Abstract
Biological invasions threaten global biodiversity, altering landscapes, ecosystems, and mutualistic relationships like pollination. Orchids are one of the most threatened plant families, yet the impact of invasive bees on their reproduction remains poorly understood. We conduct a global literature survey on the incidence of invasive honeybees (Apis mellifera) on orchid pollination, followed by a study case on Australian orchids. Our literature survey shows that Apis mellifera is the primary alien bee visiting orchids worldwide. However, in most cases, introduced honeybees do not deposit orchid pollen. We also test the extent to which introduced honeybees affect orchid pollination using Diuris brumalis and D. magnifica. Diuris brumalis shows higher fruit set and pollination in habitats with both native and invasive bees compared to habitats with only introduced bees. Male and female reproductive success in D. magnifica increases with native bee abundance, while conversely pollinator efficiency decreases with honeybee abundance and rises with habitat size. Our results suggest that introduced honeybees are likely involved in pollen removal but do not effectively deposit orchid pollen, acting as pollen wasters. However, Apis mellifera may still contribute to pollination of Diuris where native bees no longer exist. Given the global occurrence of introduced honeybees, we warn that certain orchids may suffer from pollen depletion by these invaders, especially in altered habitats with compromised pollination communities.
Keywords: Habitat alteration, Introduced honeybees, Invasive species, Orchids, Pollination, Native bees
Subject terms: Ecology, Evolution
Introduction
Biological invasions are one of the leading threats to global biodiversity1, impacting the structure and dynamics of landscapes, communities, and ecosystems2. The cascading effects of alien species can adversely affect mutualistic relationships among plant and animals, including pollination2. Particularly, invasive bees can change the original plant-pollinator networks and even harm both plant and pollinator partners3. By competing with native pollinators for floral resources and nesting sites4–6, invasive bees can impact pollinator fitness and population dynamics7–9. Through altering pollen flow, alien pollinators are in general expected to compromise plant reproductive success10, limit pollen availability to native pollinators2,10,11 and increase heterospecific pollen deposition2,12.
European honeybees (Apis mellifera) have become principal floral visitors of plant species of ecosystems around the world13, but their effect on plant reproductive success is complex to detect14 and to assess3. Honeybees are generalist pollinators and frequent plant visitors but may not necessarily benefit plant reproduction of all species15, especially when they competitively replace native pollinators and become ineffective surrogates16. Conversely, in cases where native pollinators are rare or locally extinct, honeybees often boost pollination17,18 or can even recover plant fitness from reproductive collapse in fragmented habitat19. However, most studies have documented how honeybees impact native bee communities through floral resource competition, whilst their effect on plant reproduction remains poorly documented3,14.
Orchids present highly specialised pollination mechanisms and given the great adaptability of honeybees to floral resources, the impact of invasive honeybees on the fitness of these plants might be important. Studies evaluating the effect of introduced Apis mellifera on orchid pollination success are very scarce. Beyond their renowned diversity of pollination systems, orchids can attract pollinators with nonrewarding flowers via various modes of deception20–24. About 46% of all orchid species globally are thought to lack reward25,26, typically resulting in lower insect visitation rates compared to rewarding ones27,28, deserving careful consideration for their conservation biology. Given that orchids offer pollen in discrete pollinia, instead of unpacked pollen grains as occurs in most other flowering plants, it is even more important to maximise the pollen transfer and deposition among flowers during pollinator visits29. A measure of the effectiveness of pollen transfer is pollination efficiency (PE) that is typically measured as the ratio of pollinated flowers on flowers with pollinia removed30,31. During transfer by pollinators, pollen losses in orchids are expected to be high when mediated by generalist pollinators and by a range of pollinator types27,32. For these reasons, pollinator efficiency in orchids might be hampered by exotic and generalist honeybees that manage to collect the pollinia but are not morphologically configurated to successfully deposit the pollinia and guarantee reproduction of the plant. Whilst in most cases pollinia removal and fruit set are similar across populations33–35, in some orchid species these trends can diverge. For example, the food deceptive Australian orchid species Diuris brumalis shows diverse responses of male and female reproductive success in relation to model plants’ abundance, with the first according to an exponential growth and the second to a logarithmic growth36. This variation can be attributed to the improved ‘learning behaviour’ of bees that have encountered deceptive orchids before and removed pollinia, so these are more likely to distinguish deceptive orchids from model plants4,37. As a result, the success of deception may stabilise with more abundance of rewarding plants36.
Our overall objective is to understand how introduced honeybees impact orchid pollination. We address this objective using (a) a literature search, to identify how often honeybees remove and deposit pollinia from orchid flowers, and (b) a case study which compares pollination success and efficiency of orchids in sites where native bee pollinators and honeybees co-occur and sites where native bee pollinators were fully replaced by invasive honeybees.
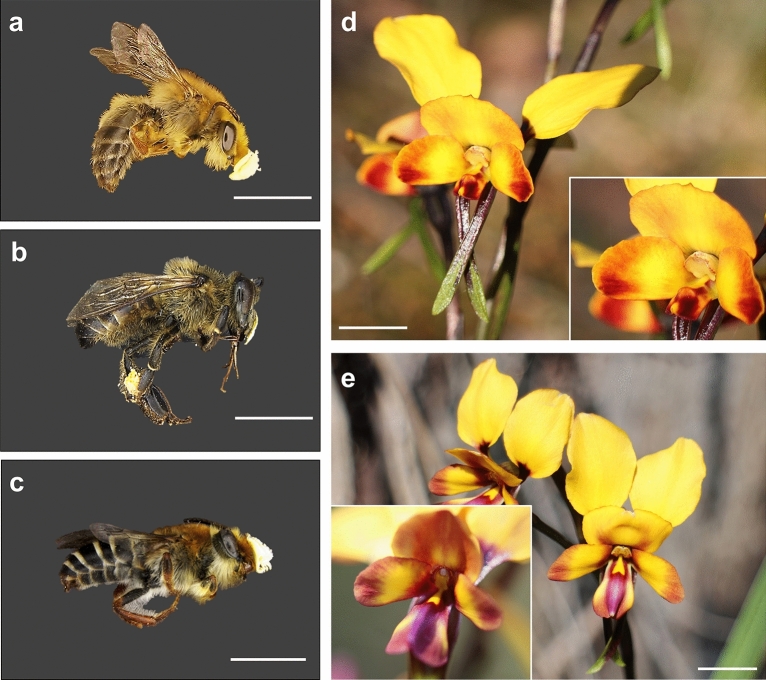
To conduct our empirical study, we focus on two orchid species in the genus Diuris (Orchidaceae) with analogous pollination strategies (food deception) and occupying different habitats that are subjected to different levels of human alteration (Fig. 1). As D. brumalis and D. magnifica populations inhabit sites subjected to anthropogenic alteration, we hypothesise that in disturbed sites lacking native pollinators36, alien honeybees can act as surrogates for pollinia removal but not for pollen transfer. Both species are generally pollinated by native bees of the genus Trichocolletes and are occasionally visited by the introduced Apis mellifera that potentially acts as a sub-optimal pollinator29,36 (Fig. 2). Whilst Apis mellifera is ubiquitous in all study sites, native bees (Trichocolletes) are patchily distributed across the sites. Given that pollination success for D. brumalis varies according to habitat type (forest vs disturbed woodland)36 and is also related to habitat size (Banksia woodland) for D. magnifica38, we hypothesise that the presence and abundance of native and exotic pollinators alter pollination success and efficiency in response to these habitat conditions.
Figure 1.
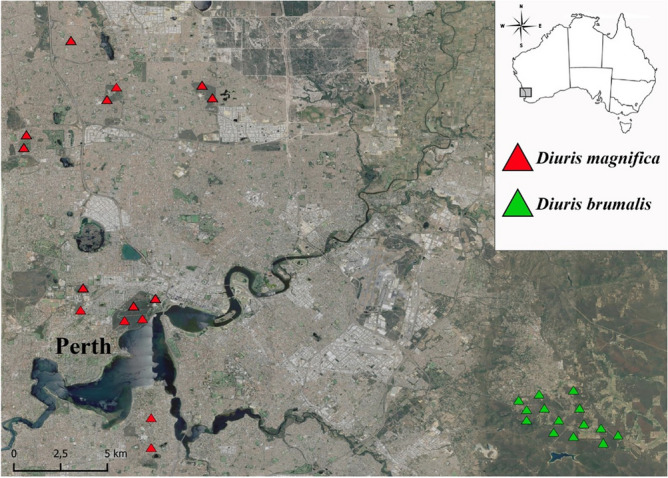
Sites where populations of Diuris brumalis (red triangles) and D. magnifica (green triangles) were studied in the metropolitan area of Perth and Perth hills respectively, Southwestern Australia. Geographic coordinates are reported in Datafile S1. Satellite imagery was obtained from Google Maps, and the map was created using QGIS 3.10 (2019) - QGIS Geographic Information System. Open-Source Geospatial Foundation Project: http://qgis.org.
