Abstract
A network meta-analysis of randomized controlled trials was conducted to compare and rank the effectiveness of various noninvasive brain stimulation (NIBS) for Parkinson's disease (PD). We searched PubMed, Web of Science, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP), and Chinese Biomedical Literature Service System (SinoMed) databases from the date of database inception to April 30th, 2024. Two researchers independently screened studies of NIBS treatment in patients with PD based on inclusion and exclusion criteria. Two researchers independently performed data extraction of the included studies using an Excel spreadsheet and assessed the quality of the literature according to the Cochrane Risk of Bias Assessment Tool (RoB2). Network meta-analysis was performed in StataMP 17.0. A total of 28 studies involving 1628 PD patients were included. The results showed that HF-rTMS over the SMA (SMD = − 2.01; 95% CI [− 2.87, − 1.15]), HF-rTMS over the M1 and DLPFC (SMD = − 1.80; 95% CI [− 2.90, − 0.70]), HF-rTMS over the M1 (SMD = − 1.10; 95% CI [− 1.55, − 0.65]), a-tDCS over the DLPFC (SMD = − 1.08; 95% CI [− 1.90, − 0.27]), HF-rTMS over the M1 and PFC (SMD = − 0.92; 95% CI [− 1.71, − 0.14]), LF-rTMS over the M1 (SMD = − 0.72; 95% CI [− 1.17, − 0.28]), and HF-rTMS over the DLPFC (SMD = − 0.70; 95% CI [− 1.21, − 0.19]) were significantly improved motor function compared with sham stimulation. The SUCRA three highest ranked were HF-rTMS over the SMA (95.1%), HF-rTMS over the M1 and DLPFC (89.6%), and HF-rTMS over the M1 (73.0%). In terms of enhanced cognitive function, HF-rTMS over the DLPFC (SMD = 0.80; 95% CI [0.03,1.56]) was significantly better than sham stimulation. The SUCRA three most highly ranked were a-tDCS over the M1 (69.8%), c-tDCS over the DLPFC (66.9%), and iTBS over the DLPFC (65.3%). HF-rTMS over the M1 (SMD = − 1.43; 95% CI [− 2.26, − 0.61]) and HF-rTMS over the DLPFC (SMD = − 0.79; 95% CI [− 1.45, − 0.12)]) significantly improved depression. The SUCRA three highest ranked were HF-rTMS over the M1 (94.1%), LF-rTMS over the M1 (71.8%), and HF-rTMS over the DLPFC (69.0%). HF-rTMS over the SMA may be the best option for improving motor symptoms in PD patients. a-tDCS and HF-rTMS over the M1 may be the NIBS with the most significant effects on cognition and depression, separately.
Trial registration: International Prospective Register of Systematic Review, PROSPERO (CRD42023456088)
Keywords: Noninvasive brain stimulation, Parkinson’s disease, Systematic review, Network meta-analysis
Subject terms: Neuroscience, Diseases of the nervous system, Parkinson's disease
Introduction
Parkinson's disease (PD) is one of the most common complex neurodegenerative disorders in humans, caused mainly by degenerative necrosis of dopaminergic neurons in the dense portion of the substantia nigra, leading to decreased dopamine levels in the striatum1–3. In addition to motor symptoms such as bradykinesia and resting tremor, PD is associated with other non-motor symptoms, such as cognitive impairment and depression4,5. Dopaminergic drug replacement therapy, represented by levodopa, can alleviate most early PD symptoms6. However, it is essential to explore effective treatment methods actively because of the apparent adverse effects of drug therapy and the reduced efficacy of long-term use7.
Noninvasive brain stimulation (NIBS), safe and convenient neuromodulation techniques, have shown efficacy in improving movement, cognitive rehabilitation, and depression in PD and are considered to be more promising modalities of treatment8–12. The main types of NIBS used for PD include repetitive transcranial magnetic stimulation (rTMS), theta-burst stimulation (TBS), and transcranial direct current stimulation (tDCS). rTMS is a therapeutic technique that repeatedly stimulates the cerebral cortex by generating a magnetic field guided by a coil13,14. rTMS with a stimulation frequency > 1 Hz is called high-frequency rTMS (HF-rTMS), and rTMS with a stimulation frequency ≤ 1 Hz is called low-frequency rTMS (LF-rTMS)15. TBS is a specific mode of rTMS that enhances cortical excitability by mimicking cortical theta wave rhythms to enhance synaptic transmission and can be categorized into intermittent TBS (iTBS) and continuous TBS (cTBS) based on the time interval16–18. tDCS is a technique that applies low-intensity direct current to the scalp's surface to modulate cortical excitability19. An anodic electrode placed above the target area is called anodic tDCS (a-tDCS), while a cathodic electrode placed above the target area is called cathodic tDCS (c-tDCS). The stimulation targets of NIBS in PD patients mainly include the supplementary motor area (SMA), primary motor cortex (M1), dorsal lateral prefrontal cortex (DLPFC), and cerebellum20–22.
However, in most clinical studies using NIBS to improve PD symptoms, the sample sizes are small, and there is a wide variety of NIBS. To comprehensively compare the therapeutic effects of different NIBS, we performed a network meta-analysis to analyze the effects of NIBS on motor, cognitive, and depressive conditions in PD patients by evaluating multiple scales to inform clinical practice.
Methods
Protocol and registration
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 guideline23,24 and A MeaSurement Tool to Assess Systematic Reviews (AMSTAR) 225. The registration of this study was completed with the International Prospective Register of Systematic Review, PROSPERO (CRD42023456088).
Search strategy
Computer searches of PubMed, Web of Science, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP), and Chinese Biomedical Literature Service System (SinoMed) databases were performed from construction to April 30th, 2024. The search languages were English and Chinese. We searched ClinicalTrials.gov for gray literature and unpublished studies. In addition, we manually searched references for included studies, review articles and meta-analysis. The whole strategy, with search terms for each database, is accessible in Supplementary Table S1.
Inclusion and exclusion criteria
The inclusion criteria included: (1) Patient: adults (≥ 18 years) with PD who meet the diagnostic criteria for PD, regardless of gender, race, or disease severity; (2) Intervention: NIBS stimulation, with an unlimited number of NIBS sessions, stimulation parameters, and target locations; (3) Comparator: sham NIBS; (4) Outcomes: indicators of motor function were the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS-III) and the motor section of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III); indicators of cognitive function assessment in non-motor function were the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA); indicators of depression assessment in non-motor function were the Beck Depression Inventory (BDI) and the Hamilton Depression Rating Scale (HDRS); (5) randomized controlled trials (RCTs).
The exclusion criteria included: (1) duplicate publications or duplicate literature data; (2) study data not available; (3) not RCT;(4) protocol but not report of study result.
Study selection and data extraction
Two researchers independently screened titles and abstracts after removing duplicates and subsequently reviewed the full text based on predetermined criteria to identify eligible studies and perform data extraction. Any disagreements were resolved through discussion with the third researcher. The following information was independently extracted for the included studies using an Excel sheet: first author, time of publication, number of study participants, gender, age, course of disease and severity, intervention modality, NIBS parameters, site of stimulation, and treatment duration, follow-up time after treatment, outcome indicators and results after treatment, and state of medication.
Risk of bias assessment
According to the Cochrane risk of bias tool (RoB2), two researchers individually assessed each of the five sections: randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of reported result26. We determined the risk of bias to be low, some concerns, or high by using the RoB2 to answer important questions for each of these sections. If each section is low risk, the overall risk of bias is "low risk"; if more than one section is "some concerns" and there is no "high risk", the overall risk of bias is "some concerns"; as long as one section is "high risk", the overall risk of bias is "high risk". Inconsistent evaluations were discussed and finalized with the third researcher.
Data synthesis and analysis
The outcome measures in this study were continuous variables, and the mean and standard deviation (SD) of the change in scores in each scale before and after treatment were calculated according to the formulas in the Cochrane Handbook for Systematic Reviews of Interventions to eliminate baseline differences26.
