Abstract
In the genetic disease cystic fibrosis, recombinant adeno-associated virus type 2 (AAV2) is being investigated as a vector to transfer CFTR cDNA to airway epithelia. However, earlier work has shown that the apical surface of human airway epithelia is resistant to infection by AAV2, presumably as a result of a lack of heparan sulfate proteoglycans on the apical surface. This inefficiency can be overcome by increasing the amount of vector or by increasing the incubation time. However, these interventions are not very practical for translation into a therapeutic airway-directed vector. Therefore, we examined the efficiency of other AAV serotypes at infecting human airway epithelia. When applied at low multiplicity of infection to the apical surface of differentiated airway epithelia we found that a recombinant AAV5 bound and mediated gene transfer 50-fold more efficiently than AAV2. Furthermore, in contrast to AAV2, AAV5-mediated gene transfer was not inhibited by soluble heparin. Recombinant AAV5 was also more efficient than AAV2 in transferring β-galactosidase cDNA to murine airway and alveolar epithelia in vivo. These data suggest that AAV5-derived vectors bind and mediate gene transfer to human and murine airway epithelia, and the tropism of AAV5 may be useful to target cells that are not permissive for AAV2.
Recombinant adeno-associated viruses (AAV) are being investigated as vectors for gene transfer to a wide variety of cells and tissues (12–14, 24, 31), and it is hoped that they may prove useful for gene therapy. Transduction with AAV2 vectors result in long-term transgene expression in vivo in several cell types, including skeletal muscle, photoreceptors, liver, and some populations of central nervous system neuronal cells (9, 16, 22, 31). Serotype 2 recombinant AAV (AAV2) are also being investigated as vectors to transfer cystic fibrosis transmembrane conductance regulator (CFTR) cDNA to airway epithelia of patients with cystic fibrosis (3, 8, 12–14, 35). However, compared to the efficiency of transfer to muscle, eye, and liver cells, the efficiency of AAV2 gene transfer to human airway epithelia is low (17, 19). The low efficiency appears to be due to the limited binding of AAV2 to the apical surface of human airway epithelia (8, 34, 36).
The molecular mechanism responsible for AAV binding has been advanced by the identification of several candidate receptors for AAV2. However, the relative importance of the various receptors is uncertain. Summerford et al. reported that AAV2 transduction is inefficient in the absence of heparan sulfate proteoglycans (HSP) on the cell surface and that transduction can be inhibited by soluble heparin (33). However, the binding of AAV2 to HSP seems to have a low affinity (26). A novel 150-kDa protein has also been identified on the surface of permissive cells for AAV2 (23). In addition to HSP and the 150-kDa protein, two molecules, FGF-R1 and the β5 subunit of αvβ5 integrins, have been reported to function as coreceptors to facilitate internalization via receptor-mediated endocytosis (25, 32).
The lack of binding of AAV2 to human airway epithelia has been recently explained by the polar expression of HSP on the basolateral but not on the apical surface of airway epithelia (8). Consistent with this, airway epithelia can be more efficiently transduced if the virus is applied to the basolateral surface (8). In addition, if the tight junctions are transiently disrupted to allow access of AAV2 to the basolateral surface, transduction is markedly improved (1, 8). Although gene transfer may also be limited by other steps in the transduction process, such as inefficient intracellular trafficking and second-strand viral DNA synthesis (7, 10, 11, 30, 42), if viral binding and internalization were enhanced, gene transfer should improve. Consistent with this prediction, recent data suggest that interventions which increase virus binding also increase AAV2-mediated gene transfer both in cell lines (2) and in primary cultures of human airway epithelia (36).
AAV are members of the parvovirus family and have in common a similar size, structure, and dependence on a helper virus for replication and gene expression. To date, six primate isolates have been reported, and their genomes appear to be organized in a similar manner (5, 28, 39). AAV2 was the first primate AAV to be cloned into a plasmid and has been extensively studied (29). Recently, AAV from the other five serotypes have been cloned, and their gene transfer abilities have been studied in laboratory cell lines (5, 6, 28, 39) and in murine liver and muscle (28, 39). Sequence and biochemical comparisons of these five serotypes of AAV indicate that AAV5 is the most divergent. Not only is the capsid protein markedly different between AAV2 and AAV5, but the inverted terminal repeats and Rep protein of AAV5 are sufficiently divergent to be unable to complement the replication of AAV2 (5). Moreover, AAV5 transduction efficiency was different from AAV2 in a variety of cells and was not sensitive to soluble heparin that inhibits AAV2 binding and transduction. These data suggest that AAV5 may utilize a distinct mechanism of binding and uptake compared to AAV2. The Rep and inverted terminal repeats of AAV4 are similar to that of AAV2 and can complement the replication of AAV2 virus. However, the capsid protein is only 60% homologous, resulting in a difference in transduction efficiency in a variety of cell types compared to AAV2 and like AAV5 is not sensitive to competition by soluble heparin (6). As a result of these differences, we examined the possibility that either recombinant AAV4 or AAV5 particles may be more efficient than AAV2 at mediating gene transfer to human airway epithelial cells.
