Abstract
Background:
The legalization of cannabis use and false claims about the plant Cannabis sativa to be considered a pharmaceutical product have been found to increase consumption, lower risk perception, and lead to more health problems, without reducing criminal activity. Brain function, typically assessed by neuropsychological tests, shows abnormalities with acute marijuana use, but inconsistent results have been published after abstinence, with a maximum follow-up of 28 days. Our previous research, using neuropsychological tests and brain perfusion single photon emission computed tomography (neuroSPECT), demonstrated consistent abnormalities in brain function among schoolchildren who consume marijuana compared to their non-consuming peers. The aim of this study is to investigate whether brain function changes in 20 adult marijuana users after 6 months of abstinence.
Methodology:
Comparison of neuropsychological tests (Rey Complex Figure; Porteus Maze; Four subtests of WAIS-IV Intellectual Tests; STROOP; D2) and perfusion neuroSPECT (functional images), obtained in relation to recent consumption and after 6 months of serial drug-screening test confirmed abstinence.
Results:
In a one-year period (2020–2021) only five compliant participants were recruited. The COVID-19 pandemic was a limiting factor. Preliminary results of neuropsychological tests, functional brain perfusion images and limited statistical analysis are presented. The results of the neuropsychological tests of the three subjects who completed the abstinence period so far show some improvement in working memory and attention after abstinence. NeuroSPECT shows disorganized hypoperfusion of variable severity in relation to recent consumption, involving areas associated with cognitive function such as the posterior cingulate and temporal lobes, in our five initially enrolled patients, when compared to a normal database. Of these, only two participants have already been re-evaluated with neuroSPECT after 6 months of abstinence, one of whom showed some improvement on the post-abstinence images.
Conclusion:
We analyze the methodological challenges of this research, including the pandemic, to incorporate the appropriate corrections in the next phase of our investigation. Our final findings may provide clinicians and users with information about the long-term effects of marijuana use on brain function.
Keywords: marijuana, neuropsychological tests, brain perfusion, SPECT, brain dysfunction
Introduction
The International Narcotics Control Board (INCB), an agency subordinate to the World Health Organization, warns in its 2022 report that the legalization of cannabis use for non-medical purposes leads to increased consumption, more health problems, with no reduction in criminal activity. The report confirms that, in the United States, for the period 2019–2020 among those over 12 years of age, the average consumption in the last year reaches 24.5% in those states that have legalized its use compared to 16.5% for the states that have not legalized it [1]. The high prevalence of consumption among adolescents, both in Chile and throughout the region in the Americas and Europe, is particularly alarming. The United States and Canada have the highest prevalence of juvenile consumption. In the 18–25 age group, the United States has an average prevalence of 35% for marijuana use in the last year while Canada has a prevalence of 52% for the 20–24 age group [1]. In addition, an increase has been observed in the percentage of people who are dependent on this substance, from 9% to 11%, with a significant increase in the number of subjects who develop psychosis as a result of its consumption [2, 3, 4].
In addition, starting consumption before the age of 18 increases the likelihood of developing a drug abuse disorder by four to seven times compared to starting in adulthood [5]. This may be related to the fact that marijuana used in the 1980s had an average Tetrahydrocannabinol (THC) concentration of 3%, whereas nowadays it has a concentration in the order of 16% [1, 6]. Thus, among the factors that explain the increase in marijuana consumption, especially in adolescents, are: (1) the progressive decrease in risk perception together with the idea of an possible therapeutic effect; (2) greater availability related to legalization; and (3) that the marijuana currently available is more powerful than that existing in previous decades due to a higher THC concentration, which is explained by the genetic manipulation of the cannabis plant [1, 7, 8].
In our previous studies, we found that young marijuana users showed cognitive dysfunction associated with decreased cerebral perfusion as determined by neuropsychological tests and functional neuroimaging with brain perfusion single photon emission computed tomography (SPECT). These abnormalities varied in location, extent and severity among users [9].
Perfusion brain SPECT is recognized as a highly sensitive method for functional brain imaging. The tracer Tc99m-HMPAO is fixed in the gray matter with an only blood-flow-dependent distribution, which in turn is an indirect representation of function. On the other hand, the effects of abstinence on cognitive functions and memory, have been mostly investigated only with neuropsychological tests and have shown that in the short term there can be an improvement in memory, but not in attention [10]. However, the investigations we reviewed reported variable findings and were limited to follow-up periods of a maximum of 28 days of abstinence [11]. Given that there is individual susceptibility, thus variability in distribution and severity of the abnormalities associated with consumption of marijuana, and differences in post-abstinence outcomes, we decided to determine whether there are individual changes in brain function with a longer period of abstinence than the one studied so far.
