Abstract
Loneliness is recognised as a risk factor for cardiovascular disease development. However, it is unclear whether loneliness itself or other closely related mental health symptoms, such as depression and social anxiety, are associated with the development of cardiovascular disease. In the present study, we examined the relationship between loneliness and several early cardiovascular disease markers in young adults, after controlling for depression and social anxiety. Sixty-six young adults (18–35 years old, Mage = 22.70; 75.8% females) completed psychological questionnaires and took part in several physiological tests assessing cardiovascular health (e.g., vascular function). Results revealed higher loneliness was significantly associated with shorter pulse transit time (β = − 0.70, p = 0.002; shorter pulse transit time is a subclinical marker for arterial stiffness). Additionally, results show that while loneliness and depression were both related to vascular dysfunction in young adults, the underlining physiological mechanisms through which they affect vascular function may be different. Specifically, higher loneliness was associated with increased arterial stiffness, whereas depression was associated with increased endothelial dysfunction (β = − 0.43, p = 0.04). Our findings indicate that presence of loneliness and depression in young adults may be accompanied by early indicators of poor cardiovascular health, such as arterial stiffness and endothelial dysfunction. Results from the study further support the link between loneliness and cardiovascular disease development.
Subject terms: Psychology, Human behaviour
Introduction
Loneliness has been identified as a social determinant of health and a major public health issue1–3. It is characterised as a subjective distressing feeling of disconnect from others, accompanied by a desire for either more, or more fulfilling social relationships4. Subsequently, it is more closely related to the quality of one’s social relationships, as opposed to the quantity of social connections5,6. Furthermore, it is also a relatively common experience3,7,8. Indeed, a 2018 survey found 51% of Australian adults felt lonely at least 1 day a week9. For the majority of people, feelings of loneliness are transient and part of an adaptive experience that motivates them to form new social connections and maintain existing ones10. However, when left unchecked, temporary feelings of loneliness can develop into problematic levels of loneliness and have a negative impact on health7,11,12.
Substantial evidence from cross-sectional and longitudinal studies have established the link between loneliness and higher incidence of physical health diseases, especially cardiovascular disease (CVD)13–16. CVDs are the global leading cause for mortality17 and loneliness has been identified as a risk factor for a number of CVDs, including coronary heart disease and stroke13–16. Studies in this area have primarily focused on older adults as they were believed to be the age group most vulnerable to the negative effects of loneliness, and most likely to present with related CVD issues13,15. However, more recent research shows loneliness to be equally pervasive in younger adults (< 35 years old)18–21. Primary and secondary CVD prevention (e.g., individual lifestyle changes, early diagnosis) have been identified as necessary steps to help relieve the disease burden on individuals and the healthcare system22,23. In order to better understand how loneliness may be related to the development of CVD, it may be prudent to focus on young adults and assess their risk of developing CVD by utilising early markers of CVD. By using this approach, we can facilitate early diagnosis and intervention, thereby promoting better health outcomes.
CVD typically develops later in life, however, progression to CVD can be detected decades before (i.e., in young adulthood) in forms of metabolic abnormalities, such as increased blood pressure, higher body mass index (BMI) as well as early impaired vascular function and autonomic nervous system function (referred to as autonomic function)22,24. Hence, to better understand the relationship between loneliness and CVD progression, in the present study, we focus on early indicators of poor cardiovascular health (CVH, the term ‘CVH indicator’ is also referred to as ‘CVD marker’ or ‘CVD indicator’ throughout the article) which have been shown to be accurate and consistent predictors of CVD22,24–27. Previous studies investigating the relationship between loneliness and some more common CVH indicators (i.e., blood pressure, BMI, and heart rate variability (HRV), 25–27) have reported mixed results. Some studies found a relationship between higher loneliness, increased blood pressure, higher BMI (obesity), and lower HRV14,28–32. Other studies reported no associations between indicators of poor CVH and loneliness33–36. Notably, very few of these studies focused on young adults29,32. To the best of our knowledge, no studies have examined the association between loneliness and specific early markers of CVD, such as vascular and autonomic function in young adults.
Loneliness has a multifaceted and possibly confounding relationship with social anxiety and depressive symptomology (referred here as depression and social anxiety respectively). Loneliness may be an antecedent to depression30,37 but the relationship between loneliness and social anxiety has been shown to be bi-directional38,39. While some studies have controlled for either depression or social anxiety when investigating the influence of loneliness on more common CVH indicators (e.g., blood pressure, HRV14,29,31,34) in older adults, they have reported mixed findings, thus necessitating further investigation. Moreover, there has been little to no research differentiating the influence of depression and social anxiety on the relationship between loneliness and certain early markers of CVD (e.g., vascular and autonomic function) in young adults. To fully understand the unique relationship between loneliness and CVH markers in young adults, it is imperative to consider the influence of highly correlated mental health symptoms such as depression and social anxiety.
The primary study aim was to investigate the relationship between loneliness and CVH indicators (i.e., vascular function, autonomic function, BMI, and blood pressure) in young adults, after accounting for social anxiety and depression. We also examine potential differences in the associations between loneliness, social anxiety, and depression, and various CVH indicators in young adults.
Method
Ethical approval was granted by the Swinburne University Human Research Ethics Committee (SUHREC) and the study was conducted in accordance with the SUHREC guidelines. Sixty-six young adults (18–35 years old) were recruited via social media advertisements, word of mouth, online forums, and a research training program for first year Bachelor of Psychology students. We adopted a cross-sectional study design. Participation was voluntary, an explanatory statement was provided, and informed consent was obtained before participants could partake in the study. The Bachelor of Psychology students involved in this study received course credit in return for participation, whereas community participants received a monetary reimbursement.
