Abstract
Introduction:
This study aimed to distinguish isolated hypogonadotropic hypogonadism (IHH) from constitutional delay in growth and puberty (CDGP) by various hormonal tests in both sexes.
Methods:
Boys with testicular volume (TV) <4 ml (14–18 years) and girls with breast B1 stage (13–18 years) were enrolled in this study. A detailed history, clinical examination and hormonal analysis including basal luteinising hormone (LH), follicle-stimulating hormone (FSH), inhibin B, anti-Mullerian hormone (AMH), testosterone (boys), oestradiol (girls), triptorelin stimulation test and 3-day human chorionic gonadotropin (HCG) stimulation test (boys) were performed. All patients were followed for 1.5 years or till 18 years of age. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-offs with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for various hormones to distinguish IHH from CDGP.
Results:
Of 34 children (male: 22 and female: 12), CDGP and IHH were diagnosed in 21 and 13 children, respectively. 4 hours post-triptorelin LH had the highest sensitivity (100%) and specificity (100%) for identifying IHH in both sexes. Basal inhibin B had good sensitivity (male: 85.7% and female: 83.8%) and specificity (male: 93.3% and female: 100%) for diagnosing IHH. 24 hours post-triptorelin testosterone (<34.5 ng/dl), day 4 post-HCG testosterone (<99.7 ng/dl) and 24 hours post-triptorelin oestradiol (<31.63 pg/ml) had reasonable sensitivity and specificity for identifying IHH. Basal LH, FSH and AMH were poor discriminators for IHH in both sexes.
Conclusion:
The best indicator was post-triptorelin 4-hour LH followed by inhibin B, which had a reasonable diagnostic utility to distinguish IHH from CDGP in both boys and girls.
Keywords: Constitutional delay in growth and puberty, anti-Mullerian hormone, HCG stimulation test, inhibin B, isolated hypogonadotropic hypogonadism, triptorelin stimulation test
INTRODUCTION
Puberty consists of a series of events that transform a sexually immature individual to a mature individual capable of reproduction, and it is tightly regulated by genetic, hormonal and environmental factors. Delayed puberty is commonly defined as the lack of development of secondary sexual characteristics, i.e. absence of testicular enlargement by the age of 14 years in boys and lack of breast development by the age of 13 years in girls.[1,2] The most common cause of delayed puberty is a constitutional delay in growth and puberty (CDGP) followed by hypogonadotropic hypogonadism (HH) and hypergonadotropic hypogonadism.[3] It is often very difficult clinically to distinguish CDGP from isolated HH (IHH). CDGP is a normal variant of growth and puberty characterised by delayed bone age, delayed adrenarche, short stature and delayed but spontaneous pubertal development before the age of 18 years. IHH is a cause of HH due to deficiency in or insensitivity to gonadotropin-releasing hormone (GnRH), where the function and anatomy of the anterior pituitary are otherwise normal and secondary causes of HH are absent. Attainment of puberty by the age of 18 years is the gold standard to differentiate between CDGP and IHH. Basal luteinising hormone (LH) and follicle-stimulating hormone (FSH) are poor diagnostic tests to distinguish CDGP from IHH.[4,5,6,7] Prior studies report inhibin B, anti-Mullerian hormone (AMH) and various stimulation tests as diagnostic tools to distinguish these two conditions but with varying results.[4,5,6,8] Very few studies reported human chorionic gonadotropin (HCG) stimulation test to differentiate IHH from CDGP.[9,10] The diagnostic cut-offs indicated by various studies varied widely with marked difference in sensitivity and specificity. To the best of our knowledge, there are only two studies in girls with delayed puberty to distinguish IHH from CDGP.[5,7]
This study aimed to study objectively the diagnostic utility of inhibin B, AMH, GnRH agonist (GnRHa) stimulation test (triptorelin) in both boys and girls and 3-day HCG stimulation test in boys to distinguish IHH from CDGP.
MATERIAL AND METHODS
This study was conducted in the clinics of the Department of Endocrinology, MKCG Medical College, Berhampur, from February 2021 till January 2023. Boys with a testicular volume (TV) <4 ml in the age group of 14–18 years and girls with breast Tanner B1 stage in the age group of 13–18 years were included in the study. Patients with chronic systemic illness and central nervous system (CNS) conditions such as structural and anatomical defects of hypothalamic–pituitary region, intracranial tumours, history of (h/o) cranial irradiation, head trauma and pituitary surgery were excluded from the study. Subjects with multiple pituitary hormone deficiency (MPHD) were excluded as the study was focused on IHH. Subjects with sex steroid replacement within the last 6 months were also excluded.
A total of 40 patients meeting the inclusion and exclusion criteria were enrolled. A detailed history regarding family h/o delayed puberty in parents, cryptorchidism, micropenis, anosmia, chronic medical illness, CNS pathologies, head injury and pituitary surgery was enquired in all subjects. In all subjects, detailed clinical examination including height, weight, body mass index (BMI), arm span, upper segment-to-lower segment (US/LS) ratio, stretched penile length (SPL) and TV were measured by standard procedures.
