Abstract
Objective
Despite advances in the treatment of psychiatric diseases, currently available therapies do not provide sufficient and durable relief for as many as 30–40% of patients. Neuromodulation, including deep brain stimulation (DBS), has emerged as a potential therapy for persistent disabling disease, however it has not yet gained widespread adoption. In 2016, the American Society for Stereotactic and Functional Neurosurgery (ASSFN) convened a meeting with leaders in the field to discuss a roadmap for the path forward. A follow-up meeting in 2022 aimed to review the current state of the field and to identify critical barriers and milestones for progress.
Design
The ASSFN convened a meeting on June 3, 2022 in Atlanta, Georgia and included leaders from the fields of neurology, neurosurgery, and psychiatry along with colleagues from industry, government, ethics, and law. The goal was to review the current state of the field, assess for advances or setbacks in the interim six years, and suggest a future path forward. The participants focused on five areas of interest: interdisciplinary engagement, regulatory pathways and trial design, disease biomarkers, ethics of psychiatric surgery, and resource allocation/prioritization. The proceedings are summarized here.
Conclusion
The field of surgical psychiatry has made significant progress since our last expert meeting. Although weakness and threats to the development of novel surgical therapies exist, the identified strengths and opportunities promise to move the field through methodically rigorous and biologically-based approaches. The experts agree that ethics, law, patient engagement, and multidisciplinary teams will be critical to any potential growth in this area.
Keywords: Deep brain stimulation (DBS), treatment resistant depression, obsessive compulsive disorder, Tourette syndrome, neuromodulation
Introduction
Psychiatric disorders such as major depressive disorder (MDD) and obsessive-compulsive disorder (OCD) are prevalent, debilitating, and sometimes lethal conditions.1–4 While a number of treatment options and modalities exist, resistance or intolerance to these treatments is common. Treatment resistant depression (TRD) is usually defined as the persistence of MDD despite at least two adequate treatment trials. Nearly 30–40% of patients exhibit treatment resistance.5–9 While OCD is not as prevalent as MDD, treatment resistance in the OCD patient population is similarly common as treatment resistance in the MDD patient population.10 Given the significant burden of untreated disease, novel approaches are necessary for treatment resistant psychiatric disorders.
Deep brain stimulation (DBS) is a well-established treatment for a number of neurological disorders including essential tremor and Parkinson disease.11,12 This neuromodulatory therapy utilizes electrical stimulation via implanted electrodes and pulse generators in an effort to ameliorate disease symptoms by modulating brain activity. Given its successful application in treating neurological disorders, DBS has been studied as a novel therapeutic approach for treatment resistant psychiatric disorders. DBS targeting regions near the anterior limb of the internal capsule (ALIC), including the nucleus accumbens (NAc), bed nucleus of the stria terminalis (BNST), and ventral capsule/ventral striatum (VC/VS), has been explored in treatment resistant OCD patients.13–17 DBS of more distant targets, such as the anteromedial subthalamic nucleus (amSTN), has also been studied as a therapy for treatment resistant OCD.18–20 The aforementioned studies were double-blind randomized trials and provide high quality evidence supporting the efficacy of DBS for OCD. As summarized in a recent meta-analysis including 352 patients across 34 qualifying studies, DBS produced a median 47% reduction (p<0.01) in the main symptom score and a 66% treatment response rate. Additionally, there was a favorable adverse event profile.21 Recent imaging studies have also demonstrated that the DBS targets mentioned above are likely nodes within a common pathological circuit, thus providing a parsimonious explanation for the similarity in benefit profile across these otherwise anatomically distinct list of targets.22–24 Based on early promising results, a Humanitarian Device Exemption (HDE) was granted by the United States Food and Drug Administration (FDA) to Medtronic and a Conformité Européenne (CE Mark) was granted in Europe for the use of ALIC DBS to treat OCD in 2009.25,26 Despite these data and regulatory approvals, DBS for OCD is still vastly underutilized.26,27
Along with treatment resistant OCD, DBS has also been investigated as a therapy for TRD. Multiple brain areas have been explored including the inferior thalamic peduncle28, lateral habenula29, medial forebrain bundle30,31, subcallosal cingulate (SCC)32–37, and the ALIC38/VC/VS39. Despite a large body of literature supporting the efficacy of DBS for TRD, two large double-blind randomized trials (BROADEN and RECLAIM) did not demonstrate a difference between active and sham stimulation over a short blinded period at interim analysis time points.32,39 These studies dampened the enthusiasm of DBS for TRD. Further investigation in the form of multiple meta-analyses, however, have supported the efficacy of DBS for TRD.40–43 Additionally, multiple long-term open-label studies have demonstrated that DBS for TRD becomes more effective over time and provides durable benefit in patients treated for up to 10 years.44–46 Insights and advances in imaging have also changed the field. The use of diffusion magnetic resonance imaging (MRI) to reconstruct brain connectomes has grown in sophistication and popularity in recent years. These techniques have been applied to DBS for TRD with encouraging results.47–53 For example, retrospective analysis has demonstrated that stimulation of a particular subregion of the SCC in which four white matter bundles (forceps minor, uncinate fasciculus, cingulum and fronto-striatal fibers) converge was associated with response to DBS treatment.48,49 The above findings and scientific advancements have encouraged continued investigation of DBS for TRD.
Given the underutilization of DBS for OCD, the need for additional clinical evidence of DBS efficacy in the TRD patient population, and the importance of refining both of these therapies for psychiatric applications, the American Society for Stereotactic and Functional Neurosurgery (ASSFN) convened a meeting to discuss these issues and to describe the future path for surgical treatment of psychiatric diseases. This meeting was convened on June 3, 2022 in Atlanta, Georgia and included leaders from the fields of neurology, neurosurgery, psychology, psychiatry, and engineering along with colleagues from industry, government, ethics, and law. The proceedings from our last meeting in 2016 have been previously published.54 The goal of the latest meeting was to review the current state of the field, assess for advances or setbacks in the interim six years, and update our recommendations.
The meeting participants focused on five major areas: interdisciplinary engagement, regulatory pathways and trial design, disease biomarkers, ethics of psychiatric surgery, and resource allocation/accessibility. The discussions were guided by strengths, weaknesses, opportunities, and threats (SWOT) analyses. While initially developed for use in business, this form of analysis has been increasingly adopted in the realm of healthcare.55 In this report, we summarize the findings of this meeting and present opportunities for future development of surgical therapies for psychiatric disease.
Interdisciplinary Engagement
Surgery for psychiatric disease requires a multidisciplinary approach. Critical to the successful development and utilization of surgical treatments for psychiatric disorders is a working relationship between and among neurosurgeons, psychiatrists, neurologists, psychologists, neuropsychologists, engineers, payors, regulators, and industry partners.56 During the workshop, we discussed the value of and need for these relationships along with a path toward fostering these relationships further. The highlights of our interdisciplinary engagement SWOT analysis are shown in Table 1.
Table 1.
Interdisciplinary Engagement SWOT
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
The advantages to developing strong interdisciplinary teams for the selection and surgical treatment of people with psychiatric disease cannot be underscored enough. Integrated, multi-specialty, team-based approaches are widely used for treating movement disorder patients via DBS57 and such multidisciplinary care teams are often considered standard of care for the management of neuro-oncologic diseases.58 By extension, a similar approach for DBS for psychiatric disorders is not unreasonable, particularly given the complexity of these patients. These interdisciplinary care teams have been implemented in centers around the world for the management of a wide variety of disorders, yielding promising results.59–61
Along with engagement across specialties, engagement within fields is necessary. One barrier to the development and use of novel surgical therapies for debilitating psychiatric disorders is insufficient engagement of general psychiatrists, who can apply their expertise to manage patients with depression. Building awareness among general psychiatrists regarding novel and emerging therapies, including the spectrum of neuromodulatory therapies, was deemed a critical step to increasing engagement. Indeed, survey results have demonstrated a desire for increased neuroscience education within psychiatry.62 Engagement of psychiatrists outside of academia could potentially mitigate concerns that these psychiatrists may have about no longer being able to provide care to patients with treatment resistant diseases. Additional barriers to psychiatry engagement include insufficient time and resources for clinicians to care for these patients. This burden may be more noticeable for psychiatrists in private practice settings, outside of larger academic institutions.
Engagement could be encouraged by including psychiatrists and other subspecialty providers in research opportunities, highlighting that surgical trials are focused on addressing unmet clinical needs. Educational opportunities via rotations in training programs and/or fellowships following residency training could be better developed throughout academic training programs around the world. Various medical specialties could also consider delivering courses at their annual meetings to address this unmet need.
Additionally, robust referral systems that decrease barriers to engagement (for both psychiatrists and prospective patients) will need to be structured to ensure that patients have the opportunity to receive this care. Analogously, referral patterns and systems have been studied in the context of DBS for movement disorders.63–65 These studies have suggested that increased referral may be preferable to using screening methods to determine candidates for referral. Information gleaned from these prior studies can be used as a basis to develop referral systems for DBS for psychiatric disorders. An increased number of patient referrals would facilitate data generation and eventual support for regulatory and payor approval (Figure 1).
Figure 1.
