Abstract
Canine liposarcoma is an uncommon tumor that shares morphological similarities with its human counterpart. In dogs, the genetic features of this tumor are unknown and, based on immunohistochemical studies, amplification of the gene MDM2 and the mutation of TP53 are suspected. In this study 51 cases of primary liposarcomas were immunohistochemically stained for MDM2 and p53 and subjected to fluorescent in situ hybridization and next-generation sequencing to detect MDM2 amplification and TP53 mutations, respectively. MDM2 and p53 were expressed in 21 and 6 cases, respectively. MDM2 amplification and TP53 mutations were identified in 10 and 15 cases, respectively. Statistical analysis revealed an association of the myxoid subtype and the mitotic count with p53 expression and TP53 mutation. No association was found between MDM2 amplification and MDM2 expression or tumor subtype. These results suggest that despite morphological similarities, canine liposarcoma differs from its human counterpart, for which MDM2 amplification is diagnostic for well differentiated and de-differentiated variants, and TP53 mutations are more common in pleomorphic liposarcoma rather than the myxoid one as occur in our cases. Furthermore, canine myxoid liposarcoma likely represents a distinct disease rather than a mere morphological variant.
Subject terms: Genetics, Cancer, Sarcoma
Introduction
Canine liposarcoma is a relatively rare sarcoma of dogs. It predominantly originates in the subcutaneous tissue of the body’s trunk and the proximal regions of the limbs. However, there have been reports of its occurrences in other sites, such as deep soft tissue (deeper than subcutis) and the spleen1–3.
Canine liposarcoma can be categorized into four morphological variants that bear a striking resemblance to their human counterparts: well-differentiated, dedifferentiated, myxoid, and pleomorphic1,2,4. In dogs, these variants primarily serve as morphological distinctions, while in humans, they represent distinct neoplastic entities with specific genetic alterations5. Well-differentiated and dedifferentiated human liposarcomas are characterized by the presence of a giant ring chromosome that carries amplified copies of the MDM2 and CDK4 genes, which play essential roles in cell cycle regulation. These two tumor types are regarded as two ends of a morphological spectrum of the same neoplastic entity, distinct from myxoid and pleomorphic liposarcoma, with the dedifferentiated variant showing metastatic potential5. Human myxoid liposarcoma is known to exhibit a reciprocal translocation between chromosomes 12 and 16: t(12;16)(q13;p11), resulting in the fusion of the DDIT3 gene with the FUS gene. Pleomorphic liposarcoma displays complex genetic rearrangements, and dysregulation of several tumor suppressor pathways (e.g., p53 and Rb1) is common in this subtype5,6.
Immunohistochemical studies have recently expanded our understanding of canine liposarcoma, revealing an overexpression of tyrosine kinase receptors (TRKs), MDM2, and p53. This suggests that TRK pathways may be involved in tumor progression, that the MDM2 gene may be amplified as in humans, and that the TP53 gene could be mutated in the myxoid variant1,7,8. However, our knowledge regarding the genetic status of canine liposarcoma remains limited, and the few cases examined in the literature have failed to demonstrate anomalies in these genes9.
The aim of this study is to investigate the presence of MDM2 gene amplification and TP53 gene mutations in a larger number of canine liposarcomas. We used fluorescent in situ hybridization (FISH) to assess MDM2 amplification and next-generation sequencing (NGS) to detect TP53 and MDM2 gene mutations.
Results
Fifty-one cases of canine liposarcoma were included in this study. Among these cases, fifteen dogs were female (4 spayed and 11 intact), thirty-two were male (1 castrated and 31 intact), and in 4 cases, sex was not reported. The age was available for 47 out of the 51 cases, ranging from 6 to 16 years, with a median age of 11 years. Among the liposarcomas, 41 affected the soft tissue, with 36 being subcutaneous, 3 intramuscular, and 2 intracavitary. Six cases were splenic, and in 4 cases, the specific site was not recorded. The distribution of subtypes included 26 well-differentiated, 10 myxoid, 8 pleomorphic, and 7 dedifferentiated cases. The mitotic count varied from 1 to 36, with a median of 5. Tumor grading classified 18 cases as grade 1, 28 as grade 2, and 5 as grade 3.