Routine biochemical investigations such as fasting plasma glucose (FPG), 2 hours post-prandial plasma glucose (PPG), serum creatinine, complete blood count (CBC) and liver function test were performed in all patients using a biochemical analyser (Siemens Autopak 300 APK). In all subjects after an overnight fasting of at least 8 hours, blood was collected at 8–9 AM for the estimation of hormones such as T3, T4, TSH, prolactin, cortisol, LH, FSH, testosterone (boys) and oestradiol (girls). A separate 4 ml of venous blood was collected for the estimation of inhibin B and AMH. It was immediately centrifuged at 2000 rpm and stored at -80°C till analysis. Subsequently, the GnRHa stimulation test was administered by injecting triptorelin acetate (decapeptyl 0.1 mg/ml) at a dose of 100 mgm/m2 (maximum 100 mgm) subcutaneously and blood samples were collected at 4 hours for the measurement of LH and FSH in both sexes and at 24 hours for the measurement of testosterone in boys or oestradiol in girls.[4,5] After 48 hours, the HCG stimulation test was performed in boys by administering HCG 1500 IU/day intramuscularly for 3 days and blood samples were collected 24 hours after the last dose for the estimation of testosterone.[10]
T3, T4, TSH, prolactin, cortisol, LH, FSH, testosterone and oestradiol were estimated by the chemiluminescent immunoassay (CLIA) method (Siemens ADVIA Centaur CP). Inhibin B was estimated using an ELISA Kit, Inhibin B GENLISA, Krishgen Pudgala LLP, with intra-assay and inter-assay coefficients of variation <10% and <12%, respectively. AMH was measured using an ELISA Kit, AMH ELISA, Calbiotech Inc., with intra-assay and inter-assay coefficient of variation <3.75% and <7.86%, respectively. Both were analysed using ELISA Reader Bio-Rad, PR 4100, at 450 nm absorbance.
Bone age was estimated in all by Greulich and Pyle’s chart. Magnetic resonance imaging (MRI) focusing on the hypothalamic–pituitary region, olfactory bulb, sulcus and tract was performed in all cases. All patients were followed at 6-month interval, and detailed clinical examinations such as auxological, pubertal staging and TV were measured.
The diagnosis of CDGP was made if the TV reached ≥8 ml[5,11,12] in boys and either spontaneous menarche or progressive breast enlargement up to B3 stage in girls at any point during a follow-up of 1.5 years.[4,13,14] IHH in males was assumed if the TV remained <5 ml and in females if there is non-progression of B1 stage during the follow-up period.[4,5,15,16] Boys with TV of 5 to 7 ml and girls with nonprogressive breast B2 stage during the follow-up were excluded from the final analysis as they could not be classified either into IHH or CDGP. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of hormonal tests were determined by generating the ROC curves to distinguish IHH from CDGP.
Either boys and girls or their parents who were anxious about their delayed sexual maturation were prescribed gonadal steroid therapy. Boys were administered testosterone enanthate 50 mg/month, intramuscularly for consecutive 3 months, and were followed for any increase in TVs.[17] If they remained <5 ml during follow-up or attained ≥8 ml during follow-up, they were assigned to the IHH or CDGP group, respectively. Those parents who were concerned about the short stature of children were offered oxandrolone at a dose of 0.1 mg/kg/day orally to promote height. In girls’ oestradiol valerate, 0.25 mg orally was administered daily for 3 months and followed later for any pubertal progression.[18]
Statistical analysis
The normality distribution was checked using the Shapiro–Wilk test. Continuous variables were expressed in mean ± Standard deviation (SD). Nonparametric test (Mann-Whitney U-test) and parametric test (independent t-test) were performed to compare the parameters between groups. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cut-off for the different diagnostic tests. To compare the diagnostic accuracy of different tests, the area under the curve (AUC) was calculated with 95% confidence limits. A P value <0.05 was considered significant. All data were analysed by IBM Statistical Package for the Social Sciences (SPSS) version 25 statistical software.
Ethical Aspect
Written informed consent was obtained from all the participants and the study was conducted in accordance with the Declaration of Helsinki.This study was approved by the Institutional Ethics Committee. (Institutional Ethical Committee approval number:869/M.K.C.G Medical college, Berhampur-4, dated 20.07.2021.).
RESULTS
There were 40 cases of delayed puberty (male: 26 and female: 14) enrolled in the study. Of these, six cases were excluded from the study because one male child was lost to follow-up and five children (male: 3 and female: 2) could not be classified either to IHH or CDGP as per the protocol. So, the final analysis included 34 children. Of 34 (male: 22 and female: 12) patients, CDGP constitutes 21 cases (61.7%) and IHH constitutes 13 cases (38.3%) [Figure 1].
Figure 1.