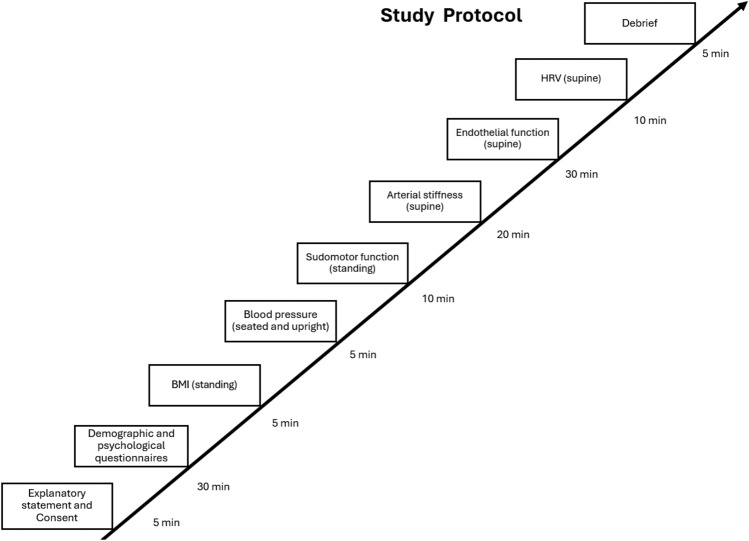
Study data was collected by a single investigator between September 2021 and July 2022. Respondents attended a two-hour laboratory session where after providing consent, they completed a short questionnaire which included demographic questions and the psychological measures. Next, participants height, weight, and BMI measurements were taken. After a 5-min rest, blood pressure measurements were taken while the participant was in an upright seated position (as per the guidelines of the National Heart Foundation of Australia). Specific details or steps for how the tests for each CVH/CVD indicator were carried out are included below, under each test description. Following blood pressure measurement, the participant completed the assessment for sudomotor function (measure for autonomic function), which took approximately 10 min to complete. Then, the participant was instructed to lie down on the clinic bed in a supine position, where they completed the test for arterial stiffness (measure for vascular function). This assessment took approximately 20 min to complete. Endothelial function (another measure for vascular function) was measured next. The test for this was 30 min long. Next, while in supine position, participants’ blood pressure, heart rate, and electrocardiogram were recorded for 10 min (measure for HRV). An illustration detailing the study protocol is included below in Fig. 1.
Figure 1.
A visual representation of the laboratory session which illustrates the study protocol, the tests conducted, their duration, and the employed testing modality.
Demographic information was obtained, including age, gender, marital status, ethnicity, employment status, education level, living status, chronic CVD conditions (see Table 1).
Table 1.
Participant characteristics (N = 66).
| Percentage/mean (SD) | |
|---|---|
| Age (years) | 22.70 (4.50) |
| Gender | |
| Female | 75.8 |
| Male | 24.2 |
| Marital status | |
| Single/never married | 72.7 |
| Married/domestic/defacto relationship | 27.3 |
| Ethnicity | |
| Asian Australian or Asian (includes Indian, Indian Australian) | 40.0 |
| White (includes Caucasian, European Australian) | 33.8 |
| Multiple board categories/others | 26.2 |
| Employment status | |
| Working part time/student/not working | 84.8 |
| Working full time | 15.2 |
| Level of education completed | |
| Secondary school | 53.0 |
| Vocational education/undergraduate/postgraduate degree | 47.0 |
| Living status | |
| Living with at least one other person | 88.5 |
| Living alone | 11.5 |
| Chronic CVD conditions | |
| No CVD present | 100.0 |
| At least have one CVD | 0.0 |
Psychological questionnaires
Loneliness
Loneliness was measured using the UCLA Loneliness Scale-Version 3 (UCLA-LS)40. The UCLA-LS includes 20 questions which assess feelings of loneliness over a one-month period. Responses are given on a 1 (Never) to 4 (Always) Likert-type scale. Composite scores are calculated by reverse coding the nine positively worded items and then adding the scores for all the questions. Score range is between 20 and 80, with higher score suggesting higher levels of loneliness. The UCLA-LS has been found to be a valid and reliable measure of loneliness across diverse populations (e.g., students, nurses, teachers, older adults40). Cronbach’s α for UCLA-LS in the present study was 0.95.
Social anxiety
Social anxiety symptoms were measured using the Social Interaction Anxiety Scale (SIAS)41. We used the straightforward version (S-SIAS) of the SIAS which consists of only the 17 negatively worded items, as opposed to the original SIAS, which includes 20 negative and positive items. S-SIAS is a more appropriate scale to use in the present study as the negatively worded statements have shown to be better indicators of social interaction anxiety, whereas the positively worded questions align more closely with constructs such as extraversion42,43. S-SIAS utilises a 0 (Not at all characteristic of me) to 4 (Extremely characteristic of me) Likert-type scale. The S-SIAS measure a person’s general levels of anxiety when initiating and maintain social interaction. Total scores are calculated by adding the individual scores for all 17 items. Score range is between 0 and 68, with higher scores indicating higher social interaction anxiety or increased social anxiety symptoms. SIAS has demonstrated excellent psychometric utility as a measure of social anxiety41. Cronbach’s α for S-SIAS in the current study was 0.94.
Depression
Depression symptoms were measured using the Centre for Epidemiological Studies-Depression (CES-D) scale44. The CES-D consists of 20 statements which assess individuals' experience of depressive symptoms on a 0 (rare or none of the time) to 3 (Most or all of the time) Likert-type scale over a 1-week period44. Composite scores range from 0 to 60, with higher scores indicating higher experience of depression symptoms. The CES-D has demonstrated excellent internal reliability44. Cronbach’s α for CES-D in the present study was 0.94.
Physiological tests for CVH/CVD indicators
BMI
BMI was measured with the Tanita scale which uses Bioelectrical Impedance Technology to determine body composition. BMI values were calculated using the participants' weight and height measurements and reported in kg/m2. Higher BMI is associated with poorer CVH45.
Blood pressure
Systolic and diastolic blood pressure was measured using a calibrated automated sphygmomanometer (Omron sphygmometer model HEM-7121). Prior to measurement, participants were asked to remove any restrictive clothing which could affect their blood flow. They were also asked to refrain from talking and using their mobile phones during testing. Participants were in an upright seated position and, after 5 min rest, three consecutive readings were taken from the right arm over a five-minute period. If large differences in readings were observed, additional measurements were taken. Mean systolic and diastolic blood pressure values in mmHg were calculated from the readings. Higher systolic and diastolic blood pressure are indicators of poor CVH and a risk factor for CVDs45.