Immunohistochemistry detected the expression of MDM2 in 21 cases (including 11 well-differentiated, 5 dedifferentiated, 3 myxoid, and 2 pleomorphic cases), and the expression of p53 in 6 cases, all of which were myxoid.
NGS
In four cases, there was an insufficient amount of tissue available in the paraffin blocks for NGS analysis, thus 47 cases were included in the study. These comprised 20 well-differentiated liposarcomas, 10 myxoid liposarcomas, 8 pleomorphic liposarcomas, and 8 dedifferentiated liposarcomas. A TP53 mutation was identified in 15 out of the 47 liposarcomas (31.9%), which included 2 well-differentiated, 9 myxoid, 3 pleomorphic, and 1 dedifferentiated neoplasms. Out of these, ten variants were missense single nucleotide variants (SNV) mutations, and five were small indels (Table 1). Three additional variants were single nucleotide indels within a homopolymer stretch and were thus excluded from further analysis.
Table 1.
Mutation of TP53identified in canine liposarcoma.
| Mutation | Exon | # of cases | PolyPhen-2 | Ratio PolyPhen-2 |
|---|---|---|---|---|
| p.Pro137Val | 4 | 2 | Possiblydamaging | 0.688 |
| p.Thr244Ter | 6 | 1 | Damaging | |
| g.32565105insC | 1 | 2 | Damaging | |
| p.Ser229Tyr | 6 | 1 | Probablydamaging | 1 |
| p.S228_N235delinsILE | 6 | 1 | Damaging | |
| p.Val204Leu | 5 | 1 | Probablydamaging | 0.999 |
| p.Met231delT | 6 | 1 | Damaging | |
| p.Arg323Cys | 9 | 1 | Probablydamaging | 0.994 |
| p.Ser343Ter | 9 | 1 | Damaging | |
| p.Asp195Asn | 5 | 1 | Benign | 0.079 |
| p.Pro129Leu | 4 | 1 | Probablydamaging | 1 |
| p.Arg162Leu | 4 | 1 | Probablydamaging | 0.999 |
| p.Leu357Met | 1 | Probablydamaging | 0.989 | |
| g.32565125delTC | – | 1 | Damaging | |
| p.Leu14insCfs | 1 | 1 | Damaging |
Of the 15 mutations (2 well-differentiated, 9 myxoid, 3 pleomorphic, and 1 dedifferentiated), all except one were classified as "damaging" or "possibly damaging" using the Polyphen2 tool (http://genetics.bwh.harvard.edu/pph2/). Even if PolyPhen2 was developed for the determination of variants in human beings, the analysis was conducted by performing the query with the amino acid sequence of canine reference and the corresponding positions of the variants, thus utilizing the in silico prediction of PolyPhen2. PolyPhen2 has been previously used for this type of evaluation in canine specimens in the literature10–12. One variant was categorized as "benign". The variant allele frequency (VAF) for these 15 mutations ranged from 8 to 76%. In two cases a nonsense mutation was detected, leading to a truncated protein at the amino acid T244 and S343; in two cases a frameshift mutation was identified (Table 1). Among these 15 variants, 5 cases were immunohistochemically positive for p53 (IHC), while 10 were negative. Interestingly, one myxoid liposarcoma that tested p53 positive by IHC showed no mutation. Notably, no missense or small indel variants were detected in the MDM2 gene.
FISH
Out of the total of 51 cases subjected to FISH analysis, 8 cases were found to be technically inadequate and were therefore considered indeterminate and subsequently excluded.
Among the remaining 43 cases, ten exhibited MDMI2 amplification (23%), with MDM2/GMCL1 ratios ranging from 2.1 to 6.5. This subset included 6 well-differentiated liposarcomas, 2 dedifferentiated, 1 myxoid, and 1 pleomorphic. The amplification pattern in 6 out of these 10 cases (60%) was identified as cluster-type (Fig. 1), characterized by closely stippled, adjacent, and numerous signals forming a large cluster within a specific area of the nucleus. In contrast, the remaining 4 cases (40%) exhibited a non-clustered amplification pattern, with signals scattered equally throughout the nucleus. Among the 10 amplified cases, 4 tested positive for MDM2 by immunohistochemistry, while 6 were negative.