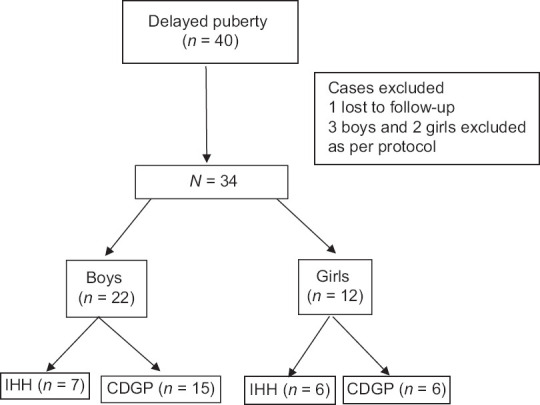
Overview of the study. IHH—isolated hypogonadotropic hypogonadism, CDGP—constitutional delay in growth and puberty
Comparing the phenotypic features between IHH and CDGP patients, it was found that the family history of delayed puberty (33% vs 15%) and personal history of delayed pubarche (95% vs 7%) were more common in CDGP than in IHH patients. However, IHH patients had higher rates of high arched palate (61%), short neck (23%), anosmia (15%), sensory neural hearing loss (15%), short 4th and 5th metacarpal (15%), achromatic iris (7%) and unilateral renal agenesis (7%) compared with CDGP patients. Micropenis (86%) and cryptorchidism (43%) were more common in male IHH patients than in male CDGP patients. In the present study, bimanual synkinesia, ataxia, nystagmus, clinodactyly and syndactyly were not found in any of IHH or CDGP patients. All the IHH patients had normal levels of thyroid hormone, serum cortisol and prolactin levels.
Boys with delayed puberty
Of 22 boys, seven had IHH and 15 had CDGP. The baseline characters of IHH and CDGP cases are shown in Table 1. There was no difference in the mean age between groups. IHH patients were taller and had a lower US/LS ratio compared with CDGP. There was no difference in the BMI and TV; however, SPL was lower in IHH than in CDGP patients. Bone age was delayed compared with chronological age in both IHH and CDGP patients; however, the difference (D) between chronological age and bone age was not statistically significant in male IHH compared with CDGP patients (2.96 ± 1.64 vs 1.99 ± 0.55 year, P = 0.174) [Table 1].
Table 1.
Baseline characteristics of IHH and CDGP patients (male)
| Parameters | IHH (n=7) (Mean±SD) | CDGP (n=15) (Mean±SD) | P |
|---|---|---|---|
| Age (years) | 16.29±2.05 | 14.92±0.69 | 0.407 |
| Height (cm) | 156.71±6.5 | 150.28±4.68 | 0.039 |
| BMI (Kg/m2) | 22±5.2 | 19.64±2.12 | 0.378 |
| US/LS ratio | 0.83±0.08 | 0.94±0.06 | 0.002 |
| Bone age (years) | 13.3±0.69 | 12.93±0.88 | 0.333 |
| CA-BA (D) (years) | 2.96±1.64 | 1.99±0.55 | 0.174 |
| Mean TV (ml) | 1.78±0.8 | 2.53±0.85 | 0.087 |
| SPL (cm) | 5.42±0.88 | 7.33±0.97 | 0.001 |
| Basal LH (U/L) | 0.30±0.28 | 0.52±0.62 | 0.34 |
| Basal FSH (U/L) | 1.36±1.31 | 2.35±0.9 | 0.072 |
| Basal inhibin B (pg/ml) | 62.74±42.49 | 117.94±26.62 | 0.008 |
| Basal AMH (ng/ml) | 17.89±6.16 | 16.82±5.35 | 0.75 |
| Basal testosterone (ng/dl) | 9.98±4.31 | 27.22±15.9 | 0.005 |
| Post-triptorelin 4-hour LH (U/L) | 3.03±1.74 | 10.45±4.66 | <0.001 |
| Post-triptorelin 4-hour FSH (U/L) | 8.34±5.3 | 9.85±4.04 | 0.370 |
| Post-triptorelin 24-hour testosterone (ng/dl) | 19.69±14.44 | 60.74±35.84 | 0.005 |
| Post-HCG day 4 testosterone (ng/dl) | 48.53±44.57 | 210.67±105.9 | 0.001 |
BMI - Body mass index, US/LS - Upper segment/lower segment, TV - Testicular volume, SPL - Stretched penile length, LH - Luteinising hormone, FSH - Follicle-stimulating hormone, AMH - Anti-Mullerian hormone, HCG - Human chorionic gonadotropin, CA - Chronological age, BA - Bone age, D - Difference, SD - Standard deviation
The basal hormones such as LH, FSH and AMH were lower in IHH than in CDGP but could not attain statistical significance. However, basal testosterone (9.98 ± 4.31 vs 27.22 ± 15.9 ng/dl) and inhibin B (62.74 ± 42.49 vs 117.94 ± 26.62 pg/ml) were significantly lower in IHH than in CDGP. The post-triptorelin 4-hour LH (3.03 ± 1.74 vs 10.45 ± 4.66 U/L) and 24-hour testosterone (19.69 ± 14.44 vs 60.74 ± 35.84 ng/dl) were significantly lower in IHH than in CDGP. Post-HCG day 4 testosterone was significantly lower in IHH than in CDGP (48.53 ± 44.57 vs 210.67 ± 105.9 ng/dl) [Table 1].