Vascular function
Endothelial function
Endothelial function was measured non-invasively using a pulse amplitude tonometry device—EndoPat (2000 Itamar). Use of EndoPat to measure endothelial function has been well validated against industry standard—flow-mediated dilation method46,47. Endothelial dysfunction is a reliable predictor of CVH issues, and CVD. In fact, endothelial dysfunction has been identified as the earliest precursor for atherosclerosis (type of CVD)48,49. Prior to testing, participants were instructed to lie in a supine position on the laboratory room bed. A deflated blood pressure cuff was placed on the left arm of the participants and two EndoPat finger probes were placed on the tip of each index finger (or middle finger if more suitable) and then inflated. The test took 15 min to complete, and the participants were instructed to stay still and to be quiet for the duration of the test. Baseline or pre-occlusion measurements were recorded for five minutes, followed by five-minute occlusion of the left arm. Occlusion was achieved by inflating the cuff on the participants' upper arm to supra-systolic pressure (60 mmHg above participant’s systolic pressure or to a maximum of 200 mmHg). Occlusion was released after five minutes to induce reactive hyperemia (i.e., interruption of blood flow). Post-occlusion measurements were taken for five minutes. Reactive Hyperemia Index (RHI) was used as an indicator for vascular function. RHI is calculated by dividing the pre-to-post occlusion signal amplitude index in the occluded arm to the corresponding ratio in the control arm to calculate RHI. Lower RHI value is indicative of endothelial dysfunction and poor CVH50,51.
Arterial stiffness
Arterial stiffness was assessed using a SphygmoCor XCEL AtCor Medical device which utilises central arterial pressure waveform analysis (pulse wave analysis) and carotid-femoral pulse wave velocity as measures of arterial stiffness. Arterial stiffness is a well-established predictor for CVD52.
At the start of the test, the participant was asked to lie in a supine position and to minimise movement and speaking during the test. For pulse wave analysis, a brachial cuff was placed on the participants' upper right arm to capture brachial systolic and diastolic blood pressure, and brachial waveform. Results for pulse wave analysis were automatically calculated by SphygmoCor XCEL software. Repeated measurements (minimum two) were taken to ensure consistency. This test took five minutes to complete. Pulse wave analysis results were reported as Augmentation Index (Alx) in this study. Alx is widely accepted as a surrogate measure for arterial stiffness and increased or higher Alx value is indicative of poor CVH53,54.
For pulse wave velocity, a cuff was placed around the participants' femoral artery to capture the femoral waveform, and a tonometer was placed on the carotid artery to capture the carotid waveform. In this study, pulse wave velocity results were reported using Pulse Transit Time (PTT) in milliseconds and Carotid-femoral Pulse Wave Velocity (cfPWV) in m/s. The distance between the carotid and femoral arteries was measured, and the velocity automatically determined by dividing the distance by the PTT. Shorter or lower PTT and higher or faster cfPWV suggest arterial stiffness, and potential vascular damage55. Both PTT and cfPWV are well established indicators for arterial stiffness55–57.
Autonomic function
Sudomotor function
Sudomotor function was assessed using SUDOSCAN (Impeto Medical). It is a quick and non-invasive test which can detect abnormality in autonomic neuropathy or dysfunction as well as predict diabetes development58,59. SUDOSCAN measures sweat chloride concentrations (i.e., sweat gland function) through electrochemical skin conductance (ESC) of hands and feet using reverse ionphoresis. Participants placed their hands and feet on two low voltage (≤ 4 V) stainless steel electrodes for approximately two minutes. Direct current is applied to the electrodes, stimulating the sweat glands to release electrically charged chloride ions onto the skin's surface. Sudomotor function indicators used in the present study are mean feet ESC (µS) and mean hands ESC (µS). Vinik et al. demonstrated the efficacy of SUDOSCAN in measuring hands and feet ESC as indicators of sudomotor function. Lower feet and hands ESC (µS) are indicative of poorer sudomotor function60.
HRV
HRV was measured using beat-to-beat blood pressure and heart rate readings. Participants were in supine position; readings were captured over a 5-min period using three electrocardiogram leads and PowerLab—a physiological data acquisition device. HRV indicators were calculated in LabChart—a physiological data analysis software. For brevity, we have only included two HRV indicators in the present study—% low frequency power (%LF) and % high frequency power (%HF). Both LF power and HF power are commonly used and well-established indicators of HRV; increase in %LF value and decrease in %HF value are indicative of poor CVH61,62.
Data analytic approach
Both the first research aim—investigate the relationship between loneliness and CVH indicator in young adults, after accounting for social anxiety and depression, and the second study aim—examining differences in the pathways through which loneliness, social anxiety, and depression affect CVH indicators in young adults were answered using Hierarchical Multiple Regression. For research aim 1 –in the hierarchical multiple regression model, the predictor variable was loneliness and outcome variables were CVH indicators. Covariates included age, gender, social anxiety, and depression scores. In Step 1 of the model, age and gender were entered as covariates, followed by the inclusion of social anxiety and depression scores (as covariates—guided by previous empirical research30,37–39) in Step 2. Finally, loneliness scores were added in Step 3. This process was repeated for each outcome variable: BMI, blood pressure, vascular function (i.e., endothelial function and arterial stiffness), and autonomic function (sudomotor function and HRV). The findings from this model are presented in Table 4, Model 1.
Table 4.
