Abstract
The immunoglobulin-like receptors that mediate entry of herpes simplex virus type 1 (HSV-1) into human cells were found to mediate the direct cell-to-cell spread of wild-type virus. The receptors here designated Nectin1α and -δ and Nectin2α were originally designated HIgR, PRR1/HveC, and PRR2α/HveB, respectively. We report the following. (i) Wild-type HSV-1 spreads from cell to cell in J cells expressing nectin1α or nectin1δ but not in parental J cells that are devoid of entry receptors. A monoclonal antibody to nectin1, which blocks entry, also blocked cell-to-cell spread in nectin1-expressing J cells. Moreover, wild-type virus did not spread from a receptor-positive to a receptor-negative cell. (ii) The antibody to nectin1 blocked transmission of wild-type virus in a number of human cell lines, with varying efficiencies, suggesting that nectin1 is the principal mediator of wild-type virus spread in a variety of human cell lines. (iii) Nectin1 did not mediate cell fusion induced by the syncytial strains HSV-1(MP) and HFEM-syn. (iv) Nectin2α could serve as a receptor for spread of a mutant virus carrying the L25P substitution in glycoprotein D, but not of wild-type virus, in agreement with its ability to mediate entry of the mutant but not of wild-type virus.
In cell culture, herpes simplex virus type 1 (HSV-1) infects cells through initial attachment and subsequent fusion of the virion envelope with the plasma membrane, or through contiguous cell-to-cell spread, by a mechanism as yet poorly understood. A very similar situation probably occurs in human tissues. At the time of primary infection, HSV replicates in mucosal tissues and then enters nerve endings for retrograde transport to dorsal root neurons. Upon reactivation from latency, the virus replicates in neuronal cells and then is transported in an anterograde direction to cells innervated by the neuron. The anterograde transport and subsequent lesions occur in the presence of neutralizing antibody. Cell-to-cell transmission represents, therefore, a major route for virus spread to tissues. A central question is whether entry of virions into cells and cell-to-cell spread of virus represent distinct pathways.
Relevant to this report are the following.
(i) The receptors for HSV entry broadly expressed in human cell lines belong to an immunoglobulin family. For nomenclature, see the Appendix and Table 1. Nectin1δ, also named PRR1 (poliovirus receptor-related 1), or HveC (herpesvirus entry mediator C), mediates entry of all HSV-1 and -2 strains tested (15, 29, 50). Its splice variant isoform HIgR (herpesvirus immunoglobulin-like receptor) (hereafter nectin1α) shares with nectin1δ the ectodomain, made of one V and two C2 domains (7). Nectin1α and nectin1δ interact with the virion glycoprotein D (gD) (7, 15, 24). The region with gD-binding activity and functional in HSV entry is located in the V domain (6, 25). The C2 domain is involved in oligomerization (25). Nectin2α (also known as PRR2α or HveB) and nectin2δ (PRR2δ) are homologs of nectin1 and are 32% identical to nectin1 in the ectodomain region. They serve as low-efficiency receptors for entry of HSV-1 mutants carrying substitutions in gD at residues 25 to 27, but not of wild-type virus (4, 11, 27, 28, 50, 53). The cellular function of nectin1δ, nectin2α, and nectin2δ is that of intercellular adhesion molecules. They are anchored to the actin cytoskeleton and are recruited to cadherin-based adherens junctions through binding to l-afadin (27, 50).
TABLE 1.
The immunoglobulin-like receptors of alphaherpesviruses: past and present nomenclaturea
| Name | Alternative designation(s) | Virus for which the immunoglobulin serves as a receptor
|
Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|
| HSV-1wt | HSV-1 (gD-unrestricted) | HSV-1syn | HSV-2 | PrV | BHV-1 | |||
| Nectin1δ | PRR1, HveC | + | + | + | + | + | + | 15, 29 |
| Nectin1α | HIgR | + | + | + | + | ND | + | 7 |
| Nectin2α | PRR2α, HveB | − | + | − | +/− | + | − | 11, 28, 50, 53 |
| Nectin2δ | PRR2δ | − | + | − | +/− | ND | − | 11, 28, 50 |
| PVRα and -δ, HveD | − | − | − | − | + | + | 15, 32 | |
BHV-1, bovine herpesvirus 1; ND, not done; +, serves as receptor; −, does not serve as receptor; +/−, serves as low-efficiency receptor.
(ii) There is evidence that molecules that mediate HSV attachment to cells, and its entry, participate in cell-to-cell spread. Thus, heparan sulfate proteoglycan, a cell surface glycoprotein carrying glycosaminoglycans, enhances the initial attachment of HSV to cells (49) and enables the spread of syncytial strains (46) but is dispensable for spread of wild-type virus (17). Cells defective in entry receptors do not allow cell-to-cell transmission of virus; this is enabled by transfection of HveA (herpesvirus entry mediator A) (34), a mediator of HSV entry that belongs to the tumor necrosis factor receptor family (43, 51).
(iii) HSV strains may be divided into two groups with respect to the mechanism of cell-to-cell transmission. Wild-type strains (also designated syn+) spread across the junctions between the membranes of adjacent cells and cause infected cells to aggregate into clumps. Syncytial mutant strains (syn−) spread by fusion of the infected cell with adjacent uninfected cells and form multinucleated polykaryocytes or syncytia (see references 42 and 48). Both strains enter cells by the same mechanisms (7, 15). A key question is whether cell-to-cell spread of cytoaggregating strains and cell fusion by syncytial strains involves identical or different cellular functions.