MATERIALS AND METHODS
Human airway epithelia.
Airway epithelial cells were obtained from surgical polypectomies of non-cystic fibrosis patients or from trachea and bronchi of lungs removed for organ donation. Cells were isolated by enzyme digestion as previously described (27). Freshly isolated cells were seeded at a density of 5 × 105 cells/cm2 onto collagen-coated, 0.6-cm2-area, 0.4-μm-pore-size Millicell polycarbonate filters (Millipore Corp., Bedford, Mass.). The cells were maintained at 37°C in a humidified atmosphere of 7% CO2 and air. Twenty-four hours after plating, the mucosal media was removed and the cells were allowed to grow at the air-liquid interface (20, 41). The culture medium consisted of a 1:1 mixture of Dulbecco modified Eagle medium and Ham's F-12, 5% Ultroser G (Biosepra SA, Cedex, France), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1% nonessential amino acids, and 0.12 U of insulin per ml. Airway epithelia were allowed to reach confluence and develop a transepithelial electrical resistance, indicating the development of tight junctions and an intact barrier. Epithelia were allowed to differentiate by culturing for at least 14 days after seeding, and the presence of a ciliated surface was tested by scanning electron microscopy (43).
Recombinant AAV.
Recombinant AAV vectors expressing β-galactosidase, AAV2/βGal, AAV4/βGal, and AAV5/βGal, were prepared by using high efficiency electroporation and packaging initiated by adenovirus infection. The resulting virus was purified by CsCl banding and characterized as described previously (5). Briefly, recombinant AAV particles were produced by electroporating 108 exponentially growing Cos cells with 400 μg of a 1:1 mixture of pAAV2RnLacZ and pSV40oriAAV2 for production of AAV2, pAAV2RnLacZ and pSV40oriAAV4 for production of AAV4, or pAAV5RnLacZ and pSV40oriAAV5 for production of AAV5 in 1× RPMI medium (2.5 ml of 2× RPMI medium, 1 ml of fetal calf serum, 1.5 ml of H2O, and 50 μl of 1 M HEPES [pH 7.4]) and were incubated on ice for 10 min prior to electroporation. Electroporation was performed in a 4-mm-gap cuvette (Bio-Rad, Richmond, Calif.) containing 0.5 ml of the cell DNA mixture by using a BTX 600 electroporator. Conditions used for electroporation were 300 V, 2,100 μF, 48 Ω. Following electroporation, the cells were incubated on ice for 10 min then plated into 10 15-cm dishes. The following day the medium was replaced, and the cells were allowed to recover. Approximately 30 to 50% of the cells which were initially electroporated reattached to the plates, and 90% of these cells showed strong expression of the β-galactosidase reporter gene. Two days later, the plates were infected with approximately 5 × 109 PFU (multiplicity of infection [MOI] of 10) of wild-type adenovirus type 5 for 1 h in serum-free media and then were supplemented with D10 media. Seventy-two hours postinfection, the cells were harvested by scraping, and the virus and the cells were pelleted by low-speed centrifugation. The pellet was resuspended in 7.5 ml of TD buffer (140 mM NaCl, 5 mM KCl, 0.7 mM K2HPO4, 25 mM Tris-HCl [pH 7.4]) for every 10 plates. Sodium deoxycholate (0.5 volumes of 10%) was added to the suspension, which was gently mixed and then incubated at 37°C for 30 min. The lysate was then homogenized thoroughly (approximately 20 strokes in a Wheaton B homogenizer). CsCl was then added to a final density of 1.4 g/cm3, and the homogenate was distributed into two polyallomer tubes and was centrifuged in a SW40.1 swinging bucket rotor at 38,000 rpm for 65 h at 20°C. The pellicle at the top of the gradient is removed by using a pasture pipette, and the gradients were fractionated by side puncture. Fractions with a refractive index of 1.373 to 1.371 were pooled for AAV2 and AAV5 and functions with a refractive index of 1.378 to 1.376 were pooled for AAV4, and the gradients were centrifuged again with an SW50.1 rotor and were fractionated as described above. Refractive indices were determined by using a Zeiss refractometer.
