Abstract
Antibodies clear Sindbis virus from infected animals through an unknown mechanism. To determine whether interferon-induced pathways are required for this clearance, we examined mice which are unable to respond to alpha/beta interferon or gamma interferon. Although extremely susceptible to infection, such mice survived and completely cleared virus if antibodies against Sindbis virus were given.
Sindbis virus (SV) is an arbovirus of the Togaviridae family and Alphavirus genus which can cause fatal encephalitis in mice. SV virions contain an encapsidated RNA genome which acquires a lipid envelope by attaching to the cytoplasmic tails of viral transmembrane glycoproteins and then budding from the plasma membrane. During infection of mice, antiviral antibodies are required for clearance of SV from the central nervous system (CNS), and exogenous antibodies against SV can protect against infections that would otherwise be fatal (9, 15). Treatment of persistently infected SCID mice with antibody against the E2 glycoprotein of SV results in the gradual, noncytopathic removal of viral RNA from neurons, a process which requires neither T cells nor complement (9). Antibodies can also clear SV from persistently infected neuronal cultures (9, 18); neutralization alone is insufficient to explain this clearance, because antibody does not need to be continuously present in culture. The isotype of antibody is unimportant, but divalency is required (18). It appears that clearance involves a novel mechanism triggered when antibody cross-links SV glycoproteins expressed on infected cells (5, 18).
The replication of SV is highly sensitive to alpha/beta interferon (IFN-α/β) in cultured cells (2), and SV is also known to induce the production of large amounts of IFN-α/β in animals, particularly in neonatal mice, where the virus is able to replicate to high levels (7, 14, 20). Mice deficient in the receptor for IFN-α/β show extreme susceptibility to many viruses, including the alphaviruses Semliki Forest virus and Venezuelan equine encephalitis virus (4, 8, 12). In these mice virus replicates to extremely high levels within a short period of time, indicating a vital role for IFN-α/β in controlling viral replication during the early stages of infection. A previous study of normal mice has shown that inducing an IFN response can synergize with antibodies to protect against fatal infection with Semliki Forest virus (1). One possible means for control of infection by antibody might be through some of the same antiviral pathways induced by IFN-α/β, and in vitro experiments performed in our laboratory indicate that antibody against SV can improve the response of infected cells to IFN-α/β (2). We therefore examined the behavior of SV in mice unable to respond to IFN and determined whether antibodies against SV could effectively control viral replication and protect such mice from death.
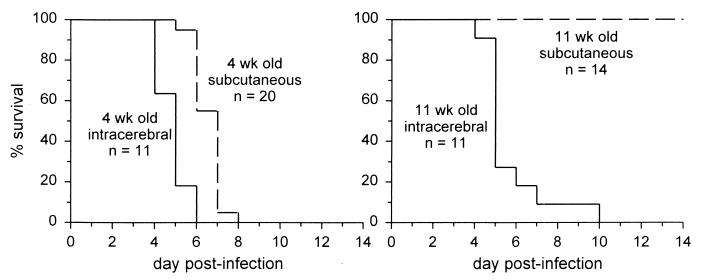
SV strain Toto 1101 (13) was grown and titers were determined on BHK cells. A129 mice on the 129/SvEv genetic background (12) were obtained from B&K Universal Ltd., Hull, United Kingdom, and bred in a specific-pathogen-free facility. A129 mice lack a functional receptor for IFN-α/β but have normal antibody responses following immunization or viral infection (16, 19). Four-week-old control 129/SvEv mice were obtained from Taconic (Germantown, N.Y.) and were found to survive infection with 1,000 PFU of Toto 1101–a relatively avirulent strain of SV (10, 14)–whether virus was given by subcutaneous (s.c.) injection in the back or by intracerebral (i.c.) injection (10 mice per group). By contrast, 4-week-old A129 mice all died after infection (Fig. 1), with significantly faster death after an i.c. infection than after an s.c. infection.
FIG. 1.