Figure 2.
Native and non-native pollinators carrying Diuris (Orchidaceae) pollinia. Pollinia placement on Trichocolletes capillosus, native pollinator for Diuris brumalis (a), on Apis mellifera, pollen remover for D. brumalis and D. magnifica (b); and on Trichocolletes gelasinus, native pollinator of D. magnifica (c); flower morphology of D. brumalis (d) and D. magnifica (e) flowers showing the column where the pollinia is placed at the top and the labellum, insect platform. Scale bar of 5 mm. Credit: Daniela Scaccabarozzi.
For D. brumalis, we expect that: (i) the presence of native pollinators and exotic bees varies according to habitat type (forest vs disturbed woodland); (ii) removal of pollinia does not differ in sites where native pollinators occur relative to sites where native pollinators are absent; (iii) fruit set and pollen efficiency are higher in sites with native pollinators. For D. magnifica, we expect that: (i) removal of pollinia, fruit set and PE increase with the abundance of native pollinators; (ii) PE decreases with the abundance of introduced honeybees; (iii) and PE also decreases with habitat size.
Results
Literature review: incidence of honeybees in pollination of orchids
A total of 124 publications were included in the literature survey, covering 120 different orchid species overall (see Table 1, Table S1, Fig. S1) that were visited by honeybees, either observed to remove pollinaria (potential pollination) or pollinate flowers (depositing pollinia). These included all continents where orchids occur. Europe represented the 36% of total cases, followed by Asia (34%), America (14%), Oceania (12%) and Africa (0.04%) (Fig. S2). Of the total number of orchid species visited, pollinated, or potentially pollinated by honeybees, introduced honeybees represented 32% of cases. Pollination or pollinaria removal by introduced bees was recorded most often for the Epidendroideae (46%), followed by Orchidoideae subfamily (31%), Vanilloideae (17%) and Cypripedioideae (0.06%), as expected given the size of each subfamily. The introduced honeybee (Apis mellifera) was observed to act as a: visitor (V, when only observed landing on a flower) in 15% of cases; a pollen depositor (PD, when successfully depositing a pollinia at least once) in 31% of cases or pollen remover (PR, when removing pollinia at least once) in 54% of cases. Of the total number of orchid species visited by honeybees, honeybees were non-native in 32% of cases. In a few cases, A. mellifera was accompanied by other introduced bee genera such as Bombus, Centris and Euglossa.
Table 1.
Literature survey presenting the incidence of non-native species Apis mellifera (Apidae) in orchid pollination across continents (species by alphabetic order), according to the following described categories: V: visitor; PR: pollen remover; PD: pollen depositor; n.a.: not available.
| Continent | Country | Subfamily | Plant species | Native or alien plant species | Native bee or other native pollinators | Introduced bee species | Pollination by introduced species | Literature source |
|---|---|---|---|---|---|---|---|---|
| America | Puerto Rico | Epidendroideae | Arundina graminifolia | Native | Megachile yaeyamaensi, Thyreus takaonis | Apis mellifera (Africanized honeybee) | PR | Ackerman39; Sugiura40 |
| Asia | Japan, South Korea | Epidendroideae | Bletilla striata | Native | likely Tetralonia nipponensis | Apis mellifera (Africanized honeybee) | PD | Sugiura41; Ogawa and Miyake42 |
| America | Chile, Argentina Andes | Orchidoideae | Brachystele unilateralis | Native | Bombus dahlbomii | Apis mellifera, Bombus terrestris, Bombus ruderarius | PD | Sanguinetti and Singer43 |
| Oceania | Western Australia | Orchidoideae | Caladenia flava | Native | Neophyllotocus, native bee | Apis mellifera | V | Adams and Lawson44; Fig S1 and Daniela Scaccabarozzi personal observation |
| Oceania | Western Australia | Orchidoideae | Caladenia xantha | Native | n.a | Apis mellifera | V | Photo Mark Brundrett, Brundrett et al.45; Fig. S1 |
| America | North America | Epidendroideae | Calopogon pallidus | Native | Bombus sp., Xylocopa virginica | Apis mellifera | PD | Luer46; Argue47 |
| America | North America | Epidendroideae | Calopogon tuberosus | Native | Bombus americanorum, B. grisecollis, B. vagans, B. fervidus B. nevadensis, B. ternarius, B. terricola, Xylocopa virginica, X. micans, Augochlora sp., Megachile melanophea | Apis mellifera | PD | Luer46; Thien and Marcks48; Heinrich49 |
| America | Chile, Argentina Andes | Orchidoideae | Chloraea virescens | Native | Bombus dahlbomii | Apis mellifera, Bombus terrestris, Bombus ruderarius | PD | Sanguinetti and Singer43 |
| America | North America | Vanilloideae | Cleistesiopsis divaricata | Native | Megachile sp, Bombus pennsylvanicus, B. fervidus, B. bimaculatus, B. vagans, B. impatiens, B. bimaculatus | Apis mellifera | PD | Gregg50,51 |
| Asia | India | Epidendroideae | Cymbidium pendulum | Native | Apis cerana | Apis mellifera | PD | Attri and Kant52; Verma et al.53 |
| Asia | Japan | Cypripedioideae | Cypripedium macranthos | Native | Andrena ruficrus, Bombus pseudobaicaiensis | Apis mellifera | PR | Sugiura et al.54,55 |
| America | USA | Cypripedioideae | Cypripedium candidum | Native | likely Andrena sp., Odontomyia pubescens (Diptera) | Apis mellifera | PR | Pearn56; Grantham et al.57 |
| America | USA | Cypripedioideae | Cypripedium parviflorum | Native | likely Andrena sp., Odontomyia pubescens (Diptera), Lasioglossum zonulum | Apis mellifera | PR | Pearn56; Grantham et al.57 |
| America | USA, Canada | Cypripedioideae | Cypripedium reginae | Native | likely Anthophora; Megachile spp. | Apis mellifera | PR | Edens-Meier et al.58 |
| America | Mexico | Epidendroideae | Cyrtopodium macrobulbon | Native | likely Centris or Xylocopa | Apis mellifera | PR | Miranda-Molina et al.59 |
| Asia | China | Epidendroideae | Cyrtopodium polyphyllum | Alien | Centris tarsata; Centris labrosa | Apis mellifera, Centris nitida, Centris errans | PR | Liu and Pemberton60; Pansarin et al.61 |
| America | Florida, USA | Epidendroideae | Cyrtopodium punctatum | Native | Xylocopa sp. | Apis mellifera, Euglossa dilemma, Centris errans | V | Pemberton and Liu62; Dutra et al.63 |
| America | Puerto Rico | Epidendroideae | Dendrobium crumenatum | Alien | Apis cerana | Apis mellifera (Africanized honeybee) | PD | Leong and Wee64; Meurgey65; Ackerman66 |
| Oceania | Eastern Australia | Epidendroideae | Dendrobium kingianum | Native | n.a. | Apis mellifera | PR | Photo Rudie Kruiter; Fig. S1 |
