Abstract
Background
This phase II nonrandomized study evaluated the efficacy and safety of AZD4635 in combination with durvalumab (Arm A) or durvalumab plus cabazitaxel (Arm B) in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel and ≥1 novel hormonal agent.
Patients and methods
The primary endpoint was radiographic progression-free survival (rPFS) per RECIST v1.1 (soft tissue) or the Prostate Cancer Clinical Trials Working Group 3 (bone). Secondary endpoints included safety, tolerability, overall survival, confirmed prostate-specific antigen (PSA50) response, pharmacokinetics, and objective response rate. Enrollment in Arm A was stopped following a sponsor decision unrelated to safety. The study was stopped based on the planned futility analysis due to low PSA50 response in Arm B.
Results
In the final analysis (1 November 2021), 30 patients were treated (Arm A, n = 2; Arm B, n = 28). The median rPFS in Arm B was 5.8 months (95% confidence interval 4.2-not calculable). Median rPFS was 5.8 months versus 4.2 months for patients with high versus low blood-based adenosine signature. The most common treatment-related adverse events in Arm B were nausea (50.0%), diarrhea (46.4%), anemia and neutropenia (both 35.7%), asthenia (32.1%), and vomiting (28.6%). Overall, AZD4635 in combination with durvalumab or AZD4635 in combination with cabazitaxel and durvalumab showed limited efficacy in patients with mCRPC.
Conclusions
Although the safety profile of both combinations was consistent with known safety data of the individual agents, the results of this trial do not support further development of the combinations.
Key words: AZD4635, cabazitaxel, durvalumab, metastatic castration-resistant prostate cancer, pharmacokinetics, safety
Highlights
-
•
AZD4635 is a novel adenosine2A receptor antagonist that blocks A2aR-mediated signaling in tumor-infiltrating immune cells.
-
•
Adding cabazitaxel to the AZD4635 plus durvalumab combination may enhance antitumor activity in the post-docetaxel setting.
-
•
AZD4635 in combination with durvalumab or durvalumab plus cabazitaxel showed limited efficacy in patients with mCRPC.
-
•
AZD4635 in combination with durvalumab or cabazitaxel was generally well tolerated.
-
•
Adenosine signature signaling score may assist with optimal patient selection for adenosine pathway modulators.
Introduction
Prostate cancer is one of the most common cancers with more than 1.46 million new cases and ∼397 000 related deaths globally reported in 2022.1 Castration-resistant prostate cancer (CRPC) occurs in 10%-20% of cases.2 The current standard of care for unselected metastatic CRPC (mCRPC) patients includes taxanes and androgen receptor inhibitors based on improvement in overall survival (OS) as shown in several phase III trials.3, 4, 5, 6, 7, 8 Therapeutics such as radiopharmaceuticals (radium-223 or 177Lu-PSMA-617) and inhibitors of DNA repair [poly (ADP-ribose) polymerase (PARP) inhibitors—e.g. olaparib or rucaparib] are also treatment options based on specific imaging or molecular criteria. PARP inhibitors have shown clinical efficacy as monotherapy or in combination treatment, and are approved in the United States for patients with mCRPC who also carry certain alterations in homologous recombination-repair genes.9, 10, 11, 12, 13 Thus, therapeutic options remain limited for most patients with mCRPC and further therapeutic strategies are needed to improve the reported median OS of <3 years in this context.3,9,11,14,15
Cabazitaxel is currently approved in a post-docetaxel setting for patients with mCRPC16 based on the phase III TROPIC trial that showed an improvement in survival in patients treated with cabazitaxel versus mitoxantrone.15 The CARD study demonstrated significant improvement in the median OS, median progression-free survival (PFS), prostate-specific antigen (PSA), and tumor response with cabazitaxel compared with a novel hormonal agent (NHA) in patients with mCRPC progressing on an alternate NHA and docetaxel.14
The combination of a taxane and immune checkpoint blockade can induce clinical responses in a subset of patients with mCRPC. For example, in the phase Ib/II KEYNOTE-365 study (cohort B), the combination of docetaxel, pembrolizumab, and prednisone demonstrated antitumor activity with an objective response rate (ORR) of 23% and a PSA response of 34% in chemotherapy-naïve patients who did not respond or were intolerant to abiraterone or enzalutamide.17 However, the combination did not provide benefit across the overall tested population.