Figure 1.
Fluorescent in situ hybridization of canine liposarcomas. (A) Diploid copy number of MDM2 per nucleus (red dots). (B) MDM2 amplified liposarcoma withMDM2/GMCL1 ratio greater than 2. Increased copy number of MDM2 with an average of 8.33 per nucleus (red dots). (C) MDM2 cluster type amplification withMDM2/GMCL1ratio greater than 2. Closely stippled adjacent numerous red MDM2 signals that form large clusters. (D) Non-polysomic MDM2 amplified liposarcoma. In green is shown the diploid GMCL1 control gene.
Most of the cases, specifically 34 out of 43 (77%), showed no MDM2 amplification and exhibited a diploid signal pattern. Notably, no cases displaying polysomy were observed. Among the 33 cases without MDM2 amplification, 13 were immunohistochemically positive for MDM2, while 20 were negative.
Statistical analysis
The occurrence of myxoid liposarcoma was significantly higher in the spleen compared to other tissues (p = 0.003), whereas the other subtypes were predominantly observed in the subcutis. Myxoid liposarcoma and dedifferentiated liposarcoma exhibited significantly higher grades in comparison to pleomorphic liposarcoma and well-differentiated liposarcoma (p = 0.0004). However, there was no significant association between the histological grade and the tumor site (p = 0.79).
A statistically significant association was found between immunohistochemical p53 positivity and TP53 mutation (p = 0.015). However, there was no statistically significant association between MDM2 positivity and MDM2 amplification or between MDM2 and p53 immunohistochemical expression (p = 0.92 and p = 0.38, respectively).
Statistical analysis revealed a strong association of the myxoid subtype (ML) with p53 immunohistochemical expression (p = 0.00003) and TP53 mutation (p = 0.00005) (Figs. 2 and 3). No association was found between histological subtypes and MDM2 immunohistochemical expression or gene amplification (p = 0.29 and p = 0.50, respectively). Similarly, there was no association between histological grade and MDM2 anomalies as detected by IHC and FISH (p = 0.79 and p = 0.91, respectively). However, the histological grade was significantly higher in p53-positive cases (p = 0.005), but not in cases with TP53 mutation (p = 0.18).
Figure 2.
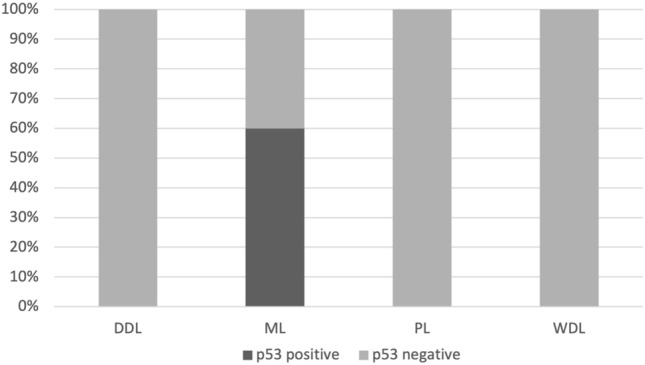
Percentage of cases immunohistochemically expressing p53 in canine liposarcoma, comparing the histotype: DDL = de-differentiated; ML = myxoid; PL = pleomorphic; WDL = well differentiated.
Figure 3.
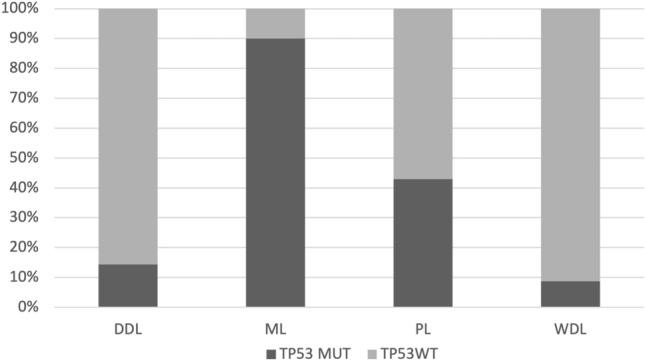
Percentage of cases harboring TP53 mutation in canine liposarcoma, comparing the histotype: DDL = de-differentiated; ML = myxoid; PL = pleomorphic; WDL = well differentiated.