| Oceania | Australia | Epidendroideae | Dendrobium speciosum var. hillii | Native | likely Trigona sp., Homalictus sp., Lassioglossum, Hylaeus | Apis mellifera | V | Slater and Calder67 |
| Oceania | Western Australia | Orchidoideae | Diuris brumalis | Native | Tichocolletes capillosus, Trichocolletes leucogenys | Apis mellifera | PR | Scaccabarozzi et al.36 |
| Oceania | Eastern Australia | Orchidoideae | Diuris maculata | Native | Trichocolletes venustus | Apis mellifera | PD | Beardsell et al.68; Indsto et al.69 |
| Oceania | Western Australia | Orchidoideae | Diuris magnifica | Native | Tichocolletes gelasinus, T.dives | Apis mellifera | PD | Scaccabarozzi et al.38 |
| Oceania | Australia | Orchidoideae | Diuris sulphurea | Native | Paracolletes sp., Amegilla sp., Lipotriches sp. | Apis mellifera | PD | Rayment70; Kruiter,71 |
| Oceania | Western Australia | Orchidoideae | Eriochilus dilatatus | Native | Halictidae bees | Apis mellifera | PR | Bundrett28; Daniela Scaccabarozzi personal observation |
| America | Brazil | Orchidoideae | Ionopsis utricularioides | Native | Ceratinini, Meliponini, Tapinotaspidini, Halictidae bees | Apis mellifera scutellata | V | Aguiar and Pansarin72 |
| America | Cayman Islands | Epidendreae | Myrmecophila thomsoniana | Native | Coereba flaveola (Aves), Gymnettis lanius, Lachnopus vanessablockae (Coleoptera), Anolis conspersus (Reptilia) | Apis mellifera | PD | Rose-Smyth73 |
| America | North America | Orchidoideae | Platanthera blephariglottis | Native | Bombus fervidus, B. vagans and various Lepidoptera | Apis mellifera | PR | Smith and Snow74; Cole and Firmage75 |
| Oceania | Australia | Orchidoideae | Prasophyllum alpinum | Native | Pterocormus promissorius (Ichneumonidae) | Apis mellifera | PR | Jones76 |
| Oceania | Australia | Orchidoideae | Prasophyllum elatum | Native | native bee | Apis mellifera | V | Photo Rudie Kruiter; Fig. S1 |
| Oceania | Australia | Orchidoideae | Prasophyllum sp. | Native | native bees and wasps | Apis mellifera | PR | Photo and personal observation by Rudie Kuiter Fig. S1 |
| Africa | South Africa | Orchidoideae | Satyrium cristatum | Native | Amegilla natalensis, A. spilostoma, A. sp. A. natalensis | Apis mellifera | PR | Johnson et al.77 |
| Africa | South Africa | Orchidoideae | Satyrium erectum | Native | Anthophora diversipies, A. praecox | Apis mellifera | PR | Ellis and Johnson78 |
| Africa | South Africa | Orchidoideae | Satyrium jacottetiae | Native | Philoliche rostrata (Diptera), Theretra capensis (Lepidoptera) | Apis mellifera | PD | Botes et al.79 |
| Africa | South Africa | Orchidoideae | Schizochilus flexuosus | Native | Lasioglossum sp., Patellapis zonalictus (Halictidae), Scoliidae | Apis mellifera | PR | van der Niet et al.80 |
| Oceania | Eastern Australia | Orchidoideae | Spiranthes australis | Native | Amegilla asserta (likely primary pollinator) | Apis mellifera | PR | Ren personal observation; Kuiter71 |
| Asia | Japan | Orchidoideae | Spiranthes australis | Native | Megachile nipponica, M. japonica, Halictidae sp. | Apis mellifera | PD | Suetsugu and Abe81; Iwata et al.82 |
| Oceania | Australia | Orchidoideae | Spiranthes sinensis | Native | guild of native bees | Apis mellifera | PR | Coleman83 |
| America | USA | Orchidoideae | Spiranthes vernalis | Native | native bee | Apis mellifera | PR | Catling84 |
Personal observations and photos are included to support evidence especially focusing on Australian orchid species. Refer to Table S1 for the literature survey summarising the global incidence of Apis bees as a native species.
Case study on Diuris brumalis and D. magnifica
Pollination in relation to the occurrence of native and alien honeybees
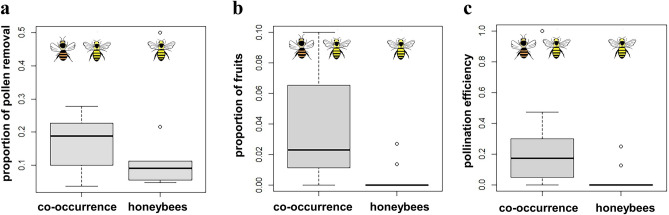
In D. brumalis we found an effect of sampling year on pollinia removal and fruit set. Specifically, the pollinia removal was higher in 2017 (χ2 = 7.4677, p = 0.006), whilst fruit set was higher in 2016 (χ2 = 4.6356, p = 0.03). Overall pollination efficiency was lower in 2017 than in 2016 (χ2 = 4.1719, p = 0.04). For D. brumalis, pollinia removal did not vary between sites characterized by honeybees only and sites with honeybees and native bees co-occurring (Fig. 3a) (χ2 = 2.8637, p = 0.091), but sites with only honeybees had significantly lower fruit set (χ2 = 5.4698, p = 0.019; Fig. 3b). Pollination efficiency (measured as the proportion of flowers with pollinia removed that also received pollen) was significantly lower where native bees were absent (disturbed woodland) relative to sites with only honeybees (forest) (χ2 = 6.1869, p = 0.012) (Fig. 3c).
Figure 3.
Boxplot of co-occurrence (minimum and maximum value) of honeybees and native bees vs. honeybees alone on pollinia removal (a), fruit set (b), and pollination efficiency (c) in Diuris brumalis (Orchidaceae).
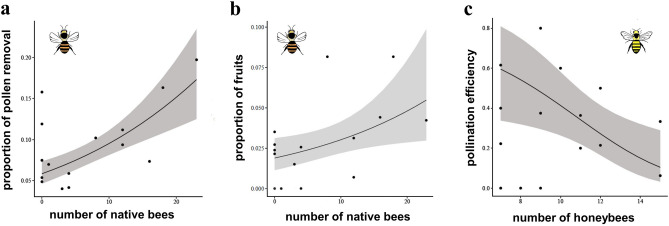
In D. magnifica, the abundance of native bees was significantly linked to an increase of both pollinia removal (χ2 = 19.572, p < 0.001) and fruit set (χ2 = 5.1371, p = 0.023) (Fig. 4a,b; Table S2). In particular, the abundance of honeybees led to a decrease of pollination efficiency (χ2 = 7.2195, p = 0.007) (Fig. 4 c; Table S2), whilst abundance of native bees did not affect pollination efficiency (Table S2).
Figure 4.
Effect of number of native bees and non-native honeybees quantified during transects for Diuris magnifica (Orchidaceae) reproductive success. The number of native bees influences pollinia removal (a), and fruit set (b) and number of non-native honeybees impacts orchid pollination efficiency (c).
Effect of habitat type and size on pollination success and efficiency
In D. brumalis populations, as native bee pollinator and introduced honeybee occurrence varied by habitat (forest vs disturbed habitat) it was not possible to untangle the direct effect of habitat from the other correlated variables. In fact, only honeybees were found in disturbed woodland, whereas honeybees and native bees occurred together in the forest habitat (Fig. 3a–c). In D. magnifica populations, habitat remnant size was positively associated with pollination efficiency (χ2 = 6.7399, p = 0.009) in a logarithmic manner (Fig. 5).
Figure 5.
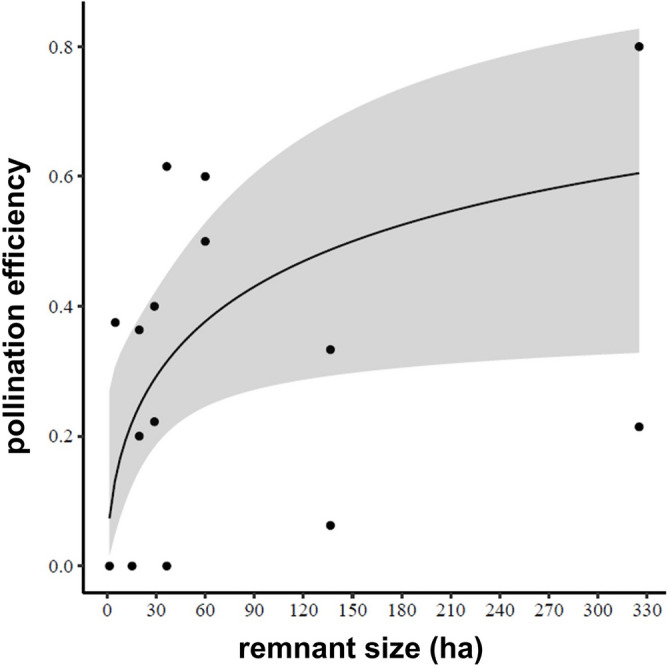
Pollination efficiency of Diuris magnifica in relation to bushland reserve area (habitat size).
Discussion
Our study combined an analysis of experimental data on Diuris reproductive success with a literature survey that addresses the role of Apis bee species in orchid pollination, focusing on introduced honeybees (Apis mellifera). We also examined whether alien honeybees adversely affect pollination success or have the capacity to contribute to orchid pollination in altered landscapes.
