Abstract
Background
Graves' disease is an autoimmune disease caused by the production of auto‐antibodies against the thyroid‐stimulating hormone receptor, which stimulates follicular cell production of thyroid hormone. It is the commonest cause of hyperthyroidism and may cause considerable morbidity with increased risk of cardiovascular and respiratory adverse events. Five per cent of people with Graves' disease develop moderate to severe Graves' ophthalmopathy. Thyroid surgery for Graves' disease commonly falls into one of three categories: 1) total thyroidectomy, which aims to achieve complete macroscopic removal of thyroid tissue; 2) bilateral subtotal thyroidectomy, in which bilateral thyroid remnants are left; and 3) unilateral total and contralateral subtotal thyroidectomy, or the Dunhill procedure. Recent American Thyroid Association guidelines on treatment of Graves' hyperthyroidism emphasised the role of surgery as one of the first‐line treatments. Total thyroidectomy removes target tissue for the thyroid‐stimulating hormone receptor antibody. It controls hyperthyroidism at the cost of lifelong thyroxine replacement. Subtotal thyroidectomy leaves a thyroid remnant and may be less likely to lead to complications, however a higher rate of recurrent hyperthyroidism is expected and revision surgery would be challenging. The choice of the thyroidectomy technique is currently largely a matter of surgeon preference, and a systematic review of the evidence base is required to determine which option offers the best outcomes for patients.
Objectives
To assess the optimal surgical technique for Graves' disease and Graves' ophthalmopathy.
Search methods
We searched the Cochrane Library, MEDLINE and PubMed, EMBASE, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). The date of the last search was June 2015 for all databases. We did not apply any language restrictions.
Selection criteria
Only randomised controlled trials (RCTs) involving participants with a diagnosis of Graves' disease based on clinical features and biochemical findings of hyperthyroidism were eligible for inclusion. Trials had to directly compare at least two surgical techniques of thyroidectomy. There was no age limit to study inclusion.
Data collection and analysis
Two review authors independently extracted and cross‐checked the data for analysis, evaluation of risk of bias and establishment of 'Summary of findings' tables using the GRADE instrument. The senior review authors reviewed the data and reconciled disagreements.
Main results
We included five RCTs with a total of 886 participants; 172 were randomised to total thyroidectomy, 383 were randomised to bilateral subtotal thyroidectomy, 309 were randomised to the Dunhill procedure and 22 were randomised to either bilateral subtotal thyroidectomy or the Dunhill procedure. Follow‐up ranged between six months and six years. One trial had three comparison arms. All five trials were conducted in university hospitals or tertiary referral centres for thyroid disease. All thyroidectomies were performed by experienced surgeons. The overall quality of the evidence ranged from low to moderate. In all trials, blinding procedures were insufficiently described. Outcome assessment for objective outcomes was blinded in one trial. Surgeons were not blinded in any of the trials. One trial blinded participants. Attrition bias was a substantial problem in one trial, with 35% losses to follow‐up. In one trial the analysis was not carried out on an intention‐to‐treat basis.
Total thyroidectomy was more effective than subtotal thyroidectomy techniques (both bilateral subtotal thyroidectomy and the Dunhill procedure) at preventing recurrent hyperthyroidism in 0/150 versus 11/200 participants (OR 0.14 (95% CI 0.04 to 0.46); P = 0.001; 2 trials; moderate quality evidence). Total thyroidectomy was also more effective than bilateral subtotal thyroidectomy at preventing recurrent hyperthyroidism in 0/150 versus 10/150 participants (odds ratio (OR) 0.13 (95% confidence interval (CI) 0.04 to 0.44); P = 0.001; 2 trials; moderate quality evidence). Compared to bilateral subtotal thyroidectomy, the Dunhill procedure was more likely to prevent recurrent hyperthyroidism in 20/283 versus 8/309 participants (OR 2.73 (95% CI 1.28 to 5.85); P = 0.01; 3 trials; low quality evidence). Total thyroidectomy compared with subtotal thyroidectomy conferred a greater risk of permanent hypocalcaemia/hypoparathyroidism in 8/172 versus 3/221 participants (OR 4.79 (95% CI 1.36 to 16.83); P = 0.01; 3 trials; low quality evidence). Effects of the various surgical techniques on permanent recurrent laryngeal nerve palsy and regression of Graves' ophthalmopathy were neutral. One death was reported in one study in year three of follow‐up. No study investigated health‐related quality of life or socioeconomic effects.
Authors' conclusions
Total thyroidectomy is more effective than subtotal thyroidectomy (both bilateral subtotal thyroidectomy and the Dunhill procedure) at preventing recurrent hyperthyroidism in Graves' disease. The type of surgery performed does not affect regression of Graves’ ophthalmopathy. There was some evidence that total thyroidectomy compared with subtotal thyroidectomy conferred a greater risk of permanent hypocalcaemia/hypoparathyroidism, which however, was not seen in comparison with bilateral subtotal thyroidectomy. Permanent recurrent laryngeal nerve palsy did not seem to be affected by type of thyroidectomy. Health‐related quality of life as a patient‐important outcome measure should form a core determinant of any future trial on the effects of thyroid surgery for Graves' disease.
Keywords: Humans, Graves Disease, Graves Disease/surgery, Graves Ophthalmopathy, Graves Ophthalmopathy/surgery, Randomized Controlled Trials as Topic, Recurrence, Remission Induction, Remission Induction/methods, Reoperation, Secondary Prevention, Thyroidectomy, Thyroidectomy/adverse effects, Thyroidectomy/methods
Plain language summary
Thyroid surgery for Graves' disease and Graves' opthalmopathy
Review question
The aim of this review was to compare different surgical techniques for treatment of Graves' disease. We wanted to address whether surgically removing the whole thyroid (total thyroidectomy) gland is better than removing most of the gland (subtotal thyroidectomy) at controlling increased activity of the thyroid (hyperthyroidism) and eye symptoms associated with Graves' disease.
Background
Graves' disease is a common condition affecting the thyroid gland, which has an important role in controlling the body's metabolism. The body's own immune system attacks the thyroid gland and causes it to work much harder. This overactivity can lead to increased risk of heart attacks and strokes if left untreated for a long period of time. Graves' disease also causes protrusion of the eye and may limit eye movement (Graves' ophthalmopathy). Graves' disease is generally treated by medication alone or sometimes with radiation or surgery. In recent years, there has been increasing interest in surgery for Graves' disease and other thyroid problems, as results of surgery and health‐related quality of life outcomes appear promising. However, there is controversy over which surgical technique is best, as total thyroidectomy may give better control of disease long term, compared with subtotal thyroidectomy, but carry a greater risk of postoperative complications, such as damage to the so‐called recurrent laryngeal nerve (causing vocal cord dysfunction).
Study characteristics
We identified five randomised controlled studies that were relevant to the review question that directly compared different thyroidectomy surgical techniques. A total of 886 participants were included in the studies and follow‐up was between six months and six years. All five studies were conducted in university hospitals or tertiary referral centres for thyroid disease. The countries involved were Germany, Sweden, Poland and Taiwan. No study was performed in an outpatient setting.
Key results
Total thyroidectomy is likely to give better control of hyperthyroidism compared to subtotal thyroidectomy (8 or 9 people out of 1000 will experience recurrence of hyperthyroidism after total thyroidectomy compared with 55 to 67 people out of 1000 after subtotal thyroidectomy). There is some evidence to suggest permanently low blood calcium (hypoparathyroidism) is more likely in total thyroidectomy compared to subtotal thyroidectomy (59 people out of 1000 after total thyroidectomy compared with 14 people out of 1000 after subtotal thyroidectomy will experience this side effect). Eye symptoms as well as permanent damage to the recurrent laryngeal nerve appear to be unrelated to the choice of surgical technique. Deaths were rarely reported in the included studies. No study evaluated health‐related quality of life or socioeconomic effects (such as absence from work).
Quality of the evidence
The studies in this review suffer from a lack of high quality evidence. Several risk of bias problems were visible such as objective measurements of outcomes without knowing the allocation of participants to the intervention groups (total or subtotal thyroidectomy) and difficulties in finding out if all planned outcomes in studies were really reported in the publications of these studies. Also, rates of outcomes (event rates) were rather low, making our estimation of the differences between the surgical techniques imprecise. Finally, all thyroidectomies were performed by experienced surgeons and it is unknown whether the described results will be the same in other settings.
Currentness of evidence
This plain language summary is current as of 22 June 2015.
Summary of findings
Summary of findings for the main comparison. Total thyroidectomy compared to subtotal thyroidectomy for Graves disease and Graves' opthalmopathy.
| Total thyroidectomy compared to subtotal thyroidectomy for Graves disease/Graves opthalmopathy | ||||||
| Patient: patients with Graves' disease/Graves' opthalmopathy Settings: university hospitals in Poland, Germany and Sweden Intervention: total thyroidectomy Comparison: subtotal thyroidectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| Subtotal thyroidectomy | Total thyroidectomy | |||||
| Rate of recurrent hyperthyroidism [measured by thyroid function tests] Follow‐up: 6 months and 6 years | 55 per 1000 | 8 per 1000 (2 to 26) | OR 0.14 (0.04 to 0.46) | 350 (2) | ⊕⊕⊕⊝ moderateb | ‐ |
| Regression of Graves' ophthalmopathy [ophthalmology assessment] Follow‐up: 6 months and 6 years | 782 per 1000 | 805 per 1000 (697 to 882) | OR 1.15 (0.64 to 2.08) | 229 (2) | ⊕⊕⊝⊝ lowc |
‐ |
| Permanent recurrent laryngeal nerve palsy [patient reported, confirmation by laryngoscopy] Follow‐up: 6 months to 6 years | 18 per 1000 | 26 per 1000 (7 to 93) | OR 1.45 (0.38 to 5.59) | 393 (3) | ⊕⊕⊝⊝ lowd | ‐ |
| Permanent hypocalcaemia/hypoparathyroidism [measured by serum calcium levels] Follow‐up: 6 months to 6 years | 14 per 1000 | 59 per 1000 (16 to 190) | OR 4.79 (1.36 to 16.83) | 393 (3) | ⊕⊕⊝⊝ lowd | ‐ |
|
All‐cause mortality Follow‐up: 6 months to 6 years |
See comment | See comment | Not estimable | 350 (1) | See comment | Only one death reported in total thyroidectomy group |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the intervention comparator groups. bDowngraded by one level because of high risk of performance bias in both trials and reporting bias in one trial. cDowngraded by two levels because of high risk of performance bias in both trials and imprecision. dDowngraded by two levels because of high risk of performance bias in all trials, imprecision and low event rates.
Summary of findings 2. Total thyroidectomy compared to bilateral subtotal thyroidectomy for Graves disease and Graves' opthalmopathy.
| Total thyroidectomy compared to bilateral subtotal thyroidectomy for Graves disease/Graves' opthalmopathy | ||||||
| Patient: patients with Graves' disease/Graves' opthalmopathy Settings: university hospitals in Germany and Poland Intervention: total thyroidectomy Comparison: bilateral subtotal thyroidectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| Bilateral subtotal thyroidectomy | Total thyroidectomy | |||||
| R ate of recurrent hyperthyroidism [measured by thyroid function tests] Follow‐up: 6 months and 6 years | 67 per 1000 | 9 per 1000 (3 to 30) | OR 0.13 (0.04 to 0.44) | 300 (2) | ⊕⊕⊕⊝ moderateb | ‐ |
| Regression of Graves' ophthalmopathy [ophthalmology assessment] Follow‐up: 6 months and 6 years | 791 per 1000 | 818 per 1000 (707 to 893) | OR 1.19 (0.64 to 2.21) | 260 (2) | ⊕⊕⊝⊝ lowc |
‐ |
| Permanent recurrent laryngeal nerve palsy [patient reported, confirmation by laryngoscopy] Follow‐up: 6 months and 6 years | 13 per 1000 | 13 per 1000 (2 to 88) | OR 1.00 (0.14 to 7.15) | 300 (2) | ⊕⊕⊝⊝ lowd | ‐ |
| Permanent hypocalcaemia/hypoparathyroidism [measured by serum calcium levels] Follow‐up: 6 months and 6 years | 20 per 1000 | 40 per 1000 (11 to 138) | OR 2.04 (0.53 to 7.85) | 300 (2) | ⊕⊕⊝⊝ lowd | ‐ |
|
All‐cause mortality Follow‐up: 6 months and 6 years |
See comment | See comment | Not estimable | 300 (2) | See comment | Only one death reported in total thyroidectomy group |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the intervention comparator groups. bDowngraded by one level because of high risk of performance bias in both trials. cDowngraded by two levels because of high risk of performance bias in both trials and imprecision. dDowngraded by two levels because of high risk of performance bias in both trials, imprecision and low event rates.
Summary of findings 3. Bilateral subtotal thyroidectomy compared to the Dunhill procedure for Graves disease and Graves' opthalmopathy.
| Bilateral subtotal thyroidectomy compared to the Dunhill procedure for Graves disease/Graves' opthalmopathy | ||||||
| Patient: patients with Graves disease/Graves' opthalmopathy Settings: university hospitals in Taiwan and Germany Intervention: bilateral subtotal thyroidectomy Comparison: Dunhill procedure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| Dunhill procedureb | Bilateral subtotal thyroidectomy | |||||
| R ate of recurrent hyperthyroidism [measured by thyroid function tests] Follow‐up: 6 months to 6 years | 26 per 1000 | 68 per 1000 (33 to 135) | OR 2.73 (1.28 to 5.85) | 592 (3) | ⊕⊕⊝⊝ lowc | ‐ |
| Regression of Graves' ophthalmopathy [ophthalmology assessment] Follow‐up: 6 months and 6 years | 689 per 1000 | 633 per 1000 (415 to 810) | OR 0.78 (0.32 to 1.92) | 90 (2) | ⊕⊕⊝⊝ lowd |
‐ |
| Permanent recurrent laryngeal nerve palsy [patient reported, confirmation by laryngoscopy] Follow‐up: 6 months to 6 years | 3 per 1000 | 0 per 1000 (0 to 22) | OR 0.14 (0.00 to 6.82) | 592 (3) | ⊕⊕⊝⊝ lowc | ‐ |
| Permanent hypocalcaemia/hypoparathyroidism [measured by serum calcium levels] Follow‐up: 6 months to 6 years | 10 per 1000 | 18 per 1000 (5 to 70) | OR 1.90 (0.47 to 7.71) | 592 (3) | ⊕⊕⊝⊝ lowc | ‐ |
| All‐cause mortality Follow‐up: 6 months to 6 years | See comment | See comment | Not estimable | 642 (3) |
See comment | No deaths reported in any study |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the intervention comparator groups bDunhill procedure: unilateral total and contralateral subtotal thyroidectomy cDowngraded by two levels because of high risk of performance bias in all trials, attrition bias in one of three trials, imprecision and low event rates dDowngraded by two levels because of high risk of performance bias in both trials, attrition bias in one of two trials, imprecision and low number of participants
Background
Description of the condition
Graves' disease accounts for 50% to 80% of cases of hyperthyroidism and has a population prevalence of 0.5% to 2%. It is more common in women, with a female to male ratio of 5:1 (Genovese 2012; Ponto 2012). Five per cent of patients with Graves' disease develop moderate to severe Graves' ophthalmopathy, with older age groups (40 to 80 years) at higher risk of developing eye signs (Laurberg 2012).