Differences in percentages of survival of young and old A129 mice. A129 mice lack the receptor for IFN-α/β. Mice were injected s.c. or i.c. with 1,000 PFU of Toto 1101 in 30 μl of Hanks' balanced salt solution. Survival was assessed daily. For 4-week-old mice, there was a significant difference between the survival curves following s.c. and i.c. infection (P < 0.05, log rank test). When 11-week-old mice were injected s.c. with virus, all mice survived, and this was significantly different from the result with 11-week-old mice injected i.c. as well as with 4-week-old mice injected s.c. (P < 0.05).
Because susceptibility to alphaviruses is known to decrease with age (6, 7), we repeated these experiments in 11-week-old A129 mice. Although after an i.c. infection these older A129 mice died with a time course similar to that seen in 4-week-old mice, all 11-week-old A129 mice were able to survive s.c. infection (Fig. 1). These s.c. infected older mice showed transient signs of illness, such as ruffled fur and reduced movement (although paralysis was never seen), and then completely recovered. Clearance of virus to below detectable levels (Fig. 2B and C) demonstrated that endogenous production of antibody was sufficient to control infection even in the absence of an IFN-α/β response. Recovered mice were able to survive a later i.c. infection with SV (not shown), further indicating that protective immunity had developed.
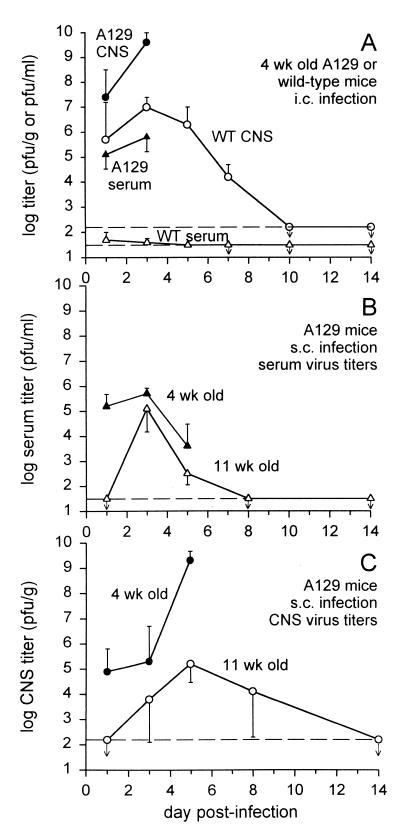
FIG. 2.
Viral titers in the serum or CNS. (A) Four-week-old A129 or wild-type 129/SvEv mice were infected i.c., and viral titers were assessed by plaque assay on BHK cells (three to four mice per time point; geometric means ± standard deviations). Mice were perfused with phosphate-buffered saline to remove blood-borne virus before brains were harvested. While wild-type (WT) mice cleared virus completely and showed no clinical signs of infection, A129 mice had very high titers of virus and died around day 4 or 5. Horizontal dashed lines indicate the limit of sensitivity of the assay (30 PFU/ml for serum and 170 PFU/g for the CNS), and arrows indicate values below the detection limit. (B and C) Following s.c. injection of virus, replication was slower and less extensive in older mice and virus was eventually cleared. Younger mice developed extremely high titers in their CNSs and died around day 6 or 7. Wild-type mice did not have any detectable virus in their sera or CNSs after s.c. infection (not shown).
When the titers of virus were measured in the serum or CNS following i.c. infection, A129 mice were found to have much more virus than control wild-type 129/SvEv mice (Fig. 2A). Wild-type mice were able to completely clear virus after an i.c. infection, a process mediated by antiviral antibodies (9). Four-week-old A129 mice developed particularly high viral titers in the CNS (>109 PFU/g), and virus reached this level more quickly after an i.c. infection than after an s.c. infection, which correlated with faster death after an i.c. infection. Interestingly, CNS viral titers did not rise to such levels in s.c. infected 11-week-old A129 mice (Fig. 2C), suggesting that these older mice survived because virus did not replicate as extensively in the CNS. This may support previous proposals that age-related resistance to SV could be a result of decreased levels of viral receptors on neurons (17).
