Abstract
Drought stress (DS) is a significant abiotic stress that limits agricultural productivity worldwide. In semi-arid climates, one potential solution to alleviate the deleterious effects of drought is the use of soil amendments such as nanoparticles. The current research was conducted out to probe the sway of drought at critical growth stages (CGS) of wheat crop (D0: Control, D1: Drought at tillering stage, and D2: Drought at anthesis stage) and the application of Cu-nanoparticles (T0: 0 mg L−1, T1: 300 mg L−1, T2: 700 mg L−1, and T3: 950 mg L−1) in order to improve drought resilience. Results of the study revealed that DS considerably decreased the wheat growth and yield during CGS. However, Cu-nanoparticles application alleviated the detrimental backlash of DS and led to improvements in various aspects of wheat growth and yield, including plant height, spike length, 1000 grain weight, stomatal conductance, leaf chlorophyll content, water use efficiency, leaf turgor potential, relative water content, and ultimately the grain yield. The use of principal component analysis allowed us to integrate and interpret the diverse findings of our study, elucidating the impact of Cu-nanoparticle treatment on wheat growth and yield under drought. Overall, the study concluded that DS during the anthesis stage had the most significant negative impact on crop yield. However, applying Cu-nanoparticles at the rate of 300 mg L−1 proved to be an effective strategy for improving crop productivity by reducing the harmful effects of drought.
Keywords: Wheat, Drought, Cu-nanoparticles, Growth, Wheat production
Subject terms: Biochemistry, Plant sciences
Introduction
World increasing population has a significant impact on agriculture, requiring drought tolerant crops to bridge the gap between food supply and demand. Drought stress (DS) poses a significant threat to global food security, particularly in semi-arid regions1. Under this scenario, there is a dire need to develop to such strategies that can increase crop productivity and sustainability having the potential to minimize the ill effects of drought. In recent years, nanotechnology has emerged as a promising field offering novel solutions for sustainable agriculture.
Wheat (Triticum aestivum L.) is an important staple food in many regions of the world, including South Asia, America, and Europe. It is called as king of cereals and ranks first among the cereals due to its high nutritional value2,3. It has protein, fiber, fat, and carbohydrates content of 9.4–13.9, 1.8–2.3, 1.2–2.5, and 69.1–75.5, g/100, respectively4,5. It is a vital source of nutrition for humans and animals, and its production is gradually threatened by drought episodes. Therefore, it is imperative to use modern technologies to enhance wheat yield and drought resilience in order to maintain wheat's status as a staple food6.
Drought, the major abiotic stress, not only reduces development and yield, but it also diminishes global crop output7. More catastrophic drought periods are projected in the next years, with an increase in average world temperature by 1.5 °C owing to climate variation, which would severely disrupt agricultural production and the farming community8. A severe shortage of water can cause damage to plant cell membrane and cell wall architecture, as well as impede cell division and photosynthesis9. According to El-Fattah et al.10 and Raza et al.11, the exposure of crop plants to DS led to a notable decline in photochemical activities and the suppression of enzyme activities involved in the Calvin cycle. Drought and other abiotic stresses can induce considerable changes in seed protein levels and production by disrupting plant development processes12,13. DS during critical growth stages (CGS) has been observed to reduce spike length, number of grains per spike (NGPS), and thousand grain weight (TGW) in wheat14. However, several studies have published that NPs supplementation under irrigation deficit conditions can have a positive effect on the growth and development of wheat by enhancing nutrient uptake and facilitating metabolic functions15.
Nanotechnology involves the study and manipulation of atomic or molecular aggregates with sizes ranging from 1 to 100 nm as describe by Rasheed et al.16. Nanoparticles (NPs) distinct physiochemical characteristics, such as their smaller size and large surface area, have led to their extensive application in various disciplines within the field of biosciences15. Notably, NPs made of aluminum (Al), cesium oxide (Ce2O3), copper (Cu), gold (Au), magnetized iron (Fe), silver (Ag), silica (Si), titanium dioxide (TiO2), zinc (Zn), and zinc oxide (ZnO) have proven useful in agriculture16,17. NPs offer potential benefits in different aspects of agriculture, such as enhancing crop production, protecting plants, improving crop quality, and optimizing fertilizer and irrigation practices16. The utilization of NPs in the field of crop sciences has been progressively growing; leading to the identification of several beneficial impacts on crop plants18. In particular, NPs treatment has been found to increase spike length (SL), NGPS, and TGW19. These positive effects are attributed to the creation of growth-regulating substances by NPs, such as indole-3-acetic acid and cytokinins, which enhance plant growth hormones, source-sink relationship, and ultimately lead to better grain weight20,21. Venkatachalam et al.22 found that zinc oxide NPs application resulted in favorable growth and reduced fertilizer applications by half in cotton. Positive effects of different NPs application on the germination, growth, physiological activities, water and fertilizers use efficiency, root growth, branching, biomass, and photosynthetic pigments have also been reported23,24. Do Espirito Santo Pereira et al.25 found that seed priming with NPs improved nutrient use efficiency, enhanced photosynthetic activity, and improved grain quality in wheat. Nanotechnology has the potential to significantly impact various scientific and biological fields. In particular, it is anticipated that nanotechnology will play a vital role in revitalizing agriculture and emerge as a strong economic force in the coming years18. Convincingly, a nanotech-based agricultural reorientation may enhance quality food production while conserving resources and the environment. NPs may revolutionize crop science research and transform agriculture into an industry by exploring their comprehensive application profile.
