Abstract
Background
Frailty has been associated with mental illness (MI) observational studies, but the causal relationship between these factors remains uncertain. We aimed to assess the bidirectional causality between frailty and MI by two-sample Mendelian randomization (MR) analyses.
Methods
To investigate the causal relationship among them, summary statistics of frailty index (FI) and six types of MI: anxiety, depression, affective disorder, mania, schizophrenia, and obsessive-compulsive disorder (OCD) were included in this MR study. This MR analysis was performed using inverse variance weighting (IVW), MR-Egger regression, and weighted median. The stability of the results was evaluated using Cochran’s Q test, MR-Egger intercept test, Funnel Plots, and leave-one-out analysis.
Results
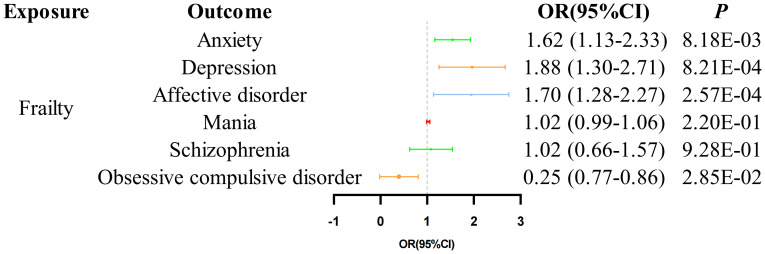
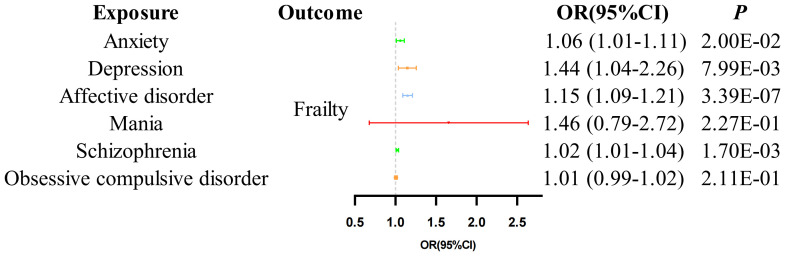
Genetic predisposition to FI was significantly associated with increased anxiety (odds ratio [OR] = 1.62, 95% confidence interval [CI] 1.13-2.33, P = 8.18E-03), depression (OR = 1.88, 95% CI 1.30-2.71, P = 8.21E-04), affective disorder (OR = 1.70, 95% CI 1.28-2.27, P = 2.57E-04). However, our study findings do not demonstrate a causal relationship between FI and mania (OR = 1.02, 95% CI 0.99-1.06, P = 2.20E-01), schizophrenia (OR = 1.02, 95% CI 0.07-0.86, P = 9.28E-01). In particular, although the IVW results suggest a potential causal relationship between FI and OCD (OR = 0.64, 95% CI 0.07-0.86, P = 2.85E-02), the directions obtained from the three methods we employed ultimately show inconsistency. Therefore, the result must be interpreted with caution. The results of the reverse MR analysis indicated a statistically significant and causal relationship between anxiety (OR = 1.06, 95% CI 1.01-1.11, P = 2.00E-02), depression (OR = 1.14, 95% CI 1.04-1.26, P = 7.99E-03), affective disorder (OR = 1.15, 95% CI 1.09-1.21, P = 3.39E-07), and schizophrenia (OR = 1.02, 95% CI 1.01-1.04, P = 1.70E-03) with FI. However, our findings do not provide support for a link between mania (OR = 1.46, 95% CI 0.79-2.72, P = 2.27E-01), OCD (OR = 1.01, 95% CI 1.00-1.02, P = 2.11E-01) and an increased risk of FI.
Conclusion
The MR results suggest a potential bidirectional causal relationship between FI and anxiety, depression, and affective disorder. Schizophrenia was found to be associated with a higher risk of FI. The evidence was insufficient to support a causal relationship between Fl and other Ml. These findings offer new insights into the development of effective management strategies for frailty and MI.
Keywords: frailty index, Mendelian randomization, mental illness, causality, older people
1. Introduction
Frailty, recognized as a prevalent geriatric syndrome, encompasses diminished physiological reserve and dysregulation across multiple systems leading to an impaired capacity to sustain a stable internal environment amidst internal and external stressors. Consequently, this condition amplifies vulnerability to adverse events (1). The frailty index (FI) is acknowledged as a sensitive and effective tool for detecting frailty (2). It is a continuous metric that quantifies frailty based on the proportion of health deficits due to aging to all the number of deficits considered. These deficits may present as symptoms, signs, diseases, disabilities, or abnormalities identified through laboratory tests, radiological imaging, and even social factors (3). By encompassing various dimensions of health, the FI serves as a robust predictor of adverse outcomes, including functional decline, physical disability, falls, and increased risk of mortality and morbidity (4). Extensive epidemiological surveys have consistently demonstrated that the prevalence of frailty is increasing globally as populations age. According to a comprehensive meta-analysis, 26.8% of the older population suffers from frailty (5), and it poses a significant public health burden due to its strong association with various adverse health outcomes, such as multimorbidity, disability, and excess mortality (1). Mental illness (MI) is a primary cause of disability worldwide, and is associated with increased all-cause mortality (6). A recent meta-analysis revealed that approximately 14.3% of global deaths, which amounts to around 8 million deaths each year, are attributed to MI (7). The common conditions include anxiety, depression, affective disorder, mania, schizophrenia, and obsessive-compulsive disorder (OCD). Indeed, Prior observational studies have suggested that frailty and MI frequently coexist as overlapping syndromes in later life (8). For instance, A study involving 297,380 participants revealed identified that the likelihood of experiencing frailty among individuals with non-MI was a mere 1.8%, whereas individuals with MI exhibited frailty traits at a rate of 4.2%-5.5% (9). Furthermore, a prospective cohort study conducted in the Netherlands involving 167,729 individuals yielded similar conclusions. This study found that frailty is associated with MI, including affective disorders, anxiety, and depression (10). However, the causal link between frailty and MI remains unclear, as existing evidence from observational studies does not rule out reverse causation and confounding effects (11). Consequently, it is challenging to ascertain whether frailty leads to MI, MI contributes to frailty, or if a bidirectional causality exists. Clarifying this relationship is essential for understanding the intricate connection between frailty and MI and for devising tailored management strategies for older adults.