The AUC for various hormones and their optimal cut-offs with sensitivity and specificity are shown in Table 2. The 4-hour LH had the highest AUC (1.00) followed by post-HCG day 4 testosterone, basal testosterone and basal inhibin B. Basal inhibin B at a cut-off of 79.2 pg/ml had a sensitivity of 85.7% and specificity of 93.3% for diagnosing IHH cases. Basal testosterone at a cut-off of 20.8 ng/dl had 100% sensitivity but had low specificity (53.3%) for identifying IHH. However, the combination of basal testosterone and basal inhibin B at a cut-off of 20.8 ng/dl and 79.2 pg/ml, respectively, had a sensitivity of 100% and specificity of 88.9% for diagnosing IHH. Basal LH and FSH had low sensitivity (57.1%) and specificity varying from 33 to 93%. Post-triptorelin 4-hour LH had the highest sensitivity (100%) and specificity (100%) for identifying IHH at a cut-off of 4.84 U/L. Post-triptorelin 24-hour testosterone (<34.5 ng/dl) and post-HCG day 4 testosterone (<99.7 ng/dl) had optimal sensitivity of 85.7% and specificity varying from 73.3 to 93.3% for identifying IHH [Table 2, Figure 2].
Table 2.
Accuracy of various hormonal tests for IHH (male)
| Hormonal parameters | Cut-off (≤) | AUC (95% CI) | P (ROC) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Basal LH (U/L) | 0.35 | 0.629 (0.351-0.90) | 0.341 | 57.1 | 33.3 | 28.57 | 62.5 |
| Basal FSH (U/L) | 1.15 | 0.743 (0.463-1.00) | 0.072 | 57.1 | 93.3 | 80 | 82.35 |
| Basal inhibin B (pg/ml) | 79.2 | 0.857 (0.615-1.00) | 0.008 | 85.7 | 93.3 | 85.7 | 93.3 |
| Basal AMH (ng/ml) | 16.84 | 0.457 (0.19-0.725) | 0.751 | 42.9 | 66.7 | 37.5 | 71.43 |
| Basal testosterone (ng/dl) | 20.82 | 0.876 (0.723-1.00) | 0.005 | 100 | 53.3 | 50 | 100 |
| Post-triptorelin 4-hour LH (U/L) | 4.84 | 1.00 | <0.001 | 100 | 100 | 100 | 100 |
| Post-triptorelin 4-hour FSH (U/L) | 9.8 | 0.638 (0.378-0.899) | 0.307 | 85.7 | 46.7 | 42.87 | 87.5 |
| Post-triptorelin 24-hour testosterone (ng/dl) | 34.52 | 0.876 (0.727-1.00) | 0.005 | 85.7 | 73.3 | 60 | 91.67 |
| Post-HCG day 4 testosterone (ng/dl) | 99.7 | 0.962 (0.88-1.00) | 0.001 | 85.7 | 93.3 | 85.7 | 93.33 |
| Basal testosterone (ng/dl) + basal inhibin B (pg/ml) | 20.82, 79.2 | - | - | 100 | 88.9 | 85.7 | 100 |
LH - Luteinising hormone, FSH - Follicle-stimulating hormone, AMH - Anti-Mullerian hormone, HCG - Human chorionic gonadotropin, AUC - Area under the curve, ROC - Receiver operating curve
Figure 2.
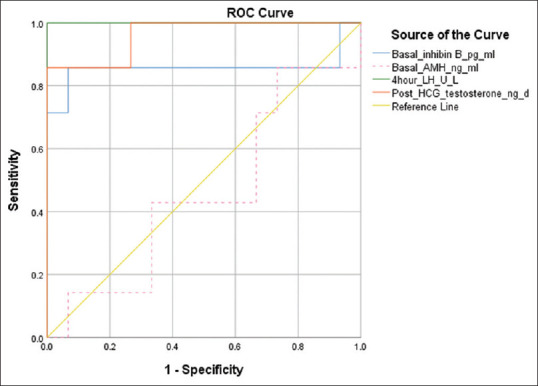
ROC plot for hormonal tests (boys)
Girls with delayed puberty
Of 12 girls, six had IHH and six had CDGP. The baseline characters of IHH and CDGP cases are shown in Table 3. The IHH patients were older compared with CDGP patients. IHH patients were significantly taller than CDGP patients. BMI and bone age were comparable, but US/LS ratio was significantly lower in IHH than in CDGP patients. Bone age was delayed compared with chronological age in both IHH and CDGP patients; however, D between chronological age and bone age was statistically significantly higher in female IHH patients than in CDGP patients (3.98 ± 1.19 vs 2.35 ± 0.35 year, P = 0.009) [Table 3].