Recombinant viruses were titered by Southern blotting, and their biological activity was tested by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining in a serial dilution on Cos-7 cells. The viral titers ranged between 4 × 1012 and 8 × 1012 particles/ml. The particle-to-transduction-unit ratio on these cells was similar to that previously reported for all three viruses on Cos cells (about 104 to 1). The recombinant viruses used were screened for wild-type AAV contamination by PCR and for wild-type adenovirus by a serial dilution assay using a fluorescein isothiocyanate-hexon antibody (less than 103 replication-competent adenoviruses/ml) (43).
Viral infection and binding assays.
Five hundred particles of the recombinant AAV per cell (in phosphate-buffered saline [PBS]) was added to the apical surface. Following the indicated incubation time, the viral suspension was removed, and the epithelia were rinsed twice with PBS. After infection, the epithelia were incubated at 37°C for an additional 14 days.
To assess binding to airway epithelia, the epithelia were incubated for 30 min at 4°C with 500 particles/cell of AAV2/βGal, AAV4/βGal, or AAV5/βGal. The epithelia were then rinsed, and cell-associated AAV DNA was measured from cell lysates of seven epithelia per dot. Samples were subjected to three freeze-thaw cycles and then blotted onto a nylon membrane (Ambion, Austin, Tex.). Detection of the AAV DNA was done by hybridizing with a 32P-labeled pCMVβgal. Unhybridized probe was washed as follows: two washes with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at room temperature for 15 min, one wash with 0.5× SSC and 0.1% SDS at 55°C for 1 h, and one final wash with 0.5× SSC and 0.1% SDS at 65°C for 30 min. Dot blots were developed and quantitated by using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) (36).
Measurement of β-galactosidase activity.
We measured total β-galactosidase activity by using a commercially available method (Galacto-Light; Tropix, Inc., Bedford, Mass.). Briefly, after rinsing with PBS, cells were removed from filters by incubation with 120 μl of lysis buffer (25 mM Tris-phosphate [pH 7.8], 2 mM dithiothreitol, 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, and 1% Triton X-100) for 15 min. Light emission was quantified in a luminometer (Analytical Luminescence Laboratory, San Diego, Calif.). To histochemically detect β-galactosidase activity, we used the chromogenic reagent X-Gal (Boehringer Mannheim). Human airway epithelia and murine lungs were fixed with 1.8% formaldehyde and 2% glutaraldehyde and were then incubated for 16 h at 37°C with 313 μl of 40 mg of X-Gal per ml in dimethyl sulfoxide dissolved in 12.5 ml of PBS (pH 7.8).
Studies in mice.
For in vivo analysis, 6- to 8-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine) were studied. Mice were lightly anesthetized by using a methoxyflurane chamber. Recombinant AAV2 and AAV5 (1010 particles) were administered intranasally in two 62.5-μl instillations delivered 5 min apart. The experiment was performed with five animals per group. Twenty-eight days after vector administration, animals were sacrificed with CO2. PBS (10 ml) was instilled into the right ventricle and then the lungs and heart were removed intact. The trachea was intubated and instilled at 10 cm of pressure with (in order) PBS, 4% paraformaldehyde, and PBS and were stained overnight with X-Gal and finally rinsed with PBS. Lungs were cryosectioned, and the sections were analyzed by two independent reviewers who were unaware of the experimental identity of the samples. The reviewers counted the blue nuclei of β-galactosidase-expressing cells from a 5-μm slice obtained every 50 μm (n = 20 fields/lung). We estimated the total number of airway epithelial cells by dividing the surface of the epithelia (π2r) by 4.9 μm, an estimate of the diameter of the airway epithelial cells (2,425.3 ± 20 airway cells/field).
RESULTS
AAV5 can mediate gene transfer through the apical surface of human airway epithelia.
