Abstract
Oligonucleotides are advancing as essential materials for the development of new therapeutics, artificial genes, or in storage of information applications. Hitherto, our capacity to write (i.e., synthesize) oligonucleotides is not as efficient as that to read (i.e., sequencing) DNA/RNA. Alternative, biocatalytic methods for the de novo synthesis of natural or modified oligonucleotides are in dire need to circumvent the limitations of traditional synthetic approaches. This Perspective article summarizes recent progress made in controlled enzymatic synthesis, where temporary blocked nucleotides are incorporated into immobilized primers by polymerases. While robust protocols have been established for DNA, RNA or XNA synthesis is more challenging. Nevertheless, using a suitable combination of protected nucleotides and polymerase has shown promises to produce RNA oligonucleotides even though the production of long DNA/RNA/XNA sequences (>1000 nt) remains challenging. We surmise that merging ligase- and polymerase-based synthesis would help to circumvent the current shortcomings of controlled enzymatic synthesis.
Subject terms: Biocatalysis, Nucleic acids
Nucleic acids are key elements in numerous applications such as therapeutics and nanotechnology. However, the synthesis of long and modified oligonucleotides remains challenging and alternative, biocatalytic approaches are needed. Here, the authors discuss recent progress in the controlled enzymatic synthesis of oligonucleotides.
Introduction
Over half a century ago, the structures as well as the biological functions of DNA and RNA have been unraveled. Since then, nucleic acids have advanced as key elements in numerous applications that markedly deviate from their natural functions. For instance, oligonucleotides are important building blocks in DNA-encoded libraries (DELs) for drug discovery campaigns1,2, storage of digital information3,4, and computing5. In addition, DNA and RNA can serve as potent catalysts6–10, binders11–17, and therapeutic agents18,19. The increasing popularity of nucleic acids is accompanied by an important surge in the demand of synthetic oligonucleotides, particularly of sequences containing chemical modifications.
Currently, two main strategies exist for the production of DNA and RNA, namely chemical, automated synthesis and polymerase-based approaches (Fig. 1). Automated synthesis using solid-phase phosphoramidite chemistry was first developed by Marvin Caruthers and Serge Beaucage in 198120,21. The activated building blocks used in this approach consist of 5’-O-DMTr protected nucleosides equipped with reactive phosphoramidite moieties located at the 3’ position of the sugar moiety. In an iterative process, phosphoramidite nucleosides are added on a growing chain which is immobilized on a solid support through a succession of cycles consisting of coupling, capping, oxidation, and deprotection steps. An interesting feat of this method is that it is directly amenable to the production of chemically modified oligonucleotides. The integration of chemical modifications is performed by substituting natural with modified phosphoramidites during solid phase synthesis. This technology is particularly useful for the crafting of therapeutic oligonucleotides (e.g., siRNAs and antisense oligonucleotides (ASOs)) that bear multiple modification patterns as well as for xeno nucleic acids (XNAs) which consist of sometimes heavily modified sugar residues (vide infra). Due to these interesting properties, solid phase synthesis has advanced as the workhorse for the preparation of therapeutic oligonucleotides in industrial settings, particularly for sequences containing phosphate and sugar alterations19,22.
Fig. 1. Pros and cons of chemical automated synthesis and enzymatic synthesis of oligonucleotides.
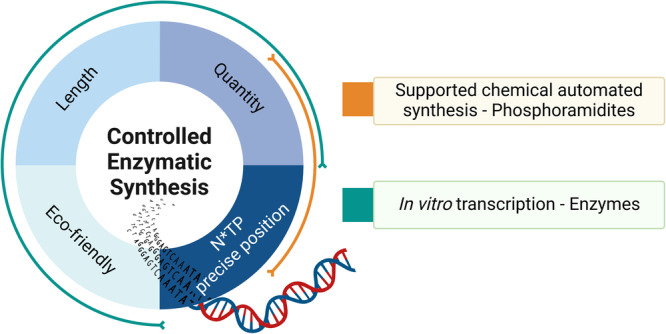
Controlled enzymatic synthesis really resides at the interface of chemical and enzymatic approaches. Allowing theoretically to reach high quantity, high length and the use of modified nucleotides at the same time.
Alternatively, oligonucleotides can be accessed via polymerase-mediated synthesis. These enzymes catalyze the co-polymerization of nucleoside triphosphates on DNA and RNA primers and produce sequences of variable lengths ranging from a few dozens to several thousands of nucleotides. This reaction can proceed either in a template-dependent or template-independent manner depending on the nature of the polymerase. Production of modified oligonucleotides is readily accessible by this approach provided the corresponding, chemically altered nucleoside triphosphates that are used in lieu of their natural counterparts are tolerated as substrates by the polymerases23. The potential of this method is showcased by the industrial production of mRNA vaccines which consist of long (>4000 nt), single-stranded RNA oligonucleotides containing modified nucleotides (mainly N1-methyl-pseudouridine)24,25.
While both production methods of (modified) oligonucleotides are well-established, they suffer from shortcomings. Indeed, chemical synthesis based on the assembly of activated phosphoramidite building blocks is limited in terms of both scalability (<10 kg batches) and sustainability (over 4000 kg waste organic solvents and reagents per kg for a 20 nt long oligonucleotide26–28). In addition, even though coupling yields of phosphoramidites are generally very high (≥99%), access to sequences larger than 150 nucleotides is difficult or impossible by this approach. This is due to the fact that the theoretical yields for larger sequences are low (e.g., 22% (0.99150 · 100%)for a 150-mer) and practical, isolated yields are much lower than these upper limits due to decreasing coupling efficiencies with increasing oligonucleotide lengths29,30. In addition, the rather harsh conditions (particularly during detritylation) imposed by solid-phase synthesis combined with errors in acetylation lead to depurination as well as single-base deletions or insertions (indels). The occurrence of such errors also increases with the length of the sequence and can have dire consequences. Even with an accuracy of 99.5% for adding a correct nucleotide, the overall fidelity for the production of a 150 nt long sequence would only be of 47% (0.995150)31. The rather harsh conditions imposed by the synthetic cycles also restrict the choice of chemical modifications that can be introduced into oligonucleotides and is for instance not compatible with modifications that are susceptible to oxidation or react with electrophiles32.
Similarly, while polymerase-mediated synthesis grants access to longer sequences (>1 kb), not all chemically modified nucleotides are tolerated by polymerases and specific introduction of single or multiple chemically altered species at discreet, user-defined locations is not possible. In addition, enantioselective synthesis with polymerases is still restricted to producing all-Rp33–35 phosphorothioate linkages and the production of the required nucleoside triphosphates is labor intensive and low yielding32,36,37.
In order to meet the exponential increase in demand of long and chemically modified oligonucleotides and to match the writing and reading speeds for storage of digital information applications, alternative, highly efficient, sustainable, and cost-affordable biocatalytic strategies are in dire need38–40.
This Perspective provides an overview of existing and emerging biocatalytic methods for the production of oligonucleotides with a particular emphasis on controlled enzymatic synthesis. We also provide a description of other enzymatic methods and comment on the evolution and future directions of the field.
Main
Controlled enzymatic synthesis
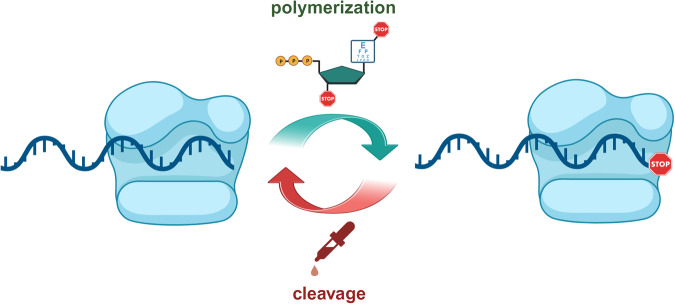
Controlled enzymatic synthesis is a method for the de novo production of oligonucleotides that combines elements of solid-phase synthesis and of enzymatic polymerization of nucleotides (Fig. 1). Conceptually, temporarily blocked nucleotides, either at the level of the nucleobase or the 3’-position of the sugar moiety, are incorporated mainly by template-independent polymerases onto a solid support bound primer (Fig. 2). After removal of excess polymerase and triphosphate, the masking groups are removed, and the extended primer can be subjected to subsequent synthetic cycles. These transiently masking groups are referred to as clippable, because they need selective and efficient covalent bond breaking reactions to be removed41.
Fig. 2. Schematic representation of de novo enzymatic synthesis of nucleic acids with template-independent polymerases.
Nucleoside triphosphates blocked at the level of the nucleobase or the sugar (mainly 3’-OH) are incorporated into single-stranded primers by polymerases. The transient blocking group prevents multiple incorporation events from occurring. Following polymerase-mediated incorporation, the masking group is removed and the system is ready for subsequent synthetic cycles.
