the interpretation of these studies, because the intracellular trafficking of these herpesvirus matrix proteins was defined in the absence of other viral functions which could markedly alter the localization of these proteins in infected cells.
Several of our results indicated that pp28 was located in a membranous compartment contiguous or interfacing with the ERGIC. These included the finding that pp28 partially colocalized with ERGIC 53, perhaps the most well-studied marker of the ERGIC, but not with markers for the ER, Golgi apparatus, TGN, or lysosomal compartments (17). ERGIC 53 is a lectin-like protein of 510 amino acids which contains a signal sequence, a transmembrane region, and a dilysine ER retrieval signal at the C terminus (17). Studies of this protein have shown that it cycles between the ER and the cis-Golgi apparatus but that under steady-state conditions it is concentrated within a compartment intermediate between the ER and Golgi apparatus, i.e., the ERGIC (2, 17, 29, 43). Under conditions which inhibited normal intracellular trafficking of ERGIC 53, such as incubation of cells at 15°C followed by rapid rewarming to 37°C, the protein has been shown to exhibit a more peripheral, spotty distribution which often completely surrounds the nucleus (23, 43). This is thought to occur secondarily to redistribution of ERGIC 53 containing vesiculotubular structures to an ER-like peripheral location. Incubation of Cos7 cells transfected with an expression plasmid bearing the gene encoding pp28 at 15°C resulted in a similar dispersion of pp28 into peripheral sites in the cell. In the same experiment, ERGIC 53 was similarly distributed in cells incubated at 15°C and partially colocalized with pp28 under these conditions. Following treatment of cells with nocadazole, pp28 and ERGIC 53 were redistributed into an intracellular compartment which resembled the distribution which was observed following incubation at 15°C, and again colocalization of these proteins was observed. The latter result suggested that the distribution of pp28 was dependent on the integrity of microtubules. Previous studies which have compared the intracellular localizations of the KDEL-R protein and ERGIC 53 have shown that nocadazole inhibited anterograde transport from the ERGIC to the Golgi apparatus but did not prevent redistribution of ERGIC 53 to the ER (43). Finally, treatments with Brefeldin A caused a subtle redistribution of pp28, suggesting that at least some of the intracellular pp28 was associated with the cis-Golgi apparatus, yet not within the same structures as the Golgi protein GM130. This finding was consistent with the distribution of proteins cycling within the ERGIC as has been shown for TAP (p115) and ERGIC 53, proteins localized to the ERGIC (29).
The finding that a herpesvirus tegument protein was targeted to a compartment which interfaced with the ERGIC was unexpected and further underscored the likelihood that HCMV did not acquire its final envelope at the nuclear membrane as was previously proposed for α-herpesviruses. The cellular ERGIC represents an early and very dynamic compartment of the secretory system (2, 17, 23, 29). Although several proteins which under steady-state conditions concentrate within the ERGIC have been described, none has been described to be a resident of this compartment because of the nature of the ERGIC. This compartment likely represents an early maturational stage of the Golgi apparatus, and proteins which do not contain ER retrieval signals, such as the C-terminal dilysine present in ERGIC 53, leave this compartment and concentrate within the Golgi stacks (2, 17). The predicted amino acid sequence of pp28 has a dilysine motif; however, it is positioned at residues 8 and 9 from the C terminus and therefore is unlikely to function as an ER retrieval signal. Although our experimental findings were consistent with the possibility that another ER retrieval signal was responsible for the apparent cycling of pp28 in the ERGIC, we cannot rule out the equally likely alternative possibilities that pp28 was interacting with a cellular protein which was cycling within the ERGIC and that this protein-protein interaction accounted for the intracellular distribution of pp28 in the absence of other viral proteins.
Previously we have shown that in virus-infected cells, three different tegument proteins (including pp28) and three different envelope proteins of HCMV accumulated late in infection in a membranous, juxtanuclear structure which could not be colocalized to the ER, ERGIC, or Golgi apparatus (36). This structure was dependent on the integrity of microtubules as demonstrated by its sensitivity to the microtubule-destabilizing drug nocadazole (36). Therefore, it was of interest that pp28 remained in a distinct, ERGIC-linked compartment and failed to traffic to a similar intracellular location when it was expressed in the absence of other viral proteins. This result indicated that either HCMV infection induced alteration of intracellular trafficking within the secretory pathway or that a specific viral protein or viral function was required for localization of pp28 to the juxtanuclear compartment in productively infected HF cells. We favor the explanation that the transit of pp28 in infected cells was facilitated by other viral proteins because this unique viral protein containing an intracellular structure failed to develop in the absence of productive virus infection. Because pp28 has been classified as a late protein during productive infection, the number of candidate viral proteins which could mediate transport of pp28 to the juxtanuclear site of viral protein localization was extensive. We propose that it was most likely a viral protein which also trafficked through the secretory pathway. If this was the case, then it follows that pp28 interacted with an envelope protein which was eventually targeted to a site of envelopment and virion assembly. Such an interaction would then suggest a role for pp28 in the intracellular budding of HCMV, perhaps similar to the role postulated for myristoylated viral matrix proteins in the assembly and budding of retroviruses.
ACKNOWLEDGMENTS
We thank Michael Mach for critical discussions and Dana Pinson for assistance in preparing the manuscript.
This work was supported by the National Institutes of Health through NIAID grant R01 AI35602 (W.J.B.).