To determine whether exogenous antibody against SV could prevent fatal infection of A129 mice, we injected mice intraperitoneally with monoclonal antibody G5 (Table 1). G5 is a neutralizing mouse immunoglobulin G1 (IgG1) antibody specific for the E2 glycoprotein of SV and can completely clear SV from persistently infected neuronal cultures (18). This antibody was derived as an isotype-switch variant of the IgG3 monoclonal antibody 209, which possesses identical antiviral properties (18). G5 and a control IgG1 antibody recognizing herpes simplex virus type 2 (3E1 from the American Type Culture Collection, Manassas, Va.) were purified from ascites fluid using a T-Gel kit from Pierce (Rockford, Ill.). In preliminary experiments, we chose a dose of 200 μg of G5 when this was found to be sufficient to protect 100% of 4-week-old A129 mice from s.c. infection with Toto 1101. The amount of antibody required was similar to amounts used in a previous study of normal mice (11), where 100 μg of antibody 209 could provide nearly complete protection against i.c. infection with a neurovirulent strain of SV. G5 antibody provided significant protection against fatal s.c. infection in A129 mice even if it was administered up to 24 h after infection (Table 1), at a point when virus was replicating extensively. G5 also protected significantly against i.c. infection, although it was less effective (Table 1). Serum viral titers were lowered significantly when i.c. infected mice were simultaneously given G5, but the effect on CNS viral titers was less pronounced (Fig. 3). G5-protected mice which survived i.c. infection were found to have undetectable levels of virus in the serum and CNS.
TABLE 1.
Survival of 4-week-old A129 mice after s.c. and i.c. infection with SVa
| Route of infection | Antibody | Time of antibody injection | No. of mice that survived/total no. of mice | % of mice that survived |
|---|---|---|---|---|
| s.c. | None | 0/20 | 0 | |
| 3E1 (control) | No delay | 0/10 | 0 | |
| G5 | No delay | 5/5* | 100 | |
| G5 | 3-h delay | 11/13* | 85 | |
| G5 | 24-h delay | 12/13* | 92 | |
| i.c. | None | 0/11 | 0 | |
| G5 | No delay | 5/17* | 29 |
Four-week-old A129 mice were infected s.c. or i.c. with 1,000 PFU of Toto 1101. Some mice were injected intraperitoneally with 200 μg of purified G5 or 3E1 monoclonal antibody. Survival was assessed daily. ∗, P < 0.05 versus results of infection without antibody (log rank test of survival curves with correction for multiple comparisons).
FIG. 3.
Treatment of i.c. infected A129 mice with antiviral antibody lowers viral titers. Mice (4 weeks old) were injected i.c. with virus, and one group was simultaneously given 200 μg of G5 intraperitoneally. Antibody lowered viral titers in the serum by more than 3 orders of magnitude (P < 0.0001, two-way analysis of variance) but had less of an effect on viral replication in the CNS (P = 0.0502).
Because A129 mice can produce and respond to IFN-γ, a cytokine which upregulates many of the same antiviral genes as IFN-α/β, we performed similar experiments with mice deficient in STAT1 (3). This protein is required for IFN signal transduction, and STAT1-deficient mice are completely unable to respond to either IFN-α/β or IFN-γ. STAT1-deficient mice on a C57B1/6 genetic background were bred in a specific-pathogen-free facility and used for experiments at 4 weeks of age. All mice died after s.c. infection (n = 13) (Fig. 4) or i.c. infection (n = 7) with 1,000 PFU of Toto 1101. Although infected STAT1-deficient mice died faster than A129 mice, it was not possible to compare results directly because the STAT1-deficient mice were on a C57B1/6 background and A129 mice were on a 129/SvEv background (wild-type C57B1/6 mice survived infection by both routes). Thus, we do not know whether IFN-γ itself plays a significant antiviral role in the absence of IFN-α/β. However, G5 antibody was able to protect STAT1-deficient mice against s.c. infection (Fig. 4). Antibody-mediated protection from SV therefore requires neither IFN-α/β nor IFN-γ.