While seemingly straightforward, this method requires extensive adjustments of the chemistry used for transitory protection of the nucleotide, the substrate tolerance of polymerases, and automation of the process. The design of the 3’-O-capping group is essential in controlled enzymatic synthesis since it will dictate the efficiency of the reaction, the viability of the process over numerous synthetic cycles, and the storage capacity of the modified nucleotide. Indeed, the transient blocking groups need to be robust enough to resist polymerase-mediated hydrolytic degradation42–45 and support longer periods of storage in buffered aqueous solutions. Concomitantly, the masking group should be labile enough to permit fast and high yielding deprotection reactions under mild conditions that do not cause any hydrolysis of phosphodiester linkages nor affect the nucleotidic scaffold of constituting nucleotides of the sequence to be produced. Besides these considerations, the modified nucleotides should not only be tolerated by polymerases but act as excellent substrates so as to enable fast (i.e., within minutes) coupling times, exclusive formation of single addition products (which avoids the need for capping steps), and quantitative yields (essential to reach longer sequences). The masking group cannot be too bulky so as to avoid steric clashes with amino acid side chains of the active sites of the polymerase, which would preclude its successful incorporation into primers46. Lastly, in order to escape synthesis in the liquid bulk phase which only permits low-scale production of short sequences, nucleotides and polymerases both need to be compatible with solid support and multiplex approaches47.
In the following section, we will describe progress made to produce natural (DNA and RNA) as well as chemically modified (XNAs) oligonucleotides with the pros and cons of each approach.
Template-independent synthesis of DNA oligonucleotides
The development of methods for de novo enzymatic DNA synthesis have benefited from progress made in DNA sequencing by synthesis (SBS), an essential element in Sanger and Illumina sequencing methods. In SBS, an immobilized primer is extended by a single, fluorescently-labeled nucleotide, the nature of which is dictated by the templating nucleotide. The identity of the templating nucleotide is then identified by reading out the color of the dye, specific to each individual nucleotide. Sequencing then proceeds by unmasking the blocking group and repeating this cycle after a washing step48–50. Each of the nucleotides used in SBS carries a fluorophore that is connected to the nucleobase via a cleavable linker and is also equipped with a 3’-O-capping group that stops the enzymatic reaction after a single incorporation event51–55. Both modifications are removed simultaneously after the read-out step so as to restore a reactive 3’-OH moiety for subsequent SBS cycles and permit a correct identification of the next fluorescently-labeled nucleotide56.
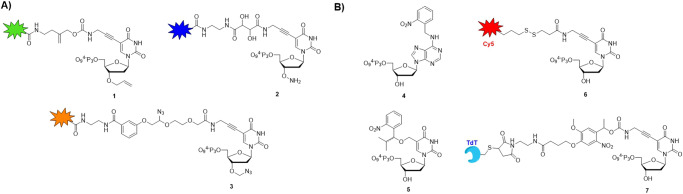
This impressive and important bulk of work allowed the identification of several capping moieties compatible with DNA polymerase synthesis and requiring only mild deprotection conditions. Amongst successful transient blocking moieties are the 3’-O-allyl group (nucleotide 1 in Fig. 3A) which are compatible with engineered polymerases such as the 9°N mutant DNA polymerase and can easily be removed in less than one minute by an in situ generated Pd0 catalyst57–60. The smallest blocking group (besides methyl) that can be affixed at position 3’ of a nucleotide is hydroxylamine (O-NH2)52. The presence of such a small alteration on a nucleotide (structure 2 in Fig. 3A) is readily tolerated by a number of DNA polymerases (vide infra) including engineered Taq variants but also common Family A (e.g., Bst and Klenow) and Family B (e.g., 9°Nm and Therminator) DNA polymerases61. Besides displaying a high degree of compatibility with polymerases, this minimal capping moiety can also be removed by application of a mild oxidant (NaNO2) that does not induce any significant oxidative damage to DNA61. Finally, the 3’-O-azidomethyl group is also capable of acting as a very potent reversible terminator (nucleotide 3 in Fig. 3A). Indeed, equipping nucleotides with such a capping moiety does not abrogate their capacity at serving as excellent substrates for the 9°Nm DNA polymerase and mutants thereof54,55. Azidomethyl groups can easily be removed by the action of mild reducing agents such as Tris(2-carboxyethyl) phosphine (TCEP) which first converts the azide to an amine via a Staudinger reaction followed by a water-mediated hydrolysis step55. Nucleotides equipped with fluorophores tethered to the nucleobases and reversibly terminated by azidomethyl groups have been commercialized by Illumina for sequencing applications. Other potentially useful, transient 3’-O-masking groups have also been proposed for such applications including carbonates56, functionalized, photocleavable ethers62, 3’-O-methyl57, and 3’-O-2-cyanoethyl51 which might be revisited in the future in the context of de novo enzymatic synthesis.
Fig. 3. Chemical structures of blocked nucleotides used in DNA sequencing by synthesis (SBS) and de novo DNA synthesis.
A Structures of common 3’-O-blocked nucleotides and (B) base-modified analogs. The colors represent different fluorophores or dyes that can be incorporated into DNA during SBS.
In addition to 3’-O-reversible terminators, modification of nucleobases at specific locations can also restrict DNA synthesis to single nucleotide insertions63,64. Initially, alkylation of 2′-deoxyadenosine with 2-nitrobenzyl bromide was believed to occur at the 3’-OH position of the nucleoside57 (thus resulting in the formation of a 3’-reversible terminator), but a subsequent study confirmed alkylation at position N6 of the nucleobase rather than at 3’-OH65. The N6-alkylated dATPs (nucleotide 4 in Fig. 3) were then shown to be excellent substrates for commercial, template-dependent DNA polymerases such as Bst and result in single incorporation events during primer extension reactions with high mismatch discrimination capacity. As for 3’-O-masking groups, removal of the base-modification (e.g., by UV light irradiation) then permits enzymatic synthesis to resume. Nucleobase modification was then extended to pyrimidines, particularly to 5-substituted-aryloxy-methyl-dUTP analogs66 and 5-propargyl-amino-dUTP scaffolds67 (nucleotides 5 and 6 in Fig. 3, respectively). The presence of a rather bulky moiety inhibits the polymerase and prevents further incorporation events from occurring. While the exact mechanism has not yet been elucidated, fine tuning of the size of the nucleobase masking groups permits a modulation of both termination and the incorporation selectivity of the incoming nucleotide66.
Collectively, development of robust SBS methods allowed to identify potent reversible terminators by modifying the 3’-O-position as well the nucleobase of nucleotides. These reversible terminators are fully compatible with enzymatic DNA synthesis, display high incorporation fidelities, lead to single incorporation events, and can easily be removed without inducing any damage to the extended DNA sequences. All these favorable features are directly exploitable and amenable to controlled enzymatic DNA synthesis. Nonetheless, fine-tuning and further engineering is still required to translate these findings to efficient and robust de novo DNA synthesis methods. Indeed, major limitations include the nature of the polymerases since SBS involves template-dependent polymerases (while de novo synthesis should preferentially rely on template-independent enzymes) and the nature of the blocking groups since nucleobase modification often leave a permanent molecular scar after deprotection (which can alter the properties of resulting oligonucleotides).
Based on these considerations, efforts at exploring controlled enzymatic DNA synthesis as a valid alternative to chemistry-based approaches for the production of oligonucleotides rapidly crystallized on the terminal deoxynucleotidyl transferase (TdT)68–70. The TdT is a member of the X family of DNA polymerases and catalyzes the addition of nucleotides at the 3’-termini of single-stranded DNA strands. In addition to being a template-independent polymerase, the TdT has also shown a rather high propensity at accepting modified nucleotides even when equipped with nucleobase surrogates71,72 or sugar-modified entities73–77. The TdT can also extend oligonucleotides as short as three nucleotides in length and of different sequence composition and chemistries78. These favorable assets were already recognized in early reports which suggested the possibility of using blocked nucleotides to synthesize DNA79 and RNA80 oligonucleotides with the TdT as polymerase. Since then, various approaches have been reported. For instance, temporary blocking the 3’-OH of dNTPs with hydroxylamine was shown to be compatible with wild-type and even more so with mutant TdT variants81, affording high yielding incorporation events (>99.5%) with all nucleotides82. The combination of 3’-O-NH2-dNTPs and TdT variants has permitted the spatial microscale enzymatic synthesis of DNA with single-base control. In this refined system, a precise silicon microelectromechanical system inkjet dispenses picoliters of the TdT enzyme alongside 3’-O-blocked nucleotides to perform a unique incorporation at the 3’-end of the DNA primer. The protecting group can then be cleaved and excess reagents removed by a washing step, liberating the 3’-OH position for further elongation steps47. This combination has also permitted the company DNA Script to launch the commercialization of the first, benchtop DNA synthesizer based on enzymatic incorporation of blocked nucleotides39.
Similarly, 3’-O-azidomethyl-blocked nucleotides combined with the TdT are currently considered by the company Nuclera Nucleics to produce long DNA oligonucleotides. These blocked nucleotides were also used as substrates for other, template-dependent DNA polymerases in a cyclic reversible termination method. In this approach, oligonucleotides are immobilized on a solid support and designed so as to transiently form hairpins or duplexes with neighboring sequences. The polymerases are then capable of extending the 3’-termini of these transient structures with 3’-O-azidomethyl-based reversible terminators with yields exceeding 98%83. Other blocking groups that have been considered for de novo DNA synthesis in conjunction with the TdT include photocleavable, aryl ethers84,85 and 3’-O-allyl86.
