Abstract
The incidence of coronary artery aneurysm is between 1.4% and 4.9% based on autopsy or angiographic series. Mycotic coronary arteries aneurysms are very rare and represent less than 3% of all coronary aneurysms. We report the case of a patient who presented with multiple coronary mycotic aneurysms.
Key Words: coronary aneurysm, coronary fistula, mycotic aneurysm
Graphical abstract
History of Presentation
A 53-year-old man presented in a referring center with increased dyspnea, malaise, low-degree fever, and signs of heart failure. His blood pressure was 123/71 mm Hg, his heart rate was 72 beats/min, and his peripheral oxygen saturation was 97%. He was afebrile with no cutaneous signs of endocarditis or skin infection. On the electrocardiogram, an anterior myocardial infarction of indeterminate age was present with abnormal ST-segment and T-wave changes suggesting lateral ischemia. His chest radiograph showed a right pleural effusion, cardiomegaly, and interstitial edema. His white blood cell count was 5,200 × 109/L (normal: 4.5-11) with an elevated C-reactive protein of 88 mg/dL (normal: <0.3).
Learning Objectives
-
•
To make the differential diagnosis of a patient presenting with fever and heart failure symptoms.
-
•
To understand the multidisciplinary investigation and management of patients with mycotic coronary aneurysms.
Medical History
The patient had hypertension, end-stage renal disease requiring hemodialysis, pulmonary embolism, and non–insulin-dependent diabetes mellitus.
Differential Diagnosis
The clinical presentation is consistent with a diagnosis of decompensated heart failure. Potential etiologies include acute coronary syndrome, ischemic cardiomyopathy, endocarditis with valvular regurgitation, inadequate dialysis, pulmonary embolism, and myocarditis.
Investigations
Biological studies were noteworthy only of a C-reactive protein of 88 mg/L and a procalcitonin of 0.09 μg/mL. Complete blood count, creatinine, and cardiac enzymes were normal. Blood cultures were negative. A transthoracic echocardiography was performed and revealed a left ventricular ejection fraction of 35% with apical and anterolateral akinesia with pleuro-pericardial effusions. A coronary angiography revealed a large aneurysm of the left anterior descending (LAD) with a fistula draining in both ventricles (Figure 1). A positron emission tomography scan suggested an inflammatory process in the anterolateral region of the right ventricle (RV) with a hypodense central zone for which an abscess could not be excluded (Figure 2A). Cardiac computed tomography revealed multiple pseudoaneurysms in the LAD, proximal right coronary artery, and the largest one originating from the distal LAD with a biventricular fistula communicating with both the RV and left ventricle (LV) (Figure 2B to 2G).
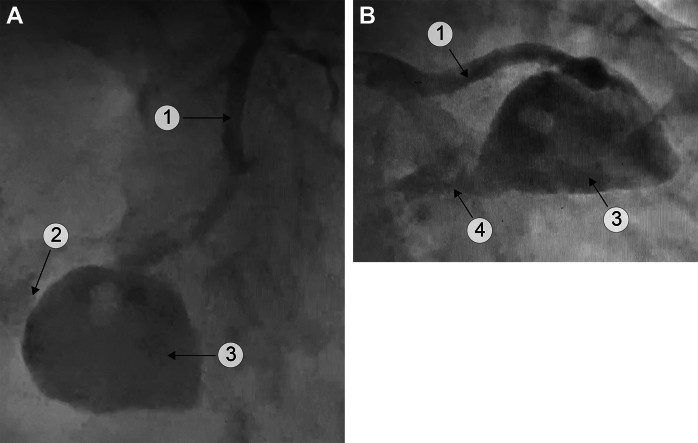
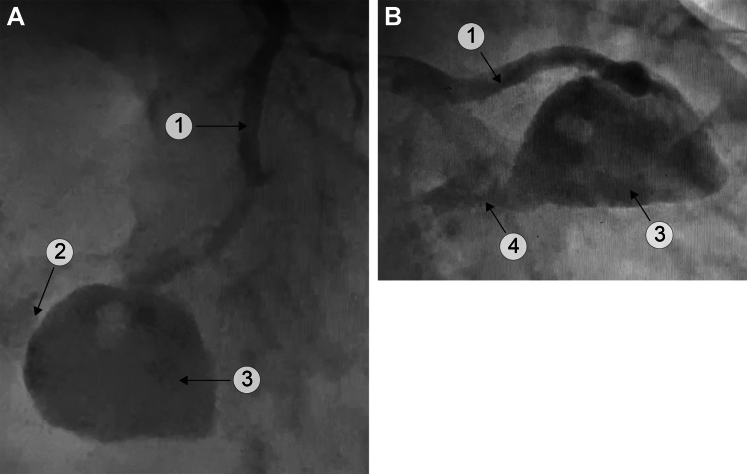
Figure 1.