This observational prospective study aims to determine the effect of marijuana use discontinuation on cognitive functions and cerebral perfusion in marijuana consumers, evaluated before and after 6 months of serial drug-screening test confirmed abstinence.
Materials and Methods
Sample Selection
The objective is a sample of 20 adults (18 to 50 years old) who are regular marijuana users as the only drug, consulting a specialist and with no other clinical history of encephalic damage. A regular user is defined as someone who reports at least four episodes of cannabis use per month, for the last 18 months.
The treating physicians administer the usual structured questionnaires to the patients before they are invited to participate voluntarily in this study, which has been approved by the Medical Ethics Committee of Clínica Las Condes (D012019) and complies with the principles of the Declaration of Helsinki. All of them sign an informed consent form prior to participation. The therapeutic interventions are decided by the treating physicians.
Instruments and Procedures
The neuropsychological tests applied are: (1) Rey Complex Figure: Copy and Memory; (2) Porteus Maze [12]; (3) Four subtests of the WAIS-IV Intellectual Test: Digits, Letter-Number Sequencing, Symbol Search and Clues [13]; (4) STROOP [14]; and (5) D2 [15]. From these tests, scores are obtained for the following specific functions: (a) cognitive development, defined as the ability to reason, form and execute a plan to solve problems (Rey’s Complex Figure Copy and Porteus Maze); (b) working memory, defined as the ability to retain and combine information, reorganizing data from long-term memory (Digits, Letter-Number Sequencing and Rey’s Complex Figure Memory); (c) processing speed, defined as the ability to process information quickly and perform mental tasks efficiently (Symbol Search, Cues, Stroop and D2); (d) attention, defined as the ability to focus on relevant stimuli and filter out distractions (Stroop and D2); (e) inhibitory control, defined as the ability to control impulses and resist the temptation to perform inappropriate or irrelevant actions (Stroop and D2).
Brain perfusion SPECT is performed on a Siemens Symbia Intevo dual head SPECT/CT system with the radiotracer Tc99m-HMPAO applying the usual protocol, previously described [9]. The results are displayed as: (1) images of the relative distribution of perfusion in the whole cortex and basal ganglia, (2) three-dimensional images of the comparison against a normal database (3D) and (3) the automatic quantification of average, minimum and maximum values of perfusion by Brodmann areas using the program Neurogam® from Segami Corporation, Columbia, MD, USA.
The comparison against the normal database is expressed in standard deviations for each of 14,000 voxels of the whole cerebral cortex in the 3D images. The colors of the accompanying scale highlight values at two, three and four standard deviations above and below the normal mean of the corresponding age database, representing areas of hyperperfusion and hypoperfusion respectively.
Urine drug tests (strip test) are performed every two weeks during the six months of abstinence in the laboratory of Clínica Las Condes with the usual protocol of nurse supervised sample collection.
Data Analysis
The variation of scores in the neuropsychological tests before versus after the cessation of consumption is analyzed only as positive or negative change considering the small sample included in this preliminary report. No other statistical analyses are applied at this stage of our investigation. We consider the use of classical statistical analysis models and non-parametric statistical contrast tests at the completion of the sample for neuropsychological tests and quantitative neuropsychological tests and brain perfusion single photon emission computed tomography (neuroSPECT) of Brodmann areas. A paired t-test will be applied to analyse neuropsychological tests conducted at baseline and six months following cessation of marijuana use when the entire group of 20 subjects is completed.
The comparison of the initial condition with respect to post-abstinence changes is qualitative for the 3D images and quantitative for the values per Brodmann’s area. The emphasis will be on the evaluation of Brodmann’s areas related to cognitive functions, applying paired Student’s t-test. We also consider analyzing the perfusion values per Brodmann area in our sample compared to the normal database with the purpose of quantifying the eventual damage in the consumers.
Preliminarily, we perform a comparative analysis between neuropsychological tests and neuroSPECT results, which will be completed when the whole sample is evaluated.
Results
The actual sample consists of five eligible adults, aged 21 to 49 years, recruited in a 12 months period. The available results of these first five patients are: (a) neuropsychological tests and neuroSPECT with a recent consumption episode for all; (b) neuropsychological tests in 3 patients after 6 months without consumption and (c) neuroSPECT after six months of abstinence in two patients. One patient resumed consumption before completing the 6-month period but agreed to have neuropsychological tests repeated. One patient was excluded from the analysis due to lack of medical follow-up and urine tests.