Copper (Cu) is an important micronutrient required in very minute quantities by plants, and is identified in improving chlorophyll formation, plant growth, development, and yield26. It is involved in various enzyme systems regulating many biochemical reactions in plants essential for photosynthesis, respiration and metabolism of proteins and carbohydrates27,28. Copper nanoparticles (Cu-NPs) are distinguished for their significant physiological advancement in regulating stomatal activity and reducing water loss under DS23,29. Cu-NPs application has been reported to enhance seed yield and quality, as well as drought resilience in soybean30. Furthermore, Van Nguyen et al.23 reported an increase in the chlorophyll content, enzyme activity, total seed number, and grain yield of maize under drought conditions by the application of Cu-NPs. However, limited research has been published to explore the potential of Cu-NPs in boosting wheat yield under drought conditions. To explore the myriad advantageous characteristics of Cu-NPs in enhancing the drought resilience and production potential of wheat, this study was designed with the objective to assess the effect of Cu-NPs on the growth, yield and physiological attributes of drought-subjected wheat.
Materials and methods
Experimental location and crop husbandry
The experimental trial was carried out at the research area of Agronomy department, The Islamia University of Bahawalpur, Pakistan situated at longitude: 71°40′59.99″E and latitude: 29°23′60.00″N. The experimental design consisted of a randomized complete block design (RCBD) with four replications in a factorial layout. Four treatments of Cu-NPs were administered at every studied growth stage as follows: T0 = control, T1 = Cu-NPs @ 300 mg L−1, T2 = Cu-NPs @ 700 mg L−1, T3 = Cu-NPs @ 950 mg L−1. The tillering (TS) and anthesis (AS) stages were subjected to DS, with full irrigation serving as the control. The acquisition of the seeds of Galaxy 2013 (wheat variety) was made from the Regional Agricultural Research Institute, Bahawalpur, ensuring originality and authenticity. After sterilizing the seeds with 70% ethanol for one minute and 3% sodium hypochlorite (NaClO) for thirty minutes, they were rinsed five times with distilled water. On November 12th, 2019, seeds were sown in plastic pots measuring 26 × 29 cm, filled with 16 kg soil that had been supplemented with NPs. The experimental soil's physiochemical analysis is presented in Table 1, while Fig. 1 displays the data concerning relative humidity, average rainfall, and temperature of the wheat growing season. A transparent plastic covering was positioned above the wire-house for shielding the crop from precipitation whenever necessary. All the pots received equal watering till complete emergence.
Table 1.
Experimental soil's physicochemical properties.
| Properties | Soil profile |
|---|---|
| Available potassium (ppm) | 112 |
| Available phosphorus (ppm) | 6.75 |
| Ammoniac N (mg g−1) | 1.58 |
| Electric conductivity (dS m−1) | 2.55 |
| Organic matter (%) | 0.92 |
| pH | 7.23 |
| Clay (%) | 11 |
| Silt (%) | 33.5 |
| Sand (%) | 61 |
| Texture class | Sandy loam soil |
Figure 1.
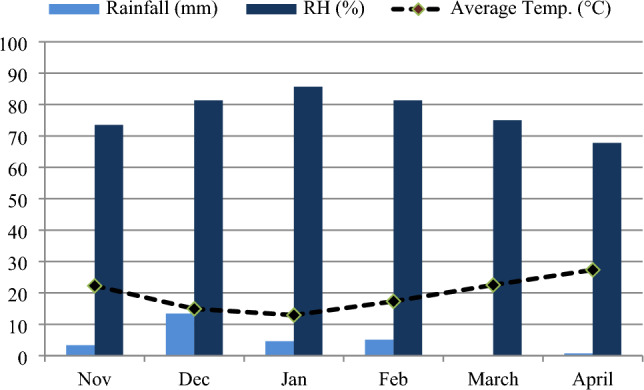
Data on relative humidity (RH), average rainfall and average temperature throughout the wheat growing season.
Drought imposition and characterization of Cu-NPs
Drought stress was imposed at tillering (TS) and anthesis (AS) stages, whereas, normal supply of water was used as control treatment. All pots received the same amount of water at 85% of soil water holding capacity (WHC), also referred as control treatment, until the commencement of DS. After that, DS was enacted at TS and AS by maintaining the soil WHC at 30%.