Randomized controlled trials (RCTs) are the gold standard for investigating causality. However, RCTs demand substantial financial and human resources, making it particularly challenging to ascertain the causal relationship between frailty and MI through such studies. In situations where conducting randomized controlled studies is not feasible, Mendelian randomization (MR) serves as an alternative technique. It utilizes genetic variation as an instrumental variable (IV) to evaluate the consistency of observational associations between risk factors and outcomes with causal effects, making it a valuable approach that provide a high level of evidence (12). Recently, the MR approaches have been successfully applied to reveal the causal role of frailty in various health outcomes (13–15). At present, there is limited research exploring the association between frailty and MI, and the existence of a causal relationship between the two remains uncertain. Therefore, this study aimed to conduct a two-sample MR analysis using published data on frailty and MI derived from genomewide association studies (GWAS). Initially, we identified single nucleotide polymorphisms (SNPs) associated with the FI, the most commonly utilized tool for frailty assessment, based on extensive GWAS. This was done to explore the causal relationship between genetic susceptibility to frailty and MI, including conditions such as anxiety, depression, affective disorders, mania, schizophrenia, and OCD. Subsequently, we carried out reverse MR analyses to assess the potential impact of MI on frailty.
2. Methods
2.1. Study design
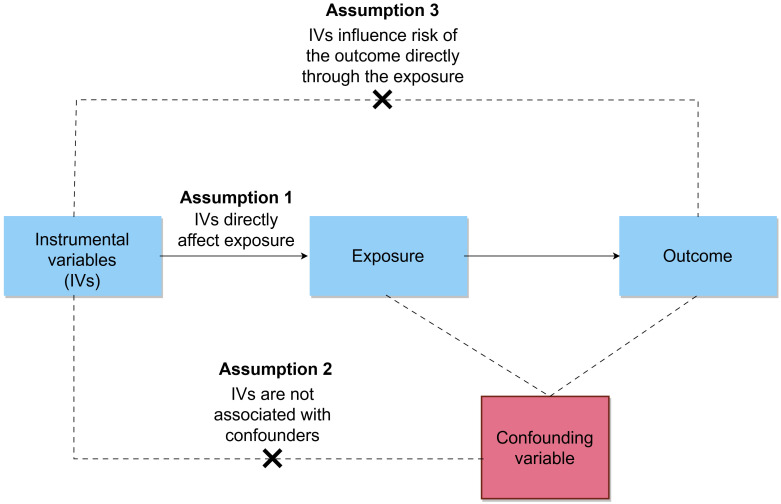
An overview of the MR framework was illustrated in Figure 1 . This study employed a two-sample MR design that extracted summarized genetic association data for the exposure and outcome variables from two independent non-overlapping populations. The study specifically focused on individuals of European ancestry to minimize bias resulting from population stratification. To ensure the validity of causal inferences drawn from MR analysis, the IVs must satisfy three core assumptions: (i) the relevance assumption, meaning the SNPs are strongly associated with the exposure; (ii) the independence assumption, indicating the SNPs should not be associated with confounding factors; (iii) the exclusion-restriction assumption, suggesting that SNPs affect the outcome solely through the exposure.
Figure 1.
Overview of the bidirectional MR study design.
MR analysis is conducted from two perspectives: (i) frailty as the exposure, assessing whether individuals with a higher FI are more likely to develop MI; (ii) frailty as the outcome, evaluating whether patients with MI are more likely to be frailty.
2.2. Data source and genetic instrument selection
Detailed information is shown in Table 1 . Summary statistics for frailty, as assessed by the FI phenotype, were acquired from a comprehensive meta-analysis of GWAS conducted within the UK Biobank and Swedish TwinGene cohorts (16). These datasets encompassed a substantial sample size of 175,226 individuals of European ancestry, enabling a robust exploration of the genetic underpinnings of frailty. FI was calculated based on 49 or 44 self-reported items according to UK Biobank and TwinGene’s defect accumulation theory, respectively (16, 17). We selected publicly available summary statistic data sets of a GWAS for anxiety (total N = 395,718; case = 27,554, control = 368,054), depression (total N = 406,986; case = 47,696, control = 359,290), affective disorder (total N = 412,181; case = 52,891, control = 359,290) in FinnGen R10 dataset. Mania data from UK Biobank (total N = 115,338; case = 4,816, control = 110,522). Schizophrenia data from a recent large GWAS study (total N = 320,404; case = 76,755, control = 243,649) (18). In addition, we obtained GWAS data for OCD from the Psychiatric Genomics Consortium (total N = 33,925; case = 26,888, control = 7,037) (19).
Table 1.
Summary of GWAS included in this study.
| Year | Trait | Population | Cases | Controls | Samplesize | Websource |
|---|---|---|---|---|---|---|
| 2021 | Frailty | European | NA | NA | 175,226 | DOI: 10.1111/acel.13459 |
| 2023 | Anxiety | European | 27,554 | 368,054 | 395,718 | www.finngen.fi/en |
| 2023 | Depression | European | 47,696 | 359,290 | 406,986 | www.finngen.fi/en |
| 2023 | Affective disorder | European | 52,891 | 359,290 | 412,181 | www.finngen.fi/en |
| 2018 | Mania | European | 4,816 | 110,522 | 115,338 | www.nealelab.is/uk-biobank |
| 2022 | Schizophrenia | European | 76,755 | 243,649 | 320,404 | https://pgc.unc.edu/ |
| 2017 | Obsessive-compulsive disorder | European | 26,888 | 7,037 | 33,925 | https://pgc.unc.edu/ |
The selection of IVs adhered strictly to the three fundamental assumptions of MR analysis. Initially, SNPs significantly associated with the exposure were identified based on genome-wide significance (P < 5E-8) and subsequently grouped to ensure SNP independence (cluster r2 cutoff = 0.001, cluster distance = 10,000 kb). In cases of linkage disequilibrium (LD) among SNPs, the SNP with the lowest p-value was retained. Palindromic SNPs, SNPs associated with the outcome (P < 0.05), and SNPs absent from the GWAS pooled data were excluded from the IV selection. Given that a portion of the GWAS data in FI and mania in this study was derived from the UK bioBank, which may lead to potential data overlap, we took steps to mitigate the impact of such overlap on the MR analysis. Specifically, only SNPs with F-statistics greater than 10 were included in subsequent analysis (20, 21). Additionally, PhenoScanner V2, an advanced tool for identifying human genotype-phenotype associations, was utilized to check if the selected SNPs were linked to confounders in the frailty and MI relationship (threshold: 1E-05). Confounders identified were adjusted for in subsequent analyses.