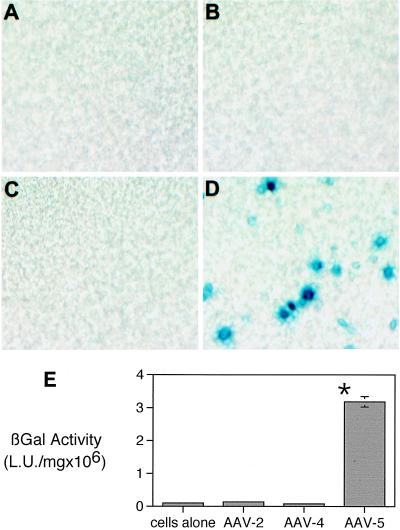
Because previous studies have shown that AAV2, AAV4, and AAV5 have different tropisms in cell lines, we compared the efficiencies of these different serotypes on primary cultures of differentiated human airway epithelia. Epithelia were transduced for 12 h at a relatively low particle-to-cell ratio (500 particles/cell) with an estimated MOI of less than 1 based on Cos cell titer. To allow for maximal expression, the epithelia were studied 2 weeks after infection. Quantification of the β-galactosidase activity showed that AAV5-transduced cells generated activity approximately 50-fold greater than that of AAV2- or AAV4-transduced cells (Fig. 1E). To histochemically detect the β-galactosidase activity, we stained the epithelia with the chromogenic reagent X-Gal. Similar to the quantitative analysis, Fig. 1B and C show only minimal gene transfer in epithelia transduced by AAV2/βGal or AAV4/βGal compared to epithelia transduced with AAV5/βGal (Fig. 1D). To rule out the possibility of pseudotransduction by protein transfer, we assayed the epithelia 1 h after the application of the AAV vectors and detected no β-galactosidase activity over background levels (data not shown).
FIG. 1.
Gene transfer to the apical surface of well-differentiated human airway epithelia by different recombinant AAV serotypes. En face images of human airway epithelia (A) and epithelia transduced with 500 particles per cell of AAV2/βGal (B), AAV4/βGal (C), and AAV5/βGal (D). The blue staining shows cells that have been transduced with vector. Panel E shows the quantitative β-galactosidase activity of airway epithelia infected with the different recombinant AAV serotypes (AAV2/βGal, AAV4/βGal, and AAV5/βGal). Data are mean β-galactosidase activities per milligram of protein ± standard errors of the means (SEMs) (n = 4 to 12). Asterisk indicates P < 0.01.
AAV5 binds to the apical surface of well-differentiated human airway epithelia.
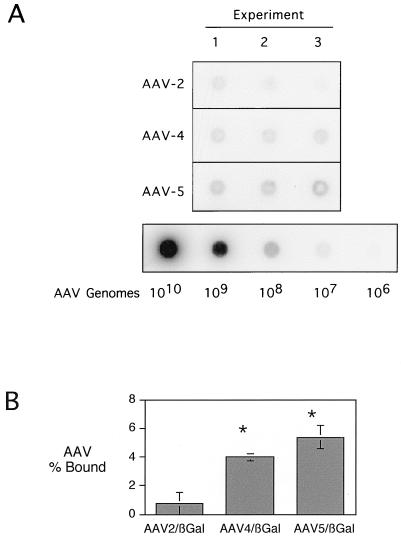
We tested the hypothesis that the improved transduction efficiency of AAV5/βGal relied on increased binding to well-differentiated airway epithelia. Epithelia were incubated for 30 min with 500 particles of AAV2/βGal, AAV4/βGal, or AAV5/βGal per cell and were then rinsed. Cell-associated AAV binding was estimated by dot blot analysis. Figure 2 shows that differentiated airway epithelia bound AAV5-derived vector approximately sevenfold more efficiently than AAV2/βGal. Of interest, AAV4/Gal also bound to the apical surface five times more efficiently than AAV2/Gal. These data may explain some of the advantages of AAV5- over AAV2-derived vectors in mediating gene transfer to the airway epithelia. Moreover, the fact that AAV4 shows increased binding but does not mediate gene transfer more efficiently than AAV2 suggests a different rate-limiting step for AAV4 that may be related to intracellular trafficking and/or binding and internalization.
FIG. 2.
Binding of AAV2/βGal, AAV4/βGal, and AAV5/βGal to organotypic cultures of ciliated human airway epithelia. (A) Dot blot of virus bound to epithelia in three experiments with seven epithelia per experiment (input of 500 virus particles/cell). For the purpose of quantitation, a dilution series of recombinant AAV plasmids was also blotted and probed in order to demonstrate the linearity of the detection system. (B) Results of the quantification of the dot blot data. The data are means ± SEMs of the percentages of total viruses added that remained epithelia-associated after a 30-min incubation. Asterisk indicates P < 0.05.
Effect of dose and incubation time on AAV5 infection of the apical surface of human airway epithelia.
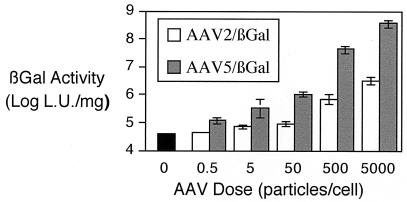
Since AAV5 appeared to bind and mediate gene transfer to the airway epithelia more efficiently than AAV2, we examined the effect of virus dose. Figure 3 shows that in a range of 0.5 to 5,000 particles/cell, AAV5 always outperformed AAV2/βGal. We also tested the course of AAV5-mediated expression of β-galactosidase in vitro over a 1-month period. We found that the level of β-galactosidase expression was stable over 28 days (3.4 × 107 ± 1.4 × 107 light units [LU]/mg of protein and 3.18 × 107 ± 1.1 × 107 LU/mg of protein for 10 and 28 days, respectively).