Network meta-analysis was performed in StataMP 17.0 using the "network meta" command. A network relationship plot was performed in which the circles indicate the sample size of included studies, and the straight lines indicate the number of studies between the two interventions. When a closed loop exists, direct and indirect comparison consistency was assessed using the node-splitting method, with P > 0.05 indicating good consistency, which can be analyzed using the consistency model, and vice versa using the inconsistency model. In addition, we evaluated the efficacy of different sham NIBS stimulations using pairwise meta-analysis with the Comprehensive Meta-Analysis software 3.7 to demonstrate the assumption of transitivity of network meta-analysis27,28. Forest plots of NIBS compared to sham stimulation were drawn. League tables for pairwise meta-analysis were made. The surface under the cumulative ranking curve (SUCRA) was calculated to perform the superiority ranking of the interventions. The closer the SUCRA value was to 100%, the higher the probability that the intervention would be optimal. Funnel plots were drawn for publication bias analysis.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating tool to assess the quality of the analyzed evidence29. We assessed quality by categorizing the outcome indicators into four levels high quality, moderate quality, low quality, and very low quality based on five dimensions: study limitations, imprecision, inconsistency, indirectness, and publication bias.
Results
Literature selection and characteristics of the included literatures
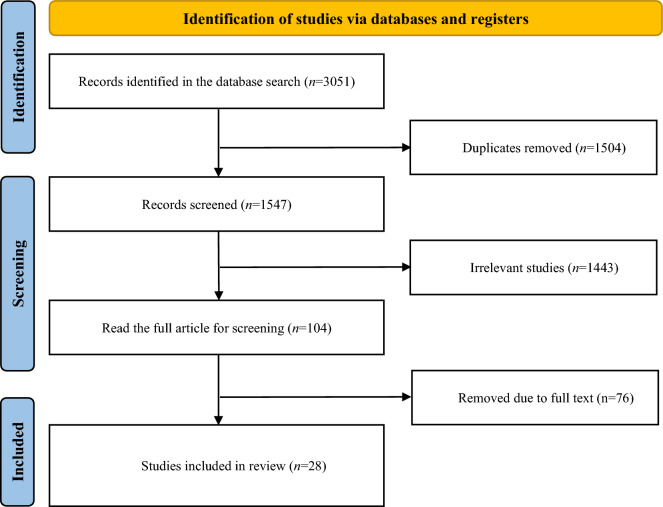
A total of 3051 articles were initially retrieved from the database. After removing 1504 duplicate articles, 1443 studies were excluded after initial screening. Of the remaining 104 articles, 76 were excluded after reviewing the full text based on inclusion and exclusion criteria. Finally, 28 studies were selected for network meta-analysis. A flowchart of the study screening process is shown in Fig. 1, and a list of excluded studies and the reasons for their exclusion are shown in Supplementary Table S2. NIBS methods for the included studies included rTMS30–46, iTBS47,48, and tDCS49–57. The studies included 1628 PD patients, the NIBS group with 966, and the sham NIBS group with 662. The sample sizes of the NIBS and sham NIBS groups ranged from 7–54 individuals. The characteristics of the included studies are shown in Table 1.
Figure 1.
The flowchart of the literature screening process.
Table 1.
The characteristics of the included studies.
| References | Sample size (E/C) | Gender (male/female) | Age (E/C, year) | Course of disease (E/C, year/month) | H&Y Stage (1/2/3/4/5) | Intervention | Site of stimulation | Treatment duration | Follow-up | Outcome | State of medication (I/E) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benninger et al.49 | 13/12 | 9/4;7/5 | (63.6 ± 9.0)/(64.2 ± 8.8) | (10.6 ± 7.1)/(9.1 ± 3.3) y | 2–4 | 2 mA tDCS | M1 + SMA | 3 d/wk, 2.5 wks | 1 mon; 3 mons | ① | On/on & off |
| Benninger47 | 13/13 | 7/6;11/2 | (62.1 ± 6.9)/(65.6 ± 9.0) | (10.8 ± 7.1)/(6.5 ± 3.4) y | 2–4 | iTBS, 80% RMT | M1 + DLPFC | 4 d/wk, 2 wks | 1 mon | ① | On/on & off |
| Shirota et al.30 | 34/36/36 | 12/22;14/22; 19/17 | (67.9 ± 8.4)/(68.8 ± 7.6)/(65.7 ± 8.5) | (7.8 ± 6.6)/(8.5 ± 7.3)/(7.6 ± 4.4) y | 0/9/21/4/0; 0/10/21/5/0; 0/10/21/5/0 | 10 Hz rTMS, 110% RMT; 1 Hz rTMS, 110% RMT | SMA | 8 wks | 12 wks | ⑤ | On/on |
| Biundo et al.50 | 7/9 | 6/1;8/1 | (69.1 ± 7.6)/(72.3 ± 4.1) | – | 1–3 | 2 mA tDCS | DLPFC | 20 min/d, 4 d/wk, 4 wks | 16 wks | ①④⑥ | On/– |
| Li31 | 30/30/30 | 15/15;16/14; 16/14 | (65.3 ± 8.1)/(66.1 ± 7.6)/(66.5 ± 7.5) | (6.6 ± 5.3)/(6.1 ± 5.2)/(6.4 ± 4.9) y | – | 5 Hz rTMS, 90–100% RMT;0.5 Hz rTMS, 90–100% RMT | DLPFC | 2 d/wk,4 wks | – | ①⑤ | On/– |
| Yu et al.32 | 31/33 | 14/17;16/17 | (67.25 ± 6.71)/(68 ± 7.56) | (2.76 ± 1.56)/(2.64 ± 1.49) y | 1–2 | 5 Hz rTMS | DLPFC | 10 days | 1 mon | ①⑤ | On/off |
| Li et al.51 | 28/28 | 14/14;15/13 | (64.32 ± 5.59)/(64.39 ± 5.5) | (1.19 ± 0.57)/(1.28 ± 0.56) y | (1.3 ± 0.4)/(1.2 ± 0.5) | 2 mA tDCS | M1 | 20 min/d, 8 wks | – | ④ | – |
| Khedr et al.33 | 26/26 | 40/22 | (59.58 ± 11.28)/(55.88 ± 13.84) | (4.60 ± 3.64)/(4.85 ± 3.39) y | – | 20 Hz rTMS, 90% RMT;1 Hz rTMS, 100% RMT | M1 | 10 days | 1 mon | ① | On/on |
| Yang et al.34 | 17/17 | 20/14 | 48–76 | – | – | 1 Hz rTMS, 80% RMT | M1 | 20 min/d, 20 days | – | ① | On/– |
| Mi et al.35 | 20/10 | 9/11;5/5 | (62.65 ± 10.56)/(65.60 ± 8.68) | (9.15 ± 5.82)/(7.40 ± 4.83) y | (2.60 ± 0.85)/(2.35 ± 0.91) | 10 Hz rTMS, 90% RMT | SMA | 5 d/wk, 2 wks | 2wks;4 wks | ② | On/on |
| Chung et al.36 | 17/17/16 | 10/7;9/8;7/9 | (62.7 ± 6.8)/(62.1 ± 5.7)/(62.1 ± 5.7) | (5.2 ± 3.4)/(7.5 ± 4.9)/(6.9 ± 3.3) y | (2.2 ± 0.3)/(2.2 ± 0.4)/(2.3 ± 0.3) | 25 Hz rTMS, 80% RMT;1 Hz rTMS, 80% RMT | M1 | 4 d/wk,3 wks | 1 mon;3 mons | ② | On/on |
| Guo37 | 38/38/38 | 18/20;17/21;19/19 | (65.91 ± 3.42)/(66.28 ± 3.55)/(66.57 ± 3.39) | (6.48 ± 2.08)/(6.15 ± 1.97)/(6.64 ± 2.11) y | 0/22/16/0/0;0/23/15/0/0;0/20/18/0/0 | 5 Hz rTMS, 100% RMT;1 Hz rTMS, 100% RMT | M1 | 10 days | 1 mon | ①③⑤ | On/– |