Further, although treatment with immunotherapies have demonstrated positive responses in a subset of patients with immunologically ‘activated’ tumors, they have not significantly improved survival in patients with mCRPC.18,19 In the phase III KEYNOTE-921 study, the addition of pembrolizumab to docetaxel did not significantly improve rPFS or OS in patients with mCRPC.20 Thus, the identification of predictive biomarkers may be required to elicit a more robust immune response by immunotherapies.21 Taken together, combining cabazitaxel with an immune checkpoint inhibitor may increase antitumor activity in an appropriately predictive biomarker-selected patient population.
The nucleoside adenosine has emerged as a major immuno-metabolomic checkpoint in tumors.22 Adenosine is found in higher concentrations in the tumor microenvironment (TME), and it negatively affects the immune system by signaling through the adenosine2A receptor (A2aR).22 A2AR signaling suppresses the function of effector T cells and natural killer cells (i.e. cytokine production and cytotoxic activity) while increasing the function of regulator T cells and myeloid-derived suppressor cells.22,23 AZD4635 is a novel A2aR antagonist that blocks A2aR-mediated signaling in tumor-infiltrating immune cells.23 In preclinical studies, AZD4635 in combination with an anti-programmed death-ligand 1 (PD-L1) antibody enhanced antitumor immunity and inhibited tumor growth.23 AZD4635 has shown antitumor activity (reduction of tumor burden compared with control mice) in PTEN-deficient prostate cancer models showing evidence of immune modulation by extracellular adenosine in prostate cancer.24 In the phase I study, AZD4635 has demonstrated clinical activity in mCRPC, and appears to have increased efficacy in combination with anti-PD-L1 in mCRPC due to increased immune activation compared with single-agent AZD4635.25 Taken together, AZD4635 treatment results in a less immunosuppressive TME and may complement other immune-targeting drugs to enhance clinical response.
The current study assessed AZD4635 in combination with durvalumab (Arm A) or durvalumab plus cabazitaxel (Arm B) in patients with mCRPC previously treated with docetaxel and/or an NHA. This study was carried out under the hypothesis that AZD4635 combined with durvalumab and cabazitaxel may lead to an increased rPFS in the post-docetaxel setting by decreasing the immunosuppressive environment and counteracting the effects of adenosine that may limit anti-PD-L1 activity.
Patients and methods
Patients
The study was conducted at 16 centers in Belgium, France, South Korea, Spain, and the United States. Eligible patients were ≥18 years old with measurable disease and disease progression detected either by imaging or PSA, inoperable, metastatic, castration-resistant (testosterone level < 50 ng/dl) adenocarcinoma of the prostate with no evidence of small-cell histology. Patients had disease progression ≤6 months before study entry as measured by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, a bone lesion on bone scan per the Prostate Cancer Clinical Trials Working Group 3 (PCWG3), or rising PSA level at least in two consecutive measures taken at least 1 week apart. In the case of non-measurable disease per RECIST v1.1, patients must have had measurable PSA ≥ 1.0 ng/ml as the minimum starting level. Patients had adequate bone marrow, renal, and liver organ function. Arm A consisted of patients with mCRPC previously treated with one or more approved NHAs (e.g. abiraterone acetate, enzalutamide, apalutamide, and/or darolutamide) and one or more taxanes, unless taxane-ineligible. Arm B consisted of patients with mCRPC previously treated with docetaxel and one prior NHA (either abiraterone acetate or enzalutamide, but not both; prior apalutamide was not permitted).
Key exclusion criteria included active brain metastases or leptomeningeal metastases <28 days before the first scheduled dose. Treatment with systemic corticosteroids (>10 mg/day prednisone/equivalent) was prohibited for at least 2 weeks before study enrollment. Patients were excluded if they had a history of pneumonitis, active second malignancy ≤3 years, allogeneic organ transplantation, uncontrolled systemic disease or active hepatobiliary or gastrointestinal infection, uncontrolled cardiovascular diseases, active or prior autoimmune or inflammatory disorders, or active primary immunodeficiency. Patients with creatine clearance <40 ml/min and prior exposure to immune-mediated therapy were excluded. Arm B-specific exclusion criteria were active grade ≥2 peripheral neuropathy, active grade ≥2 stomatitis, treatment with cytochrome P450 3A4/5 inducers or inhibitors <2 weeks before day 1 dosing, and recent radiotherapy (wide field, 4 weeks; limited field, <2 weeks of first dose).
The study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and were consistent with the International Conference on Harmonisation/Good Clinical Practice guidelines, and applicable regulatory requirements. The study protocol was approved by an institutional review board or independent ethics committee before initiation of the study. All patients provided written informed consent before participation in the study.