FIG. 3.
Effect of dose on AAV2/βGal- and AAV5/βGal-mediated gene transfer to human airway epithelia. Human airway epithelia were exposed to increasing concentrations of AAV2/βGal and AAV5/βGal from the apical surface. The epithelia were then rinsed after 1 h and were incubated for 2 weeks prior to analysis of β-galactosidase activity. Data are β-galactosidase activities per mg of protein ± SEMs (n = 4).
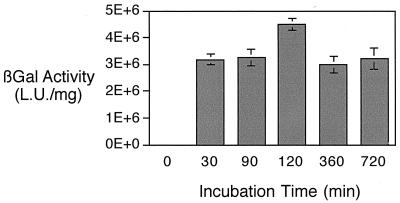
We had previously shown that resistance to adenovirus-mediated gene transfer to the airway epithelia could be at least partially overcome by prolonging the incubation time during which the adenovirus was in contact with the epithelia or by increasing the virus concentration (43). In contrast, in cells that expressed the fiber receptor (coxsackie adenovirus receptor [4]), adenovirus infection occurred very quickly and did not require prolonged incubation times. Similarly, Teramoto et al. showed a time-dependent increase in gene transfer to human airway epithelia with AAV2 (34). Therefore, we tested the effect of incubation time for the vector (AAV5/βGal) on airway epithelia. Figure 4 shows that, contrary to what is seen with recombinant adenovirus and AAV2, incubation of airway epithelia with recombinant AAV5 resulted in similar levels of gene transfer after a short incubation, a 30-min incubation, or a prolonged (12-h) incubation. This is in agreement with the increased affinity found for AAV5 compared to AAV2 and adenoviruses, and more importantly it suggests that there may be an apical receptor for AAV5.
FIG. 4.
Effect of incubation time on AAV5/βGal-mediated gene transfer to human airway epithelia. Human airway epithelia were exposed to 500 particles of AAV5/βGal per cell from the apical surface. The epithelia were then rinsed after 30, 60, 90, 360, and 720 min and were incubated for 2 weeks prior to analysis of β-galactosidase activity. Data are β-galactosidase activities per milligram of protein ± SEMs (n = 4). Asterisk indicates P < 0.01.
AAV5 infection of the apical surface of human airway epithelia is not sensitive to heparin competition.
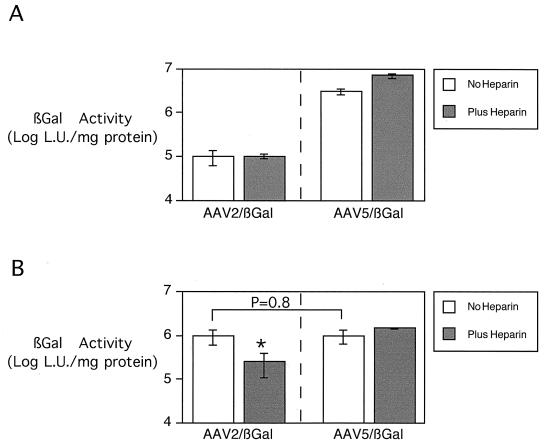
The low-level transduction of airway epithelia by AAV2/βGal is thought to be the result of poor virus binding because the apical membrane of airway epithelia expresses very low levels of HSP and αVβ5 integrins that may mediate AAV2 binding (8, 32). Previous studies have reported that AAV5 transduction occurs via an HSP-independent pathway. To test the effect of heparin competition on AAV2/βGal and AAV5/βGal transduction of human airway epithelia, the viruses were preincubated with 20 μg of soluble heparin per ml. Competition with soluble heparin had minimal effect on the already low level of AAV2/βGal-mediated gene transfer, suggesting that the observed low-level transduction was not receptor mediated (Fig. 5A). However, more importantly, heparin competition did not inhibit AAV5/βGal-mediated gene transfer to airway epithelia. These data suggest a novel receptor-mediated pathway for AAV5 binding and infection via the apical surface of human airway epithelia.
FIG. 5.
Effect of soluble heparin on AAV gene transfer to human airway epithelia. To compete off AAV binding and gene transfer, in some conditions the viruses were pretreated with 20 μg of soluble heparin per ml for 30 min. The figure shows the effect of heparin on AAV gene transfer to human airway epithelia from the apical side (A) and from the basolateral side (B). Five hundred particles per cell of either AAV2/βGal or AAV5/βGal were added for 30 min at 4°C. β-Galactosidase activity was measured 14 days later. Data are means ± SEMs (n = 8 in each group). Asterisk indicates P = 0.018.