Graves' disease may be diagnosed by evidence of extra‐thyroidal manifestations on examination and biochemical hyperthyroidism (suppressed thyroid‐stimulating hormone, high tri‐iodothyronine (T3), thyroxine (T4) or both). If the diagnosis is uncertain, a radionuclide uptake test may be performed which shows diffusely increased uptake throughout the thyroid. Assays for the thyroid‐stimulating hormone receptor antibody have 98% sensitivity and 100% specificity for Graves' disease, but their use in clinical practice remains variable (Lalwani 2012).
Hyperthyroid states are associated with significant cardiovascular and respiratory adverse events such as atrial fibrillation, congestive cardiac failure, hypercoagulability and stroke. A recent meta‐analysis suggested a 20% increase in mortality in patients diagnosed with hyperthyroidism (Brandt 2011).
Description of the intervention
Thyroid surgery for Graves' disease commonly falls into one of three categories: 1) total thyroidectomy, which aims to achieve complete macroscopic removal of thyroid tissue; 2) bilateral subtotal thyroidectomy, in which bilateral thyroid remnants are left; and 3) unilateral total and contralateral subtotal thyroidectomy, or the Dunhill procedure. In subtotal thyroidectomy techniques, 1 mL to 4 mL of thyroid remnant per lobe is recommended, to reduce the risk of recurrent hyperthyroidism (Chi 2005; Hermann 1998).
Adverse effects of the intervention
Thyroidectomy for benign disease carries significantly less risk than for thyroid malignancies. Two recent large retrospective case series for total thyroidectomy in benign disease (Bellantone 2002;Efremidou 2009) reported the risk of permanent recurrent laryngeal nerve palsy as 0% to 0.4%, and temporary recurrent laryngeal nerve palsy at 1.3%. Temporary hypocalcaemia was seen in 7.3% of patients, and permanent hypocalcaemia was seen in 0.3% to 3.4%. Haemorrhage requiring repeat surgery was reported to be in the region of 0.2% to 1.5%. The trend was for a decrease in complications over recent years, suggesting surgeon experience and caseload may be factors in complication rates. Subtotal thyroidectomy has been reported to give lower rates of temporary recurrent laryngeal nerve palsy and hypocalcaemia, but outcomes are similar in the long term (Barczynski 2011).
How the intervention might work
Total thyroidectomy removes target tissue for the thyroid‐stimulating hormone receptor antibody. It controls hyperthyroidism at the cost of lifelong thyroxine replacement. Subtotal thyroidectomy leaves a thyroid remnant and aims to achieve euthyroidism without the need for thyroxine replacement, however a higher rate of recurrent hyperthyroidism is expected.
Graves' ophthalmopathy is thought to be due to common antigens shared between the thyroid and orbit that are both targeted by the thyroid‐stimulating hormone receptor antibody and a G‐protein G2sAb. Total thyroidectomy has been shown to be associated with regression of Graves' opthalmopathy, associated with a concurrent decrease in the thyroid‐stimulating hormone receptor antibody and G2sAb postsurgery (De Bellis 2012; Leo 2012).
Why it is important to do this review
Thyroidectomy for Graves' disease may be indicated in patients who have persistent hyperthyroidism after a trial of anti‐thyroid medication; when a rapid return to euthyroidism is desired; or when radioactive iodine treatment is contraindicated (e.g. in pregnancy).
In recent years we have seen a resurgence of interest in surgical management of Graves' disease. The American Thyroid Association's Hyperthyroidism Management Guidelines 2011 named surgery as one of three first‐line treatments for hyperthyroidism associated with Graves' disease alongside radioactive iodine and anti‐thyroid drugs (ATA 2011). In studies conducted in patients who have persistent hyperthyroidism after medical treatment, total thyroidectomy is shown to be more cost‐effective than radioactive iodine or lifelong anti‐thyroid medication (In 2009; Zanocco 2012). Health‐related quality of life after surgical ablation of the thyroid has been shown to be comparable to the general population (Al‐Adhami 2012). Morbidity associated with thyroidectomy has been shown to be less than previously thought (Bellantone 2002; Efremidou 2009).
Choice of thyroidectomy technique is largely a matter of surgeon preference, and a systematic review of the evidence base is required to determine which option offers the best outcome for patients. A previous meta‐analysis on this subject was carried out in 2000 (Palit 2000), based on a MEDLINE search. Since that time, data from several new randomised controlled trials (RCTs) have been published, which demonstrates considerable interest in this topic.
Objectives
To assess the optimal surgical technique for Graves' disease and Graves' ophthalmopathy.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs.
Types of participants
All patient age groups receiving surgical treatment for Graves' disease.
Diagnostic criteria
Our criteria for Graves' disease were clinical examination and suppressed thyroid‐stimulating hormone and elevated free thyroxine (T4), free tri‐iodothyronine (T3), or both. Additional diagnostic tests such as radionuclide uptake and thyroid‐stimulating hormone receptor antibody)/thyrotropin binding inhibiting immunoglobulins were noted where performed, but not considered essential for study inclusion.
Types of interventions
The primary aim of our evaluation was to compare total thyroidectomy with subtotal thyroidectomy for Graves' disease and Graves' opthalmopathy. However, to provide maximum information we also included all types of thyroidectomy for these conditions such as bilateral thyroidectomy versus unilateral total and contralateral subtotal thyroidectomy (the Dunhill procedure).
We excluded studies where only one trial arm contained a surgical intervention, or where surgical interventions were compared to medical interventions.
Types of outcome measures
Primary outcomes
Rate of recurrent hyperthyroidism.
Adverse events.
Secondary outcomes
Regression of Graves' ophthalmopathy.
All‐cause mortality.
Socioeconomic effects.
Health‐related quality of life.
Method and timing of outcome measurement
Rate of recurrent hyperthyroidism: biochemically confirmed elevation of T3/T4 with postoperatively suppressed thyroid‐stimulating hormone and measured at more than three months postsurgery.
Adverse events such as: permanent recurrent laryngeal nerve palsy defined as persistent recurrent laryngeal nerve palsy either objectively measured by laryngoscopy or reported by the patient, and persisting more than six months postsurgery. Permanent hypocalcaemia/hypoparathyroidism is measured as persistent corrected serum calcium below the reference range as per local laboratory assays more than six months postsurgery. Postoperative bleeding, defined as bleeding sufficiently severe to warrant intervention such as suture removal or return to theatre and measured at less than 28 days postsurgery.
Regression of Graves' ophthalmopathy: a clinically significant improvement in Graves' ophthalmopathy using a validated scoring system as reported by the authors of the study and measured at more than six months postsurgery.
All‐cause mortality: defined as death from any cause measured at any time during the study.
Health‐related quality of life: measured with a validated instrument such as SF‐36, WHOQOL and measured at any time during the study.
Socioeconomic effects: defined as any qualitative or quantitative socioeconomic measure such as cost‐benefit analyses and measured at any time during the study.
Summary of findings' table
We present 'Summary of findings' tables using the following outcomes listed according to priority.
Rate of recurrent hyperthyroidism.
Regression of Graves' ophthalmopathy.
Permanent recurrent laryngeal nerve palsy.
Permanent hypocalcaemia/hypoparathyroidism.
All‐cause mortality.
Health‐related quality of life.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date and placed no restrictions on the language of publication.
-
Cochrane Library (22 June 2015).
Cochrane Central Register of Controlled Trials (Issue 5 of 12, May 2015).
Database of Abstracts of Reviews of Effect (Issue 2 of 4, April 2015).
Health Technology Assessment Database (Issue 2 of 4, April 2015).
MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present (22 June 2015) and PubMed (subsets not available on Ovid, 22 June 2015).
EMBASE 1974 to 2015 Week 25 (22 June 2015).
ClinicalTrials.gov. (22 June 2015).
-
ICTRP Search Portal (http://apps.who.int/trialsearch/, 22 June 2015).
Australian New Zealand Clinical Trials Registry (15 June 2015).
Chinese Clinical Trial Registry (15 June 2015).
ClinicalTrials.gov (15 June 2015).
EU Clinical Trials Register (15 June 2015).
ISRCTN (15 June 2015).
The Netherlands National Trial Register (15 June 2015).
Brazilian Clinical Trials Registry (26 May 2015).
Clinical Trials Registry ‐ India (26 May 2015).
Clinical Research Information Service ‐ Republic of Korea (26 May 2015).
Cuban Public Registry of Clinical Trials (26 May 2015).
German Clinical Trials Register (1 June 2015).
Iranian Registry of Clinical Trials (26 May 2015).
Japan Primary Registries Network (26 May 2015).
Pan African Clinical Trial Registry (26 May 2015).
Sri Lanka Clinical Trials Registry (1 June 2015).
Thai Clinical Trials Register (26 May 2015).
We continuously applied a MEDLINE (via Ovid SP) email alert service established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group to identify newly published trials using the same search strategy as described for MEDLINE (for details on search strategies, see Appendix 1).
In case we detected new studies for inclusion we would have evaluated these and incorporated findings in our review before submission of the final review draft (Beller 2013). If additional keywords of relevance were detected during any of the electronic or other searches, we would have modified the electronic search strategies to incorporate these terms.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (ZWL, LM) independently scanned the abstract, title or both sections of every record retrieved to determine the studies to be assessed further. We investigated all potentially relevant articles as full text. A third party (BF, PJ or KC) resolved any differences in opinion. If resolving disagreements was not possible, we added the article to those 'awaiting assessment' and we contacted authors for clarification. We attach a PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled inclusion criteria, two review authors (ZWL, LM) independently summarised relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies; Table 4; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10) with any disagreements resolved by discussion, or, if required, by a third party (BF, PJ or KC).
1. Overview of study populations.
| Characteristic | Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | Follow‐up |
| (1) Barczynski 2012 | I: total thyroidectomy | (1) To detect an estimated absolute difference in the rate
of recurrent hyperthyroidism of 5%, it was calculated that
71 participants would be required in each treatment arm
to give the study a power of 80% (2) To detect an estimated difference in ophthalmopathy progression rate of 8%, 83 participants were required in each treatment arm to give the study a power of 80% Anticipating a 15% loss to follow‐up, a total of 200 participants were included |
298 | 100 | 100 | 95 | 95.0 | 5 years |
| C: bilateral subtotal thyroidectomy | 100 | 100 | 96 | 96.0 | ||||
| total: | 200 | 200 | 191 | 95.5 | ||||
| (2) Witte 2000 | I: total thyroidectomy | ‐ | ‐ | 50 | 50 | ‐ | ‐ | 6‐36 (58) months |
| C1: bilateral subtotal thyroidectomyb | 50 | 50 | ‐ | ‐ | ||||
| C2: Dunhill procedureb | 50 | 50 | ‐ | ‐ | ||||
| total: | 150 | 150 | ‐ | ‐ | ||||
| (3) Chi 2005 | I: bilateral subtotal thyroidectomy | ‐ | ‐ | 166 | ‐ | 166 | 100 | 3‐26.4 months |
| C: Dunhill procedure | 174 | ‐ | 174 | 100 | ||||
| total: | 340 | 340 | 100 | |||||
| (4) Jarhult 2005 | I: total thyroidectomy | ‐ | ‐ | 22 | ‐ | 21 | 100 | 3 years |
| C: subtotal thyroidectomy | 22 | ‐ | 21 | 95.5 | ||||
| total: | 44 | 43 | 97.7 | |||||
| (5) Muller 2001 | I: bilateral subtotal thyroidectomy | ‐ | ‐ | 67 | ‐ | 46 | 68.7 | 6 years |
| C: Dunhill procedure | 85 | ‐ | 53 | 62.4 | ||||
| total: | 152 | ‐ | 99 | 65.1 | ||||
| Grand total | All interventions | 405 | 368 | |||||
| All comparators | 481 | 344 | ||||||
| All interventions and comparators | 886 | 712 | ||||||
aAccording to power calculation in study publication or report. bSubtotal thyroid resection was performed in 103 patients with an attempt to leave less than 4 mL total remnant
"‐" denotes not reported.
C: comparator; I: intervention; ITT: intention‐to‐treat; N/A: not applicable.
We provide information about registered studies in the table Characteristics of ongoing studies and in the appendix 'Matrix of study endpoints (publications and trial documents'; Appendix 5).
We sent an email request to study authors of included studies to enquire whether they were willing to answer questions regarding their trials. Appendix 11 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the study authors of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we planned to maximise yield of information by collating all available data. In case of doubt, the publication reporting the longest follow‐up associated with our primary or secondary outcomes would have obtained priority.
Assessment of risk of bias in included studies
Two review authors (ZWL, LM) assessed each trial independently. Possible disagreements were resolved by consensus, or with consultation of a third party (BF, PJ or KC). In cases of disagreement, seniors authors were consulted and a consensus reached.
We assessed risk of bias using the Cochrane Collaboration’s tool (Higgins 2011a; Higgins 2011b). We used the following bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel, and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We assessed outcome reporting bias (Kirkham 2010) by integrating the results of the table 'High risk of outcome reporting bias according to ORBIT classification' (Appendix 6) and the table 'Matrix of study endpoints (publication and trial documents'; Appendix 5). This analysis formed the basis for the judgement of selective reporting (reporting bias).
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We present a 'Risk of bias' graph and a 'Risk of bias summary' table .
We assessed the impact of individual bias domains on study results at endpoint and study levels.
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
Regression of Graves' ophthalmopathy.
Health‐related quality of life.
We defined the following outcomes as objective outcomes.
Rate of recurrent hyperthyroidism.
Permanent recurrent laryngeal nerve palsy (if confirmed by laryngoscopy).
Permanent hypocalcaemia/hypoparathyroidism.
All‐cause mortality.
Socioeconomic effects.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as crossover trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from authors, if feasible, and evaluated important numerical data such as screened, eligible, randomised participants as well as intention‐to‐treat, as‐treated and per‐protocol populations. We investigated attrition rates, for example losses to follow‐up and withdrawals, and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not report study results as meta‐analytically pooled effect estimates.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1, in view of the low power of this test. We examined heterogeneity using the I² statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I² statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
Had we found heterogeneity, we would have attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
In case of 10 studies or more for a given outcome, we planned to use funnel plots to assess small study effects. Due to several explanations for funnel plot asymmetry we planned to interpret results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across studies we planned to primarily summarise low risk of bias data by means of a random‐effects model (Wood 2008). We would have interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Atkins 2004). Two review authors (ZWL, LM) independently rated the quality of evidence for each outcome. We present a summary of the evidence in the 'Summary of findings' tables. These tables provide key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and studies addressing each important outcome and rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' tables on the basis of methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) using RevMan's table editor. Otherwise, we would have used the GRADEproGDT software (GRADEproGDT 2015). We present results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we present the results in a narrative format in the 'Summary of findings' table. We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses and investigate interaction.
Age (depending on data).
Gender.
Effects of previous treatment.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to the following.
Published trials.
Taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section.
Very long or large trials to establish the extent to which they dominate the results.
Trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country.
We also planned to test the robustness of results by repeating the analysis using different measures of effect size (RR, OR, etc) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
We identified 694 records from our comprehensive literature searches; from these, we identified 46 full text papers for further examination. We excluded the other studies on the basis of their titles or abstracts because they did not meet the inclusion criteria or were not relevant to the question under study (see Figure 1 for the amended PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow diagram. After screening the full text of the selected publications, six trials (one of which is an ongoing study) met the inclusion criteria. All studies were published in English. We sought additional information from the lead author of the ongoing trial (Maschuw 2012) but unfortunately no preliminary data could be provided.
1.
