Abstract
Background
The PENELOPE-B study demonstrated that the addition of 1-year post-neoadjuvant palbociclib to endocrine therapy (ET) in patients with high-risk early breast cancer (BC) did not improve invasive disease-free survival (iDFS) compared to placebo. Here, we report results for premenopausal women.
Patients and methods
Patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative BC at high risk of relapse [defined as no pathological complete response after neoadjuvant chemotherapy and a clinical, pathological stage, estrogen receptor, grading (CPS-EG) score ≥3 or 2/ypN+] were randomized to receive 13 cycles of palbociclib or placebo + standard ET. Ovarian function (OF) was evaluated by centrally assessed estradiol, follicle-stimulating hormone and anti-Müllerian hormone serum levels.
Results
Overall, 616 of 1250 randomized patients were premenopausal; of these, 30.0% were <40 years of age, 47.4% had four or more metastatic lymph nodes, and 58.2% had a CPS-EG score ≥3. 66.1% of patients were treated with tamoxifen alone, and 32.9% received ovarian function suppression (OFS) in addition to either tamoxifen or aromatase inhibitor (AI). After a median follow-up of 42.8 months (97.2% completeness) no difference in iDFS between palbociclib and placebo was observed [hazard ratio = 0.95, 95% confidence interval (CI) 0.69-1.30, P = 0.737]. The estimated 3-year iDFS rate was marginally higher in the palbociclib arm (80.6% versus 78.3%). Three year iDFS was higher in patients receiving AI than tamoxifen plus OFS or tamoxifen alone (86.0% versus 78.6% versus 78.0%). Patients receiving tamoxifen plus OFS showed a favorable iDFS with palbociclib (83.0% versus 74.1%, hazard ratio = 0.52, 95% CI 0.27-1.02, P = 0.057). Hematologic adverse events were more frequent with palbociclib (76.1% versus 1.9% grade 3-4, P < 0.001). Palbociclib seems not to negatively impact the OF throughout the treatment period.
Conclusions
In premenopausal women, who received tamoxifen plus OFS as ET, the addition of palbociclib to ET results in a favorable iDFS. The safety profile seems favorable and in contrast to chemotherapy palbociclib does not impact OF throughout the treatment period.
Key words: early breast cancer, hormone receptor positive, HER2 negative, adjuvant CDK4/6 inhibitor, palbociclib, premenopausal, PENELOPE-B, ovarian function
Highlights
-
•
AZD4635 is a novel adenosine2A receptor antagonist that blocks A2aR-mediated signaling in tumor-infiltrating immune cells.
-
•
Adding cabazitaxel to the AZD4635 plus durvalumab combination may enhance antitumor activity in the post-docetaxel setting.
-
•
Palbociclib did not influence estradiol and FSH levels significantly added to ET in still premenopausal women after NACT.
-
•
The majority of women <40 years of age had non-fertile AMH levels at study entry (79·4%) and at end of treatment (83·1%).
-
•
Palbociclib does not seem to impact the ovarian reserve as defined by AMH levels.
Introduction
Premenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) early breast cancer (BC) at low risk of recurrence are adequately treated with tamoxifen alone.1,2 Several strategies to improve outcomes have been investigated for premenopausal patients at higher risk of recurrence, including the addition of ovarian function suppression (OFS) in combination with either tamoxifen or an aromatase inhibitor (AI). The selection of patients who will benefit most from treatment with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) is challenging. Potential benefits must be weighed against additional symptom burden resulting from the use of OFS and AIs.1,3,4 Patients younger than 35 years have the largest absolute benefit from adding OFS and especially AIs, but non-adherence to OFS is significantly higher compared to their older counterparts.4 Underlying reasons include toxicity, a desire of pregnancy and the need for monthly OFS administration.4, 5, 6, 7 Increasing the efficacy of adjuvant endocrine therapy (ET) without increasing menopausal and musculoskeletal symptoms in this age group remains an important strategy to increase adherence and improve outcomes.8
CDK4/6i have consistently shown to improve progression-free survival in metastatic HR+, HER2− BC, whilst maintaining quality of life, including in premenopausal patients.9,10 This has prompted the investigation of CDK4/6i as adjuvant therapy. So far, four large trials have reported inconsistent results.11, 12, 13, 14 The monarchE trial showed that the addition of 2 years of abemaciclib to adjuvant ET in patients with high-risk HR+, HER2− BC led to a significant improvement of invasive disease-free survival (iDFS), which was maintained throughout a median follow-up (FU) of 27 months.11,15,16 After a pronounced effect of premenopausal patients further analyses in this cohort revealed an absolute improvement at 3 years of 5.7% for iDFS and 4.4% for distant relapse-free survival rates.17 In PENELOPE-B, 13 cycles of post-neoadjuvant palbociclib did not significantly improve iDFS when added to ET. However, a transient numerical improvement in iDFS was observed through 3 years.12 A prolonged duration of palbociclib therapy for 2 years in the PALLAS trial again did not show any benefit in terms of iDFS.13 The NATALEE trial has recently reported an absolute iDFS benefit of 3.3% from adding 3 years of ribociclib to an AI [hazard ratio = 0.75, 95% confidence interval (CI) 0.665-0.91, P = 0.001].14 Differences in study design, patient selection, duration of treatment and FU might explain the contrasting results.
The impact of therapeutic interventions on ovarian function (OF) is rarely assessed in early BC trials, although these results are needed for fertility counseling in clinical practice. No trials investigating CDK4/6i have reported prespecified OF endpoints.18 The consequences of ET, including premature menopause, impaired family planning, lifestyle and sexual health, in premenopausal patients have distinct medical and psychosocial implications and mandate a special focus on premenopausal patients within large clinical trials.
Here, we report an analysis of premenopausal patients treated within the post-neoadjuvant PENELOPE-B trial, including a prospective evaluation of OFS throughout the treatment period.