(iv) The four virion glycoproteins required for virion entry into the cells, gD, gB, gH, and gL, also participate in cell-to-cell transmission. Thus, mutant viruses with deletions in each glycoprotein are defective in both processes (3, 14, 26, 44). Antibodies to the glycoproteins block both steps (16, 19, 21, 35, 38, 39). A case in point is gD. Soluble forms of gD or anti-idiotypic antibodies mimicking gD inhibit both virus entry and cell-to-cell transmission (21, 22, 36).
(v) The entry of free virion and cell-to-cell spread differ with respect to the role of the two virion glycoproteins, gE and gI, which form a heterocomplex. The complex localizes to the cell-cell junctions and favors virus spread across the junctions in cell cultures and in neurons in vivo (9, 10, 23). In its absence, the rate of HSV entry into cells is not altered (8). Also for the animal alphaherpesvirus pseudorabies virus (PrV), the gE-gI complex mediates cell-to-cell spread and transmission to specific neuronal circuits (5, 52, 54). Other HSV membrane proteins, gG, gM, and UL45, appear to play a role in cell-to-cell spread of syncytial strains but not in virion entry (2, 18, 31).
In these studies, we show that nectin1 mediates cell-to-cell spread of wild-type HSV-1, but not of syncytial strains, both in cells expressing the cDNA of the receptor as a transgene and in a variety of human cell lines. Nectin2α serves for cell-to-cell spread of a mutant virus carrying an L25P substitution in gD but not of wild-type virus.
Nectin1α and nectin1δ mediate cell-to-cell spread of HSV-1(F).
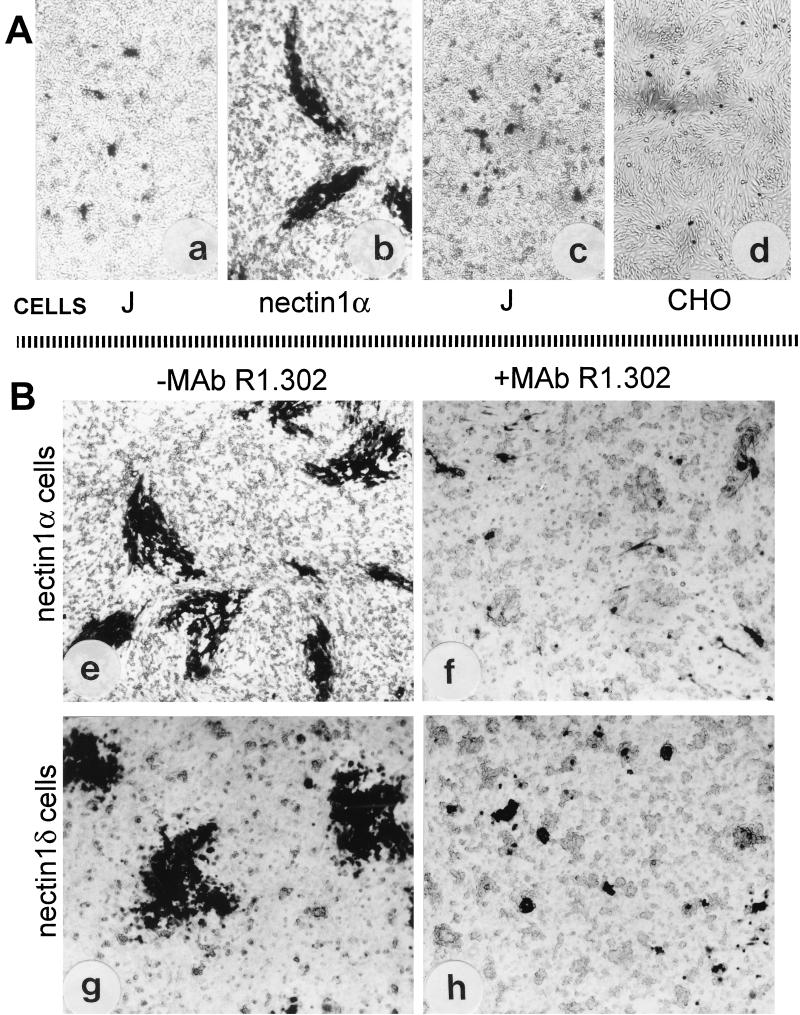
To investigate whether nectin1α and nectin1δ play a role in cell-to-cell transmission of HSV-1, we compared the abilities of HSV-1(F) (12) and its derivative R8102 (7) to form plaques in cells defective in receptors for virus entry (J cells) or in clonal derivatives of J cells, which constitutively express nectin1α or nectin1δ cDNA (designated as nectin1α or nectin1δ cells) (7). Three series of experiments were performed. In the first, since J cells cannot be infected with HSV-1 because of the lack of receptors for entry, the DNA of HSV-1(F) was transfected into J cells (1 μg of DNA/well of a six-well dish) and, for comparison, in nectin1α cells. The cultures were monitored for plaque formation 2 to 3 days later by immunostaining with a polyclonal antibody to gM (1). The results in Fig. 1A show that, in monolayers of J cells, infected cells consisted of single cells or very small aggregates and plaques were not formed, whereas in monolayers of nectin1α cells plaques were readily formed. In the second experiment, nectin1α or nectin1δ cells were infected with R8102, which carries a lacZ gene under the α27 promoter. Infection with this virus is readily monitored as β-galactosidase (β-Gal) activity by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) staining (34). In nectin1α or nectin1δ cells, plaques were readily formed (Fig. 1B) and were undistinguishable from those formed in nectin1α cells transfected with the HSV-1(F) DNA (compare panels b and e of Fig. 1). We note that throughout the study we found a general agreement between results obtained with wild-type virus and those obtained with the R8102 recombinant, whose infection can be readily quantified as β-Gal activity. In the third series of experiments, an infectious center assay was performed, as described in reference 43. Nectin1α cells, infected with R8102, were trypsinized 4 h after infection and seeded onto recipient monolayers of J, CHO (also defective in entry receptors) (34), or nectin1α cells. The seeded cells and the recipient monolayers were constantly maintained in the presence of pooled human gamma globulins, to block the infectivity of virions possibly released and to ensure