2.3. Statistical analysis
Before conducting the MR analysis, we initially employed MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) to identify and discard any abnormal IVs. The MR-PRESSO procedure was performed with a cycle number of 10,000 and P < 0.05 was used as a threshold to detect and remove outliers. Considering the significant heterogeneity of SNP effects, we applied the multiplicative random-effects model in the inverse variance weighting (IVW) approach as the main analytical method for MR, which is an extension of the Wald ratio estimator based on the principles of meta-analysis (22). The significance threshold was set at P <0.05 and the results of causality were expressed as odds ratios (OR) and 95% confidence intervals (95% CI). To further demonstrate the stability and directionality of the results, we additionally performed MR-Egger and weighted median to assess causality. These methods rely on different assumptions, so the consistent effects of multiple methods can lead to causal conclusions with greater persuasiveness (23). Next, to test the robustness of our results, we assessed heterogeneity using Cochran’s Q (24). Then, horizontal pleiotropy was tested using the MR-Egger intercept and MR-PRESSO global test (25). Finally, we also performed sensitivity analyses through funnel plots and leave-one-out methods.
We further performed reverse MR analysis to assess whether anxiety or depression affects frailty. Due to the limited number of SNPs meeting the genome-wide significance threshold (P < 5E-8) in the GWAS of the pooled dataset for part MI, we conducted a screening for SNPs meeting a more relaxed genome-wide significance threshold (P < 5E-6) to serve as IVs associated with MI (26). The subsequent method is the same.
All analyses in this study were performed based on R software (version 4.2.1). The “TwoSampleMR” R package was used in our MR study (27). All statistical tests were two tailed, and α = 0.05 was considered as the significant level.
3. Results
3.1. Instrumental variables for Mendelian randomization
This study investigates the impact of FI on MI risk using two-sample MR. A set of 15 SNPs, associated with FI and independent from other factors, were selected as IVs from the GWAS dataset. When employing PhenoScanner, IVs linked to pertinent potential confounders were identified by applying a threshold of 1E-05. These confounders were subsequently omitted from the formal MR analyses, resulting in the exclusion of 5 SNPs, the specifics of which are delineated in Supplementary Table 1 . The remaining 10 SNPs as IVs associated with frailty are shown in Supplementary Table 2 .
Examined a set of SNPs associated with anxiety, depression, affective disorders, mania, schizophrenia, and OCD. Specifically, we screened 18, 17, 21, 19, 217, and 15 significant and independent SNPs from each respective disorder to serve as IVs. PhenoScanner was used to eliminate 6, 6, 6, 6, 57, and 3 SNPs associated with confounders, the specifics of which are delineated in Supplementary Table 1 . The remaining 12,11, 14, 17, 160 and 12 SNPs as IVs associated with MI are shown in Supplementary Tables 3 - 8 .
3.2. The effect of frailty index on the risk of mental illness
The results of the MR analysis indicate a causal relationship between FI and multi-MI. The MR results using the IVW method revealed that FI was associated with a significantly increased risk of anxiety (OR = 1.62, 95% CI 1.13-2.33, P = 8.18E-03), depression (OR = 1.88, 95% CI 1.30-2.71, P = 8.21E-04) and affective disorder (OR = 1.70, 95% CI 1.28-2.27, P = 2.57E-04). However, our results do not support a causal relationship between FI on the risk of mania (OR = 1.02, 95% CI 0.99-1.06, P = 2.20E-01) and schizophrenia (OR = 1.02, 95% CI 0.66-1.57, P = 9.28E-01). In particular, the results of the IVW analysis indicated a negative correlation between FI and the risk of OCD (OR = 0.25, 95% CI 0.07-0.86, P = 2.85E-02). However, it is important to interpret these results with caution, as the three methods used in this study were not consistent in their direction.The detail results of the MR analysis are shown in Table 2 , Figure 2 . Further, the Scatter plots and forest plots of the SNP-outcome associations against the SNP-exposure associations are displayed in Supplementary Figures 1 , 2 . Cochran’s Q statistic results revealed significant heterogeneity when examining the causal effect of FI on depression (P = 0.03) and schizophrenia (P = 0.01), whereas no significant heterogeneity was observed in the effect of SNPs across the remaining studies. The MR-Egger regression analysis did not find any evidence of horizontal pleiotropy, and no significant outlier was further identified by MRPRESSO. The results of “leave one out” indicate that there is no single SNP that has a large role in driving the outcome ( Supplementary Figures 3 ). Additionally, the funnel plot provides further evidence that the study is unbiased ( Supplementary Figure 4 ). The results of the sensitivity analysis of the MR analysis are shown in Table 3 .
Table 2.
MR estimates from each method of assessing the causal effect of frailty on the risk of psychiatric illness.
| Exposure | Outcome | MR method | OR | Beta | SE | 95% confidence interval |
P value |
|---|---|---|---|---|---|---|---|
| Frailty | Anxiety | IVW | 1.62 | 0.48 | 0.18 | 1.13-2.33 | 8.18E-03 |
| MR Egger | 2.13 | 0.76 | 0.77 | 0.47-9.65 | 3.58E-01 | ||
| Weighted median | 1.37 | 0.46 | 0.20 | 1.07-2.33 | 2.19E-02 | ||
| Depression | IVW | 1.88 | 0.63 | 0.19 | 1.30-2.71 | 8.21E-04 | |
| MR Egger | 3.25 | 1.18 | 0.72 | 0.79-13.31 | 1.62E-01 | ||
| Weighted median | 2.00 | 0.69 | 0.18 | 1.40-2.84 | 1.25E-04 | ||
| Affective disorder | IVW | 1.70 | 0.53 | 0.15 | 1.28-2.27 | 2.57E-04 | |
| MR Egger | 3.57 | 1.27 | 0.47 | 1.43-8.94 | 7.24E-02 | ||
| Weighted median | 1.88 | 0.63 | 0.17 | 1.34-2.64 | 2.37E-04 | ||
| Mania | IVW | 1.02 | 0.02 | 0.02 | 0.99-1.06 | 2.20E-01 | |
| MR Egger | 1.13 | 0.13 | 0.06 | 1.01-1.27 | 6.93E-02 | ||
| Weighted median | 1.03 | 0.03 | 0.02 | 1.00-1.07 | 4.23E-02 | ||
| Schizophrenia | IVW | 1.02 | 0.02 | 0.22 | 0.66-1.57 | 9.28E-01 | |
| MR Egger | 15.44 | 2.74 | 1.70 | 0.56-429.13 | 1.51E-01 | ||
| Weighted median | 1.18 | 0.17 | 0.22 | 0.77-1.81 | 4.38E-01 | ||
| Obsessive-compulsive disorder | IVW | 0.25 | -1.40 | 0.64 | 0.07-0.86 | 2.85E-02 | |
| MR Egger | 0.94 | 1.60 | 5.87 | 5.02E-05-4.86E+05 | 7.94E-01 | ||
| Weighted median | 0.24 | -1.42 | 0.82 | 0.05-1.20 | 8.17E-02 |
Figure 2.