Hierarchal multiple regression models.
| Outcome variables | Model 1—loneliness (predictor) | Model 2—social anxiety (predictor) | Model 3—depression (predictor) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | R2change | p | β | R2change | p | β | R2change | p | |
| BMI | |||||||||
| BMI (kg/m2) | − 0.14 | 0.006 | 0.54 | 0.20 | 0.15 | 0.31 | 0.19 | 0.014 | 0.33 |
| Blood pressure | |||||||||
| Systolic blood pressure (mmHg) | 0.10 | 0.000 | 0.93 | − 0.21 | 0.017 | 0.27 | 0.15 | 0.009 | 0.42 |
| Diastolic blood pressure (mmHg) | − 0.083 | 0.002 | 0.71 | 0.068 | 0.002 | 0.74 | 0.11 | 0.005 | 0.57 |
| Vascular function | |||||||||
| Endopat—RHI | 0.30 | 0.027 | 0.20 | 0.21 | 0.16 | 0.32 | − 0.43 | 0.073 | 0.036 |
| Pulse wave analysis—Alx | 0.026 | 0.000 | 0.90 | 0.11 | 0.004 | 0.57 | − 0.037 | 0.001 | 0.84 |
| Pulse wave velocity—PTT (ms) | − 0.70 | 0.15 | 0.002 | 0.54 | 0.10 | 0.008 | 0.238 | 0.023 | 0.20 |
| Pulse wave velocity—cfPWV (m/s) | 0.28 | 0.024 | 0.20 | − 0.24 | 0.02 | 0.24 | − 0.035 | 0.000 | 0.85 |
| Autonomic function | |||||||||
| Sudomotor—Feet ESC (µS) | − 0.18 | 0.01 | 0.38 | 0.33 | 0.04 | 0.09 | 0.17 | 0.011 | 0.36 |
| Sudomotor—Hands ESC (µS) | 0.16 | 0.008 | 0.45 | 0.16 | 0.008 | 0.45 | − 0.012 | 0.000 | 0.95 |
| HRV—LF (%) | − 0.40 | 0.045 | 0.068 | 0.092 | 0.003 | 0.63 | 0.22 | 0.19 | 0.23 |
| HRV—HF (%) | 0.19 | 0.011 | 0.39 | − 0.38 | 0.053 | 0.06 | 0.089 | 0.003 | 0.65 |
N = 66. Hierarchical Multiple Regression analyses results. β = Standardised regression parameter estimates. β values specified in the table are for the last predictor variable entered in each model (italicised here). Predictors entered: Model 1—Age, gender, depression, social anxiety, loneliness. Model 2—Age, gender, loneliness, depression, social anxiety; Model 3—Age, gender, loneliness, social anxiety, depression.
Significant values are bolded, p < 0.05.
To ascertain how loneliness, social anxiety, and depression differed in their associations with different CVH indicators, we ran additional two hierarchical multiple regression models. In the first additional model, we examined the unique relationship between social anxiety scores and various CVH indicators in young adults, while controlling for age, gender, depression, and loneliness scores. The model involved three steps. In Step 1, we entered age and gender as covariates. For Step 2, loneliness and depression scores were included as covariates, followed by the addition of the predictor variable—social anxiety—in Step 3. This model was repeated for each outcome variable: BMI, blood pressure, vascular function (i.e., endothelial function and arterial stiffness), and autonomic function (sudomotor function and HRV). The results for this model are showcased in Table 4, Model 2. Likewise, in the second additional model, the unique relationship between depression scores and different CVH indicators in young adults was assessed (after controlling for age, gender, social anxiety, and loneliness scores). The model followed the same three-step process, with age and gender entered in Step 1, loneliness and social anxiety scores (covariates) in Step 2, and depression scores as the predictor variable in Step 3. This model was also applied to each outcome variable, with results presented in Table 4, Model 3.
In addition to the hierarchical multiple regression models, we also ran a correlation matrix to further showcase the interrelated nature of loneliness, social anxiety, and depression. The results for these are presented in Table 3.
Table 3.
Correlational matrix for loneliness, social anxiety, and depression scores.
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Loneliness | – | 0.74** | 0.75** |
| 2. Social anxiety | – | – | 0.69** |
| 3. Depression | – | – | – |
N = 66. Significant p-values are bolded, **p < 0.001.
Results
Participant characteristics are presented in Table 1. For categorical variables, percentages were included, and for continuous variables, mean and standard deviation (SD) were included. Majority of the participants were single or non-married females who resided with at least one other person and reported no previous diagnosis of CVD.
The data were analysed using IBM Statistical Package for Social Sciences Version 27.0. The descriptive statistics including, mean and SD values for loneliness, social anxiety, depression, and CVH indicators are presented in Table 2. We have also included the score range observed in the study for aforementioned questionaries and CVH tests in Table 2. All data cleaning, screening, and assumption testing details are included in the Supplementary Material.
Table 2.
Descriptive statistics (N = 66).
| Mean | SD | Score range | |
|---|---|---|---|
| Test | |||
| Loneliness | 52.75 | 1.85 | 28–75 |
| Social anxiety | 34.78 | 2.68 | 2–59 |
| Depression | 24.41 | 2.45 | 3–50 |
| BMI | |||
| BMI (kg/m2) | 24.95 | 5.47 | 17.6–44.7 |
| Blood pressure | |||
| Systolic blood pressure (mmHg) | 113.21 | 12.41 | 90–146 |
| Diastolic blood pressure (mmHg) | 72.14 | 8.91 | 58–104 |
| Vascular function | |||
| Endopat—RHI | 2.12 | 0.66 | 1.2–4.0 |
| Pulse wave analysis—Alx | 8.26 | 12.31 | -17.0–33.0 |
| Pulse wave velocity—PTT (ms) | 114.80 | 35.01 | 65–233 |
| Pulse wave velocity—cfPWV (m/s) | 5.36 | 1.64 | 2.30–12.4 |
| Autonomic function | |||
| Sudomotor—Feet ESC (µS) | 82.59 | 7.31 | 54–92 |
| Sudomotor—Hands ESC (µS) | 74.18 | 11.91 | 45–92 |
| HRV—LF (%) | 24.74 | 9.97 | 6.5–58.1 |
| HRV—HF (%) | 42.97 | 17.27 | 12.7–80.3 |
Correlation matrix for loneliness, social anxiety, and depression are presented in Table 3. There were strong, positive, and significant relationships between loneliness, social anxiety, and depression. These directions were as expected.