that the detected plaques were formed only by cell-to-cell spread. The seeded cells became attached to the monolayer within 5 h or less in these as well as in all subsequent infectious center assays, with an efficiency estimated at >95%. The results in Fig. 1A show that plaques were not formed when infected nectin1α cells were seeded onto recipient monolayers of J or CHO cells but were formed when they were seeded onto recipient monolayers of nectin1α cells (data not shown). Taken together, these experiments indicate that plaque formation does not ensue in cells defective in virus entry receptors (J or CHO), that nectin1α or nectin1δ can mediate cell-to-cell spread of HSV-1, and that virus was not transmitted from a receptor-positive to a receptor-negative cell. No major difference was observed between cells expressing the α and cells expressing the δ isoform of nectin1.
FIG. 1.
Cell-to-cell spread of HSV-1 occurs in nectin1α- or nectin1δ-expressing cells (B) but not in the receptor-negative J or CHO cells (A). (a) Micrograph of J cells transfected with HSV-1(F) DNA shows only singly infected cells or small aggregates. (b) Micrograph of stable transformants of J cells expressing nectin1α transfected with HSV-1(F) DNA shows presence of plaques. (c and d) Infectious center assay. Monolayers of nectin1α cells infected with R8102 were trypsinized and seeded onto recipient monolayers of J (c) or CHO (d) cells. Note the absence of plaques. (B) Plaque formation in nectin1α or nectin1δ cells infected with R8102 and inhibition by MAb R1.302. (e and f) Micrographs of nectin1α-expressing J cells infected with R8102, unexposed (e) or exposed to MAb R.1302 (f). (g and h) Micrographs of nectin1δ-expressing J cells infected with R8102, unexposed (g) or exposed to MAb R1.302 (h). Note that plaque size is greatly reduced in panels f and h relative to size in panel e or g. Infected cells were detected by immunostaining with polyclonal antibody to gM (a and b) or by X-Gal staining (c to h). Pictures were taken in an Axioplan Zeiss microscope. All pictures are at the same magnification.
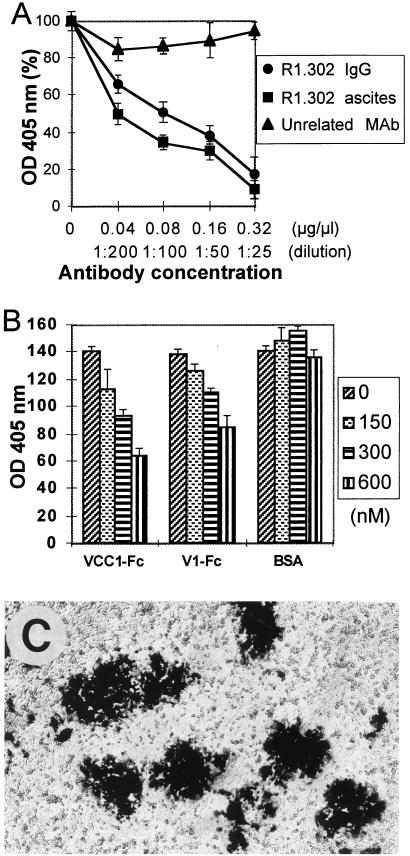
Plaque formation in nectin1α and nectin1δ cells was indeed dependent upon expression of the receptors, as incubation of infected cells with monoclonal antibody (MAb) R1.302 to nectin1 (30), from 4 h after infection till fixation, reduced the plaque size to small aggregates (Fig. 1B). An irrelevant MAb (MAb 6E2 to p48 of human herpesvirus 6B), which does not cross-react to any cellular or HSV protein (13), had almost no effect (data not shown). As expected, the number of plaques in nectin1α and nectin1δ cells exposed to MAb R1.302, scored by counting the single cells and the small aggregates, did not differ from the number of plaques scored in the cultures not treated or exposed to the irrelevant MAb (data not shown). The effect of MAb R1.302 was quantified by exposing R8102-infected nectin1α cells, grown in 96 wells, to increasing amounts of purified immunoglobulins G (IgGs) from MAb R1.302, added at 4 h after infection. Plaque formation was monitored 48 h later as β-Gal expression, with ONPG (o-nitrophenyl-β-d-galactopyranoside) as substrate (34). Figure 2A shows that MAb R1.302 inhibited plaque formation in a dose-dependent fashion; 50% inhibition occurred at 0.08 mg/ml. At the same concentration, the control pooled mouse immunoglobulins had no significant effect. We note that the MAb concentrations required to inhibit plaque formation were higher than those required to block HSV infectivity in the same cells (7). In replicate samples, MAb R1.302 (ascites fluid, 1:25) inhibited plaque formation approximately to the same extent as did purified IgGs at 0.32 mg/ml. The remaining experiments were carried out under the former conditions.