The importance of introduced Apis mellifera, as a pollinator for orchid species remains unresolved because most studies on interactions between introduced and native bees have focused on other plant families. In our literature survey, Apis mellifera is the principal alien bee observed visiting orchids (Table 1). Pollination (or potential pollination) by Apis bees (native and introduced) is not common among orchids, resulting in 10% (120 cases in our literature survey) of the ~ 1200 cases of orchid pollination by Hymenoptera26. This is a relatively small number compared to the prevalence (~ 60%) of other corbiculate Apidae (including orchid bees and bumblebees) which are specialist pollinators of numerous orchid species20,26,85,86 (Table 1 and S1). Despite the widespread distribution of honeybees in Eurasia and Africa87, most orchids rely on specific foragers rather than super-generalist pollinators such as honeybees88. Even though honeybees are the most frequently observed native pollinator of Mediterranean orchids, no Mediterranean species specialises on Apis mellifera89. Pollination by introduced Apis mellifera accounts for over 3% of the documented cases of orchid pollination by Hymenoptera86 and commonly occurs in Cypripedium, and Cyrtopodium in both Asia and America, in the Australian genus Diuris, in Satyrium from South Africa and in Spiranthes across Australia, Asia and North America (Table1). In these cases, introduced Apis are similar in size to at least some of the natural pollinators (i.e., Amegilla, Bombus, Megachile)90 (Table 1). In the South American orchid species Brachystele unilateralis and Chloraea virescens, introduced bees like Bombus terrestris, B. ruderarius and Apis mellifera were successful in displacing the natural pollinator Bombus dahlbomii43. Therefore, a requisite to be an alien surrogate pollinator seems to be the level of morphological fit between the alien bee and the newly acquired floral resource. According to our review, in two orchid species, the American Cyrtopodium polyphyllum and the Asian Dendrobium crumenatum, invasive to China and Puerto Rico respectively, introduced species such as Apis mellifera acted as pollen removers and pollen depositors respectively43. This raises concerns regarding the impact of introduced bees in facilitating the invasion of non-native orchids. Our literature search shows that in most cases introduced Apis mellifera have been ineffective in replacing the role of native pollinators. In 69% of recorded cases introduced honeybees were observed as a visitor or a pollen remover but in only 30% of cases they were recorded as a pollinator (pollen depositor).
An important caveat is that, thus far, we have only documented the impact of introduced honeybees on pollinia removal and deposition. However, the generalist foraging behaviour of Apis mellifera15 may have further implications, including the breakdown of pre-pollination reproductive barriers among coexisting orchid species15,32,91. Such hybridization can have evolutionary biological implications for the plant life cycle, the persistence of future generations, diversification, and speciation in Orchidaceae.
In our empirical study, western honeybees occurred in all study sites for both target species (D. brumalis and D. magnifica) whilst the occurrence of native bees (Trichocolletes spp.) was patchy across sites. In D. brumalis, honeybees predominantly occurred along with native Trichocolletes36, but in the absence of native bees, orchid fruit set showed the lowest values at 0% (Fig. 3b). Notably, there was no difference on orchid pollinia removal between sites where honeybees occurred alone (10%) and sites where they co-occurred with native bees (20%) (Fig. 3a), indicating that honeybees led to comparable level of pollinia removal to native bees. Thus, European honeybees are capable of successfully removing pollinia from flowers of D. brumalis (Fig. 2a–d), but because fruit set and pollination efficiency were lowest at 0% when honeybees occurred alone, we hypothesise that they deplete pollen supplies available to native pollinators39 and are ineffective at pollen deposition. This highlights the value of native pollinator specificity in orchid pollen deposition. According to the lock and key hypothesis a set of European food deceptive species show higher levels of correlation between pollinarium and stigmatic cavity lengths comparing to sexual deceptive species92, to avoid heterospecific pollen deposition of sympatric species. This pollinator specificity seems very crucial in food deceptive species globally26.
In D. magnifica both pollinia removals and fruit set exponentially increased with native bee abundance (Trichocolletes gelasinus; Fig. 4a,b) from 0 to 20% and from 0 to 8% respectively and they were not impacted by the abundance of Apis mellifera among study sites. The output was similar among pollinia removal and fruit set and conforms with our expectations that optimal pollinator frequency would enhance orchid reproductive success. Interestingly, in D. magnifica, the increase of honeybee abundance reduced the orchid pollination efficiency from 80 to 0% likely because they withdraw pollinia without successfully depositing them on the next flower3,39 (Figs. 2b,c,e; 4c) as argued in D. brumalis. However, the abundance of native bees did not influence the PE for this species. This could be explained by the patchy occurrence of Trichocolletes species across the bushland remnants, especially in smaller bushland reserves. It is also plausible that other factors might interfere with the ability of native pollinators to fulfil their pollination service, i.e., pollinia depletion by honeybees during their visits to the orchids, presence of suboptimal pollinators such as beetles, that were observed to remove pollinia and deposit it on the same flowers on few occasions38, and competition between honeybees and wild bees for access to floral resources3,14. In addition, plant reproductive success often relies more on bee assemblage and diversity than abundance per se93. However, the significant impact of honeybees’ abundance on D. magnifica pollination efficiency suggested a detrimental effect of honeybees’ abundance on orchid pollination effectiveness. The honeybee is well known for its modest efficiency in pollination service21,94 and in some cases its role may be an antagonistic one where costs (i.e., associated with nectar replenishment, pollen discounting or damage to flowers) exceed the benefits for the plant95. One explanation for the potential failure of introduced honeybees in depositing pollen is linked to the mimicry system and the foraging behaviour of native pollinators. Trichocolletes bees, the native pollinators of D. brumalis and D. magnifica, are specialised pollinators of Faboideae species96. They are tricked by reward-less orchids via specific floral visual signals that mimic those of Faboideae flowers97. In contrast, honeybees, being generalists, visit various flowering plants, including these deceptive orchids, potentially extracting orchid pollinia. However, because they do not exclusively target pea plants like Trichocolletes bees, and because orchids do not offer nectar, honeybees are less likely to consistently visit them to deposit pollen. On the other hand, we occasionally observed fruit set of D. magnifica and D. brumalis in populations where only Apis mellifera was present suggesting a local benefit where pollinator networks are compromised (Datafile S1). However, to determine this, assessing seed viability might be necessary. Notably, Apis mellifera was observed both removing and depositing pollinia on the same flowers in D. magnifica, indicating potential self-pollination rather than pollen transfer between different plants38. We suggest that management strategies for beekeeping activities should consider the abundance of alien bees relative to native ones and be designed to reduce antagonistic costs for the plants. We also note that our study sites did not include orchid populations with native bees only, as honeybees have become ubiquitous.
To conclusively test the effect of native bees and introduced honeybees on orchid pollination, and to determine if this effect is influenced by resource overlap between native and introduced bees, it would be necessary to: (i) isolate the effects of native bee occurrence from honeybee occurrence (if feasible); (ii) assess whether the absence of native bees is primarily due to habitat change or competition with honeybees, and (iii) investigate honeybee abundance in intact and altered habitats, respectively.
In our study, habitat type (wild vs disturbed) influenced orchid reproductive success in D. brumalis, but it was not possible to untangle the direct effect of habitat from the co-occurrence of honeybees and native bees (Fig. 3b,c), because only honeybees occurred in the disturbed woodland site.
We were not able to determine the causes of lack of native pollinators in some study sites, but we hypothesise that anthropogenic habitat alteration (disturbance linked to urban development) might have led to their decline96,98. Given that Trichocolletes native bees are ground-nesting bees97, habitat change might interfere with nesting and foraging sites4,99,100, eventually leading to their local loss. This primarily impacts species that employ Batesian floral mimicry such as D. brumalis that rely on specialised pollinators101.
Our results highlight the importance of conservation of specialised native bee fauna and associated habitats. For D. magnifica, larger bushland reserves led to an increase of pollination efficiency (Fig. 4). Specifically, the increase was greatest in the lower half of the predicted trend, where values ranged from 0 to 50% PE and were linked to habitats within a range of 1–60 ha. This means that even relatively small bush fragments can sustain effective pollination service. However, only bigger bushland reserves (over 100 ha) showed PE > 50%, suggesting that the continuous habitat provided more optimal pollination service. This trend might be explained by the expectation that larger habitat sizes sustain a higher biodiversity of native bees (number and richness)102.