Patients and methods
This exploratory subgroup analysis included premenopausal women treated within the PENELOPE-B (NCT01864746) trial. PENELOPE-B is a prospective, multicenter, multinational, randomized, double-blind, placebo-controlled, phase III study investigating the addition of 1 year of palbociclib to standard adjuvant ET in patients with HR+/HER2− early BC with residual invasive disease after standard neoadjuvant chemotherapy (NACT) and high risk of relapse defined as a clinical, pathological stage, estrogen receptor, grading (CPS-EG) score of ≥3 or 2 with ypN+.19,20 ET with either tamoxifen or an AI with or without OFS was given according to local guidelines for a minimum duration of 5 years and could have been started before the enrollment into the study. Estrogen receptor (ER) and progesterone receptor positivity was centrally assessed and defined as ≥1% positively stained cells and HER2 negativity as an immunohistochemistry score of 0-1 or fluorescence in situ hybridization test ratio <2.0. Details on the PENELOPE-B trial and primary results have been previously published.12 This analysis was based on the menopausal status reported by the investigator.
Objectives and endpoints
We report iDFS in premenopausal patients stratified by study treatment and type of ET. iDFS was defined as the time in months between randomization and first event including ipsilateral invasive in-breast or locoregional recurrence, invasive contralateral BC, distant recurrence, second primary invasive (non-breast) cancer or death of any cause.21 Safety was analyzed in all randomized patients.
Additionally, we assessed the impact of palbociclib on OF. Estradiol (E2), follicle-stimulating hormone (FSH) and the anti-Müllerian hormone (AMH) were centrally assessed in serum samples collected at baseline, before cycle (C) 7 and at the end of treatment (EOT, 30 days after last intake of study drug). FSH >12.4 IU/l and E2 <52.2 ng/l were defined as postmenopausal hormone levels; fertile levels of AMH were defined as ≥0.22 ng/ml.22 Patients were defined as pre- or postmenopausal in compliance with the current local guidelines (last menstrual period >12 months at study entry before receiving chemotherapy). The median level of FSH, E2 and AMH, the rate of pre- versus postmenopausal hormone levels and fertile versus non-fertile AMH levels were compared between treatment arms at baseline, before C7 and at EOT. Subgroup analyses were carried out in patients with pre- versus postmenopausal FSH/E2 levels at baseline, according to age <40 versus ≥40 years and the use of OFS (yes versus no) during the trial.
Statistical analysis
The Kaplan–Meier method was used to estimate the survival probability at specific time points together with a two-sided 95% CI; univariate Cox proportional hazards models were used to calculate hazard ratios with two-sided 95% CIs. Survival probabilities were compared using the log-rank test and/or Wald P value from Cox regressions. To test interaction between subgroups and treatment, Cox models including subgroup variable, treatment and their interaction were used. The safety population consists of all patients receiving at least one dose of study treatment. Fisher’s exact test was used to compare rates of all-grade as well as grade (G) 3-4 adverse events (AEs). Rates (rate of pre-/postmenopausal hormone levels, AMH fertile/non-fertile levels) were reported per time point/subgroup in frequency tables with number and percent of patients in each category; rates were compared using Fisher’s exact test. The statistical analysis is exploratory. All reported P values are two-sided and to be considered descriptive. CIs symmetrically span 95%. Adjustment for multiple testing was not planned or carried out.
Results
Patients and treatment
In PENELOPE-B, 1250 patients were randomized to either palbociclib or placebo in addition to ET. Overall, 616 patients (49.3%) were defined as premenopausal after surgery before enrollment into PENELOPE-B. Baseline characteristics of premenopausal patients were well balanced between the treatment arms (Table 1). In this cohort, median age at diagnosis was 43 years (range 19-56 years); 25 of 616 (4.1%) patients were aged <30 years, 160 of 616 (26.0%) 30-39 years, 368 of 616 (59.7%) 40-49 years and 63 of 616 (10.2%) ≥50 years. 57.5% were included with a CPS-EG score ≥3 and 47.4% had ≥4 metastatic axillary lymph nodes at surgery.
Table 1.
Baseline characteristics
| Parameter | Category | Palbociclib n = 300 n (valid %) |
Placebo n = 316 n (valid %) |
Overall n = 616 n (valid %) |
P value |
|---|---|---|---|---|---|
| Age, years | Median (min, max) | 43.0 (22.0, 55.0) | 43.0 (19.0, 56.0) | 43.0 (19.0, 56.0) | 0.589 |
| <30 | 11 (3.7) | 14 (4.4) | 25 (4.1) | 0.799 | |
| 30 to <40 | 83 (27.7) | 77 (24.4) | 160 (26.0) | ||
| 40 to <50 | 176 (58.7) | 192 (60.8) | 368 (59.7) | ||
| 50 to <60 | 30 (10.0) | 33 (10.4) | 63 (10.2) | ||
| ECOG performance status | ECOG 0 | 259 (86.3) | 273 (86.4) | 532 (86.4) | 0.983 |
| ECOG 1 | 41 (13.7) | 43 (13.6) | 84 (13.6) | ||
| Tumor focality by sonography | Unifocal | 192 (66.2) | 187 (61.5) | 379 (63.8) | 0.412 |
| Multifocal | 65 (22.4) | 73 (24.0) | 138 (23.2) | ||
| Multicentric | 33 (11.4) | 44 (14.5) | 77 (13.0) | ||
| Clinical tumor stage by sonography | cT1 | 14 (4.7) | 28 (8.9) | 42 (6.8) | 0.207 |