Mycotic Aneurysm of the LAD With LV and RV Fistula
(A) Cranial left anterior oblique view. (B) Caudal right anterior oblique view. A perfusion defect in the aneurysm is present. 1 = LAD; 2 = RV fistula; 3 = mycotic aneurysm; 4 = LV fistula (see Videos 1A, 1B, and 1C). LAD = left anterior descending; LV = left ventricle; RV = right ventricle.
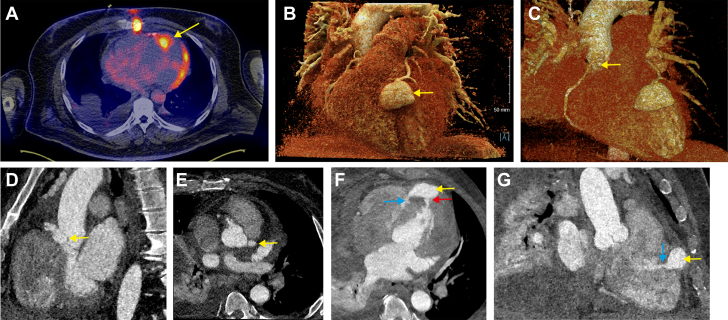
Figure 2.
Mycotic Aneurysm Imaging
(A) Axial slice of fused positron emission tomography and CT scan. Abnormal uptake at the end of the LAD coronary representing the mycotic aneurysm is present (yellow arrow). Additional uptake of the left ventricular lateral wall and at the sternum are present. (B-E) Cardiac CT angiography (Siemens SOMATOM Force) with prospective electrocardiographic-gating and intravenous contrast (iopamidol 370) in arterial phase. The yellow arrow indicates a 3D volume rendering and 2D of the (B) LAD, (C and D) right coronary artery, and (E) left main mycotic aneurysms. (F and G) The red arrow indicates the fistula to the LV and the blue arrow, the fistula to the RV (see Video 2). 2D = 2-dimensional; 3D = 3-dimensional; CT = computed tomography; other abbreviations as in Figure 1.
Management
Cardiac surgery was consulted for aneurysm exclusion, LAD coronary artery bypass grafting, and fistulas closure. The patient’s EuroSCORE 2 was 14%. After informed consent was obtained, the patient was scheduled for surgery. Intraoperatively, a midesophageal 4-chamber view demonstrated a systolic jet originating from the RV apical cavity (Figure 3A) close to the septum. Doppler interrogation revealed a systolo-diastolic continuous-wave Doppler signal with a 41 mm Hg systolic peak gradient, consistent with an RV fistula (Figure 3B). In addition, a diastolic velocity signal in the LV cavity was also identified corresponding to the LV fistula (Figure 3C and 3D). A midesophageal long-axis aortic view also revealed the presence of a fluid-filled cavity near the septum (Figure 4). A median sternotomy was performed. The pleurae were drained (1,400 mL of serosanguinous liquid). The LAD pseudoaneurysm was identified, and another thrombosed and calcified aneurysm of the circumflex artery was also noted (Figure 5). After opening of the LAD aneurysm, the 2 fistulous orifices communicating with the RV and LV were identified and closed (Figure 6A to 6C). The LV fistula was opened up and content sent for culture, which came back positive for Staphylococcus epidermidis. A venous bypass was performed at the distal end of the LAD (Figure 6D). Weaning from cardiopulmonary bypass was challenging, with RV dysfunction and distributive shock. No residual fistula was present on transesophageal echocardiography. RV dysfunction was treated with inhaled milrinone and noradrenaline, while distributive shock required epinephrine, vasopressin, methylene blue, and meropenem. The aortic cross-clamp and cardiopulmonary bypass times were 57 min and 78 min, respectively. No extracardiac source of infection was identified postoperatively.