AAV5 mediates gene transfer through the basolateral surface in a heparin-sulfate-independent manner.
The binding of AAV5 to the apical membrane suggests a novel receptor. However, AAV2 can infect via HSP receptors present on the basolateral surface. Duan et al. found that, similar to retroviruses and adenovirus (37, 38), AAV2 could infect human airway epithelia via the basolateral side. To test if the receptor for AAV5 is present on the basolateral surface, the transduction experiments were repeated as described in the previous section, but vector was applied from the basolateral side. Because AAV2 can infect via the basolateral side, this experimental design also allows us to ask if AAV5 had an advantage over AAV2 once they were both in the cell. Briefly, the epithelia were turned upside down, and we carefully applied 500 particles of AAV5/βGal or AAV2/βGal per cell in a volume of 25 μl to the bottom of the Millipore filter. After 30 min, the epithelia were rinsed thoroughly. To allow for maximal expression, the epithelia were studied 2 weeks after infection. Figure 5B shows that similar levels of β-galactosidase activity were detected in airway epithelia transduced with AAV2/βGal and AAV5/βGal. These data suggest that both viruses work equally well when applied to the basolateral side. To test the mechanism of uptake, the studies were repeated in the presence of soluble heparin. As previously reported, basolateral infection of the airway epithelia by AAV2 was competed off by soluble heparin (8). However, the AAV5/βGal transduction via the basolateral surface was not blocked by heparin competition. These data suggest that AAV5 binds to an as-yet-unidentified receptor present both on the apical and basolateral surfaces of human airway epithelia.
AAV5-mediated gene transfer to the airways in vivo.
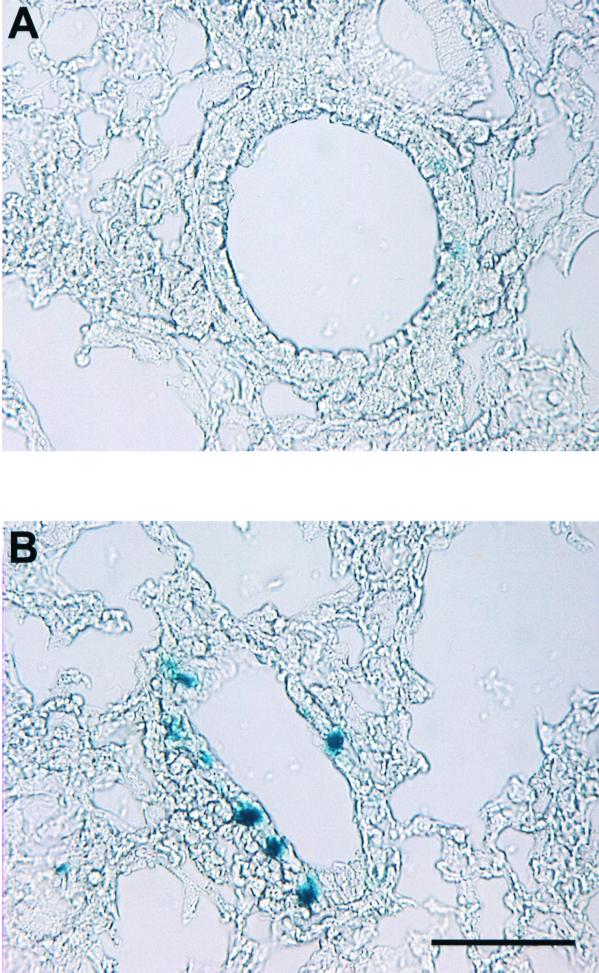
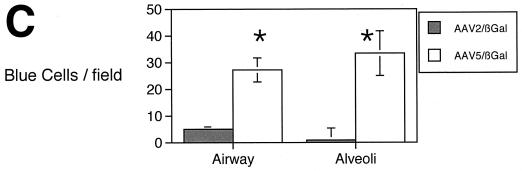
These data demonstrate improved gene transfer of AAV5 compared to AAV2 on human ciliated airway epithelia. To compare the transduction efficiency of AAV5 and AAV2 in vivo, we administered either AAV2 or AAV5 (1010 particles) to 6- to 8-week-old C57BL/6 mice in a total volume of 125 μl via nasal instillation. After 30 days, the mice were sacrificed, and the lungs were fixed and stained with X-Gal as previously described (36). We chose a relatively low viral input to maximize the difference between specific receptor binding and nonspecific binding that may occur when the viral concentrations are very high (8, 18, 34, 43). We observed only minimal transduction in mice treated with AAV2/βGal (Fig. 6). In contrast, we found a significant increase in the number of blue cells in the lungs of mice treated with AAV5. These data confirm the in vitro observation that AAV5 is more efficient than AAV2 at mediating gene transfer to the luminal surface of airway epithelia and suggest that murine airway epithelia express the receptor for AAV5.