| cT2 | 150 (50.2) | 154 (48.9) | 304 (49.5) | ||
| cT3 | 101 (33.8) | 96 (30.5) | 197 (32.1) | ||
| cT4 | 34 (11.4) | 37 (11.7) | 71 (11.6) | ||
| Histological tumor stage at surgery | ypT0 | 13 (4.3) | 6 (1.9) | 19 (3.1) | 0.163 |
| ypTis | 1 (0.3) | 2 (0.6) | 3 (0.5) | ||
| ypT1 | 103 (34.3) | 103 (32.7) | 206 (33.5) | ||
| ypT2 | 115 (38.3) | 148 (47.0) | 263 (42.8) | ||
| ypT3 | 60 (20.0) | 50 (15.9) | 110 (17.9) | ||
| ypT4 | 8 (2.7) | 6 (1.9) | 14 (2.3) | ||
| Clinical nodal status by sonography | cN0 | 23 (7.7) | 36 (11.4) | 59 (9.6) | 0.253 |
| cN1 | 221 (73.7) | 211 (66.8) | 432 (70.1) | ||
| cN2 | 33 (11.0) | 42 (13.3) | 75 (12.2) | ||
| cN3 | 23 (7.7) | 27 (8.5) | 50 (8.1) | ||
| Histological nodal status at surgery | ypN0 | 13 (4.4) | 16 (5.1) | 29 (4.8) | 0.941 |
| ypN1 | 140 (47.1) | 152 (48.6) | 292 (47.9) | ||
| ypN2 | 110 (37.0) | 110 (35.1) | 220 (36.1) | ||
| ypN3 | 34 (11.4) | 35 (11.2) | 69 (11.3) | ||
| Tumor grading, local (core biopsy) | G1 | 19 (6.4) | 24 (7.7) | 43 (7.1) | 0.228 |
| G2 | 171 (57.6) | 158 (50.6) | 329 (54.0) | ||
| G3 | 107 (36.0) | 130 (41.7) | 237 (38.9) | ||
| Histological tumor type | Ductal or ductal–lobular invasive | 263 (87.7) | 286 (90.5) | 549 (89.1) | 0.272 |
| Lobular invasive carcinoma | 24 (8.0) | 24 (7.6) | 48 (7.8) | ||
| Mucinous carcinoma | 4 (1.3) | 2 (0.6) | 6 (1.0) | ||
| Invasive micropapillary carcinoma | 1 (0.3) | 2 (0.6) | 3 (0.5) | ||
| Other | 8 (2.7) | 2 (0.6) | 10 (1.6) | ||
| Histological lymph node status at surgery documented at randomization | ypN 0-1 | 156 (52.0) | 168 (53.2) | 324 (52.6) | 0.809 |
| ypN 2-3 | 144 (48.0) | 148 (46.8) | 292 (47.4) | ||
| Ki-67% centrally at randomizationa | ≤15% | 229 (76.3) | 245 (77.5) | 474 (76.9) | 0.774 |
| >15% | 71 (23.7) | 71 (22.5) | 142 (23.1) | ||
| Global region of participating site | Non-Asian | 268 (89.3) | 288 (91.1) | 556 (90.3) | 0.498 |
| Asian | 32 (10.7) | 28 (8.9) | 60 (9.7) | ||
| Risk status | CPS-EG score 2 and ypN+ | 124 (41.3) | 138 (43.7) | 262 (42.5) | 0.569 |
| CPS-EG score ≥3 | 176 (58.7%) | 178 (56.3) | 354 (57.5) |
AI, aromatase inhibitor; CPS-EG, clinical, pathological stage, estrogen receptor, grading; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; ET, endocrine treatment; HER2, human epidermal growth factor receptor 2; ITT, intent-to-treat.
Central pathology, preferably based on surgical tissue and if not available based on biopsy.
Overall, 99.2% of the premenopausal patients were treated with anthracycline- and taxane-based NACT and 98.2% received adjuvant radiotherapy. Tamoxifen alone was used in 66.1% and OFS in 32.9% of the patients as part of their initial ET, together with tamoxifen in 19.3%, and an AI in 13.6% of the patients. 4.5% of the patients started OFS during study treatment (Table 2). Separated by age, the rate of OFS together with tamoxifen was 32.4% in patients aged <40 years and 13.7% in patients aged ≥40 years (P < 0.001). In comparison, the rate of OFS together with AI was 17.8% in patients aged <40 years and 11.8% in patients aged ≥40 years (P < 0.055). The rate of OFS varied significantly by age and was 50.3% in patients aged <40 years, but only 25.5% in patients aged ≥40 years (P < 0.001). In the age group <35 years, OFS was used in 65.4% as part of the initial ET (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103466). Only 32 (5.2%) of the premenopausal patients switched ET during the trial, most of them from tamoxifen to an AI (25 patients) and 2 patients from an AI to tamoxifen.
Table 2.
Endocrine treatment
| Parameter | Category | Palbociclib N = 300 n (valid %) |
Placebo N = 316 n (valid %) |
Overall N = 616 n (valid %) |
P value |
|---|---|---|---|---|---|
| Start of ET | Before palbociclib/placebo | 272 (90.7) | 286 (90.5) | 558 (90.6) | 1.000 |
| Concomitantly with palbociclib/placebo | 28 (9.3) | 30 (9.5) | 58 (9.4) | ||
| First ET | Tamoxifen alone | 199 (66.3) | 208 (66.8) | 407 (66.1) | 0.932 |
| Tamoxifen plus OFS | 61 (20.3) | 58 (18.4) | 119 (19.3) | 0.542 | |
| AI plus OFS | 37 (12.3) | 47 (14.9) | 84 (13.6) | 0.411 | |
| Letrozole plus OFS | 18 (6.0) | 20 (6.3) | 38 (6.2) | ||
| Exemestane plus OFS | 15 (5.0) | 20 (6.3) | 35 (5.7) | ||
| Anastrozole plus OFS | 4 (1.3) | 7 (2.2) | 11 (1.8) | ||
| AI alonea | 3 | 3 | 6 | 1.000 | |
| OFS | 98 (32.7) | 105 (33.2) | 203 (33.0) | ||
| Type of OFS | Goserelin | 90 (30.0) | 94 (29.7) | 184 (29.9) | |
| Other GnRHa | 7 (2.3) | 11 (3.5) | 18 (2.9) | ||
| Surgical | 1 | 0 | 1 | ||
| Radiologic | 0 | 0 | 0 | ||
| Start of GnRHa during study therapy | 11 (3.7) | 17 (5.4) | 28 (4.5) | ||
Type of first ET in premenopausal patients.
AI, aromatase inhibitor; ET, endocrine therapy; GnRHa, gonadotropin-releasing hormone analogue; OFS, ovarian function suppression.
Patients receiving AI alone and were excluded from analyses according to ET treatment.
Efficacy
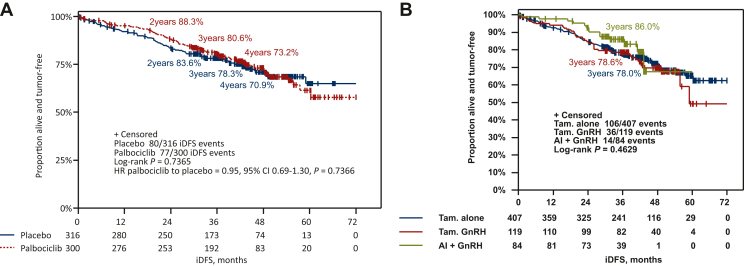
After a median FU of 42.8 months (97.2% completeness), 157 iDFS events (25.5%) have been documented in premenopausal patients, mainly distant recurrences (74.5%). There was no significant difference in iDFS between the treatment arms (hazard ratio = 0.95, 95% CI 0.69-1.30, P = 0.737; Figure 1A). The estimated 3-year iDFS rate was 80.6% (95% CI 75.5% to 84.8%) in the palbociclib arm and 78.3% (95% CI 73.1% to 82.5%) in the placebo arm (Figure 1A).