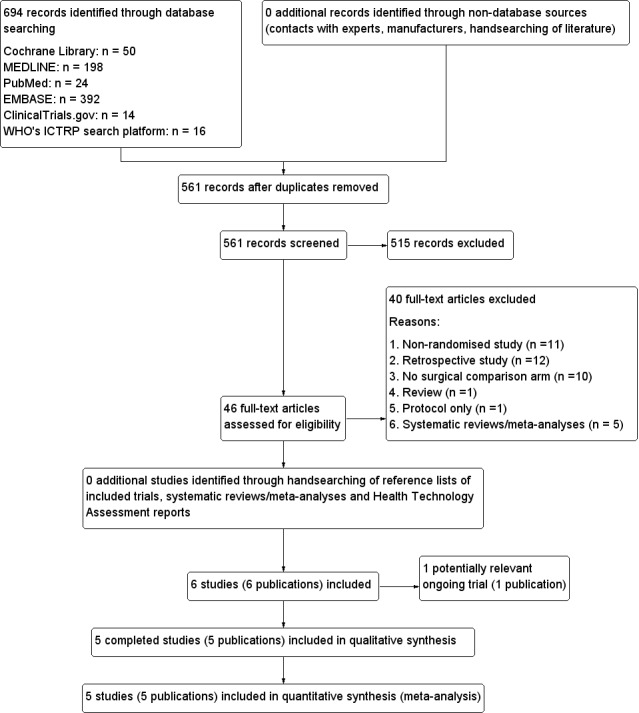
Study flow diagram.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies and appendices). The following is a succinct overview.
Source of data
We obtained the data presented in the review from published literature and information in trial registers. We contacted authors for clarification and to obtain preliminary data from ongoing studies, however no new data were made available by correspondence.
Comparisons
Three thyroidectomy techniques were compared in the identified trials. Total thyroidectomy is removal of all macroscopic thyroid tissue. Two techniques of subtotal thyroidectomy are a) bilateral subtotal thyroidectomy, in which small tissue remnants (1 mL to 4 mL) are left on both sides of the thyroid gland, with the theoretical advantage of preventing postoperative hypothyroidism and permanent hypocalcaemia/hypoparathyroidism; and b) the Dunhill procedure, in which one lobe of the thyroid is entirely removed and a small remnant (1 mL to 4 mL) is left on the contralateral side.
Comparisons identified in the review fall into the following categories.
Total thyroidectomy versus bilateral subtotal thyroidectomy versus. Dunhill procedure (one RCT).
Total thyroidectomy versus bilateral subtotal thyroidectomy (one RCT).
Bilateral subtotal thyroidectomy versus Dunhill procedure (three RCTs).
Overview of study populations
A total of 886 participants were included in the five trials; 172 participants were randomised to total thyroidectomy, 383 were randomised to bilateral subtotal thyroidectomy, 309 were randomised to the Dunhill procedure and 22 were randomised to either bilateral subtotal thyroidectomy or the Dunhill procedure (not specified which procedure had been carried out).
A total of 165 (96%) participants completed the study in the total thyroidectomy group. Altogether 362 (94.5%) participants finished in the bilateral subtotal thyroidectomy group. A total of 274 (88.7%) completed follow‐up in the Dunhill procedure group and 22 (100%) completed follow‐up in the subtotal thyroidectomy group (surgical technique unspecified).
The individual sample size ranged from 44 to 340.
Study design
Studies were RCTs. Five trials adopted a parallel group superiority design and no studies contained a placebo group. There were no multicentre trials. One study had three comparison arms (Witte 2000).
In terms of blinding, there were no double‐blinded studies. One study was single‐blinded for participants (Barczynski 2012), and in four studies, blinding was not defined (Chi 2005; Jarhult 2005; Muller 2001; Witte 2000). Outcome assessors were blinded in two studies (Barczynski 2012; Jarhult 2005).
Studies were performed between the years 1988 to 2004. The duration of follow‐up ranged from three months to six years, with a mean follow‐up period of 40 months. No studies had a run‐in period. No studies were terminated prematurely.
Settings
All five studies were conducted in university hospitals or tertiary referral centres for thyroid disease. The countries involved were Germany, Sweden, Poland and Taiwan. None were performed in an outpatient setting.
Participants
The participating population consisted of the following: patients diagnosed with Graves' disease undergoing surgical treatment. All trials included participants from economically developed countries. There was no breakdown of ethnic group data. Most patients received a course of medical treatment for Graves' disease. This was reported in five trials (Barczynski 2012; Chi 2005; Jarhult 2005; Muller 2001; Witte 2000), with the mean duration of prior treatment being 9 to 34.2 months. Participants’ gender showed a 6:1 female predominance consistent with the population prevalence of Graves' disease. The mean age of participants in the trials ranged from 30 to 46 years. Comorbidities were not reported for the included studies. Five trials described cointerventions in participants in the form of preoperative anti‐thyroid medication to render the patients euthyroid (Barczynski 2012; Chi 2005; Jarhult 2005; Muller 2001; Witte 2000).
Criteria for entry into the individual trials are outlined in the Characteristics of included studies. Major exclusion criteria were 1) suspicion of thyroid malignancy, 2) thyroid remnant less than 2.5 x 1 x 1cm (Chi 2005) or previous total thyroidectomy (for studies that only compared subtotal thyroidectomy techniques), 3) preoperative recurrent laryngeal nerve palsy and 4) patient refusal.
Diagnosis
Participants were diagnosed with Grave's disease in all trials. All trials confirmed the diagnosis of Graves' disease on clinical and biochemical criteria, which is hyperthyroidism with endocrine ophthalmopathy and thyroid‐stimulating hormone receptor antibodies or thyrotropin binding inhibiting immunoglobulin antibodies. Two trials used an ultrasound scan in addition to the criteria above (Chi 2005; Muller 2001). Histological confirmation was used in two trials (Chi 2005; Jarhult 2005).
Interventions
All five trials reported treatment before the start of the trial which included anti‐thyroid medication (e.g. carbimazole) and beta‐blockers. All participants were rendered euthyroid prior to surgery. The duration of the intervention was the time taken to perform the specified type of thyroidectomy (knife to skin to surgical closure of wound). All trials used adequate interventions and comparators.
Outcomes
One study explicitly stated primary and secondary endpoints in the publication (Barczynski 2012), four studies reported endpoints without specifying whether they were primary or secondary. The most commonly defined primary outcomes in publications were recurrent hyperthyroidism and change in Graves' ophthalmopathy. Other commonly reported outcomes were temporary/permanent hypocalcaemia and temporary/permanent recurrent laryngeal nerve palsy.
Reporting of endpoints
A total of five trials collected a median of six (range six to eight) outcomes. Recurrent hyperthyroidism was measured in four trials (Barczynski 2012; Chi 2005; Muller 2001; Witte 2000). Grave's ophthalmopathy was assessed in three trials (Barczynski 2012; Jarhult 2005; Witte 2000). Postoperative autoimmune antibody levels were reported in two trials (Jarhult 2005; Witte 2000). Surgical complications such as postoperative hypoparathyroidism and recurrent laryngeal nerve palsy were measured in all trials; three trials also reported haematoma and wound complication rates (Chi 2005; Jarhult 2005; Muller 2001). One death was reported which occurred three years after surgery and was not reported to be related to the surgery or Graves' disease. No trial specifically reported health‐related quality of life or socioeconomic effects. For a summary of all outcomes assessed in each trial, see Appendix 5.
Excluded studies
We had to excluded a total of 38 trials (40 publications) after careful evaluation of the full publication (see Figure 1).
The main reasons for exclusion were lack of randomisation (n = 11), retrospective study (n = 12) and no direct comparison of two surgical techniques (n = 10). For further details, see Characteristics of excluded studies.
Risk of bias in included studies
For details of risk of bias in included trials see Characteristics of included studies. For an overview of review authors' judgements about each risk of bias item for individual studies and across all trials see Figure 2 and Figure 3. We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures. 'Objective outcome' measures were defined as rate of recurrent hyperthyroidism, permanent recurrent laryngeal nerve palsy (if confirmed by laryngoscopy), permanent hypocalcaemia/hypoparathyroidism, all‐cause mortality and socioeconomic effects.
2.
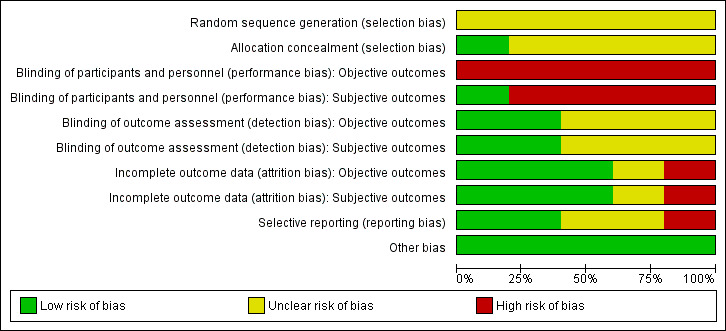
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
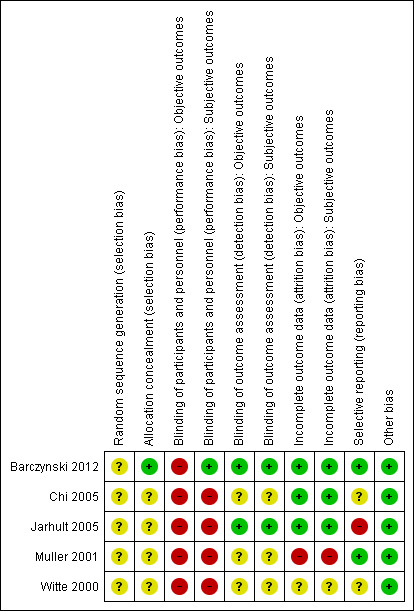
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'Subjective outcome' measures were defined as regression of Graves' ophthalmopathy and health‐related quality of life.
Allocation
All five trials were described as randomised, although only one study (Barczynski 2012) specified that random numbers were computer generated and sealed envelopes were used for allocation concealment. We could not obtain any more information on randomisation methods for the remaining trials.
Blinding
Only one trial explicitly stated that blinding of the participants was undertaken (Barczynski 2012). The surgical team was not blinded in any of the studies. One trial reported blinding of outcome assessment for some outcome measures (Barczynski 2012). External, blinded personnel undertook thyroid function, calcium measurements and assessment of recurrent laryngeal nerve status. Two trials reported blinding of subjective outcome assessment (Barczynski 2012; Jarhult 2005). An external blinded ophthalmologist undertook assessment of Graves' ophthalmopathy. In one trial it was unclear whether any of the outcome assessments were blinded (Muller 2001). None of the trials provided sufficient information about blinding procedures.
Incomplete outcome data
Participant withdrawal figures were described in four trials that had losses to follow‐up (Barczynski 2012; Chi 2005; Jarhult 2005; Muller 2001). Analysis was reported as intention‐to‐treat in four trials (Barczynski 2012; Jarhult 2005; Muller 2001; Witte 2000). No intention‐to‐treat analysis was undertaken in the trial by Chi 2005. One trial did not report losses to follow‐up (Witte 2000). Detailed descriptions of participants' withdrawals and reasons underpinning them were not provided in any of the included trials. One trial had attrition rates with possible impact on the outcomes (Muller 2001, overall loss to follow‐up 35%).
Selective reporting
Risk of reporting bias was considered to be low in two trials (Barczynski 2012; Muller 2001). Where the trial measured primary outcomes of the review (recurrent hyperthyroidism and adverse events), the results were reported in all but one trial (Jarhult 2005) as thyroid function was measured but not reported. Three trials did not explicitly report all‐cause mortality (Chi 2005; Jarhult 2005; Witte 2000).
Other potential sources of bias
We did not identify any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4.
1. Total thyroidectomy compared to subtotal thyroidectomy (both bilateral subtotal and the Dunhill procedure)
For recurrent hyperthyroidism and regression of Graves' ophthalmopathy, the results are based on a meta‐analysis of two studies (Barczynski 2012; Witte 2000). For hypoparathyroidism and recurrent laryngeal nerve palsy, the results are based on a meta‐analysis of three studies (Barczynski 2012; Jarhult 2005; Witte 2000).
Primary outcomes
Rate of recurrent hyperthyroidism
Total thyroidectomy was more effective than subtotal thyroidectomy at preventing recurrent hyperthyroidism: OR 0.14 (95% confidence interval (CI) 0.04 to 0.46); P = 0.001; 350 participants; 2 trials; moderate quality evidence (Figure 4). These findings translate into 8 (95% CI 2 to 26) per 1000 versus 55 per 1000 individuals with recurrent hyperthyroidism, if treated by total versus subtotal thyroidectomy, respectively.
4.
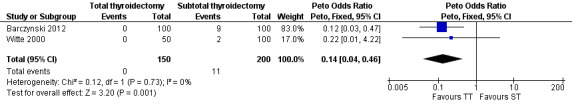
Forest plot of comparison: 2 Total thyroidectomy versus subtotal thyroidectomy (both bilateral subtotal thyroidectomy and the Dunhill procedure), outcome: 2.1 Recurrent hyperthyroidism.
TT: total thyroidectomy, ST: subtotal thyroidectomy
Adverse events: permanent hypocalcaemia/hypoparathyroidism, permanent recurrent laryngeal nerve palsy and wound complications
Total thyroidectomy conferred a greater risk of permanent hypocalcaemia/hypoparathyroidism: OR 4.79 (95% CI 1.36 to 16.83); P = 0.01; 393 participants; 3 trials; low quality evidence (Analysis 1.2). These findings translate into 59 (95% CI 16 to 190) per 1000 versus 14 per 1000 individuals with permanent hypocalcaemia/hypoparathyroidism, if treated by total versus subtotal thyroidectomy, respectively.
1.2. Analysis.
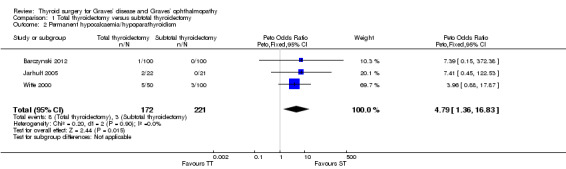
Comparison 1 Total thyroidectomy versus subtotal thyroidectomy, Outcome 2 Permanent hypocalcaemia/hypoparathyroidism.
There were no marked differences in rates of recurrent laryngeal nerve palsy between the total and subtotal thyroidectomy groups: OR 1.45 (95% CI 0.38 to 5.59); P = 0.59; 393 participants; 3 trials; low quality evidence (Analysis 1.3).
1.3. Analysis.
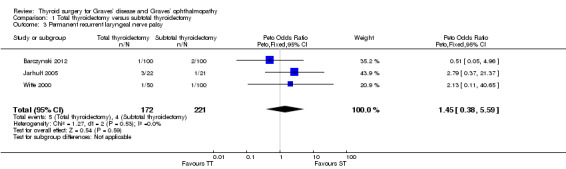
Comparison 1 Total thyroidectomy versus subtotal thyroidectomy, Outcome 3 Permanent recurrent laryngeal nerve palsy.
One trial reported a postoperative haematoma rate of 4.5% in the total thyroidectomy group versus 9.5% in the subtotal thyroidectomy group (Jarhult 2005). However, the absolute numbers in this trial were small.
Regression of Graves' ophthalmopathy
There were no marked differences in postoperative regression of Graves' ophthalmopathy between the total and subtotal thyroidectomy groups: OR 1.15 (95% CI 0.64 to 2.08); P = 0.64; 229 participants; 2 trials; low quality evidence (Analysis 1.4).