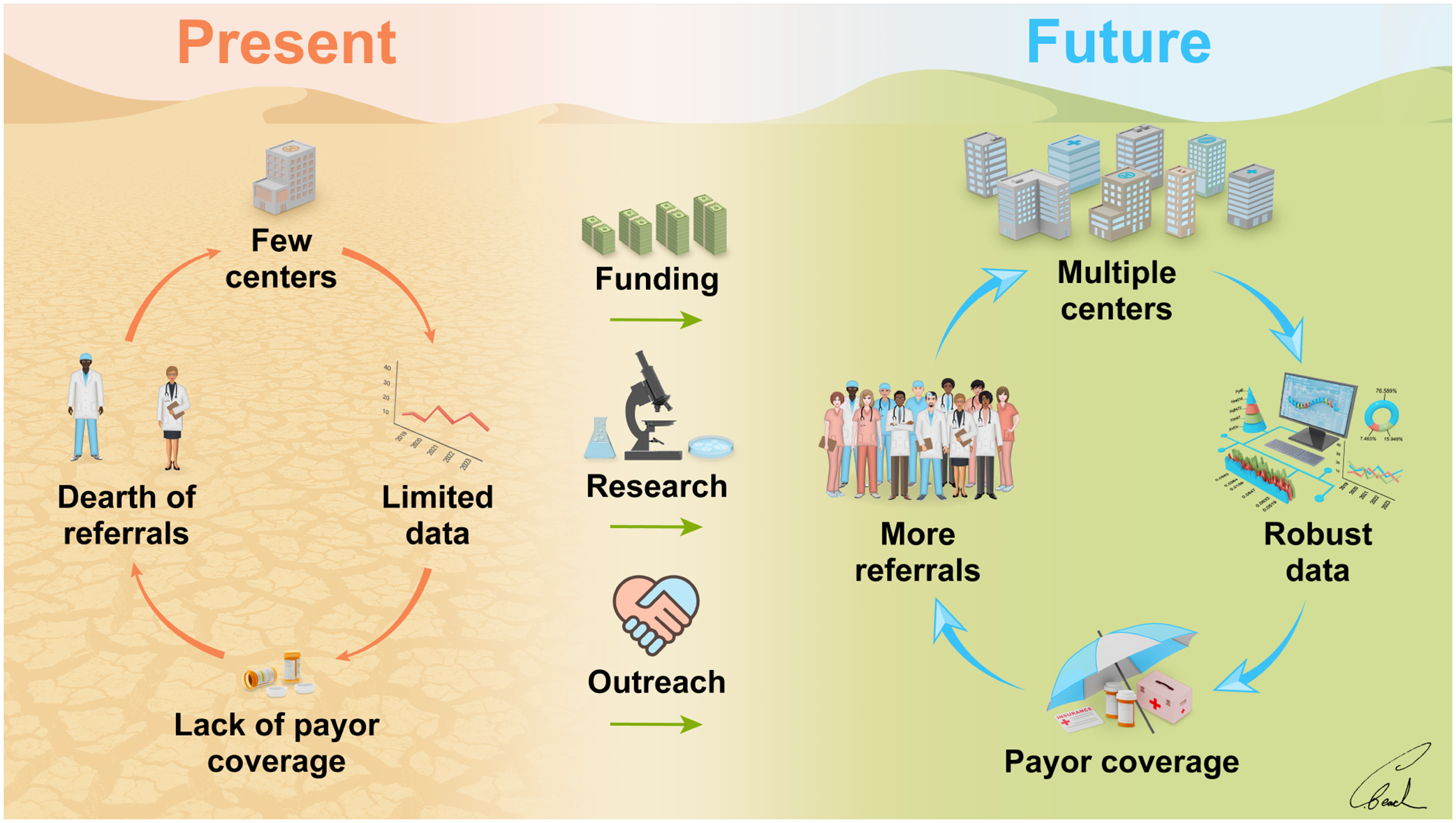
A path forward for psychiatric neurosurgery. Through additional funding, research, and outreach, the field aims to transition from the present limiting cycle of psychiatric neurosurgery to a future, more productive cycle.
To address the issue of insufficient resources, the inclusion of providers such as clinical psychologists, physician assistants, and nurse practitioners should be considered. The engagement of psychologists will be integral to the success of surgical treatments for psychiatric disease. These providers must be engaged for both recruitment and for post-operative patient care. For treatment approaches such as DBS, patients require multimodal care, commonly including post-operative psychotherapy.66,67 Furthermore, neuromodulation-induced brain state changes may enable previously failed psychotherapeutic approaches to transform into potentially effective post-operative therapies. In the example of OCD, it is believed that many of the circuitry modifications that occur with DBS involve the reduction of inflexible and rigid behavior and thought patterns.15 However, without regular cognitive behavioral therapy to optimize behavioral modifications following DBS, the chances of improvement are less likely.
Physician extenders are a key resource that are currently underutilized. Nurse practitioners frequently partake in DBS programming for movement disorders and can similarly be a valuable resource for psychiatric patients.
Given the early stage of therapy development, the consensus of the multidisciplinary group at this meeting was that therapy development trials and associated referrals should be initially focused at high-volume DBS centers. This is due to the complexity and incomplete knowledge of patient selection and because, at this time, the treatment of psychiatric disease with DBS requires extensive experience with device programming and care. This centralization of care has been facilitated, in part, by the relatively recent development of remote device programming and interventional psychiatry programs.68 Ideally, patient assessment and data collection could be standardized across centers. Management of difficult pathology such as TRD likely requires multimodal therapy with DBS representing only one aspect of care. Management of patient expectations is also an important aspect of their care, and this requires experience and proactive management.69 For example, prior to surgery, patients should be advised that neuromodulation will likely be coupled with continued psychotherapy and medication.
While surgical treatment of psychiatric disorders should be initially focused at highvolume/expert centers, the ultimate goal will be to make the treatment more accessible by increasing the number of qualified centers (Figure 1). Technique refinement and standardization, for example, could enable more widespread adoption of these therapies. An increase in patient referrals could be accommodated by an increase in the number of centers offering this therapy (Figure 1).
Movement disorders are currently the most common indication for treatment with DBS. As a consequence, neurologists who manage patients with DBS systems for movement disorders, such as Parkinson’s disease and essential tremor, have vast experience with DBS programming and may be key partners in centers and in studies of neuromodulation for psychiatric disease. Furthermore, many neurological disorders are comorbid with psychiatric disease.70–72 Thus, there is a clear need for close collaboration between neurology and psychiatry, including specialized multi-disciplinary training for both fields. Such focused training may be facilitated by targeted enrichment for small groups (in the form of specialized residency tracks or fellowships or focused coursework), and expanding to larger groups as indications for psychiatric neurosurgery grow.
Advances in medical device technologies are integral to augmenting the current surgical neuromodulatory approaches, including vagus nerve stimulation (VNS), responsive neurostimulation (RNS), and DBS. Engagement of engineering and industry partners is therefore essential to the success of psychiatric neurosurgery. Collaboration with engineers will drive device development with capabilities for network-based brain sensing and modulation, customized stimulation patterns, increased usability/practicality, and miniaturization which collectively can lead to more personalized therapeutic approaches and increased adoption.73 Engineers also play a critical role in advancing methods and approaches for the analysis of neural activity. Regulating brain activity dynamically and algorithmically is a promising path forward, given that symptoms fluctuate over time, and the development of adaptive closed loop systems will require engagement of sophisticated engineers with experience modeling systems and identifying useful neurophysiological biomarkers.
From the industry perspective, challenges include the slow rate of growth in the adoption of DBS in the movement disorder space, which can ultimately hamper investor confidence. This dampened confidence is compounded by the limited growth of neuromodulation for indications such as OCD and the high cost of device maintenance due to the requirement for industry representative device support. From the device manufacturers’ perspective, therapeutic development and investment should consider focusing on therapies for TRD as the prevalence of TRD is greater compared to other psychiatric disorders such as OCD. The relative prevalence will facilitate economic models for therapy development and deployment as well as the ease of patient recruitment for pivotal trials. The field has gained a lot of knowledge since the RECLAIM39 and BROADEN32 trials missed their primary endpoints, such as the importance of patient selection, trial design, and electrode targeting. Given the clear need for novel TRD therapies and evidence from institutional series with longer follow-up to support future trials, the FDA has granted breakthrough designation to Abbott for a further large scale trial of DBS for TRD.74 The breakthrough designation will potentially offer a sponsor better access to FDA resources and more expedited review. These designations granted when there is a “reasonable expectation” that the investigational therapy is more effective than standard of care.
In sum, speakers and panelists at this meeting identified a need for increased engagement of psychiatrists and psychologists, and referral of treatment resistant patients to high volume centers, especially given the complexity and multidisciplinary nature of their care. There is also a need to engage neurologists with extensive experience in device programming, and engage engineers for the development of DBS features to study and support psychiatric disorder treatment. Close collaboration with industry is needed regarding funding and providing/manufacturing the systems necessary for this advanced care. Though industry is most interested in TRD, the experts felt it important that other indications such as OCD, Tourette syndrome, addiction, and others also be co-developed.