| Lai et al.38 | 20/20 | 12/8;14/6 | (69.55 ± 1.64)/(71.2 ± 1.67) | (4.23 ± 0.61)/(5.5 ± 1.28) y | – | 10 Hz rTMS, 80% RMT | SMA | 5 d/wk,4 wks | – | ① | On/– |
| Spagnolo et al.39 | 19/20/20 | 12/7;15/5;14/6 | (63.9 ± 10)/(60.4 ± 8.1)/(64.2 ± 5.5) | (7.6 ± 4.9)/(5.8 ± 2.1)/(7.2 ± 3) y | 2(2–2.5);2(2,2);2(2,2) | 10 Hz rTMS, M1: 90% RMT; PFC:100% RMT | M1 + PFC; M1 | 3 d/wk,4 wks | – | ②③⑥ | On/off |
| Sun et al.52 | 11/11 | 4/7;9/2 | (62 ± 14.73)/(65 ± 12.67) | (8.2 ± 3.8)/(7.6 ± 3.2) y | 1–3 | 2 mA tDCS | DLPFC | 20 min/d,5 d/wk,4 wks | – | ③④ | On/– |
| Wu et al.53 | 28/26 | 16/12;14/12 | (61 ± 11.6)/(62.6 ± 12.2) | (5.8 ± 2.6)/(5.7 ± 3.5) y | (2.4 ± 0.8)/(2.5 ± 0.6) | 1.2 mA tDCS | DLPFC | 20 min/d,5 d/wk,4 wks | – | ⑤ | On/on |
| Aftanas et al.40 | 23/23 | 12/11;9/14 | (63.7 ± 8.8)/(62.9 ± 7.1) | (7.0 ± 4.0)/(5.6 ± 4.0) y | 0/10/13/0/0;0/11/12/0/0 | 10 Hz rTMS, M1: 100% RMT; DLPEC:110% RMT | M1 + DLPFC | 40 min/d,3 wks | – | ②③⑤⑥ | On/– |
| He et al.48 | 20/15 | 13/7;10/5 | (70.0 ± 6.3)/(74.8 ± 6.9) | (2.7 ± 1.5)/(2.5 ± 1.1) y | (2.7 ± 1.1)/(2.5 ± 1.0) | iTBS, 100% RMT | DLPFC | 5 d/wk,2 wks | 3 mons | ④ | On/on |
| Hu et al.54 | 49/49 | 30/19;28/21 | (64.23 ± 4.78)/(63.68 ± 5.22) | (33.02 ± 10.65)/(32.32 ± 12.44) mon | – | 2 mA tDCS | DLPFC | 45 min/d, 12 wks | – | ③④ | On/– |
| Lee and Kim55 | 15/15 | 6/9;8/7 | (70.00 ± 3.76)/(71.33 ± 3.27) | (6.27 ± 1.03)/(7.00 ± 1.41) mon | (2.47 ± 0.52)/(2.80 ± 0.41) | 2 mA tDCS | M1 | 20 min/d, 5 d/wk,4 wks | 2 wks | ① | On/– |
| Liao et al.41 | 30/30 | 17/13;16/14 | (59.03 ± 6.84)/(60.43 ± 6.94) | (1.89 ± 0.63)/(1.92 ± 0.59) y | 1–2.5 | 10 Hz rTMS, 90% RMT | DLPFC | 25 min/d,5 d/wk,4 wks | – | ④ | – |
| Chen et al.42 | 32/32 | 19/13;18/14 | (65.21 ± 5.32)/(65.32 ± 5.24) | (2.59 ± 0.61)/(2.65 ± 0.63) y | (2.16 ± 0.5)/(2.19 ± 0.52) | 1 Hz rTMS | DLPFC | 20 min/d,5 d/wk,4 wks | – | ② | On/– |
| Dong et al.43 | 49/49 | 27/22;26/23 | (66.02 ± 4.83)/(65.73 ± 4.97) | (5.81 ± 1.41)/(5.89 ± 1.35) y | 2–3 | 10 Hz rTMS, 90% RMT;1 Hz rTMS, 90% RMT | M1 | 5 d/wk,4 wks | – | ①⑤ | On/– |
| Hong et al.57 | 30/30 | 17/13;18/12 | (68.16 ± 3.97)/(68.34 ± 4.29) | (2.38 ± 0.72)/(2.34 ± 0.86) y | 8/8/7/7/0;9/7/8/6/0 | 2 mA tDCS | DLPFC | 20 min/d,5 d/wk,2 wks | – | ①④ | On/– |
| Wang et al.56 | 43/42 | 26/17;23/19 | (64.41 ± 5.65)/(63.96 ± 6.49) | – | – | 2 mA tDCS | DLPFC | 25 min/d,5 d/wk,3 mons | – | ③④ | On/– |
| Zheng et al.45 | 54/54 | 26/28;24/30 | (66.83 ± 7.86)/(67.70 ± 8.41) | – | 1–3 | 5 Hz rTMS, 110% RMT | DLPFC | 5 d/wk,4 wks | 1 mon | ①③⑤ | On/on |
| Zhou et al.44 | 12/12 | 5/7;6/6 | (70.75 ± 7.83)/(70.42 ± 8.99) | 5(3.25, 9.75);5.5(2.5, 9.75) y | 1/7/2/2/0;2/5/3/2/0 | 1 Hz rTMS, 120% RMT | M1 | 1d/wk, 4wks | – | ① | On/on |
| Wang et al.46 | 41/41 | 30/11;32/9 | (60.52 ± 2.35)/(60.15 ± 2.32) | – | – | 25 Hz rTMS, 90% RMT | DLPFC | 5 d/wk,4 wks | 3 mons | ④⑤ | – |
Data presented as mean ± SD or median (interquartile range, IQR); E/C, E, experiment group/control group; I/E, intervention/evaluation; PFC, prefrontal cortex; RMT, resting motor threshold; ① UPDRS-III; ② MDS-UPDRS-III; ③MMSE; ④ MoCA; ⑤ HDRS; ⑥ BDI-II
Risk of bias of included literatures
42.9% of studies33,35,36,40,45,47–50,53 showed a low overall risk of bias. 53.6%30–32,34,37–39,42–44,46,51,52,54–57 of studies expressed some concerns about the risk of bias. 3.6% of studies41 showed a high overall risk of bias. The risk of bias was mainly due to unclear randomization methods or allocation processes32,39,41,42,51,54, inability to ensure blinding of intervention implementers due to research needs30,31,34,37,42,46,52,56, and uncertainty as to whether the study blinded the outcome assessors31,32,34,37,38,41–44,46,55,57. A summary of the risk of bias is shown in Fig. 2.
Figure 2.
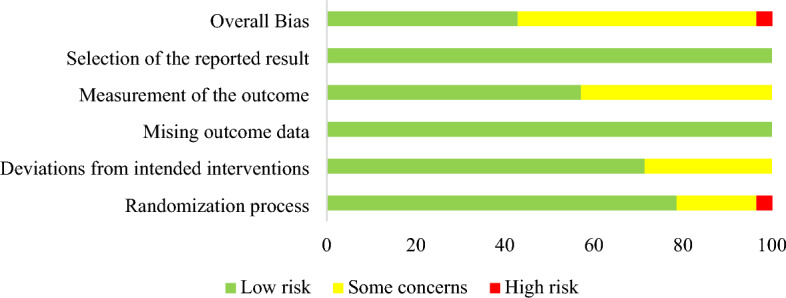
Risk of bias summary.
Assessment of motor function improvement
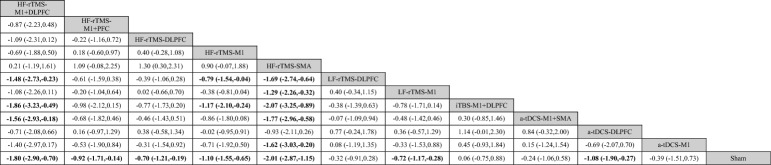
As shown in Fig. 3A, the network meta-analysis reporting motor function in patients with PD contains 12 interventions that form 14 pairs of direct comparisons. The node-splitting method reports that this closed-loop local inconsistency is not significant (Supplementary Table S3). The sham NIBS treatment effect was not statistically different between sham iTBS, sham rTMS, and sham tDCS treatments (P = 0.378) (Supplementary Figure 1). The pairwise meta-analysis of NIBS compared with sham stimulation showed that HF-rTMS over the SMA (SMD = − 2.01; 95% CI [− 2.87, − 1.15]), HF-rTMS over the M1 and DLPFC (SMD = − 1.80; 95% CI [− 2.90, − 0.70]), HF-rTMS over the M1 (SMD = − 1.10; 95% CI [− 1.55, − 0.65]), a-tDCS over the DLPFC (SMD = − 1.08; 95% CI [− 1.90, − 0.27]), HF-rTMS over the M1 and PFC (SMD = − 0.92; 95% CI [− 1.71, − 0.14]), LF-rTMS over the M1 (SMD = − 0.72; 95% CI [− 1.17, − 0.28]), and HF-rTMS over the DLPFC (SMD = − 0.70; 95% CI [− 1.21, − 0.19]) significantly improved motor function (Fig. 4A, Table 2). According to SUCRA, HF-rTMS over the SMA (95.1%) ranked the highest probability of being the best therapy, followed by HF-rTMS over the M1 and DLPFC (89.6%) and HF-rTMS over the M1 (73.0%) (Fig. 5A, Table 3).