Conclusions
Our literature survey highlights the importance of conducting studies on the interaction of native and alien pollinator species globally. Because many members of the orchid family are at high risk of extinction, resolving their pollination ecology in areas occupied by introduced honeybees is vital for their conservation through effective land management. We empirically showed that honeybees are ineffective substitutes for native bees as pollinators of Diuris orchids. In D. brumalis pollination was higher in the wild habitat where native and alien honeybees co-occurred and was lower in the altered habitats with only introduced honeybees. Pollination was also positively impacted by habitat type and size respectively for D. brumalis and D. magnifica. Our study provides evidence that biological invasion by Apis mellifera can impact orchid pollination and that this effect is exacerbated or even might be triggered by habitat disturbance (altered and fragmented habitats). However, Apis mellifera might provide a limited pollinator service for D. brumalis and D. magnifica where native bees no longer exist, such as disturbed and small fragments of habitat. This indicates that the impact of introduced honeybees on orchid pollination varies with context, necessitating individual evaluations of their effects in each case study. Our findings recommend an accurate and considered management of beekeeping in natural areas and caution against introduction of honeybees to new areas, without carefully determining the minimum ‘safe’ distance of hives to orchid populations and monitoring the number of honeybees relative to native bees in the sites where hives are located. This knowledge is required for ensuring the survival of many orchid species, especially where the habitat is altered and the effect of introduced honeybees on orchid reproductive success is likely to be most severe.
Methods
Literature review: incidence of honeybees in pollination of orchids
We searched the global literature to identify and summarise studies in which native and introduced honeybees have been reported as visitors, pollen removers, and depositors in orchid species. In Google Scholar and Web of Science Core Collection we searched the following key words: ‘Apis’, ‘pollinat’, ‘visitor’, ‘introduced bee’, ‘invasive bee’ and ‘honeybee’ and ‘orchid’. The first search was conducted on 1st July 2022 and repeated on 1st March 2023 any paper that mentioned an orchid-honeybee interaction was included. In addition, we included records from available orchid pollination databases86,89, books, our photos, and personal observations in which invasive honeybees were reported as a pollen removers of Australian orchid species. During the survey, the introduced honeybee was recorded as a visitor (V, when only observed landing on a flower); pollen depositor (PD, when successfully pollinating the flowers at least once or determined by assessing the configurational features between the flower reproductive structures and bee) or pollen remover (PR, when removing pollinia at least once).
Study species
Diuris (Orchidaceae) comprises approx. 120 species distributed principally in Australia, with centres of diversity in south-western and south-eastern Australia103. Diuris are terrestrial geophytes, producing a solitary scape per plant yearly; some species within the genus seem capable of clonal reproduction through vegetative propagation of tubers104. We selected two allogamous and self-compatible species, Diuris brumalis and D. magnifica, pollinated via mimicry of co-flowering rewarding legumes by native bees of genus Trichocolletes36,38. Apis was observed to act as a pollen remover of both species (Fig. 2a–e).
Endemic to southwestern Australia, Diuris brumalis, is very common in the Darling Range, to the immediate east of Perth, and produces yellow and reddish nectar-less flowers during July and August, with between three and 15 flowers per inflorescence105. Diuris magnifica is endemic to the Swan Coastal Plain in Western Australia, with its main distribution centred on the Perth metropolitan area105 (Fig. 1). Flowering occurs from late winter to early spring, with between three and nine yellow and purple flowers per inflorescence105. Given that the species were visited by introduced honeybees and occupied two different habitats, subject to anthropogenic alteration, they were chosen as model species to test for our hypothesis.
Study sites
We studied 14 populations of D. brumalis in the Darling Range, near Perth in Western Australia (Fig. 1). The populations were selected across two different habitat types: Jarrah Forest (hereafter referred to as ‘forest’) dominated by Eucalyptus marginata with Corymbia calophylla and open Jarrah Forest with Eucalyptus marginata and Allocasuarina fraseriana highly subjected to fragmentation due to urbanization (hereafter referred to as ‘disturbed woodland’). Populations of D. magnifica were distributed across 15 sites in bushland remnants within the metropolitan area of the city of Perth (Fig. 1). Habitat was uniform across populations and characterised by Banksia woodland, an ecological community adjacent to the Swan Coastal Plain of Perth with a tree layer of Banksia with scattered Eucalyptus or Allocasuarina species and a diverse understorey including sclerophyllous shrubs, graminoids and forbs. Both the orchid species co-flowered with a range of Faboideae that represent a conspicuous component of the understorey vegetation.
Orchid pollination success
The proportion of pollen removed (proxy of male fitness) and fruits (proxy of female fitness) come from previously published studies36,38 for D. brumalis and D. magnifica respectively (Datafile S1). Data from two additional populations were included to increase the sample size for D. magnifica. For D. brumalis the proportion of flowers with pollinia removal and the proportion of pollinated flowers at the end of the flowering period (i.e., the number of flowers found with at least one pollen massula on the stigma) was quantified in 2016 and in 2017, using a 30 × 30 m quadrat centred in each population. As per D. brumalis, at the end of flowering period in 2017, the proportion of D. magnifica flowers with pollinia removal and the proportion of pollinated flowers was recorded.
Observational transects on pollinator occurrence
We carried out observations along transects of 100 m length for 10 sites (populations) in September 2016 and 14 sites in September 2017 during D. brumalis flowering period. We recorded the occurrence of the native pollinator, Trichocolletes spp. (Colletidae) bees, and the introduced honeybee by observing all the flowering species of the understory vegetation along the transect and habitat type. Transects were centred on the same quadrats used to quantify pollination success of D. brumalis (see former paragraph). Observations along transects lasted 40 min for both species, spending approximately 3 min per flowering plant. Transects were repeated one week after the initial survey, following the same route. For D. magnifica we carried out two observation transects for all the bushland reserves, from 5th to 15th September 2017, by recording the frequency (number of insects) of native Trichocolletes spp. bees, and the introduced honeybee during 3 min of observation per flowering plant. Beetles of Neophyllotocus sp. (Scarabeideae; Coleoptera) were included too because they have been observed to extract pollinia and deposit it on the stigma of the same orchid flower on two occasions38. Sizes of bushland reserves were obtained from Scaccabarozzi et al.38. To quantify the effectiveness of pollen transfer, we calculated pollination efficiency (PE) for each population of both species, expressed as a ratio of Fp/Fr where Fp is the number of pollinated flowers and Fr is the number of flowers found with one or both pollinia removed30,31. The value of PE potentially ranges between 0 and 1, with 1 representing the maximum and 0 the lowest efficiency.
Statistical analysis
We analysed the relationship between the proportion of pollinia removed, proportion of fruits, and pollination efficiency with the following independent variables via generalised linear mixed models (GLMs): co-occurrence of honeybees and native bees, lack of co-occurrence (for D. brumalis), and abundance of honeybees and abundance of native bees (for D. magnifica). Year was included in each model as a fixed factor, while population was included as a random effect to account for repeated measures over time.
We also evaluated the effect of pollinator occurrence and year on (i) the proportion of pollinia removed, (ii) the proportion of fruits on number of flowering plants and (iii) the pollination efficiency in D. brumalis. To do so, we employed generalized linear regression models (GLMs) with binomial or quasi-binomial distributions of the response variables, depending on the overdispersion parameter. We first evaluated the role of the factor sampling site on the response variables to avoid possible data dependency. Regression models were evaluated for collinearity among covariates using the VIF criterion (VIF < 3). All the models were subjected to a backward regression approach to remove non-significant variables through the AICc criterion (delta AICc > 2). For D. magnifica we wanted to assess the effect of habitat size on orchid pollination success (pollinia removed and fruit set) and pollination efficiency. To do so, we tested the effects of number of plants, native and honeybee abundance, beetle abundance, and remnant size on the same response variables analysed for D. brumalis. The statistical analyses followed the same workflow described above. Honeybee and native abundance were not collinear. Furthermore, the relationship between remnant size and native bee abundance was evaluated through a negative binomial GLM to account for the overdispersion of the residuals occurring in the Poisson model. All the analyses were carried out in R ver 4.2.0 (R Core Team 2022) exploiting the following packages “ggplot2”, “plyr”, “MuMIn”, “mass”106–109.
Supplementary Information
Acknowledgements
We thank Rudie Hermann Kuiter for providing photos to complement the literature survey with documented observations, Massimo Labra and Andrea Galimberti for the collaboration, and two anonymous reviewers for suggestions that greatly improved the final manuscript.
Author contributions
D.S., Z.-X.R., S.C. contributed to the study conception and design. Material preparation, data collection, graphics and analysis were performed by D.S., L.G., E.P., A.A., Z.-X.R., M.B., M.V.-M. and G.P. The first draft of the manuscript was written by D.S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Uppsala University. DS was funded by the Endeavor Fellowship Program [5117_2016] and the Università degli Studi di Napoli Federico II via Short Mobility Program [D.M. 976_2017] during data acquisition. Z-XR is funded by a Talent Young Scientist Program of Yunnan Province (YNWRQNBJ-2019–055).