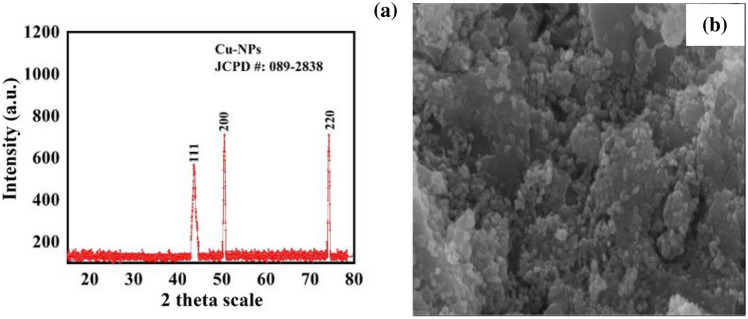
Cu-NPs were obtained from Sigma Aldrich Company, USA. The X-ray diffraction spectroscopy (XRD) test was performed to analyse the crystalline nature and purity of Cu-NPs. The typical size of the Cu-NPs was observed to be 21.86 nm using Debye Scherrer's equation. The findings of the XRD study of Cu-NPs are shown in Fig. 2a which indicates that the Cu-NPs are completely pure and crystalline with a uniform structure as described by Betancourt-Galindo et al.31 and Phul et al.32. Furthermore, scanning electron microscope (SEM) analysis was performed to determine the shape and topology of the Cu-NPs. Figure 2b determined the SEM image of Cu-NPs and demonstrates that the Cu-NPs have a round spherical shape as reported earlier by Mali et al.33.
Figure 2.
The XRD analysis (a) to determine the crystalline nature and scanning electron microscope analysis (b) to determine the shape of Cu-NPs.
Plant height and yield-related parameters
The plant height (cm) and yield related parameters i.e., SL (cm), NGPS, TGW (g), biological (BY) and grain yield (GY) plant−1 (g), and harvest index (HI) were measured as per established protocols/procedures.
Water use efficiency and leaf chlorophyll contents
Hussain and Al. Jaloud34 proposed a formula to calculate the water use efficiency (WUE, g pot−1 mm−1) given as under:
A chlorophyll meter (CL-01, USA) was used for the measurement of leaf chlorophyll contents.
Water relations
The water potential of leaf (-MPa) was measured by employing a portable water potential tool (Chas W. Cook Div., made up of England) on the completely extended freshest leaf, specifically the fourth leaf from the top.
The calculation of excised leaf water loss (ELWL) and relative water content (RWC) was performed according to the formulae described by Haider et al.14.
Here FW represents fresh weight, WW denotes wilted weight, DW represents dry weight, and TW stands for turgid weight.
To determine leaf turgor potential (TP, MPa) the following equation was used;
where WP = water potential (ψw) and OP = osmotic potential (ψs).
Stomatal conductance
Measurements of stomatal conductance (mmol of H2O m−2 s−1) were recorded using an automated equipment MK-3, Hertford, Herts (Delta-T Devices, Burwell Cambridge, made up of England).
Statistical analysis
STATISTIX (version 8.1) software was employed to do the analysis of variance and compare the mean values at the 5% probability level using the least significant difference (LSD) technique, as stated by Sharma35. A PCA (principal component analysis) was also done on the data, and the findings were visualized using a biplot graph based on the PCA main components (PC 1 and PC 2).
Ethics approval and consent to participate
This study does not include human or animal subjects.
Statement on guidelines
All experimental studies and experimental materials involved in this research are in full compliance with relevant institutional, national and international guidelines and legislation.
Results
Growth and yield parameters
Drought stress (DS) significantly affected all the growth, yield and related parameters (SL, NGPS, TGW, HI) of wheat (Table 2). All the aforementioned parameters were considerably decreased when DS was enacted at TS and AS, while higher values of PH, SL, NGPS, TGW, BY, GY and HI were noted in control treatment (D0). However, the application of Cu-NPs significantly mitigated the adverse effects of drought and improved the studied parameters of wheat both under control and DS conditions. An increase in PH, SL, NGPS, TGW, BY, GY and HI was observed when Cu-NPs were applied @ 300 mg L−1 at TS and AS, in comparison to control treatment. However, higher doses of Cu-NPs (700 and 950 mg L−1) reduced all these parameters when compared with control treatment (T0) as presented in Table 2.
Table 2.
Plant height and yield related traits of wheat as affected by Cu-NPs under drought.
| Treatments | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Drought | Cu-NPs | Plant height | Spike length | Number of Grains/Spike | 1000-grain weight | Biological yield | Grain yield | Harvest index |
| D0 = Control | T0 | 66.12b | 13.4ab | 36.66a | 29.41b | 16.77b | 10.56b | 52.51b |
| T1 | 68.13a | 13.56a | 35.33b | 30.26a | 17.2a | 11.71a | 55.46a | |
| T2 | 65.43bc | 13.16b | 34.66c | 28.81c | 15.52bc | 10.41c | 50.24c | |
| T3 | 63.76d | 13.06c | 34 0.56c | 28.41c | 15.2c | 10.26c | 49.54d | |
| D1 = Drought at tillering | T0 | 65.16b | 12.96b | 35.66a | 28.22b | 15.88b | 9.17b | 53.45a |
| T1 | 65.5a | 13.53a | 34.33b | 29.07a | 16.3a | 10.32a | 50.34b | |
| T2 | 64.03c | 12.83b | 33.66c | 27.62c | 14.6bc | 9.02b | 48.12c | |
| T3 | 63.43d | 12.66c | 33.0 cd | 27.22d | 14.38c | 8.87c | 47.42d | |
| D2 = Drought at anthesis | T0 | 61.76b | 12.8b | 35.06a | 27.01b | 14.78b | 7.96b | 50.5 7a |
| T1 | 62.43a | 13.36a | 33.66b | 27.86a | 15.26a | 9.11a | 47.45b | |
| T2 | 59.73c | 12.53c | 33.21bc | 26.41c | 13.53c | 7.81c | 45.23c | |
| T3 | 59.26c | 12.26d | 32.33c | 26.0 cd | 13.28c | 7.66d | 44.53d | |
T0, T1, and T2, T3 denotes control, 300 mg L−1, 700 mg L−1, and 950 mg L−1 Cu-NPs, respectively. The means that are sharing similar letter case does not exhibit significant differences at 5% level of probability.