Table 3.
Baseline characteristics of IHH and CDGP patients (female)
| Parameters | IHH (n=6) Mean±SD | CDGP (n=6) Mean±SD | P |
|---|---|---|---|
| Age (years) | 16.65±1.76 | 13.84±0.78 | 0.037 |
| Height (cm) | 153.83±7.36 | 141.83±3.4 | 0.024 |
| BMI (Kg/m2) | 19.5±3 | 18.72±0.75 | 0.423 |
| US/LS ratio | 0.86±0.07 | 0.94±0.05 | 0.03 |
| Bone age (years) | 12.67±0.98 | 11.5±0.83 | 0.053 |
| CA-BA (Δ) (years) | 3.98±1.19 | 2.35±0.35 | 0.009 |
| Right OV (ml) | 0.94±0.4 | 0.93±0.4 | 0.522 |
| Left OV (ml) | 0.7±0.27 | 0.95±0.25 | 0.297 |
| Uterine volume (ml) | 2.28±0.87 | 2.1±1.03 | 1.0 |
| Basal LH (U/L) | 0.5±0.76 | 0.68±1 | 0.575 |
| Basal FSH (U/L) | 1.94±1.41 | 3±0.87 | 0.171 |
| Basal inhibin B (pg/ml) | 44.17±47.35 | 104.68±37.14 | 0.037 |
| Basal AMH (ng/ml) | 2.62±1.15 | 3.31±2.26 | 0.749 |
| Basal oestradiol (pg/ml) | 13.81±2.13 | 16.71±5.82 | 0.470 |
| Post-triptorelin 4-hour LH (U/L) | 3.3±2.98 | 16.75±7.79 | 0.004 |
| Post-triptorelin 4-hour FSH (U/L) | 10.5±7.18 | 21.61±8.68 | 0.025 |
| Post-triptorelin 24-h oestradiol (pg/ml) | 30.11±15.66 | 59.02±54.52 | 0.262 |
BMI - Body mass index, US/LS - Upper segment/lower segment, OV - Ovarian volume, LH - Luteinising hormone, FSH - Follicle-stimulating hormone, AMH - Anti-Mullerian hormone, HCG - Human chorionic gonadotropin, CA - Chronological age, BA - Bone age, Δ - Difference, SD - Standard deviation
The basal LH, FSH, AMH and oestradiol were lower in IHH but could not attain statistical significance. However, basal inhibin B was significantly lower in IHH than in CDGP (44.17 ± 47.35 vs 104.68 ± 37.14 pg/ml). The post-triptorelin 4-hour LH (3.3 ± 2.98 vs 16.75 ± 7.79 U/L) and 4-hour FSH (10.5 ± 7.18 vs 21.61 ± 8.68 U/L) were significantly lower in IHH than in CDGP. Post-triptorelin 24-hour oestradiol was lower in IHH than in CDGP but could not attain statistical significance [Table 3].
The AUC for various hormones and their cut-offs with sensitivity and specificity are shown in Table 4. The AUC for 4-hour LH was highest (1.00) followed by 4-hour FSH (0.889) and basal inhibin B (0.861). Basal hormones such as LH, FSH, AMH, oestradiol and post-triptorelin 24-hour oestradiol showed insignificant and lower AUC. Basal inhibin B at a cut-off of 45.7 pg/ml had a sensitivity of 83.3% and specificity of 100% for identifying IHH. Basal oestradiol (<16.79 pg/ml) had good sensitivity (100%) but poor specificity (50%), whereas basal AMH at a cut-off of 1.97 pg/ml had poor sensitivity (33.3%) and optimal specificity (83.3%) for diagnosing IHH. However, the combination of basal inhibin B (45.7 pg/ml) and basal oestradiol (16.79 pg/ml) had 100% sensitivity and 100% specificity for detecting IHH. Basal LH and FSH were poor discriminators of IHH. Post-triptorelin 4-hour LH had the highest sensitivity (100%) and specificity (100%) for identifying IHH at a cut-off of 9.3 U/L. Post-triptorelin 24-hour oestradiol (<31.63 pg/ml) had reasonable sensitivity (83.3%) and specificity (66.7%) for identifying IHH in females [Table 4, Figure 3].
Table 4.