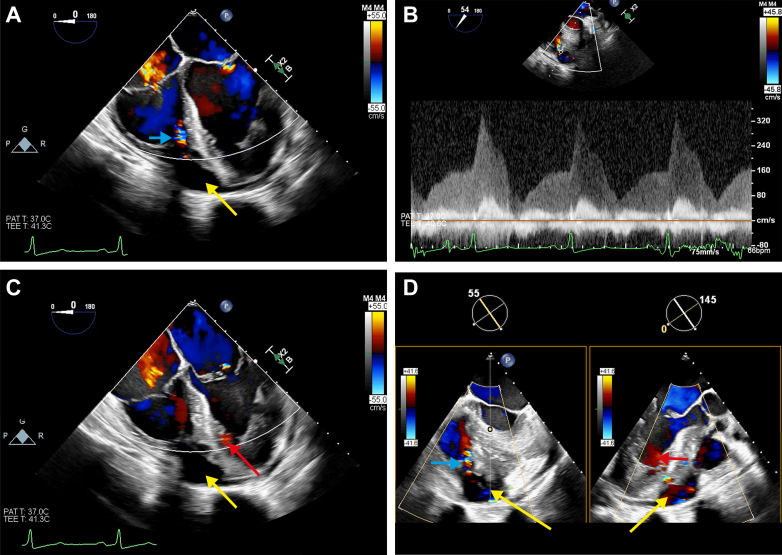
Figure 3.
Intraoperative TEE
(A) ME 4-chamber view with an anechoic cavity at the apical region (yellow arrow). A systolic color Doppler signal (blue arrow) is directed in the RV. (B) Continuous-wave Doppler interrogation of the RV fistula signal with a diastolo-systolic peak gradient of 3.2 m/s (41 mm Hg). (C) ME 4-chamber view showing the mycotic aneurysm (yellow arrow) and a lower velocity diastolic signal (red arrow) are seen in the LV. (D) Biplane RV outflow tract and ME aortic long-axis view in which the mycotic aneurysm is seen (yellow arrow). Both the RV fistula (blue arrow) and the LV fistula (red arrow) are present (see Video 3). ME = midesophageal; TEE = transesophageal echocardiography; other abbreviations as in Figure 1.
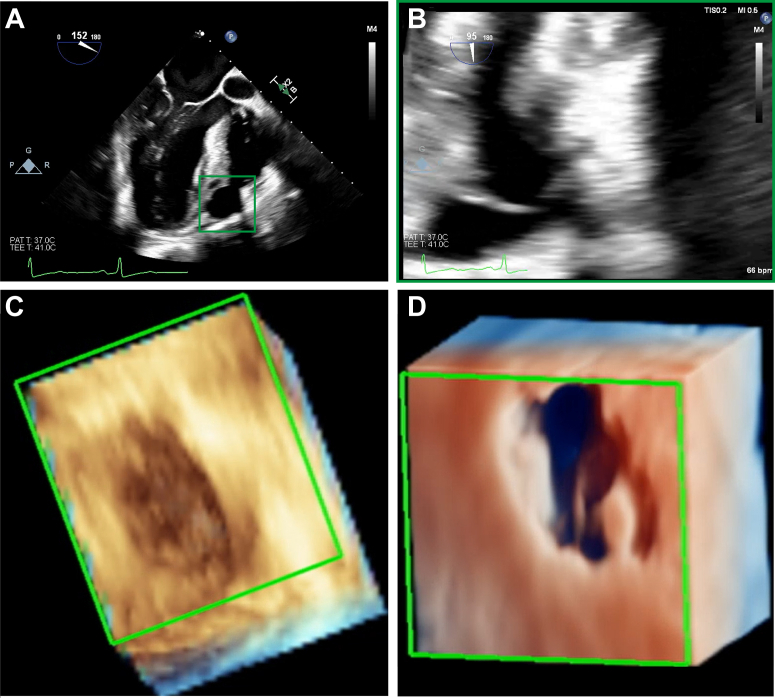
Figure 4.
Mycotic Coronary Aneurysm
(A and B) ME aortic long-axis view with a zoomed view on the mycotic aneurysm (note the central membrane). (C and D) 3D zoomed view and true view of the mycotic aneurysm (see Video 4A, Video 4B, Video 4C, Video 4D). Abbreviations as in Figures 2 and 3.