MR analysis of the causal correlation between FI on the risk of MI.
Table 3.
Sensitivity analysis of the MR analysis results of exposures and outcomes.
| Exposure | Outcome | Heterogeneity test | Pleiotropy test | MR-PRESSO* | |
|---|---|---|---|---|---|
| Cochran’s Q test (P value) IVW |
Egger intercept (P value) MR-egger |
Distortion test Outliers |
Global test P Value |
||
| Frailty | Anxiety | 0.11 | 0.73 | NA | 0.15 |
| Depression | 0.03 | 0.46 | NA | 0.07 | |
| Affective disorder | 0.29 | 0.20 | NA | 0.37 | |
| Mania | 0.07 | 0.11 | NA | 0.10 | |
| Schizophrenia | 0.01 | 0.15 | NA | 0.02 | |
| Obsessive-compulsive disorder | 0.68 | 0.63 | NA | 0.69 | |
| Anxiety | Frailty | 0.33 | 0.61 | NA | 0.39 |
| Depression | 0.05 | 0.86 | NA | 0.08 | |
| Affective disorder | 0.31 | 0.77 | NA | 0.36 | |
| Mania | 0.05 | 0.71 | NA | 0.05 | |
| Schizophrenia | <0.001 | 0.93 | NA | <0.001 | |
| Obsessive-compulsive disorder | 0.13 | 0.48 | NA | 0.12 | |
*Results of MR-PRESSO global test are presented here. For MR analyses with a global test, P < 0.05, no significant outlier was detected by MR-PRESSO.
3.3. Results of reverse Mendelian randomization analysis
The investigation into the causal relationship between multi-MI and the risk of FI reveals compelling evidence through MR analysis. According to the primary IVW, genetically predicted anxiety (OR = 1.06, 95% CI 1.01-1.11, P = 2.00E-02), depression (OR = 1.14, 95% CI 1.04-1.26, P = 7.99E-03), affective disorder (OR = 1.15, 95% CI 1.09-1.21, P = 3.39E-07), and schizophrenia (OR = 1.02, 95% CI 1.01-1.04, P = 1.70E-03) were identified as risk factors for FI. However, according to the IVW results, there was no evidence of a causal relationship between mania (OR = 1.46, 95% CI 0.79-2.72, P = 2.27E-01) and OCD (OR = 1.01, 95% CI 0.99-1.02, P = 2.11E-01) with FI. The detail results of the MR analysis are shown in Table 4 , Figure 3 . Further, the scatter plots and forest plots of the SNP-outcome associations against the SNP-exposure associations are displayed in Supplementary Figures 5 , 6 . The results of Cochran’s Q statistic results revealed significant heterogeneity when examining the causal effect of schizophrenia (P < 0.001) on FI, whereas no significant heterogeneity was observed in the effect of SNPs across the remaining studies. The MR-Egger regression analyses indicated that the presence of multiplicity affecting the results was unlikely, and no significant outlier was further identified by MRPRESSO. Additionally, we visually examined sensitivity using “leave one out” ( Supplementary Figure 7 ) and funnel plots ( Supplementary Figure 8 ), which confirmed the robustness of our results. The results of the sensitivity analysis of the MR analysis are shown in Table 3 .
Table 4.
MR estimates from each method of assessing the causal effect of psychiatric illness on the risk of frailty.
| Exposure | Outcome | MR method | OR | Beta | SE | 95% confidence interval |
P value |
|---|---|---|---|---|---|---|---|
| Anxiety | Frailty | IVW | 1.06 | 0.06 | 0.02 | 1.01-1.11 | 2.00E-02 |
| MR Egger | 1.01 | 0.01 | 0.10 | 0.84-1.22 | 9.42E-01 | ||
| Weighted median | 1.05 | 0.06 | 0.03 | 0.99-1.12 | 9.46E-02 | ||
| Depression | IVW | 1.14 | 0.13 | 0.05 | 1.04-1.26 | 7.99E-03 | |
| MR Egger | 1.21 | 0.20 | 0.34 | 0.62-2.39 | 5.91E-01 | ||
| Weighted median | 1.14 | 0.13 | 0.05 | 1.03-1.27 | 1.51E-02 | ||
| Affective disorder | IVW | 1.15 | 0.14 | 0.03 | 1.09-1.21 | 3.39E-07 | |
| MR Egger | 1.07 | 0.07 | 0.24 | 0.67-1.72 | 7.80E-01 | ||
| Weighted median | 1.15 | 0.14 | 0.04 | 1.08-1.24 | 7.52E-05 | ||
| Mania | IVW | 1.46 | 0.38 | 0.32 | 0.79-2.72 | 2.27E-01 | |
| MR Egger | 1.16 | 0.15 | 0.68 | 0.31-4.38 | 6.77E-01 | ||
| Weighted median | 0.88 | -0.12 | 0.34 | 0.46-1.71 | 3.38E-01 | ||
| Schizophrenia | IVW | 1.02 | 0.01 | 0.02 | 1.01-1.04 | 1.70E-03 | |
| MR Egger | 1.03 | 0.03 | 0.02 | 0.97-1.07 | 4.36E-01 | ||
| Weighted median | 1.02 | 0.01 | 0.02 | 1.00-1.04 | 3.30E-02 | ||
| Obsessive-compulsive disorder | IVW | 1.01 | 0.01 | 0.01 | 0.99-1.02 | 2.10E-01 | |
| MR Egger | 1.02 | 0.02 | 0.02 | 0.98-1.07 | 3.12E-01 | ||
| Weighted median | 1.01 | 0.01 | 0.01 | 0.99-1.02 | 4.00E-01 |
Figure 3.