FIG. 2.
MAb R1.302 (A) and soluble nectin1 (B) inhibit plaque formation in nectin1α-expressing J cells infected with R8102. (A) Nectin1α-expressing J cells in 96 wells, infected with R8102, were exposed to the indicated concentrations of purified IgGs from MAb R1.302 (●), control mouse IgG (▴), or R1.302 ascites fluid (■), from 4 h after infection. Infection was detected 48 h later by permeabilization with 0.5% NP-40 and quantitative detection of β-galactosidase activity with ONPG, followed by reading of the optical density (OD) at 405 nm in a Bio-Rad enzyme-linked immunosorbent assay reader. The reading in untreated cultures was in the linear range (around 1 optical density unit) and was made equal to 100%. Each point represents the average of triplicate samples. The values in the abscissa represent the concentrations of purified IgGs (● and ▴) or dilution of ascites fluid (■). (B) Nectin1α-expressing J cells infected with R8102 in 96 wells were exposed to the indicated concentrations of the soluble form of nectin1 containing the entire ectodomain fused to the Fc portion of human IgG (VCC1-Fc) or containing the single V domain (V1-Fc) or to bovine serum albumin (BSA), from 4 h after infection. The soluble forms of nectin1 were described previously (6). Infection was detected as described for panel A. The values in the ordinate represent the optical densities (OD) at 405 nm (×100). Each point represents the average of triplicate samples. (C) Micrograph of R8102-infected J cells expressing a chimeric form of nectin1 [V(HIgR)-PVRα] that contains the V domain of nectin1 fused to C domains and the transmembrane and cytoplasmic regions of poliovirus receptor.
The V domain of nectin1 is functional in cell-to-cell spread.
The region of nectin1α and nectin1δ functional in binding to gD and in virion entry is located in the V domain (6, 25). The first evidence that the V domain of nectin1 also plays a key role in the spread of the virus was provided by the inhibitory effect of MAb R1.302, which recognizes an epitope located in the V domain (6). Additional evidence was provided in two series of experiments. First, cell-to-cell spread occurred in cells which expressed a chimeric form of nectin1, originally named V(HIgR)-PVRα (6), consisting of the V domain of nectin1 fused to the C domains and transmembrane and cytoplasmic regions of the poliovirus receptor (PVR; also CD155 or HveD) (15, 32) (Fig. 2C). Second, a soluble form of nectin1 carrying the entire ectodomain (VCC1-Fc), or the single V domain (V1-Fc) fused to the Fc portion of IgG (6), could compete with cell-bound receptor and thereby block cell-to-cell spread of virus. In this experiment, infected nectin1α cells were incubated with VCC1-Fc or V1-Fc from 4 h after infection. This caused a dose-dependent inhibition and about 50% reduction at the highest concentration used (600 nM) (Fig. 2B). Higher concentrations of VCC1-Fc or V1-Fc were not tested, because of limitations in the quantities of recombinant proteins that could be produced. As noted above for MAb R1.302, the concentrations of VCC1-Fc and V1-Fc which inhibit cell-to-cell spread were higher than those required to inhibit virus infectivity in the same cells (6). This may reflect a limited accessibility of the antibodies, or soluble receptor, to receptors located at adherens junctions, as opposed to full accessibility to receptors located in regions of the plasma membrane not engaged in cell-cell junctions.
Nectin1α or nectin1δ mediates cell-to-cell spread of wild-type virus in a variety of human cell lines.