Trial Design Considerations
Crucial to the approval and adoption of novel therapies are clinical trials to demonstrate the efficacy of experimental treatments. Various trial designs offer distinct advantages and disadvantages in the context of surgical treatment of psychiatric disorders. Randomized parallel trial designs compare groups of patients that have been randomized to receive either sham or active treatment over a period of time. These trial designs are favorable because they produce the highest quality of data, but they are resource intensive and may not be the most efficient way to demonstrate the efficacy of a novel therapy. Indeed, many trials designed this way are underpowered to reveal the efficacy of an experimental treatment.75 The pivotal RECLAIM39 and BROADEN32 trials were designed in this manner; the trial design, which is relatively rigid, may have contributed to the lack of success.43,54 For example, the parallel design did not enable within-subject comparisons to be made. Within-subject statistical analysis may be particularly important in psychiatric trials because disease heterogeneity results in increased variability between patients.
In contrast, when using crossover trials, all participants receive both active and sham treatment. Half of the cohort receives active followed by sham treatment while the other half receives sham followed by active treatment. A “washout” period between the crossover may be used to reestablish the patient’s baseline. Crossover trials are valuable because each patient can serve as their own control, thereby enabling within-subject comparisons as opposed to between-subject comparisons which increases statistical power.76 This trial design has successfully been applied in DBS for OCD19 and DBS for TRD.28,31,36,38 A potential downside to a crossover trial is that of unequal crossover (i.e. if a patient is unable to tolerate switching from active to sham treatment due to clinical deterioration). Furthermore, a “washout” period may not result in a complete return to baseline. Finally, crossovers in psychiatric disease device trials can lead to dropouts at mid-point or refusal to crossover especially when meaningful benefit is perceived.
An alternative trial design discussed is a delayed-onset trial, also known as a staggered-onset trial. In this design, half of the cohort begins active treatment at a later time point relative to the other half of the cohort. This design is a combination of a parallel design with a long-term open-label study. It therefore has a mixture of the advantages and disadvantages of both. An advantage of this design is the ability to compare active surgical treatment to sham surgical treatment with standard of care therapy during the initial delayed-onset period. It was used to investigate DBS for OCD in a pilot study.77 In a discontinuation study, patients are given active therapy titrated to maximum effect (e.g. DBS stimulation parameter optimization) followed by randomized treatment discontinuation, to ensure benefits cannot be attributed to a placebo effect.
An augmentation trial is a design in which one therapy is added to another. This was used to test the efficacy of stimulating two targets simultaneously for the treatment of OCD.18 While there is no one optimal design for every study, the workshop highlighted how critical careful selection of trial design is before a study is initiated and that it is important for regulatory agencies to be receptive to trial designs other than randomized controlled trials when considering the efficacy of DBS for psychiatric indications. Further work will be required to define acceptable and optimal trial designs given the evolving knowledge base in this field.
The group discussed some unique challenges in designing a DBS for TRD trial. One challenge is that each patient requires individualized programming, which can be a prolonged process. To accommodate personalized programming, it may be necessary to include an optimization phase before randomization. Of note, this has been performed in prior trials.38,53 Transitioning from an optimization phase to the randomized phase may be difficult if patients do not tolerate stimulation discontinuation. Another challenge in trial design is that DBS therapies often have insertional effects and long washout periods. While a rational decision-making process for stimulation target selection has not been fully established, there are inherent differences in adverse effects, programming, and speed of efficacy between stimulation targets.43 Trial designs should account for these differences. For example, the group suggested that SCC stimulation may require fewer programming adjustments than VC/VS stimulation. Furthermore, due to more rapid symptom recurrence with discontinuation of VC/VS stimulation, trials targeting this area may be more amenable to crossover and discontinuation studies compared to other targets. Some warned of the danger of rapid symptom recurrence and advocated for a slower down titration of stimulation rather than a complete discontinuation in crossover or withdrawal studies.
Other challenges of trial design include determining the ideal length of the trial. Future trials should likely be of longer duration given the outcomes of the BROADEN and RECLAIM trials. Another important consideration is standardization of device implantation. Targeting variability between surgeons could impact the success of trials and there should be standardization of implantation techniques. Variability among patients (co-morbidities, prior and current medical/ECT treatments, etc.), postoperative medication selection, involvement of cognitive behavioral therapy, and frequency of follow-up, could all contribute to heterogeneity of investigations and potentially poor outcomes with larger studies.
Another important consideration in trial design is outcome measure selection. Traditional outcome measures of disease severity, such as the Montgomery–Åsberg Depression Rating Scale, represent single clinical timepoints. Since most patients have a fluctuating disease course, single snapshots in time of disease severity don’t represent true disease burden. Area under the curve (AUC) analysis can be employed to measure disease severity over a period of time, and this form of analysis can be used for any clinical outcome measure. This computation has been dubbed the Illness Density Index (IDI) and has been utilized in recent studies.44,45,78,79 Furthermore, outcome measure selection may also inform trial design. Specifically, the group discussed the potential value of biomarkers, such as changes in brain activity that can be measured with electrophysiology or functional imaging, that can be used to guide therapy or to predict outcomes, potentially leading to truncation of the duration of trials.
As a complement to clinical trials, registries can serve a particularly valuable role in developing and collating evidence of treatment efficacy. Given that most single centers have a small sample size of patients, registries can help enhance the power of observations. Defining uniform data collection methods, common data elements, and outcome measures will make registries more feasible and impactful. Furthermore, these collaborations could increase the number of stakeholders to improve care. Maintaining registries is challenging as registries can be costly to develop and maintain and credit for the work is diluted. The “collective action problem” of registries could potentially be solved by implementing reporting requirements. Another significant challenge is that the data gleaned from registries may not be sufficiently compelling to gain favor of regulatory agencies. Registries may still play a pivotal role as randomized trials for DBS for psychiatric disease may not be feasible or optimal, particularly for uncommon diseases. Of note, other treatments such as laser interstitial thermal therapy (LITT) for epilepsy have received regulatory approval without randomized trials.80 There is data from the well maintained International Deep Brain Stimulation Database and Registry that have demonstrated efficacy of DBS for Tourette syndrome, but to date it has not yet received regulatory approval mainly due to reluctance of industry partners to pursue HDE’s in small disease populations.81 A key distinction here is that DBS systems are FDA class III implantable devices. This distinction may preclude approval with registry data. Another challenge for registry data collection is garnering funding for the devices, procedures, and data collection.
In summary, speakers and panelists at this meeting identified a need to strongly consider alternatives to the standard parallel trial design, such as a crossover design with an initial optimization phase. There is a need for the formation of registries and the implementation of standardized reporting requirements to enhance the feasibility of these registries.
Regulatory Pathways
FDA representatives participated in the meeting to shed light on the regulatory process in the context of novel surgical treatments for psychiatric disease. The regulatory approval process is broken down into three classes based on risk of the intervention, with Class I treatments regarded as low risk and Class III treatments regarded as high risk. Surgical therapies such as DBS, RNS, and VNS are considered Class III and require premarket approval (PMA), which is the FDA’s most rigorous type of device application.82 Before any study is begun, an investigational device exemption (IDE) must be obtained.83 FDA recommends that potential investigators work with them prior to IDE/PMA submissions. This pre-submission process is voluntary, but it increases the likelihood that the FDA will receive acceptable data for future potential approval. Study design considerations include the disease studied, adjunct versus standalone treatment, a risk-benefit analysis (surgical risk, other options, uncertainty), and the target population (age, treatment resistance, DSM diagnosis). New brain targets require some type of safety data, which could include preclinical data. Studies should have a sufficient sample size for both safety and efficacy. Safety parameters, including adverse events and long-term plans, should be defined. The measure of efficacy should be pre-defined with definite time frames and statistical plans. Clinical meaningfulness should be the goal. Studies should conform to safety monitoring guidelines and should include informed consent and data safety monitoring boards. An HDE may be pursued if the annual incidence of a disease is less than 8,000. However, whereas DBS for treatment resistant OCD is currently FDA approved under an HDE, its adoption has faced hurdles with insurance coverage of the treatment despite the high-quality evidence of its benefit.26,27
In brief, working closely with the FDA, even prior to IDE/PMA submission, is expected to streamline device approval. The bullet points of our regulatory pathways and trial design SWOT analysis are summarized in Table 2.
Table 2.