Study design and treatment
This was a phase II international, open-label, two-arm, nonrandomized study (Clinical Trials.gov identifier: NCT04495179). Patients in each arm were stratified by the presence of measurable soft-tissue metastasis per RECIST v1.1 or bone-only metastasis per PCWG3 criteria.
Patients were allocated to one of the two treatment arms. In the phase Ia/b study of AZD463525, the recommended phase II dose for AZD5635 was 75 mg once daily, as monotherapy or in combination with durvalumab. Patients in Arm A were treated with AZD4635 75 mg orally (PO) daily plus durvalumab 1500 mg intravenously (IV) every 4 weeks (Q4W). Enrollment in Arm A was stopped after two patients received treatment following sponsor decisions unrelated to any safety issues. Patients in Arm A continued treatment if they were experiencing clinical benefit and did not meet any discontinuation criteria. Patients in Arm B were treated with AZD4635 75 mg PO daily plus durvalumab 1500 mg IV every 3 weeks (Q3W) plus cabazitaxel 20 or 25 mg/m2 IV Q3W as per local prescribing guidelines. Cabazitaxel was administered as per local prescribing guidelines for a maximum of 10 cycles. After cycle 10, durvalumab + AZD4635 was administered Q4W to harmonize with Arm A treatment-cycle length. Patients received prednisone [10 mg daily continuously (or equivalent steroid)] and prophylactic granulocyte colony-stimulating factor (according to product label)16 for the duration of cabazitaxel administration.
A ‘no-go’ futility interim analysis was planned for Arm B based on whether the proportion of patients achieving a ≥50% decrease in PSA from baseline (PSA50) response was <35%, once the arm had 30 assessable patients. Of these patients, ∼15 patients were to have RECIST v1.1 measurable disease at baseline, and the remainder of the patients may have had bone-only disease or non-measurable disease.
Study endpoints
The primary efficacy endpoint was rPFS, defined as the time from the first dose until radiographic progression per RECIST v1.1 (soft tissue), and PCWG3 (bone) or death from any cause, whichever occurred first. Patients who did not progress [defined as complete response (CR), partial response (PR), stable disease (SD) by RECIST v1.1 for soft-tissue disease, or non-progression of disease (PD) for bone disease] at the time of analysis were censored at the time of the last evaluable RECIST v1.1 assessment or bone scan.
Secondary endpoints included safety and tolerability, OS, ORR per RECIST v1.1 and PCWG3 by the investigator, rPFS by adenosine-signaling gene expression, PSA50 response, and pharmacokinetics (PK). Patients in Arm A had disease assessments/imaging at baseline and every 8 weeks (±7 days) from the start of dosing for the first 24 weeks and then every 12 weeks (±7 days) thereafter. Patients in Arm B had disease assessments/imaging at baseline and every 9 weeks (±7 days) from the start of dosing for the first 27 weeks and then every 12 weeks (±7 days) thereafter.
Safety
Safety and tolerability endpoints included assessment of adverse events (AEs), serious AEs (SAEs), laboratory assessments, vital signs, standard 12-lead electrocardiogram, echocardiogram, and physical examination. AEs were summarized by the Medical Dictionary for Regulatory Activities version 24.1 system organ class and preferred term, with further categorization by maximum Common Terminology Criteria for Adverse Events (CTCAE) grade (version 5.0), potential causal relationship to any study medication, AEs associated with dose interruptions or modifications, and AEs classed as CTCAE grade 3 or higher.
Dose-limiting toxicity (DLT) criteria were used to assess safety for Arm B in the first six assessable patients. DLTs were graded according to CTCAE version 5.0. A DLT-assessable patient was defined as a patient who had received AZD4635 in Arm B, and either had completed the minimum safety evaluation requirements and had received at least 75% of the specified AZD4635 doses concomitantly with durvalumab and cabazitaxel during cycle 1, or had experienced a DLT during cycle 1, the DLT evaluation period, for Arm B.
Pharmacokinetics
Single-dose PK parameters were determined from venous blood samples (2 ml) for AZD4635, its metabolites (SSP-005173 and SSP-005174), and cabazitaxel. Durvalumab PK parameters derivation was not possible due to a sparse sample collection schedule. Sparse PK samples for AZD4635 (pre-dose) were collected from Arm A patients on cycles 1, 3, 5, and 7. Venous blood samples were drawn pre-dose and 0.5, 1, 2, 4, 6, 8, and 24 h post-dose for cycle 1, and pre-dose for cycle 2 in the first 12 assessable Arm B patients. Subsequent patients entering the study had sparse PK schedules (cycle 1, 3, 5, 7, day 1; pre-dose). PK samples for the same 12 assessable Arm B patients for cabazitaxel were collected pre-dose, 5 min before the end of infusion, and 0.5, 1, 2, 4, 6, 8, and 24 h post-dose for cycle 1.