Figure 1.
Kaplan–Meier curves for iDFS in premenopausal patients. Kaplan–Meier estimates for iDFS in premenopausal patients (A) according to treatment arm and (B) by endocrine treatment backbone.
AI, aromatase inhibitor; CI, confidence interval; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; iDFS, invasive disease-free survival; Tam., tamoxifen.
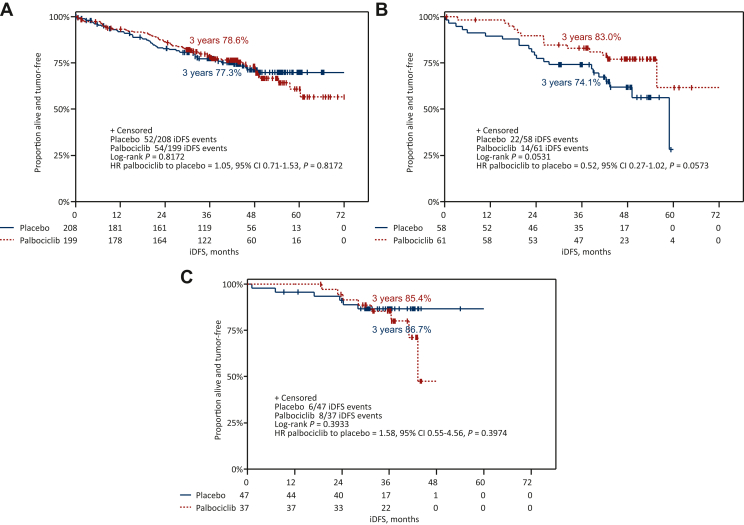
The 3-year iDFS rate in premenopausal patients receiving an AI + OFS as initial ET was 86.0% compared with 78.6% in patients receiving tamoxifen + OFS (hazard ratio = 1.48, 95% CI 0.79-2.75) and 78.0% in patients treated with tamoxifen alone (hazard ratio = 1.34, 95% CI 0.77-2.35, P = 0.463; Figure 1B), irrespective of the addition of palbociclib. A numerically favorable 3-year iDFS was observed for patients receiving palbociclib compared to placebo in addition to tamoxifen + OFS (83.0% versus 74.1%, hazard ratio = 0.52, 95% CI 0.27-1.02, P = 0.053; Figure 2). A test for interaction between type of ET and study treatment arm was not significant (P = 0.124).
Figure 2.
Kaplan–Meier curves for iDFS in premenopausal patients. iDFS by treatment arm in patients receiving (A) tamoxifen alone, (B) tamoxifen + GnRHa and (C) AI + GnRHa.
AI, aromatase inhibitor; CI, confidence interval; GnRHa, gonadotropin-releasing hormone analogue; HR, hazard ratio; iDFS, invasive disease-free survival.
Safety
All patients except one in each treatment arm experienced at least one AE. G3-4 AEs were significantly more frequent in the palbociclib arm compared to the placebo arm (81.1% versus 18.5%, P < 0.001), especially G3-4 hematologic AEs (76.1% versus 1.9%, P < 0.001; G1-4 99.0% versus 83.8%, P < 0.001; Table 3). Non-hematologic AEs did not differ significantly between treatment arms (G3-4 18.9% versus 16.6%, P = 0.461; G1-4 99.3% versus 99.4%, P = 1.000). More patients in the palbociclib arm experienced G1-4 hypocalcemia (43.9% versus 33.1%, P = 0.008), constipation (24.9% versus 14.6%, P = 0.002), dyspnea (10.6% versus 5.7%, P = 0.028), fatigue (67.4% versus 51.3%, P < 0.001), infections (61.1% versus 52.9%, P = 0.042) and stomatitis (32.9% versus 7.6%, P < 0.001; Table 3). There was no difference in terms of serious AEs (8.0% versus 9.2%, P = 0.667) between treatment arms. Details on AEs by treatment arm according to first ET are given in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103466.
Table 3.