Regulatory Pathways and Trial Design SWOT
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
Biomarkers for Psychiatric Neurosurgery
Biomarkers of disease can be important in both diagnosis and treatment. If validated, biomarkers could be used to personalize treatment, assess treatment efficacy, reduce side effects of treatment, and to provide insights into disease and treatment mechanisms. Biomarkers may be particularly valuable in the setting of psychiatric disease because changes in symptom severity can lag behind stimulation titration. Electrophysiological biomarkers may be particularly attractive because they are directly accessible via the implanted device and could be used for closed-loop neuromodulation. Electrophysiological activity recorded by macroelectrodes or subdural strip electrodes has been used as surrogates of symptom severity in Parkinson’s disease (PD)84, Tourette syndrome85, TRD86, and OCD15,87,88 for objectively guiding stimulation titration and in some cases with the ultimate goal of developing a closed-loop therapy algorithm driven by these electrophysiological biomarkers. Affective dysfunction is often comorbid with PD and there has been exploration into the measurement of electrophysiological biomarkers of depressive symptomatology in these patients as well.89 Exploring electrophysiological biomarkers in TRD using a stereoelectroencephalography (sEEG) approach, which is commonly used to localize the seizure onset zone in epilepsy patients, is under active investigation at two centers.53,90–92 These trials have tested personalized stimulation for TRD and the knowledge gleaned from this work has the potential to be generalizable to future TRD patients. It is possible that in the future patients may not need sEEG studies if information can be gleaned with alternative methods. There are already a number of available devices that could be used to modulate stimulation based on electrophysiological biomarkers including the Neuropace RNS system that is approved for use in epilepsy and the Medtronic Percept™ DBS device platform that is approved for use in movement disorder treatment.
In addition to electrophysiological biomarkers, various imaging modalities may serve as potential biomarkers of psychiatric disease states. MRI can be used to identify anatomic and morphological predictors of response to intervention. Furthermore, MR diffusion imaging can be used to reconstruct fiber bundles in the intact human brain that can be used for direct targeting of DBS electrode implantation or to predict response to neuromodulation.48 Future studies could examine whether characteristics of certain tracts could serve as biomarkers for TRD patients that would benefit most from DBS. Imaging can be further used to guide and facilitate personalized programming of stimulation parameters. The ability to see images of the patient’s own anatomy, targets for stimulation, and volumes of tissue activated can facilitate targeting the correct anatomical structures to promote the desired treatment effect while avoiding brain structures that could result in side effects.93 Positron emission tomography (PET) studies that demonstrated hyperactivity of the SCC in TRD patients provided the initial motivation for selecting the SCC as a DBS target,94 and a recent study has used PET imaging to evaluate treatment response in patients undergoing medial forebrain bundle DBS for TRD.95 The most accurate treatment and diagnosis of these complex psychiatric disorders likely requires the combined use of multiple modalities and biomarkers, including thoughtful symptom classification. Combining these various modalities may require a machine learning approach for accurate diagnosis and treatment.
The National Institutes of Health (NIH) Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative recently hosted the Brain Behavior Quantification and Synchronization (BBQS) workshop. In this workshop, participants spoke about the importance of measuring behavior in a way that would enable synchronization with brain activity, with the same level of temporal resolution. Current neurostimulators, such as the Neuropace RNS and Medtronic Percept™ DBS, do not easily provide this brain-behavior data synchronization outside of the laboratory and in the ecological setting. In this regard, industry partners can play a critical role in allowing development on their platforms. Public-private interactions, perhaps facilitated by the NIH Blueprint program96,97, could help foster these partnerships. Measurements of behavior could be achieved with novel devices and sensors or already existing devices such as smartphones and smartwatches could be leveraged.15 This high-dimensionality data may require advanced data science techniques to utilize, but this is a clear opportunity for future development. Participants commented that behavioral biomarkers are difficult to assess, but very important to measure. For psychiatric disorders, facial affect recognition may prove useful as a study behavioral biomarker, but further enhancements in accuracy may be necessary.98,99
A critical challenge in biomarker development is that psychiatric disorders are internally heterogeneous. Individual patients diagnosed with TRD may have very different underlying circuit dysfunctions despite some overlap in their clinical symptoms.100,101 It is unlikely that there will be a single biomarker that reflects symptom severity in all patients.102 It may be necessary to first phenotype patients at a more granular psychological or behavioral level, e.g. through assessments based in the NIH Research Domain Criteria framework.101,103,104 Closed loop algorithms can even be designed to operate directly on those psychological constructs, which may be a more robust approach to closed loop psychiatric treatment.103,105
In sum, speakers and panelists at this meeting identified a need for further active investigation of electrophysiological, imaging, and behavioral biomarkers of disease severity and subtype. Furthermore, the NIH BRAIN Initiative has issued funding opportunity announcements seeking to support the development of devices capable of synchronizing behavior with recorded brain activity [RFA-MH-22–240: BRAIN Initiative: Brain Behavior Quantification and Synchronization (R61/R33 Clinical Trial Optional)]. Table 3 outlines our disease biomarkers SWOT analysis.
Table 3.
Disease Biomarkers SWOT
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
Ethics of Psychiatric Surgery
Research into the ethics of surgical psychiatric treatments should be conducted in parallel with the development of novel treatments.56 One area of investigation should be timing of surgery. Namely, how many failed treatment trials should a patient undergo before being considered for surgery and how long should those treatment trials be? We have decades of clinical experience with neurostimulation11,45, and devices can be implanted at a young age for dystonia and epilepsy, in which early intervention is generally considered advantageous.106,107 Surgical intervention is typically considered after multiple prior treatment failures, so patients may view surgical treatments as their “last resort” for improvement. It is important to reframe this perception pre-operatively with psychoeducation and adequate discussion of post-operative treatment options, because treatment failure could increase the risk of hopelessness.
Another open question is whether and how to differentiate invasive vs non-invasive treatments and how to compare their relative desirability. While DBS is clearly invasive, its value and tolerability is well established in movement disorders, where it has become standard of care for certain populations of patients. In contrast, while medications are often perceived as “non-invasive,” there is growing appreciation of the broader impact and “invasiveness” of medications, since side effects can greatly impact a patient’s life. Furthermore, the risks of invasive procedures are generally frontloaded, whereas medication side effects frequently persist throughout the course of treatment.
Additionally, informed consent deserves ethical scrutiny. It is difficult to determine if patients achieve an adequate understanding of the risks and benefits of surgical treatment when it is discussed pre-operatively and these attitudes may change over time with therapy.108 In the treatment resistant patient population, desperation for novel therapies may distort the informed consent process. Conversely, patients may have an inflated sense of the risk of surgery. Indeed, these types of misunderstandings extend to all surgery, not just psychiatric surgery. Patients should be asked directly about their goals and expectations of the treatment as these may not be directly aligned with a physician’s goals. Including patients’ perspectives is important not just in clinical decision-making, but also in the funding and development of future studies. These discussions can be facilitated by community leaders, disease-specific support groups, and/or ethicists skilled in community engagement.69,109
The role of patients and patient-groups was suggested to extend beyond informed consent. Meeting participants recommended that patients be included in discussions of study design (i.e., designing with, not merely for patients.) Other challenges include equitable access to these treatments. Some patient groups may be less likely to participate in trials due to mistrust of physicians and preference for established therapies. Ethnography should therefore be integrated into neuroethical discussions to best protect patients’ interests. Thoughtfully considering these issues during trial design may mitigate critical ethical concerns.
In addition to ethical issues, the group discussed legal issues relevant to psychiatric surgery. There is legislation in place around the world prohibiting or limiting eligibility for some invasive procedures for psychiatric disease.110 The laws are complex, however, and some have exceptions for neuromodulatory therapies like DBS.110 In light of the development of novel surgical therapies, the meeting participants emphasized the importance of re-reviewing these older laws to determine what is optimal to protect patients while enabling the development of novel treatments. Consent practices, risk disclosure, and continued patient management are all areas ripe for legal review. Many devices allow for partial or full remote programming, and soon all devices will likely have this capability.111 This feature could enable patients that live far from advanced centers to benefit from these novel therapies. There may be legal impediments to such a strategy if, for example, physicians are licensed in a different state. If traveling for psychiatric surgery becomes common, there may be legal implications to this “psychiatric neurosurgical tourism.” Changing or enacting laws is difficult, so participants suggested that physicians start with position/consensus statements.
In sum, meeting participants identified a need for further investigation of the ethics of psychosurgery including, but not limited to, the consent process and patient eligibility for treatment. There is a need for discussions with patients and patient advocacy groups regarding study design and participation. Meeting participants also noted that laws concerning the surgical treatment of psychiatric diseases could benefit from review and reform. The results of our ethics of psychiatric surgery SWOT analysis are listed in Table 4.
Table 4.
Ethics of Psychiatric Surgery SWOT
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
Resource Allocation, Prioritization, and Access
Funding is necessary for the development of novel therapies, but is, of course, a limited resource. NIH speakers reported on the growing number of funding opportunities for trials of surgical and non-surgical interventions for psychiatric disease, and the robust NIH device development pipeline. New funding opportunities such as the Blueprint MedTech program aim to foster collaboration between academia and industry.97
Another potential avenue for public-private collaboration is the Accelerating Medicines Partnership® (AMP®) program managed through the Foundation for the National Institutes of Health (FNIH). As an example, an AMP program currently exists for schizophrenia research (AMP SCZ). This partnership developed as a response to the waning development of novel treatments for this disease. In this program, private companies provide an asset (in the case of AMPSCZ, a drug), and the FNIH provides funding for the research study. While this program is focused on drug development, it represents a model for what might be possible for future public/private partnerships in the area of devices.
Many previous trials relied on subjective self-reported measures that led to high heterogeneity between subjects and did not clearly connect behavior to brain circuit function. NIH resources will be allocated to future trials that favor transdiagnostic patient selection. Furthermore, trials structured to provide valuable knowledge (e.g. brain state alterations during stimulation) in addition to symptom-based outcomes will be prioritized to safeguard against trial “failure.” Resources will be preferentially allocated towards trials designed to develop disease classifications based on brain states as outlined above in the section on biomarkers.