1.4. Analysis.
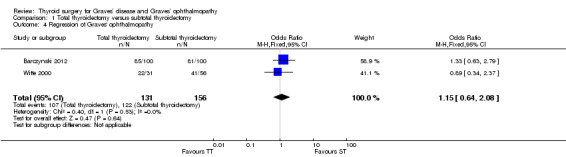
Comparison 1 Total thyroidectomy versus subtotal thyroidectomy, Outcome 4 Regression of Graves' ophthalmopathy.
All‐cause mortality
Only a single death was reported in the total thyroidectomy group which was seen as unrelated to the surgical intervention (myocardial infarction three years after surgery) (Barczynski 2012).
Socioeconomic effects
None of the trials reported information relevant to these outcomes.
Health‐related quality of life
None of the trials reported information relevant to health‐related quality of life.
2. Total thyroidectomy compared to bilateral subtotal thyroidectomy
This is based on a meta‐analysis of two trials with a total of 300 participants (Barczynski 2012; Witte 2000).
Primary outcomes
Rate of recurrent hyperthyroidism
Total thyroidectomy was more effective than bilateral subtotal thyroidectomy at preventing recurrent hyperthyroidism: OR 0.13 (95% CI 0.04 to 0.44); P = 0.001; 300 participants; 2 trials; moderate quality evidence (Figure 5). These findings translate into 9 (95% CI 3 to 30) per 1000 versus 67 per 1000 individuals with recurrent hyperthyroidism, if treated by total versus bilateral subtotal thyroidectomy, respectively.
5.
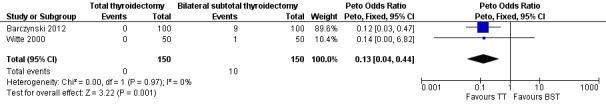
Forest plot of comparison: 1 Total thyroidectomy versus bilateral subtotal thyroidectomy, outcome: 1.1 Recurrent hyperthyroidism.
TT: total thyroidectomy, BST: bilateral subtotal thyroidectomy
Adverse events: permanent hypocalcaemia/hypoparathyroidism and permanent recurrent laryngeal nerve palsy
No marked differences were seen in rates of permanent hypocalcaemia/hypoparathyroidism: OR 2.04 (95% CI 0.53 to 7.85); P = 0.30; 300 participants; 2 trials low quality evidence (Analysis 2.2) or permanent recurrent laryngeal nerve palsy between the comparator groups: OR 1.00 (95% CI 0.14 to 7.15); P = 1.0; 300 participants; 2 trials; low quality evidence (Analysis 2.3).
2.2. Analysis.
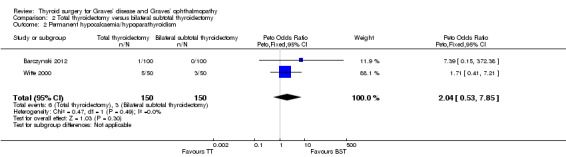
Comparison 2 Total thyroidectomy versus bilateral subtotal thyroidectomy, Outcome 2 Permanent hypocalcaemia/hypoparathyroidism.
2.3. Analysis.
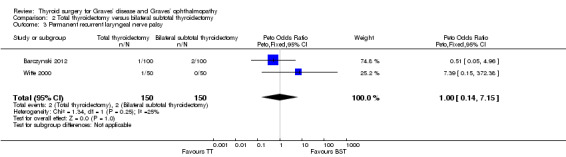
Comparison 2 Total thyroidectomy versus bilateral subtotal thyroidectomy, Outcome 3 Permanent recurrent laryngeal nerve palsy.
Secondary outcomes
Regression of Graves' ophthalmopathy
There were no marked differences in postoperative regression of Graves' ophthalmopathy between the total and bilateral subtotal thyroidectomy groups: OR 1.19 (95% CI 0.64 to 2.21); P = 0.58; 260 participants; 2 trials; low quality evidence (Analysis 2.4).
2.4. Analysis.
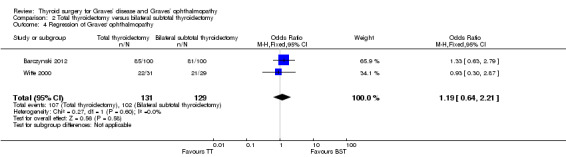
Comparison 2 Total thyroidectomy versus bilateral subtotal thyroidectomy, Outcome 4 Regression of Graves' ophthalmopathy.
All‐cause mortality
Only a single death was reported in the total thyroidectomy group (Barczynski 2012) which was seen as unrelated to the surgical intervention (myocardial infarction three years after surgery).
Socioeconomic effects
None of the trials reported information relevant to these outcomes.
Health‐related quality of life
None of the trials reported information relevant to health‐related quality of life.
3. Bilateral subtotal thyroidectomy compared to the Dunhill procedure
Three trials provided data on outcome measures (Chi 2005; Muller 2001; Witte 2000).
Primary outcomes
Rate of recurrent hyperthyroidism
Patients in the bilateral subtotal thyroidectomy groups were more likely than those in the Dunhill procedure group to experience recurrent hyperthyroidism: OR 2.73 (95% CI 1.28 to 5.85); P = 0.01; 592 participants; 3 trials; low quality evidence (Figure 6). These findings translate into 68 (95% CI 33 to 135) per 1000 versus 26 per 1000 individuals with recurrent hyperthyroidism, if treated by bilateral subtotal thyroidectomy versus the Dunhill procedure, respectively.
6.
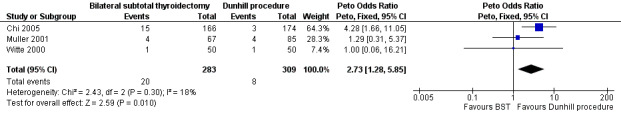
Forest plot of comparison: 3 Bilateral subtotal thyroidectomy versus the Dunhill procedure, outcome: 3.1 Recurrent hyperthyroidism.
BST: bilateral subtotal thyroidectomy
Adverse events: permanent hypocalcaemia/hypoparathyroidism, permanent recurrent laryngeal nerve palsy and wound complications
There were no marked differences between the bilateral subtotal thyroidectomy and the Dunhill procedure groups in rates of permanent hypocalcaemia/hypoparathyroidism: OR 1.90 (95% CI 0.47 to 7.71); P = 0.37; 592 participants; 3 trials; low quality evidence (Analysis 3.2).
3.2. Analysis.
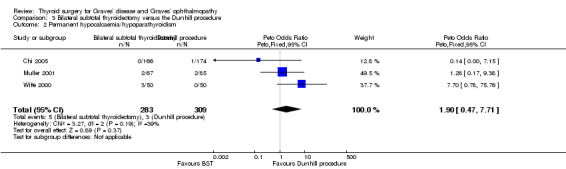
Comparison 3 Bilateral subtotal thyroidectomy versus the Dunhill procedure, Outcome 2 Permanent hypocalcaemia/hypoparathyroidism.
There were no marked differences between the bilateral subtotal thyroidectomy and the Dunhill procedure groups in rates of permanent recurrent laryngeal nerve palsy: (OR 0.14 (95% CI 0.00 to 6.82); P = 0.32; 592 participants; 3 trials; low quality evidence (Analysis 3.3).
3.3. Analysis.
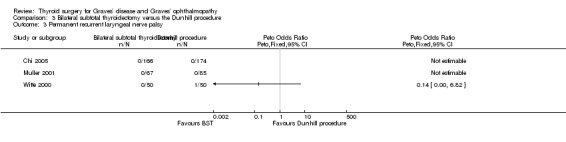
Comparison 3 Bilateral subtotal thyroidectomy versus the Dunhill procedure, Outcome 3 Permanent recurrent laryngeal nerve palsy.
Overall rates of haematoma and other wound complications related to surgery were low. Postoperative haematoma requiring drainage or return to the theatre were in the range of 0.5% to 3% (Chi 2005), and seroma rates were 3% to 4.7% (Muller 2001). Direct comparison between the studies was not possible due to different reporting criteria.
Secondary outcomes
Regression of Graves' ophthalmopathy
There were no marked differences between the bilateral subtotal thyroidectomy and the Dunhill procedure groups in regression of Graves' ophthalmopathy postoperatively: OR 0.78 (95% CI 0.32 to 1.92); P = 0.59; 90 participants; 2 trials; low quality evidence (Analysis 3.4).
3.4. Analysis.
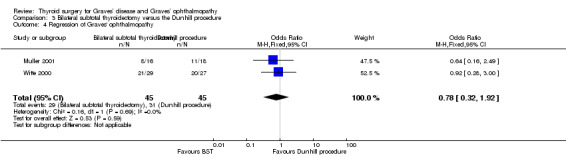
Comparison 3 Bilateral subtotal thyroidectomy versus the Dunhill procedure, Outcome 4 Regression of Graves' ophthalmopathy.
All‐cause mortality
No cases of mortality were reported in any of the relevant trials.
Socioeconomic effects
None of the trials reported information relevant to these outcomes.
Health‐related quality of life
None of the trials reported information relevant to health‐related quality of life.
Subgroup analyses
We did not perform subgroups analyses because there were not enough trials to estimate effects in various subgroups.
Sensitivity analyses
We did not perform any sensitivity analyses because there were not enough trials.
Assessment of reporting bias
We did not draw funnel plots due to the limited number of trials (n = 5).
Discussion
Summary of main results
Total thyroidectomy was more effective than subtotal thyroidectomy techniques (both bilateral subtotal thyroidectomy and the Dunhill procedure (unilateral total and contralateral subtotal thyroidectomy)) at preventing recurrent hyperthyroidism (moderate quality evidence). There were neutral effects for regression of Graves' ophthalmopathy and permanent recurrent laryngeal nerve palsy when comparing total with subtotal thyroidectomy, but increased rates of hypocalcaemia/hypoparathyroidism when comparing total with subtotal thyroidectomy (all low quality evidence).
The Dunhill procedure compared with bilateral subtotal thyroidectomy was more effective in preventing recurrent hyperthyroidism (low quality evidence). There were neutral effects for regression of Graves' opthalmopathy, permanent recurrent laryngeal nerve palsy and permanent hypocalcaemia/hypoparathyroidism when comparing these two techniques (all low quality evidence).
Deaths were rarely reported, health‐related quality of life and socioeconomic effects were not investigated in any of the included studies.
Overall completeness and applicability of evidence
We identified five studies and one ongoing study in our comprehensive literature search that fit the criteria for inclusion. We carried out a meta‐analysis to compare outcomes from three types of interventions We did not exclude any studies based on language of publication. We sought data from the authors where study results were incompletely reported.
The main conclusions from the meta‐analysis can be extrapolated to general clinical practice, however it is noted that all studies were carried out in institutions with high case throughput in thyroidectomies. It is possible that complication rates (such as permanent recurrent laryngeal nerve palsy or permanent hypocalcaemia/hypoparathyroidism) may be higher where surgeons do not perform thyroidectomy for Graves' disease on a regular basis.
Quality of the evidence
Overall quality of the evidence ranged from low to moderate. All trials were randomised. However, in all the trials blinding procedures were insufficiently described. Objective outcome assessment was blinded in two trials (Barczynski 2012; Jarhult 2005). Surgeons were not blinded in any of the trials. One trial blinded participants (Barczynski 2012). Attrition bias applied to one trial (Muller 2001), with 35% loss to follow‐up. In one trial the analysis was not intention‐to‐treat (Chi 2005).
Quality of evidence was highest when surgical techniques were compared in terms of postoperative recurrent hyperthyroidism, as the outcome is objective. Quality of evidence was lowest when rates of complications (recurrent laryngeal nerve palsy and permanent hypocalcaemia/hypoparathyroidism) were considered (as event rates were low across all studies). Overall, the main reasons for downgrading decisions in the 'Summary of findings' tables were high risk of performance bias, imprecision and low event rates.
Potential biases in the review process
The search for this topic was comprehensive and fully up‐to‐date, allowing for identification of all appropriate studies. We were unable to obtain additional information from authors on methodology; therefore risk of bias is unclear in some studies. We were only able to compare one study protocol with its publication (Barczynski 2012), therefore evaluation of reporting bias was limited.
Agreements and disagreements with other studies or reviews
Our results are consistent with the systematic review and meta‐analysis conducted by Palit et al, who found an 8% rate of recurrent hyperthyroidism with subtotal thyroidectomy (compared with total thyroidectomy), with no marked differences in complication rates (Palit 2000). A systematic review by Guo et. al. came to similar conclusions indicating that total thyroidectomy was more effective than subtotal thyroidectomy at preventing recurrent hyperthyroidism, although total thyroidectomy was associated with a higher rates of temporary hypoparathyroidism (Guo 2013). No marked differences were found in rates of permanent hypocalcaemia/hypoparathyroidism or recurrent laryngeal nerve palsy.
Authors' conclusions
Implications for practice.
Total thyroidectomy is more effective than subtotal thyroidectomy (both bilateral subtotal thyroidectomy and the Dunhill procedure (unilateral total and contralateral subtotal thyroidectomy)) at preventing recurrent hyperthyroidism in Graves' disease. Complications like permanent recurrent nerve palsy or permanent hypocalcaemia/hypoparathyroidism were not clearly associated with one of these techniques. However, there was some evidence for increased rates of permanent hypocalcaemia/hypoparathyroidism when comparing total with subtotal thyroidectomy. Effects were neutral for regression of Graves' ophthalmopathy. The Dunhill procedure compared with bilateral subtotal thyroidectomy was more effective in preventing recurrent hyperthyroidism, effects were neutral for complications and regression of Graves' ophthalmopathy. Deaths were rarely reported, health‐related quality of life and socioeconomic effects were not investigated in any of the included studies.
Implications for research.
There was a lack of high quality evidence on postoperative complications of thyroidectomy to conclude whether total thyroidectomy carries a higher risk of permanent hypocalcaemia/hypoparathyroidism or recurrent laryngeal nerve palsy compared to subtotal thyroidectomy techniques. In our included studies, there were no measures of health‐related quality of life or socioeconomic effects associated with thyroid surgery for Graves' disease. Well designed trials that are randomised, adequately powered and have suitable follow‐up are required to assess the benefit‐risk ratio of treatments for control of Graves hyperthyroidism and Graves' opthalmopathy. Of particular interest are adverse events and morbidity; standardised reporting will be important in this respect. Blinding of participants and personnel as well as outcome assessors for most outcome measures appears essential and all trials should be prospectively registered to analyse reporting bias amongst other things. Finally, health‐related quality of life is an important outcome and should form a core determinant of any future study.
Notes
We have based parts of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review on a standard template established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group.
Acknowledgements
We acknowledge the very helpful contributions made by the editorial team of the Cochrane Metabolic and Endocrine Disorders Group. We are also very grateful for the support of the Cambridge Biomedical Research Campus in conducting this review.