FIG. 6.
AAV5/βGal-mediated gene transfer to murine conducting airway epithelia and alveolar epithelia in vivo. Mice were exposed to 1010 particles of either AAV2/βGal or AAV5/βGal via nasal instillation. After 30 days, the mice were sacrificed, and the lungs were fixed and stained with X-Gal. The figure shows representative photomicrographs showing ciliated and nonciliated cells transduced by AAV2/βGal (A) and AAV5/βGal (B). Panel C shows quantitation of gene transfer by number of blue nuclei of β-galactosidase-expressing bronchial and alveolar cells per microscopic field (n = 5 mice per group). Asterisk indicates P < 0.01.
DISCUSSION
Recombinant AAV have been widely used for gene transfer to a variety of cells in vitro and several organs in vivo. AAV has several advantages that make it a promising vector for gene therapy, including the lack of human pathology associated with wild-type AAV, prolonged expression of the transgene, and the lack of genes that encode viral proteins. Furthermore, there is already a significant amount of safety data both from animal experiments for diseases that require targeting to liver, muscle, lung, eye, and central nervous system cells (12, 13, 16, 22, 31) and from human experiments in which the vectors have been targeted to the nasal, sinus, and intrapulmonary airway epithelia (35). However, the lack of efficiency results in two significant problems. The first is a limited therapeutic index. Second, how does a low transduction efficiency result in a problem with vector production?
In order to improve the transduction efficiency of AAV2 for a particular target cell, several groups have reported the augmentation of the natural tropism of the virus by genetic modification of the capsid designed to retarget the vector (15), by bispecific antibodies (2), or by incorporation of AAV in a calcium phosphate coprecipitate in order to increase nonspecific binding (36). In this work, we used a different approach and examined the tropism of other naturally occurring AAV isolates for airway epithelia.
Of the six primate isolates, AAV2 has been the most extensively studied. AAV1 and AAV4 were isolated from nonhuman primates (6, 39). Xiao et al. (39) showed that recombinant AAV1 was better than AAV2 in transducing muscle, but AAV2 outperformed AAV1 in liver. Furthermore, Xiao et al. pointed to an additional advantage of using a different serotype of AAV. They found that AAV1 could be used in mice that had been immunized with recombinant AAV2. Recently, recombinant AAV3 and AAV6 vectors have also been shown to have different tropisms than AAV2, and in the case of AAV6, they have been shown to be resistant to neutralizing antibodies directed against AAV2 (28).
Sequencing data indicate that AAV5, in particular the capsid protein open reading frame, is the most divergent of the group of primate AAV. Based upon the published X-ray crystal structure of the canine parvovirus, we predicted the four loop regions on the capsid surfaces of AAV2 and AAV5 (40). The sequence homologies between the predicted loops 1, 2, 3, and 4 are 12, 10, 42, and 17%, respectively. This predicted divergence in the capsid protein probably accounts for the different tropism of AAV5.
The data presented in this manuscript suggest that the capsid of AAV5 is sufficiently different from that of AAV2 to allow for efficient binding and infection of human airway epithelia. While previous research has demonstrated transduction of airway epithelial cells with AAV2, those studies have required very high MOIs and/or prolonged incubation times. We found that both human and murine airway epithelia could be more efficiently transduced by AAV5. Furthermore, the data suggest a novel receptor present in both the apical and basolateral surfaces of airway epithelia.
ACKNOWLEDGMENTS
We thank Pary Weber, Phil Karp, Tom Moninger, Janice Launspach, David Welsh, Geri Traver, Theresa Mayhew, and Christine McLennan for excellent assistance. We especially appreciate the help of ISOPO and IIAM for the human lungs. We appreciate the support of the University of Iowa Gene Transfer Vector Core, In Vitro Cell Models Core, and Morphology Core.
This work was supported by National Heart, Lung, and Blood Institute (grant HL58340), the Cystic Fibrosis Foundation, and the Roy J. Carver Charitable Trust.