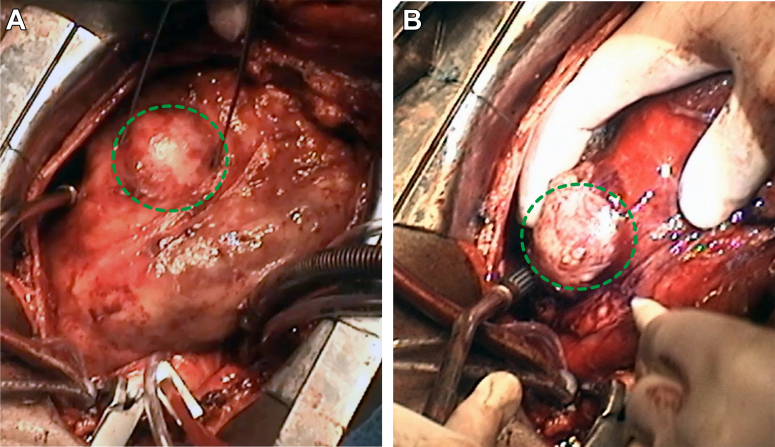
Figure 5.
Intraoperative Imaging
Aspect of the LAD (green dotted circle) (A) and thrombosed circumflex (B) coronary artery aneurysm (green dotted circle) (see Video 4A, Video 4B, Video 4C, Video 4D). Abbreviation as in Figure 1.
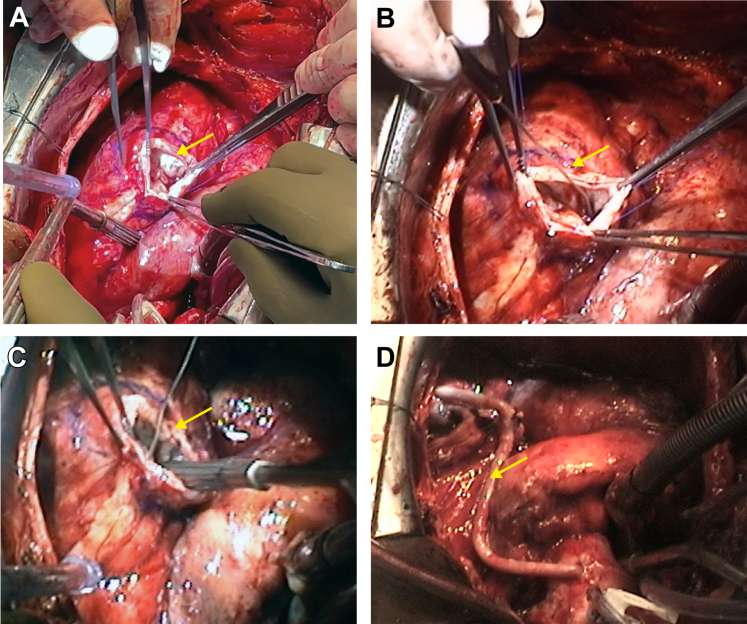
Figure 6.
Surgical Procedure
(A) The mycotic aneurysm on the LAD is opened (arrow). (B) A rod or probe (arrow) identifies the RV and (C) the LV fistula (arrow). (D) A bypass graft to the LAD (arrow) is shown (see Video 4A, Video 4B, Video 4C, Video 4D). Abbreviations as in Figure 1.