MR analysis of the causal correlation between MI on the risk of FI.
4. Discussion
Using several large-scale GWAS data, we performed a bidirectional MR analysis to estimate the causal relationship between frailty and MI. We observed bidirectional causality between FI and anxiety, depression, and affective disorders. FI had no significant effect on the risk of mania, schizophrenia and OCD. In contrast, schizophrenia was associated with higher FI, and there was no reliable evidence to support genetically predicted effects of manic and OCD on FI. Although several previous MR studies have explored the causal relationship between frailty and some MI (17, 28–31), our study possesses several strengths worth noting. First, this study is the first to explore the causal relationship between FI and affective disorders, mania, and OCD through MR. Secondly, our data were sourced from the latest version of the database and enhancing the reliability of our results and updating for previous findings. This finding offers valuable insights into the association between FI and MI, laying a theoretical groundwork for future development of public health policies.
Previous observational studies have indicated a possible link between the vulnerability index, a clinical metric of biological age in the field of psychiatry, and MI (32). Both individuals classified as frail and pre-frail exhibited significantly diminished scores in mental and physical quality of life compared to non-frail individuals (33). A 25-year study utilizing replicated data revealed that individuals experiencing frailty exhibited significantly lower scores in the Short-Form 36 General Health Survey (SF-36) in comparison to non-frailty individuals (34). For instance, a prior study conducted in western China, involving 4,103 community residents aged 60 years and older, revealed that individuals grappling with co-morbidities of depression and anxiety exhibited elevated odds of pre-frailty (OR=1.86, 95% CI=1.41-2.45) and frailty (OR=7.03, 95% CI=4.48-11.05) in contrast to individuals without depressive and anxiety symptoms (35). In addition, Recent studies have shown a correlation between affective disorders and frailty, as well as an increased risk of relapse in frail patients (10, 36). Furthermore, the COVID-19 pandemic appears to have resulted in a heightened severity of frailty and MI. Studies have demonstrated that pre-existing frailty in older adults was correlated with increased likelihood of enduring and abrupt MI during the initial wave of the COVID-19 pandemic, and this association persisted (37, 38). Notably, several studies have indicated a high prevalence of frailty among individuals diagnosed with schizophrenia, including those in younger age groups (39, 40). Recent studies also have demonstrated strikingly similar patterns of gene activity in aging and schizophrenic patients, particularly in neurons and astrocytes in the prefrontal cortex (41). This indicates a potential association between frailty and schizophrenia. Similarly, our findings indicate a link between FI and schizophrenia, suggesting that a state of schizophrenia may elevate the risk of frailty, though the evidence for reverse causality is weak. However, it has to be recognized that when examining the impact of schizophrenia on the risk of frailty onset, the OR was only 1.02 with a confidence interval of 1.01-1.04, which was also confirmed by the Weighted median approach. This suggests that schizophrenia may not significantly heighten the risk of frailty. Theoretically, MR evaluates the lifelong impact of genetically predicted exposures on the incidence of an outcome over an extended duration, often yielding results with more pronounced effect sizes compared to traditional observational studies (42, 43). This aspect should be considered when interpreting our findings, leading us to adopt a conservative stance in observing a modest association between frailty and schizophrenia. Future observational studies examining the relationship between frailty and schizophrenia should be conducted in larger populations, and a more extensive population-based GWAS for mania is warranted. Previous observational studies have given little attention to OCD in older adults. Some studies suggest that individuals with OCD experience accelerated brain aging, as well as shortening of mitochondrial DNA copy number (mtDNAcn) and telomere length in the blood (44, 45). However, our study did not observe a bidirectional relationship between FI and OCD. We consider the lack of association found in our analysis to be relatively reliable. Observational studies can be confounded by confounders and reverse causation, making it difficult to determine a causal relationship between variables. Our study utilized the MR method, which is less susceptible to confounding bias than traditional observational designs (46). To confirm our findings, Further studies based on GWAS data with larger sample sizes and representative participants are needed.
The exact mechanisms underlying the relationship between frailty and MI have yet to be fully understood. Various hypotheses can explain the bidirectional relationship between frailty and MI. First, physical weakness can result in diminished physical activity and social interaction, as well as increased sedentary behavior, ultimately contributing to the onset of MI (47, 48). Conversely, MI can give rise to unfavorable symptoms, such as reduced social interaction, weight loss, and malnutrition (49, 50). Secondary, frailty and MI share numerous risk factors, including chronic inflammation (51, 52), cardiovascular disease (53, 54), and unhealthy lifestyle choices (23, 55). Thirdly, treatments for frailty or MI that had beneficial effects can also protect each other. For instance, it was well-known that incorporating physical activity as a strategy can enhance physical functioning in older and frail individuals while also benefiting reasoning and problem-solving abilities in individuals with MI, improving their symptoms in the process (56–58). Therefore, the presence of a bidirectional relationship between frailty and MI is not coincidental, as all the evidence substantiates this hypothesis.
The escalating global burden of frailty underscores the urgent need to slow down its progression and enhance the well-being and quality of life of older adults (59). A recent study has revealed a significant co-occurrence of frailty and mental disorders, leading to heightened mortality rates (9). This study’s results comprehensively evaluated the bidirectional causal relationship between FI and MI, minimizing potential confounding biases. These reciprocal findings on FI and MI carry critical implications for public health and clinical practice. Primarily, there is a crucial necessity to fortify efforts in identifying and managing frailty, implementing timely interventions in its early stages. Additionally, our results underscore the importance of psychologically relevant strategies, including routine screening for psychological disorders, social support, and targeted psychological interventions, essential for both primary and secondary prevention of frailty to avert unfavorable outcomes and break vicious cycles. From a public health perspective, our findings could inform the development of management strategies addressing common risk factors and interventions to prevent frailty and MI in older adults, thereby alleviating adverse outcomes and enhancing their overall quality of life.