(A) Neuropsychological Tests
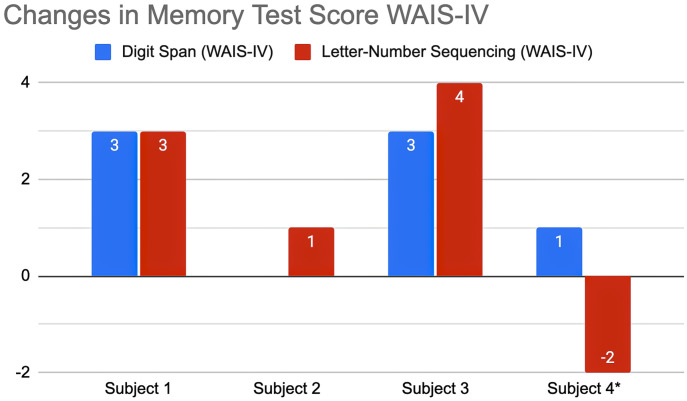
We observed an improvement in Working Memory in all three compliant participants. The patient who resumed consumption, although showing improvement in some tests, is the only one who decreased his score in the WAIS-IV Sequence sub-test (Fig. 1, case 4). This test, which measures working memory, involves a more complex processing that requires prioritizing and reordering information.
Fig. 1.
Memory WAIS-IV Digits (in blue) and Sequencing (in red): each bar represents the absolute score value change after abstention when compared to the score obtained with recent consumption. A positive value indicates an improvement in performance after abstention. (*subject 4 resumed marijuana use before the final neuropsychological assessment).
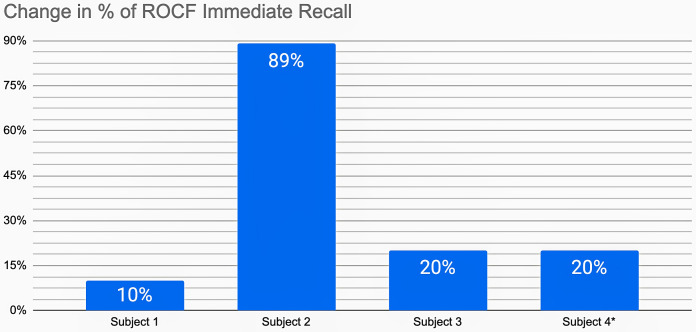
Overall an increase in the scores of the memory tests is seen. There was a notorious increase in the Rey Complex Figure Memory score of examinee 2, and a decrease in the sequencing score of examinee 4, who was the one who had resumed consumption (Fig. 2).
Fig. 2.
Rey Memory test score changes are expressed as percentage of increase (improvement) in score after abstinence compared to the score obtained with recent marijuana consumption. (*subject 4 resumed marijuana use before the final neuropsychological assessment). ROCF, Rey Complex Figure.
Regarding cognitive development, a function that fundamentally aims at problem solving, no homogeneous variation was observed in the scores of the participants. Thus, these results do not allow us to draw preliminary conclusions.
Attention improved in all participants who did not resume consumption.
In processing speed, all participants improved their scores. However, the one who resumed consumption had a more discrete improvement.
Regarding inhibitory control, the results show a considerable improvement in a participant who had abstained from consuming marijuana. Although an improvement is observed in the rest of the participants, it is discreet.
(B) NeuroSPECT
In Fig. 3 disorganized lateral temporal hypoperfusion is demonstrated in both hemispheres, of greater extent and severity on the left, deep hypoperfusion of the right occipital pole and of both posterior cingulated gyri. These abnormalities are clearly less severe post-abstinence, allowing a qualitative analysis.
Fig. 3.
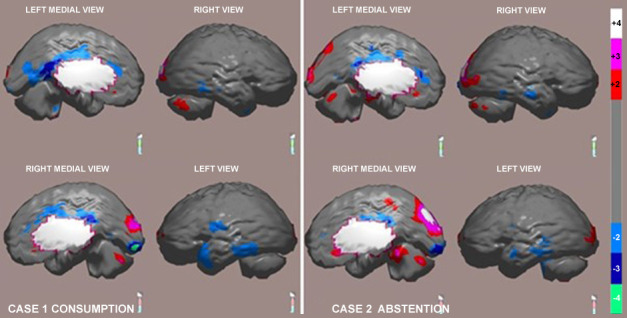
NeuroSPECT brain images. The comparison of 3D images of a patient in a state of recent consumption (left) and after 6 months of abstinence (right), expressed in standard deviations from the normal mean and representing hypoperfusion at two, three and four standard deviations below the normal mean with the colors light blue, dark blue and green respectively. NeuroSPECT, neuropsychological tests and brain perfusion single photon emission computed tomography.