Discussion
To our knowledge this is the first case report describing a mycotic LAD aneurysm with biventricular fistulas and a thrombosed circumflex aneurysm. The infectious nature of the aneurysm could only be determined after surgery. Coronary aneurysm can result from 2 mechanisms. The first mechanism is an internal mechanism as a result of vascular endothelial injury caused by foreign or infectious agent.1 The second mechanism can result from an extrinsic cause such as septic emboli of the vasa vasorum by immune complex, leading to inflammatory dilatation of the arterial wall.2, 3, 4 This can lead to coronary rupture, distal embolization, or thrombosis. Their incidence is higher in immunocompromised patients and patients with neoplasm but also in diabetic individuals.5 The incriminated germs are typically Staphylococcus aureus and Streptococcus viridans. Most patients present with symptoms such as fever, chills, or lethargy with no pathognomonic features. Patients can be asymptomatic or present with myocardial ischemia and sudden death secondary to aneurysm rupture.1 Coronary angiography is the gold standard in the diagnosis of this condition but multidetector computed tomography allow a more detailed anatomy and relationship with adjacent structures.4 Pre-existing stents, coronary bypass grafting, and bacteriemia may represent risk factors,5,6 but the natural history of coronary aneurysms remains unknown.1 The prognosis of mycotic coronary aneurysms is very poor with perioperative mortality up to 38.9%, with half being intraoperative death, particularly if treatment is not undertaken early.7 The treatment follows 2 principles: small aneurysms are treated with broad-spectrum antibiotic therapy, whereas larger aneurysms require surgical intervention with antibiotic therapy.5 Evaluation seeking other sites of mycotic aneurysms should also be considered.8 Currently there are no guidelines regarding the optimal management of this rare condition.
Follow-up
The immediate postoperative period was marked by RV dysfunction and persistence of a distributive shock. Intraoperative cultures came back positive for S epidermidis treated with intravenous vancomycin for 3 weeks. The RV failure and distributive shock subsequently resolved. The patient was discharge from the hospital on day 10. The patient was asymptomatic at the 3-month follow-up.
Conclusions
Mycotic coronary aneurysms can present with new-onset heart-failure and lead to serious complications such as rupture or ventricular fistulation. Early recognition of this condition, and prompt surgical intervention, followed by antibiotic therapy, may improve outcomes.
Funding Support and Author Disclosures
Vincent Chauvette is a Vanier Scholarship recipient. Guillaume Marquis-Gravel is a Junior Clinical Research Scholar of the Fonds de Recherche du Québec–Santé. André Denault is supported by the Richard I. Kaufman Endowment Fund in Anesthesia and Critical Care. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
CT angiography.
TEE ME 4C view. 4
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
Intraoperative view: LAD aneurysm.
Intraoperative view: RV and LV fistula.
References
- 1.Syed M., Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40:77–84. doi: 10.1016/s0033-0620(97)80024-2. [DOI] [PubMed] [Google Scholar]
- 2.Yeen W., Panza A., Cook S., Warrell C., Sun B., Crestanello J.A. Mycotic coronary artery aneurysm from fungal prosthetic valve endocarditis. Ann Thorac Surg. 2007;84:280–282. doi: 10.1016/j.athoracsur.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Padmakumar E.A., Jariwala P. Mycotic aneurysm of the left anterior descending coronary artery induced by 'mother-and-child' catheter assisted percutaneous coronary angioplasty. Indian Heart J. 2017;69:663–665. doi: 10.1016/j.ihj.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buono A., Maloberti A., Bossi I.M., et al. Mycotic coronary aneurysms. J Cardiovasc Med (Hagerstown) 2019;20:10–15. doi: 10.2459/JCM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 5.Ali U., Stamp N., Larbalestier R. Resection of a large mycotic aneurysm of the left anterior descending coronary artery. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-232894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto Y., Ushijima T., Yamaguchi S., et al. Mycotic aneurysm of the left anterior descending coronary artery after coronary artery bypass graft surgery. Ann Thorac Surg. 2011;91:1601–1603. doi: 10.1016/j.athoracsur.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Bosman W.M., Borger van der Burg B.L., Schuttevaer H.M., Thoma S., Hedeman Joosten P.P. Infections of intravascular bare metal stents: a case report and review of literature. Eur J Vasc Endovasc Surg. 2014;47:87–99. doi: 10.1016/j.ejvs.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kalangos A., Julia P.L., Ozler A., Jebara V.A., Fabiani J.N., Sezerman O. Successful surgical treatment of a coronary artery mycotic aneurysm. Ann Thorac Surg. 1994;58:1521–1523. doi: 10.1016/0003-4975(94)91947-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
(A) LAO cranial. (B) RAO caudal. (C) Right coronary artery.
CT angiography.
TEE ME 4C view. 4
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
(A) TEE ME LAX view. (B) Zoomed TEE ME LAX view. (C) TEE 3D cavity zoomed view. (D) TEE 3D cavity true view.
Intraoperative view: LAD aneurysm.
Intraoperative view: RV and LV fistula.