AEs >10% (and SAEs <10%) in either treatment arm in premenopausal patients by treatment arm (all causality)
| AE | Grade | Palbociclib N = 301 n (valid %) |
Placebo N = 314 n (valid %) |
Overall N = 615 n (valid %) |
P value |
|---|---|---|---|---|---|
| Patients with AE | Any | 300 (99.7) | 313 (99.7) | 613 (99.7) | 1.00 |
| Patients with grade 3/4 AE | 3-4 | 244 (81.1) | 58 (18.5) | 302 (49.1) | <0.001 |
| Patients with hematologic AE | Any | 298 (99.0) | 263 (83.8) | 561 (91.2) | <0.001 |
| Patients with hematologic grade 3/4 AE | 3-4 | 229 (76.1) | 6 (1.9) | 235 (38.2) | <0.001 |
| Patients with non-hematologic AE | Any | 299 (99.3) | 312 (99.4) | 611 (99.3) | 1.00 |
| Patients with non-hematologic grade 3/4 AE | 3-4 | 57 (18.9) | 52 (16.6) | 109 (17.7) | 0.461 |
| Patients with SAEs | 24 (8.0) | 29 (9.2) | 53 (8.6) | 0.667 | |
| Anemia | Any | 230 (76.4) | 105 (33.4) | 335 (54.5) | <0.001 |
| 3-4 | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 | |
| Leukopenia | Any | 298 (99.0) | 232 (73.9) | 530 (86.2) | <0.001 |
| 3-4 | 173 (57.5) | 3 (1.0) | 176 (28.6) | <0.001 | |
| Neutropenia | Any | 286 (95.0) | 78 (24v8) | 364 (59.2) | <0.001 |
| 3-4 | 218 (72.4) | 5 (1.6) | 223 (36.3) | <0.001 | |
| Febrile neutropenia | Any | 6 (2.0) | 1 (0.3) | 7 (1.1) | 0.064 |
| Thrombocytopenia | Any | 185 (61.5) | 59 (18.8) | 244 (39.7) | <0.001 |
| 3-4 | 2 (0.7) | 0 (0.0) | 2 (0.3) | 0.239 | |
| Non-hematologic toxicities | |||||
| ALAT increased | Any | 57 (18.9) | 69 (22.0) | 126 (20.5) | 0.370 |
| 3-4 | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 | |
| Blood AP increased | Any | 43 (14.3) | 49 (15.6) | 92 (15.0) | 0.653 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| ASAT increased | Any | 58 (19.3) | 54 (17.2) | 112 (18.2) | 0.532 |
| 3-4 | 2 (0.7) | 1 (0.3) | 3 (0.5) | 0.617 | |
| Hyperkalemia | Any | 26 (8.6) | 37 (11.8) | 63 (10.2) | 0.232 |
| 3-4 | 2 (0.7) | 2 (0.6) | 4 (0.7) | 1.000 | |
| Hypernatremia | Any | 33 (11.0) | 28 (8.9) | 61 (9.9) | 0.420 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Hypocalcemia | Any | 132 (43.9) | 104 (33.1) | 236 (38.4) | 0.008 |
| 3-4 | 3 (1.0) | 0 (0.0) | 3 (0.5) | 0.117 | |
| Hypomagnesemia | Any | 88 (29.2) | 92 (29.3) | 180 (29.3) | 1.000 |
| 3-4 | 2 (0.7) | 0 (0.0) | 2 (0.3) | 0.239 | |
| Alopecia | Any | 31 (10.3) | 22 (7.0) | 53 (8.6) | 0.153 |
| Arthralgia | Any | 114 (37.9) | 112 (35.7) | 226 (36.7) | 0.616 |
| 3-4 | 2 (0.7) | 2 (0.6) | 4 (0.7) | 1.000 | |
| Back pain | Any | 34 (11.3) | 39 (12.4) | 73 (11.9) | 0.709 |
| 3-4 | 3 (1.0) | 0 (0.0) | 3 (0.5) | 0.117 | |
| Bone pain | Any | 47 (15.6) | 52 (16.6) | 99 (16.1) | 0.826 |
| 3-4 | 1 (0.3) | 0 (0.0) | 1 (0.2) | 0.489 | |
| Blood creatinine increased | Any | 32 (10.6) | 25 (8.0) | 57 (9.3) | 0.269 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Constipation | Any | 75 (24.9) | 46 (14.6) | 121 (19.7) | 0.002 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Cough | Any | 51 (16.9) | 53 (16.9) | 104 (16.9) | 1.000 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Dyspnea | Any | 32 (10.6) | 18 (5.7) | 50 (8.1) | 0.028 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Diarrhea | Any | 58 (19.3) | 44 (14.0) | 102 (16.6) | 0.084 |
| 3-4 | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 | |
| Fatigue | Any | 203 (67.4) | 161 (51.3) | 364 (59.2) | <0.001 |
| 3-4 | 8 (2.7) | 3 (1.0) | 11 (1.8) | 0.135 | |
| Headache | Any | 73 (24.3) | 87 (27.7) | 160 (26.0) | 0.358 |
| 3-4 | 2 (0.7) | 2 (0.6) | 4 (0.7) | 1.000 | |
| Hot flushes | Any | 157 (52.2) | 172 (54.8) | 329 (53.5) | 0.519 |
| 3-4 | 3 (1.0) | 4 (1.3) | 7 (1.1) | 1.000 | |
| Vulvovaginal dryness | Any | 33 (11.0) | 36 (11.5) | 69 (11.2) | 0.899 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Infection | Any | 184 (61.1) | 166 (52.9) | 350 (56.9) | 0.042 |
| 3-4 | 7 (2.3) | 15 (4.8) | 22 (3.6) | 0.129 | |
| Pyrexia | Any | 38 (12.6) | 27 (8.6) | 65 (10.6) | 0.116 |
| 3-4 | 2 (0.7) | 1 (0.3) | 3 (0.5) | 0.617 | |
| Insomnia | Any | 47 (15.6) | 57 (18.2) | 104 (16.9) | 0.452 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Myalgia | Any | 63 (20.9) | 50 (15.9) | 113 (18.4) | 0.119 |
| 3-4 | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 | |
| Nausea | Any | 80 (26.6) | 73 (23.2) | 153 (24.9) | 0.352 |
| 3-4 | 0 (0.0) | 1 (0.3) | 1 (0.2) | 1.000 | |
| Vomiting | Any | 28 (9.3) | 34 (10.8) | 62 (10.1) | 0.593 |
| 3-4 | 0 (0.0) | 2 (0.6) | 2 (0.3) | 0.499 | |
| Edema peripheral | Any | 57 (18.9) | 54 (17.2) | 111 (18.0) | 0.601 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Lymphedema | Any | 34 (11.3) | 30 (9.6) | 64 (10.4) | 0.511 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Pain in extremity | Any | 36 (12.0) | 31 (9.9) | 67 (10.9) | 0.439 |
| 3-4 | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1.000 | |
| Muscle spams | Any | 33 (11.0) | 23 (7.3) | 56 (9.1) | 0.125 |
| 3-4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | |
| Stomatitis | Any | 99 (32.9) | 24 (7.6) | 123 (20.0) | <0.001 |
| 3-4 | 2 (0.7) | 1 (0.3) | 3 (0.5) | 0.617 |
AEs, adverse events; ALAT, alanine aminotransferase; AP, alkaline phosphatase; ASAT, aspartate aminotransferase; NA, not applicable; SAEs, serious adverse events.
Comparing AEs according to first ET, anemia G1-4 was significantly less frequent with AI + OFS (39.3%) compared to tamoxifen alone (56.7%) or tamoxifen + OFS (59.7%), as was thrombocytopenia G1-4 (23.8% versus 41.6% versus 45.4%), as these side-effects are caused by tamoxifen. The ATAC study, which compared the efficacy and safety of anastrozole and tamoxifen as adjuvant treatment for postmenopausal women with early-stage BC, also showed more frequent thrombocytopenia and anemia with tamoxifen than with the AI.23 Arthralgia G1-4 was significantly more frequent in patients receiving an AI + OFS (69.0%) compared to tamoxifen alone (34.0%) or tamoxifen + OFS (22.7%), as were G1-4 hot flushes (71.4% versus 51.5% versus 47.1%), vulvovaginal dryness (20.2% versus 9.6% versus 10.9%) and fatigue (71.4% versus 59.6% versus 49.6%; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103466).