Quantitative neuroSPECT analysis. Given the limited preliminary sample, only a percentage comparison between baseline (with consumption) and control (in abstinence) values is performed, considering the baseline value as 100%. A positive variation means that perfusion increased in that Brodmann area post-abstinence (Fig. 4). With this methodology, increases of more than 10% have been observed in some areas, as well as notable decreases, all of which points to the need to increase the study base in order to explore these preliminary results in greater depth.
Fig. 4.
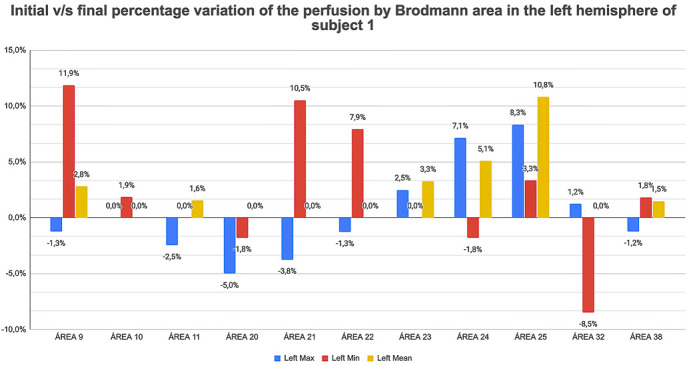
Percentage of change in post-abstinence perfusion compared to the value during consumption for selected Brodmann areas of the left cerebral hemisphere of one patient. There is a marked increase of the minimum values (in red), suggestive of a recovery in post-abstinence function, in Brodmann area 9 which is related to higher executive functions, in areas 21 and 22 associated with auditory processing, and in the average perfusion values (in yellow) in area 25, fundamental in emotional integration and regulation.
Discussion
The small preliminary sample precludes making an inferential statistic to obtain general conclusions. However, once we obtain a larger study base we will be able to test if there is a consistent difference between pre- and post-abstinence perfusion. For this, a paired mean difference t-test will be used. This test is performed to measure the effect of some action on a group of individuals. Our final objective is to test whether there is a significant difference in perfusion for each individual before and after abstinence.
It is of interest to correlate the results of neuropsychological tests with the information yielded by neuroSPECT, considering that perfusion is indirectly related to brain functions. Thus, it has already been established that the blood supply observed in certain areas of the brain has a relationship with some brain functions that are specifically measured with neuropsychological tests, allowing to investigate a possible coincidence in the findings with both instruments.
There is ample knowledge regarding brain dysfunction associated with marijuana use, but we lack sufficient information regarding the harms that persist even after a prolonged discontinuation of consumption. Given the current scenario of legalization of marijuana use, which is associated with decreased risk perception, an increase in users and the availability of plants with increasingly higher THC concentrations, it is essential to determine how brain functional damage changes after cessation of use, which is the objective of the present study [1, 2, 3, 11].
Although the results of this research are preliminary, they already show improvements in working memory and attention after prolonged abstinence from marijuana. In one case, neuroSPECT imaging and Brodmann area quantification also confirm fewer post-abstinence abnormalities. Upon completion of the target group we will evaluate the coincidence between changes in neuropsychological testing and cerebral perfusion. In this first sample, two of the participants who managed to discontinue consumption had noticeable vital changes, recognized by them and their environment. We remark that, in addition to the normal difficulties in recruiting and following up patients for a research project, the COVID-19 pandemic reduced psychiatric consultations, made the application of neuropsychological tests in person more problematic, and complicated the collection of urine samples under strict surveillance. The original two-year time frame for a sample of 20 participants proved impossible to meet under these conditions being this the main limitation of our study.
Generally, functional problems due to marijuana, as assessed by neuropsychological tests, lack anatomical damage demonstrable on magnetic resonance imaging (MRI) or computed tomography (CT) scans, so that these methods may discourage discontinuation of use due to a false sense of “absence of damage”. It is essential to apply functional methods such as brain perfusion SPECT, positron emission tomography with fludeoxyglucose-18 (PET-FDG) or functional MRI in the evaluation of brain damage caused by marijuana and other drugs of abuse.
Our results obtained so far using neuropsychological tests combined with neuroSPECT are in accordance with the findings of Darley CF, et al. [16] (1973) and Hanson et al. [17] (2010), which suggest improvement in brain functions following abstinence of marijuana . Consequently, it is imperative to continue this line of research, as the prospect of restoring functions previously considered irrecoverable damaged, motivates consumers to cease marijuana use.