Water use efficiency (g pot−1 mm−1) and leaf chlorophyll contents (%)
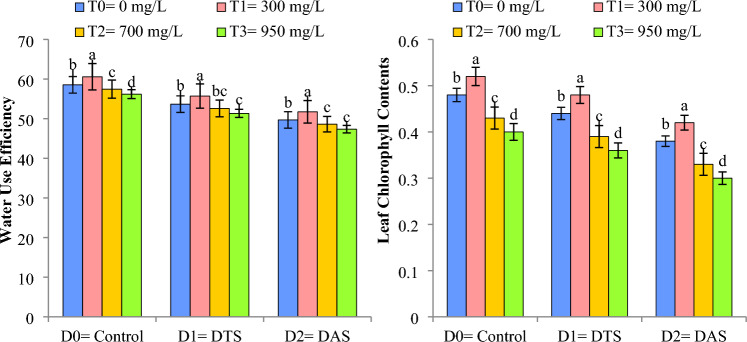
Cu-NPs application at CGS (TS and AS) demonstrated enhanced WUE and leaf chlorophyl contents (LCC) of wheat during water deficit conditions. Under DS conditions, the WUE and leaf chlorophyl contents shows a decline during the TS (D1) and AS (D2), respectively, when compared with control treatment (D0). Cu-NPs (300 mgL−1) significantly reduced the drought impact and increased the WUE and LCC by 3.78% and 10%, respectively, in comparison to control treatment (T0), as depicted in Fig. 3.
Figure 3.
Impact of Cu-NPs application on the WUE and leaf chlorophyll contents of wheat under drought. D0: control, D1: drought at tillering stage (DTS), D2: drought at anthesis stage (DAS). The error bars denote the standard error (n = 4).
Stomatal conductance (mmol m−2 s−1)
Figure 4 illustrated that DS negatively affected the stomatal conductance (SC) of wheat. During DS the SC shows a decline during the TS and AS, in comparison to the control treatment. Cu-NPs application at CGS demonstrated enhanced wheat WUE during water scarce (D1 and D2) and control (D0) conditions. Maximum values of SC (442.93 mmol m−2 s−1) were noted by the application of Cu-NPs (300 mg L−1) under control conditions (D0) and minimum values for SC (401.21 mmol m−2 s−1) were observed when Cu-NPs were applied @ 950 mg L−1 (T3) at AS (D2).
Figure 4.
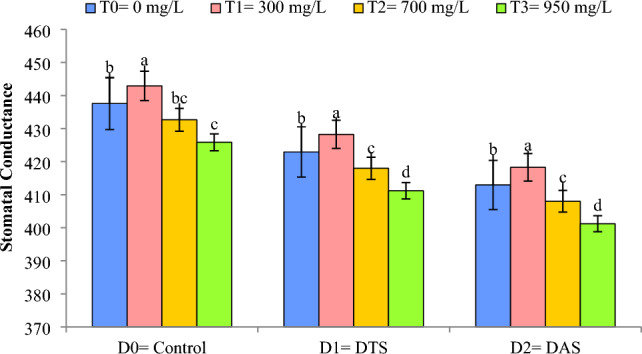
Impact of Cu-NPs application on the SC of wheat under drought. D0: control, D1: drought at tillering stage (DTS), D2: drought at anthesis stage (DAS). The error bars denote the standard error (n = 4).
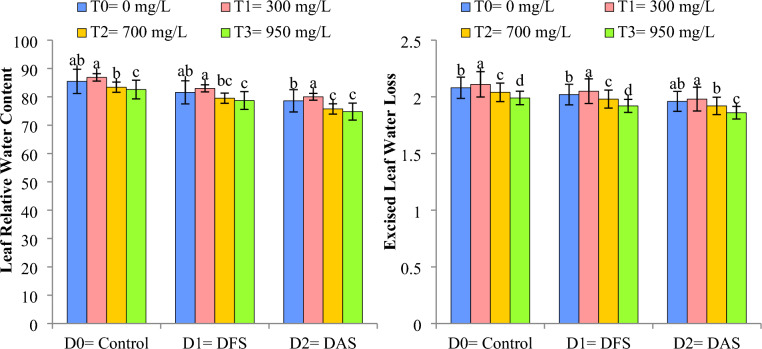
Leaf relative water contents (%) and excised leaf water loss (%)
The application of Cu-NPs significantly improved the leaf relative water contents (LRWC) and excised leaf water loss (ELWL) in wheat both under control and DS situations as depicted in Fig. 5. Reduction in LRWC and ELWL was recorded when DS was imposed at AS while maximum values for both parameters was noticed in under normal irrigation (D0). However, Cu-NPs when applied @ 300 mg L−1 (T1) resulted in an increase in LRWC and ELWL followed by T0 and reduced values for LRWC and ELWL were recorded when Cu-NPs were applied @ 950 mg L−1 (T3) as compared to control treatment (T0).