This study is subject to certain limitations. Firstly, it exclusively considers individuals of European descent, thus failing to address potential genetic variations across different races, countries, and regions. Secondly, the reliance on pooled data limits the availability of detailed clinical information, which restricts our ability to perform analyses to gender specificity. Thirdly, the limited number of SNPs meeting the genome-wide significance threshold for the GWAS of the pooled dataset for MI may have impacted the reverse MR analysis. Fourthly, It is crucial to acknowledge that MR analyses inherently offer less robust evidence of causality compared to RCTs. Fifthly, part of the FI data and mania’s summarized GWAS data came from the same database, which led to an overlap in the sample populations between the studies, as some participants were included in both studies. To address these limitations, future large-scale GWAS studies should be conducted across diverse ethnicities and differentiate between their sexes. In addition, there is a pressing need for additional high-quality RCTs to corroborate and fortify these findings. However, it is ethically challenging to explore the causal relationship between FI and MI through RCTs; therefore, extended prospective cohort studies may serve as a viable alternative. Furthermore, exploring the efficacy of interventions tailored to target common risk factors for FI and MI in enhancing patient care management warrants further investigation in forthcoming trials.
5. Conclusion
In summary, this study identified a reciprocal association between frailty and risk of MI using MR methods. On the basis of our findings, it is reasonable to consider promoting routine frailty screening in MI patients. In addition, proper management of MI is also essential for downregulating the risk of frailty.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics statement
All data analyzed in this study were obtained from publicly available databases in which ethical approval was obtained for each cohort, and informed consent was obtained from all participants prior to participation.
Author contributions
LM: Data curation, Formal analysis, Writing – original draft. ZL: Data curation, Writing – original draft. LF: Formal analysis, Writing – original draft. JF: Formal analysis, Writing – original draft, Writing – review & editing. CK: Writing – original draft. TW: Writing – original draft. HB: Writing – review & editing. QL: Writing – review & editing. JY: Conceptualization, Writing – review & editing. XF: Conceptualization, Project administration, Writing – review & editing.
Acknowledgments
This research has been conducted using the Psychiatric Genomics Consortium, IEU Open GWAS project, UK biobank and FinnGen Consortium. The authors thank the participants and coordinators for this unique dataset. The Figure 1 in this article were drawn by Figdraw.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Key Research and Development Program of China(2020YFC2008400); Medical Education Research Program of Henan Province (WILX2023017); The Medical Science and Technology Program of Henan Province (Provincial-Ministry JointProject) (SBGJ202302052); The National Natural Science Foundation of China (Grant No.82371235).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1397813/full#supplementary-material
Scatter plots of single SNP effect and estimates from multiple two-sample MR analyses for the causal effect of FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) in replication analysis.
Forest plots of causal effects of FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F). The bars indicate the confidence interval of MR estimates.
Leave-one-out plots of two-sample Mendelian randomization analysis for genetically predicted FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) outcomes. The dots indicate MR estimates for using inverse-variance weighted method when the SNP was removed. The bars indicate the confidence interval of MR estimates.
Funnel plots assess the presence of potential heterogeneity across genetic instruments for FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F), which indicates possible pleiotropic effects. The causal effect of each genetic instrument was presented by dots, and combined causal effect by inverse variance weighted and MR Egger were depicted by lines.
Scatter plots of single SNP effect and estimates from multiple two-sample MR analyses for the causal effect of anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI in replication analysis.
Forest plots of causal effects of anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI. The bars indicate the confidence interval of MR estimates.
Leave-one-out plots of two-sample Mendelian randomization analysis for genetically predicted anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI outcomes. The dots indicate MR estimates for using inverse-variance weighted method when the SNP was removed. The bars indicate the confidence interval of MR estimates.
Funnel plots assess the presence of potential heterogeneity across genetic instruments for anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI, which indicates possible pleiotropic effects. The causal effect of each genetic instrument was presented by dots, and combined causal effect by inverse variance weighted and MR Egger were depicted by lines.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London England). (2013) 381:752–62. doi: 10.1016/s0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin FC, O'Halloran AM. Tools for assessing frailty in older people: general concepts. Adv Exp Med Biol. (2020) 1216:9–19. doi: 10.1007/978-3-030-33330-0_2 [DOI] [PubMed] [Google Scholar]
- 3. Palliyaguru DL, Moats JM, Di Germanio C, Bernier M, de Cabo R. Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions. Mech Ageing Dev. (2019) 180:42–8. doi: 10.1016/j.mad.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao G, Wang H, Hu J, Liu L, Zhang T, Zhou M, et al. Estimating the causal effect of frailty index on vestibular disorders: A two-sample Mendelian randomization. Front Neurosci. (2022) 16:990682. doi: 10.3389/fnins.2022.990682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Veronese N, Custodero C, Cella A, Demurtas J, Zora S, Maggi S, et al. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res Rev. (2021) 72:101498. doi: 10.1016/j.arr.2021.101498 [DOI] [PubMed] [Google Scholar]
- 6. De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. (2018) 20:31–40. doi: 10.31887/DCNS.2018.20.1/mdehert [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. (2015) 72:334–41. doi: 10.1001/jamapsychiatry.2014.2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pearson E, Siskind D, Hubbard RE, Gordon EH, Coulson EJ, Warren N. Frailty and severe mental illness: A systematic review and narrative synthesis. J Psychiatr Res. (2022) 147:166–75. doi: 10.1016/j.jpsychires.2022.01.014 [DOI] [PubMed] [Google Scholar]