Accuracy of various hormonal tests for IHH (female)
| Hormonal parameters | Cut-off (≤) | AUC (95% CI) | P (ROC) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Basal LH (U/L) | 0.39 | 0.579 (0.246-0.949) | 0.575 | 66.7 | 33.3 | 54.55 | 100 |
| Basal FSH (U/L) | 1.09 | 0.736 (0.445-1.00) | 0.173 | 33.3 | 100 | 100 | 60 |
| Basal inhibin B (pg/ml) | 45.7 | 0.861 (0.604-1.00) | 0.037 | 83.3 | 100 | 100 | 85.71 |
| Basal AMH (ng/ml) | 1.97 | 0.556 (0.206-0.905) | 0.749 | 33.3 | 83.3 | 66.67 | 55.56 |
| Basal oestradiol (pg/ml) | 16.79 | 0.625 (0.277-0.973) | 0.471 | 100 | 50 | 66.67 | 100 |
| Post-triptorelin 4-hour LH (U/L) | 9.3 | 1.00 | 0.004 | 100 | 100 | 100 | 100 |
| Post-triptorelin 4-hour FSH (U/L) | 11.58 | 0.889 (0.67-1.00) | 0.025 | 83.3 | 100 | 100 | 85.71 |
| Post-triptorelin 24-hour oestradiol (pg/ml) | 31.63 | 0.694 (0.377-1.00) | 0.262 | 83.3 | 66.7 | 71.43 | 80 |
| Basal inhibin B (pg/ml) + basal oestradiol (pg/ml) | 45.7, 16.97 | - | - | 100 | 100 | 100 | 100 |
LH - Luteinising hormone, FSH - Follicle-stimulating hormone, AMH - Anti-Mullerian hormone, HCG - Human chorionic gonadotropin, AUC - Area under the curve, ROC - Receiver operating curve
Figure 3.
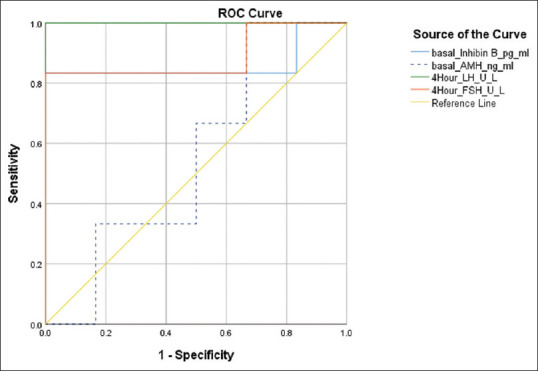
ROC plot for hormonal tests (girls)
DISCUSSION
Distinguishing IHH from CDGP is often very difficult from the clinical ground. CDGP is a delayed maturation of growth and puberty, which often results in normal adult stature, whereas IHH requires gonadal steroid replacement for pubertal induction and maturation and hence the importance of correctly identifying the disease. In the present study, both boys and girls were prospectively followed till the end of the study with the estimation of basal and stimulated hormones to distinguish IHH from CDGP.
In boys with delayed puberty, basal hormones such as LH and FSH were not efficient in distinguishing IHH from CDGP. In the present study, basal LH at a cut-off of 0.35 U/L had poor sensitivity and specificity contrary to the study by Binder et al. who found that LH cut-off of 0.3 U/L had good sensitivity (100%) but similar specificity of 28.5%.[5] Coutant et al.[6] with LH at a higher cut-off of 0.8 U/L found a sensitivity of 86% and specificity of 83% for IHH. In the present study, FSH at a cut-off of 1.15 U/L had good specificity (93.45%) with poor sensitivity (57.1%) for the diagnosis of IHH. Similar to the present study, Grinspon et al.[19] found that basal FSH with a cut-off of 1.2 U/L had similar sensitivity and specificity of 100%. Coutant et al.[6] found that FSH at a cut-off of 0.7 U/L had 100% sensitivity and specificity in a study of boys (n = 16) for the diagnosis of IHH. The variation in cut-off levels, sensitivity and specificity among basal LH and FSH makes them unreliable for distinguishing IHH from CDGP. Inhibin B was reported earlier and had better diagnostic accuracy for distinguishing IHH from CDGP. In our study, inhibin B at a cut-off of 79.2 pg/ml had good sensitivity (85.7%) and good specificity (93.3%). Binder et al.[5] in a study of 61 boys report similar result for inhibin B at a cut-off of 111 pg/ml and had 100% sensitivity and 92.3% specificity. These results contrasted with an earlier study by Coutant et al.[6] who reported inhibin B at a cut-off of 35 pg/ml and had sensitivity and specificity of 100% each. A similar study by Mishra et al.[20] found that inhibin B at a cut-off of 105 pg/ml had 100% sensitivity and 83% specificity. These results show that inhibin B had good sensitivity and specificity to distinguish IHH from CDGP. The diagnostic cut-off varied from 28.5 pg/ml to 113 pg/ml across studies reported in boys.[21] This result may be due to variation in ethnicity of studied subjects, criteria for diagnosis of IHH and CDGP and duration of follow-up among various studies. It may be reasonable to opt for region-specific inhibin B cut-offs for the diagnosis of IHH. In the present study, post-triptorelin 4-hour LH had the highest sensitivity, specificity, PPV and NPV (100% each) for the diagnosis of IHH. To the best of our knowledge, there are two studies that used the triptorelin stimulation test to distinguish IHH from CDGP.[5,7] Binder et al. demonstrated that post-triptorelin 4-hour LH at a cut-off of 5.3 U/L had 100% sensitivity and specificity in diagnosing IHH, which is similar to the present study. Others have studies on leuprolide or GnRH as a stimulatory agent for distinguishing both.[9,19,20] The present study also found that basal testosterone had good sensitivity (100%) and poor specificity (53.3%) at a cut-off of 20.8 ng/dl. The study by Coutant et al.[6] found that basal testosterone at a cut-off of 9 ng/dl had 100% sensitivity and specificity, whereas the study by Mishra et al.[20] at a cut-off of 19.71 ng/dl found sensitivity and specificity of 67% and 78%, respectively. These test results show that basal testosterone is not a specific test to distinguish IHH from CDGP in boys. The results on post-HCG-stimulated testosterone varied across studies. The present study found that post-HCG day 4 testosterone at a cut-off of 99.7 ng/dl had a sensitivity of 85.7% and specificity of 93.3%, which was similar to the study by Mishra et al.,[20] which had 100% sensitivity and 91% specificity at a cut-off of 110 ng/dl.