Mitotic count (MC) was significantly higher in myxoid (mean 16.5 ± 11.6) and dedifferentiated liposarcoma (mean 15.8 ± 11.09) in comparison to pleomorphic (mean 5.1 ± 2.03) and well differentiated liposarcoma (2.8 ± 1.67). Furthermore, MC was significantly higher in p53-positive liposarcomas (p = 0.00001) and in those with TP53 mutation (p = 0.04).
Discussion
In this study, we have conducted the analysis of MDM2 and TP53 genes in a case series of canine liposarcoma. The cases examined encompassed 51 cases and comprised all the histologic variants reported in dogs. This investigation revealed some notable clinical features that differ from liposarcoma in humans. It is well-established that liposarcoma is a relatively rare tumor in dogs compared to humans, where it represents one of the most frequent soft tissue sarcomas5. Interestingly, in humans myxoid liposarcoma is more commonly found in the lower extremities, particularly affecting the thighs while intra-abdominal cases are less common13. In contrast, in our dataset, half of the myxoid liposarcomas developed in the spleen, with only 2 out of 10 occurring in the soft tissue of the extremities. Furthermore, the majority of splenic liposarcomas (5 out of 6) were myxoid, suggesting that the spleen may be a preferred site for this specific sarcoma in dogs.
TP53 mutations were prevalent in 9 out of 10 (90%) myxoid liposarcomas, while they were less frequent in other subtypes (7.7% of well-differentiated, 37.5% of pleomorphic, and 14.3% of dedifferentiated cases). Among these 9 mutations, eight were predicted to be "damaging" or "probably damaging," while one was categorized as "benign." This differs from findings in human liposarcoma, where TP53 mutations are described in 60% of pleomorphic liposarcomas and in approximately 20% of myxoid liposarcomas6. Notably, a study assessing p53 immunohistochemical expression in human myxoid liposarcomas found staining predominantly in highly cellular and round-cell areas, more frequently in high-grade tumors than low-grade ones13. Contrarily in this caseload we did not observe a preferential p53 staining in round cell areas and a statistically significant association between p53 overexpression and grade was identified. Supporting the impact of p53 alterations on tumor biological aggressiveness is the statistically significant association of the MC with both p53 staining and TP53 mutation. However, it should be noted that in our study, 10 out of 15 cases with a TP53 mutation did not exhibit p53 overexpression at the immunohistochemical level. Interestingly, none of these p53-negative cases were bearing a nonsense or frameshift mutation. Therefore, this discrepancy may be attributed to reduced antibody sensitivity, although it has been shown to cross-react with the canine species14. The same antibody yielded similar results in a study examining p53 alterations in canine malignancies15 and should therefore be interpreted with caution when used to infer the mutation status of TP53 incanine tumors. Furthermore, in one immunopositive case, a TP53 variant was not identified. This incongruity may also be related to an antibody-related issue or less likely, to other molecular anomalies leading to p53 stabilization within the nucleus of neoplastic cells. Interestingly, while the expression of p53 and TP53 mutation has been well-documented in canine osteosarcoma16, studies in canine soft tissue sarcomas are limited and have not reported associations with specific subtypes9,17.
The 15 mutations identified within our cohort were uniformly distributed across all the exons of the TP53 gene. Interestingly, no variants were detected in only three exons (2, 7, 8), while in all other exons, at least one substitution was identified. This emphasizes the significance of analyzing the entire coding sequence (CDS) of the TP53 gene and highlights that focusing solely on specific exons may result in an underestimation of potential mutations.