Figure 5.
Impact of Cu-NPs application on the LRWC and ELWL of wheat under drought. D0: control, D1: drought at tillering stage (DTS), D2: drought at anthesis stage (DAS). The error bars denote the standard error (n = 4).
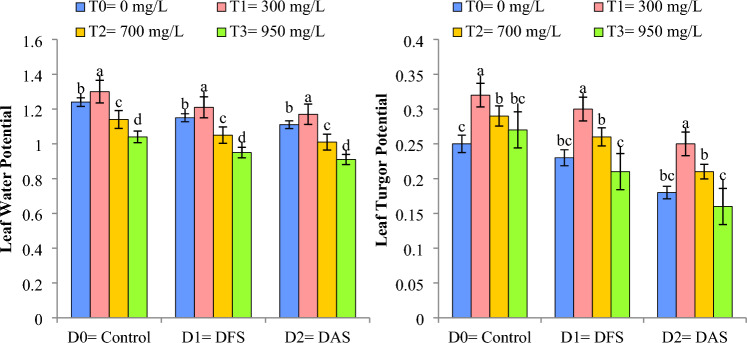
Leaf water potential (-MPa) and Leaf turgor potential (MPa)
Significant reduction in leaf water potential (LWP) and leaf turgor potential (LTP) of wheat were observed under DS circumstances. DS reduced the LWP and LTP when imposed at TS (D1) and AS (D2) in comparison to control (D0). The application of Cu-NPs @ 300 mg L−1 significantly reduced the drought impact on LTP and LWP by 18% and 5.6%, respectively, when compared to control conditions. Higher values of LWP and LTP were recorded in T1 followed by T0 under control conditions (D0), whereas, reduction in both parameters was noticed in T3 when drought was imposed at AS (D2) as depicted in Fig. 6.
Figure 6.
Impact of Cu-NPs application on the LWP and LTP of wheat under drought. D0: control, D1: drought at tillering stage (DTS), D2: drought at anthesis stage (DAS). The error bars denote the standard error (n = 4).
Principal component analysis
The variability and relationships between the morpho-physiological characteristics of wheat under various drought and Cu-NPs treatments were investigated using principal component analysis (PCA) as depicted in Fig. 7. Results revealed that the first two PCs captured 96% of the total variations. Out of these PCs the PC1 and PC2 captured 86.8% and 9.2% variations. Variation in PC1 is mainly due to PH, SL, NGPS, TGW, GY, BY, HI, LWP, LRW, LCC, WUE, SC, and ELWL. While in PC2 main variation was due to LTP. Our results demonstrated that T1 (300 mg L−1) is better because it is clustered near the origin.
Figure 7.
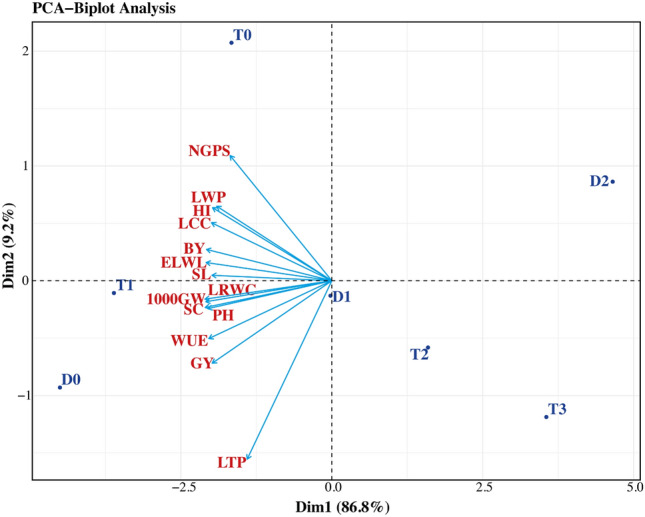
PCA of morpho-physiological attributes of wheat crop under Cu-NPs and drought. D0: control, D1: drought at TS, D2: drought at AS and T0, T1, T2, and T3 signposts control, 300 mg L−1, 700 mg L−1, and 950 mg L−1 Cu-NPs, respectively.
Discussion
Drought stands out as a primary obstacle to achieving sustainable crop production11,36. Significant decrease in the growth and yield of wheat during drought underscore the need to develop and identify strategies to alleviate its impact. The current study demonstrate that the application of Cu-NPs significantly influences the growth, yield, and related factors under both normal and drought conditions. These results align with previous findings suggesting that application of Cu-NPs can effectively mitigate drought stress, leading to improved wheat growth and yield, especially under controlled conditions37.
Drought stress significantly reduced the chlorophyll contents in wheat due to the over production of reactive oxygen species (ROS) that leads to lipid peroxidation and consequently chlorophyll destruction11,38,39. Similarly, Gill and Tuteja40 and Aslam et al.7 described that DS decreased the chlorophyll contents in wheat and quinoa, respectively, which are in line with our findings. However, Cu-NPs improved the leaf chlorophyll contents when applied in lower concentration (300 mg L−1) as it increases cytokinin’s production that increases the metabolic activity and cell growth and reduces the ROS production and so helps to enhance chlorophyll contents41,42 whereas, higher concentrations (700 mg L−1, 950 mg L−1) reduced the chlorophyll contents by decreasing photosynthetic performance and antioxidant activities16,43.