Palbociclib and ovarian function
Overall, 576 patients had serum samples available at baseline, 526 before C7 and 541 at EOT, respectively. At baseline, 58.7% of patients in the palbociclib arm and 58.4% of the patients in the placebo arm had postmenopausal E2 and FSH levels (P = 1.000). Of these, 80.4% remained postmenopausal at C7 and 77.2% at EOT with no significant differences between treatment arms (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103466).
Of the patients with premenopausal E2/FSH levels at baseline, 9.1% under palbociclib versus 13.5% under placebo (P = 0.387) developed postmenopausal hormone levels at C7 and 17.6% versus 14.5% (P = 0.587) at EOT, respectively. Among patients aged <40 years, 28.1% in the palbociclib arm versus 24.7% in the control arm (P = 0.728) had postmenopausal hormone levels at baseline, 19.2 versus 12.5% at C7 (P = 0.276) and 27.4 versus 14.5% (P = 0.054) at EOT, respectively. The majority of patients not receiving OFS had postmenopausal hormone levels at baseline (80.3%) and remained postmenopausal throughout the study treatment (72.2% at C7 and 71.4% at EOT) without any significant differences between treatment arms at any time point. Although there was no overall difference in the fertility level of AMH, the group receiving palbociclib had slightly higher postmenopausal levels at the EOT.
Overall, the rate of non-fertile AMH levels at baseline was high (92.7%) and remained stable throughout the study (94.6% at EOT). No significant differences in the rate of non-fertile AMH levels were observed between treatment arms and subgroups at any time point (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103466).
Especially in the subgroup of patients aged <40 years, the rate of non-fertile AMH levels was not significantly influenced by palbociclib.
Discussion
In this exploratory subgroup analysis of the PENELOPE-B trial, the addition of palbociclib to standard ET did not improve iDFS in premenopausal patients at high risk of recurrence after NACT. These results are consistent with the previously published main overall results of the PENELOPE-B trial.12
The monarchE trial showed an improvement in iDFS with 2-year abemaciclib together with ET in the overall cohort and suggested a greater benefit in premenopausal patients.15,16 Considering that younger age is correlated with higher risk of relapse, it seemed reasonable to explore survival within the premenopausal subgroup. However, our current analysis does not support an interaction between menopausal status and adjuvant palbociclib. One possible explanation might be that a longer or even more intensive CDK4/6i therapy is needed to elicit cytotoxic rather than cytostatic effects. PENELOPE-B had the shortest treatment duration but the longest FU of all the adjuvant CDK4/6i trials, although an FU of 42.8 months is defined as relatively short for an ER+/HER2− BC population. This hypothesis, however, does not explain the lack of benefit from 2 years of adjuvant palbociclib in the PALLAS study.13 A subgroup analysis of PALLAS according to menopausal status has not been reported yet. However, benefit from the addition of palbociclib to ET was not seen in patients younger or older than 50 years, respectively. There are speculations whether the observed iDFS benefit in the monarchE trial was in part caused by informative censoring caused by the open-label design.24 In fact, the PENELOPE-B trial was the only placebo-controlled study compared to open-label design used in the PALLAS, monarchE and NATALEE trials. Moreover, the NATALEE trial is equipped with a comparably high power of 93% for the final analysis and interim analysis was reported at a very early phase since 79.2% of the patients had not yet completed the planned therapy.25 This might be of major relevance for subsequent analyses. The publication of positive results in an open-label trial with the majority of patients still having to complete therapy will presumably lead to enhanced therapy adherence in the experimental arm and pronounced censoring in the control arm, which ultimately is able to influence the final survival analysis. However, the different observations in NATALEE, monarchE and PENELOPE-B trials might also be drug-specific.
The toxicity profile of palbociclib in premenopausal women was consistent with the intent-to-treat population, with a significantly higher rate of hematologic AEs but similar rate of non-hematologic AEs. However, some low-grade but relevant non-hematologic toxicities like fatigue and stomatitis were observed more frequently with palbociclib. As expected, the type of ET also impacted AEs, with the combination of an AI + OFS in PENELOPE-B leading to significantly higher rates of particularly bothersome side-effects compared to tamoxifen alone or tamoxifen + OFS, including arthralgias, bone pain, hot flushes and vulvovaginal dryness, irrespective of the treatment arm. The arthralgia rate in the PENELOPE-B trial was lower in patients treated with palbociclib (41.2%) compared to the placebo group (46.8%).12 This trend can be confirmed in all CDK4/6i trials (PALLAS: 38.2% palbociclib + ET versus 45.0% ET and monarchE: 26.5% abemaciclib + ET versus 37.8% ET).26,27 An exact cause for this is still unknown; however, based on the current data, a protective effect of CDK4/6i against joint inflammation and the occurrence of arthralgias can be assumed.28 At the same time, patients treated with an AI + OFS had a numerically superior 3-years iDFS compared to patients receiving tamoxifen ± OFS. PENELOPE-B was not designed to investigate differences between ET in premenopausal women since the choice of the ET was not randomized and is subject to a selection bias, especially by age as described in the results. However, these data are in line with data from SOFT and TEXT studies.1 In a high-risk population of premenopausal women, the additional benefit from AI + OFS over tamoxifen might justify higher rates of side-effects to some extent. The addition of palbociclib to tamoxifen + OFS in premenopausal women did not increase side-effects compared to AI + OFS and appeared highly effective. Further studies are needed in this case to evaluate the potential benefit of palbociclib in premenopausal patients receiving tamoxifen + OFS as a potential alternative to AI + OFS for better tolerability, especially in patients who cannot tolerate the side-effects of AI.