Immunohistochemical expression of MDM2 was identified in 21 out of 51 cases (41.17%), with the majority found in well-differentiated and dedifferentiated cases, accounting for approximately 70% of these two histotypes. This result aligns partially with the human literature, where immunohistochemistry for MDM2 is often regarded as a surrogate marker for the detection of MDM2 gene amplification, which is diagnostic for these two liposarcoma subtypes5. The immunohistochemical expression of MDM2 in canine liposarcoma has been reported, and similar to what is described in people it was interpreted as suggestive of gene amplification1. Nevertheless, data regarding MDM2 amplification in canine soft tissue sarcomas are scarce, and only a few cases of amplification have been demonstrated by Southern blot in rhabdomyosarcoma and malignant nerve sheath tumors, while the few cases of canine liposarcoma tested did not exhibit MDM2 amplification9. In this study, MDM2 amplification status was assessed for the first time in canine liposarcoma using FISH. MDM2 amplification was detected in 10 out of 43 cases (23%), with the majority found in well-differentiated and dedifferentiated cases; however, 6 of these cases were negative for MDM2 immunohistochemistry. Conversely, 13 out of 33 non-amplified cases were positive at the immunohistochemistry level. These findings lead to the conclusion that neither the assessment of MDM2 amplification by FISH nor the immunohistochemical evaluation of MDM2 protein expression can be considered specific for well-differentiated and dedifferentiated liposarcoma in dogs. These discrepancies may be attributed to sensitivity and specificity issues related to the use of probes and antibodies designed for human tissues, which, despite their cross-reactivity, may not perform optimally in canine tissues. Another hypothesis is that canine liposarcomas, despite their morphological similarities to the human counterpart, harbor a different set of genetic and proteomic alterations distinct from those of the human counterpart and that MDM2 protein expression may result, rather than from genetic amplification, from increased transcription or reduced degradation.
In summary, our findings indicate that, despite the morphological similarities between canine and its human counterpart, it appears that MDM2 amplification is not a defining feature of canine liposarcoma, although it may occur in a minority of cases, and MDM2 protein expression could potentially contribute to its oncogenic processes. Furthermore, canine myxoid liposarcoma likely represents a distinct disease rather than a mere morphological variant, which is characterized by TP53 mutations and a preference for involvement in the spleen.
Materials and methods
Case selection and histologic evaluation
Cases of histologically diagnosed canine liposarcoma were retrospectively retrieved from the archives of multiple institutions (three universities and one private diagnostic laboratory). Cases lacking sufficient paraffin-embedded tissue were excluded from the study. Subsequently, all the slides were collaboratively reviewed by two veterinary pathologists (GA and VP) using a multiheaded microscope. The diagnosis was confirmed and tumors were categorized based on histomorphology, according to the most recent classification system4. Mitotic count was determined as the total number of mitotic figures within 10 consecutive, non-overlapping high-power fields (HPF), corresponding to the standard area of 2.37 mm2 in the most cellular and proliferative regions of the tumor. The grade was determined using the grading system currently used in veterinary and human medicine18.
Immunohistochemistry
Three-micrometer-thick sections underwent dewaxing and rehydration. To block endogenous peroxidase activity, they were immersed in a 3% H2O2 solution in methanol for 30 min. Subsequently, the sections were rinsed in Tris buffer (pH 7.0).
For antigen retrieval, the sections were placed in citrate buffer (pH 6.0) and heated in a microwave oven at 750 W for 2 cycles of 5 min each. Afterward, they were allowed to cool at room temperature for 20 min.
Specific antibodies for MDM2 (mouse monoclonal, clone 2A10, dilution 1:100, Abcam, Cambridge, UK) and p53 (mouse monoclonal, clone PAb 240, dilution 1:100, BD Bioscience) were applied and allowed to incubate overnight at 4 °C. Following this, the sections were incubated for 30 min at room temperature with the appropriate biotin-conjugated secondary antibody (dilution 1:200, Dako, Glostrup, Denmark).
To enhance the reaction, an avidin–biotin method (ABC kit elite, Vector, Burlingame, CA, USA) was employed, and visualization was achieved using 3,3ʹ-diaminobenzidine (0.04% for 4 min). The sections were counterstained with Harris hematoxylin, rinsed in tap water, dehydrated, and cover-slipped.