- 9. Mutz J, Choudhury U, Zhao J, Dregan A. Frailty in individuals with depression, bipolar disorder and anxiety disorders: longitudinal analyses of all-cause mortality. BMC Med. (2022) 20:274. doi: 10.1186/s12916-022-02474-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borges MK, Jeuring HW, Marijnissen RM, van Munster BC, Aprahamian I, van den Brink RHS, et al. Frailty and affective disorders throughout adult life: A 5-year follow-up of the Lifelines Cohort Study. J Am Geriatr Soc. (2022) 70:3424–35. doi: 10.1111/jgs.18021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgess S, Daniel RM, Butterworth AS, Thompson SG, Consortium EP-I. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. (2015) 44:484–95. doi: 10.1093/ije/dyu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 13. Gao L, Di X, Gao L, Liu Z, Hu F. The Frailty Index and colon cancer: a 2-sample Mendelian-randomization study. J Gastrointest Oncol. (2023) 14:798–805. doi: 10.21037/jgo-23-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Chen H, He W, Luo L, Guo X. Frailty index and risk of cardiovascular diseases: a mendelian randomization study. Ann Transl Med. (2022) 10:1007. doi: 10.21037/atm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renedo D, Acosta JN, Koo AB, Rivier C, Sujijantarat N, de Havenon A, et al. Higher hospital frailty risk score is associated with increased risk of stroke: observational and genetic analyses. Stroke. (2023) 54:1538–47. doi: 10.1161/STROKEAHA.122.041891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atkins JL, Jylhava J, Pedersen NL, Magnusson PK, Lu Y, Wang Y, et al. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell. (2021) 20:e13459. doi: 10.1111/acel.13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma T, Chen M, Cheng X, Bai Y. Assessment of bidirectional relationships between frailty and mental disorders: A bidirectional Mendelian randomization study. J Am Med dir Assoc. (2024) 25:506–13.e529. doi: 10.1016/j.jamda.2023.10.009 [DOI] [PubMed] [Google Scholar]
- 18. Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. (2022) 604:502–8. doi: 10.1038/s41586-022-04434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. (2018) 23:1181–8. doi: 10.1038/mp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu S, Tan JS, Hu MJ, Guo TT, Chen L, Hua L, et al. The causality between diabetes and venous thromboembolism: A bidirectional two-sample Mendelian randomization study. Thromb haemostasis. (2023) 123:913–9. doi: 10.1055/a-2040-4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dan YL, Wang P, Cheng Z, Wu Q, Wang XR, Wang DG, et al. Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study. Rheumatology. (2021) 60:940–6. doi: 10.1093/rheumatology/keaa506 [DOI] [PubMed] [Google Scholar]
- 22. Pagoni P, Dimou NL, Murphy N, Stergiakouli E. Using Mendelian randomisation to assess causality in observational studies. Evid Based Ment Health. (2019) 22:67–71. doi: 10.1136/ebmental-2019-300085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lv J, Wu L, Sun S, Yu H, Shen Z, Xu J, et al. Smoking, alcohol consumption, and frailty: A Mendelian randomization study. Front Genet. (2023) 14:1092410. doi: 10.3389/fgene.2023.1092410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. (1997) 315:1533–7. doi: 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeng Y, Cao S, Yang H. The causal role of gastroesophageal reflux disease in anxiety disorders and depression: A bidirectional Mendelian randomization study. Front Psychiatry. (2023) 14:1135923. doi: 10.3389/fpsyt.2023.1135923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu J, Zhou D, Nie Y, Wang J, Yang Y, Chen D, et al. Assessment of the bidirectional causal association between frailty and depression: A Mendelian randomization study. J cachexia sarcopeni. (2023) 14:2327–34. doi: 10.1002/jcsm.13319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng MG, Liu F, Liang Y, Wang K, Nie JQ, Liu J. Association between frailty and depression: A bidirectional Mendelian randomization study. Sci Adv. (2023) 9:eadi3902. doi: 10.1126/sciadv.adi3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen JH, Lei H, Wan YF, Zhu XC, Zeng LY, Tang HX, et al. Frailty and psychiatric disorders: A bidirectional Mendelian randomization study. J Affect Disord. (2024) 356:346–55. doi: 10.1016/j.jad.2024.04.024 [DOI] [PubMed] [Google Scholar]
- 31. Luo X, Ruan Z, Liu L. Causal relationship between depression and aging: a bidirectional two-sample Mendelian randomization study. Aging Clin Exp Res. (2023) 35:3179–87. doi: 10.1007/s40520-023-02596-4 [DOI] [PubMed] [Google Scholar]
- 32. Bersani FS, Canevelli M, Cesari M, Maggioni E, Pasquini M, Wolkowitz OM, et al. Frailty Index as a clinical measure of biological age in psychiatry. J Affect Disord. (2020) 268:183–7. doi: 10.1016/j.jad.2020.03.015 [DOI] [PubMed] [Google Scholar]
- 33. Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. (2016) 70:716–21. doi: 10.1136/jech-2015-206717 [DOI] [PubMed] [Google Scholar]
- 34. Landré B, Ben Hassen C, Kivimaki M, Bloomberg M, Dugravot A, Schniztler A, et al. Trajectories of physical and mental functioning over 25 years before onset of frailty: results from the Whitehall II cohort study. J cachexia sarcopenia muscle. (2023) 14:288–97. doi: 10.1002/jcsm.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao W, Zhang Y, Liu X, Yue J, Hou L, Xia X, et al. Comorbid depressive and anxiety symptoms and frailty among older adults: Findings from the West China health and aging trend study. J Affect Disord. (2020) 277:970–6. doi: 10.1016/j.jad.2020.08.070 [DOI] [PubMed] [Google Scholar]
- 36. Kessing LV, Olsen EW, Andersen PK. Recurrence in affective disorder: analyses with frailty models. Am J epidemiol. (1999) 149:404–11. doi: 10.1093/oxfordjournals.aje.a009827 [DOI] [PubMed] [Google Scholar]
- 37. Szlejf C, Suemoto CK, Goulart AC, Santos IS, Bacchi PS, Fatori D, et al. A pandemic toll in frail older adults: Higher odds of incident and persistent common mental disorders in the ELSA-Brasil COVID-19 mental health cohort. J Affect Disord. (2023) 325:392–8. doi: 10.1016/j.jad.2023.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aryaie M, Sokout T, Moradi S, Abyad A, Asadollahi A. Frailty and mental health disorders before and during COVID-19 occurrence in older population in Iran: A longitudinal repeated-measures study. J primary Care Community Health. (2022) 13:21501319221126979. doi: 10.1177/21501319221126979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pearson E, Siskind D, Hubbard R, Gordon E, Coulson E, Arnautovska U, et al. Frailty and treatment-resistant schizophrenia: A retrospective cohort study. Community Ment Health J. (2023) 59:105–9. doi: 10.1007/s10597-022-00998-8 [DOI] [PubMed] [Google Scholar]