Figure 3.
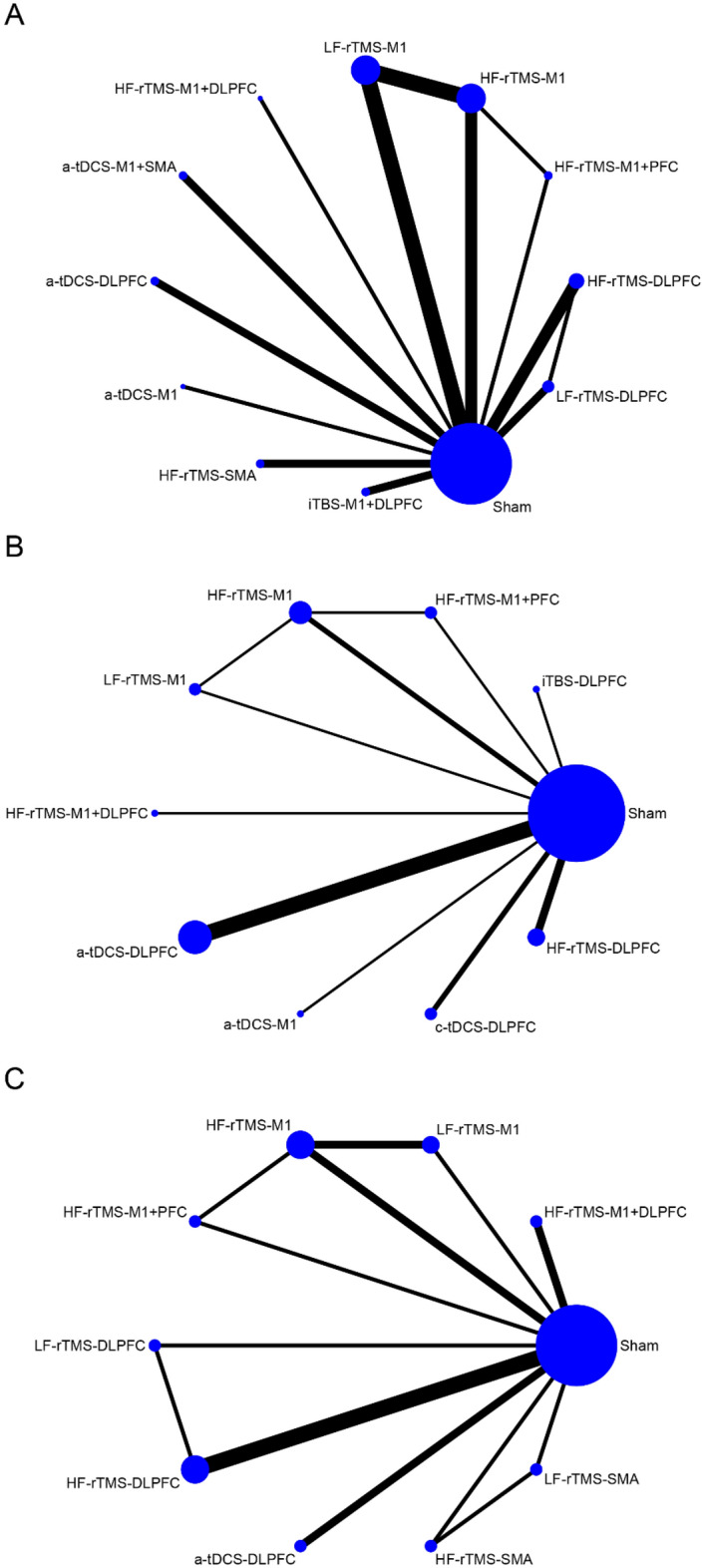
Network relationship plots. (A) motor function (B) cognitive function (C) depression.
Figure 4.
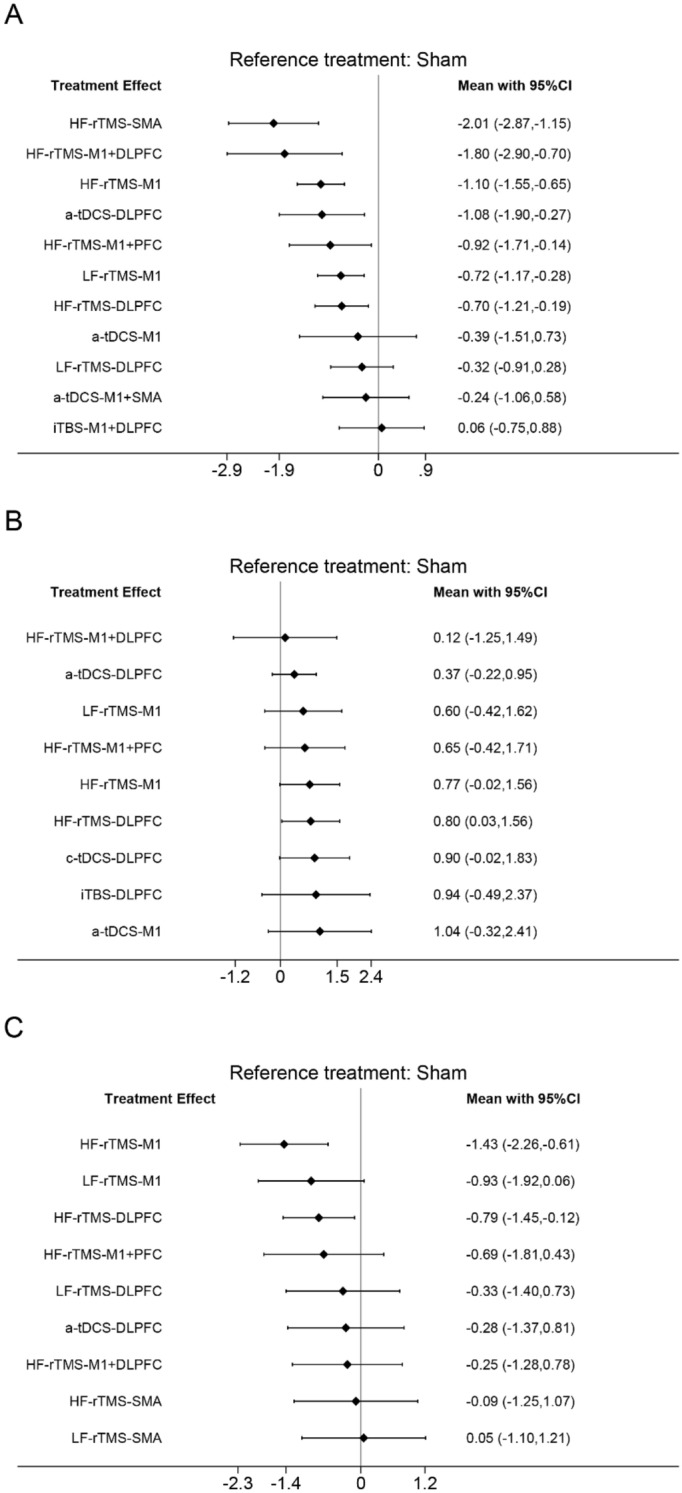
Forest plots for direct comparison with sham stimulation. (A) motor function (B) cognitive function (C) depression.
Table 2.
League table of the changes of motor function.
Bold results marked with indicate statistical significance.
Figure 5.