Positive controls consisted of sections from normal canine testis for MDM2 and sections from canine mammary carcinoma, where p53 expression was known, for p53. Negative controls included slides incubated with a non-specific antibody or the omission of the primary antibody.
MDM2 positivity was defined as the presence of at least one positive nucleus per high-power field (40× magnification; 0.237 mm2), while p53 positivity was determined when more than 5% of neoplastic cells exhibited nuclear staining, as previously documented1,8.
Next generation sequencing
DNA was extracted from 2 to 4 tissue sections, each measuring 10 µm in thickness, and mounted on slides. The samples were manually scraped using a sterile blade, focusing on the area selected by the pathologist as the most representative on the hematoxylin and eosin-stained slide.
DNA amplification was carried out using an amplicon-based laboratory-developed NGS panel, enabling the amplification and sequencing of the entire Coding Sequence (CDS) of the TP53 and MDM2 genes (reference Canisfamiliaris 3, 48 amplicons, size 4.73 kb). TP53 CDS and MDM2 CDS were the only regions included in the panel. Using a targeted NGS panel has allowed to have a analytical sensitivity (10%) and costs not exceeding €300 per sample. Moreover, this panel could be easily carried into clinical practice. The median coverage obtained in this cohort using this panel was 2425×. The obtained results were analyzed using Variant Caller tool (Thermo Fisher Scientific), Integrative Genomics Viewer (IGV—v.2.12.2) and GenomeBrowse tools (https://www.goldenhelix.com/products/GenomeBrowse/index.html). Only mutations with a variant allele frequency (VAF) exceeding 5% were considered for mutational calls. Bam files of the obtained sequences were loaded on the BioPrject dataset (BioProject ID: PRJNA1118484).
Tissue microarray
Tissue microarrays (TMA) were constructed following a previously published method19–21. Two cores were sampled for each case, each core measuring 3 mm in diameter. Care was taken to select areas devoid of necrosis and inflammation. Nine double-core TMA blocks were assembled, with each one containing six cases, except for the last TMA which contained three cases, and one orientation core consisting of normal hepatic parenchyma.
Fluorescence in situ hybridization
To adapt commercially available FISH probes designed for human tissue for use with canine samples, the homology between the sequences of the canine and human MDM2 gene was assessed using the database BLAST (Basic Local Alignment Search Tool—NCBI). The alignment revealed a 92.46% homology between the MDM2 gene sequences of both species, thus allowing the use of human commercial probes.
Furthermore, to rule out polysomy, a suitable housekeeping gene on the same MDM2 chromosome (CFA 10) was sought. The selection criteria included:
No known involvement in the tumorigenesis of human and canine liposarcoma.
Location on CFA10.
High nucleotide sequence homology between canine and human species.
The GMCL1 gene (germ cell-less, spermatogenesis-associated 1) met these criteria, demonstrating a 95.55% sequence homology between human and canine species according to BLAST analysis.
The tumors underwent fluorescence in situ hybridization (FISH) using a dual-core tissue microarray. The Easy FISH Pretreatment Kit (OACP IE LTD, Cork, Ireland) was utilized.
The sections were initially incubated at 75 °C for 5 min in the hybridization plate. Subsequently, the sections underwent dewaxing, dehydration, air-drying, and then incubation with a permeation solution in a water bath at 90 °C for 8 min, followed by incubation in pepsin and HCl solution at 37 °C for 19 min. The sections were then washed in a washing buffer for 5 min, and dehydration was carried out in 2-min steps using 70%, 85%, and 100% ethanol. Finally, the sections were air-dried at room temperature.
The MDM2 gene copy number was identified using the MDM2 spectrum Orange FISH probe (catalogue number FP-054, TITAN FISH probe, OACP IE LTD, Cork, Ireland) and the Smart-ISH Solve buffer OACP IE LTD, Cork, Ireland). The GMCL1 probe, combined with its corresponding buffer (GMCL1 probe set spectrum green, catalogue number GMCL1-10-GR, Empire Genomic), was used as a control to confirm the presence of polysomy. The hybridization area was then cover-slipped and sealed with rubber cement (Supplementary Information).