REFERENCES
- 1.Bals R, Xiao W, Sang N, Weiner D J, Meegalla R L, Wilson J M. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol. 1999;73:6085–6088. doi: 10.1128/jvi.73.7.6085-6088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett J S, Kleinschmidt J, Boucher R C, Samulski R J. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′gamma)2 antibody. Nat Biotechnol. 1999;17:181–186. doi: 10.1038/6185. [DOI] [PubMed] [Google Scholar]
- 3.Beck S E, Jones L A, Chesnut K, Walsh S M, Reynolds T C, Carter B J, Askin F B, Flotte T R, Guggino W B. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol. 1999;73:9446–9455. doi: 10.1128/jvi.73.11.9446-9455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Chiorini J A, Kim F, Yang L, Kotin R M. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan D, Yan Z, Yue Y, Engelhardt J F. Structural analysis of adeno-associated virus transduction circular intermediates. Virology. 1999;261:8–14. doi: 10.1006/viro.1999.9821. [DOI] [PubMed] [Google Scholar]
- 8.Duan D, Yue Y, Yan Z, McCray P B, Jr, Engelhardt J F. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 9.During M J, Samulski R J, Elsworth J D, Kaplitt M G, Leone P, Xiao X, Li J, Freese A, Taylor J R, Roth R H, Sladek J R, Jr, O'Malley K L, Redmond D E., Jr In vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector. Gene Ther. 1998;5:820–827. doi: 10.1038/sj.gt.3300650. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 13.Flotte T R, Afione S A, Solow R, Drumm M L, Markakis D, Guggino W B, Zeitlin P L, Carter B J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 14.Flotte T R, Carter B J. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995;2:357–362. [PubMed] [Google Scholar]
- 15.Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, Deleage G, Hallek M. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1052–1056. doi: 10.1038/12491. [DOI] [PubMed] [Google Scholar]
- 16.Guy J, Qi X, Muzyczka N, Hauswirth W W. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117:929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 17.Halbert C L, Alexander I E, Wolgamot G M, Miller A D. Adeno-associated virus vectors transduce primary cells much less efficiently than immortalized cells. J Virol. 1995;69:1473–1479. doi: 10.1128/jvi.69.3.1473-1479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo M, Finkbeiner W E, Widdicombe J H. Simple technique for culture of highly differentiated cells from dog tracheal epithelium. Am J Physiol. 1991;261:L106–L117. doi: 10.1152/ajplung.1991.261.2.L106. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Dressman D, Tsao Y P, Sakamoto A, Hoffman E P, Xiao X. RAAV vector-mediated sarcoglycan gene transfer in a hamster model for limb girdle muscular dystrophy. Gene Ther. 1999;6:74–82. doi: 10.1038/sj.gt.3300830. [DOI] [PubMed] [Google Scholar]
- 22.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 24.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 25.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 26.Qui J, Mizukami H, Brown K E. Adeno-associated virus 2 co-receptors? Nat Med. 1999;5:467–468. doi: 10.1038/8328. [DOI] [PubMed] [Google Scholar]
- 27.Rich D P, Couture L A, Cardoza L M, Guiggio V M, Armentano D, Espino P C, Hehir K, Welsh M J, Smith A E, Gregory R J. Development and analysis of recombinant adenoviruses for gene therapy of cystic fibrosis. Hum Gene Ther. 1993;4:461–476. doi: 10.1089/hum.1993.4.4-461. [DOI] [PubMed] [Google Scholar]
- 28.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samulski R J, Berns K I, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanlioglu S, Duan D, Engelhardt J F. Two independent molecular pathways for recombinant adeno-associated virus genome conversion occur after UV-C and E4orf6 augmentation of transduction. Hum Gene Ther. 1999;10:591–602. doi: 10.1089/10430349950018661. [DOI] [PubMed] [Google Scholar]
- 31.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 32.Summerford C, Bartlett J S, Samulski R J. AlphaVbeta5 integrin: a coreceptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 33.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teramoto S, Bartlett J S, McCarty D X X, Samulski R J, Boucher R C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner J A, Reynolds T, Moran M L, Moss R B, Wine J J, Flotte T R, Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 36.Walters R W, Duan D, Engelhardt J F, Welsh M J. Incorporation of adeno-associated virus in a calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J Virol. 2000;74:535–540. doi: 10.1128/jvi.74.1.535-540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Davidson B L, Melchert P, Slepushkin V A, van Es H H, Bodner M, Jolly D J, McCray P B., Jr Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie Q, Chapman M S. Canine parvovirus capsid structure, analyzed at 2.9 Å resolution. J Mol Biol. 1996;264:497–520. doi: 10.1006/jmbi.1996.0657. [DOI] [PubMed] [Google Scholar]
- 41.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Zhou W, Zhang Y, Zidon T, Ritchie T, Engelhardt J F. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]