The hierarchal multiple regressions results are presented below in Table 4. As mentioned previously, Model 1 includes the results for study aim 1—the relationship between loneliness and CVH indicators in young adults after accounting for social anxiety and depression. The results revealed that after controlling for age, gender, social anxiety, and depression, higher loneliness was significantly associated with lower or shorter PTT (i.e., measure for arterial stiffness) in young adults. Additionally, the R2change statistic also shows that loneliness scores explain 15% of the unique variance in PTT among young adults.
Results for two additional hierarchal multiple regressions assessing study aim two (investigating whether loneliness, social anxiety, and depressions differ in their associations with different CVH indicators in young adults) are presented in Table 4—Models 2 and 3. Higher social anxiety was associated with higher PTT (Table 4, Model 2), after controlling for covariates. Lastly, we found higher scores for depression to be significantly associated with lower RHI (i.e., measure of endothelial function) among young adults, after controlling for age, gender, loneliness, and social anxiety (Table 4, Model 3). Depression scores also accounted for 7.3% of the variance in RHI scores.
Discussion
The aim of the present study was to investigate the unique relationship between loneliness and CVH indicators in young adults after controlling for confounding effects of depression and social anxiety. We also examined how loneliness, social anxiety, and depression differ in their associations with various CVH indicators in young adults.
We found a unique and significant relationship between loneliness and one of the vascular function indicators in young people. Higher loneliness in young adults was associated with lower PTT, after controlling for social anxiety and depression (Model 1 in Table 4). Shortened PTT is an early sign of arterial stiffness and vascular dysfunction55. A lower PTT may occur due to a sudden increase in blood pressure, leading to a rise in vascular tone and subsequent arterial wall stiffness57,63. The results of this study make a unique contribution to the literature by demonstrating that young adults with loneliness may already be exhibiting signs of vascular dysfunction or damage, evident as early as in their twenties (mean age for the present study was 22.70 years). These findings are alarming as they indicate that young adults experiencing higher levels of loneliness may also have shorter PTT, potentially contributing to arterial stiffness and reducing the arteries' ability to adapt to changes in blood pressure and flow. Consequently, prolonged arterial stiffness and vascular dysfunction may result in arterial damage and increase their risk of developing CVDs64–66.
Additionally, some differences in the pathways through which loneliness, depression, and social anxiety influence vascular function in young adults were noted, mainly between loneliness and depression. Higher depression was significantly associated with lower RHI—an indicator for endothelial dysfunction, even after controlling for loneliness and social anxiety (Table 4 Model 3). The endothelium is a membrane surrounding the insides of the heart and blood vessels and its main role is to maintain homeostasis by regulating vasodilation and vasoconstriction of the blood vessels67. Arterial stiffness, on the other hand, refers to the rigidity or stiffness of the arterial wall52. Both endothelial dysfunction and arterial stiffness are related to vascular damage/dysfunction and have been identified as independent risk factors for several CVDs48,52,65. Indeed, endothelial dysfunction is widely acknowledged as one of the earliest indicators of atherosclerosis (i.e., type of major CVD)48,49, although it can potentially be reversible in early stages68. On the other hand, arterial stiffness is a more progressed form of vascular damage and a contributing factor to a number of other CVDs, such as myocardial infarction, heart failure, and even mortality66. It is possible that CVH abnormalities such as endothelial dysfunction manifest earlier in young people with depression symptoms. Indeed, several studies have found an association between depression and endothelial dysfunction69,70. However, it should be noted that we did not control for any other known covariates of depression (e.g., genetics, previous diagnosis of major depressive disorder). These findings indicate that young people with more depressive symptoms are more likely to present with earlier indicators for poor CVH, whereas young people who report higher loneliness may present equally concerning impairment in CVH, although in a more progressed form.
While previous research suggests that loneliness may be an antecedent to depression30,37, it is possible that the relationship between these constructs and how they affect CVH may be more complex and closely intertwined than we anticipated (e.g., reciprocal or cyclic in nature). Interestingly, in the current study, higher social anxiety was associated with higher PTT in young adults (after controlling for loneliness and depression, Table 4 Model 2). In other words, higher social anxiety was related to improved vascular function. This finding was unexpected, as we would assume social anxiety to follow the same trajectory and directionality as loneliness in relation to PTT, given that previous research indicates a reciprocal relationship between the two constructs38,39. Similar to depression, it should be noted that we did not account for the influence of any confounding variables for social anxiety (e.g., previous social anxiety disorder diagnosis) which may have affected the results. Future research with a broader consideration of the confounding variables could help validate and extent these findings. Nonetheless, ultimately, both higher loneliness and depression are related to impaired vascular function and poor CVH in young adults. Future research focusing on loneliness and depression, and how they impact CVH and increase risk for CVD, especially in young adults, would be a pertinent and strategic approach to facilitate early intervention and disease prevention.
We found no relationship between loneliness and autonomic function, BMI, and blood pressure in young adults after controlling for social anxiety and depression (Model 1 in Table 4). While these results are inconsistent with some of the reviewed studies28–32, they are congruent with some other research findings33–36. There are several possible explanations for the non-significant findings. For instance, hardly any of the abovementioned studies with inconsistent results accounted for the influence of concomitant mental health issues such as social anxiety and depression. Thus, it is plausible that some of the predictive and explanatory power attributed to loneliness in regard to CVH indicators in previous research was in fact the influence of concurrent mental health symptoms, such as social anxiety and depression. Indeed, findings from some of the congruent studies demonstrate that a positive relationship between BMI and loneliness is only present when relevant covariates are not considered35,36. Our findings further emphasise the importance of controlling for highly correlated mental health symptoms, such as social anxiety and depression, when investigating the influence of loneliness on health or CVH, especially in young adults.