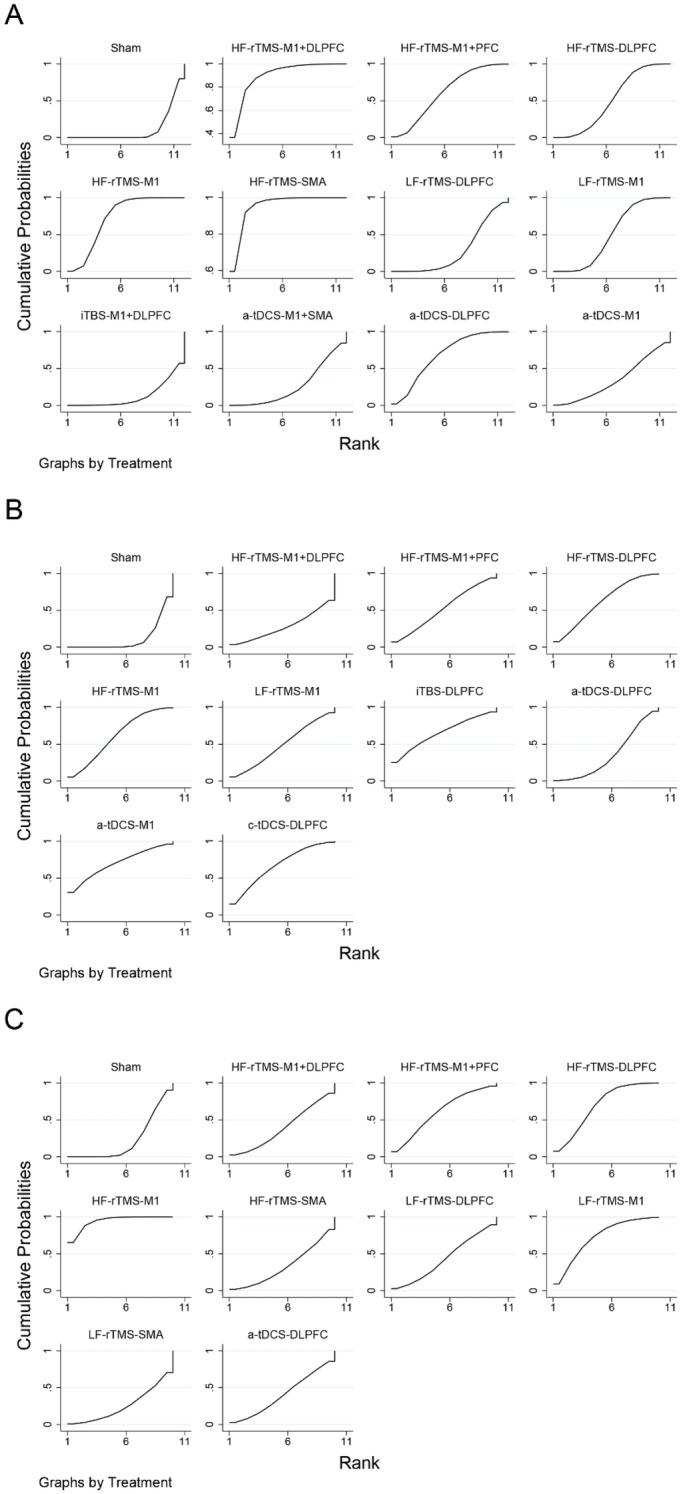
Probability rankings based on SUCRA. (A) motor function (B) cognitive function (C) depression.
Table 3.
SUCRA of the changes of motor function.
| Treatment | SUCRA (%) |
|---|---|
| HF-rTMS-SMA | 95.1 |
| HF-rTMS-M1 + DLPFC | 89.6 |
| HF-rTMS-M1 | 73.0 |
| a-tDCS-DLPFC | 67.7 |
| HF-rTMS-M1 + PFC | 61.1 |
| HF-rTMS-DLPFC | 50.4 |
| LF-rTMS-M1 | 50.0 |
| a-tDCS-M1 | 34.5 |
| LF-rTMS-DLPFC | 28.3 |
| a-tDCS-M1 + SMA | 26.3 |
| iTBS-M1 + DLPFC | 12.6 |
| Sham | 11.3 |
Assessment of cognitive function improvement
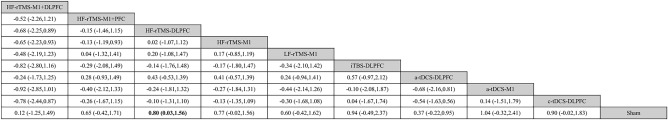
As shown in Fig. 3B, the network meta-analysis reporting cognitive functioning in patients with PD contains 10 interventions that form 11 pairs of direct comparisons. The node-splitting method shows no significant local inconsistency in this network plot (Supplementary Table S4). The difference in the efficacy of sham NIBS treatment was not significant between sham iTBS, sham rTMS, and sham tDCS treatments (P = 0.055) (Supplementary Figure 2). However, the efficacy was significant in the sham tDCS group (SMD = 1.052; 95% CI [0.599, 1.504]). The pairwise meta-analysis with sham stimulation showed that HF-rTMS over the DLPFC (SMD = 0.80; 95% CI [0.03,1.56]) significantly enhanced cognitive function (Fig. 4B, Table 4). The probability of a-tDCS over the M1 (69.8%) being the optimal therapy is the highest according to SUCRA, followed by c-tDCS over the DLPFC (66.9%) and iTBS over the DLPFC (65.3%) (Fig. 5B, Table 5).
Table 4.
League table of the changes of cognitive function.
Bold result marked with indicate statistical significance.
Table 5.
SUCRA of the changes of cognitive function.
| Treatment | SUCRA (%) |
|---|---|
| a-tDCS-M1 | 69.8 |
| c-tDCS-DLPFC | 66.9 |
| iTBS-DLPFC | 65.3 |
| HF-rTMS-DLPFC | 61.5 |
| HF-rTMS-M1 | 60.8 |
| HF-rTMS-M1 + PFC | 52.1 |
| LF-rTMS-M1 | 48.7 |
| a-tDCS-DLPFC | 35.5 |
| HF-rTMS-M1 + DLPFC | 27.9 |
| Sham | 11.3 |
Assessment of depression improvement
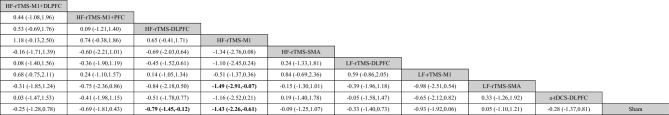
As shown in Fig. 3C, the network meta-analysis reporting depression in patients with PD contained 10 interventions that formed 13 pairwise direct comparisons. The node-splitting method shows that local inconsistency is insignificant in this closed loop (Supplementary Table S5). The sham NIBS treatment effect was not significantly different between sham rTMS and sham tDCS treatments (P = 0.875) (Supplementary Figure 3). The NIBS and sham stimulation pairwise meta-analysis showed that HF-rTMS over the M1 (SMD = − 1.43; 95% CI [− 2.26, − 0.61]) and HF-rTMS over the DLPFC (SMD = − 0.79; 95% CI [− 1.45, − 0.12)]) significantly improved depression (Fig. 4C, Table 6). Based on SUCRA, HF-rTMS over the M1 (94.1%) has the highest probability of being the optimal treatment followed by LF-rTMS over the M1 (71.8%) and HF-rTMS over the DLPFC (69.0%) (Fig. 5C, Table 7).
Table 6.
League table of the changes of depression.
Bold results marked with indicate statistical significance.
Table 7.
SUCRA of the changes of depression.
| Treatment | SUCRA (%) |
|---|---|
| HF-rTMS-M1 | 94.1 |
| LF-rTMS-M1 | 71.8 |
| HF-rTMS-DLPFC | 69.0 |
| HF-rTMS-M1 + PFC | 60.7 |
| LF-rTMS-DLPFC | 43.1 |
| a-tDCS-DLPFC | 40.6 |
| HF-rTMS-M1 + DLPFC | 39.5 |
| HF-rTMS-SMA | 33.2 |
| LF-rTMS-SMA | 25.6 |
| Sham | 22.5 |
Publication bias
Funnel plots using motor function, cognitive function, and depression status as outcome indicators were all generally symmetrical, suggesting no significant publication bias (Fig. 6A–C).
Figure 6.