WUE decreased under DS when imposed at CGS, i.e., tillering (TS) and anthesis (AS) stages. Likewise, Muhammad et al.42 and Raza et al.44 confirmed that DS decrease the WUE in wheat. Cu-NPs application helps to improve plant functions such as water availability and glycolysis metabolism and hence the WUE in wheat crop as also reported by Elshayb et al.41 and Raza et al.44. Figure 7 indicates that WUE is a significant determinant for wheat yield vacillations at critical growth stages.
Stomatal conductance (SC) is an important parameter to explore the drought resilience in a crop. DS abridged the water uptake from the soil which ultimately leads to stomatal closure and hence reduced the SC as perceived in current study. Similar outcomes have also been reported in wheat by Haider et al.14 and Raza et al.45. Lower concentration of Cu-NPs improved the SC both under control and DS conditions. Likewise, Raza et al.44 reported an increase in SC by the application of NPs in wheat under DS conditions because NPs increase the production of phytohormones, accumulation of osmolytes, improve water uptake and photosynthetic efficiency thus offering better resistance to wheat crop against water deficit conditions16,46.
Limited water availability significantly decreased the LRWC, ELWL, LWP and LTP at TS and AS in comparison to control treatment. Similar findings have been reported by others under limited water supply probably due to reduced soil moisture, cell division SC, and disability of plants to nutrient absorption11,41,42. Cu-NPs application improved the aforementioned attributes both under control and irrigation deficit situations. The findings of our study are in-line with others who reported that NPs improved the water relations (LRWC, ELWL, LWP and LTP) as the application of NPs increases the root biomass, lateral roots formation and hormonal signaling, thereby improving water uptake and maintaining better water relations under DS conditions47,48.
Significant reduction in plant height was noticed under water deficit conditions when compared with control treatment. Similarly, Raza et al.2 and Raza et al.11 reported a decrease in plant height of wheat and quinoa, respectively, under DS due to the dehydration of plant cell, low turgidity and reduced cell division. However, application of Cu-NPs in low concentration (300 mg L−1) improved the plant height of wheat crop by improving the source sink relationship, water and nutrient uptake and photosynthetic activity44. Similarly, Ahmed et al.38 and Raza et al.11 reported a positive effect of NPs application on plant height of rice and wheat under both control and DS conditions, respectively.
The yield contributing attributes (SL, NGPS, TGW), biological yield, grain yield and harvest index were significantly affected under water deficit conditions. The decrease in SL, NGPS, TGW and grain yield was found to be more pronounced as DS was imposed at anthesis stage. Raza et al.11 and Muhammad et al.42 observed similar outcomes that DS reduce the yield contributing attributes in wheat and quinoa, respectively, due to decrease in plant metabolic processes resulting from insufficient water availability, inadequate absorption and distribution of photosynthates16. Likewise, Aslam et al.7 and Raza et al.45 also reported a reduction in grain yield and associated parameters in quinoa and wheat, respectively, under short supply of water. The application Cu-NPs significantly improved the yield components, biological and grain yield both under control and drought conditions. The lower concentration of Cu-NPs (300 mg L−1) significantly enhanced the aforementioned yield components and hence the biological and grain yield as NPs improves water and nutrient uptake, photosynthetic efficiency, osmolytes accumulation, antioxidant activities, and gene expression and attenuated the adverse effects of drought stress49,50. Similarly, Yasmeen et al.19 reported an increase in SL, NGPS and grain yield of wheat by the application of Cu-NPs under DS conditions. However, the higher concentration of Cu-NPs (700 mg L−1, 950 mg L−1) negatively affected the growth, yield and yield components as the excessive use of NPs cause oxidative stress and physiological abnormalities in plants, resulting in decreased antioxidant activities and gas exchange characteristics16,50. Similar findings about the toxicity of NPs in plants have been reported earlier by Wang et al.43 and Hou et al.51. The biplot graph of PCA revealed several significant factors, including SC, leaf chlorophyll contents and WUE, as key explanatory variables contributing to resilience of wheat to drought, consistent with findings of Aslam et al.7 (Fig. 7). Concludingly, the anthesis stage of wheat was found most sensitive to DS due to limited water availability and compromised nutrient transport to developing seeds, resulting in reduced seed size, and ultimately lowering overall wheat yield. It's worth noting that only the appropriate amount of Cu-NPs shows a positive response, while excessive levels of Cu-NPs can induce toxicity by disrupting plant metabolic processes, triggering oxidative stress and cellular damage, inhibiting photosynthesis, impairing nutrient uptake and transport, ultimately inhibiting growth and reducing the yield of wheat16,23,50.