The slides were incubated at 85 °C for 5 min for DNA denaturation and at 42 °C overnight for hybridization with the MDM2 probe; at 83 °C for 3 min for DNA denaturation and at 37 °C overnight for hybridization with the GMCL1 probe. Following this, the slides were washed in NP40 0.5%/2 × SSC (pH 7.0–7.5) at 75 °C for 2 min and in washing buffer for 2 min at room temperature. The slides were then dehydrated and counterstained using DAPI counterstaining solution (OACP IE LTD, Cork, Ireland).
The specificity of in-situ hybridization was further evaluated by considering the euploidy of the fibroblasts and lymphocytes adjacent to the neoplastic cells.
Evaluation of the signals' numbers and quality was made by using an Olympus BX61 fluorescent microscope equipped with relevant filters and objectives by 2 independent operators (EDO, LVM), and then images were obtained. The Cytovision Image analysis software (The CytoVision®) was employed to additionally count the number of gene copies per nucleus in the available nuclei with visible signals.
FISH assessment was conducted in accordance with the MDM2 amplification patterns observed in human liposarcoma22. A MDM2/GMCL1 ratio higher than 2 was considered indicative of MDM2 amplification.
Samples were deemed indeterminate for MDM2 if technical issues prevented them from being reported as either positive or negative.
Statistical analysis
Categorical variables were reported as percentages, while for continuous variables, mean, median, standard deviation (SD), and range were provided. Fisher's test was performed to assess associations between categorical variables. One-way ANOVA was employed to determine associations between mitotic count (MC) and other categorical variables. Results were considered significant at a threshold of p ≥ 0.05.
Statistical analysis was conducted using R (version 4.2.0).
Supplementary Information
Author contributions
Conception of the work: GA Design of the work: LVM, DDB, GA Data acquisition/analysis: LVM, DDB, TM, EDO, VP, BB, PR, EL, LC, GA Data interpretation: LVM, DDB, TM, EDO, GT, VP, BB, GA Manuscript drafting: GA Manuscript substantial revision: all the authors
Data availability
The datasets generated and/or analysed during the current study are available in the BioProject repository, ID 1118484-BioProject-NCBI (nih.gov), accession number: PRJNA1118484.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luisa Vera Muscatello and Dario de Biase.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64963-z.
References
- 1.Avallone G, et al. Histological classification and immunohistochemical evaluation of MDM2 and cdk4 expression in canine liposarcoma. Vet. Pathol. 2016;53:773–780. doi: 10.1177/0300985815626573. [DOI] [PubMed] [Google Scholar]
- 2.LaDouceur EEB, Stevens SE, Wood J, Reilly CM. Immunoreactivity of canine liposarcoma to muscle and brown adipose antigens. Vet. Pathol. 2017;54:885–891. doi: 10.1177/0300985817723691. [DOI] [PubMed] [Google Scholar]
- 3.Gower KLA, et al. Splenic liposarcoma in dogs: 13 cases (2002–2012) J. Am. Vet. Med. Assoc. 2015;247:1404–1407. doi: 10.2460/javma.247.12.1404. [DOI] [PubMed] [Google Scholar]
- 4.Roccabianca, P. et al. Surgical Pathology of Tumors of Domestic Animals Volume3: Tumors of Soft Tissue. (Foundation, C. L. Davis and S. W. Thompson, 2020).
- 5.Goldblum, J. R., Folpe, A. L. & Weiss, S. W. Liposarcoma. in Enzinger & Weiss’s Soft Tissue Tumors (eds. Goldblum, J. R., Folpe, A. L. & Weiss, S. W.) 520–563 (Elsevier, 2020).