Appendices
Appendix 1. Search strategies
| Cochrane Library |
| 1. [mh "Graves disease"] 2. (grave* near/7 (diseas* or thyrotoxicos* or hyperthyr*)):ti,ab,kw 3. (grave* near/7 (orbitopath* or ophthalmopath*)):ti,ab,kw 4. (basedow* near/7 (diseas* or syndrom*)):ti,ab,kw 5. {or #1‐#4} 6. [mh Thyroidectomy] 7. thyroidectom*:ti,ab,kw 8. #6 or #7 9. #5 and #8 |
| MEDLINE (Ovid SP) |
| 1. exp Graves Disease/ 2. (grave* adj6 (diseas* or thyrotoxicos* or hyperthyr*)).tw. 3. (grave* adj6 (orbitopath* or ophthalmopath*)).tw. 4. (basedow* adj6 (diseas* or syndrom*)).tw. 5. or/1‐4 6. exp Thyroidectomy/ 7. thyroidectom*.tw. 8. 6 or 7 9. 5 and 8 [10‐19:Lefebvre 2011RCT filter ‐ max. sensitivity version, without "drug therapy.fs."] 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. or/ 10‐16 18. exp animals/ not humans/ 19. 17 not 18 20. 9 and 19 |
| PubMed (subsets not available on Ovid) |
| 1. grave*[tw] AND (diseas*[tw] OR thyrotoxicos*[tw] OR hyperthyr*[tw] OR orbitopath*[tw] OR ophthalmopath*[tw]) 2. basedow*[tw] AND (diseas*[tw] OR syndrom*[tw]) 3. #1 OR #2 4. thyroidectom*[tw] OR surg*[tw] 5. #3 AND #4 6. random*[tw] OR trial[tw] OR groups[tw] 7. #5 AND #6 8. #7 NOT medline[sb] NOT pmcbook |
| EMBASE (Ovid SP) |
| 1. exp Graves disease/
2. (grave* adj6 (diseas* or thyrotoxicos* or hyperthyr*)).tw.
3. (grave* adj6 (orbitopath* or ophthalmopath*)).tw.
4. (basedow* adj6 (diseas* or syndrom*)).tw.
5. or/1‐ 4
6. exp subtotal thyroidectomy/ or exp thyroidectomy/
7. thyroidectom*.tw.
8. 6 or 7
9. 5 and 8
[10:Wong 2006"treatment studies" filter ‐ SDSSGS version] 10. random*.tw. or clinical trial*.mp. or exp treatment outcome/ 11. 9 and 10 12. limit 11 to embase |
| ICTRP Search Portal (Standard search) |
| grave* AND thyroidectom* OR basedow* AND thyroidectom* OR grave* AND surg* OR basedow* AND surg* |
| ClinicalTrials.gov (Advanced search) |
| Conditions: grave OR graves OR basedow OR basedows OR "exophthalmic goiter" OR "exophthalmic goiters" OR "autoimmune hyperthyroidism" Interventions: thyroidectomy OR thyroidectomies OR surgery OR surgical OR surgeries |
Appendix 2. Description of interventions
| Intervention | Comparator intervention | |
| Barczynski 2012 | Total thyroidectomy | Bilateral subtotal thyroidectomy |
| Witte 2000 | Total thyroidectomy | Bilateral subtotal thyroidectomy |
| Unilateral total and contralateral subtotal thyroidectomy (Dunhill procedure) | ||
| Jarhult 2005 | Total thyroidectomy | Subtotal thyroidectomy |
| Chi 2005 | Bilateral subtotal thyroidectomy | Unilateral total and contralateral subtotal thyroidectomy (Dunhill procedure) |
| Muller 2001 | Bilateral subtotal thyroidectomy | Unilateral total and contralateral subtotal thyroidectomy (Dunhill procedure) |
Appendix 3. Baseline characteristics (I)
| Intervention and comparator intervention | Duration of follow‐up | Participating population | Study period [year to year] | Country | Setting | Ethnic groups [%] | Duration of Graves' disease | |
| Barczynski 2012 | I: total thyroidectomy | 5 years | Adults diagnosed with Graves' disease and mild active ophthalmopathy | 2000 to 2004 | Poland | Teaching hospital | ‐ | < 24 months |
| C: bilateral subtotal thyroidectomy | < 24 months | |||||||
| Witte 2000 | I: total thyroidectomy | 6 months | Participants with clinically or biochemically proven Graves' disease | 1993 to 1995 | Germany | Teaching hospital | ‐ | 2‐300 months |
| C1: bilateral subtotal thyroidectomy | 2‐300 months | |||||||
| C2: Dunhill procedure | 2‐300 months | |||||||
| Jarhult 2005 | I: total thyroidectomy | 3 years | Participants with Graves' disease and moderate to severe ophthalmopathy | ‐ | Sweden | University/county hospitals | ‐ | ‐ |
| C: subtotal thyroidectomy | ||||||||
| Chi 2005 | I: bilateral subtotal thyroidectomy | 26.4 (SD 1.1) months | Participants undergoing subtotal thyroidectomy for Graves' hyperthyroidism | 1998 to 2002 | Taiwan | Teaching hospital | ‐ | ‐ |
| C: Dunhill procedure | ||||||||
| Muller 2001 | I: bilateral subtotal thyroidectomy | 6 years | Diagnosed with Graves' disease if participants had hyperthyroidism and had ophthalmopathy or TSH‐R antibodies, or based on US scan | 1988 to 1992 | Germany | Tertiary referral centre | ‐ | ‐ |
| C: Dunhill procedure | ||||||||
| ‐ denotes not reported C: comparator; I: intervention; SD: standard deviation; TSH‐R: thyroid‐stimulating hormone receptor; US: ultrasound | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention and comparator intervention | Sex [female %] | Age [mean years (CI/SD/range)] | Comedications/Cointerventions | Comorbidities | |
| Barczynski 2012 | I: total thyroidectomy | 89 | 46.2 (CI 43.5 to 48.9) | Antithyroid drugs/beta blockers | ‐ |
| C: bilateral subtotal thyroidectomy | 89 | 45.5 (CI 43.1 to 47.9) | |||
| Witte 2000 | I: total thyroidectomy | 82 | 38 (19 to 68) | Antithyroid drugs/beta blockers (40%), thyroid hormone substitution (40.7%) | ‐ |
| C1: bilateral subtotal thyroidectomy | 86 | 35.5 (12 to 73) | |||
| C2: Dunhill procedure | 82 | 41 (17 to 66) | |||
| Jarhult 2005 | I: total thyroidectomy | 95 | 44 (21 to 62) | Antithyroid drugs for at least 3 months preoperatively Postoperative thyroxine supplementation 5 participants in each group received cortisone to decrease the activity of the EO |
‐ |
| C: subtotal thyroidectomy | 86 | 42 (24 to 69) | |||
| Chi 2005 | I: bilateral subtotal thyroidectomy | 84 | 33.3 (SD 0.73) | Propylthiouracil/methimazole | ‐ |
| C: Dunhill procedure | 87 | 34.5 (SD 0.93) | |||
| Muller 2001 | I: bilateral subtotal thyroidectomy | ‐ | 30.8 (14 to 78) | Antithyroid medication | ‐ |
| C: Dunhill procedure | ‐ | 30.8 (14 to 78) | |||
| ‐ denotes not reported C: comparator; CI: confidence interval; EO: endocrine ophthalmopathy; I: intervention; SD: standard deviation | |||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a |
Study results posted in trial register [Yes/No] |
Publications specified in trial register | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Barczynski 2012 |
Source: NCT01408368 Primary outcome measure(s): long term control of Graves' disease (time frame: up to 60 months postoperatively), recurrence rate of hyperthyroidism and change in Graves' ophthalmopathy |
No | Yes | Primary outcome measure(s): prevalence of recurrent hyperthyroidism during follow‐up and changes in Graves’ ophthalmopathy | Primary outcome measure(s): prevalence of recurrent hyperthyroidism and changes in Graves’ ophthalmopathy |
|
Secondary outcome measure(s): morbidity rate (time frame: up to 12 months postoperatively), RLN injury and hypoparathyroidism |
Secondary outcome measure(s): postoperative hypoparathyroidism and recurrent RLN injury | Secondary outcome measure(s): postoperative transient and permanent paresis of the RLN, postoperative hypocalcaemia and hypoparathyroidism | |||
| Other outcome measure(s): ‐ | Other outcome measure(s): serum free triiodothyronine, free thyroxine and thyroid‐stimulating hormone, serum thyrotropin binding inhibitory immunoglobulin (TBII) levels, ophthalmological assessment, clinical activity score to evaluate the activity of the ophthalmopathy, high‐resolution Doppler ultrasonography of the neck, duration of operation, parathyroid autotransplantation, parathyroid glands identified by histology in thyroid specimens, incidental papillary thyroid cancer, diameter of thyroid cancer | Other outcome measure(s): | |||
| Chi 2005 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | ||
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||||
| Other outcome measure(s): operative time, triiodothyronine (T3), thyroxine (T4), thyrotropin (TSH), and antimicrosomal antibodies, postoperative serum calcium, permanent hypoparathyroidism, complications (postoperative mortality, RLN palsy, wound haematoma or bleeding after thyroidectomy, need of calcium medication), recurrent hyperthyroidism, hypothyroidism, incidental carcinoma | Other outcome measure(s): operative time, recurrent hyperthyroidism, hypothyroidism, postoperative complications | ||||
| Witte 2000 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | ||
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||||
| Other outcome measure(s): thyroid‐stimulating hormone receptor antibodies (TSH‐RAb), postoperative total remnant size measured by ultrasonography, clinical courses of Graves’ ophthalmopathy using the modified scale system of the American Thyroid Association (ATA), early postoperative complications (RLN palsy, hypothyroidism, wound infection) until discharge from hospital, long‐term results of changes in endocrine orbitopathy, serum calcium levels, vocal cord motility, and TSH‐RAb titers were measured between 18 and 58 months after thyroid operation | Other outcome measure(s): postoperative Graves’ ophthalmopathy changes, postoperative TSH‐RAb titers, postoperative complication rates | ||||
| Jarhult 2005 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | ||
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||||
| Other outcome measure(s): evaluation of ophthalmopathy, patients overall opinion about the actual eye problems was expressed on a visual analogue scale, thyroid remnant, operating time, reoperation due to bleeding, hospital stay, permanent vocal cord palsy, permanent hypothyroidism, TSH, free triiodothyronine (T3) and free thyroxine (T4), thyrotropin receptor antibodies (TRAB) titre, calcium | Other outcome measure(s): development of ophthalmopathy, thyrotropin (TSH)‐receptor antibody levels, surgical complication rate (permanent RLN paresis and permanent hypoparathyroidism) | ||||
| Muller 2001 | N/T | Primary outcome measure(s): ‐ | Primary outcome measure(s): ‐ | ||
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | ||||
| Other outcome measure(s): recurrent hyperthyroidism, regression of Graves' ophthalmopathy, all‐cause mortality, adverse events | Other outcome measure(s): mortality, recurrent laryngeal nerve palsy, hypocalcaemia, secondary haemorrhage, wound infections, recurrence rate | ||||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers) bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study) cOther outcome measures refer to all outcomes not specified as primary or secondary outcome measures EMA: European Medicines Agency; FDA: Food and Drug Administration (US); N/T: no trial document available; RLN: recurrent laryngeal nerve | |||||
Appendix 6. High risk of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Barczynski 2012 | N/A | ||||
| Chi 2005 | All‐cause mortality | x | |||
| Jarhult 2005 | All‐cause mortality | x | |||
| Rate of recurrent hyperthyroidism | x | ||||
| Witte 2000 | All‐cause mortality | x | |||
| Muller 2001 | N/A | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant
(Classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported (Classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010).
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials. | |||||
Appendix 7. Definition of endpoint measurement
| Rate of recurrent hyperthyroidism | Permanent recurrent laryngeal nerve palsy | Permanent hypocalcaemia/hypoparathyroidism | Regression of Graves' ophthalmopathy | Health‐related quality of life | Socioeconomic effects | Severe/serious adverse events | |
| Barczynski 2012 | Laboratory tests included measurement of serum free tri‐iodothyronine (fT3; reference range 0.22 to 6.78 pmol/L), free thyroxine (fT4; reference range 12 to 30 pmol/L) and thyroid‐stimulating hormone (TSH, thyrotropin; reference range 0.4 to 4.2 units/L). A serum thyrotropin binding inhibitory immunoglobulin (TBII) level > 1.5 units/L was defined as positive | Vocal cord paresis for more than 12 months after the operation was regarded as permanent Indirect laryngoscopy was performed before surgery |
Persistent hypocalcaemia with serum PTH level below 10 ng/L for more than 12 months | The following definitions were used: a change in TES and/or CAS of fewer than 2 points from the preoperative baseline value was regarded as ‘no change’, an increase in TES and/or CAS of 2 points or more was regarded as‘progression’, and a decrease in TES and/or CAS of 2 points or more was regarded as ‘improvement’ Assessed by blinded ophthalmologist Measured at yearly intervals for 5 years postop |
N/I | N/I | One death reported (due to a myocardial infarction 3 years after surgery) |
| Chi 2005 | Suppressed TSH (human TSH radioimmunoassay kit measured between 3‐26.4 months after surgery | RLN palsy defined as permanent if longer than 6 months Based on independent ENT specialist assessment and patient report |
Permanent hypocalcaemia if longer than 6 months postop.. Assessed by external laboratory | N/I | N/I | N/I | N/I |
| Jarhult 2005 | N/I | RLN palsy considered permanent if persisting > 9 months (unclear whether laryngoscopy was performed) | Persistent hypocalcaemia > 9 months | 1. Improvement in ophthalmological score (based on author experience, not validated) 2. Improvment in objective and subjective evaluation of eye symptoms by patient and ophthalmologist |
N/I | N/I | N/I |
| Witte 2000 | N/I | RLN palsy defined as permanent if longer than 6 months Assessed by independent ENT specialist |
Permanent hypocalcaemia if longer than 6 months postop.. Assessed by external laboratory | GO measured from > 18‐58 months postop using standardised scale Both patient reported and ophthalmology assessment scores are reported |
N/I | N/I | N/I |
| Muller 2001 | Biochemical tests of TSH, fT3 and fT4 with reference levels as per local laboratory. Suppressed TSH and elevated fT3/fT4 was taken to be evidence of hyperthyroidism (measured at 6 years) | Recurrent laryngeal nerve palsy defined by impaired vocal cord movement seen on laryngoscopy (preop and postop laryngoscopy routine). Considered permanent if persisting more than 1 year | Hypocalcaemia persisting more than 1 year was considered to be permanent | Surgical team/physician assessment Unclear what scale was used or whether an ophthalmologist was involved Outcome measured at 6 years |
N/I | N/I | No deaths |
| CAS: clinical activity score; ENT: ear, nose & throat; fT3: free tri‐iodothyronine; fT4: free thyroxine; GO: Graves' ophthalmopathy; N/I: not investigated; PTH: parathyroid hormone; RLN: recurrent laryngeal nerve; TES: total eye score; TT: total thyroidectomy; TSH: thyroid‐stimulating hormone | |||||||
Appendix 8. Adverse events (I)
| Intervention and comparator intervention | Randomised/safety [N] | Deaths [N] | Deaths [%] | All adverse events [N] | All adverse events [%] | Severe/serious adverse events [N] | Severe/serious adverse events [%] | |
| Barczynski 2012 | I: total thyroidectomy | 100 | 1 | 1 | 21 | 21 | 2 | 2 |
| C: bilateral subtotal thyroidectomy | 100 | 0 | 0 | 35 | 35 | 3 | 3 | |
| Witte 2000 | I: total thyroidectomy | 50 | ‐ | ‐ | 10 | 20 | 1 | 2 |
| C1: bilateral subtotal thyroidectomy | 50 | ‐ | ‐ | 16 | 32 | 0 | 0 | |
| C2: Dunhill procedure | 50 | ‐ | ‐ | 9 | 18 | 1 | 2 | |
| Jarhult 2005 | I: total thyroidectomy | 22 | ‐ | ‐ | 5 | 23 | ‐ | ‐ |
| C: subtotal thyroidectomy | 21 | ‐ | ‐ | 1 | 5 | ‐ | ‐ | |
| Chi 2005 | I: bilateral subtotal thyroidectomy | 166 | ‐ | ‐ | 22 | 13.3 | 0 | 0 |
| C: Dunhill procedure | 174 | ‐ | ‐ | 55 | 31.6 | 1 | 0.6 | |
| Muller 2001 | I: bilateral subtotal thyroidectomy | 67 | 0 | 0 | 14 | 21 | 1 | 1.5 |
| C: Dunhill procedure | 85 | 0 | 0 | 18 | 21 | 2 | 2.3 | |
| ‐ denotes not reported C: comparator intervention; I: intervention | ||||||||
Appendix 9. Adverse events (II)
| Intervention and comparator intervention | Randomised/safety [N] | Left study due to adverse events [N] | Left study due to adverse events [%] | Hospitalisation [N] | Hospitalisation [%] | Outpatient treatment [N] | Outpatient treatment [%] | |
| Barczynski 2012 | I: total thyroidectomy | 100 | 1 | 1 | ‐ | ‐ | ‐ | ‐ |
| C: bilateral subtotal thyroidectomy | 100 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Witte 2000 | I: total thyroidectomy | 50 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C1: bilateral subtotal thyroidectomy | 50 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C2: Dunhill procedure | 50 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Jarhult 2005 | I: total thyroidectomy | 22 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: subtotal thyroidectomy | 21 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Chi 2005 | I: bilateral subtotal thyroidectomy | 166 | ‐ | ‐ | 9 | 5.4 | ‐ | ‐ |
| C: Dunhill procedure | 174 | ‐ | ‐ | 15 | 8.6 | ‐ | ‐ | |
| Muller 2001 | I: bilateral subtotal thyroidectomy | 67 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: Dunhill procedure | 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||||||
Appendix 10. Adverse events (III)
| Intervention and comparator intervention | Randomised/safety [N] | Specific adverse events [description] | Specific adverse events [N] | Specific adverse events [%] | |
| Barczynski 2012 | I: total thyroidectomy | 100 | ‐ | ‐ | ‐ |
| C: bilateral subtotal thyroidectomy | 100 | ‐ | ‐ | ‐ | |
| Witte 2000 | I: total thyroidectomy | 50 | ‐ | ‐ | ‐ |
| C1: bilateral subtotal thyroidectomy | 50 | ‐ | ‐ | ‐ | |
| C2: Dunhill procedure | 50 | ‐ | ‐ | ‐ | |
| Jarhult 2005 | I: total thyroidectomy | 22 | ‐ | ‐ | ‐ |
| C: subtotal thyroidectomy | 21 | ‐ | ‐ | ‐ | |
| Chi 2005 | I: bilateral subtotal thyroidectomy | 166 | ‐ | ‐ | ‐ |
| C: Dunhill procedure | 174 | ‐ | ‐ | ‐ | |
| Muller 2001 | I: bilateral subtotal thyroidectomy | 67 | ‐ | ‐ | ‐ |
| C: Dunhill procedure | 85 | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | |||||
Appendix 11. Survey of study authors providing information on included trials
| Study author contacted | Study author replied | Study author asked for additional information | Study author provided data | |
| Barczynski 2012 | 04/04/2014 | 05/04/2014 | Yes | Yes |
| Witte 2000 | 04/04/2014 | Did not reply | Yes | No |
| Jarhult 2005 | 24/11/2012 | 25/11/2012 | Yes | Yes |
| Chi 2005 | 04/04/2014 | Did not reply | Yes | No |
| Muller 2001 | 04/04/2014 | Did not reply | Yes | No |
Data and analyses
Comparison 1. Total thyroidectomy versus subtotal thyroidectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of recurrent hyperthyroidism | 2 | 350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.04, 0.46] |
| 2 Permanent hypocalcaemia/hypoparathyroidism | 3 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.79 [1.36, 16.83] |
| 3 Permanent recurrent laryngeal nerve palsy | 3 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.38, 5.59] |
| 4 Regression of Graves' ophthalmopathy | 2 | 287 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.64, 2.08] |