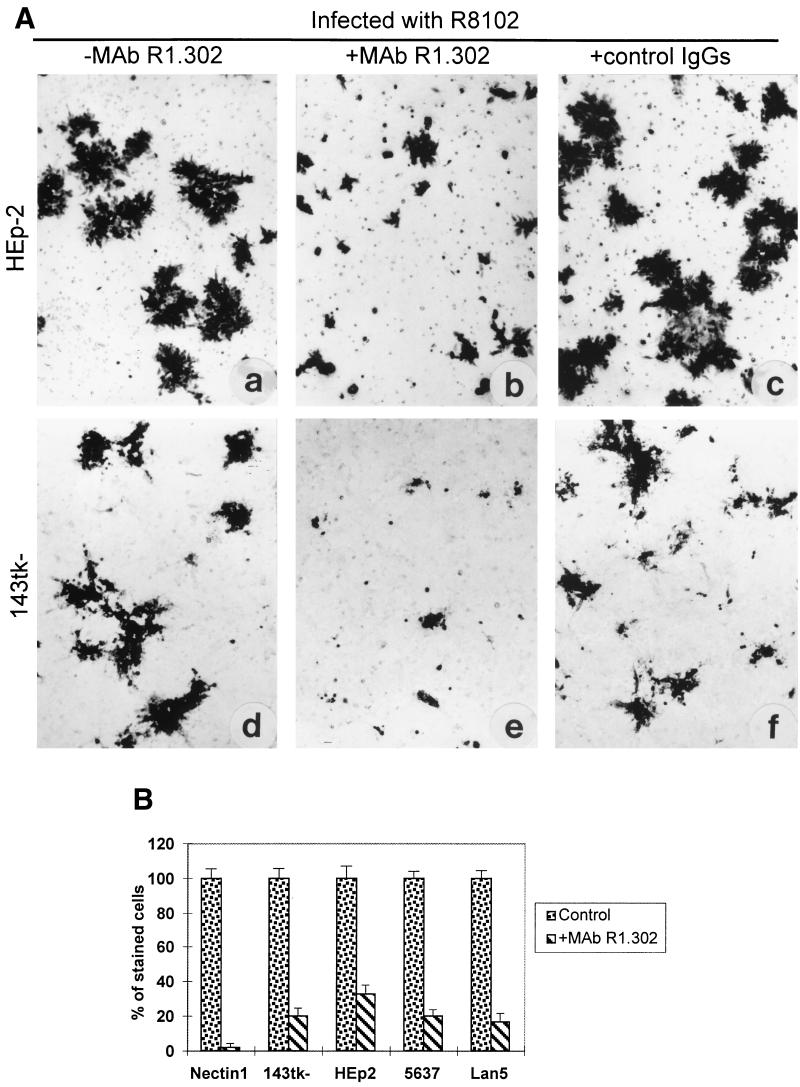
A variety of human cell lines express nectin1 and are susceptible to HSV infection (7, 15). The pathway of entry is via nectin1, and infection is inhibited by MAb R1.302 (7). The antibody could be expected to block virus transmission, provided that nectin1 is the principal receptor serving also for virus spread. The effect of antibody R1.302 on formation of plaques and infectious centers was assessed in a number of human cell lines of different origin, namely, HEp-2 (epithelial carcinoma), 143tk− (a derivative of human sarcoma), 5637 (epithelial bladder carcinoma), and Lan5 (neuroblastoma). Representative results in Fig. 3A show that, in 143tk− and HEp-2 cells, R8102 plaques were decreased in size, although to a lower extent in HEp-2 cells than in the nectin1α cells or in 143tk− cells. The inhibitory effect of MAb R1.302 was quantified in an infectious center assay. Infected nectin1α cells were trypsinized at 4 h after infection and seeded onto recipient monolayers of human cell lines in the presence or absence of MAb R1.302. The number of stained cells in the monolayer was determined by means of Photoshop software, as detailed in the figure legend. This type of measure accounts for the overall number of stained cells in a monolayer and was expressed as percentage relative to the overall number of stained cells in the corresponding monolayer not exposed to the antibody. As shown in Fig. 3B, the extent of inhibition was 98% in recipient monolayers of nectin1α cells and ranged from 80 to 67% in the other recipient monolayers. While the partial inhibition may reflect the concomitant activity of additional receptors, the results clearly indicate that nectin1 is the principal receptor available for virus transmission in a number of human cell lines.
FIG. 3.
(A) MAb R1.302 inhibits R8102 plaque formation in HEp-2 and 143tk− cells. Shown are micrographs of HEp-2 (a to c) and 143tk− (d to f) cells infected with R8102, unexposed (a and d) or exposed to MAb R1.302 (b and e) or to an irrelevant MAb (c and f), from 4 to 48 h after infection. Plaques were detected by X-Gal straining. (B) MAb R1.302 reduces infectious center formation in human cell lines. Nectin1α cells infected with R8102 were trypsinized at 4 h after infection and plated onto monolayers of recipient cells in the absence (control) (left) or presence (right) of MAb R1.302 in six-well plates. Recipient cells were nectin1α cells, 143tk−, HEp-2, 5637, and Lan5 cells. Monolayers were stained with X-Gal. The stained six-well dishes were scanned in an Agfa StudioStar scanner, equipped with incident light, and the image was imported by means of Photoshop software. The number of stained cells was determined by the histogram program. This measure accounts for all the stained cells in the monolayer. The overall number of stained cells in the monolayer treated with MAb R1.302 was expressed as a percentage relative to the corresponding untreated monolayer. All pictures are at the same magnification.
Nectin1α and nectin1-δ do not mediate cell fusion induced by syncytial strains.