In girls with delayed puberty, basal LH at a cut-off of 0.39 U/L had poor sensitivity (66.7%) and specificity (33.3%), while basal FSH at a cut-off of 1.09 U/L had 100% specificity with poor sensitivity (33.3%) in distinguishing IHH from CDGP. Chaudhary et al.[7] found that basal LH at a cut-off of 0.3 U/L had the same sensitivity of 66% and specificity of 60%. Binder et al.[4] taking a higher cut-off for FSH at 1.6 U/L had a specificity of 91.7% with a higher sensitivity of 88.9%. One of the best indicators to distinguish IHH from CDGP was 4-hour LH and 4-hour FSH following the triptorelin stimulation test in the present study. The 4-hour LH at a cut-off of 9.3 U/L had 100% sensitivity and 100% specificity, whereas 4-hour FSH at a cut-off of 11.58 U/L had 100% specificity with 83% sensitivity. Binder et al.[4] reported that 4-hour LH at a cut-off of 9 U/L had a sensitivity of 100% and specificity of 83% similar to the present study. Binder et al.[4] also reported that FSH at a cut-off of 11 U/L similar to our study had 100% sensitivity but a higher specificity of 100% in detecting HH in females. Chaudhary et al.[7] reported that 4-hour LH at a cut-off of 14 U/L had a sensitivity of 66% and specificity of 100% to predict entering into puberty in girls. Basal oestradiol and 24-hour oestradiol had poor specificity varying from 50% to 66%. The combination of basal oestradiol and inhibin B improved the sensitivity and specificity to 100%.
From the present study, the best indicator to distinguish IHH from CDGP was post-triptorelin 4-hour LH in both boys and girls, whereas the combination of basal inhibin B and basal oestradiol performed well in girls. AMH had poor sensitivity and specificity and hence cannot be used as a distinguishing test. Others have tried FSH-stimulated inhibin B as a good predictor of entering into puberty, but it is not available in all centres.[7] The Kisspeptin stimulation test was also reported to be able to distinguish IHH and CDGP, but it is very cumbersome to do that.[22]
Limitations
In our study, CDGP was presumed to be present in girls who attained breast B3 stage although there were no previous studies on it. The higher sample size and longer duration could have strengthened the study.
CONCLUSION
The present study showed that the best indicator to distinguish IHH from CDGP was post-triptorelin 4-hour LH in both boys and girls. The combination of basal inhibin B and oestradiol is also a useful marker to distinguish IHH from CDGP in girls. Basal inhibin B as a single marker is a reasonably good marker to distinguish IHH from CDGP in both boys and girls.
Authors’ contribution
Ravikumar P. conceptualized the study, designed the research, managed recruitment, wrote the manuscript, and proofread it. Sahoo BK. contributed to recruitment, performed statistical analysis, and participated in manuscript writing. Pattanaik SR aided in recruitment and intervention. Dash DK. contributed in recruitment, intervention, and proofreading. Patro D and Telagareddy R contributed to case recruitment.
Financial support and sponsorship
This study was supported by the ESI, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Endocrine Society of India for the research grant and Dept of Radiology,M.K.C.G MCH, Berhampur for performing MRI study of pituitary and brain.