Instead of using reversible terminators with 3’-O-masking groups, the TdT can be kinetically controlled by adding a competing enzyme. Particularly, the enzyme apyrase hydrolyzes nucleoside triphosphates into the corresponding di- and mono-phosphates and thus, depletes the pool of available TdT substrates in a controlled manner, in terms of number and sequence of nucleotide transitions69. While directly amenable to storage of digital information in DNA applications, this strategy could potentially be combined with blocked or partially modified nucleotides for de novo DNA synthesis.
Progress to further improve synthetic yields and reach yet longer sequences by this approach, will certainly involve exploring other template-independent polymerases such as polymerase theta87. In addition, numerous engineered TdT variants have already been screened and tested but their sequences and properties remain unknown due to intellectual property rights. Undoubtedly, the identification of yet more robust88 and proficient TdT mutants81 will also improve the effectiveness of controlled enzymatic DNA synthesis.
As for SBS methods, nucleobase-modified nucleotides can be used instead of 3’-O-reversible terminators. A prime example, pioneered by ANSA Biotechnologies, consists of TdT enzymes directly tethered to the nucleobase of a nucleotide via a linker arm (nucleotide 7 in Fig. 3). Directly connecting the TdT to the nucleobase permits the incorporation of a single, specific nucleotide into DNA and concomitantly accelerates the incorporation rate89. The exact mechanism underlying single incorporation events is still unclear but could stem from multiple origins. For instance, it is possible that the steric bulk caused by the presence of the tethered TdT prevents further addition from occurring. Also, rate acceleration (and hence single incorporation) can be favored because the entropic penalty is less for an intra- vs. an inter-molecular reaction. Finally, the use of the TdT-nucleotide conjugate potentially increases the local concentration of both reaction partners and might coerce the active site of the TdT into a more suitable conformation. Some of these elements may also explain why other nucleobase-modified substrates such as 4 and 5 (Fig. 3) produce exclusively single incorporation events.
The insertion of photocleavable89 or electrochemically90 cleavable moieties between the TdT and the nucleobase then permits the removal of the polymerase under mild and efficient conditions. Average coupling times are below 2 min for C, G, and T and around 3 min for A, coupling yields of the TdT-modified nucleotides are in the 97–98% range, and deprotection is achieved by UV light irradiation within 1 min89. These highly advantageous features have recently been exploited for the production of a 1005-nucleotide long, unmodified DNA sequence91. Other base-blocked nucleotides, for instance photocaged deoxypseudouridine92 or 5-hydroxymethyl-pyrimidines93,94 have been reported recently but their compatibility with TdT-mediated enzymatic synthesis has not yet been evaluated.
Collectively, highly efficient approaches exist for controlled enzymatic DNA synthesis, either relying on 3’-O- or nucleobase-protection patterns, combined with variants of the template-independent DNA polymerase TdT. Undoubtedly, these robust technologies will be further extended for the production of very long (>1000 nt) or nucleobase-modified DNA oligonucleotides95 in the near future.
Template-independent synthesis of RNA oligonucleotides
Most research efforts for the development of controlled enzymatic synthesis have been devolved to DNA and de novo RNA synthesis by this approach remains an uncharted field of investigation. A structural key feature of RNA nucleotides is the presence of a cis-diol pattern where the 3’-OH is complemented by an additional hydroxyl moiety at position 2’ of the sugar. Hence, the choice of the protection pattern in the design of reversible RNA terminators is not as straightforward as for DNA. Indeed, reversible terminators can consist of nucleotides either blocked at 3’-OH (and with a free 2’-OH) or at both positions (Fig. 4). Protection at a single 3’-OH position is sufficient once the nucleotide is installed on the primer since this group is directly involved in the SN2 reaction with the α-phosphate moiety of the incoming nucleotide. However, such a single and selective protection pattern is synthetically more demanding and requires additional manipulation of nucleoside intermediates. Also, not all protecting groups are compatible with single protection patterns since they need to be resistant against 2′,3′-migration during chemical and enzymatic synthesis and most protocols have been devised for the 2’-position rather than 3’-OH96. Despite more demanding synthetic campaigns, a single protection of the 3’-hydroxyl grants access to 2’-O-modified nucleotides which are prevalent in most therapeutic oligonucleotides18. These features have successfully been harnessed by the laboratory of Georges Church, who introduced 3’-O-propargyl- and 3’-O-allyl-masking groups on unmodified and 2’-O-modified ribonucleotides (structures 8 and 9 in Fig.4, respectively)97. These reverse terminators acted as excellent substrates for a mutant variant of the Poly(U) Polymerase (PUP). High coupling yields (>95%) could be achieved within a few minutes of primer extension reactions with all canonical and with 2’-O-modified nucleotides. Deblocking of the allyl moiety could be achieved in high yields in rather short periods of time (12 min). This method was successfully applied to multi-cycle enzymatic RNA synthesis, both in solution and on solid-support-bound RNA primers97. Up to 10 synthetic cycles could be achieved, which is a remarkable feature. Nonetheless, Pd-mediated deprotection steps are not compatible with phosphorothioate linkages and the presence of a free 2’-OH moiety is involved in the formation of 2’-5’-branched by-products, suggesting a small room for improvement of this approach.
Fig. 4. Chemical structures of blocked RNA nucleotides.
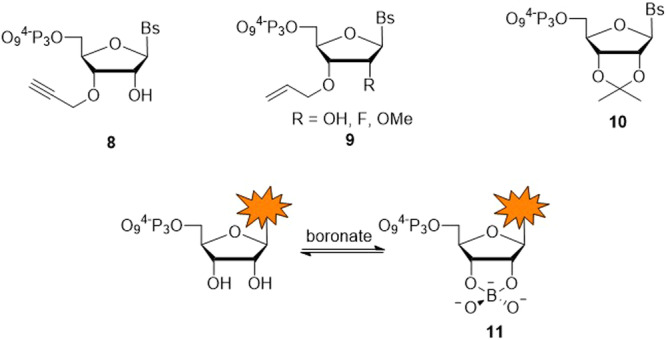
The protection pattern can involve only 3’-O-protection (structures 8 and 9) or cis-diol protection (nucleotides 10 and 11). Bs represents any nucleobase.
On the other hand, a single, cis-2’,3’-diol-protection step is synthetically much more affordable and also will prevent 2’-5’-branched RNA from forming during de novo RNA synthesis. However, this protection scheme abrogates the possibility of introducing therapeutically relevant, 2’-altered nucleotides. Concomitantly, our laboratory has identified a number of potential cis-diol protecting groups for the design of RNA reversible terminators98. Pyrimidine nucleotides equipped with a 2′,3′-O-isopropylidene masking group (structure 10 in Fig. 4) acted as excellent substrates for wild-type PUP and afforded high coupling yields (>95%) in less than 5 min of primer extension reactions, but purines equipped with a similar blocking scaffold were not tolerated by wild-type PUP or the corresponding polyadenosine polymerase (PAP). A conceptually related approach was reported by Kyoung et al. who used the in situ formation of a transient ribonucleotide–borate complex (structure 11 in Fig. 4) to 3’-terminally label RNA oligonucleotides with the TdT99.
Collectively, these recent reports clearly underscore the possibility of using RNA reversible terminators combined with the template-independent RNA polymerases PAP and PUP for de novo RNA synthesis. While the TdT has shown a limited yet non negligible degree of compatibility with modified ribonucleotides68,99–101, PUP/PAP appear as more suitable enzymes for controlled enzymatic RNA synthesis. Masking scaffolds consisting of single, 3’-O-ether moieties or cis-diol protecting groups can both be considered for the construction of reversible RNA terminators. These recent reports will certainly serve as robust foundations for the construction of longer (100-200 nt) RNA oligonucleotides with or without modified residues in the near future.
Extension to sugar-modified nucleic acids (XNAs)
Xeno nucleic acids (XNAs) are a class of synthetic genetic polymers that entail a substantially different sugar composition compared to that of canonical DNA and RNA, and their replication is orthogonal to that of the natural counterparts (Fig. 5)102,103. Besides the creation of genetically modified organisms, XNAs are key elements in the development of potent therapeutic oligonucleotides (i.e., antisense therapy, aptamers, and XNA enzymes) due to their enhanced nuclease resistance10,15,104 and in other applications such as mediation of cellular aggregation via their orthogonal base-pairing nature105,106 or the construction of smart biomaterials107. Given the increasing popularity of XNAs, alternative methods to chemical synthesis and the use of engineered polymerases are in dire need.
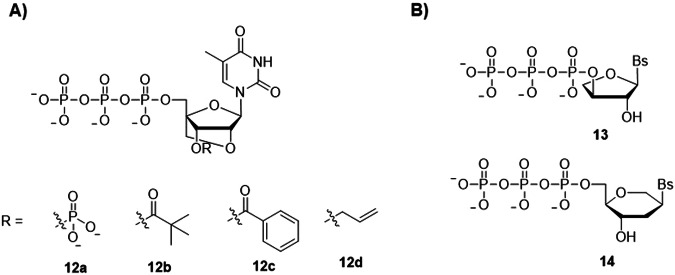
Fig. 5. Chemical structures of XNA nucleotides.
A Structures of 3’-O-blocked locked nucleic acids (LNAs) and B structures of threose nucleic acids (TNAs) and hexitol nucleic acids (HNAs) nucleotides. Bs represents any nucleobase.