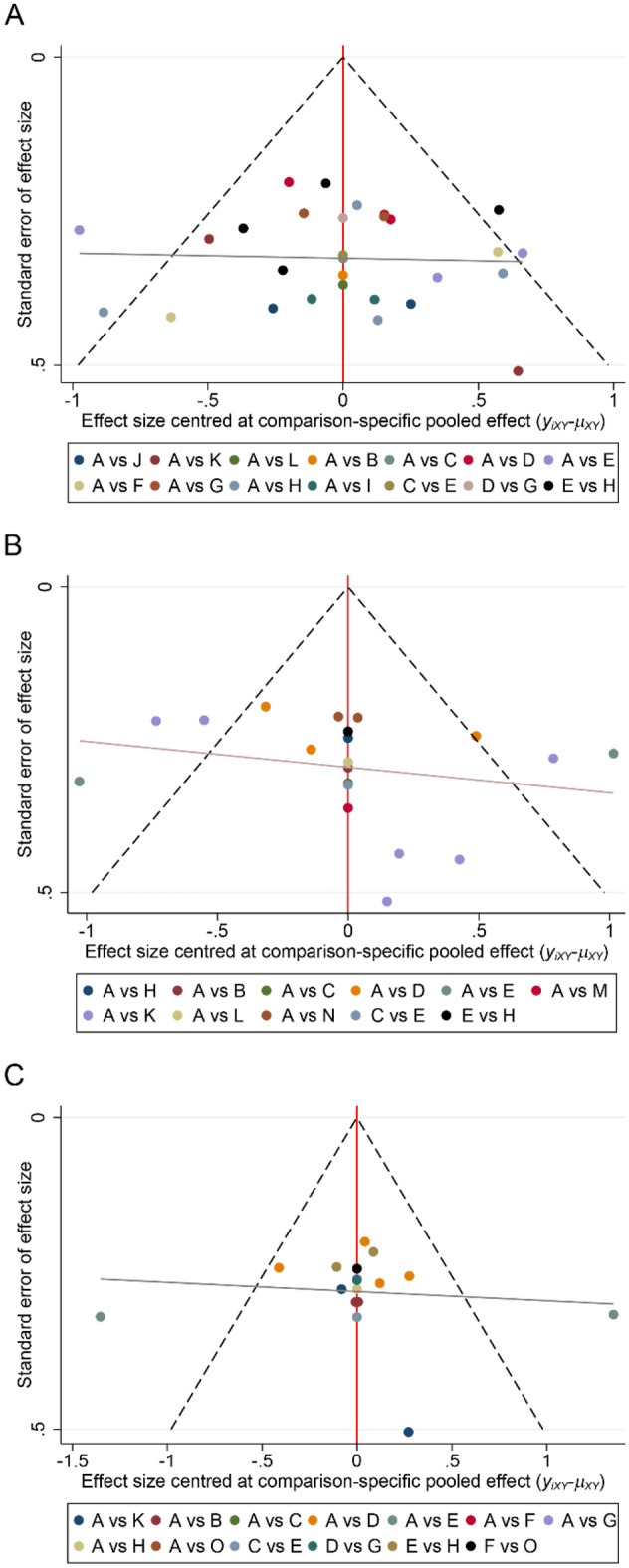
Funnel plots. (A) motor function (B) cognitive function (C) depression. A, Sham; B, HF-rTMS-M1 + DLPFC; C, HF-rTMS-M1 + PFC; D, HF-rTMS-DLPFC; E, HF-rTMS-M1; F, HF-rTMS-SMA; G, LF-rTMS-DLPFC; H, LF-rTMS-M1; I, iTBS-M1 + DLPFC; J, a-tDCS-M1 + SMA; K, a-tDCS-DLPFC; L, a-tDCS-M1; M, iTBS-DLPFC; N, c-tDCS-DLPFC; O, LF-rTMS-SMA.
GRADE ratings
The results of the GRADE evaluation are shown in Table 8. In summary, the overall quality of the overall evidence was low to moderate. It was mainly due to some risk of bias in the included studies, 95% confidence intervals crossing the clinical decision threshold, and some heterogeneity among the combined studies, which affected the scientific validity of the research methodology and the reliability of the findings.
Table 8.
GRADE evaluation quality of evidence.
| Comparisons | Study limitations | Imprecision | Inconsistency | Indirectness | Publication bias | GRADE |
|---|---|---|---|---|---|---|
| HF-rTMS-M1 + DLPFC versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| HF-rTMS-M1 + PFC versus Sham | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| HF-rTMS-M1 + PFC versus HF-rTMS-M1 | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| HF-rTMS-DLPFC versus Sham | No downgrade | No downgrade | Downgraded because I2 > 50% | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| HF-rTMS-DLPFC versus LF-rTMS-DLPFC | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| HF-rTMS-M1 versus Sham | No downgrade | No downgrade | Downgraded because I2 > 50% | No downgrade | Downgraded because of incomplete symmetry of scatter points in the funnel plot |
⊕⊕◯◯ Low |
| HF-rTMS-M1 versus LF-rTMS-M1 | No downgrade | Downgraded because 95% CI passes through the equivalence line | Downgraded because I2 > 50% | No downgrade | Downgraded because of incomplete symmetry of scatter points in the funnel plot |
⊕◯◯◯ Very Low |
| HF-rTMS-SMA versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | Downgraded because I2 > 50% | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| HF-rTMS-SMA versus LF-rTMS-SMA | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| LF-rTMS-DLPFC versus Sham | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| LF-rTMS-M1 versus Sham | Downgraded because moderate RoB2 comparisons > 70% | No downgrade | Downgraded because I2 > 50% | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| iTBS-M1 + DLPFC versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| a-tDCS-M1 + SMA versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| a-tDCS-DLPFC versus Sham | Downgraded because moderate RoB2 comparisons > 70% | No downgrade | Downgraded because I2 > 50% | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| a-tDCS-M1 versus Sham | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| iTBS-DLPFC versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
| c-tDCS-DLPFC versus Sham | Downgraded because moderate RoB2 comparisons > 70% | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕◯◯ Low |
| LF-rTMS-SMA versus Sham | No downgrade | Downgraded because 95% CI passes through the equivalence line | No downgrade | No downgrade | No downgrade |
⊕⊕⊕◯ Moderate |
We grade based on the following criteria estimates.
(1) Study limitations: We downgraded by one level when the contributions from low RoB2 comparisons were less than 30% and contributions from moderate RoB2 comparisons were 70% or greater.
(2) Imprecision: We determined whether the confidence intervals crossed the clinical decision thresholds for recommended and non-recommended treatments. If it crossed it was downgraded for imprecision.
(3) Inconsistency: We based our ratings on heterogeneity tests and inconsistency tests. Downgrade if there is significant heterogeneity (I2 > 50%) or inconsistency (P < 0.05).
(4) Indirectness: We analyzed the efficacy of different sham NIBS by pairwise meta-analysis methods to ensure network transitivity. The results of our analysis proved the transitivity (P > 0.05).
(5) Publication bias: We assessed this based on the symmetry of the comparison-correction funnel plot and the funding sources and stakes of the included study.
Discussion
This study is based on 28 RCTs using network meta-analysis to assess the efficacy of different NIBS in the treatment of PD and to help in choosing the best option for clinical treatment. We found that most NIBS protocols improved motor function in patients with PD. Specifically, HF-rTMS over the SMA was found to be most effectively associated with improved motor function. In terms of cognitive function, SUCRA results showed that a-tDCS over the M1 was considered most effectively associated with its improvement. Notably, the results of pairwise meta-analysis showed that only HF-rTMS over the DLPFC was significantly more efficacious than the sham stimulation group in the different NIBS. HF-rTMS over the M1 was found to be most effectively associated with improved depression.
A primary finding of the study results was that HF-rTMS was effective in improving motor dysfunction in patients with PD, which is consistent with the conclusions of a previous network meta-analysis58. We further comparatively investigated the target areas of action of rTMS and found that SMA may be more effective in the treatment of motor disorders. SMA is a key brain region that connects the motor and cognitive nervous systems and plays an important role in motor preparation and control59. SMA dysfunction is considered to be an important cause of continuous motor abnormalities and gait disturbances in PD patients. Resting-state functional magnetic resonance imaging study showed significant differences in functional connectivity in sensorimotor, insula, and cerebellum networks between PD patients and healthy individuals60.
The second primary finding of the study results is that a-tDCS over the M1 and HF-rTMS over the M1 may be better for cognition and depression separately. However, there was no statistically significant difference in efficacy between a-tDCS over the M1 compared to the sham stimulation group. Therefore, these findings should be interpreted cautiously to ensure that future large-scale randomized controlled trials provide additional evidence. Patients with PD suffer from dopamine neuronal damage in the dense midbrain substantia nigra and dopamine deficiency in the striatum61. The substantia nigra contains the largest network of dopaminergic cells in the brain and is involved in the regulation of motor, emotional and cognitive behavior62. It was found that rTMS over the M1 region induced endogenous dopamine release in the ventral striatum, which may be its intrinsic mechanism for the treatment of PD63. In addition, HF-rTMS over the DLPFC demonstrated favorable improvement in cognition and depression. DLPFC is a core brain region of the central executive network, which is closely related to executive function, attention, and visuospatial ability. It was shown that mood changes in PD patients may be closely related to decreased activity in the left DLPFC. There is still a need for in-depth research on the mechanism of action of NIBS to improve PD, to reveal the scientific basis of its efficacy from neurophysiological and biochemical perspectives, and to conduct large-scale comparative efficacy studies on different targets.