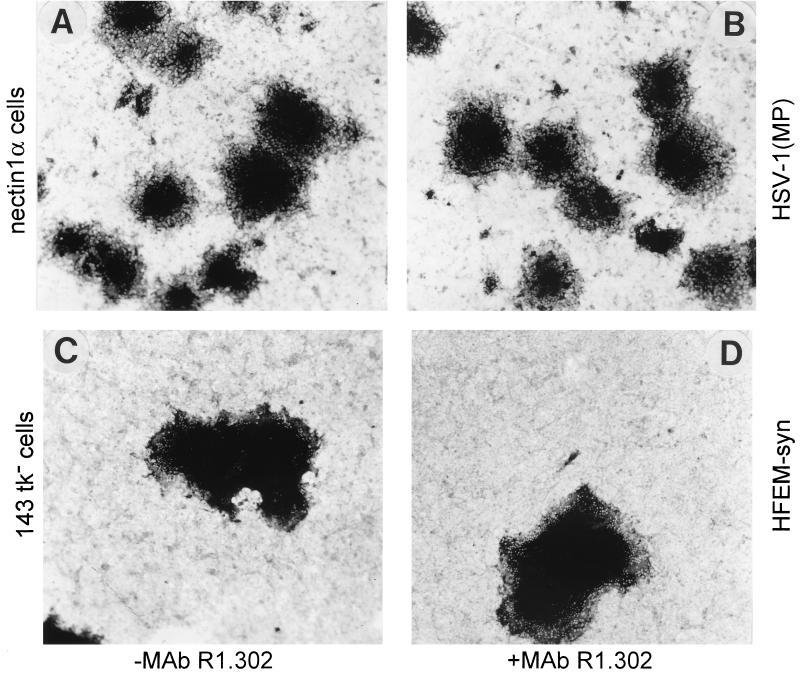
Nectin1α, 143tk−, and HEp-2 cells were infected with the syncytial strain HSV-1(MP) (20) or HFEM-syn (55), and incubated with MAb R1.302, from 4 h after infection. Representative results illustrated in Fig. 4 show that the two syncytial strains behaved quite differently from the wild-type virus. The antibody did not significantly affect the plaque size of either virus in any of the cells tested. The number of plaques was also not affected (data not shown). The results indicate that cell fusion by HSV-1(MP) and HFEM-syn is mostly independent of nectin1.
FIG. 4.
MAb R1.302 does not reduce plaque formation by HSV-1(MP) or HFEM-syn. Shown are micrographs of nectin1α (A and B) and 143tk− (C and D) cells infected with HSV-1(MP) or HFEM-syn, respectively, and unexposed (A and C) or exposed to MAb R1.302 (B and D) from 4 h after infection till fixation. Plaques were detected by immunostaining with polyclonal antibody to gM. All pictures are at the same magnification.
Nectin2α mediates cell-to-cell spread of an HSV-1 gD-mutant.
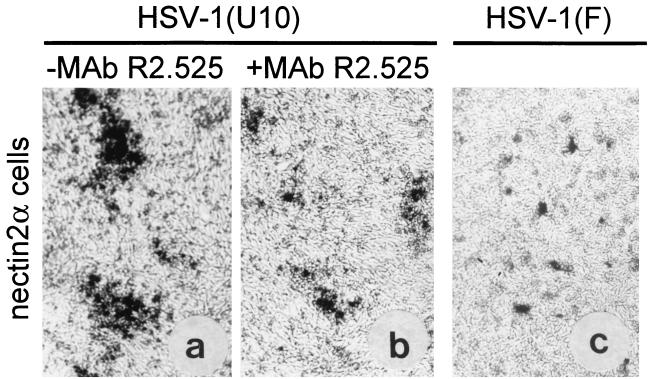
Nectin2α and nectin1δ can serve as entry receptors for gD-unrestricted mutants carrying substitutions in gD at residues 25 to 27, but not for wild-type virus (28, 53) (Table 1). To test the ability of nectin2α to serve in cell-to-cell spread, nectin2α cells (28) were infected with HSV-1(U10), which carries the L25P substitution in gD and can enter nectin2 cells (4, 28). The infected cultures were incubated in the absence or presence of MAb R2.525, a MAb to nectin2 which blocks HSV-1(U10) entry (28, 30). The results in Fig. 5 show that HSV-1(U10) could form plaques in nectin2α cells. They were reduced in size by MAb R2.525. These results were confirmed and extended in an infectious center assay, where HSV-1(U10)-infected nectin1α cells were trypsinized and seeded onto a monolayer of nectin2α cells in the absence or presence of MAb R2.525. Plaques were formed and were reduced in size by the antibody (data not shown). To ascertain whether nectin2 can serve as a receptor for the spread of wild-type virus, nectin2α cells were transfected with the DNA of HSV-1(F), to overcome the need for a receptor. This resulted in singly infected cells or small aggregates but not in plaques (Fig. 5C). Taken together, the results indicate that nectin2α can serve as a receptor for cell-to-cell spread of HSV-1(U10) but not of wild-type HSV-1.
FIG. 5.
(a and b) Plaque formation in nectin2α-expressing cells infected with HSV-1(U10) and inhibition by MAb R2.525. Shown are micrographs of nectin2α cells, which harbor a lacZ gene driven by the α27 promoter, infected with HSV-1(U10) and maintained in the absence (a) or presence (b) of MAb R2.525 from 4 h after infection. (c) Micrograph of nectin2α cells transfected with HSV-1(F) DNA. Note singly infected cells or small aggregates and the absence of plaques. (a and b) Infection was monitored as β-Gal activity. (c) Cells were stained with polyclonal antibody to gM.
The results presented in this paper show the following.