Industry representatives in the group shared their enthusiasm for working with NIH to optimize resource allocation and suggested standing meetings to foster collaboration. Their goal is to “transform patients’ lives,” which means not only development of novel therapies but also adoption of those therapies. Increased treatment access requires increased adoption, and participants at the meeting discussed facilitating programming (e.g. via automation or visualization) to enhance adoption. Proper access to care requires robust referral networks and reimbursement for the treatment as well. For increased access to this type of care, adoption of these therapies will be required by all relevant parties including prescribers and referring physicians, implanters, hospitals, and payors.
Work toward improving access could start with advocating for the enforcement of already existing mental health parity laws.27 Insurance company approval is critical for access to these treatments and may be facilitated by developing a centralized database of insurance authorizations, appeals, and denials.112 Maintaining an existing HDE, for example the DBS for OCD HDE, is essential for the care of both new patients and patients already implanted with DBS systems. Collaboration with groups such as the International OCD Foundation (IOCDF), Hope for Depression foundation, and the Tourette Association of America to develop guidelines and advocate for patients may serve to improve access to care.
In sum, meeting participants identified a need for further private-public partnership and collaboration that build off the success of AMP programs. There is also a need for resource allocation towards trials that include transdiagnostic patient selection and outcome measures beyond disease severity. We propose that insurance companies be included more frequently in discussions and meetings regarding the development of novel therapies. The findings of our resource allocation/prioritization and access SWOT analysis are enumerated in Table 5.
Table 5.
Resource Allocation/Prioritization and Access SWOT
| Strengths | Weaknesses |
|---|---|
|
|
| Opportunities | Threats |
|
|
Future Directions, Action Items, and Conclusions
The expert discussion yielded a realization that there continues to be a ‘push-pull’ phenomenon between the increasing complexity of research studies versus the need to simplify novel clinical treatments. We will need to address the challenges inherent to the ‘push-pull’ in order to facilitate widespread adoption of psychiatric DBS. The two aims are not at odds, and future research could use complex strategies to ascertain mechanisms that may be used, for example, to simplify programming, for example. Increased collaboration between disciplines was discussed throughout the meeting and an important future direction discussed was increased education and meeting attendance by collaborating specialties (e.g. neurosurgeons attending psychiatry focused meetings, such as meetings held by the American Psychiatric Association). Furthermore, increased interdisciplinary collaboration between psychiatry and psychology was considered essential and integration of psychotherapy in conjunction with neuromodulation could be important in the future.66,67
Fostering an increase in private-public collaboration was another important action item. As outlined above, the Blueprint MedTech and AMP programs are potential future avenues for NIH-industry partnership. While the meeting focused on the development of DBS for psychiatric disorders, VNS was also considered as a viable option for some patients. VNS may offer certain advantages, such as decreased cost and simpler implementation. The results of an ongoing prospective, multi-center, randomized controlled blinded trial of VNS for TRD (NCT03887715) may further elucidate the utility and indications for VNS. Overall, the group felt that patient populations would benefit from many types of neuromodulation and more work will need to be performed to clarify who should receive what treatment. Additionally, working with industry partners to establish appropriate market size estimates will be important for future indication expansion proposals.
Another priority of the group is to improve access to mental health care and promote insurance coverage of effective new neuromodulation therapies. To this end, we propose inviting insurance providers and Medicare/Medicaid representatives to future meetings to discuss the path toward coverage of surgical treatments for psychiatric disease. Furthermore, there is a need to increase awareness and understanding among patients and the public in general about these novel therapies in order to reduce prior stigma associated with surgical psychiatry. Foundation representatives or community leaders could be invited to future meetings to address patient concerns. Ensuring that patients have access to the care they need for treatment resistant psychiatric diseases will be an important future topic and area for increased emphasis. While some weakness and threats to the development and adoption of surgical treatments for psychiatric diseases exist, meeting participants anticipate that the strengths and opportunities of these approaches will prevail.
Highlights.
The American Society for Stereotactic and Functional Neurosurgery (ASSFN) convened a meeting of leaders in the field to discuss a path forward for psychiatric neurosurgery.
The participants were experts in the fields of neurology, neurosurgery, psychiatry, industry, government, ethics, and law.
The meeting focused on interdisciplinary engagement, regulatory pathways and trial design, disease biomarkers, ethics of psychiatric surgery, and resource allocation/prioritization.
We present our strengths, weaknesses, opportunities, and threats analyses of the current state of psychiatric neurosurgery.
Acknowledgements:
We thank Anita Bajaj, Doe Kumsa, John Marler, David McMullen, Pamela Scott, and Vivek Pinto from the FDA for their expertise on device regulatory pathways. The views expressed are the authors’ own and do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, FDA, or the US government.
Conflicts of Interest:
Sarah Bick receives funding from the Neurosurgery Research and Career Development Program (K12 NS080223) and consulting honoraria from Varian Medical Systems. Rafael Carbunaru owns stock options and is an employee of Boston Scientific, a manufacturer of DBS devices. Jennifer Chandler receives funding from CIHR (Canadian Institutes of Health Research) through the ERANET-Neuron program. Binith Cheeran owns stock options and is an employee of Abbott, a manufacturer of DBS devices. Rachel Davis receives consulting honoraria from Medtronic and speaker fees from Baylor for an OCD conference. Darin Dougherty’s research has been funded by the International OCD Foundation, Brain and Behavior Research Foundation, National Institute of Mental Health, Tiny Blue Dot Foundation and Medtronic; he has received honoraria and consultation fees from Medtronic, Sage, and Celanese and has equity in Neurable, Innercosmos, and Intrinsic Powers. Ashley Feinsinger receives funding from the NIH (RF1MH121373 and UH3NS103442), received honoraria for her work on the NIH Neuroethics Workgroup, she is on an advisory board of Vivani Medical Products (Orion Early Feasibility Study), and she is on the data safety monitoring board of R01 MH122431. Kelly Foote reported grants from the National Institutes of Health during the conduct of the study; nonfinancial support from Medtronic (donation of closed-loop DBS devices) outside the submitted work; and grants from Medtronic, Boston Scientific, and Functional Neuromodulation outside the submitted work. Wayne Goodman receives funding from NIH (UH3NS100459), the McNair Foundation, and Biohaven. WG receives royalties from Nview, LLC and OCDscales, LLC as well as consulting honoraria from Biohaven. Aysegul Gunduz receives investigational device donations from Medtronic under the NIH BRAIN Public-Private Partnership agreements, and her research is funded by NIH grants UH3NS095553, R01NS096008, UH3NS119844. Casey Halpern has patents related to sensing and brain stimulation for the treatment of neuropsychiatric disorders, and he works as a consultant for Boston Scientific Neuromodulation and Insightec. Brian Kopell has received consulting honoraria from Abbott and Medtronic. Cynthia Kubu receives grant funding from the NIH (5RO1MH114853, 5RC1NS068086, 3RF1MH123407-01S1) and participates on the data safety monitoring boards for studies investigating the use of DBS for pain (UHS3 BRIAN/UH3 HEAL, 3UH3NS113661). She is the president of the Society for Clinical Neuropsychology. Sarah Lisanby receives funding from the NIMH (1ZIAMH002955) and has a role on the Scientific Advisory Boards of the Aalto University School of Science and the German Center for Brain Stimulation. Cameron McIntyre is a paid consultant for Boston Scientific Neuromodulation, receives royalties from Hologram Consultants, Neuros Medical, Qr8 Health, and is a shareholder in the following companies: Hologram Consultants, Surgical Information Sciences, BrainDynamics, CereGate, Autonomic Technologies, Cardionomic, Enspire DBS. Michael Okun serves as Medical Advisor for the Parkinson’s Foundation, and has received research grants from NIH, Parkinson’s Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Michael Okun’s research is supported by: NIH R01 NR014852, R01NS096008, UH3NS119844, U01NS119562. Michael Okun is PI of the NIH R25NS108939 Training Grant. Michael Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). Nader Pouratian receives research funding from NIH (R24 MH114796, UH3 NS103442, UH3 NS103549, R01 NS097782, UH3 NS113661, R01 GM135420, RF1 MH121373), consulting/presentation honoraria from Abbott, Sensoria Therapeutics, Boston Scientific, and BrainLab. Nader Pouratian is on an advisory board at Abbott Laboratories and has leadership positions in the Congress of Neurological Surgeons and American Society of Stereotactic and Functional Neurosurgery. Robert Raike owns stock options and is an employee of Medtronic, a manufacturer of DBS devices. Patricio Riva-Posse has received honoraria for consulting for Janssen Pharmaceuticals, Abbott Neuromodulation, and LivaNova. Sameer Sheth received funding from the McNair Foundation for this work. SS receives consulting honoraria from Boston Scientific, Neuropace, Zimmer Biomet, and Koh Young. Nora Vanegas-Arroyave receives research funding from NIH and the Michael J. Fox foundation. Alik Widge has received honoraria for consulting for Abbott, he has received device donations from Medtronic, and he has unlicensed patents in the area of deep brain stimulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Frederick Hitti: Conceptualization, Project Administration, Writing – Original Draft Preparation, Writing – Review & Editing. Alik Widge: Writing – Review & Editing. Patricio Riva-Posse: Writing – Review & Editing. Donald Malone Jr.: Writing – Review & Editing. Michael Okun: Writing – Review & Editing. Maryam Shanechi: Writing – Review & Editing. Kelly Foote: Writing – Review & Editing. Sarah Lisanby: Writing – Review & Editing. Elizabeth Ankudowich: Writing – Review & Editing. Srinivas Chivukula: Writing – Review & Editing. Edward Chang: Writing – Review & Editing. Aysegul Gunduz: Writing – Review & Editing. Clement Hamani: Writing – Review & Editing. Ashley Feinsinger: Writing – Review & Editing. Cynthia Kubu: Writing – Review & Editing. Winston Chiong: Writing – Review & Editing. Jennifer Chandler: Writing – Review & Editing. Rafael Carbunaru: Writing – Review & Editing. Binith Cheeran: Writing – Review & Editing. Robert Raike: Writing – Review & Editing. Rachel Davis: Writing – Review & Editing. Casey Halpern: Writing – Review & Editing. Nora Vanegas-Arroyave: Writing – Review & Editing. Dejan Markovic: Writing – Review & Editing. Sarah Bick: Writing – Review & Editing. Cameron McIntyre: Writing – Review & Editing. R. Mark Richardson: Writing – Review & Editing. Darin Dougherty: Writing – Review & Editing. Brian Kopell: Writing – Review & Editing. Jennifer Sweet: Writing – Review & Editing. Wayne Goodman: Writing – Review & Editing. Sameer Sheth: Conceptualization, Project Administration, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing. Nader Pouratian: Conceptualization, Project Administration, Supervision, Writing – Original Draft Preparation, Writing – Review & Editing.