Theoretically, controlled enzymatic XNA synthesis using reversible terminators should be feasible. However, XNAs face a number of additional challenges in comparison to DNA/RNA and nucleic acids with less imposing modification patterns. Indeed, more intricate and demanding synthetic routes are required for the production of suitably protected XNA nucleosides and nucleotides18 and even alternate phosphorylation protocols might be necessary108,109. Moreover, given the important alterations in the chemical nature of the backbone, XNA nucleotides are often poor substrates for existing DNA and RNA polymerases.
Our laboratory has explored several transient 3’-O-masking groups for locked nucleic acids (LNA) nucleotides (structures 12a-12d in Fig. 5A) and evaluated their capacity at serving as substrates for DNA polymerases for controlled enzymatic XNA synthesis35,44,86,110. The installation of a 3’-phosphate moiety on LNA-TTP (nucleotide 12a in Fig. 5A) decreased the substrate tolerance of engineered, template-dependent DNA polymerases as well as the TdT. Nonetheless, the major hurdle caused by the presence of an additional phosphate moiety, even on canonical DNA nucleotides, was the inherent phosphatase activity of a number of DNA polymerases, suggesting that such a transient protecting group was not compatible with de novo nucleic acid synthesis44. In order to act against the esterase/phosphatase activity of polymerases, more robust esters (nucleotides 12b and 12c in Fig. 5A) as well as ethers (nucleotide 12d in Fig. 5A) were introduced into LNA nucleotides86,110. These sturdy groups successfully resisted against polymerase-mediated hydrolysis and were compatible with template-dependent DNA polymerases. Primer extension reactions revealed that mainly single LNA incorporation events could be achieved, albeit after rather long (>5 h) incubation times. Interestingly, even though both the TdT and the PUP readily accepted a number of 3’-O-blocked LNA nucleotides as substrates86,110, both polymerases stalled after the introduction of a single, protected or unprotected, LNA nucleotide75. Threose nucleic acids (TNAs) nucleotides (structure 13 in Fig. 5B) have recently been evaluated as substrates for the TdT107. While being better substrates than LNA nucleotides, only a few TNA nucleotides can be incorporated at the 3’-termini of DNA primers by the TdT, even after several hours of reaction. Unlike other XNAs, hexitol nucleic acids (HNA, structure 14 in Fig. 5B) are rather well-tolerated as substrates by the TdT, even better than ribonucleotides, since up to 15 purine HNA nucleotides and four pyrimidine HNA nucleotides can be incorporated into DNA primers111. Nonetheless, these studies suggest that engineered TdT and PUP polymerases will be required for de novo XNA synthesis applications.
Template-dependent enzymatic approaches for nucleic acid synthesis
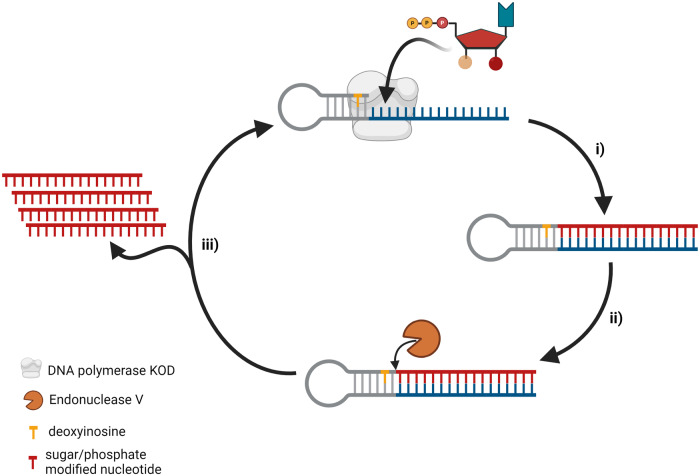
In parallel to controlled enzymatic synthesis of oligonucleotides, various biocatalytic approaches based on template-dependent polymerases have been reported. For instance, asymmetric PCR112, Nicking Enzyme Amplification Reaction113,114, and primer extension reactions followed by magnoseparation115 or digestion116 have been proposed. More recently, an elegant approach for the crafting of short, modified therapeutic oligonucleotides is based on the polymerization of modified nucleotides on a catalytic self-priming hairpin template (Fig. 6)117. Briefly, the strategy developed by the Lovelock group is based on the use of a DNA polymerase (Thermococcus kodakarensis KOD1) with a rather large tolerance for modified nucleoside triphosphate building blocks for the extension of a self-priming hairpin template. The fully extended template is then divided into expected, modified product and initial template via the action of an endonuclease (Thermotoga neapolitana TnEndoV) which cleaves the second phosphodiester moiety after a deoxyinosine moiety installed on the template. This cleavage reaction regenerates the hairpin template which can be used for another one-pot enzymatic cascade cycle. Advantages of this approach encompass a minimum amount of by-products, with only pyrophosphate expelled after primer extension reactions and compatibility with a large number of chemical modifications including phosphorothioates, 2’-fluoro-, 2’-OMe, and LNA. Application of this biocatalytic approach allowed to produce several clinically relevant oligonucleotides in up to 100 mg scales. Finally, the process mass intensity (PMI) was estimated at 530 kg of raw material for 1 kg of oligonucleotide product compared to >4000 kg for phosphoramidite-based synthesis. Potentially, some of the 3’-O-blocked nucleotides described in the above sections could be compatible with this isothermal biocatalytic approach for the introduction of XNAs at site-specific positions in oligonucleotides.
Fig. 6. Alternative biocatalytic method for the production of modified nucleic acids.
This isothermal biocatalytic approach based on the polymerase-mediated incorporation of modified nucleotides into a self-priming hairpin template (gray–blue). (i) Template-dependent synthesis using sugar and phosphate-modified nucleotides and the KOD polymerase; (ii) endonuclease V cleaves the second phosphodiester linkage 3’-downstream of a deoxyinosine moiety present in the template and generates the modified sequence (in red); (iii) after endonuclease-cleavage, the template is available for subsequent rounds of catalytic synthesis.
Another, potentially useful approach relies on the use of a trinucleotide codon rather than a single nucleotide as substrates for polymerases (Fig. 7). The replacement of internal phosphodiester bonds of trinucleotide triphosphates with phosphorothioates moieties permits to resist against the phosphatase activity of DNA polymerases and concomitantly supports enzymatic synthesis of DNA three-by-three118. While this method has only been evaluated on rather simple nucleotidic scaffolds so far, it could be extended to XNA-containing trinucleotides or codons capped with transient blocking groups at the 3’-termini for controlled enzymatic XNA synthesis.
Fig. 7. Enzymatic synthesis of DNA three-by-three rather than one-by-one.
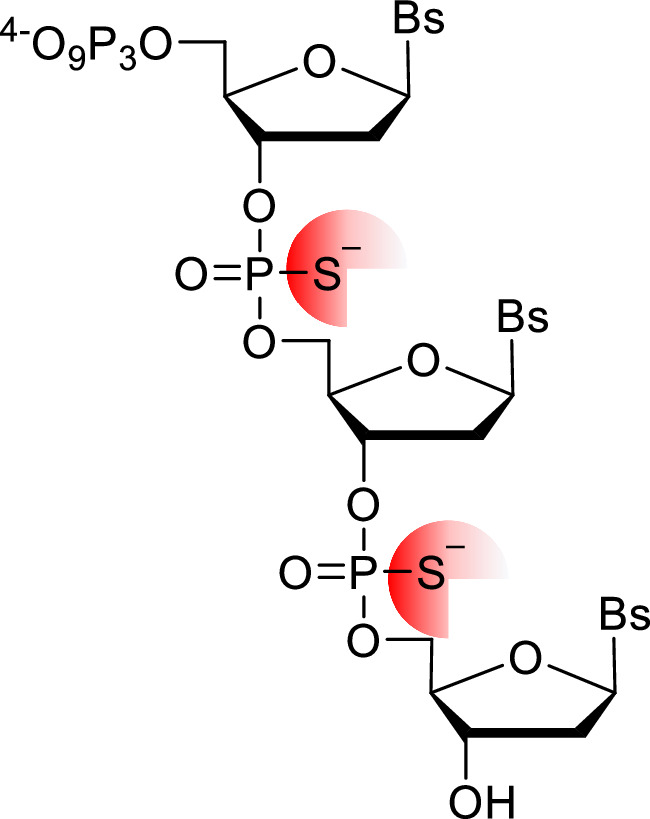
Chemical structure of a trinucleotide triphosphate stabilized by phosphorothioate linkages. Bs represents any nucleobase.
In addition to these alternative, biocatalytic approaches, engineering of more potent template-independent polymerases, either by rational design119–121 or evolution experiments122,123, will certainly permit controlled enzymatic synthesis of RNA and XNA oligonucleotides in the near future.