1.1. Analysis.
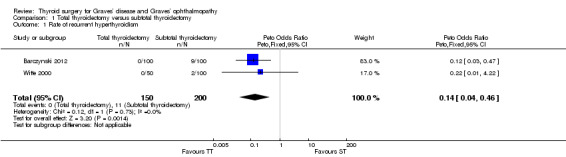
Comparison 1 Total thyroidectomy versus subtotal thyroidectomy, Outcome 1 Rate of recurrent hyperthyroidism.
Comparison 2. Total thyroidectomy versus bilateral subtotal thyroidectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of recurrent hyperthyroidism | 2 | 300 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.04, 0.44] |
| 2 Permanent hypocalcaemia/hypoparathyroidism | 2 | 300 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.53, 7.85] |
| 3 Permanent recurrent laryngeal nerve palsy | 2 | 300 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.15] |
| 4 Regression of Graves' ophthalmopathy | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.64, 2.21] |
2.1. Analysis.
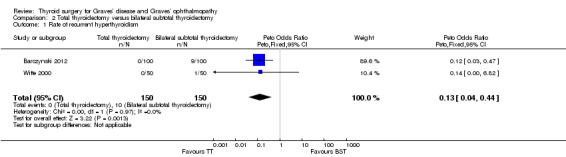
Comparison 2 Total thyroidectomy versus bilateral subtotal thyroidectomy, Outcome 1 Rate of recurrent hyperthyroidism.
Comparison 3. Bilateral subtotal thyroidectomy versus the Dunhill procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of recurrent hyperthyroidism | 3 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [1.28, 5.85] |
| 2 Permanent hypocalcaemia/hypoparathyroidism | 3 | 592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [0.47, 7.71] |
| 3 Permanent recurrent laryngeal nerve palsy | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Regression of Graves' ophthalmopathy | 2 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.92] |
3.1. Analysis.
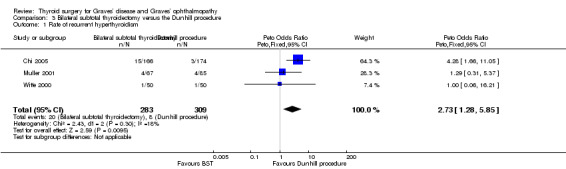
Comparison 3 Bilateral subtotal thyroidectomy versus the Dunhill procedure, Outcome 1 Rate of recurrent hyperthyroidism.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barczynski 2012.
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1 Intervention model: parallel group superiority design Masking: single‐blinded for participants Primary purpose: treatment of Graves' disease |
|
| Participants |
Condition: Graves' disease Enrolment: 200 in total Inclusion criteria: patients diagnosed with Graves’ disease and mild active ophthalmopathy undergoing surgical treatment Exclusion criteria: previous thyroid or parathyroid surgery, recurrent hyperthyroidism after radioiodine ablation, a history of Graves’ disease of more than 24 months, thyroid nodules within the posterior aspect(s) of the thyroid lobe(s), suspicion of thyroid cancer, preoperative recurrent laryngeal nerve palsy, pregnancy or lactation, age below 18 years, American Society of Anesthesiologists fitness grade IV, and inability to comply with the follow‐up protocol Diagnostic criteria: clinical features of Graves' disease and TFT supportive of the diagnosis |
|
| Interventions |
Number of study centres: 1 Treatment before study: patients were rendered euthyroid with thiamazole Titration period: none Intervention(s): bilateral subtotal thyroidectomy Comparator(s): total thyroidectomy |
|
| Outcomes |
Primary outcome(s): resolution of Graves ophthalmopathy; recurrence of hyperthyroidism Secondary outcome(s): temporary/permanent hypoparathyroidism; temporary/permanent RLN paresis Other outcome(s): ‐ |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no Study start date: 2000 Study completion date: 2004 |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal, full article |
|
| Stated aim for study | "To evaluate long term results of bilateral subtotal thyroidectomy compared with total thyroidectomy in patients with Graves' disease and mild active ophthalmopathy" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The randomisation sequence was generated by the computer. Sequencing was based on permutated blocks of two and three to balance the number of patients in the treatment groups" Comment: computer generated random numbers. Does not state if randomisation after consent obtained for surgery; sequence may be guessed easily with use of permutated blocks of two and three |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Information on the type of intervention remained in consecutively numbered and sealed envelopes that were stored in the operating theatre. An envelope containing the allocation was added to the patient's file in the operating room. The envelope was opened and the surgeon performed the assigned intervention" Comment: sealed envelope opened by surgeon in theatre |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "the patients and ophthalmologist involved in this study were blinded to the group assignment" Comment: patients blinded, surgical team not blinded |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote from publication: "the patients and ophthalmologist involved in this study were blinded to the group assignment" Comment: ophthalmology assessment blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "biochemical evaluation consisted of measurement of serum concentrations of TSH, fT3 and fT4 at each visit and serum TBII levels yearly" Comment: objective outcomes assessed by independent third party; thyroid status and calcium status monitored by regular blood tests (externally referenced laboratory) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "ophthalmologists involved in this study were blinded to the group assignment" and "indirect laryngoscopy was performed before surgery and on the first day after operation by external ENT specialist" Comment: subjective outcomes assessed by independent third party; (1) ophthalmology assessment of thyroid eye status at regular intervals, (2) RLN palsy checked by external ENT specialist |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "CONSORT diagram: five lost to follow up in bilateral subtotal thyroidectomy group, four lost to follow up in total thyroidectomy group" Comment: nine out of 200 lost to follow‐up |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote from publication: "CONSORT diagram: 5 lost to follow up in bilateral subtotal thyroidectomy group, 4 lost to follow up in total thyroidectomy group" Comment: nine out of 200 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in the methods section reported in the results section (except for mean difference in TSH concentration postoperatively) |
| Other bias | Low risk | Comment: no other sources of bias detected |
Chi 2005.
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1 Intervention model: parallel group superiority design Masking: no blinding of patients or personnel Primary purpose: treatment of Graves' disease |
|
| Participants |
Condition: Graves' disease Enrolment: 340 in total Inclusion criteria: diagnosis of Graves disease and informed consent to study Exclusion criteria: 1) Previous recurrent hyperthyroidism 2) Large thyroid tumour that prohibited subtotal thyroidectomy 3) Less than 2.5 x 1 x 1 cm thyroid remnant (not suitable for further surgery) 4) Minimum follow‐up period less than 3 months (early loss to follow‐up) Diagnostic criteria: Graves' hyperthyroidism diagnosed with clinical manifestations, TFT, thyroid scan, histology (postoperatively) |
|
| Interventions |
Number of study centres: 1 Treatment before study: patients were rendered euthyroid with propylthiouracil or methimazole (treatment duration from one month to 144 months) Titration period: none Intervention(s): bilateral subtotal thyroidectomy Comparator(s): unilateral total lobectomy and contralateral subtotal thyroidectomy |
|
| Outcomes |
Primary outcome(s): 1. Postop thyroid status (TFTs, TSH) Secondary outcome(s): 2. Hypoparathyroidism 3. Recurrent laryngeal nerve injury 4. Wound haematoma/bleeding Other outcome(s): ‐ |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no Study start date: 1998 Study completion date: 2002 |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer‐reviewed journal, full article |
|
| Stated aim for study | To compare the results of bilateral subtotal thyroidectomy and unilateral total and contralateral subtotal thyroidectomy in control of hyperthyroidism in Graves' disease | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "patients who underwent subtotal thyroidectomy for hyperthyroidism were prospectively randomised into two groups" Comment: randomisation after consent for surgery; method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clearly stated whether allocation concealment took place |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "surgery was performed by one surgeon. The standard surgical technique of subtotal thyroidectomy under general anaesthesia with intact inferior thyroid artery was used" Comment: all operations performed by single surgeon; surgeon could not be blinded to group allocation; no blinding of participants |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote from publication: "surgery was performed by one surgeon. The standard surgical technique of subtotal thyroidectomy under general anaesthesia with intact inferior thyroid artery was used" Comment: all operations performed by single surgeon; surgeon could not be blinded to group allocation; no blinding of participants |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote from publication: " T3 and T4 were measured with radioimmunoassay kit ... total calcium level was measured routinely one day after operation... permanent hypoparathyroidism was defined as symptomatic hypocalcaemia for more than 6 months" Comment: (1) evaluation of thyroid function: laboratory standard tests; (2) operation time: not blinded; (3) blood loss: not blinded; (4) postoperative calcium level: laboratory standard tests; (5) recurrent laryngeal nerve palsy: assessed by laryngoscopy, not clear if blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No subjective outcomes are measured in this study |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "we excluded patients for whom the follow up period was less than 3 months" Comment: outcome data reported for all 340 participants; however participants lost at an early stage of follow‐up were excluded from study, therefore not intention‐to‐treat analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote from publication: "we excluded patients for whom the follow up period was less than 3 months" Comment: outcome data reported for all 340 participants; however participants lost at an early stage of follow‐up were excluded from study, therefore not intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: participants lost at an early stage of follow‐up were excluded from study, therefore not intention‐to‐treat analysis; mortality not reported |
| Other bias | Low risk | Comment: none detected |
Jarhult 2005.
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1 Intervention model: parallel group superiority design Masking: no blinding of patients or personnel Primary purpose: treatment of Graves' disease |
|
| Participants |
Condition: Graves' disease Enrolment: 44 in total Inclusion criteria: 1. Diagnosis of Graves disease 2. Preop treatment with antithyroid drugs for at least 3 months 3. Euthyroidism at time of operation 4. Active Graves opthalmopathy of at least moderate degree Exclusion criteria: 1. Previous thyroid or parathyroid surgery 2. Previous treatment with radioiodine 3. Elevation of TSH value more than 200% above normal during antithyroid treatment and within three months prior to surgery Diagnostic criteria: Graves' disease with ophthalmopathy, TFT, TSH antibodies and histological diagnosis based on tissue obtained form surgery. |
|
| Interventions |
Number of study centres: 10 Treatment before study: preoperative treatment with antithyroid medications for at least 3 months. Titration period: none Intervention(s): total thyroidectomy (complete macroscopic removal of thyroid tissue) Comparator(s): subtotal thyroidectomy (either subtotal bilateral resection or hemithyroidectomy plus unilateral subtotal resection leaving no more than a total of 4 g of remnant tissue) |
|
| Outcomes |
Primary outcome(s): 1. Change in Graves' ophthalmopathy Secondary outcome(s): 2. Permanent hypoparathyroidism 3. Recurrent laryngeal nerve palsy Other outcome(s): TRAb measurements |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no Study start date: not stated Study completion date: not stated |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding Publication status: peer‐reviewed journal, full article |
|
| Stated aim for study | This study enrolled patients with moderate‐severe endocrine ophthalmopathy and discussed long term results of a prospective, randomised trial of treatment with total or subtotal thyroidectomy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Forty‐four patients with Graves' disease (GD) and a moderate‐severe Grave's ophthalmopathy were randomly allocated to undergo total or subtotal thyroidectomy" Comment: method of randomisation not specified; took place after consent for surgery |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated whether allocation concealment took place |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "The design of the study left decisions about the surgical and postoperative care to the attending surgeon" Comment: surgeon could not be blinded to group allocation; note that multiple centres were involved in recruitment for the study |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: not stated that patients were blinded to the intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "the follow up consisted of ... biochemical analyses of TSH, free T3 and free T4, TRAB titre and calcium" Comment: evaluation of TRAb, thyroid function and calcium: laboratory standard tests |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "Evaluation of eye disease activity was carried out by an ophthalmologist... the ophthalmologist was not informed of the type of thyroid surgery performed" Comment: ophthalmopathy ‐ assessed by blinded ophthalmologist |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote from publication: "one patient in the subtotal resection group... refused to come for evaluation after the first two post operative check ups" Comment: one patient lost to follow‐up out of 44; all other outcomes reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote from publication: "one patient in the subtotal resection group... refused to come for evaluation after the first two post operative check ups" Comment: one patient lost to follow‐up out of 44; all other outcomes reported |
| Selective reporting (reporting bias) | High risk | Comment: postoperative thyroid function (recurrent hyperthyroidism) was measured but not reported; mortality not reported |
| Other bias | Low risk | Comment: none identified |
Muller 2001.