Additionally, it is also possible that some of the CVH indicators used in previous studies were not sufficiently sensitive. Most research on loneliness and CVD has utilised CVH indicators such as blood pressure, HRV, and BMI. It may be that these CVH indicators are not sensitive or discernible enough to detect small differences or associations concerning loneliness and its influence on CVH, particularly in otherwise healthy young adults with no existing CVD issues, as was the case in the present study. These findings further highlight the necessity of using early or subclinical markers for CVD/CVH such as vascular function, especially when working with young adults, as they are unlikely to present with significant CVD/CVH issues. These recommendations are also supported by our results for loneliness and PTT. As mentioned previously, PTT refers to the time it takes for a pulse wave to travel between two arterial sites; the speed at which this arterial pulse wave travel’s is inversely proportional to blood pressure71,72. PTT is understood to be strongly influenced by blood pressure71–75. However, the pulse wave, from which PTT is calculated, moves much faster than blood. Consequently, pulse wave and PTT can detect minute and subtle physiological or cardiovascular changes that may not be discernible when using more common CVH such as direct blood pressure measurement. Our findings suggest that PTT, which is a subclinical indicator for CVH/CVD, may be a better predictor for impaired vascular function and poor CVH than more traditionally used measures such as blood pressure, HRV, and BMI, especially in research involving young adults. Future research should focus on the use of subclinical CVH indicators as they may help predict CVH issues earlier in life52 and as such, facilitate primary and secondary prevention of cardiovascular events. An alternate explanation for the observed lack of relationship between loneliness and some of the CVH indicators (e.g., blood pressure) could be due to the diverse roles these factors play in influencing CVH outcomes. Rather than directly influencing CVH, it is possible that some of the CVH indicators may have a moderating or mediating effect on the relationship between loneliness and CVH markers.
The present study has some limitations. It is important to consider the cross-sectional study design, small sample size, generalisability, and ecological validity of our findings. For example, our participants were primarily female university students who spoke English as a first language, resided in the state of Victoria, and who were living with other people. As such, our sample may not be representative of all Australian young adults. Additionally, use of cross-sectional study design restricted our ability to draw causal inferences or ascertain directionality between loneliness and various CVH indicators in young adults. Future researchers should adopt a longitudinal study design to potentially replicate and extend the findings from the current study and establish causality. For instance, future research could focus on investigating the influence of other common covariates related to CVDs (e.g., genetics and medical history). Other methodological and statistical limitations include the different time periods over which loneliness and depression symptoms were measured (i.e., loneliness was assessed over the past month using UCLA-LS, while depression was assessed over the past seven days using CES-D). Furthermore, it is important to note that no additional corrections for multiple comparisons were applied to the data, as hierarchical multiple regression was utilised. In this approach, covariates and predictors were entered into the model in a stepwise manner (see Table 4), and the study was not exploratory in nature, as only one a-priori hypothesis was tested at a given time76. Nonetheless, these methodological and statistical shortcomings should be taken into account when interpreting the results. Additionally, we do not know how different levels of loneliness, depression, and social anxiety (e.g., low loneliness and high depression) interact to influence different CVH markers in young adults over time. Investigating these relationships will help establish causality and better elucidate the multifaceted relationship between loneliness, depression, and social anxiety, and their combined and individual effects on CVH in young adults.
Clinical implications
While we were limited by our ability to generalise the results to a broader young adult population and establish causality, we believe our findings have important clinical implications, particularly in delivering early intervention for CVD. Our findings suggest that young adults with loneliness are at a higher risk for developing CVD. As such, we would urge healthcare professionals to measure loneliness when assessing a patient’s risk of developing CVD. Doing so would allow healthcare professionals to develop more personalised and holistic interventions, focusing on many of the interdependent factors that may contribute to the relationship between higher loneliness and poorer CVH in young adults, as well as primary and secondary CVD prevention. The clinical implications and applications of our findings are more grounded in preventative healthcare, rather than the more traditional approach of curative healthcare77. Long term studies are urgently needed to clearly establish the link between loneliness and risk of developing CVD in young adults, after accounting for the role of various highly correlated mental health issues. In particular, how does more persistent states of loneliness (i.e., chronicity) influence the development of CVD?
Conclusion
We demonstrated higher loneliness is associated with poorer CVH, in particular impaired vascular function (i.e., a well-established risk factor for several CVDs) in young adults, after controlling for the influence of known confounding mental health issues. Additionally, we also highlight the different pathways through which loneliness and depression may affect CVH in young adults, namely arterial stiffness and endothelial function respectively.
Supplementary Information
Author contributions
SV was involved in study conceptualisation, data collection and analysis, writing the first draft of manuscript. MHL, NE, and EL were involved in project supervision, study conceptualisation, data interpretation, as well as reviewing the manuscript.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due ethical restrictions, but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-65039-8.
References
- 1.Hunter D. Loneliness: A public health issue. Perspect. Public Health. 2012;132:153. doi: 10.1177/1757913912449564. [DOI] [PubMed] [Google Scholar]
- 2.Lim MH, Eres R, Vasan S. Understanding loneliness in the twenty-first century: An update on correlates, risk factors, and potential solutions. Soc. Psychiatry. Psychiatr. Epidemiol. 2020 doi: 10.1007/s00127-020-01889-7. [DOI] [PubMed] [Google Scholar]
- 3.Surkalim DL, et al. The prevalence of loneliness across 113 countries: Systematic review and meta-analysis. BMJ. 2022;376:e067068. doi: 10.1136/bmj-2021-067068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badcock, J. C., Holt-Lunstad, J., Garcia, E., Bombaci, P. & Lim, M. H. Position statement: Addressing social isolation and loneliness and the power of human connection. https://www.gilc.global/_files/ugd/410bdf_74fffc2d18984b0e8217288b1b12d199.pdf
- 5.Asher SR, Paquette JA. Loneliness and peer relations in childhood. Curr. Dir. Psychol. Sci. 2003;12:75–78. doi: 10.1111/1467-8721.01233. [DOI] [Google Scholar]
- 6.de Jong Gierveld J, Havens B. Cross-national comparisons of social isolation and loneliness: Introduction and overview. Can. J. Aging. 2004;23:109–113. doi: 10.1353/cja.2004.0021. [DOI] [PubMed] [Google Scholar]
- 7.Cacioppo, J. T. & Cacioppo, S. In Advances in Experimental Social Psychology Vol. 58 (ed. Olson, J.) 127–197 (Academic Press, 2018).