Ligase-based strategies
Despite impressive advancements in controlled enzymatic synthesis of nucleic acids, this approach still needs to overcome serious limitations. While robust protecting groups for reversible terminators have been identified for DNA, the situation is less clear for RNA, modified DNA/RNA, and particularly for XNAs. What is more, the absence of suitable protecting groups and especially of template-independent polymerases capable of recognizing XNAs and to a certain extent, modified RNAs, pushes controlled enzymatic synthesis of such biopolymers back into a relative stage of infancy. Finally, even though a >1 kb DNA fragment could be achieved by controlled enzymatic DNA synthesis, automation, larger scale production, and extension to yet larger oligonucleotides still remain important challenges. To by-pass a number of these hurdles, biocatalytic approaches relying on other enzymes than polymerases have been considered40. Amongst interesting enzymes are DNA and RNA ligases which catalyze, often via the help of ATP, the formation of covalent phosphodiester bonds between the 3’-OH of an acceptor strand and a 5’-phosphate moiety of a donor sequence. Surprisingly, DNA ligases are rather lax in terms of substrate requirements and tolerate the presence of modifications on the nucleobase124–126, but also on the sugar moiety of oligonucleotides127. This rather high permissiveness can be exploited to generate longer, chemically modified oligonucleotides by joining together carefully designed precursor fragments. This technology has been successful for sequences containing 2’-O-modifications but also XNAs such as HNA and TNA (Fig. 5B)127,128. As for polymerases, the substrate scope and catalytic promiscuity of ligases for modified systems can be improved by molecular evolution or rational design approaches129. For instance, the introduction of a single glycine residue at the hinge region at the interface between the oligonucleotide binding and the nucleotidyl-transferase domains massively improved the ligation capacity of the enzyme which could connect two, fully modified XNA (2’-OMe and HNA) oligonucleotides on an XNA template, a feature not accessible to any existing ligase129. Alternatively, our laboratory has shown that short (i.e., pentanucleotide) monophosphorylated fragments containing a variety of sugar, phosphate, and nucleobase modifications could be combined together using a suitably designed DNA template and the T3 DNA ligase130. This isothermal, one-step approach permits the production of short, clinically relevant oligonucleotides, but also much larger (>150 nt), heavily modified (>50 chemically modified nucleotides) fragments which are not readily accessible by any existing chemical or enzymatic approach. A related strategy was recently reported for the construction of short RNA sequences bearing 2’-O-modifications131.
In the future, a combination of ligases and polymerases, as reported for the MEGAA method for DNA variant synthesis132 or the Gibson assembly133, might be leveraged to access long XNA oligonucleotides.
Summary and outlook
Collectively, nucleic acids and their chemically modified counterparts have advanced as major players in numerous fields including therapeutics, diagnostics, storage technology, and nanotechnology. Given the importance of these biopolymers, the demand for oligonucleotides is exponentially increasing. Moreover, these needs increase not only in terms of number of sequences, but also with respect to length of oligonucleotides and presence and specific localization of an increasing variety of chemical modifications. Traditional chemical and enzymatic methods are overwhelmed by the sheer demand but are also impinged by technical limitations due to increasing complexity of the chemical nature of desired oligonucleotides. For instance, the production of a 150 nt RNA sequence equipped with dozens of individual modifications at user-defined, specific locations on a reasonable scale (>10 nmol) is a daunting task for any existing method. Consequently, alternative, biocatalytic approaches are in dire need. Amongst various approaches, controlled enzymatic synthesis is certainly the most advanced. In this chemoenzymatic nucleic acid synthesis method, transiently blocked nucleotides are incorporated by (mainly template-independent) polymerases and after installation of a single nucleotide and deprotection of the masking group, additional synthetic cycles are performed. This method is particularly well-developed for unmodified DNA since a first DNA synthesizer prototype has been commercialized recently and oligonucleotides of up to 1 kb are accessible. Nonetheless, challenges in the field still need to be lifted. Particularly, suitable protecting groups for RNA, and more importantly for modified nucleic acids such as XNAs still need to be identified. Additionally, more processive polymerases, particularly with XNA substrates need to be engineered for this method to be generally applicable. In parallel to controlled enzymatic synthesis, other polymerase-based approaches are currently evaluated and research interest shifts towards yet unrelated enzymes such as ligases.
With recent progress in enzyme engineering and design combined with medicinal chemistry type of approaches for the identification of potential nucleobase or sugar masking groups, controlled enzymatic synthesis has a bright future. Progress in the field will also make this approach more cost-affordable and eventually cheaper than existing, traditional methods. The potential of this method can be further enhanced by combining other enzymes such as ligases and potentially will enable the production of any type and size of oligonucleotide, modified or not, on industrial scales in the not-so-distant future.
Supplementary information
Acknowledgements
The authors acknowledge generous funding from DARRI and Institut Carnot “Pasteur Microbes and Health” Call 2021 (grant # INNOV-99-2, including a postdoctoral fellowship to M.P.). Institut Pasteur is acknowledged for funding.
Author contributions
M.P. and M.H. wrote and edited the manuscript and M.H. conceived the scope and constructed the manuscript.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-024-01216-0.
References
- 1.Fitzgerald PR, Paegel BM. DNA-encoded chemistry: drug discovery from a few good reactions. Chem. Rev. 2021;121:7155–7177. doi: 10.1021/acs.chemrev.0c00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixit A, Barhoosh H, Paegel BM. Translating the genome into drugs. Acc. Chem. Rev. 2023;56:489–499. doi: 10.1021/acs.accounts.2c00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H, et al. Photon-directed multiplexed enzymatic DNA synthesis for molecular digital data storage. Nat. Commun. 2020;11:5246. doi: 10.1038/s41467-020-18681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doricchi A, et al. Emerging approaches to DNA data storage: challenges and prospects. ACS Nano. 2022;16:17552–17571. doi: 10.1021/acsnano.2c06748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieffer C, Genot AJ, Rondelez Y, Gines G. Molecular computation for molecular classification. Adv. Biol. 2023;7:2200203. doi: 10.1002/adbi.202200203. [DOI] [PubMed] [Google Scholar]
- 6.Kennebeck MM, et al. DNAzyme-catalyzed site-specific N-acylation of DNA oligonucleotide nucleobases. Angew. Chem. Int. Ed. 2024;63:e202317565. doi: 10.1002/anie.202317565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aranda J, Terrazas M, Gómez H, Villegas N, Orozco M. An artificial DNAzyme RNA ligase shows a reaction mechanism resembling that of cellular polymerases. Nat. Catal. 2019;2:544–552. doi: 10.1038/s41929-019-0290-y. [DOI] [Google Scholar]
- 8.Nguyen K, Malik TN, Chaput JC. Chemical evolution of an autonomous DNAzyme with allele-specific gene silencing activity. Nat. Commun. 2023;14:2413. doi: 10.1038/s41467-023-38100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenstein M. Nucleic acid enzymes based on functionalized nucleosides. Curr. Opin. Chem. Biol. 2019;52:93–101. doi: 10.1016/j.cbpa.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AI, Wan CJK, Donde MJ, Peak-Chew S-Y, Holliger P. A modular XNAzyme cleaves long, structured RNAs under physiological conditions and enables allele-specific gene silencing. Nat. Chem. 2022;14:1295–1305. doi: 10.1038/s41557-022-01021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang LF, Ling M, Kacherovsky N, Pun SH. Aptamers 101: aptamer discovery and in vitro applications in biosensors and separations. Chem. Sci. 2023;14:4961–4978. doi: 10.1039/D3SC00439B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zutterling C, et al. The forkhead DNA-binding domain binds specific G2-rich RNA sequences. Nucleic Acids Res. 2023;51:12367–12380. doi: 10.1093/nar/gkad994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegl J, et al. Split–combine click-SELEX reveals ligands recognizing the transplant rejection biomarker CXCL9. ACS Chem. Biol. 2022;17:129–137. doi: 10.1021/acschembio.1c00789. [DOI] [PubMed] [Google Scholar]
- 14.Husser C, Vuilleumier S, Ryckelynck M. FluorMango, an RNA-based fluorogenic biosensor for the direct and specific detection of fluoride. Small. 2023;19:2205232. doi: 10.1002/smll.202205232. [DOI] [PubMed] [Google Scholar]