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1 Intervention model: parallel group superiority design Masking: no blinding of patients or personnel Primary purpose: treatment of Graves' disease |
|
| Participants |
Condition: Graves' disease Enrolment: 152 in total Inclusion criteria: 1. Diagnosis of Graves disease 2. Completed six years follow‐up 3. Euthyroidism at time of operation Exclusion criteria: not specified Diagnostic criteria: hyperthyroidism with endocrine ophthalmopathy or TSH‐R antibodies and characteristics of Graves' disease on ultrasound scan |
|
| Interventions |
Number of study centres: 1 Treatment before study: preoperative treatment with antithyroid medications if hyperthyroid Titration period: none Intervention(s): 1. Bilateral subtotal thyroidectomy with 3 mL of remaining tissue Comparator(s): 2. Ipsilateral lobectomy and contralateral subtotal resection with 3 mL thyroid remnant per side (6 mL total) |
|
| Outcomes |
Primary outcome(s): 1. Recurrent hyperthyroidism Secondary outcome(s): 2. Permanent hypoparathyroidism 3. Recurrent laryngeal nerve palsy 4. Wound complications (seroma, infection, haematoma) 5. Progression of Graves' Ophthalmopathy Other outcome(s): ‐ |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no Study start date: 1988 Study completion date: 1992 |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer‐reviewed journal, full article |
|
| Stated aim for study | In this prospective randomised study, the two methods (bilateral subtotal resection and the Dunhill operation) are evaluated with respect to their operative and postoperative complications and their long term results [in treatment of Grave's disease] | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "We examined 152 patients... in a prospective randomised study" Comment: the study is prospectively randomised although randomisation techniques not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clearly stated whether allocation concealment took place |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Quote from publication: "The surgeons were specialised surgeons or were directly supervised by specialised surgeons" Comment: not stated whether surgeons were blinded to group allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Comment: not stated whether patients were blinded to group allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote from publication: "Following surgery, patients were started on a thyroxine replacement regimen with iodine and levothyroxine, and the regimen was later adjusted according to their free T3, T4 and TSH levels. All patients had a repeat ... measurement of their plasma calcium" Comment: evaluation of TRAb, thyroid function and calcium: laboratory standard tests |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote form publication: "the 6 year follow up period ended with a questionnaire sent to the physician currently treating the patient" Comment: unclear whether physician completing follow‐up questionnaire was blinded to intervention; not clear who assessed eye involvement |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Quote from publication: "after 6 years, 99 patients returned the questionnaire" Comment: outcomes only reported in 99/152 (63%) participants |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Quote from publication: "after 6 years, 99 patients returned the questionnaire" Comment: outcomes only reported in 99/152 (63%) participants |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported appear to be standard for studies addressing this type of question |
| Other bias | Low risk | Comment: no other risk of bias detected |
Witte 2000.
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1:1 Intervention model: parallel group superiority design (three arms) Masking: no blinding of patients or personnel Primary purpose: treatment of Graves' disease |
|
| Participants |
Condition: Graves' disease Enrolment: 150 in total Inclusion criteria: diagnosis of Graves disease + informed consent to study Exclusion criteria: No diagnosis of Graves disease Does not consent to study Not medically fit for surgical management Diagnostic criteria: clinical features of Graves' disease with TSH‐R antibodies and hyperthyroidism |
|
| Interventions |
Number of study centres: 1 Treatment before study: preoperative treatment with antithyroid medications, beta‐blockers and thyroid hormone substitution. Titration period: none Intervention(s): total thyroidectomy Comparator(s): Dunhill procedure (total lobectomy + contralateral subtotal thyroidectomy) Bilateral subtotal thyroidectomy |
|
| Outcomes |
Primary outcome(s): Graves' ophthalmopathy Postoperative TSH‐R antibody level Secondary outcome(s): RLN palsy Hypoparathyroidism Other outcome(s): Wound complications/infection rate |
|
| Study details |
Run‐in period: none Study terminated before regular end (for benefit/because of adverse events): no Study start date: 1993 Study completion date: 1995 |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer‐reviewed journal, full article |
|
| Stated aim for study | We investigated whether total thyroidectomy might be superior to subtotal thyroidectomy (bilateral or unilateral remnants of less than 4 mL) in respect to the adverse effect on immunologic parameters and endocrine orbitopathy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "After the patients consented to the prospective randomised trial, 50 were randomised to total thyroidectomy, 50 to unilateral total and contralateral subtotal thyroid resection, and 50 to bilateral subtotal thyroid resection" Comment: randomisation after consent for surgery; method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported whether allocation concealment took place |
| Blinding of participants and personnel (performance bias) Objective outcomes | High risk | Comment: not stated that surgeons were blinded to group allocation |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote from publication: "in case of specific technical demands, surgeons were allowed to change to recommended procedure individually for the patient's advantage" Comment: in some cases group allocation changed intra‐operatively for technical reasons |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Quote from publication: "pre‐operative and post operative TSH‐Ab titres were measured"; "All patients were checked by certified ear, nose and throat physicians for recurrent laryngeal nerve paralysis" Comment: thyroid status and calcium status were assessed with standardised referenced assays; recurrent laryngeal nerve status was assessed by ENT specialty review, unclear whether blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Quote from publication: "GO was estimated clinically, using the modified scale system of the American Thyroid Association, by general practitioners, ophthalmologists and by the patients themselves" Comment: Graves' ophthalmopathy was assessed by ophthalmologist and patient; unclear whether blinding took place |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "the TSH‐R antibodies were preoperatively measured in 128 of the 150 patients... post operative TSH‐R‐Abs were measured in 107 of the 150 patients" Comment: preoperative/postoperative TSH‐R antibodies not measured in 14.7% and 28.7% of participants; GO/TSH‐R antibodies/long term calcium all measured from > 18‐58 months postoperatively |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Quote from publication: "GO was estimated clinically, using the modified scale system of the American thyroid association, by general practitioners, ophthalmologists and by the patients themselves" Comment: all Graves' ophthalmopathy patients underwent a standardised assessment pre‐ and postoperatively using ATA criteria |
| Selective reporting (reporting bias) | Unclear risk | Quote from publication: "the TSH‐R antibodies were preoperatively measured in 128 of the 150 patients... post operative TSH‐R‐Abs were measured in 107 of the 150 patients" Comment: a large number of participants had no assessment of the TSH‐R antibodies postoperatively |
| Other bias | Low risk | Comment: no other risk of bias detected |
ATA: American Thyroid Association; ENT: ear, nose and throat; fT3: free tri‐iodothyronine; fT4: free thyroxine; GO: Graves' opthalmopathy; RLN: recurrent laryngeal nerve; TBII: thyrotropin binding inhibiting immunoglobulins; TFT: thyroid function tests; TRAb: anti‐thyroid receptor antibody; TSH: thyroid‐stimulating hormone; TSH‐R: thyroid‐stimulating hormone receptor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 1998 | Non randomised, no surgical comparison arm |
| Al‐Adhami 2012 | Retrospective study |
| Barakate 2002 | Retrospective study |
| Boostrom 2007 | Prospective study, non‐randomised |
| Campos 1994 | Only one surgical arm |
| Candela 2006 | Retrospective case series |
| De Bellis 2012 | Non randomised, no surgical comparison arm |
| Fernandez‐Sanchez 1993 | Only one surgical arm |
| Feroci 2014 | Review |
| Guo 2013 | Review |
| Hughes 2011 | Total thyroidectomy both arms, non‐randomised |
| Hussain 2008 | Non‐randomised, no surgical comparison arm |
| Jarhult 2012 | Non randomised |
| Jukic 2010 | Radio‐iodine versus surgery, no surgical comparison arm |
| Khandra 1992 | Retrospective study |
| Ku 2005 | Historical control group, non‐randomised |
| Kurihara 2002 | Retrospective study |
| Leo 2012 | No surgical comparison arm |
| Lepner 2008 | Prospective study, non‐randomised |
| Ljunggren 1998 | No surgical comparison arm |
| Lofthouse 2006 | Review |
| Marcocci 1992 | Retrospective study, no surgical comparison arm |
| Maschuw 2011 | Protocol only, recruitment not complete due to lack of funding |
| Menconi 2007 | Subtotal thyroidectomy in both arms, no comparison of alternative surgical technique |
| Miccoli 1996 | Non‐randomised |
| Morosini 1988 | Retrospective study |
| Robert 2001 | Retrospective study |
| Rosato 2002 | Retrospective study |
| Sanchez 1999 | Retrospective study |
| Sugino 2008 | Non‐randomised, no comparison of alternative surgical techniques |
| Tallstedt 1992 | No alternative surgical technique comparison |
| Tallstedt 1997 | Only one surgical arm |
| Tian 2012 | No surgical comparison arm |
| Torring 1996 | Only one surgical arm |
| Welch 2011 | Consecutive, non‐randomised participants |
| Woods 2014 | Meta‐analysis |
| Yalcin 2010 | Retrospective study |
| Zaraca 2000 | Retrospective study |
Characteristics of ongoing studies [ordered by study ID]
Maschuw 2012.
| Trial name or title | Total versus near‐total thyroidectomy in Graves' disease and their outcome on postoperative transient hypoparathyroidism: study protocol for a randomised controlled trial |
| Methods |
Type of study: randomised controlled clinical trial Allocation: 1:1 Intervention model: parallel group superiority Masking: blinding of patients and observer Primary purpose: to compare rates of transient postoperative hypoparathyroidism in total and subtotal thyroidectomy |
| Participants |
Condition: Graves' disease, for definite surgery Inclusion criteria: diagnosis of Graves disease and informed consent to study Exclusion criteria: conservative treatment, malignancy, previous thyroid surgery, coincident hypoparathyroidism |
| Interventions |
Intervention: bilateral subtotal thyroidectomy, remnants of < 1mL on each side Control: total thyroidectomy |
| Outcomes |
Primary outcome: transient postoperative hypoparathyroidism Secondary outcomes: reoperations due to bleeding, recurrent laryngeal nerve palsy, permanent hypoparathyroidism, recurrent disease, changes of endocrine orbitopathy, quality of life within a one‐year of follow‐up period |
| Starting date | Recruitment not started |
| Contact information | maschuw@med.uni‐marburg.de |
| Study identifier | German clinical trials register (DRKS) DRKS00004161 |
| Official title | Total versus near‐total thyroidectomy in Graves' disease and their outcome on postoperative transient hypoparathyroidism: study protocol for a randomised controlled trial |
| Stated purpose of study | The trial is designed as a prospective randomised controlled patient and observer blinded multi‐centred trial in a parallel design including an active comparator and an intervention group. The intervention addresses the surgical procedure: near‐total thyroidectomy leaving bilateral remnants of <= 1 mL on each side in the intervention group and total thyroidectomy in the control group. The occurrence of transient postoperative hypoparathyroidism is defined as primary endpoint |
| Notes |
Differences between protocol and review
Originally we planned to only investigate total versus subtotal thyroidectomy for Graves' disease and Graves' ophthalmopathy. However, to provide maximum information for patients, clinicians and decision makers we also included all types of thyroidectomy for these conditions, such as bilateral thyroidectomy versus unilateral total and contralateral subtotal thyroidectomy (Dunhill procedure). Therefore, the title was changed from ‘Total thyroidectomy versus subtotal thyroidectomy for Graves' disease’ to 'Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy'.
We were not able to conduct analyses of health‐related quality of life or socioeconomic effects (health economic outcomes were changed to the broader term socioeconomic effects) related to thyroidectomy in Graves' disease due to insufficient data from the included studies. We were also not able to conduct a meta‐analysis of some postoperative complications (e.g. bleeding/wound infection), as reporting criteria were not sufficiently similar between studies.
We inserted a new paragraph 'Quality of evidence' under 'Data synthesis' in the 'Methods' section.
Contributions of authors
All review authors read and approved the final review draft.
Zi Wei Liu (ZWL): protocol draft, search strategy development, acquiring trial copies, trial selection, data extraction, data analysis, data interpretation, and future review update.
Liam Masterson (LM): protocol draft, search strategy development, acquiring of trial copies, trial selection, data extraction, data analysis, data interpretation, and future review update.
Brian Fish (BF): protocol draft, trial selection, data interpretation, and future review update.
Piyush Jani (PJ): protocol draft, trial selection, data interpretation, and future review update.
Krishna Chatterjee (KC): protocol draft, trial selection, data interpretation, and future review update.
Declarations of interest
Zi Wei Liu: none known.
Liam Masterson: none known.
Piyush Jani: none known.
Brian Fish: none known.
Krishna Chatterjee: none known.