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLOS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steel Z, Marnane C, Iranpour C, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol. 2014;43(2):476–493. doi: 10.1093/ije/dyu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini L, Maietti E, Rucci P, et al. Suicide attempts and suicidal ideation in patients with obsessive-compulsive disorder: A systematic review and meta-analysis. J Affect Disord. 2020;276:1001–1021. doi: 10.1016/j.jad.2020.07.115 [DOI] [PubMed] [Google Scholar]
- 5.Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and Medication in the Treatment of Adult and Geriatric Depression: Which Monotherapy or Combined Treatment? J Clin Psychiatry. 2005;66(4):455–468. [DOI] [PubMed] [Google Scholar]
- 6.Thase ME, Friedman ES, Biggs MM, et al. Cognitive Therapy Versus Medication in Augmentation and Switch Strategies as Second-Step Treatments: A STAR*D Report. Am J Psychiatry. 2007;164(5):739–752. doi: 10.1176/ajp.2007.164.5.739 [DOI] [PubMed] [Google Scholar]
- 7.Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67 Suppl 6:16–22. [PubMed] [Google Scholar]
- 8.Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62 Suppl 16:26–31. [PubMed] [Google Scholar]
- 9.Taipale H, Reutfors J, Tanskanen A, et al. Risk and risk factors for disability pension among patients with treatment resistant depression- a matched cohort study. BMC Psychiatry. 2020;20(1):232. doi: 10.1186/s12888-020-02642-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181–191. doi: 10.1017/S1461145702002900 [DOI] [PubMed] [Google Scholar]
- 11.Hitti FL, Ramayya AG, McShane BJ, Yang AI, Vaughan KA, Baltuch GH. Long-term outcomes following deep brain stimulation for Parkinson’s disease. J Neurosurg. Published online January 18, 2019:1–6. doi: 10.3171/2018.8.JNS182081 [DOI] [PubMed] [Google Scholar]
- 12.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, Applications, and Mechanisms of Deep Brain Stimulation. JAMA Neurol. 2013;70(2):163–171. doi: 10.1001/2013.jamaneurol.45 [DOI] [PubMed] [Google Scholar]
- 13.Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061–1068. doi: 10.1001/archgenpsychiatry.2010.122 [DOI] [PubMed] [Google Scholar]
- 14.Luyten L, Hendrickx S, Raymaekers S, Gabriels L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21(9):1272–1280. doi: 10.1038/mp.2015.124 [DOI] [PubMed] [Google Scholar]
- 15.Provenza NR, Sheth SA, Dastin-van Rijn EM, et al. Long-term ecological assessment of intracranial electrophysiology synchronized to behavioral markers in obsessive-compulsive disorder. Nat Med. 2021;27(12):2154–2164. doi: 10.1038/s41591-021-01550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet Lond Engl. 1999;354(9189):1526. doi: 10.1016/S0140-6736(99)02376-4 [DOI] [PubMed] [Google Scholar]
- 17.Nuttin BJ, Gabriëls LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263–1272; discussion 1272–1274. doi: 10.1227/01.neu.0000064565.49299.9a [DOI] [PubMed] [Google Scholar]
- 18.Tyagi H, Apergis-Schoute AM, Akram H, et al. A Randomized Trial Directly Comparing Ventral Capsule and Anteromedial Subthalamic Nucleus Stimulation in Obsessive-Compulsive Disorder: Clinical and Imaging Evidence for Dissociable Effects. Biol Psychiatry. 2019;85(9):726–734. doi: 10.1016/j.biopsych.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121–2134. doi: 10.1056/NEJMoa0708514 [DOI] [PubMed] [Google Scholar]
- 20.Welter ML, Alves Dos Santos JF, Clair AH, et al. Deep Brain Stimulation of the Subthalamic, Accumbens, or Caudate Nuclei for Patients With Severe Obsessive-Compulsive Disorder: A Randomized Crossover Controlled Study. Biol Psychiatry. 2021;90(10):e45–e47. doi: 10.1016/j.biopsych.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 21.Gadot R, Najera R, Hirani S, et al. Efficacy of deep brain stimulation for treatment-resistant obsessive-compulsive disorder: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93(11):1166–1173. doi: 10.1136/jnnp-2021-328738 [DOI] [PubMed] [Google Scholar]
- 22.Haber SN, Yendiki A, Jbabdi S. Four Deep Brain Stimulation Targets for Obsessive-Compulsive Disorder: Are They Different? Biol Psychiatry. 2021;90(10):667–677. doi: 10.1016/j.biopsych.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Baldermann JC, Kibleur A, et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nat Commun. 2020;11(1):3364. doi: 10.1038/s41467-020-16734-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldermann JC, Schüller T, Kohl S, et al. Connectomic Deep Brain Stimulation for Obsessive-Compulsive Disorder. Biol Psychiatry. 2021;90(10):678–688. doi: 10.1016/j.biopsych.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 25.Garnaat SL, Greenberg BD, Sibrava NJ, et al. Who Qualifies for Deep Brain Stimulation for OCD? Data from a Naturalistic Clinical Sample. J Neuropsychiatry Clin Neurosci. 2014;26(1):81–86. doi: 10.1176/appi.neuropsych.12090226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser-Vandewalle V, Andrade P, Mosley PE, et al. Deep brain stimulation for obsessive–compulsive disorder: a crisis of access. Nat Med. 2022;28(8):1529–1532. doi: 10.1038/s41591-022-01879-z [DOI] [PubMed] [Google Scholar]
- 27.Davis RA, Giordano J, Hufford DB, et al. Restriction of Access to Deep Brain Stimulation for Refractory OCD: Failure to Apply the Federal Parity Act. Front Psychiatry. 2021;12:706181. doi: 10.3389/fpsyt.2021.706181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raymaekers S, Luyten L, Bervoets C, Gabriels L, Nuttin B. Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and long-term follow-up. Transl Psychiatry. 2017;7(10):e1251. doi: 10.1038/tp.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartorius A, Kiening KL, Kirsch P, et al. Remission of Major Depression Under Deep Brain Stimulation of the Lateral Habenula in a Therapy-Refractory Patient. Biol Psychiatry. 2010;67(2):e9–e11. doi: 10.1016/j.biopsych.2009.08.027 [DOI] [PubMed] [Google Scholar]
- 30.Coenen VA, Bewernick BH, Kayser S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2019;44(7):1224–1232. doi: 10.1038/s41386-019-0369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenoy AJ, Schulz PE, Selvaraj S, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8(1):111. doi: 10.1038/s41398-018-0160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839–849. doi: 10.1016/S2215-0366(17)30371-1 [DOI] [PubMed] [Google Scholar]
- 33.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–158. doi: 10.1001/archgenpsychiatry.2011.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merkl A, Schneider GH, Schonecker T, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol. 2013;249:160–168. doi: 10.1016/j.expneurol.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 35.Merkl A, Aust S, Schneider GH, et al. Deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: A double-blinded randomized controlled study and long-term follow-up in eight patients. J Affect Disord. 2018;227:521–529. doi: 10.1016/j.jad.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 36.Puigdemont D, Portella M, Perez-Egea R, et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiatry Neurosci JPN. 2015;40(4):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZHT. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J Psychiatry Neurosci JPN. 2013;38(5):325–332. doi: 10.1503/jpn.120160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergfeld IO, Mantione M, Hoogendoorn MLC, et al. Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73(5):456–464. doi: 10.1001/jamapsychiatry.2016.0152 [DOI] [PubMed] [Google Scholar]
- 39.Dougherty DD, Rezai AR, Carpenter LL, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biol Psychiatry. 2015;78(4):240–248. doi: 10.1016/j.biopsych.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Zhang H, Qin Y, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:224–232. doi: 10.1016/j.pnpbp.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 41.Kisely S, Li A, Warren N, Siskind D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468–480. doi: 10.1002/da.22746 [DOI] [PubMed] [Google Scholar]
- 42.Berlim MT, McGirr A, Van den Eynde F, Fleck MPA, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31–38. doi: 10.1016/j.jad.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 43.Hitti FL, Yang AI, Cristancho MA, Baltuch GH. Deep Brain Stimulation Is Effective for Treatment-Resistant Depression: A Meta-Analysis and Meta-Regression. J Clin Med. 2020;9(9). doi: 10.3390/jcm9092796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crowell AL, Riva-Posse P, Holtzheimer PE, et al. Long-Term Outcomes of Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Am J Psychiatry. Published online October 4, 2019:appi.ajp.2019.18121427. doi: 10.1176/appi.ajp.2019.18121427 [DOI] [PubMed] [Google Scholar]