- 15.Lozoya-Colinas A, Yu Y, Chaput JC. Functionally enhanced XNA aptamers discovered by parallelized library screening. J. Am. Chem. Soc. 2023;145:25789–25796. doi: 10.1021/jacs.3c09497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niogret G, et al. Interrogating aptamer chemical space through modified nucleotide substitution facilitated by enzymatic DNA synthesis. ChemBioChem. 2024;25:e202300539. doi: 10.1002/cbic.202300539. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland C, et al. The selection of a hydrophobic 7-phenylbutyl-7-deazaadenine-modified DNA aptamer with high binding affinity for the Heat Shock Protein 70. Commun. Chem. 2023;6:65. doi: 10.1038/s42004-023-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenzie LK, El-Khoury R, Thorpe JD, Damha MJ, Hollenstein M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 2021;50:5126–5164. doi: 10.1039/D0CS01430C. [DOI] [PubMed] [Google Scholar]
- 19.Egli M, Manoharan M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023;51:2529–2573. doi: 10.1093/nar/gkad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaucage SL, Caruthers MH. Deoxynucleoside phosphoramidites—a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett. 1981;22:1859–1862. doi: 10.1016/S0040-4039(01)90461-7. [DOI] [Google Scholar]
- 21.Sinha ND, Biernat J, Köster H. β-Cyanoethyl N,N-dialkylamino/N-morpholinomonochloro phosphoamidites, new phosphitylating agents facilitating ease of deprotection and work-up of synthesized oligonucleotides. Tetrahedron Lett. 1983;24:5843–5846. doi: 10.1016/S0040-4039(00)94216-3. [DOI] [Google Scholar]
- 22.Narasipura EA, VanKeulen-Miller R, Ma Y, Fenton OS. Ongoing clinical trials of nonviral siRNA therapeutics. Bioconjugate Chem. 2023;34:1177–1197. doi: 10.1021/acs.bioconjchem.3c00205. [DOI] [PubMed] [Google Scholar]
- 23.Huber LB, Betz K, Marx A. Reverse transcriptases: from discovery and applications to xenobiology. ChemBioChem. 2023;24:e202200521. doi: 10.1002/cbic.202200521. [DOI] [PubMed] [Google Scholar]
- 24.Nance KD, Meier JL. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 2021;7:748–756. doi: 10.1021/acscentsci.1c00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Chen J, Xu Q. Current developments and challenges of mRNA vaccines. Annu. Rev. Biomed. Eng. 2022;24:85–109. doi: 10.1146/annurev-bioeng-110220-031722. [DOI] [PubMed] [Google Scholar]
- 26.Van Giesen KJD, Thompson MJ, Meng Q, Lovelock SL. Biocatalytic synthesis of antiviral nucleosides, cyclic dinucleotides, and oligonucleotide therapies. JACS Au. 2023;3:13–24. doi: 10.1021/jacsau.2c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews BI, et al. Sustainability challenges and opportunities in oligonucleotide manufacturing. J. Org. Chem. 2021;86:49–61. doi: 10.1021/acs.joc.0c02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrazzano L, Corbisiero D, Tolomelli A, Cabri W. From green innovations in oligopeptide to oligonucleotide sustainable synthesis: differences and synergies in TIDES chemistry. Green. Chem. 2023;25:1217–1236. doi: 10.1039/D2GC04547H. [DOI] [Google Scholar]
- 29.Choi H, et al. Purification of multiplex oligonucleotide libraries by synthesis and selection. Nat. Biotechnol. 2022;40:47–53. doi: 10.1038/s41587-021-00988-3. [DOI] [PubMed] [Google Scholar]
- 30.Kosuri S, Church GM. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods. 2014;11:499–507. doi: 10.1038/nmeth.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Zhao C, Ma B, Liu H. Uncertainties in synthetic DNA-based data storage. Nucleic Acids Res. 2021;49:5451–5469. doi: 10.1093/nar/gkab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hocek M. Enzymatic synthesis of base-functionalized nucleic acids for sensing, cross-linking, and modulation of protein–DNA binding and transcription. Acc. Chem. Rev. 2019;52:1730–1737. doi: 10.1021/acs.accounts.9b00195. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, DeBrosse C, Benkovic SJ. Template-prime-dependent turnover of (Sp)-dATP alpha S by T4 DNA polymerase. The stereochemistry of the associated 3’ goes to 5’-exonuclease. J. Biol. Chem. 1982;257:7689–7692. doi: 10.1016/S0021-9258(18)34436-3. [DOI] [PubMed] [Google Scholar]
- 34.Burgers PM, Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J. Biol. Chem. 1979;254:6889–6893. doi: 10.1016/S0021-9258(18)50258-1. [DOI] [PubMed] [Google Scholar]
- 35.Flamme M, et al. Towards the enzymatic synthesis of phosphorothioate containing LNA oligonucleotides. Bioorg. Med. Chem. Lett. 2021;48:128242. doi: 10.1016/j.bmcl.2021.128242. [DOI] [PubMed] [Google Scholar]
- 36.Singh J, et al. Synthesis of modified nucleoside oligophosphates simplified: fast, pure, and protecting group free. J. Am. Chem. Soc. 2019;141:15013–15017. doi: 10.1021/jacs.9b08273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benčić P, et al. Non-canonical nucleosides: biomimetic triphosphorylation, incorporation into mRNA and effects on translation and structure. FEBS J. 2023;290:4899–4920. doi: 10.1111/febs.16889. [DOI] [PubMed] [Google Scholar]
- 38.Campos KR, et al. The importance of synthetic chemistry in the pharmaceutical industry. Science. 2019;363:eaat0805. doi: 10.1126/science.aat0805. [DOI] [PubMed] [Google Scholar]
- 39.Hoose A, Vellacott R, Storch M, Freemont PS, Ryadnov MG. DNA synthesis technologies to close the gene writing gap. Nat. Chem. Rev. 2023;7:144–161. doi: 10.1038/s41570-022-00456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buller R, et al. From nature to industry: harnessing enzymes for biocatalysis. Science. 2023;382:eadh8615. doi: 10.1126/science.adh8615. [DOI] [PubMed] [Google Scholar]
- 41.Shieh P, Hill MR, Zhang W, Kristufek SL, Johnson JA. Clip chemistry: diverse (Bio)(macro)molecular and material function through breaking covalent bonds. Chem. Rev. 2021;121:7059–7121. doi: 10.1021/acs.chemrev.0c01282. [DOI] [PubMed] [Google Scholar]
- 42.LinWu S-W, et al. Thermococcus sp. 9°N DNA polymerase exhibits 3′-esterase activity that can be harnessed for DNA sequencing. Commun. Biol. 2019;2:224. doi: 10.1038/s42003-019-0458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canard B, Cardona B, Sarfati RS. Catalytic editing properties of DNA polymerases. Proc. Natl Acad. Sci. USA. 1995;92:10859–10863. doi: 10.1073/pnas.92.24.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flamme M, et al. Evaluation of 3 ‘-phosphate as a transient protecting group for controlled enzymatic synthesis of DNA and XNA oligonucleotides. Commun. Chem. 2022;5:68. doi: 10.1038/s42004-022-00685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krayevsky AA, Victorova LS, Arzumanov AA, Jasko MV. Terminal deoxynucleotidyl transferase: catalysis of DNA (oligodeoxynucleotide) phosphorylation. Pharmacol. Ther. 2000;85:165–173. doi: 10.1016/S0163-7258(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase β, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. doi: 10.1126/science.7516580. [DOI] [PubMed] [Google Scholar]
- 47.Verardo D, et al. Multiplex enzymatic synthesis of DNA with single-base resolution. Sci. Adv. 2023;9:eadi0263. doi: 10.1126/sciadv.adi0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shendure J, et al. DNA sequencing at 40: past, present and future. Nature. 2017;550:345–353. doi: 10.1038/nature24286. [DOI] [PubMed] [Google Scholar]
- 49.Guo J, Yu L, Turro NJ, Ju J. An integrated system for DNA sequencing by synthesis using novel nucleotide analogues. Acc. Chem. Res. 2010;43:551–563. doi: 10.1021/ar900255c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez R, Krishnan Y. The chemistry of next-generation sequencing. Nat. Biotechnol. 2023;41:1709–1715. doi: 10.1038/s41587-023-01986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knapp DC, et al. Fluoride-cleavable, fluorescently labelled reversible terminators: synthesis and use in primer extension. Chem. Eur. J. 2011;17:2903–2915. doi: 10.1002/chem.201001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen F, et al. Reconstructed evolutionary adaptive paths give polymerases accepting reversible terminators for sequencing and SNP detection. Proc. Natl Acad. Sci. USA. 2010;107:1948–1953. doi: 10.1073/pnas.0908463107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, et al. A photocleavable fluorescent nucleotide for DNA sequencing and analysis. Proc. Natl Acad. Sci. USA. 2003;100:414–419. doi: 10.1073/pnas.242729199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentley DR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo J, et al. Four-color DNA sequencing with 3’-O-modified nucleotide reversible terminators and chemically cleavable fluorescent dideoxynucleotides. Proc. Natl Acad. Sci. USA. 2008;105:9145–9150. doi: 10.1073/pnas.0804023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turcatti G, Romieu A, Fedurco M, Tairi AP. A new class of cleavable fluorescent nucleotides: synthesis and optimization as reversible terminators for DNA sequencing by synthesis. Nucleic Acids Res. 2008;36:e25. doi: 10.1093/nar/gkn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metzker ML, et al. Termination of DNA synthesis by novel 3’-modified deoxyribonucleoside 5’-triphosphates. Nucleic Acids Res. 1994;22:4259–4267. doi: 10.1093/nar/22.20.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju J, et al. Four-color DNA sequencing by synthesis using cleavable fluorescent nucleotide reversible terminators. Proc. Natl Acad. Sci. USA. 2006;103:19635–19640. doi: 10.1073/pnas.0609513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, et al. 3’-O-modified nucleotides as reversible terminators for pyrosequencing. Proc. Natl Acad. Sci. USA. 2007;104:16462–16467. doi: 10.1073/pnas.0707495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruparel H, et al. Design and synthesis of a 3’-O-allyl photocleavable fluorescent nucleotide as a reversible terminator for DNA sequencing by synthesis. Proc. Natl Acad. Sci. USA. 2005;102:5932–5937. doi: 10.1073/pnas.0501962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutter D, et al. Labeled nucleoside triphosphates with reversibly terminating aminoalkoxyl groups. Nucleosides Nucleotides Nucleic Acids. 2010;29:879–895. doi: 10.1080/15257770.2010.536191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu G, Burgess K. A diversity oriented synthesis of 3′-O-modified nucleoside triphosphates for DNA ‘sequencing by synthesis. Bioorg. Med. Chem. Lett. 2006;16:3902–3905. doi: 10.1016/j.bmcl.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 63.Ménová P, et al. Polymerase synthesis of oligonucleotides containing a single chemically modified nucleobase for site-specific redox labelling. Chem. Commun. 2013;49:4652–4654,. doi: 10.1039/c3cc41438h. [DOI] [PubMed] [Google Scholar]