New
References
References to studies included in this review
Barczynski 2012 {published data only}
- Barczyński M, Konturek A, Hubalewska‐Dydejczyk A, Gołkowski F, Nowak W. Randomized clinical trial of bilateral subtotal thyroidectomy versus total thyroidectomy for Graves' disease with a 5‐year follow‐up. British Journal of Surgery 2012;99(4):515‐22. [DOI] [PubMed] [Google Scholar]
- Niederle B. Randomized clinical trial of bilateral subtotal thyroidectomy versus total thyroidectomy for Graves' disease with a 5‐year follow‐up. British Journal of Surgery 2012; Vol. 99:515‐22. [DOI] [PubMed]
Chi 2005 {published data only}
- Chi SY, Hsei KC, Sheen‐Chen SM, Chou FF. A prospective randomized comparison of bilateral subtotal thyroidectomy versus unilateral total and contralateral subtotal thyroidectomy for graves' disease. World Journal of Surgery 2005;29(2):160‐3. [DOI] [PubMed] [Google Scholar]
Jarhult 2005 {published data only}
- Jarhult J, Rudberg C, Larsson E, Selvander H, Sjövall K, Winsa B, et al. TEO Study Group. Graves' disease with moderate‐severe endocrine ophthalmopathy‐long term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid 2005;15(10):1157‐64. [DOI] [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Müller PE, Bein B, Robens E, Bein HS, Spelsberg F. Thyroid surgery according to Enderlen‐Hotz or Dunhill: a comparison of two surgical methods for the treatment of Graves' disease. International Surgery 2001;86(2):112‐6. [PubMed] [Google Scholar]
Witte 2000 {published data only}
- Witte J, Goretzki PE, Dotzenrath C, Simon D, Felis P, Neubauer M, et al. Surgery for Graves' disease: total versus subtotal thyroidectomy‐results of a prospective randomized trial. World Journal of Surgery 2000;24(11):1303‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 1998 {published data only}
- Abe Y, Sato H, Noguchi M, Mimura T, Sugino K, Ozaki O, et al. Effect of subtotal thyroidectomy on natural history of ophthalmopathy in graves' disease. World Journal of Surgery 1998;22:714‐17. [DOI] [PubMed] [Google Scholar]
Al‐Adhami 2012 {published data only}
- Al‐Adhami A, Craig W, Krukowski ZH. Quality of life after surgery for graves' disease:Comparison of those having surgery intended to preserve thyroid function with those having ablative surgery. Thyroid 2012;22:494‐500. [DOI] [PubMed] [Google Scholar]
Barakate 2002 {published data only}
- Barakate MS, Agarwal G, Reeve TS, Barraclough B, Robinson B, Delbridge LW. Total thyroidectomy is now the preferred option for the surgical management of graves' disease. ANZ Journal of Surgery 2002;72:321‐24. [DOI] [PubMed] [Google Scholar]
Boostrom 2007 {published data only}
- Boostrom S, Richards ML. Total thyroidectomy is the preferred treatment for patients with Graves' disease and a thyroid nodule. Otolaryngology ‐ Head and Neck Surgery 2007;136:278‐81. [DOI] [PubMed] [Google Scholar]
Campos 1994 {published data only}
- Campos Pastor MM, Fernandez Soto ML, Escobar‐Jimenez F, Barredo Acedo F, Ruiz de Almodovar M. Long‐term evolution of immunologic and thyroid function in Graves disease according different therapeutic options. [Spanish] [Evolucion inmunologica y de la funcion tiroidea a largo plazo en la enfermedad de Graves segun distintas opciones terapeuticas]. Medicina clinica 1994;102:446‐50. [PubMed] [Google Scholar]
Candela 2006 {published data only}
- Candela G, Varriale S, Libero L, Maschio A, Giordano M, Manetta F, et al. Nearly total thyroidectomy: Versus total thyroidectomy: Our experience [Tiroidectomia quasi totale versus tiroidectomia totale: Nostra esperienza]. Minerva Chirurgica 2006;61:17‐24. [PubMed] [Google Scholar]
De Bellis 2012 {published data only}
- Bellis A, Conzo G, Cennamo G, Pane E, Bellastella G, Colella C, et al. Time course of Graves' ophthalmopathy after total thyroidectomy alone or followed by radioiodine therapy: A 2‐year longitudinal study. Endocrine 2012;41:320‐6. [DOI] [PubMed] [Google Scholar]
Fernandez‐Sanchez 1993 {published data only}
- Fernandez Sanchez JR, Rosell Pradas J, Carazo Martinez O, Torres Vela E, Escobar Jimenez F, Garbin Fuentes I. Graves' ophthalmopathy after subtotal thyroidectomy and radioiodine therapy. British Journal of Surgery 1993;80:1134‐6. [DOI] [PubMed] [Google Scholar]
Feroci 2014 {published data only}
- Feroci F, Rettori M, Borrelli, A, Coppola A, Castagnoli A, Perigli, G, et al. A systematic review and meta‐analysis of total thyroidectomy versus bilateral subtotal thyroidectomy for Graves' disease. Surgery 2014;155:529‐40. [DOI] [PubMed] [Google Scholar]
Guo 2013 {published data only}
- Guo Z, Yu P, Liu Z, Si Y, Jin M. Total thyroidectomy vs bilateral subtotal thyroidectomy in patients with Graves' diseases: A meta‐analysis of randomized clinical trials. Clinical Endocrinology 2013;79:739‐46. [DOI] [PubMed] [Google Scholar]
Hughes 2011 {published data only}
- Hughes OR, Scott‐Coombes DM. Hypocalcaemia following thyroidectomy for treatment of Grave's disease: Implications for patient management and cost‐effectiveness. Journal of Laryngology and Otology 2011;125:849‐52. [DOI] [PubMed] [Google Scholar]
Hussain 2008 {published data only}
- Hussain M, Hisham AN. Total thyroidectomy: The procedure of choice for toxic goitre. Asian Journal of Surgery 2008;31:59‐62. [DOI] [PubMed] [Google Scholar]
Jarhult 2012 {published data only}
- Jarhult J, Andersson PO, Duncker L. Alternating from subtotal thyroid resection to total thyroidectomy in the treatment of Graves' disease prevents recurrences but increases the frequency of permanent hypoparathyroidism. Langenbeck's Archives of Surgery 2012;397:407‐12. [DOI] [PubMed] [Google Scholar]
Jukic 2010 {published data only}
- Jukic T, Stanicic J, Petric V, Kusic Z. Radioiodine versus surgery in the treatment of Graves' hyperthyroidism [Radioaktivni jod‐131 ili kirurski zahvat u lijecenju Gravesove hipertireoze (Croatian)]. Lijecnicki vjesnik 2010;132:355‐60. [PubMed] [Google Scholar]
Khandra 1992 {published data only}
- Khadra M, Delbridge L, Reeve TS, Poole AG, Crummer P. Total thyroidectomy: its role in the management of thyroid disease. The Australian and New Zealand Journal of Surgery 1992;62:91‐5. [DOI] [PubMed] [Google Scholar]
Ku 2005 {published data only}
- Ku CF, Lo CY, Chan WF, Kung AWC, Lam KSL. Total thyroidectomy replaces subtotal thyroidectomy as the preferred surgical treatment for Graves' disease. ANZ Journal of Surgery 2005;75:528‐31. [DOI] [PubMed] [Google Scholar]
Kurihara 2002 {published data only}
- Kurihara H. Total thyroidectomy for the treatment of hyperthyroidism in patients with ophthalmopathy. Thyroid 2002;12:265‐7. [DOI] [PubMed] [Google Scholar]
Leo 2012 {published data only}
- Leo M, Marcocci C, Pinchera A, Nardi M, Megna L, Rocchi R, et al. Outcome of Graves' orbitopathy after total thyroid ablation and glucocorticoid treatment:follow‐up of a randomized clinical trial. Journal of Clinical Endocrinology & Metabolism 2012;97:E44‐8. [DOI] [PubMed] [Google Scholar]
Lepner 2008 {published data only}
- Lepner U, Seire I, Palmiste V, Kirsimagi U. Surgical treatment of Graves' disease: subtotal thyroidectomy might still be the preferred option. Medicina (Kaunas, Lithuania) 2008;44:22‐6. [PubMed] [Google Scholar]
Ljunggren 1998 {published data only}
- Ljunggren JG, Torring O, Wallin G, Taube A, Tallstedt L, Hamberger B, et al. Quality of life aspects and costs in treatment of Graves' hyperthyroidism with antithyroid drugs, surgery, or radioiodine: results from a prospective, randomized study. Thyroid 1998;8:653‐9. [DOI] [PubMed] [Google Scholar]
Lofthouse 2006 {published data only}
- Lofthouse M. Subtotal versus total thyroidectomy for patients with Graves' eye disease. Nature Clinical Practice Endocrinology and Metabolism 2006;2:63‐4. [Google Scholar]
Marcocci 1992 {published data only}
- Marcocci C, Bartalena L, Bogazzi F, Bruno‐Bossio G, Pinchera A. Relationship between Graves'ophthalmopathy and type of treatment of Graves' hyperthyroidism. Thyroid 1992;2:171‐8. [DOI] [PubMed] [Google Scholar]
Maschuw 2011 {published data only}
- Maschuw K, Schlosser K, Sitter H, Bartsch DK. Total versus near‐total thyroidectomy in Graves'disease (TONIG) ‐ A potential prospective randomized controlled multicentered trial of the CAEK society. Langenbeck's Archives of Surgery 2011;396(8):1300. [Google Scholar]
Menconi 2007 {published data only}
- Menconi F, Marino M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effectsof total thyroid ablation versus near‐total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. Journal of Clinical Endocrinology & Metabolism 2007;92:1653‐8. [DOI] [PubMed] [Google Scholar]
Miccoli 1996 {published data only}
- Miccoli P, Vitti P, Rago T, Iacconi P, Bartalena L, Bogazzi F, et al. Surgical treatment of Graves' disease: Subtotal or total thyroidectomy?. Surgery 1996;120:1020‐5. [DOI] [PubMed] [Google Scholar]
Morosini 1988 {published data only}
- Morosini PP, Fianchini A, Landi E. Surgical treatment of hyperthyroidism: total thyroidectomy versus subtotal thyroidectomy [Italian] [La terapia chirurgica dell'ipertiroidismo: tiroidectomia totale "versus" tiroidectomia subtotale]. Giornale di chirurgia 1988;9:210‐2. [PubMed] [Google Scholar]
Robert 2001 {published data only}
- Robert J, Mariethoz S, Pache JC, Bertin D, Caulfield A, Murith N, et al. Short‐ and long‐term results of total vs subtotal thyroidectomies in the surgical treatment of Graves' disease. Swiss Surgery 2001;7:20‐4. [DOI] [PubMed] [Google Scholar]
Rosato 2002 {published data only}
- Rosato L, Avenia N, Palma M, Gulino G, Nasi PG, Pezzullo L. Complications of total thyroidectomy: incidence, prevention and treatment [Complicanze della tiroidectomia totale: incidenza, prevenzione e trattamento (Italian)]. Chirurgia italiana 2002;54:635‐42. [PubMed] [Google Scholar]
Sanchez 1999 {published data only}
- Sánchez J, Lamata F, Lagos J, Cerdán R, García FA, Martínez M, et al. Total versus subtotal thyroidectomy in the surgical treatment of Graves' disease. Cirugía Española 1999;65(2):110‐5. [Google Scholar]
Sugino 2008 {published data only}
- Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ito K. Surgical management of Graves' disease ‐10‐year prospective trial at a single institution. Endocrine Journal 2008;55:161‐7. [DOI] [PubMed] [Google Scholar]
Tallstedt 1992 {published data only}
- Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. The Thyroid Study Group. New England Journal of Medicine 1992;326:1773‐8. [DOI] [PubMed] [Google Scholar]
Tallstedt 1997 {published data only}
- Tallstedt L, Lundell G. Radioiodine treatment, ablation, and ophthalmopathy: a balanced perspective. Thyroid 1997;7:241‐5. [DOI] [PubMed] [Google Scholar]
Tian 2012 {published data only}
- Tian JL, Chen SF, Du YH, Li CL, Wang W, Li YS, et al. A comparative study of the long‐term effect of thyroid arterial embolization with surgical thyroidectomy in treating Graves' disease. Journal of Interventional Radiology (China) 2012;21:194‐7. [Google Scholar]
Torring 1996 {published data only}
- Torring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves' hyperthyroidism: Treatment with antithyroid drugs, surgery, or radioiodine ‐ A prospective, randomized study. Journal of Clinical Endocrinology and Metabolism 1996;81:2986‐93. [DOI] [PubMed] [Google Scholar]
Welch 2011 {published data only}
- Welch KC, McHenry CR. Total thyroidectomy: Is morbidity higher for graves' disease than non‐toxic goiter?. Journal of Surgical Research 2011;170:96‐9. [DOI] [PubMed] [Google Scholar]
Woods 2014 {published data only}
- Woods R. Total thyroidectomy versus bilateral subtotal thyroidectomy in patients with Graves' diseases: A meta‐analysis of randomized clinical trials. Clinical Endocrinology 2014;80(2):316. [DOI] [PubMed] [Google Scholar]
Yalcin 2010 {published data only}
- Yalcin Y, Topaloglu O, Berker D, Isik S, Guler S. Evaluation of therapeutic modalities in Graves' hyperthyroidism. Endocrine Abstracts 2010;22:815. [Google Scholar]
Zaraca 2000 {published data only}
- Zaraca F, Paola M, Gossetti F, Proposito D, Filippoussis P, Montemurro L, et al. Benign thyroid disease: 20‐year experience in surgical therapy. [Italian] [Malattia tiroidea benigna: esperienza ventennale di terapia chirurgica]. Chirurgia italiana 2000;52:41‐7. [PubMed] [Google Scholar]
References to ongoing studies
Maschuw 2012 {published data only}
- Maschuw K, Schlosser K, Lubbe D, Nies C, Bartsch DK. Total versus near‐total thyroidectomy in Graves' disease and their outcome on postoperative transient hypoparathyroidism: study protocol for a randomized controlled trial?. Trials 2012;13:234. [DOI: 10.1186/1745-6215-13-234] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ATA 2011
- Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. https://www.aace.com/files/hyper‐guidelines‐2011.pdf (accessed 26 June 2015). [DOI] [PubMed]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barczynski 2011
- Barczyński M, Konturek A, Stopa M, Cichoń S, Richter P, Nowak W. Total thyroidectomy for benign thyroid disease: is it really worthwhile?. Annals of Surgery 2011;254(5):724‐9. [PUBMED: 22005150] [DOI] [PubMed] [Google Scholar]
Bellantone 2002
- Bellantone R, Lombardi CP, Bossola M, Boscherini M, Crea C, Alesina P. Total thyroidectomy for management of benign thyroid disease: review of 526 cases. World Journal of Surgery 2002;26(12):1468‐71. [PUBMED: 12360381] [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2:36. [2046‐4053: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandt 2011
- Brandt F, Green A, Hegedüs L, Brix TH. A critical review and meta‐analysis of the association between overt hyperthyroidism and mortality. European Journal of Endocrinology/European Federation of Endocrine Societies 2011;165(4):491‐7. [PUBMED: 21724839] [DOI] [PubMed] [Google Scholar]
Efremidou 2009
- Efremidou EI, Papageorgiou MS, Liratzopoulos N, Manolas KJ. The efficacy and safety of total thyroidectomy in the management of benign thyroid disease: a review of 932 cases. Canadian Journal of Surgery 2009;52(1):39‐44. [PUBMED: 19234650] [PMC free article] [PubMed] [Google Scholar]
Genovese 2012
- Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for Graves' disease? A systematic review of the existing literature. Annals of Surgical Oncology 2012;20(2):660‐7. [DOI: 10.1245/s10434-012-2606-x] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Hermann 1998
- Hermann M, Roka R, Richter B, Freissmuth M. Early relapse after operation for Graves' disease: postoperative hormone kinetics and outcome after subtotal, near‐total, and total thyroidectomy. Surgery 1998;124(5):894‐900. [9823404] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
In 2009
- In H, Pearce EN, Wong AK, Burgess JF, McAneny DB, Rosen JE. Treatment options for Graves disease: a cost‐effectiveness analysis. Journal of the American College of Surgeons 2009;209(2):170‐9. [PUBMED: 19632593] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Lalwani 2012
- Lalwani K. Lange Current Diagnosis and Treatment: Otolaryngology ‐ Head and Neck Surgery. 3rd Edition. McGraw‐Hill, 2012. [Google Scholar]
Laurberg 2012
- Laurberg P, Berman DC, Bülow Pedersen I, Andersen S, Carlé A. Incidence and clinical presentation of moderate to severe graves' orbitopathy in a Danish population before and after iodine fortification of salt. Journal of Clinical Endocrinology and Metabolism 2012;97(7):2325‐32. [PUBMED: 22518849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palit 2000
- Palit TK, Miller CC 3rd, Miltenburg DM. The efficacy of thyroidectomy for Graves' disease: a meta‐analysis. Journal of Surgical Research 2000;90(2):161‐5. [PUBMED: 10792958] [DOI] [PubMed] [Google Scholar]
Ponto 2012
- Ponto Ka, Kahaly GJ. Autoimmune thyrotoxicosis: diagnostic challenges. American Journal of Medicine 2012;125(9):S1. [PUBMED: 22938935] [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SSL, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanocco 2012
- Zanocco K, Heller M, Elaraj D, Sturgeon C. Is subtotal thyroidectomy a cost‐effective treatment for Graves disease? A cost‐effectiveness analysis of the medical and surgical treatment options. Surgery 2012;152(2):164‐72. [PUBMED: 22503512] [DOI] [PubMed] [Google Scholar]