Potential limitations of this study are: (1) inconsistencies in patient age, duration of illness, and severity among the studies included in the analysis may have increased study heterogeneity and affected the results of the analysis; (2) most of the included studies did not explicitly report or implement allocation concealment processes, and more than half of the studies did not implement evaluator blinding; (3) due to language limitations, the literature included in the present study covered only the English and Chinese literature, there is a possibility of incomplete search.
Conclusions
In summary, HF-rTMS over the SMA may be the best option for improving motor symptoms in PD patients. a-tDCS and HF-rTMS over the M1 may be the NIBS with the most significant effects on cognition and depression, separately. A large number of future RCTs are needed to investigate the efficacy of NIBS in patients with Parkinson's disease and the optimal combination of appropriate parameters, including stimulation frequency and stimulation target.
Supplementary Information
Abbreviations
- NIBS
Noninvasive brain stimulation
- PD
Parkinson’s disease
- rTMS
Repetitive transcranial magnetic stimulation
- iTBS
Intermittent theta-burst stimulation
- tDCS
Transcranial direct current stimulation
- UPDRS-III
The motor section of the Unified Parkinson's Disease Rating Scale
- MDS-UPDRS-III
The motor section of the Movement Disorder Society Unified Parkinson's Disease Rating Scale
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- BDI
Beck Depression Inventory
- HDRS
Hamilton Depression Rating Scale
Author contributions
Y.W. conceived the theme of the study. Y.W. and Y.D. performed the systematic search, reviewed the literature, and extracted the data. Y.W. and C.G. analyzed data and wrote the first draft of the paper. Y.W. and Y.D. checked and modified the manuscript. All authors read and approved the final manuscript.
Funding
Shandong Traditional Chinese Medicine Science and Technology Development Programs (Q-2023067, Q-2023069).
Data availability
Data is provided within the manuscript or supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Ding, Email: 1253038007@qq.com.
Chenchen Guo, Email: 1071955451@qq.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64196-0.
References
- 1.Hayes MT. Parkinson's Disease and Parkinsonism. Am. J. Med. 2019;132:802–807. doi: 10.1016/j.amjmed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, et al. Efficacy of repetitive transcranial magnetic stimulation in Parkinson's disease: A systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine. 2022;52:101589. doi: 10.1016/j.eclinm.2022.101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsworth JD. Parkinson's disease treatment: past, present, and future. J. Neural. Transm (Vienna) 2020;127:785–791. doi: 10.1007/s00702-020-02167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. 2021;397:2284–2303. doi: 10.1016/s0140-6736(21)00218-x. [DOI] [PubMed] [Google Scholar]
- 5.Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin. Geriatr. Med. 2020;36:1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong MJ, Okun MS. Diagnosis and treatment of parkinson disease: A review. Jama. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J, Tan EK. Parkinson's disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 8.Sun C, Armstrong MJ. Treatment of Parkinson's disease with cognitive impairment: current approaches and future directions. Behav. Sci. (Basel) 2021;11:54. doi: 10.3390/bs11040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino G, Todisco M, Blandini F. Noninvasive neuromodulation in Parkinson's disease: Neuroplasticity implication and therapeutic perspectives. Handb. Clin. Neurol. 2022;184:185–198. doi: 10.1016/b978-0-12-819410-2.00010-2. [DOI] [PubMed] [Google Scholar]
- 10.Mosilhy EA, et al. Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sci. 2022;307:120869. doi: 10.1016/j.lfs.2022.120869. [DOI] [PubMed] [Google Scholar]
- 11.Udupa K, Bhattacharya A, Bhardwaj S, Pal PK, Chen R. Parkinson's disease: Alterations of motor plasticity and motor learning. Handb. Clin. Neurol. 2022;184:135–151. doi: 10.1016/b978-0-12-819410-2.00007-2. [DOI] [PubMed] [Google Scholar]
- 12.Xiao H, et al. Non-invasive brain stimulation for treating catatonia: a systematic review. Front Psychiatry. 2023;14:1135583. doi: 10.3389/fpsyt.2023.1135583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randver R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: A review and clinical implications. J. Neurol. Sci. 2018;393:88–99. doi: 10.1016/j.jns.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Xu N, Wang R, Zai W. Systematic review and network meta-analysis of effects of noninvasive brain stimulation on post-stroke cognitive impairment. Front. Neurosci. 2022;16:1082383. doi: 10.3389/fnins.2022.1082383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hett D, Marwaha S. Repetitive transcranial magnetic stimulation in the treatment of bipolar disorder. Ther. Adv. Psychopharmacol. 2020;10:2045125320973790. doi: 10.1177/2045125320973790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuteboom D, et al. Accelerated intermittent theta burst stimulation in major depressive disorder: A systematic review. Psychiatry Res. 2023;327:115429. doi: 10.1016/j.psychres.2023.115429. [DOI] [PubMed] [Google Scholar]
- 17.Chu M, et al. Efficacy of intermittent theta-burst stimulation and transcranial direct current stimulation in treatment of post-stroke cognitive impairment. J. Integr. Neurosci. 2022;21:130. doi: 10.31083/j.jin2105130. [DOI] [PubMed] [Google Scholar]
- 18.Rachid F. Safety and efficacy of theta-burst stimulation in the treatment of psychiatric disorders: A review of the literature. J Nerv Ment Dis. 2017;205:823–839. doi: 10.1097/nmd.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 19.Sadler CM, Kami AT, Nantel J, Lommen J, Carlsen AN. Transcranial direct current stimulation over motor areas improves reaction time in Parkinson's disease. Front Neurol. 2022;13:913517. doi: 10.3389/fneur.2022.913517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A. Noninvasive brain stimulation for Parkinson's disease and dystonia. Neurotherapeutics. 2008;5:345–361. doi: 10.1016/j.nurt.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu YL, Xi C. Application of different stimulation targets in the rehabilitation of Parkinson′s disease mediated by transcranial direct current stimulation. Rehabil. Med. 2023;33:180–185. doi: 10.3724/SP.J.1329.2023.02014. [DOI] [Google Scholar]
- 22.Chen KS, Chen R. Invasive and noninvasive brain stimulation in Parkinson's disease: Clinical effects and future perspectives. Clin. Pharmacol. Ther. 2019;106:763–775. doi: 10.1002/cpt.1542. [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutton B, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern Med. 2015;162:777–784. doi: 10.7326/m14-2385. [DOI] [PubMed] [Google Scholar]
- 25.Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023). Available at: www.training.cochrane.org/handbook. Accessed August 21, 2023.