- 45.Hitti FL, Cristancho MA, Yang AI, O’Reardon JP, Bhati MT, Baltuch GH. Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Treatment-Resistant Depression: A Decade of Clinical Follow-Up. J Clin Psychiatry. 2021;82(6):21m13973. doi: 10.4088/JCP.21m13973 [DOI] [PubMed] [Google Scholar]
- 46.van der Wal JM, Bergfeld IO, Lok A, et al. Long-term deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. J Neurol Neurosurg Psychiatry. 2020;91(2):189–195. doi: 10.1136/jnnp-2019-321758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noecker AM, Choi KS, Riva-Posse P, Gross RE, Mayberg HS, McIntyre CC. StimVision Software: Examples and Applications in Subcallosal Cingulate Deep Brain Stimulation for Depression. Neuromodulation J Int Neuromodulation Soc. 2018;21(2):191–196. doi: 10.1111/ner.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riva-Posse P, Choi KS, Holtzheimer PE, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23(4):843–849. doi: 10.1038/mp.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsolaki E, Espinoza R, Pouratian N. Using probabilistic tractography to target the subcallosal cingulate cortex in patients with treatment resistant depression. Psychiatry Res Neuroimaging. 2017;261:72–74. doi: 10.1016/j.pscychresns.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coenen VA, Sajonz B, Reisert M, et al. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. NeuroImage Clin. 2018;20:580–593. doi: 10.1016/j.nicl.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coenen VA, Schlaepfer TE, Reinacher PC, Mast H, Urbach H, Reisert M. Machine learning-aided personalized DTI tractographic planning for deep brain stimulation of the superolateral medial forebrain bundle using HAMLET. Acta Neurochir (Wien). 2019;161(8):1559–1569. doi: 10.1007/s00701-019-03947-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatia KD, Henderson L, Ramsey-Stewart G, May J. Diffusion tensor imaging to aid subgenual cingulum target selection for deep brain stimulation in depression. Stereotact Funct Neurosurg. 2012;90(4):225–232. doi: 10.1159/000338083 [DOI] [PubMed] [Google Scholar]
- 53.Sheth SA, Bijanki KR, Metzger B, et al. Deep Brain Stimulation for Depression Informed by Intracranial Recordings. Biol Psychiatry. 2022;92(3):246–251. doi: 10.1016/j.biopsych.2021.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bari AA, Mikell CB, Abosch A, et al. Charting the road forward in psychiatric neurosurgery: proceedings of the 2016 American Society for Stereotactic and Functional Neurosurgery workshop on neuromodulation for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2018;89(8):886–896. doi: 10.1136/jnnp-2017-317082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghazinoory S, Abdi M, Azadegan-Mehr M. SWOT methodology: a state-of-the-art review for the past, a framework for the future. J Bus Econ Manag. 2011;12(1):24–48. doi: 10.3846/16111699.2011.555358 [DOI] [Google Scholar]
- 56.Nuttin B, Wu H, Mayberg H, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2014;85(9):1003–1008. doi: 10.1136/jnnp-2013-306580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radder DLM, Nonnekes J, van Nimwegen M, et al. Recommendations for the Organization of Multidisciplinary Clinical Care Teams in Parkinson’s Disease. J Park Dis. 2020;10(3):1087–1098. doi: 10.3233/JPD-202078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzales AA, Mastrolonardo A, Winget K, Ragulojan M, Fleming AJ, Singh SK. The Role of a Longitudinal, Multidisciplinary Clinic in Building a Unique Research Collaborative. Front Oncol. 2022;12:857699. doi: 10.3389/fonc.2022.857699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redpath P, Searle A, Wall C, et al. Guided Self-Help for People with Chronic Pain: Integrated Care in a Public Tertiary Pain Clinic-A Pilot Study. Pain Ther. Published online January 3, 2023. doi: 10.1007/s40122-022-00464-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray KK, Ference BA, Séverin T, et al. World Heart Federation Cholesterol Roadmap 2022. Glob Heart. 2022;17(1):75. doi: 10.5334/gh.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vilendrer S, Saliba-Gustafsson EA, Asch SM, et al. Evaluating clinician-led quality improvement initiatives: A system-wide embedded research partnership at Stanford Medicine. Learn Health Syst. 2022;6(4):e10335. doi: 10.1002/lrh2.10335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fung LK, Akil M, Widge A, Roberts LW, Etkin A. Attitudes toward neuroscience education in psychiatry: a national multi-stakeholder survey. Acad Psychiatry J Am Assoc Dir Psychiatr Resid Train Assoc Acad Psychiatry. 2015;39(2):139–146. doi: 10.1007/s40596-014-0183-y [DOI] [PubMed] [Google Scholar]
- 63.Oyama G, Rodriguez RL, Jones JD, et al. Selection of deep brain stimulation candidates in private neurology practices: referral may be simpler than a computerized triage system. Neuromodulation J Int Neuromodulation Soc. 2012;15(3):246–250; discussion 250. doi: 10.1111/j.1525-1403.2012.00437.x [DOI] [PubMed] [Google Scholar]
- 64.Katz M, Kilbane C, Rosengard J, Alterman RL, Tagliati M. Referring patients for deep brain stimulation: an improving practice. Arch Neurol. 2011;68(8):1027–1032. doi: 10.1001/archneurol.2011.151 [DOI] [PubMed] [Google Scholar]
- 65.Thomas NJ, Mertens P, Danaila T, et al. Optimizing the deep brain stimulation care pathway in patients with Parkinson’s disease. J Neurol. 2017;264(7):1454–1464. doi: 10.1007/s00415-017-8548-2 [DOI] [PubMed] [Google Scholar]
- 66.Denys D, Graat I, Mocking R, et al. Efficacy of Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Refractory Obsessive-Compulsive Disorder: A Clinical Cohort of 70 Patients. Am J Psychiatry. 2020;177(3):265–271. doi: 10.1176/appi.ajp.2019.19060656 [DOI] [PubMed] [Google Scholar]
- 67.Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychol Med. 2014;44(16):3515–3522. doi: 10.1017/S0033291714000956 [DOI] [PubMed] [Google Scholar]
- 68.Trapp NT, Williams NR. The Future of Training and Practice in Neuromodulation: An Interventional Psychiatry Perspective. Front Psychiatry. 2021;12:734487. doi: 10.3389/fpsyt.2021.734487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goering S, Klein E, Dougherty DD, Widge AS. Staying in the Loop: Relational Agency and Identity in Next-Generation DBS for Psychiatry. AJOB Neurosci. 2017;8(2):59–70. doi: 10.1080/21507740.2017.1320320 [DOI] [Google Scholar]
- 70.Zahodne LB, Marsiske M, Okun MS, Rodriguez RL, Malaty I, Bowers D. Mood and motor trajectories in Parkinson’s disease: multivariate latent growth curve modeling. Neuropsychology. 2012;26(1):71–80. doi: 10.1037/a0025119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller KM, Okun MS, Fernandez HF, Jacobson CE, Rodriguez RL, Bowers D. Depression symptoms in movement disorders: comparing Parkinson’s disease, dystonia, and essential tremor. Mov Disord Off J Mov Disord Soc. 2007;22(5):666–672. doi: 10.1002/mds.21376 [DOI] [PubMed] [Google Scholar]
- 72.Okun MS, Watts RL. Depression associated with Parkinson’s disease: clinical features and treatment. Neurology. 2002;58(4 Suppl 1):S63–70. doi: 10.1212/wnl.58.suppl_1.s63 [DOI] [PubMed] [Google Scholar]
- 73.Wendt K, Denison T, Foster G, et al. Physiologically informed neuromodulation. J Neurol Sci. 2022;434:120121. doi: 10.1016/j.jns.2021.120121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abbott Receives FDA’s Breakthrough Device Designation to Explore Use of Deep Brain Stimulation to Manage Severe Depression. Abbott MediaRoom. Accessed August 30, 2022. https://abbott.mediaroom.com/2022-07-12-Abbott-Receives-FDAs-Breakthrough-Device-Designation-to-Explore-Use-of-Deep-Brain-Stimulation-to-Manage-Severe-Depression [Google Scholar]
- 75.Moher D, Dulberg CS, Wells GA. Statistical power, sample size, and their reporting in randomized controlled trials. JAMA. 1994;272(2):122–124. [PubMed] [Google Scholar]
- 76.Jones B, Lewis JA. The case for cross-over trials in phase III. Stat Med. 1995;14(9–10):1025–1038. doi: 10.1002/sim.4780140921 [DOI] [PubMed] [Google Scholar]