Conclusion
DS severely abridged the wheat growth, water relations, physiological attributes, and yield when administered at CGS. The application of NPs reduced the negative consequences of DS by enhancing water and nutrient retention capacity and fertility of soil, and the source to sink relationship. Under both control and drought conditions, the application of Cu-NPs enhanced wheat growth, WUE, physiological parameters, water-related characteristics, and yield. Furthermore, the AS seems to be more vulnerable stage to drought, and Cu-NPs (300 mg L−1) soil treatment is the most effective strategy for mitigating the deleterious sways of drought and to upsurge the productivity of wheat under controlled pot conditions. Therefore, further field trials are necessary to validate the applicability and effectiveness of Cu-NPs in improving drought resilience in wheat under real-world agricultural settings.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R483), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Writing—original draft, conceptualization, investigation, M.A.S.R., J.A. and M.U.A. Data collection, script evaluation, Software, and editing, M.V., B.Z., and R.I. Writing—review and editing, A.A.A., M.S.E., M.T., H.H.E., J.I., and M.V. Data curation and formal analysis, M.T., J.A., F.M., M.A.I., Visualization and validation, R.I., F.M. Methodology and references, H.H.E., J.A. M.U.A. Supervision, M.A.S.R.
Funding
We would like to acknowledge LAMMC for funding RTO project KarboIzotopas “Application of labelled 13C and 15N isotope methods for research of climate change mitigation potential of recent winter wheat genotypes and common mugwort”.
Data availability
The datasets analysed during this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Aown Sammar Raza, Email: aown.sammar@iub.edu.pk.
Rashid Iqbal, Email: rashid.iqbal@iub.edu.pk.
Heba H. Elsalahy, Email: heba.elsalahy@zalf.de
References
- 1.Alabdallah NM, Hasan MM. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021;28:5631–5639. doi: 10.1016/j.sjbs.2021.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raza MAS, et al. Integrating biochar, rhizobacteria and silicon for strenuous productivity of drought stressed wheat. Commun. Soil Sci. Plant Anal. 2021;52:338–352. doi: 10.1080/00103624.2020.1853149. [DOI] [Google Scholar]
- 3.Ijaz, M., Afzal, A., Shabbir, G., Iqbal, J. & Rafique, M. Breeding wheat for leaf rust resistance: past, present and future. Asian J. Agric. Biol.1 (2023).
- 4.Hussan, M. u., Farrukh Saleem, Muhammad, Bilal Hafeez, M., Ramzan, Y. & Nadeem, M. Impact of soil applied humic acid, zinc and boron supplementation on the growth, yield and zinc translocation in wheat. Asian J. Agric. Biol.1 (2022).
- 5.Mathur, S., Raikalal, P. & Jajoo, A. Physiological responses of wheat to environmental stresses. In Wheat Production in Changing Environments: Responses, Adaptation and Tolerance, 31–61 (2019).
- 6.Chanu, T. T. & Upadhyaya, H. In Nanomaterials in Plants, Algae and Microorganisms, 43–64 (Elsevier, 2019).
- 7.Aslam MU, et al. Improving strategic growth stage-based drought tolerance in quinoa by rhizobacterial inoculation. Commun. Soil Sci. Plant Anal. 2020;51:853–868. doi: 10.1080/00103624.2020.1744634. [DOI] [Google Scholar]
- 8.Allen, M. et al. Technical Summary: Global warming of 1.5 C. An IPCC Special Report on the impacts of global warming of 1.5 C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (2019).
- 9.Ganie SA, Ahammed GJ. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice. Plant Cell Rep. 2021;40:437–459. doi: 10.1007/s00299-020-02649-2. [DOI] [PubMed] [Google Scholar]
- 10.El-Fattah, A., Ahmed, D., Elhamid Hashem, F. A. & Abd-Elrahman, S. H. Impact of applying organic fertilizers on nutrient content of soil and lettuce plants, yield quality and benefit-cost ratio under water stress conditions. Asian J. Agric. Biol.2 (2021).
- 11.Raza MAS, et al. Seed priming with selenium improves growth and yield of quinoa plants suffering drought. Sci. Rep. 2024;14:886. doi: 10.1038/s41598-024-51371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizwan, M., Khalil, A., Khalid Bhatti, M., Muhammad, S. & Naseem, R. Effect of sowing techniques and tillage practices on paddy yield of direct seeded rice in salt affected soils. Asian J. Agric. Biol.1 (2022).
- 13.Victoria, O. et al. Seed treatment with 24-epibrassinolide improves wheat germination under salinity stress. Asian J. Agric. Biol.3 (2023).