- 40. Yang C, Hou X, Ma X, Wu D. Frailty among inpatients with Schizophrenia: Status, influencing factors, and their correlation with quality of life. Front Psychiatry. (2022) 13:1067260. doi: 10.3389/fpsyt.2022.1067260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ling E, Nemesh J, Goldman M, Kamitaki N, Reed N, Handsaker RE, et al. A concerted neuron-astrocyte program declines in ageing and schizophrenia. Nature. (2024) 627:604–11. doi: 10.1038/s41586-024-07109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labrecque JA, Swanson SA. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am J epidemiol. (2019) 188:231–8. doi: 10.1093/aje/kwy204 [DOI] [PubMed] [Google Scholar]
- 43. Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. Bmj. (2021) 375:n2233. doi: 10.1136/bmj.n2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu L, Liu J, Yang L, Wen B, Zhang X, Cheng J, et al. Accelerated brain aging in patients with obsessive-compulsive disorder. Front Psychiatry. (2022) 13:852479. doi: 10.3389/fpsyt.2022.852479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang JI, Park CI, Lin J, Kim ST, Kim HW, Kim SJ. Alterations of cellular aging markers in obsessive- compulsive disorder: mitochondrial DNA copy number and telomere length. J Psychiatr Neurosci. (2021) 46:E451–e458. doi: 10.1503/jpn.200238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cui G, Li S, Ye H, Yang Y, Jia X, Lin M, et al. Gut microbiome and frailty: insight from genetic correlation and mendelian randomization. Gut Microbes. (2023) 15:2282795. doi: 10.1080/19490976.2023.2282795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naslund JA, Aschbrenner KA, Marsch LA, Bartels SJ. The future of mental health care: peer-to-peer support and social media. Epidemiol Psych Sci. (2016) 25:113–22. doi: 10.1017/S2045796015001067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katayama O, Lee S, Bae S, Makino K, Chiba I, Harada K, et al. The association between social activity and physical frailty among community-dwelling older adults in Japan. BMC Geriatr. (2022) 22:870. doi: 10.1186/s12877-022-03563-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mehrabi F, Béland F. Frailty as a moderator of the relationship between social isolation and health outcomes in community-dwelling older adults. Int J Environ Res Public Health. (2021) 18:null. doi: 10.20944/preprints202102.0091.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen C, Winterstein AG, Fillingim RB, Wei YJ. Body weight, frailty, and chronic pain in older adults: a cross-sectional study. BMC Geriatr. (2019) 19:143. doi: 10.1186/s12877-019-1149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goh XX, Tang PY, Tee SF. Meta-analysis of soluble tumour necrosis factor receptors in severe mental illnesses. J Psychiatr Res. (2023) 165:180–90. doi: 10.1016/j.jpsychires.2023.07.014 [DOI] [PubMed] [Google Scholar]
- 52. McKechnie DGJ, Papacosta AO, Lennon LT, Ramsay SE, Whincup PH, Wannamethee SG. Associations between inflammation, cardiovascular biomarkers and incident frailty: the British Regional Heart Study. Age ageing. (2021) 50:1979–87. doi: 10.1093/ageing/afab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goldfarb M, De Hert M, Detraux J, Di Palo K, Munir H, Music S, et al. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 80:918–33. doi: 10.1016/j.jacc.2022.06.017 [DOI] [PubMed] [Google Scholar]
- 54. Ko D, Bostrom JA, Qazi S, Kramer DB, Kim DH, Orkaby AR. Frailty and cardiovascular mortality: A narrative review. Curr Cardiol Rep. (2023) 25:249–59. doi: 10.1007/s11886-023-01847-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mallet J, Le Strat Y, Schürhoff F, Mazer N, Portalier C, Andrianarisoa M, et al. Tobacco smoking is associated with antipsychotic medication, physical aggressiveness, and alcohol use disorder in schizophrenia: results from the FACE-SZ national cohort. Eur Arch Psychiatry Clin Neurosci. (2019) 269:449–57. doi: 10.1007/s00406-018-0873-7 [DOI] [PubMed] [Google Scholar]
- 56. Rossi PG, Carnavale BF, Farche ACS, Ansai JH, de Andrade LP, Takahashi ACM. Effects of physical exercise on the cognition of older adults with frailty syndrome: A systematic review and meta-analysis of randomized trials. Arch Gerontol Geriatr. (2021) 93:104322. doi: 10.1016/j.archger.2020.104322 [DOI] [PubMed] [Google Scholar]
- 57. Tavares VDO, Rossell SL, Schuch FB, Herring M, Menezes de Sousa G, Galvão-Coelho NL, et al. Effects of exercise on cognitive functioning in adults with serious mental illness: A meta analytic review. Psychiatry Res. (2023) 321:115081. doi: 10.1016/j.psychres.2023.115081 [DOI] [PubMed] [Google Scholar]
- 58. Kleemann E, Bracht CG, Stanton R, Schuch FB. Exercise prescription for people with mental illness: an evaluation of mental health professionals' knowledge, beliefs, barriers, and behaviors. Rev Bras psiquiatria (Sao Paulo Brazil 1999). (2020) 42:271–7. doi: 10.1590/1516-4446-2019-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots of single SNP effect and estimates from multiple two-sample MR analyses for the causal effect of FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) in replication analysis.
Forest plots of causal effects of FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F). The bars indicate the confidence interval of MR estimates.
Leave-one-out plots of two-sample Mendelian randomization analysis for genetically predicted FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) outcomes. The dots indicate MR estimates for using inverse-variance weighted method when the SNP was removed. The bars indicate the confidence interval of MR estimates.
Funnel plots assess the presence of potential heterogeneity across genetic instruments for FI on anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F), which indicates possible pleiotropic effects. The causal effect of each genetic instrument was presented by dots, and combined causal effect by inverse variance weighted and MR Egger were depicted by lines.
Scatter plots of single SNP effect and estimates from multiple two-sample MR analyses for the causal effect of anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI in replication analysis.
Forest plots of causal effects of anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI. The bars indicate the confidence interval of MR estimates.
Leave-one-out plots of two-sample Mendelian randomization analysis for genetically predicted anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI outcomes. The dots indicate MR estimates for using inverse-variance weighted method when the SNP was removed. The bars indicate the confidence interval of MR estimates.
Funnel plots assess the presence of potential heterogeneity across genetic instruments for anxiety (A), depression (B), affective disorder (C), mania (D), schizophrenia (E), and OCD (F) on FI, which indicates possible pleiotropic effects. The causal effect of each genetic instrument was presented by dots, and combined causal effect by inverse variance weighted and MR Egger were depicted by lines.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.