REFERENCES
- 1.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee PA. Normal ages of pubertal events among American males and females. J Adolesc Health Care. 1980;1:26–9. doi: 10.1016/s0197-0070(80)80005-2. [DOI] [PubMed] [Google Scholar]
- 3.Sedlmeyer IL, Palmert MR. Delayed puberty: Analysis of a large case series from an academic center. J Clin Endocrinol Metab. 2002;87:1613–20. doi: 10.1210/jcem.87.4.8395. [DOI] [PubMed] [Google Scholar]
- 4.Binder G, Schweizer R, Haber P, Blumenstock G, Braun R. Accuracy of endocrine tests for detecting hypogonadotropic hypogonadism in girls. J Pediatr. 2015;167:674–8. doi: 10.1016/j.jpeds.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Binder G, Schweizer R, Blumenstock G, Braun R. Inhibin B plus LH vs GnRH agonist test for distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism in boys. Clin Endocrinol (Oxf) 2015;82:100–5. doi: 10.1111/cen.12613. [DOI] [PubMed] [Google Scholar]
- 6.Coutant R, BietteDemeneix E, Bouvattier C, Bouhours-Nouet N, Gatelais F, Dufresne S, et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty. J Clin Endocrinol Metab. 2010;95:5225–32. doi: 10.1210/jc.2010-1535. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary S, Walia R, Bhansali A, Dayal D, Sachdeva N, Singh T, et al. FSH-stimulated Inhibin B (FSH-iB): A Novel marker for the accurate prediction of pubertal outcome in delayed puberty. J Clin Endocrinol Metab. 2021;109:3495–505. doi: 10.1210/clinem/dgab357. [DOI] [PubMed] [Google Scholar]
- 8.Rohayem J, Nieschlag E, Kliesch S, Zitzmann M. Inhibin B, AMH, but not INSL3, IGF1 or DHEAS support differentiation between constitutional delay of growth and puberty and hypogonadotropic hypogonadism. Andrology. 2015;3:882–7. doi: 10.1111/andr.12088. [DOI] [PubMed] [Google Scholar]
- 9.Degros V, Cortet-Rudelli C, Soudan B, Dewailly D. The human chorionic gonadotropin test is more powerful than the gonadotropin-releasing hormone agonist test to discriminate male isolated hypogonadotropic hypogonadism from constitutional delayed puberty. Eur J Endocrinol. 2003;149:23–2. doi: 10.1530/eje.0.1490023. [DOI] [PubMed] [Google Scholar]
- 10.Segal TY, Mehta A, Anazodo A, Hindmarsh PC, Dattani MT. Role of gonadotropin-releasing hormone and human chorionic gonadotropin stimulation tests in differentiating patients with hypogonadotropic hypogonadism from those with constitutional delay of growth and puberty. J Clin Endocrinol Metab. 2009;94:780–5. doi: 10.1210/jc.2008-0302. [DOI] [PubMed] [Google Scholar]
- 11.Mul D, Fredriks AM, van Buuren S, Oostdijk W, Verloove-Vanhorick SP, Wit JM, et al. Pubertal development in The Netherlands 1965-1997. Pediatr Res. 2001;50:479–86. doi: 10.1203/00006450-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lawaetz JG, Hagen CP, Mieritz MG, Blomberg Jensen M, Petersen JH, Juul A, et al. Evaluation of 451 Danish boys with delayed puberty: Diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. J Clin Endocrinol Metab. 2015;100:1376–85. doi: 10.1210/jc.2014-3631. [DOI] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, et al. Progression through puberty in girls enrolled in a contemporary British cohort. J Adolesc Health. 2010;47:282–9. doi: 10.1016/j.jadohealth.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarfati J, Guiochon-Mantel A, Rondard P, Arnulf I, Garcia-Piñero A, Wolczynski S, et al. A comparative phenotypic study of kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab. 2010;95:659–69. doi: 10.1210/jc.2009-0843. [DOI] [PubMed] [Google Scholar]
- 16.Aydogdu A, Bolu E, Sonmez A, Tasci I, Haymana C, Acar R, et al. Effects of three different medications on metabolic parameters and testicular volume in patients with hypogonadotropic hypogonadism:3-year experience. Clin Endocrinol (Oxf) 2013;79:243–51. doi: 10.1111/cen.12135. [DOI] [PubMed] [Google Scholar]
- 17.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366:443–53. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- 18.Raivio T, Miettinen PJ. Constitutional delay of puberty versus congenital hypogonadotropic hypogonadism: Genetics, management and updates. Best Pract Res Clin Endocrinol Metab. 2019;33:101316. doi: 10.1016/j.beem.2019.101316. [DOI] [PubMed] [Google Scholar]
- 19.Grinspon RP, Ropelato MG, Gottlieb S, Keselman A, Martínez A, Ballerini MG, et al. Basal folliclestimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95:2811–8. doi: 10.1210/jc.2009-2732. [DOI] [PubMed] [Google Scholar]
- 20.Mishra PK, Mishra I, Choudhury AK, Baliarsinha AK, Mangaraj SS, Jena S, et al. Accuracy of various tests alone and in combination to differentiate IHH from CDGP. Indian J Endocr Metab. 2022;26:160–6. doi: 10.4103/ijem.ijem_448_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Du Q, Liu L, Liao Z. Serum inhibin B for differentiating between congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty: A systematic review and meta-analysis. Endocrine. 2021;72:633–43. doi: 10.1007/s12020-020-02582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan Y, Lippincott M, Barroso P, Alleyn C, Brodsky J, Granados H, et al. Using kisspeptin to predict pubertal outcomes for youth with pubertal delay. J Clin Endocrinol Metab. 2020;105:2717–25. doi: 10.1210/clinem/dgaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]