- 27.Tseng PT, et al. The beneficial effect on cognition of noninvasive brain stimulation intervention in patients with dementia: A network meta-analysis of randomized controlled trials. Alzheimers Res. Ther. 2023;15:20. doi: 10.1186/s13195-023-01164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng YC, et al. Effectiveness and acceptability of noninvasive brain and nerve stimulation techniques for migraine prophylaxis: A network meta-analysis of randomized controlled trials. J. Headache Pain. 2022;23:28. doi: 10.1186/s10194-022-01401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani A, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/s0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y. Supplementary motor area stimulation for Parkinson disease: A randomized controlled study. Neurology. 2013;80:1400–1405. doi: 10.1212/WNL.0b013e31828c2f66. [DOI] [PubMed] [Google Scholar]
- 31.Li B. Evaluation on curative effect and safety of low frequency and high frequency repetitive transcranial magnetic stimulation in treatment of Parkinson disease. China Foreign Med. Treat. 2016;14:188–190. doi: 10.16662/j.cnki.1674-0742.2016.14.188. [DOI] [Google Scholar]
- 32.Yu WW, et al. Clinical investigation of repetitive transcranial magnetic stimulation on treating depression and sleep disorder in patients with Parkinson’s disease in early stage. J. Clin. Neurol. 2017;30:341–345. doi: 10.3969/j.issn.1004-1648.2017.05.007. [DOI] [Google Scholar]
- 33.Khedr EM, et al. The effect of 20 Hz versus 1 Hz repetitive transcranial magnetic stimulation on motor dysfunction in Parkinson's disease: Which is more beneficial? J Parkinsons Dis. 2019;9:379–387. doi: 10.3233/jpd-181540. [DOI] [PubMed] [Google Scholar]
- 34.Yang HW, Liu YH, Zhang YL. Clinical study of the effect of transcranial magnetic stimulation on Parkinson's syndrome after stroke. Chin. J. Integr. Med. Cardio/Cerebrovasc. Dis. 2019;17:453–455. doi: 10.12102/j.issn.1672-1349.2019.03.038. [DOI] [Google Scholar]
- 35.Mi TM, et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson's disease: A randomized controlled trial. Parkinsonism Relat. Disord. 2019;68:85–90. doi: 10.1016/j.parkreldis.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Chung CL, Mak MK, Hallett M. Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann. Neurol. 2020;88:933–945. doi: 10.1002/ana.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F. Observation on the application effect of high-frequency repetitive transcranial magnetic stimulation in patients with Parkinson's disease. J. Med. Forum. 2020;41:98–101. [Google Scholar]
- 38.Lai JH, et al. Effects of high-frequency rtms on limb movement and sleep in patients with Parkinson’s disease. World J. Sleep Med. 2020;7:1861–1863. doi: 10.3969/j.issn.2095-7130.2020.11.001. [DOI] [Google Scholar]
- 39.Spagnolo F, et al. Bilateral repetitive transcranial magnetic stimulation with the H-coil in Parkinson's disease: A randomized, Sham-controlled study. Front. Neurol. 2020;11:584713. doi: 10.3389/fneur.2020.584713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aftanas LI, et al. Clinical and neurophysiological effects of the therapeutic combination of high-frequency rhythmic transcranial magnetic stimulation of the motor and frontal cortex in Parkinson’s disease. Neurosci. Behav. Physiol. 2021;51:135–141. doi: 10.1007/s11055-021-01048-8. [DOI] [Google Scholar]
- 41.Liao ZZ, Yuan LJ, Tang XY, Chen ZS, Jiang W. Clinical observation of high frequency repetitive transcranial magnetic stimulation on mild cognitive impairment in early Parkinson's disease. J. Clin. Neurol. 2021;34:32–36. doi: 10.3969/j.issn.1004-1648.2021.01.009. [DOI] [Google Scholar]
- 42.Chen, Y., Zhao, J., Wan, Z. H. & Miao, G. Z. The effect of low frequency repetitive transcranial magnetic stimulation on anxiety and insomnia in patients with Parkinson’s disease. Neural Injury Funct. Reconstr.17, 449–451, 475. 10.16780/j.cnki.sjssgncj.20211034 (2022).
- 43.Dong LL, Liu BW, Xie Y. Comparison of the clinical effects of low-frequency and high-frequency repetitive transcranial magnetic stimulation in the treatment of patients with Parkinson's disease. China Health Care Nutr. 2022;32:64–66. [Google Scholar]
- 44.Zhou ZC, et al. Effect of low-frequency repetitive transcranial magnetic stimulation on Parkinson's disease patient's movement disorder. China Modern Med. 2023;30:87–89. doi: 10.3969/j.issn.1674-4721.2023.04.022. [DOI] [Google Scholar]
- 45.Zheng XQ, et al. Effects of high-frequency transcranial magnetic stimulation on cellular senescence in Parkinson′s disease. Chin. J. Phys. Med. Rehabil. 2022;44:427–432. doi: 10.3760/cma.j.issn.0254-1424.2022.05.010. [DOI] [Google Scholar]
- 46.Wang YS, Ma H, Zhang K. Clinical effect of high-frequency repetitive transcranial magnetic stimulation in the treatment of cognitive dysfunction in patients with Parkinson's disease. Clin. Res. 2024;32:82–85. doi: 10.12385/j.issn.2096-1278(2024)02-0082-04. [DOI] [Google Scholar]
- 47.Benninger DH, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology. 2011;76:601–609. doi: 10.1212/WNL.0b013e31820ce6bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He W, Wang JC, Tsai PY. Theta burst magnetic stimulation improves Parkinson's-related cognitive impairment: A randomised controlled study. Neurorehabil. Neural Repair. 2021;35:986–995. doi: 10.1177/1545968321104131. [DOI] [PubMed] [Google Scholar]
- 49.Benninger DH, et al. Transcranial direct current stimulation for the treatment of Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2010;81:1105–1111. doi: 10.1136/jnnp.2009.202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biundo R, et al. Double-blind randomized trial of tDCS versus sham in Parkinson patients with mild cognitive impairment receiving cognitive training. Brain Stimul. 2015;8:1223–1225. doi: 10.1016/j.brs.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 51.Li X, et al. Transcranial direct current stimulation on cognitive function in patients with early untreated Parkinson's disease and auditory event-related potentials. Chin. J. Phys. Med. Rehabil. 2018;40:198–201. doi: 10.3760/cma.j.issn.0254-1424.2018.03.009. [DOI] [Google Scholar]
- 52.Sun L, Wang S, Ye W, Dan M. Clinical efficacy of transcranial direct current stimulation combined with cognitive training in the improvement of cognitive impairment in Parkinson disease. Chin. J. Rehabil. 2020;35:308–311. doi: 10.3870/zgkf.2020.06.007. [DOI] [Google Scholar]
- 53.Wu SP, Li X, Qi YW, Wang H, Ma JJ. The influence of transcranial stimulation on rapid eye movement sleep disorders among persons with Parkinson′s disease. Chin. J. Phys. Med. Rehabil. 2020;42:50–54. doi: 10.3760/cma.j.issn.0254-1424.2020.01.012. [DOI] [Google Scholar]
- 54.Hu XL, Xue CP, Liu ZS. Effectiveness of transcranial direct current stimulation-assisted functional rehabilitation training on the rehabilitation of patients with Parkinson's disease. Chin. J. Gerontol. 2021 doi: 10.3969/j.issn.1005-9202.2021.17.027. [DOI] [Google Scholar]
- 55.Lee SA, Kim MK. The effect of transcranial direct current stimulation combined with visual cueing training on motor function, balance, and gait ability of patients with Parkinson's disease. Medicina (Kaunas) 2021;57:1. doi: 10.3390/medicina57111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C, Niu DW, Wu WB, Hu CS. The effect of transcranial direct current stimulation combined with personalized rehabilitation education on mood, cognitive function and three-dimensional gait in patients with Parkinson's disease. J. Int. Psychiatry. 2022;49:904–907. [Google Scholar]
- 57.Hong, D. H. et al. Effects of rehabilitation exercise training combined with transcranial direct current stimulation on walking function, balance function and cognitive function in patients with Parkinson’s disease. Prog. Mod. Biomed.22, 2575–2578, 2563. 10.13241/j.cnki.pmb.2022.13.034 (2022).
- 58.Liu X, Li L, Liu Y. Comparative motor effectiveness of non-invasive brain stimulation techniques in patients with Parkinson's disease: A network meta-analysis. Medicine (Baltimore) 2023;102:e34960. doi: 10.1097/md.0000000000034960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li RZ, et al. Research progress on potential brain stimulation targets of rTMS for alleviating motor symptoms in Parkinson's disease. Chin. J. Biomed. Eng. 2023;42:345–352. doi: 10.3969/j.issn.0258-8021.2023.03.011. [DOI] [Google Scholar]
- 60.Wang S, Zhang Y, Lei J, Guo S. Investigation of sensorimotor dysfunction in Parkinson disease by resting-state fMRI. Neurosci. Lett. 2021;742:135512. doi: 10.1016/j.neulet.2020.135512. [DOI] [PubMed] [Google Scholar]
- 61.McGregor MM, Nelson AB. Circuit mechanisms of Parkinson's disease. Neuron. 2019;101:1042–1056. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, He YJ, Ji XX, Chen XG. Advances in the correlation between pathological changes in the brainstem and symptomatology in Alzheimer's disease. J. Clin. Neurol. 2019;32:231–233. doi: 10.3969/j.issn.1004-1648.2019.03.019. [DOI] [Google Scholar]
- 63.Ohnishi T, et al. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol. Psychiatry. 2004;55:484–489. doi: 10.1016/j.biopsych.2003.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.