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files. Supplementary material associated with this article includes Figure S1, Figure S2, Table S1, Table S2 and Datafile S1. Experimental research and field studies on study plants comply with relevant institutional, national, and international guidelines and legislation. Permission to collect Diuris brumalis and D. magnifica for identification purposes were obtained and collected specimens were vouchered and identified by the Herbarium of Western Australia, Perth. The material is publicly available at the Herbarium. Vaucher numbers: DS004, DS009, DS010, DS013, DS018.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64218-x.
References
- 1.Bellard C, Cassey P, Blackburn TM. Alien species as a driver of recent extinctions. Biol. Lett. 2016;12:20150623. doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traveset A, Richardson DM. Mutualistic interactions and biological invasions. Ann. Rev. Ecol. Evol. Syst. 2014;45:89–113. doi: 10.1146/annurev-ecolsys-120213-091857. [DOI] [Google Scholar]
- 3.Agüero JI, Pérez-Méndez N, Torretta JP, Garibaldi LA. Impact of invasive bees on plant-pollinator interactions and reproductive success of plant species in mixed Nothofagus Antarctica forests. Neotrop. Entomol. 2020;49:557–567. doi: 10.1007/s13744-020-00787-6. [DOI] [PubMed] [Google Scholar]
- 4.Goulson D. Effects of introduced bees on native ecosystems. Ann. Rev. Ecol. Evol. Syst. 2003;34:1–26. doi: 10.1146/annurev.ecolsys.34.011802.132355. [DOI] [Google Scholar]
- 5.Agüero JI, et al. Impactos de la abeja melífera sobre plantas y abejas silvestres en hábitats naturales. Ecosistemas. 2018;27:60–69. doi: 10.7818/ECOS.1365. [DOI] [Google Scholar]
- 6.Thomson DM, Page ML. The importance of competition between insect pollinators in the Anthropocene. Curr. Opin. Insect Sci. 2020;38:55–62. doi: 10.1016/j.cois.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Thomson D. Competitive interactions between the invasive European honeybee and native bumble bees. Ecology. 2004;85:458–470. doi: 10.1890/02-0626. [DOI] [Google Scholar]
- 8.Paini DR, Roberts JD. Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus) Biol. Conserv. 2005;123:103–112. doi: 10.1016/j.biocon.2004.11.001. [DOI] [Google Scholar]
- 9.Hudewenz A, Klein AM. Red mason bees cannot compete with honeybees for floral resources in a cage experiment. Ecol. Evol. 2015;5:5049–5056. doi: 10.1002/ece3.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohzono I, Yokoyama J. Impacts of alien bees on native plant-pollinator relationships: A review with special emphasis on plant reproduction. Appl. Entomol. Zool. 2010;45:37–47. doi: 10.1303/aez.2010.37. [DOI] [Google Scholar]
- 11.Do Carmo RM, Franceschinelli EV, da Silveira FA. Introduced honeybees (Apis mellifera) reduce pollination success without affecting the floral resource taken by native pollinators. Biotropica. 2004;36:371–376. doi: 10.1111/j.1744-7429.2004.tb00329.x. [DOI] [Google Scholar]
- 12.Marrero HJ, Medan D, Zarlavsky GE, Torretta JP. Agricultural land management negatively affects pollination service in Pampean agro-ecosystems. Agric Ecosyst. Environ. 2016;218:28–32. doi: 10.1016/j.agee.2015.10.024. [DOI] [Google Scholar]
- 13.Herrera CM. Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years. Proc. R. Soc. B. 2020;287:20192657. doi: 10.1098/rspb.2019.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page ML, Williams NM. Honey bee introductions displace native bees and decrease pollination of a native wildflower. Ecology. 2022;104:e3939. doi: 10.1002/ecy.3939. [DOI] [PubMed] [Google Scholar]
- 15.Ollerton J, et al. Overplaying the role of honeybees as pollinators: A comment on Aebi and Neumann. Trends Ecol. Evol. 2012;27:141–142. doi: 10.1016/j.tree.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Page ML, et al. A meta-analysis of single visit pollination effectiveness comparing honeybees and other floral visitors. Am. J. Bot. 2021;108:2196–2207. doi: 10.1002/ajb2.1764. [DOI] [PubMed] [Google Scholar]
- 17.Lomov B, Keith DA, Hochuli DF. Pollination and plant reproductive success in restored urban landscapes dominated by a pervasive exotic pollinator. Landsc. Urban Plan. 2010;96:232–239. doi: 10.1016/j.landurbplan.2010.03.009. [DOI] [Google Scholar]
- 18.Hanna C, Foote D, Kremen C. Invasive species management restores a plant–pollinator mutualism in Hawaii. J Appl. Ecol. 2013;50:147–155. doi: 10.1111/1365-2664.12027. [DOI] [Google Scholar]
- 19.Dick WC. Genetic rescue of remnant tropical trees by an alien pollinator. Proc. R. Soc. B. 2001;268:2391–2396. doi: 10.1098/rspb.2001.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Pijl L, Dodson H. Orchid Flowers: Their Pollination and Evolution. 1. Coral Gable: The Fairchild Tropical Garden and the University of Miami Press; 1966. [Google Scholar]
- 21.Dressler RL. The Orchids: Natural History and Classification. Cambridge: Harvard University Press; 1981. [Google Scholar]
- 22.Dafni A. Mimicry and deception in pollination. Ann. Rev. Ecol. Evol. Syst. 1984;15:259–278. doi: 10.1146/annurev.es.15.110184.001355. [DOI] [Google Scholar]
- 23.Schiestl FP. On the success of a swindle: pollination by deception in orchids. Sci. Nat. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- 24.Jersáková J, Johnson SD, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006;81:219–235. doi: 10.1017/S1464793105006986. [DOI] [PubMed] [Google Scholar]
- 25.Shrestha M, Dyer AG, Dorin A, Ren ZX, Burd M. Rewardlessness in orchids: How frequent and how rewardless? Plant Biol. 2020;22:555–561. doi: 10.1111/plb.13113. [DOI] [PubMed] [Google Scholar]
- 26.Ackerman JD, et al. Beyond the various contrivances by which orchids are pollinated: global patterns in orchid pollination biology. Bot. J. Linn. Soc. 2023;202:295–324. doi: 10.1093/botlinnean/boac082. [DOI] [Google Scholar]
- 27.Scopece G, Cozzolino S, Johnson SD, Schiestl FP. Pollination efficiency and the evolution of specialized deceptive pollination systems. Am. Nat. 2010;175:98–105. doi: 10.1086/648555. [DOI] [PubMed] [Google Scholar]
- 28.Brundrett M. Identification and Ecology of Southwest Australian Orchids. Perth: Western Australian Naturalists' Club Inc; 2014. [Google Scholar]
- 29.Johnson SD, Edwards TJ. The structure and function of orchid pollinaria. Plant Syst. Evol. 2000;222:243–269. doi: 10.1007/BF00984105. [DOI] [Google Scholar]
- 30.Johnson SD, Peter CI, Ågren J. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proc. R. Soc. B. 2004;271:803–809. doi: 10.1098/rspb.2003.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Linn. Soc. 2005;84:1–54. doi: 10.1515/biorc-2015-0024. [DOI] [Google Scholar]
- 32.Cozzolino S, et al. Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: absence of premating barriers? Proc. R. Soc. B. 2005;272:1271–1278. doi: 10.1098/rspb.2005.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schemske DW. Evolution of floral display in the orchid Brassavola nodosa. Evolution. 1980;1:489–493. doi: 10.1111/j.1558-5646.1980.tb04838.x. [DOI] [PubMed] [Google Scholar]
- 34.Ackerman JD, Montalvo AM. Short-and long-term limitations to fruit production in a tropical orchid. Ecology. 1990;71:263–272. doi: 10.2307/1940265. [DOI] [Google Scholar]
- 35.Li P, Huang BQ, Pemberton RW, Luo YB, Cheng J. Floral display influences male and female reproductive success of the deceptive orchid Phaius delavayi. Plant Syst. Evol. 2011;296:21–27. doi: 10.1007/s00606-011-0473-8. [DOI] [Google Scholar]