- 77.Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535–542. doi: 10.1016/j.biopsych.2009.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelley ME, Franco AR, Mayberg HS, Holtzheimer PE. The Illness Density Index (IDI) : A longitudinal measure of treatment efficacy. Clin Trials Lond Engl. 2012;9(5):596–604. doi: 10.1177/1740774512450099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bewernick BH, Kayser S, Gippert SM, Switala C, Coenen VA, Schlaepfer TE. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimulat. 2017;10(3):664–671. doi: 10.1016/j.brs.2017.01.581 [DOI] [PubMed] [Google Scholar]
- 80.LaRiviere MJ, Gross RE. Stereotactic Laser Ablation for Medically Intractable Epilepsy: The Next Generation of Minimally Invasive Epilepsy Surgery. Front Surg. 2016;3. Accessed September 2, 2022. https://www.frontiersin.org/articles/10.3389/fsurg.2016.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, et al. Efficacy and Safety of Deep Brain Stimulation in Tourette Syndrome: The International Tourette Syndrome Deep Brain Stimulation Public Database and Registry. JAMA Neurol. 2018;75(3):353–359. doi: 10.1001/jamaneurol.2017.4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Health C for D and R. Premarket Approval (PMA). FDA Published March 15, 2022. Accessed September 1, 2022. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma
- 83.Health C for D and R. Investigational Device Exemption (IDE). FDA Published November 30, 2021. Accessed September 1, 2022. https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/investigational-device-exemptionide
- 84.Gilron R, Little S, Perrone R, et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat Biotechnol. 2021;39(9):1078–1085. doi: 10.1038/s41587-021-00897-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cagle JN, Okun MS, Opri E, et al. Differentiating tic electrophysiology from voluntary movement in the human thalamocortical circuit. J Neurol Neurosurg Psychiatry. 2020;91(5):533–539. doi: 10.1136/jnnp-2019-321973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veerakumar A, Tiruvadi V, Howell B, et al. Field potential 1/f activity in the subcallosal cingulate region as a candidate signal for monitoring deep brain stimulation for treatment-resistant depression. J Neurophysiol. 2019;122(3):1023–1035. doi: 10.1152/jn.00875.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vissani M, Nanda P, Bush A, Neudorfer C, Dougherty D, Richardson RM. Toward Closed-Loop Intracranial Neurostimulation in Obsessive-Compulsive Disorder. Biol Psychiatry. Published online September 16, 2022:S0006–3223(22)01432–9. doi: 10.1016/j.biopsych.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 88.Olsen ST, Basu I, Bilge MT, et al. Case Report of Dual-Site Neurostimulation and Chronic Recording of Cortico-Striatal Circuitry in a Patient With Treatment Refractory Obsessive Compulsive Disorder. Front Hum Neurosci. 2020;14:569973. doi: 10.3389/fnhum.2020.569973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Hemptinne C, Chen W, Racine CA, et al. Prefrontal Physiomarkers of Anxiety and Depression in Parkinson’s Disease. Front Neurosci. 2021;15:748165. doi: 10.3389/fnins.2021.748165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scangos KW, Makhoul GS, Sugrue LP, Chang EF, Krystal AD. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med. Published online January 18, 2021:1–3. doi: 10.1038/s41591-020-01175-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scangos KW, Khambhati AN, Daly PM, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27(10):1696–1700. doi: 10.1038/s41591-021-01480-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allawala A, Bijanki KR, Goodman W, et al. A Novel Framework for Network-Targeted Neuropsychiatric Deep Brain Stimulation. Neurosurgery. 2021;89(2):E116–E121. doi: 10.1093/neuros/nyab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adkinson JA, Tsolaki E, Sheth SA, et al. Imaging versus electrographic connectivity in human mood-related fronto-temporal networks. Brain Stimul Basic Transl Clin Res Neuromodulation. 2022;15(3):554–565. doi: 10.1016/j.brs.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 95.Conner CR, Quevedo J, Soares JC, Fenoy AJ. Brain metabolic changes and clinical response to superolateral medial forebrain bundle deep brain stimulation for treatment-resistant depression. Mol Psychiatry. 2022;27(11):4561–4567. doi: 10.1038/s41380-022-01726-0 [DOI] [PubMed] [Google Scholar]
- 96.Mott MC, Austin CP, Bianchi DW, et al. The NIH Blueprint for Neuroscience Research Seeks Community Input on Future Neuroscience Investments. J Neurosci Off J Soc Neurosci. 2019;39(5):774–775. doi: 10.1523/JNEUROSCI.2742-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Home | Blueprint. Accessed September 5, 2022. https://neuroscienceblueprint.nih.gov/
- 98.Dupré D, Krumhuber EG, Küster D, McKeown GJ. A performance comparison of eight commercially available automatic classifiers for facial affect recognition. PLOS ONE. 2020;15(4):e0231968. doi: 10.1371/journal.pone.0231968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ertugrul IO, Jeni LA, Ding W, Cohn JF. AFAR: A Deep Learning Based Tool for Automated Facial Affect Recognition. In: 2019 14th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2019).; 2019:1–1. doi: 10.1109/FG.2019.8756623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105 [DOI] [PubMed] [Google Scholar]
- 101.Sullivan CRP, Olsen S, Widge AS. Deep brain stimulation for psychiatric disorders: From focal brain targets to cognitive networks. NeuroImage. 2021;225:117515. doi: 10.1016/j.neuroimage.2020.117515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Widge AS, Bilge MT, Montana R, et al. Electroencephalographic Biomarkers for Treatment Response Prediction in Major Depressive Illness: A Meta-Analysis. Am J Psychiatry. 2019;176(1):44–56. doi: 10.1176/appi.ajp.2018.17121358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Widge AS, Ellard KK, Paulk AC, et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp Neurol. 2017;287(Pt 4):461–472. doi: 10.1016/j.expneurol.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 104.Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Rashed Ahmed AP, Samara Z, Williams LM. Transdiagnostic Symptom Clusters and Associations With Brain, Behavior, and Daily Function in Mood, Anxiety, and Trauma Disorders. JAMA Psychiatry. 2018;75(2):201–209. doi: 10.1001/jamapsychiatry.2017.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basu I, Yousefi A, Crocker B, et al. Closed-loop enhancement and neural decoding of cognitive control in humans. Nat Biomed Eng. Published online November 1, 2021. doi: 10.1038/s41551-021-00804-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gelineau-Morel R, Kruer MC, Garris JF, et al. Deep Brain Stimulation for Pediatric Dystonia: A Review of the Literature and Suggested Programming Algorithm. J Child Neurol. Published online September 2, 2022:8830738221115248. doi: 10.1177/08830738221115248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hartnett SM, Greiner HM, Arya R, et al. Responsive neurostimulation device therapy in pediatric patients with complex medically refractory epilepsy. J Neurosurg Pediatr. Published online August 26, 2022:1–8. doi: 10.3171/2022.7.PEDS2281 [DOI] [PubMed] [Google Scholar]
- 108.Kubu CS, Frazier T, Cooper SE, Machado A, Vitek J, Ford PJ. Patients’ shifting goals for deep brain stimulation and informed consent. Neurology. 2018;91(5):e472–e478. doi: 10.1212/WNL.0000000000005917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klein E, Goering S, Gagne J, et al. Brain-computer interface-based control of closed-loop brain stimulation: attitudes and ethical considerations. Brain-Comput Interfaces. 2016;3(3):140–148. doi: 10.1080/2326263X.2016.1207497 [DOI] [Google Scholar]
- 110.Chandler JA, Cabrera LY, Doshi P, et al. International Legal Approaches to Neurosurgery for Psychiatric Disorders. Front Hum Neurosci. 2020;14:588458. doi: 10.3389/fnhum.2020.588458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Merola A, Singh J, Reeves K, et al. New Frontiers for Deep Brain Stimulation: Directionality, Sensing Technologies, Remote Programming, Robotic Stereotactic Assistance, Asleep Procedures, and Connectomics. Front Neurol. 2021;12:694747. doi: 10.3389/fneur.2021.694747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinckard-Dover H, Ward H, Foote KD. The Decline of Deep Brain Stimulation for Obsessive-Compulsive Disorder Following FDA Humanitarian Device Exemption Approval. Front Surg. 2021;8:642503. doi: 10.3389/fsurg.2021.642503 [DOI] [PMC free article] [PubMed] [Google Scholar]