(i) They identify the immunoglobulin-like receptors nectin1α and nectin1δ as cellular functions involved in cell-to-cell transmission of wild-type HSV-1, both in cells expressing nectin1 cDNA as a transgene and in a variety of human cell lines. Cell-to-cell spread, like entry, involved the V domain of nectin1. In addition, transmission could not take place from receptor-positive to receptor-negative cells. Nectin1 is expressed in human tissues, including central nervous system and tissues of epithelial origin (7). Taken together, these properties make this molecule a likely candidate that mediates virus spread to tissues that are the target of HSV infection in humans. With respect to the function of nectin1, HSV differs from PrV. In the case of PrV, which needs gD for virus entry, but not for cell-to-cell transmission (40, 41, 45), the human HveC/nectinδ, HveB/nectin2α, and HveD enabled entry of virions into the resistant CHO cells, but typical plaques were not formed (37). The small clusters of infected cells formed in cultures expressing these receptors were similar to those formed in CHO cells lacking receptors (37).
(ii) They identify nectin2α as a mediator of cell-to-cell transmission for an HSV gD mutant carrying a substitution at residue 25, but not for wild-type virus. Nectin2α shows, therefore, the same restricted range as a mediator of virus transmission and as a mediator of virion entry (28, 53). Both activities are consistent with its ability to interact physically with mutant gD and failure to interact with wild-type gD (28).
(iii) They show that cell fusion induced by syncytial strains occurs independently of nectin1. This was a surprising result, in view of the ability of the syncytial viruses to enter cells via nectin1 (7, 15). Syncytial and cell-aggregating strains were reported to be able to use HveA for cell-to-cell transmission in cells transfected with the cDNA of the mediator (43, 51). However, given that its expression is limited to highly specific cell lineages, this molecule cannot represent the principal mediator for transmission of syncytial strains in the majority of human cell lines. Recently, 3-O-sulfated heparan sulfate has been shown to provide a binding site for gD and initiate HSV entry (47). Whether human cell lines can actually be infected with HSV through this moiety has not been determined. It remains also to be determined whether it can mediate cell fusion of syncytial strains. In the past, it has been speculated that cell fusion induced by syncytial strains of HSV mimics and represents a good model for the fusion event between virion and plasma membrane in the entry process. The results presented here underscore a notable difference between the two activities and identify the usage of cellular receptors as yet another difference between the two processes. Current findings raise the possibility that cell fusion induced by the syncytial strains involves different cellular components than does the spread of wild-type cell-aggregating HSV.
(iv) Nectin1δ, nectin2α, and nectin2δ are intercellular adhesion molecules that act by homophilic interaction and are recruited to adherens junctions (27, 50). Nectin1α differs from nectin1δ, nectin2α, and nectin2δ in that it does not carry the conserved C-terminal motif (A/ExYV) that binds the latter molecules to the PDZ domain of l-afadin, an F-actin binding protein. It is nonetheless expressed at the cell surface and also at cell-cell contact sites, although at reduced efficiency (our unpublished observations). Consistently, engineered forms of nectin1δ and nectin2α that lack the C-terminal motif maintain the ability to localize at cell-cell contact sites (33, 50). Current data indicate that nectin1α serves in cell-to-cell spread of HSV, as does its isoform nectin1δ. Therefore, binding to l-afadin does not seem to be a prerequisite to promote cell-to-cell spread of HSV. Of note, the two viral glycoproteins that play a role in cell-to-cell transmission of virus, but not in entry of free virions, gE and gI, also localize at cell-cell junctions (10). Previously, one of our laboratories showed that homophilic interaction of nectin2α, or nectin2δ, correlates with the cis dimerization of the molecules at the cell surface and that this function requires a domain different from that functional in HSV entry (27, 28). It can be proposed that the nectin1α and nectin1δ and nectin2α and nectin1δ molecules promote cell-to-cell spread by establishing the necessary intercellular contacts between juxtaposed cells, both by engaging in homophilic interaction and by interaction with viral gD, the viral ligand of nectin1 and nectin2. In turn, the viral gD that engages with nectin1, or nectin2, may be expressed at the surface of the infected cell and/or in progeny virions. The intercellular contacts created by nectin1, or nectin2, might contribute to encasing the progeny virus within the intercellular junctions and to shielding it, in part, from molecules present in medium. These may be the human gamma globulins normally used to allow plaque formation or the molecules used in this study, i.e., antibodies to nectin1 or nectin2 or soluble forms of nectin1. This hypothesis would explain why high concentrations of the antibodies or soluble receptor were necessary to exert an inhibitory effect.
Acknowledgments
We thank Elisabetta Romagnoli for invaluable assistance with cell cultures. We thank N. S. Markovitz and B. Roizman (Chicago, Ill.) for the gift of R8102.
The work done at the University of Bologna was supported by grants from Target Project in Biotechnology/CNR, Telethon grant A141, MURST 40%, University of Bologna 60%, and pluriannual plan. The studies at INSERM U119, Marseille, were aided by INSERM, the Association pour la Récherche Contre le Cancer (ARC), and the Ligue Nationale Française Contre le Cancer (LNFCC).