As there are no data on the effect of CDK4/6i on OF, a prospective evaluation of hormone levels was carried out in PENELOPE-B. As expected, after NACT most patients had postmenopausal hormone levels at randomization, even if defined as premenopausal by the investigator based on the menstrual and medical history. The addition of palbociclib to the ET after NACT did not significantly influence E2 and FSH levels in women who were reported premenopausal by investigators at study entry. More importantly, no effect was seen in patients with proven premenopausal hormone levels at baseline. Among patients not receiving OFS, the majority had postmenopausal hormone levels at baseline and remained postmenopausal throughout the study, without difference between treatment arms. We cannot make any conclusions as to a potential resumption of OF beyond the 12 months’ treatment period, as no blood was collected beyond EOT. In PENELOPE-B, 50.3% of patients aged <40 years and 65.4% <35 years were already receiving OFS at baseline, rendering them functionally postmenopausal, although it is generally recommended to start OFS only in patients with proven OF. This led to interferences in the analysis of E2 and FSH levels. Consequently, this also led to the manageable proportion of premenopausal patients of 33% who received OFS.
Fertility preservation is an important issue in premenopausal patients undergoing treatment for early BC. As shown in prospective analyses, AMH levels are a surrogate of persistent ovarian dysfunction.29, 30, 31 Our data demonstrate no significant impact of palbociclib on fertile AMH levels. However, the high rate of non-fertile AMH levels at baseline even in patients with premenopausal hormone levels or in patients aged <40 years is intriguing. This is in line with our previously published data on AMH levels in patients aged ≤45 years treated with (neo)adjuvant chemotherapy for early BC.32 In PENELOPE-B, no additional impact of palbociclib on fertility as assessed via AMH levels was seen in patients with proven premenopausal hormone levels or <40 years of age at baseline. These results underline the importance of fertility-preserving measure before induction of chemotherapy whenever indicated.
As this analysis is based on a 1-year treatment period, we cannot firmly conclude that longer treatment with CDK4/6i as used in the other adjuvant trials may not have an impact on OF or on ovarian reserve.
In conclusion, in PENELOPE-B, the addition of 1-year of palbociclib to adjuvant ET did not improve iDFS in premenopausal patients. Ongoing trials will inform about the efficacy of a longer duration of adjuvant CDK4/6 inhibition in premenopausal patients. PENELOPE-B reports the first efficacy and safety results of palbociclib in combination with tamoxifen from a large adjuvant phase III trial and is the first analysis of adjuvant CDK4/6i therapy providing substantial information regarding fertility counseling. It is important to consider the high rate of non-fertile AMH levels in premenopausal patients after state-of-the-art NACT when counseling about fertility preservation and to take measures before start of chemotherapy.
Acknowledgements
We thank all patients and their families participating in the PENELOPE-B trial, the network of investigators, the study personnel and the team at the GBG headquarters.
Funding
This work was supported by the German Breast Group, which received funding from Pfizer (no grant number). Pfizer supported the conduct of the trial financially and provided the drug. Pfizer was not involved in the design of the health-economic study, collection, analysis or interpretation of data; or in the writing of the report; or in the decision to submit the article for publication.
Disclosure
SL employee (CEO) of GBG Forschungs GmbH; receive grants from AbbVie, AstraZeneca, Celgene, Daiichi-Sankyo, Immunomedics/Gilead, Molecular Health, Novartis, Pfizer and Roche; honoraria for advisory board from Abbvie, Amgen, AstraZeneca, BMS, Celgene, DSI, EirGenix, Gilead, GSK, Lilly, Merck, Novartis, Olema, Pfizer, Pierre Fabre, Relay Therapeutics, Roche, Sanofi and Seagen; honoraria as invited speaker from AstraZeneca, DSI, Gilead, Novartis, Pfizer, Roche, Seage and Medscape. SL reports non-financial interest as advisory role in AGO Kommission Mamma, as principal investigator (Aphinity), as member in AGO, ASCO, DKG, ESMO and other non-financial interest from AstraZeneca, Daiichi-Sankyo, immunomedica/Gilead, Novartis, Pfizer, Roche and Seagen. GBG Forschungs GmbH has following royalties/patents: EP14153692.0, EP21152186.9, EP15702464.7, EP19808852.8 and VM Scope GmbH. MG reports personal fees/travel support from AstraZeneca, Daiichi Sankyo, EliLilly, Menarini-Stemline, MSD, Novartis, PierreFabre, Veracyte; an immediate family member is employed by Sandoz. JF and VN GBG Forschungs GmbH employee. GBG Forschungs GmbH received funding for research grants from Abbvie, AstraZeneca, BMS, Daiichi-Sankyo, Gilead, Novartis, Pfizer and Roche (paid to the institution); other (non-financial/medical writing) from Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen (paid to the institution). GBG Forschungs GmbH has following royalties/patents: EP14153692.0, EP21152186.9, EP15702464.7, EP19808852.8 and VM Scope GmbH. HBe reports grants or contracts from Merck (support for clinical trial with no relation to current work); support for attending meetings and participation on an advisory board from Merck (with no relation to current work); stock ownership options from Pfizer, Abbvie and Viatris. AM declares consulting fees from Pfizer, Roche, Lilly, Novartis and Sanofi; payment for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer, Roche, Lilly, Nanostring and Veracyte. JAGS reports grants from Menarini-Stemline; consulting fees from AstraZeneca, Daiichi-Sankyo and Novartis; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Novartis, Lilly, AstraZeneca, Daiichi-Sankyo, Seagen and MSD; payment for expert testimony from Lilly; support for attending meetings and/or travel from AstraZeneca and Daiichi-Sankyo. OV declares to be a Pfizer employee and to hold stocks in Pfizer. TR reports payment by Pfizer for presentations and for attending meetings and travel. MM declares grants from Roche, Novartis and Puma; personal fees from Roche, Novartis, Puma, Pierre Fabre, Taiho Oncology, Daiichi Sankyo, Pfizer and Lilly; support from AstraZeneca. MT reports research grants from Kansai Med Net, AFI technology, Eisai, Astellas, Nippon Kayaku, Taiho, Sanwa Syurui, Shimadzu, Chugai, Pfizer, Yakult, Zene, AstraZeneca, JBCRG; consulting fees from Bertis, Eli Lilly, Daiichi Sankyo; lecture honoraria or lecture chair from AstraZeneca, MSD, Eisai, Devicore Medical Japan, Kyowa-Kirin, Daiichi-Sankyo, Eli Lilly, Nippon Kayaku, Taiho, Exact Science, Shimadzu, Chugai, Pfizer, Yakult, Sysmex. MT is a member of the board of directors with no salary at Assoc. JBCRG, Assoc. KBCR and to be a chairman with no salary at Japanese Breast Cancer Society; and other non-financial interests as associate editor in Scientific Reports, Breast Cancer Research and Treatment and Cancer Science. MU declares honoraria from AstraZeneca, Art tempi, Amgen, Daichi Sankyo, Eli Lilly, Roche, Pfizer, MSD Oncology, Pierre Fabre, Sanofi-Aventis, Myriad, Seagen, Gilead and Novartis; honoraria for consulting or advisory role from Amgen, Eli Lilly, Roche, Pfizer, MSD Oncology, Pierre Fabre, Novartis, Agendia, Seagen, Gilead, Stemline and Genzyme. All honoraria and fees were paid to the employer/institution. ZZ declares to be a Pfizer employee and to hold stocks in Pfizer. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Pagani O., Francis P.A., Fleming G.F., et al. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2020;38(12):1293–1303. doi: 10.1200/JCO.18.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paluch-Shimon S., Cardoso F., Partridge A.H., et al. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 3.Francis P.A., Pagani O., Fleming G.F., et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha P., Regan M.M., Pagani O., et al. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the International Breast Cancer Study Group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35(27):3113–3122. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sella T., Poorvu P.D., Ruddy K.J., et al. Impact of fertility concerns on endocrine therapy decisions in young breast cancer survivors. Cancer. 2021;127(16):2888–2894. doi: 10.1002/cncr.33596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits-Seemann R.R., Kaul S., Zamora E.R., et al. Barriers to follow-up care among survivors of adolescent and young adult cancer. J Cancer Surviv. 2017;11(1):126–132. doi: 10.1007/s11764-016-0570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paranjpe R., John G., Trivedi M., Abughosh S. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat. 2019;174(2):297–305. doi: 10.1007/s10549-018-05073-z. [DOI] [PubMed] [Google Scholar]
- 8.Franzoi M.A., Agostinetto E., Perachino M., et al. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021;22(7):e303–e313. doi: 10.1016/S1470-2045(20)30666-5. [DOI] [PubMed] [Google Scholar]
- 9.Costa R., Costa R.B., Talamantes S.M., et al. Meta-analysis of selected toxicity endpoints of CDK4/6 inhibitors: palbociclib and ribociclib. Breast. 2017;35:1–7. doi: 10.1016/j.breast.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Loibl S., Turner N.C., Ro J., et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22(9):1028–1038. doi: 10.1634/theoncologist.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston S.R.D., Harbeck N., Hegg R., et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loibl S., Marme F., Martin M., et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the Penelope-B trial. J Clin Oncol. 2021;39(14):1518–1530. doi: 10.1200/JCO.20.03639. [DOI] [PubMed] [Google Scholar]
- 13.Mayer E.L., Dueck A.C., Martin M., et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–222. doi: 10.1016/S1470-2045(20)30642-2. [DOI] [PubMed] [Google Scholar]
- 14.Slamon D.J., Fasching P.A., Hurvitz S., et al. Rationale and trial design of NATALEE: a phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2− early breast cancer. Ther Adv Med Oncol. 2023;15 doi: 10.1177/17588359231178125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbeck N., Rastogi P., Martin M., et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi P., O’Shaughnessy J., Martin M., et al. Adjuvant abemaciclib plus endocrine therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative, high-risk early breast cancer: results from a preplanned monarchE overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol. 2024;42(9):987–993. doi: 10.1200/JCO.23.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paluch-Shimon S., Neven P., Huober J., et al. Efficacy and safety results by menopausal status in monarchE: adjuvant abemaciclib combined with endocrine therapy in patients with HR+, HER2−, node-positive, high-risk early breast cancer. Ther Adv Med Oncol. 2023;15 doi: 10.1177/17588359231151840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui W., Francis P.A., Loi S., et al. Assessment of ovarian function in phase III (neo)adjuvant breast cancer clinical trials: a systematic evaluation. J Natl Cancer Inst. 2021;113(12):1770–1778. doi: 10.1093/jnci/djab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marme F., Solbach C., Michel L., et al. Utility of the CPS + EG scoring system in triple-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer. 2021;153:203–212. doi: 10.1016/j.ejca.2021.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Mittendorf E.A., Jeruss J.S., Tucker S.L., et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29(15):1956–1962. doi: 10.1200/JCO.2010.31.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudis C.A., Barlow W.E., Costantino J.P., et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 22.Freeman E.W., Sammel M.D., Lin H., Gracia C.R. Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group. Buzdar A., Howell A., Cuzick J., et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7(8):633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 24.Meirson T., Goldstein D.A., Gyawali B., Tannock I.F. Review of the monarchE trial suggests no evidence to support use of adjuvant abemaciclib in women with breast cancer. Lancet Oncol. 2023;24(6):589–593. doi: 10.1016/S1470-2045(23)00165-1. [DOI] [PubMed] [Google Scholar]
- 25.Slamon D.J., Stroyakovskiy D., Yardley D.A., et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41(suppl 17):LBA500. LBA500. [Google Scholar]
- 26.Gnant M., Dueck A.C., Frantal S., et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03) J Clin Oncol. 2022;40(3):282–293. doi: 10.1200/JCO.21.02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston S.R.D., Toi M., O’Shaughnessy J., et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(1):77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skafida E., Andrikopoulou A., Terpos E., et al. Impact of CDK4/6 inhibitors on aromatase inhibitor-associated musculoskeletal syndrome (AIMSS) in the adjuvant setting. Breast J. 2023;2023 doi: 10.1155/2023/3614296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson R.A., Rosendahl M., Kelsey T.W., Cameron D.A. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013;49(16):3404–3411. doi: 10.1016/j.ejca.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnabei A., Strigari L., Marchetti P., et al. Predicting ovarian activity in women affected by early breast cancer: a meta-analysis-based nomogram. Oncologist. 2015;20(10):1111–1118. doi: 10.1634/theoncologist.2015-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su H.C., Haunschild C., Chung K., et al. Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer. 2014;120(23):3691–3698. doi: 10.1002/cncr.28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furlanetto J., Marme F., Seiler S., et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/adjuvant breast cancer trials. Eur J Cancer. 2021;152:193–203. doi: 10.1016/j.ejca.2021.04.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.