- 64.McGorman B, et al. Enzymatic synthesis of chemical nuclease triplex-forming oligonucleotides with gene-silencing applications. Nucleic Acids Res. 2022;50:5467–5481. doi: 10.1093/nar/gkac438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu W, et al. Termination of DNA synthesis by N6 -alkylated, not 3′-O-alkylated, photocleavable 2′-deoxyadenosine triphosphates. Nucleic Acids Res. 2007;35:6339–6349. doi: 10.1093/nar/gkm689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Litosh VA, et al. Improved nucleotide selectivity and termination of 3′-OH unblocked reversible terminators by molecular tuning of 2-nitrobenzyl alkylated HOMedU triphosphates. Nucleic Acids Res. 2011;39:e39–e39. doi: 10.1093/nar/gkq1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowers J, et al. Virtual terminator nucleotides for next-generation DNA sequencing. Nat. Methods. 2009;6:593–595. doi: 10.1038/nmeth.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarac I, Hollenstein M. Terminal deoxynucleotidyl transferase in the synthesis and modification of nucleic acids. ChemBioChem. 2019;20:860–871. doi: 10.1002/cbic.201800658. [DOI] [PubMed] [Google Scholar]
- 69.Lee HH, Kalhor R, Goela N, Bolot J, Church GM. Terminator-free template-independent enzymatic DNA synthesis for digital information storage. Nat. Commun. 2019;10:2383. doi: 10.1038/s41467-019-10258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motea EA, Berdis AJ. Terminal deoxynucleotidyl transferase: the story of a misguided DNA polymerase. Biochim. Biophys. Acta Proteins Proteom. 2010;1804:1151–1166. doi: 10.1016/j.bbapap.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jarchow-Choy SK, Krueger AT, Liu H, Gao J, Kool ET. Fluorescent xDNA nucleotides as efficient substrates for a template-independent polymerase. Nucleic Acids Res. 2010;39:1586–1594. doi: 10.1093/nar/gkq853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Röthlisberger P, et al. Facile immobilization of DNA using an enzymatic his-tag mimic. Chem. Commun. 2017;53:13031–13034. doi: 10.1039/C7CC07207D. [DOI] [PubMed] [Google Scholar]
- 73.Winz M-L, Linder EC, André T, Becker J, Jäschke A. Nucleotidyl transferase assisted DNA labeling with different click chemistries. Nucleic Acids Res. 2015;43:e110–e110. doi: 10.1093/nar/gkv544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winz M-L, Samanta A, Benzinger D, Jäschke A. Site-specific terminal and internal labeling of RNA by poly(A) polymerase tailing and copper-catalyzed or copper-free strain-promoted click chemistry. Nucleic Acids Res. 2012;40:e78. doi: 10.1093/nar/gks062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuwahara M, et al. Smart conferring of nuclease resistance to DNA by 3′-end protection using 2′,4′-bridged nucleoside-5′-triphosphates. Bioorg. Med. Chem. Lett. 2009;19:2941–2943. doi: 10.1016/j.bmcl.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 76.Boulé J-B, Rougeon F, Papanicolaou C. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J. Biol. Chem. 2001;276:31388–31393. doi: 10.1074/jbc.M105272200. [DOI] [PubMed] [Google Scholar]
- 77.Cavanaugh NA, et al. Molecular insights into DNA polymerase deterrents for ribonucleotide insertion. J. Biol. Chem. 2011;286:31650–31660. doi: 10.1074/jbc.M111.253401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaudy E, Lietard J, Somoza MM. Sequence preference and initiator promiscuity for de novo DNA synthesis by terminal deoxynucleotidyl transferase. ACS Synth. Biol. 2021;10:1750–1760. doi: 10.1021/acssynbio.1c00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bollum FJ. Oligodeoxyribonucleotide-primed reactions catalyzed by calf thymus polymerase. J. Biol. Chem. 1962;237:1945–1949. doi: 10.1016/S0021-9258(19)73964-7. [DOI] [PubMed] [Google Scholar]
- 80.Mackey JK, Gilham PT. New approach to the synthesis of polyribonucleotides of defined sequence. Nature. 1971;233:551–553. doi: 10.1038/233551a0. [DOI] [PubMed] [Google Scholar]
- 81.Lu X, et al. Enzymatic DNA synthesis by engineering terminal deoxynucleotidyl transferase. ACS Catal. 2022;12:2988–2997. doi: 10.1021/acscatal.1c04879. [DOI] [Google Scholar]
- 82.Eisenstein M. Enzymatic DNA synthesis enters new phase. Nat. Biotechnol. 2020;38:1113–1115. doi: 10.1038/s41587-020-0695-9. [DOI] [PubMed] [Google Scholar]
- 83.Hoff K, Halpain M, Garbagnati G, Edwards JS, Zhou W. Enzymatic synthesis of designer DNA using cyclic reversible termination and a universal template. ACS Synth. Biol. 2020;9:283–293. doi: 10.1021/acssynbio.9b00315. [DOI] [PubMed] [Google Scholar]
- 84.Mathews AS, Yang HK, Montemagno C. Photo-cleavable nucleotides for primer free enzyme mediated DNA synthesis. Org. Biomol. Chem. 2016;14:8278–8288. doi: 10.1039/C6OB01371F. [DOI] [PubMed] [Google Scholar]
- 85.Mathews AS, Yang H, Montemagno C. 3′-O-Caged 2′-deoxynucleoside triphosphates for light-mediated, enzyme-catalyzed, template-independent DNA synthesis. Curr. Protoc. Nucleic Acid Chem. 2017;71:13.17.11–13.17.38. doi: 10.1002/cpnc.41. [DOI] [PubMed] [Google Scholar]
- 86.Sabat N, et al. Towards the controlled enzymatic synthesis of LNA containing oligonucleotides. Front. Chem. 2023;11:1161462. doi: 10.3389/fchem.2023.1161462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Randrianjatovo-Gbalou I, et al. Enzymatic synthesis of random sequences of RNA and RNA analogues by DNA polymerase theta mutants for the generation of aptamer libraries. Nucleic Acids Res. 2018;46:6271–6284. doi: 10.1093/nar/gky413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chua JPS, et al. Evolving a thermostable terminal deoxynucleotidyl transferase. ACS Synth. Biol. 2020;9:1725–1735. doi: 10.1021/acssynbio.0c00078. [DOI] [PubMed] [Google Scholar]
- 89.Palluk S, et al. De novo DNA synthesis using polymerase-nucleotide conjugates. Nat. Biotechnol. 2018;36:645–650. doi: 10.1038/nbt.4173. [DOI] [PubMed] [Google Scholar]
- 90.Smith JA, et al. Spatially selective electrochemical cleavage of a polymerase-nucleotide conjugate. ACS Synth. Biol. 2023;12:1716–1726. doi: 10.1021/acssynbio.3c00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ansa Biotechnologies, Inc. https://www.businesswire.com/news/home/20230309005124/en/Ansa-Biotechnologies-Announces-Successful-de-novo-Synthesis-of-World%E2%80%99s-Longest-Oligonucleotide-at-1005-Bases#:~:text=Business%20Wire-,Ansa%20Biotechnologies%20Announces%20Successful%20de%20novo%20Synthesis%20of%20World’s%20Longest,oligonucleotide%20with%20industry%2Dleading%20accuracy (2023).
- 92.Takeshita L, Yamada Y, Masaki Y, Seio K. Synthesis of deoxypseudouridine 5′-triphosphate bearing the photoremovable protecting group at the N1 position capable of enzymatic incorporation to DNA. J. Org. Chem. 2020;85:1861–1870. doi: 10.1021/acs.joc.9b02194. [DOI] [PubMed] [Google Scholar]
- 93.Boháčová S, Vaníková Z, Poštová Slavětínská L, Hocek M. Protected 2′-deoxyribonucleoside triphosphate building blocks for the photocaging of epigenetic 5-(hydroxymethyl)cytosine in DNA. Org. Biomol. Chem. 2018;16:5427–5432. doi: 10.1039/C8OB01106K. [DOI] [PubMed] [Google Scholar]
- 94.Boháčová S, et al. Protected 5-(hydroxymethyl)uracil nucleotides bearing visible-light photocleavable groups as building blocks for polymerase synthesis of photocaged DNA. Org. Biomol. Chem. 2018;16:1527–1535. doi: 10.1039/C8OB00160J. [DOI] [PubMed] [Google Scholar]
- 95.Kawabe H, et al. Enzymatic synthesis and nanopore sequencing of 12-letter supernumerary DNA. Nat. Commun. 2023;14:6820. doi: 10.1038/s41467-023-42406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Somoza Á. Protecting groups for RNA synthesis: an increasing need for selective preparative methods. Chem. Soc. Rev. 2008;37:2668–2675,. doi: 10.1039/b809851d. [DOI] [PubMed] [Google Scholar]
- 97.Wiegand, D. J. et al. A platform for controlled template-independent enzymatic synthesis of RNA oligonucleotides and therapeutics. bioRxiv10.1101/2023.06.29.547106 (2023). This contribution demonstrates controlled enzymatic synthesis of short, natural and modified RNA oligonucleotides.