Appendix
HveC/PRR1, HIgR, HveB/PRR2α, and PRR2δ are members of an immunoglobulin family whose function has been recently identified as that of intercellular adhesion molecules (27, 50). The molecules were renamed nectin1 and nectin2, replacing PRR1 and PRR2, respectively (50). Knowledge of the natural function of these proteins may help in understanding the mechanism by which they fulfill their function as viral receptors. Furthermore, receptor designations in virology should be comprehensible to broad audiences in cell biology and not merely to the workers in the field. To this aim, we propose to maintain, for the alphaherpesvirus immunoglobulin-like receptors, the designation that describes their biological function. Examples of this rule are numerous, e.g., intercellular adhesion molecule, vascular cell adhesion molecule 1, CD4, chemokine receptors CCR5 and CXCR4, integrins, amino acid transporter, carboxypeptidase D, fibronectin, etc. Table 1 lists the names given to members of the immunoglobulin subfamily and the viruses for which they serve as receptors. The α and δ suffixes are those used in the literature to designate the different isoforms of poliovirus receptor. Where appropriate, the prefixes h, m, etc., can be used to designate the species of origin (human, murine, etc., respectively).
REFERENCES
- 1.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan P, Davis-Poynter N, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoprotein gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 3.Cai W Z, Person S, Warner S C, Zhou J H, De Luca N A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987;61:714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume G, Qi S, Avitabile E, Foà-Tomasi L, Brandimarti R, Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990;64:6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- 6.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex viruses 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell K S, Johnson D C. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberlé F, Dubreuil P, Mattei M G, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- 12.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 13.Foà-Tomasi L, Avitabile E, Campadelli-Fiume G. Selection of a monoclonal antibody specific for variant B human herpesvirus 6-infected mononuclear cells. J Virol Methods. 1995;51:289–296. doi: 10.1016/0166-0934(94)00120-6. [DOI] [PubMed] [Google Scholar]
- 14.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 16.Gompels U, Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986;153:230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 17.Gruenheid S, Gatzke L, Meadows H, Tufaro F. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J Virol. 1993;67:93–100. doi: 10.1128/jvi.67.1.93-100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haanes E J, Nelson C M, Soule C L, Goodman J L. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein-B-induced cell fusion. J Virol. 1994;68:5825–5834. doi: 10.1128/jvi.68.9.5825-5834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoggan M D, Roizman B. The isolation and properties of a variant of herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- 21.Huang T, Campadelli-Fiume G. Anti-idiotypic antibodies mimicking glycoprotein D of herpes simplex virus identify a cellular protein required for virus spread from cell to cell and viral induced polykaryocytosis. Proc Natl Acad Sci USA. 1996;93:1836–1840. doi: 10.1073/pnas.93.5.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummenacher C, Rux A H, Whitbeck J C, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty R J, Spear P G, Eisenberg R J, Cohen G H. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- 28.Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74:1267–1274. doi: 10.1128/jvi.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez M, Eberlé F, Mattei M G, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- 30.Lopez M, Jordier F, Bardin F, Coulombel L, Chabannon C, Dubreuil P. Identification of a new class of IgG superfamily antigens expressed in hemopoiesis. In: Kishimoto T, Kikutani H, von der Borne A, Mason D Y, Miyasaka M, Moretta A, Okumura K, Shaw S, Springer T A, Sugumura K, Zola H, editors. Leukocyte typing VI: white cell differentiation antigens. New York, N.Y: Garland Publishing, Inc.; 1997. pp. 1081–1083. [Google Scholar]
- 31.MacLean C A, Robertson L M, Jamieson F E. Characterization of the UL10 gene product of herpes simples virus type 1 and investigation of its role in vivo. J Gen Virol. 1993;74:975–983. doi: 10.1099/0022-1317-74-6-975. [DOI] [PubMed] [Google Scholar]
- 32.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 33.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 35.Navarro D, Paz P, Pereira L. Domains of herpes simplex virus glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992;186:99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- 36.Nicola A V, Willis S H, Naidoo N N, Eisenberg R J, Cohen G H. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70:3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nixdorf R, Schmidt J, Karger A, Mettenleiter T. Infection of Chinese hamster ovary cells by pseudorabies virus. J Virol. 1999;73:8019–8026. doi: 10.1128/jvi.73.10.8019-8026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noble A G, Lee G T, Sprague R, Parish M L, Spear P G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983;129:218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- 39.Novotny M J, Parish M L, Spear P G. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology. 1996;221:1–13. doi: 10.1006/viro.1996.0347. [DOI] [PubMed] [Google Scholar]
- 40.Peeters B, De Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 43.Roller J R, Rauch D. Herpesvirus entry mediator HVEM mediates cell-cell spread in BHK(TK−) cell clones. J Virol. 1998;72:1411–1417. doi: 10.1128/jvi.72.2.1411-1417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt J, Klupp B G, Karger A, Mettenleiter T C. Adaptability in herpesviruses: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh M T, Spear P G. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shukla D, Liu J, Blaiklock P, Shworak N W, Bai X, Esko J D, Cohen G H, Eisenberg R J, Rosenberg R D, Spear P G. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 48.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 49.Spear P G, Shieh M T, Herold B C, WuDunn D, Koshy T I. Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terry-Allison T, Montgomery R I, Whitbeck J C, Xu R, Cohen G H, Eisenberg R J, Spear P G. HveA (herpesvirus entry mediator A), a coreceptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J Virol. 1998;72:5802–5810. doi: 10.1128/jvi.72.7.5802-5810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 54.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wildy P, Russel W C, Horne R W. The morphology of herpes virus. Virology. 1960;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]