- 36.Scaccabarozzi D, et al. Masquerading as pea plants: Behavioural and morphological evidence for mimicry of multiple models in an Australian orchid. Ann. Bot. 2018;122:1061–1073. doi: 10.1093/aob/mcy166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaccabarozzi D, Galimberti A, Dixon KW, Cozzolino S. Rotating arrays of orchid flowers: a simple and effective method for studying pollination in food deceptive plants. Diversity. 2020;12:286. doi: 10.3390/d12080286. [DOI] [Google Scholar]
- 38.Scaccabarozzi D, et al. Ecological factors driving pollination success in an orchid that mimics a range of Fabaceae. Bot. J. Linn. Soc. 2020;194:253–269. doi: 10.1093/botlinnean/boaa039. [DOI] [Google Scholar]
- 39.Ackerman JD. Island invasions by introduced honeybees: What can be expected for Puerto Rico and the Caribbean? Front. Ecol. Evol. 2021;8:556744. doi: 10.3389/fevo.2020.556744. [DOI] [Google Scholar]
- 40.Sugiura N. Pollination and floral ecology of Arundina graminifolia (Orchidaceae) at the northern border of the species’ natural distribution. J. Plant Res. 2014;127:131–139. doi: 10.1007/s10265-013-0587-x. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura N. The pollination ecology of Bletilla striata (Orchidaceae) Ecol. Res. 1995;10:171–177. doi: 10.1007/BF02347939. [DOI] [Google Scholar]
- 42.Ogawa Y, Miyake T. How do rewardless Bletilla striata flowers attract pollinators to achieve pollination? Plant Syst. Evol. 2020;306:78. doi: 10.1007/s00606-020-01709-0. [DOI] [Google Scholar]
- 43.Sanguinetti A, Singer RB. Invasive bees promote high reproductive success in Andean orchids. Biol. Conserv. 2014;175:10–20. doi: 10.1016/j.biocon.2014.04.011. [DOI] [Google Scholar]
- 44.Adams PB, Lawson SD. Pollination in Australian Orchids: A Critical-Assessment of the Literature 1882–1992. Aust. J. Bot. 1993;41:553–575. doi: 10.1071/BT9930553. [DOI] [Google Scholar]
- 45.Brundrett M, Ladd P, Keighery G. Pollination strategies are exceptionally complex in Southwestern Australia—A globally significant ancient biodiversity hotspot. Aust. J. Bot. 2024;72:1–70. doi: 10.1071/BT23007. [DOI] [Google Scholar]
- 46.Luer CA. The Native Orchids of the United States and Canada Excluding Florida. New York: New York Botanical Garden; 1975. [Google Scholar]
- 47.Argue GL. The Pollination Biology of North American Orchids. New York: Springer; 2012. [Google Scholar]
- 48.Thien LB, Marcks BG. The floral biology of Arethusa bulbosa, Calopogon tuberosus and Pogonia ophioglossoides (Orchidaceae) Can. J. Bot. 1972;50:2319–2325. doi: 10.1139/b72-300. [DOI] [Google Scholar]
- 49.Heinrich B. Bumblebee Economics. Cambridge: Harvard University Press; 1979. [Google Scholar]
- 50.Gregg KB. Reproductive biology of the orchid Cleistes divaricata (L.) Ames var. bifaria growing in a West Virginia meadow. Castañea. 1989;54:57–78. [Google Scholar]
- 51.Gregg KB. Reproductive strategy of Cleistes divaricata (Orchidaceae) Am. J. Bot. 1991;78:350–360. doi: 10.1002/j.1537-2197.1991.tb15197.x. [DOI] [Google Scholar]
- 52.Attri LK, Kant R. Orchid Pollination: An observation on pollination-pollinator interaction in Cymbidium pendulum (Sw.) Roxb. Curr. Bot. 2011;2:05–08. [Google Scholar]
- 53.Verma J, et al. Pollination in Cymbidium pendulum (Roxb.) Sw. (Orchidaceae) Vegetos. 2012;25:298–302. [Google Scholar]
- 54.Sugiura N, Fujie T, Inoue K, Kitamura K. Flowering phenology, pollination, and fruit set of Cypripedium macranthos var. rebunense, a threatened Lady’s Slipper (Orchidaceae) J. Plant Res. 2001;114:171–176. doi: 10.1007/PL00013980. [DOI] [Google Scholar]
- 55.Sugiura N, Goubara M, Kitamura K, Inoue K. Bumblebee pollination of Cypripedium macranthos var. rebunense (Orchidaceae); a possible case of floral mimicry of Pedicularis schistostegia (Orobanchaceae) Plant Syst. Evol. 2002;235:189–195. doi: 10.1007/s00606-002-0229-6. [DOI] [Google Scholar]
- 56.Pearn, M. A. Pollination and comparative reproductive success of lady's slipper orchids Cypripedium candidum, C. parviflorum, and their hybrids in southern Manitoba. Dissertation (University of Manitoba, 2013).
- 57.Grantham MA, Ford BA, Worley AC. Pollination and fruit set in two rewardless slipper orchids and their hybrids (Cypripedium, Orchidaceae): Large yellow flowers outperform small white flowers in the northern tall grass prairie. Plant Biol. 2019;21:997–1007. doi: 10.1111/plb.13026. [DOI] [PubMed] [Google Scholar]
- 58.Edens-Meier R, Arduser M, Westhus E, Bernhardt P. Pollination ecology of Cypripedium reginae Walter (Orchidaceae): Size matters. Telopea. 2011;13:327–340. doi: 10.7751/telopea20116024. [DOI] [Google Scholar]
- 59.Miranda-Molina YM, Gonzalez EJ, Marquez-Guzman J, Meave JA, Perez-Garcia EA. Pollination success in three tropical dry forest orchid species from Mexico: insights from floral display, visitation rates, and flower micromorphology. Bot. Sci. 2021;99:771–790. doi: 10.17129/botsci.2785. [DOI] [Google Scholar]
- 60.Liu H, Pemberton R. Pollination of an invasive orchid, Cyrtopodium polyphyllum (Orchidaceae), by an invasive oil-collecting bee, Centris nitida, in southern Florida. Botany. 2010;88:290–295. doi: 10.1139/B10-017. [DOI] [Google Scholar]
- 61.Pansarin LM, Pansarin ER, Sazima M. Reproductive biology of Cyrtopodium polyphyllum (Orchidaceae): A Cyrtopodiinae pollinated by deceit. Plant Biol. 2008;10:650–659. doi: 10.1111/j.1438-8677.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 62.Pemberton RW, Liu H. Potential of invasive and native solitary specialist bee pollinators to help restore the rare cowhorn orchid (Cyrtopodium punctatum) in Florida. Biol. Conserv. 2008;141:1758–1764. doi: 10.1016/j.biocon.2008.04.016. [DOI] [Google Scholar]
- 63.Dutra D, Kane ME, Adams CR, Richardson L. Reproductive biology of Cyrtopodium punctatum in situ: implications for conservation of an endangered Florida orchid. Plant Species Biol. 2009;24:92–103. doi: 10.1111/j.1442-1984.2009.00242.x. [DOI] [Google Scholar]
- 64.Leong TM, Yeow CW. Observations of pollination in the pigeon orchid, Dendrobium crumenatum Swartz (Orchidaceae) in Singapore. Nat. Singap. 2013;6:91–96. [Google Scholar]
- 65.Meurgey F. Bee species and their associated flowers in the French West Indies (Guadeloupe, Les Saintes, La Désirade, Marie Galante, St Barthelemy and Martinique) (Hymenoptera: Anthophila: Apoidea) Ann. Soc. Entomol. Fr. 2016;52:209–232. doi: 10.1080/00379271.2016.1244490. [DOI] [Google Scholar]
- 66.Ackerman JD. Orchidées invasives: accélération de la colonization et de la propagation. L’Orchidophile. 2017;213:167–173. [Google Scholar]
- 67.Slater AT, Calder DM. The pollination biology of Dendrobium speciosum Smith: A case of false advertising? Aust. J. Bot. 1988;36:145–158. doi: 10.1071/BT9880145. [DOI] [Google Scholar]
- 68.Beardsell DV, Clements MA, Hutchinson JF, Williams EG. Pollination of Diuris maculata R Br (Orchidaceae) by Floral Mimicry of the Native Legumes Daviesia spp and Pultenaea scabra R Br. Aust. J. Bot. 1986;34:165–173. doi: 10.1071/BT9860165. [DOI] [Google Scholar]
- 69.Indsto JO, et al. Pollination of Diuris maculata (Orchidaceae) by male Trichocolletes venustus bees. Aust. J. Bot. 2006;54:669–679. doi: 10.1071/BT05146. [DOI] [Google Scholar]
- 70.Rayment T. Two orchids and a bee. Vic. Nat. 1932;49:140–140. [Google Scholar]
- 71.Kuiter HR. Orchid Pollinators of Victoria. 5. Victoria: Aquatic Photographics; 2023. [Google Scholar]
- 72.Aguiar JMRBV, Pansarin ER. Deceptive pollination of Ionopsis utricularioides (Oncidiinae: Orchidaceae) Flora. 2019;250:72–78. doi: 10.1016/j.flora.2018.11.018. [DOI] [Google Scholar]