- 8.Cacioppo JT, Cacioppo S, Boomsma DI. Evolutionary mechanisms for loneliness. Cogn. Emot. 2014;28:3–21. doi: 10.1080/02699931.2013.837379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim, M. & Australian Psychological Society. Australian loneliness report: A survey exploring the loneliness levels of Australians and the impact on their health and wellbeing (2018). https://psychweek.org.au/2018-archive/loneliness-study/
- 10.Hawkley LC, Cacioppo JT. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich LM, Gullone E. The clinical significance of loneliness: A literature review. Clin. Psychol. Rev. 2006;26:695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn. Sci. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosom. Med. 2009;71:836. doi: 10.1097/psy.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawkley LC, Thisted RA, Masi CM, Cacioppo JT. Loneliness predicts increased blood pressure: 5-year cross-lagged analyses in middle-aged and older adults. Psychol. Aging. 2010;25:132–141. doi: 10.1037/a0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102:1009–1016. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valtorta NK, Kanaan M, Gilbody S, Hanratty B. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur. J. Prev. Cardiol. 2020;25:1387–1396. doi: 10.1177/2047487318792696. [DOI] [PubMed] [Google Scholar]
- 17.Australian Institute of Health and Welfare. Cardiovascular disease: Australian facts 2011. (Australian Institute of Health and Welfare, Canberra, 2011). https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/cardiovascular-disease-australian-facts-2011
- 18.Lasgaard M, Friis K, Shevlin M. "Where are all the lonely people?" A population-based study of high-risk groups across the life span. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51:1373–1384. doi: 10.1007/s00127-016-1279-3. [DOI] [PubMed] [Google Scholar]
- 19.Lim, M. H., Eres, R. & Peck, C. The Young Australian Loneliness Survey: Understanding loneliness in adolescents and young adults (2019). (Swinburne University of Technology and VicHealth). https://www.vichealth.vic.gov.au/loneliness-survey.
- 20.Cigna. Loneliness and the workplace: 2020 U.S. Report (2020). https://www.cigna.com/static/www-cigna-com/docs/about-us/newsroom/studies-and-reports/combatting-loneliness/cigna-2020-loneliness-factsheet.pdf
- 21.Ernst M, et al. Loneliness before and during the COVID-19 pandemic: A systematic review with meta-analysis. Am. Psychol. 2022;77:660–677. doi: 10.1037/amp0001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SS, Pilkerton CS, Shrader CD, Frisbee SJ. Subclinical atherosclerosis, cardiovascular health, and disease risk: Is there a case for the Cardiovascular Health Index in the primary prevention population? BMC Public Health. 2018;18:429. doi: 10.1186/s12889-018-5263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017;6:2048004016687211. doi: 10.1177/2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomiyama H, Yamashina A. Non-invasive vascular function tests: Their pathophysiological background and clinical application. Circ. J. 2010;74:24–33. doi: 10.1253/circj.cj-09-0534. [DOI] [PubMed] [Google Scholar]
- 25.Lambert E, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–358. doi: 10.1161/hypertensionaha.110.155663. [DOI] [PubMed] [Google Scholar]
- 26.Lambert EA, Lambert GW. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr. Hypertens. Rep. 2011;13:244–248. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA. 1996;275:1571–1576. doi: 10.1001/jama.1996.03530440051036. [DOI] [PubMed] [Google Scholar]
- 28.Hajek A, König HH. Obesity and loneliness. Findings from a longitudinal population-based study in the second half of life in Germany. Psychogeriatr. 2019;19:135–140. doi: 10.1111/psyg.12375. [DOI] [PubMed] [Google Scholar]
- 29.Cacioppo JT, et al. Loneliness and health: Potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging. 2010;25:453. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson SJ, et al. Loneliness and telomere length: Immune and parasympathetic function in associations with accelerated aging. Ann. Behav. Med. 2019;53:541–550. doi: 10.1093/abm/kay064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roddick CM, Chen FS. Effects of chronic and state loneliness on heart rate variability in Women. Ann. Behav. Med. 2020;55:460–475. doi: 10.1093/abm/kaaa065. [DOI] [PubMed] [Google Scholar]
- 33.Das A. Loneliness does (not) have cardiometabolic effects: A longitudinal study of older adults in two countries. Soc. Sci. Med. 2019;223:104–112. doi: 10.1016/j.socscimed.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30:377–385. doi: 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- 35.Oser, T. K. et al. (2021) Loneliness in primary care patients: Relationships with body mass index and health care utilization. J. Patient Cent. Res. Rev. 8, 239–247. 10.17294/2330-0698.1808 [DOI] [PMC free article] [PubMed]
- 36.Kobayashi LC, Steptoe A. Social isolation, loneliness, and health behaviors at older ages: Longitudinal cohort study. Ann. Behav. Med. 2018;52:582–593. doi: 10.1093/abm/kax033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erzen E, Çikrikci Ö. The effect of loneliness on depression: A meta-analysis. Int. J. Soc. Psychiatry. 2018;64:427–435. doi: 10.1177/0020764018776349. [DOI] [PubMed] [Google Scholar]
- 38.Lim MH, Rodebaugh TL, Zyphur MJ, Gleeson JF. Loneliness over time: The crucial role of social anxiety. J. Abnorm. Psychol. 2016;125:620–630. doi: 10.1037/abn0000162. [DOI] [PubMed] [Google Scholar]
- 39.Maes M, et al. Loneliness and social anxiety across childhood and adolescence: Multilevel meta-analyses of cross-sectional and longitudinal associations. Devl. Psychol. 2019;55:1548–1565. doi: 10.1037/dev0000719. [DOI] [PubMed] [Google Scholar]
- 40.Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. J. Pers. Assess. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 41.Brown EJ, et al. Validation of the social interaction anxiety scale and the Social Phobia Scale across the anxiety disorders. Psychol. Assess. 1997;9:21–27. doi: 10.1037/1040-3590.9.1.21. [DOI] [Google Scholar]