Conclusion
Our preliminary results confirm the feasibility of the methodology to evaluate changes in brain function after the interruption of marijuana consumption but thus far the small number of individuals already included preclude any general conclusion based on statistical analysis. The majority of studies on marijuana users and withdrawal are limited to neuropsychological tests, which demonstrate both the damage caused by the drug and the recovery of functions. Furthermore, existing studies lack functional imaging and have a maximum withdrawal time limit of 28 days. In contrast, the present study combines neuropsychological and functional imaging assessment by neuroSPECT, thereby enhancing the validity of the results.
Acknowledgment
We thank Mrs. Magdalena Castro for her valuable contribution to the formulation of this project and Mr. Patricio Labatut who offered his disinterested and generous support.
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
SN: coordinator, in charge of neuroSPECT studies, writing the manuscript. AD: neuropsychological tests application and processing, writing the manuscript, responsibility for communication with the journal during the manuscript submission, peer-review, and publication process. SL and SV: neuropsychological tests application and processing. DS and CI: treating physicians, clinical data collection for sample selection. JR: statistical analysis. All authors contributed to the drafting or important editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of Clínica Las Condes (D012019). All patients were provided with information about the study by SN and signed a written document (informed consent).
Funding
This study was funded by the Academic Direction of Clínica Las Condes (PIDA19) and by the Department of Psychiatry and Mental Health East of the University of Chile.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].UNODC, World Drug Report 2022, booklet 3 Drug Market Trends of Cannabis and Opioids (United Nations publication) 2022. [(Accessed: 16 May 2023)]. Available at: https://www.incb.org/documents/Publications/AnnualReports/AR2022/Annual_Report_Chapters/040_Chapter_I.pdf.
- [2].Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001-2002 and 2012-2013. JAMA Psychiatry . 2015;72:1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. The Lancet. Psychiatry . 2019;6:427–436. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hjorthøj C, Posselt CM, Nordentoft M. Development Over Time of the Population-Attributable Risk Fraction for Cannabis Use Disorder in Schizophrenia in Denmark. JAMA Psychiatry . 2021;78:1013–1019. doi: 10.1001/jamapsychiatry.2021.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Winters KC, Lee CYS. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug and Alcohol Dependence . 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. The New England Journal of Medicine . 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ranganathan M, Skosnik PD, D’Souza DC. Marijuana and Madness: Associations Between Cannabinoids and Psychosis. Biological Psychiatry . 2016;79:511–513. doi: 10.1016/j.biopsych.2016.02.007. [DOI] [PubMed] [Google Scholar]
- [8].Libuy N, Ibáñez C, Mundt AP. Factors related to an increase of cannabis use among adolescents in Chile: National school based surveys between 2003 and 2017. Addictive Behaviors Reports . 2020;11:100260. doi: 10.1016/j.abrep.2020.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mena I, Dörr A, Viani S, Neubauer S, Gorostegui ME, Dörr MP, et al. Efectos del consumo de marihuana en escolares sobre funciones cerebrales demostrados mediante pruebas neuropsicológicas e imágenes de neuro-SPECT. Salud Mental . 2013;36:367–374. (In Spanish) [Google Scholar]
- [10].Schuster RM, Gilman J, Schoenfeld D, Evenden J, Hareli M, Ulysse C, et al. One Month of Cannabis Abstinence in Adolescents and Young Adults Is Associated With Improved Memory. The Journal of Clinical Psychiatry . 2018;79:17m11977. doi: 10.4088/JCP.17m11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association. Drug and Alcohol Dependence . 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- [12].Porteus SD. Laberinto de Porteus, Manual. 4th edn . TEA Ediciones; Madrid: 2006. (In Spanish) [Google Scholar]
- [13].Wechsler D, Uribe Ferrari MC, Moreno Zarco G. Wais-IV: Escala Wechsler de inteligencia para adultos-IV: manual de aplicación . El Manual Moderno; México: 2014. (In Spanish) [Google Scholar]
- [14].Golden CJ. STROOP: Test de Colores y Palabras, Manual . TEA Ediciones; Madrid: 2010. (In Spanish) [Google Scholar]
- [15].Brickenkamp R. D2: Test de Atención (adaptación española por Nicolás Seisdedos) TEA Ediciones; Madrid: 2012. (In Spanish) [Google Scholar]
- [16].Darley CF, Tinklenberg JR, Hollister TE, Atkinson RC. Marihuana and retrieval from short-term memory. Psychopharmacologia . 1973;29:231–238. doi: 10.1007/BF00414037. [DOI] [PubMed] [Google Scholar]
- [17].Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors . 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.