- 6.Ghadimi MP, et al. Pleomorphic liposarcoma. Cancer. 2011;117:5359–5369. doi: 10.1002/cncr.26195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avallone G, et al. Tyrosine kinase receptor expression in canine liposarcoma. Vet. Pathol. 2017;54:212–217. doi: 10.1177/0300985816671379. [DOI] [PubMed] [Google Scholar]
- 8.Avallone G, et al. p53 Expression in canine liposarcoma correlates with myxoid variant and higher proliferative activity. Vet. Pathol. 2020;57:620–622. doi: 10.1177/0300985820941501. [DOI] [PubMed] [Google Scholar]
- 9.Nasir L, Rutteman GR, Reid SW, Schulze C, Argyle DJ. Analysis of p53 mutational events and MDM2 amplification in canine soft-tissue sarcomas. Cancer Lett. 2001;174:83–89. doi: 10.1016/S0304-3835(01)00637-1. [DOI] [PubMed] [Google Scholar]
- 10.Stern JA, Reina-Doreste Y, Chdid L, Meurs KM. Identification of PDE5A:E90K: A polymorphism in the canine phosphodiesterase 5A gene affecting basal cGMP concentrations of healthy dogs. J. Vet. Intern. Med. 2014;28:78–83. doi: 10.1111/jvim.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D-J, Kim Y-C, Kim A-D, Jeong B-H. Novel polymorphisms and genetic characteristics of the prion protein gene (PRNP) in dogs—A resistant animal of prion disease. Int. J. Mol. Sci. 2020;21:4160. doi: 10.3390/ijms21114160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook S, et al. Canine models of Charcot-Marie-Tooth: MTMR2, MPZ, and SH3TC2 variants in golden retrievers with congenital hypomyelinating polyneuropathy. Neuromuscul. Disord. 2023;33:677–691. doi: 10.1016/j.nmd.2023.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonescu CR, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: A molecular and clinicopathologic study of 82 cases. Clin. Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- 14.Keller SM, et al. A comprehensive test system to identify suitable antibodies against p53 for immunohistochemical analysis of canine tissues. J. Comp. Pathol. 2007;137:59–70. doi: 10.1016/j.jcpa.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Brunetti B, et al. Validation of p53 immunohistochemistry (PAb240 clone) in canine tumors with next-generation sequencing (NGS) analysis. Animals. 2023;13:899. doi: 10.3390/ani13050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza S, Konishi T, Dernell WS, Withrow SJ, Miller CW. Status of the p53, Rb and MDM2 genes in canine osteosarcoma. Anticancer Res. 1998;18:4449–4453. [PubMed] [Google Scholar]
- 17.Das S, Idate R, Lana SE, Regan DP, Duval DL. Integrated analysis of canine soft tissue sarcomas identifies recurrent mutations in TP53, KMT genes and PDGFB fusions. Sci. Rep. 2023;13:10422. doi: 10.1038/s41598-023-37266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avallone G, et al. Review of histological grading systems in veterinary medicine. Vet. Pathol. 2021;58:809–828. doi: 10.1177/0300985821999831. [DOI] [PubMed] [Google Scholar]
- 19.Muscatello LV, et al. HER2 amplification status in feline mammary carcinoma: A tissue microarray-fluorescence in situ hydridization-based study. Vet. Pathol. 2019;56:230–238. doi: 10.1177/0300985818808531. [DOI] [PubMed] [Google Scholar]
- 20.Pellegrino V, Muscatello LV, Sarli G, Avallone G. Canine gastrointestinal spindle cell tumors efficiently diagnosed by tissue microarray-based immunohistochemistry. Vet. Pathol. 2018;55:678–681. doi: 10.1177/0300985818777793. [DOI] [PubMed] [Google Scholar]
- 21.Muscatello LV, et al. HER2 overexpression and cytogenetical patterns in canine mammary carcinomas. Vet. Sci. 2022;9:583. doi: 10.3390/vetsci9110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambella A, et al. FISH diagnostic assessment of MDM2 amplification in liposarcoma: potential pitfalls and troubleshooting recommendations. Int. J. Mol. Sci. 2023;24:1342. doi: 10.3390/ijms24021342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the BioProject repository, ID 1118484-BioProject-NCBI (nih.gov), accession number: PRJNA1118484.