- 98.Pichon M, Levi-Acobas F, Kitoun C, Hollenstein M. 2’,3’-protected nucleotides as building blocks for enzymatic de novo RNA synthesis. Chem. Eur. J. 2024;30:e202400137. doi: 10.1002/chem.202400137. [DOI] [PubMed] [Google Scholar]
- 99.Jang EK, Son RG, Pack SP. Novel enzymatic single-nucleotide modification of DNA oligomer: prevention of incessant incorporation of nucleotidyl transferase by ribonucleotide-borate complex. Nucleic Acids Res. 2019;47:e102–e102. doi: 10.1093/nar/gkz612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ashley J, Potts IG, Olorunniji FJ. Applications of terminal deoxynucleotidyl transferase enzyme in biotechnology. ChemBioChem. 2023;24:e202200510. doi: 10.1002/cbic.202200510. [DOI] [PubMed] [Google Scholar]
- 101.Baladi T, et al. Stealth fluorescence labeling for live microscopy imaging of mRNA delivery. J. Am. Chem. Soc. 2021;143:5413–5424. doi: 10.1021/jacs.1c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herdewijn P, Marlière P. Toward safe genetically modified organisms through the chemical diversification of nucleic acids. Chem. Biodivers. 2009;6:791–808. doi: 10.1002/cbdv.200900083. [DOI] [PubMed] [Google Scholar]
- 103.Chaput JC, Herdewijn P. What is XNA? Angew. Chem. Int. Ed. 2019;58:11570–11572. doi: 10.1002/anie.201905999. [DOI] [PubMed] [Google Scholar]
- 104.Lee EM, Setterholm NA, Hajjar M, Barpuzary B, Chaput JC. Stability and mechanism of threose nucleic acid toward acid-mediated degradation. Nucleic Acids Res. 2023;51:9542–9551. doi: 10.1093/nar/gkad716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gasse C, et al. Controlled E. coli aggregation mediated by DNA and XNA hybridization. ChemBioChem. 2023;24:e202300191. doi: 10.1002/cbic.202300191. [DOI] [PubMed] [Google Scholar]
- 106.Gavins GC, et al. Live cell PNA labelling enables erasable fluorescence imaging of membrane proteins. Nat. Chem. 2021;13:15–23. doi: 10.1038/s41557-020-00584-z. [DOI] [PubMed] [Google Scholar]
- 107.Qin, B. et al. Enzymatic synthesis of TNA protects DNA nanostructures. Angew. Chem. Int. Ed. 63, e202317334 (2024). [DOI] [PubMed]
- 108.Sau SP, Chaput JC. A one-pot synthesis of α-l-threofuranosyl nucleoside triphosphates (tNTPs) Bioorg. Med. Chem. Lett. 2016;26:3271–3273. doi: 10.1016/j.bmcl.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 109.Liu C, et al. Phosphonomethyl oligonucleotides as backbone-modified artificial genetic polymers. J. Am. Chem. Soc. 2018;140:6690–6699. doi: 10.1021/jacs.8b03447. [DOI] [PubMed] [Google Scholar]
- 110.Flamme, M. et al. Benzoyl and pivaloyl as efficient protecting groups for controlled enzymatic synthesis of DNA and XNA oligonucleotides. Asian J. Org. Chem. 1110.1002/ajoc.202200384 (2022). [DOI] [PMC free article] [PubMed]
- 111.Vastmans K, Rozenski J, Van Aerschot A, Herdewijn P. Recognition of HNA and 1,5-anhydrohexitol nucleotides by DNA metabolizing enzymes. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2002;1597:115–122. doi: 10.1016/S0167-4838(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 112.Ondruš M, Sýkorová V, Bednárová L, Pohl R, Hocek M. Enzymatic synthesis of hypermodified DNA polymers for sequence-specific display of four different hydrophobic groups. Nucleic Acids Res. 2020;48:11982–11993. doi: 10.1093/nar/gkaa999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ménová P, Hocek M. Preparation of short cytosine-modified oligonucleotides by nicking enzyme amplification reaction. Chem. Commun. 2012;48:6921–6923,. doi: 10.1039/c2cc32930a. [DOI] [PubMed] [Google Scholar]
- 114.Ménová P, Raindlová V, Hocek M. Scope and limitations of the nicking enzyme amplification reaction for the synthesis of base-modified oligonucleotides and primers for PCR. Bioconjugate Chem. 2013;24:1081–1093. doi: 10.1021/bc400149q. [DOI] [PubMed] [Google Scholar]
- 115.Brázdilová P, et al. Ferrocenylethynyl derivatives of nucleoside triphosphates: synthesis, incorporation, electrochemistry, and bioanalytical applications. Chem. Eur. J. 2007;13:9527–9533. doi: 10.1002/chem.200701249. [DOI] [PubMed] [Google Scholar]
- 116.Ondruš M, Sýkorová V, Hocek M. Traceless enzymatic synthesis of monodispersed hypermodified oligodeoxyribonucleotide polymers from RNA templates. Chem. Commun. 2022;58:11248–11251. doi: 10.1039/D2CC03588J. [DOI] [PubMed] [Google Scholar]
- 117.Moody ER, Obexer R, Nickl F, Spiess R, Lovelock SL. An enzyme cascade enables production of therapeutic oligonucleotides in a single operation. Science. 2023;380:1150–1154. doi: 10.1126/science.add5892. [DOI] [PubMed] [Google Scholar]
- 118.Sabat N, et al. Artificial nucleotide codons for enzymatic DNA synthesis. Chem. Commun. 2023;59:14547–14550. doi: 10.1039/D3CC04933G. [DOI] [PubMed] [Google Scholar]
- 119.Freund N, et al. A two-residue nascent-strand steric gate controls synthesis of 2′-O-methyl- and 2′-O-(2-methoxyethyl)-RNA. Nat. Chem. 2023;15:91–100. doi: 10.1038/s41557-022-01050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dousis A, Ravichandran K, Hobert EM, Moore MJ, Rabideau AE. An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts. Nat. Biotechnol. 2023;41:560–568. doi: 10.1038/s41587-022-01525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Y, Zhu TF. Mirror-image T7 transcription of chirally inverted ribosomal and functional RNAs. Science. 2022;378:405–412. doi: 10.1126/science.abm0646. [DOI] [PubMed] [Google Scholar]
- 122.Arangundy-Franklin S, et al. A synthetic genetic polymer with an uncharged backbone chemistry based on alkyl phosphonate nucleic acids. Nat. Chem. 2019;11:533–542. doi: 10.1038/s41557-019-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen T, et al. Evolution of thermophilic DNA polymerases for the recognition and amplification of C2ʹ-modified DNA. Nat. Chem. 2016;8:556–562. doi: 10.1038/nchem.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kong D, Yeung W, Hili R. In vitro selection of diversely functionalized aptamers. J. Am. Chem. Soc. 2017;139:13977–13980. doi: 10.1021/jacs.7b07241. [DOI] [PubMed] [Google Scholar]
- 125.Chen Z, Lichtor PA, Berliner AP, Chen JC, Liu DR. Evolution of sequence-defined highly functionalized nucleic acid polymers. Nat. Chem. 2018;10:420–427. doi: 10.1038/s41557-018-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takezawa, Y., Zhang, H., Mori, K., Hu, L. & Shionoya, M. Ligase-mediated synthesis of CuII-responsive allosteric DNAzyme with bifacial 5-carboxyuracil nucleobases. Chem. Sci. 10.1039/D1033SC05042D 10.1039/D3SC05042D (2024). [DOI] [PMC free article] [PubMed]
- 127.Kestemont D, et al. XNA ligation using T4 DNA ligase in crowding conditions. Chem. Commun. 2018;54:6408–6411. doi: 10.1039/C8CC02414F. [DOI] [PubMed] [Google Scholar]
- 128.McCloskey CM, Liao J-Y, Bala S, Chaput JC. Ligase-mediated threose nucleic acid synthesis on DNA templates. ACS Synth. Biol. 2019;8:282–286. doi: 10.1021/acssynbio.8b00511. [DOI] [PubMed] [Google Scholar]
- 129.Vanmeert M, et al. Rational design of an XNA ligase through docking of unbound nucleic acids to toroidal proteins. Nucleic Acids Res. 2019;47:7130–7142. doi: 10.1093/nar/gkz551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sabat N, et al. Template-dependent DNA ligation for the synthesis of modified oligonucleotides. ChemRxiv. 2023 doi: 10.26434/chemrxiv-22023-vwwt26435-v26432. [DOI] [Google Scholar]
- 131.Mann G, et al. Biocatalytic assembly of chemically modified oligonucleotides. Tetrahedron Lett. 2022;93:153696. doi: 10.1016/j.tetlet.2022.153696. [DOI] [Google Scholar]
- 132.Liu L, Huang Y, Wang HH. Fast and efficient template-mediated synthesis of genetic variants. Nat. Methods. 2023;20:841–848. doi: 10.1038/s41592-023-01868-1. [DOI] [PubMed] [Google Scholar]
- 133.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–U341. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.