- 73.Rose-Smyth MC. Investigating the pollination biology of a long-lived island endemic epiphyte in the presence of an adventive alien pollinator. In: Pridgeon AM, Arosemena A, editors. Proceedings of the 22nd World Orchid Conference. Guayaquil: Asociación Ecuatoriana de Orquideología; 2019. pp. 80–91. [Google Scholar]
- 74.Smith GR, Snow GE. Pollination ecology of Platanthera (Habenaria) ciliaris and P. blephariglottis (Orchidaceae) Bot. Gaz. 1976;137:133–140. doi: 10.1086/336852. [DOI] [Google Scholar]
- 75.Cole FR, Firmage DH. The floral ecology of Platanthera blephariglottis. Am. J. Bot. 1984;71:700–710. doi: 10.1002/j.1537-2197.1984.tb14177.x. [DOI] [Google Scholar]
- 76.Jones DL. The pollination of Prasophyllum alpinum R.Br. Vic. Nat. 1972;89:260–263. [Google Scholar]
- 77.Johnson SD, et al. Diverse pollination systems of the twin-spurred orchid genus Satyrium in African grasslands. Plant Syst. Evol. 2011;292:95–103. doi: 10.1007/s00606-010-0411-1. [DOI] [Google Scholar]
- 78.Ellis AG, Johnson SD. Do pollinators determine hybridization patterns in sympatric Satyrium (Orchidaceae) species? Plant Syst. Evol. 1999;219:137–150. doi: 10.1007/BF00985575. [DOI] [Google Scholar]
- 79.Botes C, van der Niet T, Cowling RM, Johnson SD. Is biodiversity underestimated by classical her arium-based taxonomy? A multi-disciplinary case study in Satyrium (Orchidaceae) Bot. J. Linn. Soc. 2020;194:342–357. doi: 10.1093/botlinnean/boaa041/5867273. [DOI] [Google Scholar]
- 80.Van der Niet T, Jürgens A, Johnson SD. Pollinators, floral morphology and scent chemistry in the southern African orchid genus Schizochilus. S. Afr. J. Bot. 2010;76:726–738. doi: 10.1016/j.sajb.2010.07.004. [DOI] [Google Scholar]
- 81.Suetsugu K, Abe Y. Unexpected contribution of the introduced honeybee Apis mellifera to high fruit set in Spiranthes australis (Orchidaceae) Entomol. News. 2021;129:559–563. doi: 10.3157/021.129.0511. [DOI] [Google Scholar]
- 82.Iwata T, Nagasaki O, Ishii HS, Ushimaru A. Inflorescence architecture affects pollinator behaviour and mating success in Spiranthes sinensis (Orchidaceae) New Phytol. 2012;193:196–203. doi: 10.1111/j.1469-8137.2011.03892.x. [DOI] [PubMed] [Google Scholar]
- 83.Coleman E. Further notes on the pollination of Spiranthes sinensis (Pers.) Ames. Vic. Nat. 1933;50:61–64. [Google Scholar]
- 84.Catling P. Pollination of northeastern North American Spiranthe (Orchidaceae) Can. J. Bot. 1983;61:1080–1093. doi: 10.1139/b83-116. [DOI] [Google Scholar]
- 85.Van der Cingel, N. A. An Atlas Of Orchid Pollination: America, Africa, Asia And Australia (Balkema, A.A., ed) (Rotterdam, 2001).
- 86.Ackerman JD, 2023. Beyond the various contrivances by which orchids are pollinated: Global patterns in orchid pollination biology. Bot. J. Linn. Soc. [DOI]
- 87.Michener, C. D. The Bees of the World (2 ed.) xvi + 953 (Johns Hopkins University Press, Baltimore, 2007).
- 88.Valido A, Rodríguez-Rodríguez MC, Jordano P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019;9:4711. doi: 10.1038/s41598-019-41271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joffard N, Massol F, Grenié M, Montgelard C, Schatz B. Effect of pollination strategy, phylogeny and distribution on pollination niches of Euro-Mediterranean orchids. J. Ecol. 2019;107:478–490. doi: 10.1111/1365-2745.13013. [DOI] [Google Scholar]
- 90.De Luca PA, Vallejo-Marín M. What's the ‘buzz’about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013;16:429–435. doi: 10.1016/j.pbi.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Zhang HP, et al. Reproductive isolation among three nocturnal moth-pollinated sympatric Habenaria species (Orchidaceae) Front. Plant Sci. 2022;13:908852. doi: 10.3389/fpls.2022.908852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lussu M, De Agostini A, Cogoni A, Marignani M, Cortis P. Does size really matter? A comparative study on floral traits in orchids with two different pollination strategies. Plant Biol. 2019;21:961–966. doi: 10.1111/plb.12993. [DOI] [PubMed] [Google Scholar]
- 93.Klein AM, Steffan-Dewenter I, Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B. 2003;270:955–961. doi: 10.1098/rspb.2002.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hung KLJ, Kingston JM, Albrecht M, Holway DA, Kohn JR. The worldwide importance of honeybees as pollinators in natural habitats. Proc. R. Soc. B. 2018;285:20172140. doi: 10.1098/rspb.2017.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aizen MA, et al. When mutualism goes bad: Density-dependent impacts of introduced bees on plant reproduction. New Phytol. 2014;12:e8697. doi: 10.1002/ece3.8697. [DOI] [Google Scholar]
- 96.Scheper J, et al. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl. Acad. Sci. U S A. 2014;111:17552–17557. doi: 10.1073/pnas.1412973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Houston TF, Dods K, Milne LA, Scaccabarozzi D. New insights into the unusual nesting biology of the bee Trichocolletes orientalis (Hymenoptera: Colletidae, Neopasiphaeinae), particularly its larval ‘oil bath’. Apidologie. 2023;54:11. doi: 10.1007/s13592-022-00981-y. [DOI] [Google Scholar]
- 98.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 99.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313(5785):351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 100.Baude M, et al. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature. 2016;530(7588):85–88. doi: 10.1038/nature16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson SD, Schiestl FP. Floral Mimicry. Oxford: Oxford University Press; 2016. [Google Scholar]
- 102.Blaauw BR, Isaacs R. Larger patches of diverse floral resources increase insect pollinator density, diversity, and their pollination of native wildflowers. Basic Appl. Ecol. 2014;15:701–711. doi: 10.1016/j.baae.2014.10.001. [DOI] [Google Scholar]
- 103.Backhouse, G. N., Copeland, L. M., Brown, A. P. & Bates, R. J. Checklist of the Orchids of Australia Including its Island Territories (Melbourne, 2019).
- 104.Jones DL. A Complete Guide to Native Orchids of Australia, Including the Island Territories. 2. Reed New Holland: Frenchs Forest; 2006. [Google Scholar]
- 105.Hoffman N, Brown A. Orchids of South-West Australia. 3. Perth: Gooseberry Hill; 2011. [Google Scholar]
- 106.Wickham, H. & Wickham, H. Getting Started with ggplot2. ggplot2: Elegant graphics for data analysis 11–31. (2016).
- 107.Barton, K. MuMIn: multi-model inference. R package version 1. 0. 0. http://r-forge.r-project.org/projects/mumin/ (2009).
- 108.Wilkinson, L. ggplot2: elegant graphics for data analysis (2011).
- 109.Ripley B, et al. Package ‘mass’. Cran r. 2013;538:113–120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ackerman JD, 2023. Beyond the various contrivances by which orchids are pollinated: Global patterns in orchid pollination biology. Bot. J. Linn. Soc. [DOI]
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information files. Supplementary material associated with this article includes Figure S1, Figure S2, Table S1, Table S2 and Datafile S1. Experimental research and field studies on study plants comply with relevant institutional, national, and international guidelines and legislation. Permission to collect Diuris brumalis and D. magnifica for identification purposes were obtained and collected specimens were vouchered and identified by the Herbarium of Western Australia, Perth. The material is publicly available at the Herbarium. Vaucher numbers: DS004, DS009, DS010, DS013, DS018.