- 14.Haider I, et al. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc. 2020;24:974–981. doi: 10.1016/j.jscs.2020.10.005. [DOI] [Google Scholar]
- 15.Behboudi F, Tahmasebi Sarvestani Z, Kassaee MZ, Modares Sanavi S, Sorooshzadeh A. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018;20:1479–1492. [Google Scholar]
- 16.Rasheed A, et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front. Plant Sci. 2022;13:976179. doi: 10.3389/fpls.2022.976179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashem AH, et al. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in faba bean plants. J. Fungi. 2021;7:195. doi: 10.3390/jof7030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues SM, et al. Nanotechnology for sustainable food production: promising opportunities and scientific challenges. Environ. Sci. Nano. 2017;4:767–781. doi: 10.1039/C6EN00573J. [DOI] [Google Scholar]
- 19.Yasmeen F, Raja NI, Razzaq A, Komatsu S. Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2017;1865:28–42. doi: 10.1016/j.bbapap.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, et al. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021;67:245–259. doi: 10.1080/03650340.2020.1723003. [DOI] [Google Scholar]
- 21.Li M, et al. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021;269:116134. doi: 10.1016/j.envpol.2020.116134. [DOI] [PubMed] [Google Scholar]
- 22.Venkatachalam P, et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.) Plant Physiol. Biochem. 2017;110:118–127. doi: 10.1016/j.plaphy.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Van Nguyen D, et al. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2022;41:364–375. doi: 10.1007/s00344-021-10301-w. [DOI] [Google Scholar]
- 24.Silva S, Dias MC, Silva AM. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a) biotic stresses? Toxics. 2022;10:172. doi: 10.3390/toxics10040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 2021;11:267. doi: 10.3390/nano11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viera I, Pérez-Gálvez A, Roca M. Green natural colorants. Molecules. 2019;24:154. doi: 10.3390/molecules24010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosini VG, et al. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol. Biochem. 2018;128:89–98. doi: 10.1016/j.plaphy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Din MI, Arshad F, Hussain Z, Mukhtar M. Green adeptness in the synthesis and stabilization of copper nanoparticles: catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017;12:1–15. doi: 10.1186/s11671-017-2399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 2016;171:1569–1580. doi: 10.1104/pp.16.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linh TM, et al. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020;2020:1–13. doi: 10.1155/2020/4056563. [DOI] [Google Scholar]
- 31.Betancourt-Galindo R, et al. Synthesis of copper nanoparticles by thermal decomposition and their antimicrobial properties. J. Nanomater. 2014;2014:10–10. doi: 10.1155/2014/980545. [DOI] [Google Scholar]
- 32.Phul R, Kaur C, Farooq U, Ahmad T. Ascorbic acid assisted synthesis, characterization and catalytic application of copper nanoparticles. Mater. Sci. Eng. Int. J. 2018;2:90–94. [Google Scholar]
- 33.Mali SC, Dhaka A, Githala CK, Trivedi R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020;27:e00518. doi: 10.1016/j.btre.2020.e00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain G, Al-Jaloud AA. Effect of irrigation and nitrogen on water use efficiency of wheat in Saudi Arabia. Agric. Water Manag. 1995;27:143–153. doi: 10.1016/0378-3774(95)91233-W. [DOI] [Google Scholar]
- 35.Sharma JR. Statistical and Biometrical Techniques in Plant Breeding. New Delhi: New Age International; 2006. [Google Scholar]
- 36.Fatemi R, Yarnia M, Mohammadi S, Vand E, Mirashkari B. Screening barley genotypes in terms of some quantitative and qualitative characteristics under normal and water deficit stress conditions. Asian J. Agric. Biol. 2023;2:2022071. [Google Scholar]
- 37.Faraji J, Sepehri A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil Sci. Plant Nutr. 2020;20:703–714. doi: 10.1007/s42729-019-00158-0. [DOI] [Google Scholar]
- 38.Ahmed T, et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021;209:111829. doi: 10.1016/j.ecoenv.2020.111829. [DOI] [PubMed] [Google Scholar]
- 39.Taratima, W., Kunpratum, N. & Maneerattanarungroj, P. Effect of salinity stress on physiological aspects of pumpkin (Cucurbita moschata duchesne.'laikaotok') under hydroponic condition. Asian J. Agric. Biol.2 (2023).
- 40.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Elshayb OM, et al. The integrative effects of biochar and ZnO nanoparticles for enhancing rice productivity and water use efficiency under irrigation deficit conditions. Plants. 2022;11:1416. doi: 10.3390/plants11111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad F, et al. Ameliorating drought effects in wheat using an exclusive or co-applied rhizobacteria and ZnO nanoparticles. Biology. 2022;11:1564. doi: 10.3390/biology11111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Li Q, Pei Z, Wang S. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018;62:801–808. doi: 10.1007/s10535-018-0813-4. [DOI] [Google Scholar]
- 44.Raza MAS, et al. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep. 2023;13:2700. doi: 10.1038/s41598-023-29784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raza MAS, et al. Exploring the recuperative potential of brassinosteroids and nano-biochar on growth, physiology, and yield of wheat under drought stress. Sci. Rep. 2023;13:15015. doi: 10.1038/s41598-023-42007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zulfiqar F, Navarro M, Ashraf M, Akram NA, Munné-Bosch S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019;289:110270. doi: 10.1016/j.plantsci.2019.110270. [DOI] [PubMed] [Google Scholar]
- 47.Dimkpa CO, et al. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019;688:926–934. doi: 10.1016/j.scitotenv.2019.06.392. [DOI] [PubMed] [Google Scholar]
- 48.Zahedi SM, Hosseini MS, Daneshvar-Hakimi-Meybodi N, Peijnenburg W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021;101:5202–5213. doi: 10.1002/jsfa.11167. [DOI] [PubMed] [Google Scholar]
- 49.Semida WM, et al. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021;10:421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akhtar N, et al. Improvement of plant responses by nanobiofertilizer: a step towards sustainable agriculture. Nanomaterials. 2022;12:965. doi: 10.3390/nano12060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou J, et al. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere. 2018;193:852–860. doi: 10.1016/j.chemosphere.2017.11.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during this study are included in this manuscript.