- 42.Rodebaugh TL, et al. More reasons to be straightforward: Findings and norms for two scales relevant to social anxiety. J. Anxiety Disord. 2011;25:623–630. doi: 10.1016/j.janxdis.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodebaugh TL, Woods CM, Heimberg RG. The reverse of social anxiety is not always the opposite: The reverse-scored items of the Social Interaction Anxiety Scale do not belong. Behav. Ther. 2007;38:192–206. doi: 10.1016/j.beth.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 45.Australian Institute of Health and Welfare. Cardiovascular disease (2019). (Australian Institute of Health and Welfare, Canberra). https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/cardiovascular-health-compendium
- 46.Haller MJ, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr. Diabetes. 2007;8:193–198. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 47.Meeme A, Buga GA, Mammen M, Namugowa A. Endothelial dysfunction and arterial stiffness in pre-eclampsia demonstrated by the EndoPAT method. Cardiovasc. J. Afr. 2017;28:23–29. doi: 10.5830/cvja-2016-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 49.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Circulation. 2007;115:1285–1295. doi: 10.1161/circulationaha.106.652859. [DOI] [PubMed] [Google Scholar]
- 50.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp. 2010 doi: 10.3791/2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuvin JT, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 52.Lee J-G, Joo S-J. Arterial stiffness and cardiovascular risk. Korean J. Intern. Med. 2019;34:504–506. doi: 10.3904/kjim.2019.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm B, et al. Increased arterial augmentation and augmentation index as surrogate parameters for arteriosclerosis in subjects with diabetes mellitus and nondiabetic subjects with cardiovascular disease. J. Diabetes Sci. Technol. 2007;1:260–263. doi: 10.1177/193229680700100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nürnberger J, et al. Can arterial stiffness parameters be measured in the sitting position? Hypertens. Rep. 2011;34:202–208. doi: 10.1038/hr.2010.196. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y-L, Zheng Y-Y, Ma Z-C, Sun Y-N. Radial pulse transit time is an index of arterial stiffness. Hypertens. Rep. 2011;34:884–887. doi: 10.1038/hr.2011.41. [DOI] [PubMed] [Google Scholar]
- 56.Doupis J, Papanas N, Cohen A, McFarlan L, Horton E. Pulse wave analysis by applanation tonometry for the measurement of arterial stiffness. Open Cardiovasc. Med. J. 2016;10:188–195. doi: 10.2174/1874192401610010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 2005;18:3s–10s. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. J. 2011;37:527–532. doi: 10.1016/j.diabet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Provitera V, et al. Evaluation of sudomotor function in diabetes using the dynamic sweat test. Neurology. 2010;74:50–56. doi: 10.1212/WNL.0b013e3181c7da4b. [DOI] [PubMed] [Google Scholar]
- 60.Vinik AI, Nevoret ML, Casellini C. The new age of sudomotor function testing: A sensitive and specific biomarker for diagnosis, estimation of severity, monitoring progression, and regression in response to intervention. Front. Endocrinol. (Lausanne) 2015;6:94. doi: 10.3389/fendo.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front. Public Health. 2017 doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lombardi F, et al. Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am. J. Cardiol. 1987;60:1239–1245. doi: 10.1016/0002-9149(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 63.Smith RP, Argod J, Pépin JL, Lévy PA. Pulse transit time: An appraisal of potential clinical applications. Thorax. 1999;54:452–457. doi: 10.1136/thx.54.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foo JY, Lim CS. Pulse transit time as an indirect marker for variations in cardiovascular related reactivity. Health Technol. 2006;14:97–108. doi: 10.3233/thc-2006-14205. [DOI] [PubMed] [Google Scholar]
- 65.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.Cir.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 66.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–943. doi: 10.1161/01.Atv.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 67.Vita JA, Keaney JF. Endothelial function. Circulation. 2002;106:640–642. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 68.Ross R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 69.Lavoie, K. L., Pelletier, R., Arsenault, A., Dupuis, J. & Bacon, S. L. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom. Med. 72 (2010). https://journals.lww.com/psychosomaticmedicine/Fulltext/2010/01000/Association_Between_Clinical_Depression_and.4.aspx [DOI] [PubMed]
- 70.Tiemeier H, et al. Relationship between atherosclerosis and late-life depression: The Rotterdam Study. Arch. Gen. Psychiatry. 2004;61:369–376. doi: 10.1001/archpsyc.61.4.369. [DOI] [PubMed] [Google Scholar]
- 71.Mukkamala R, et al. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE. Trans. Biomed. Eng. 2015;62:1879–1901. doi: 10.1109/TBME.2015.2441951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong MY, Poon CC, Zhang YT. An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: A half year study on normotensive subjects. Cardiovasc. Eng. 2009;9:32–38. doi: 10.1007/s10558-009-9070-7. [DOI] [PubMed] [Google Scholar]
- 73.Gao, M., Olivier, N. B. & Mukkamala, R. Comparison of noninvasive pulse transit time estimates as markers of blood pressure using invasive pulse transit time measurements as a reference. Physiol. Rep. 4, e12768 (2016). 10.14814/phy2.12768 [DOI] [PMC free article] [PubMed]
- 74.Gesche H, Grosskurth D, Küchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: Comparison to a cuff-based method. Eur. J. Appl. Physiol. 2012;12:309–315. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 75.Radha M, et al. Arterial path selection to measure pulse wave velocity as a surrogate marker of blood pressure. Biomed. Phys. Eng. Express. 2017;3:015022. doi: 10.1088/2057-1976/aa5b40. [DOI] [Google Scholar]
- 76.Menon V. Multiple testing and protection against Type I error using p value correction: Application in cross-sectional study designs. Indian J. Psychol. Med. 2019;41:197. doi: 10.4103/IJPSYM.IJPSYM_12_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Visseren FLJ, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due ethical restrictions, but are available from the corresponding author on reasonable request.