FIG. 4.
Antibody protects s.c. infected STAT1-deficient mice. STAT1-deficient mice do not respond to either IFN-α/β or IFN-γ. Four-week-old mice were infected with 1,000 PFU of Toto 1101 by s.c. injection. Some mice were also injected intraperitoneally with 200 μg of G5. Survival curves for both groups of G5-injected mice were significantly different from the survival curve after injection with virus alone (P < 0.05, log rank test).
From these results, the role of endogenous IFN-α/β is to limit SV replication during the few days before a specific antibody response develops. This is particularly true for the CNS, where SV replicates extremely well. We have further shown that exogenously added antiviral antibody can control SV and promote survival of mice even in the complete absence of IFNs, which therefore cannot be a necessary part of the mechanism of action of antiviral antibody.
Acknowledgments
This work was supported by a postdoctoral fellowship from the National Multiple Sclerosis Society (A.P.B.) and grant R01-NS18596 from the National Institutes of Health (D.E.G.).
REFERENCES
- 1.Coppenhaver D H, Singh I P, Sarzotti M, Levy H B, Baron S. Treatment of intracranial alphavirus infections in mice by a combination of specific antibodies and an interferon inducer. Am J Trop Med Hyg. 1995;52:34–40. doi: 10.4269/ajtmh.1995.52.34. [DOI] [PubMed] [Google Scholar]
- 2.Desprès P, Griffin J W, Griffin D E. Antiviral activity of alpha interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J Virol. 1995;69:7345–7348. doi: 10.1128/jvi.69.11.7345-7348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 4.Grieder F B, Vogel S N. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257:106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- 5.Griffin D, Levine B, Tyor W, Ubol S, Desprès P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 6.Griffin D E. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J Infect Dis. 1976;133:456–464. doi: 10.1093/infdis/133.4.456. [DOI] [PubMed] [Google Scholar]
- 7.Hackbarth S A, Reinarz A B G, Sagik B P. Age-dependent resistance of mice to Sindbis virus infection: reticuloendothelial role. J Reticuloendothel Soc. 1973;14:405–425. [PubMed] [Google Scholar]
- 8.Hwang S Y, Hertzog P J, Holland K A, Sumarsono S H, Tymms M J, Hamilton J A, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons α and β and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 10.Lustig S, Jackson A C, Hahn C S, Griffin D E, Strauss E G, Strauss J H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988;62:2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza Q P, Stanley J, Griffin D E. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J Gen Virol. 1988;70:3015–3022. doi: 10.1099/0022-1317-69-12-3015. [DOI] [PubMed] [Google Scholar]
- 12.Müller U, Steinhoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 13.Rice C M, Levis R, Strauss J H, Huang H V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherman L A, Griffin D E. Pathogenesis of encephalitis induced in newborn mice by virulent and avirulent strains of Sindbis virus. J Virol. 1990;64:2041–2046. doi: 10.1128/jvi.64.5.2041-2046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley J, Cooper S J, Griffin D E. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J Virol. 1986;58:107–115. doi: 10.1128/jvi.58.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhoff U, Müller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubol S, Griffin D E. Identification of a putative alphavirus receptor on mouse neural cells. J Virol. 1991;65:6913–6921. doi: 10.1128/jvi.65.12.6913-6921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubol S, Levine B, Lee S-H, Greenspan N S, Griffin D E. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J Virol. 1995;69:1990–1993. doi: 10.1128/jvi.69.3.1990-1993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Broek M F, Müller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilcek J. Production of interferon by newborn and adult mice infected with Sindbis virus. Virology. 1964;22:651–652. doi: 10.1016/0042-6822(64)90091-1. [DOI] [PubMed] [Google Scholar]