REFERENCES
- 1.Baines J D, Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J Virol. 1992;66:5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannykh S I, Balch W E. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battista M C, Bergamini G, Boccuni M C, Campanini F, Ripalti A, Landini M P. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J Virol. 1999;73:3800–3809. doi: 10.1128/jvi.73.5.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billstrom M A, Britt W J. Postoligomerization folding of human cytomegalovirus glycoprotein B: identification of folding intermediates and importance of disulfide bonding. J Virol. 1995;69:7015–7022. doi: 10.1128/jvi.69.11.7015-7022.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 6.Britt W J, Vugler L, Butfiloski E J, Stephens E B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990;64:1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darlington R W, Moss L H. Herpesvirus envelopment. J Virol. 1968;2:48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lazarro C, Campadelli-Fiume G, Torrisi M R. Intermediate forms of glycoconjugates are present in the envelope of herpes simplex virions during their transport along the exocytic pathway. Virology. 1995;214:619–623. doi: 10.1006/viro.1995.0073. [DOI] [PubMed] [Google Scholar]
- 11.Garoff H, Hewson R, Opstelten D J E. Virus maturation by budding. Microbiol Mol Biol Rev. 1998;62:1171–1190. doi: 10.1128/mmbr.62.4.1171-1190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gielkens A L J, Salden M H L, Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci USA. 1974;71:1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlinger H F, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow H, Weiland F, Jons A, Klupp B G, Karger A, Mettenleitner T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 17.Itin C, Schindler R, Hauri H P. Targeting of protein ERGIC-53 to the ER/ERGIC/cis-Golgi recycling pathway. J Cell Biol. 1995;131:57–67. doi: 10.1083/jcb.131.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson D C, Spear P G. Monesin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson D C, Spear P G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983;32:987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson D R, Bhatnagar R S, Knoll L J, Gordon J I. Genetic and biochemical studies of protein N-myristoylation. Annu Rev Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- 21.Jones F, Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988;62:2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerry J A, Priddy M A, Kohler C P, Staley T L, Weber D, Jones T R, Stenberg R M. Translational regulation of the human cytomegalovirus pp28 (UL99) late gene. J Virol. 1997;71:981–987. doi: 10.1128/jvi.71.2.981-987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klumperman J, Schweizer A, Clausen H, Tang B L, Hong W, Oorschot V, Hauri H P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- 24.Landini M P, Severi B, Furlini G, Badiali D G L. Human cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65-69 by immunoelectron microscopy. Virus Res. 1987;8:15–23. doi: 10.1016/0168-1702(87)90036-0. [DOI] [PubMed] [Google Scholar]
- 25.MacLean C A, Clark B, McGeoch D J. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J Gen Virol. 1989;70:3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- 26.MacLean C A, Dolan A, Jamieson F E, McGeoch D J. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J Gen Virol. 1992;73:539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- 27.Meyer H, Bankier A, Landini M P, Ruger R. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988;62:2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molloy S S, Anderson E D, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 29.Nelson D S, Alvarez C, Gao Y S, Garcia-Mata R, Fialkowski E, Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J Cell Biol. 1998;143:319–331. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster viral gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens R J, Dubay J W, Hunter E, Compans R W. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc Natl Acad Sci USA. 1991;88:3987–3991. doi: 10.1073/pnas.88.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rein A, McClure M R, Rice N R, Luftig R B, Schultz A M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci USA. 1986;83:7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roizman B. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2221–2230. [Google Scholar]
- 35.Sanchez V, Angeletti P C, Engler J A, Britt W J. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J Virol. 1998;72:3321–3329. doi: 10.1128/jvi.72.4.3321-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez V, Greis K D, Sztul E, Britt W J. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol. 2000;74:975–986. doi: 10.1128/jvi.74.2.975-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz A M, Rein A. Unmyristylated Moloney murine leukemia virus Pr65 gag is excluded from virus assembly and maturation events. J Virol. 1989;63:2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith J D, DeHarven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973;12:919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spear P G. Glycoproteins specified by herpes simplex viruses. In: Roizman B, editor. The herpesviruses. Vol. 3. New York, N.Y: Plenum Press; 1985. pp. 315–356. [Google Scholar]
- 40.Steven A C, Spear P G. Herpes capsid assembly and envelopment. In: Chiu W, Burnett R M, Garcea R L, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1997. pp. 312–351. [Google Scholar]
- 41.St. Johnston D. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 42.Suomalainen M, Hultenby K, Garoff H. Targeting of Moloney murine leukemia virus gag precursor to the site of virus binding. J Cell Biol. 1996;135:1841–1852. doi: 10.1083/jcb.135.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang B L, Low S H, Hauri H P, Hong W. Segregation of ERGIC53 and the mammalian KDEL receptor upon exit from the 15 degrees C compartment. Eur J Cell Biol. 1995;68:398–410. [PubMed] [Google Scholar]
- 44.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk H D, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 46.Wan L, Molloy S S, Thomas L, Liu G, Xiang Y, Rybak S L, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 47.Whealy M E, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of Brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whealy M E, Robbins A K, Enquist L W. The export pathway of the pseudorabies virus gB homolog gII involves oligomer formation in the endoplasmic reticulum and protease processing in the Golgi apparatus. J Virol. 1990;64:1946–1955. doi: 10.1128/jvi.64.5.1946-1955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitley A, Bruun B, Minson T, Browne H. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]