PK analysis of plasma concentration data for AZD4635 and its metabolites and cabazitaxel was determined by Covance Bioanalytical Laboratory Services Inc., (Madison, WI) using a validated bioanalytical method, and PK parameters were calculated using Pheonix® WinNonlin® version 8.1 or higher. Maximum observed concentration (Cmax) and time to reach Cmax (tmax) were determined directly by inspection of the concentration–time profiles. Terminal half-life slope (t½λz) was calculated as ln2/terminal elimination rate constant (λz). Area under the plasma concentration–time curve (AUC) from zero to last measurable concentration (AUClast) was calculated using the linear up/log down trapezoidal rule. AUClast was extrapolated to infinity using λz to obtain overall exposure (AUCinf).
rPFS by adenosine gene signature
Blood samples for RNA isolation and subsequent gene expression analysis were collected from patients in Arm B at baseline. Gene expression data were generated by nCounter® (Nanostring Technologies, Inc., Seattle, WA) gene expression assays using the PanCancer Immune Profiling panel (NanoString Technologies, Inc., Seattle, WA) and standard protocol as previously described.25 The adenosine-signaling levels were assessed using a 14-gene expression signature (PPARG, CYBB, COL3A1, FOXP3, LAG3, APP, CD81, GPI, PTGS2, CASP1, FOS, MAPK1, MAPK3, and CREB1) previously developed by Sidders and colleagues.26 Signature scores were calculated as the median of normalized, batch-corrected log2 gene expression values across the 14 genes. Subsequently, the median signature score across all patients was used as the cut-off for assigning patients to groups with high versus low levels of blood-based adenosine signaling.
Statistical analyses
Data from Arm A were reported in listings because the number of enrolled patients (n = 2) was too small for a meaningful analysis. Descriptive statistics were used to summarize patient characteristics, efficacy, PK, and safety data for Arm B. rPFS and OS were analyzed using the Kaplan–Meier method. Best overall response was summarized. ORR was summarized and presented with a two-sided 95% confidence interval (CI) using the Clopper–Pearson method. The proportion of patients achieving a PSA50 response and patients with a confirmed PSA response was calculated using the Clopper–Pearson method and presented with 95% CI. The PK analysis set included dosed patients for whom an adequate PK profile was obtained. The tumor response analysis set included dosed patients with a baseline tumor assessment and measurable disease at baseline. The assessable-for-efficacy-analysis set included all dosed patients with a baseline tumor assessment. The PSA-assessable set comprised all dosed patients with baseline PSA levels ≥1 ng/ml. All statistical analyses were carried out using (SAS Institute Inc, Cary, NC) version 9.4 or later.
Results
Futility analysis
At the interim analysis for Arm B, using the interim PSA-assessable patient population, 4/27 (14.8%) patients had a confirmed PSA50 response, and 3/27 (11.1%) patients had an unconfirmed PSA50 response. The ‘no-go’ futility analysis criterion was met with fewer than 10 of 27 patients having a confirmed response (<35% PSA50 responses). Therefore, further enrollment in Arm B was stopped. Results from the final analysis are described later in the efficacy section.
The remainder of the results section presents data from the final analysis only.
Patient demographics and baseline characteristics
As of data cut-off for the final analysis (1 November 2021), Arm A enrolled 2 patients and Arm B enrolled 31 patients, 28 of whom were treated. Three (10.7%) patients had protocol deviations related to inclusion/exclusion criteria (received > 1 NHA, n = 2; testosterone level ≥ 50 ng/dl, n = 1). A total of 27 patients discontinued treatment due to the following reasons: AEs (1/28), death (4/28), withdrawal by the investigator (18/28; 7 due to PD) or by patient (4/28; 2 due to PD); one patient was still on treatment. Patient demographics and baseline characteristics for Arm B are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103446. The median (minimum-maximum) age in Arm B was 69 years (47-78 years), and most patients (67.9%; 19/28 patients) were White. Tumors were high-grade (Gleason score ≥ 8) in 71.4% (20/28) of patients, with lymph node metastasis in 42.9% (12/28) of patients and bone metastasis in 46.4% (13/28) of patients. As of the final analysis, the median (minimum-maximum) duration of follow-up was 6 months (0-8.8 months); 21 patients were still being followed. A median number of 7.0 cycles (range: 1-10) of cabazitaxel were administered.
Efficacy
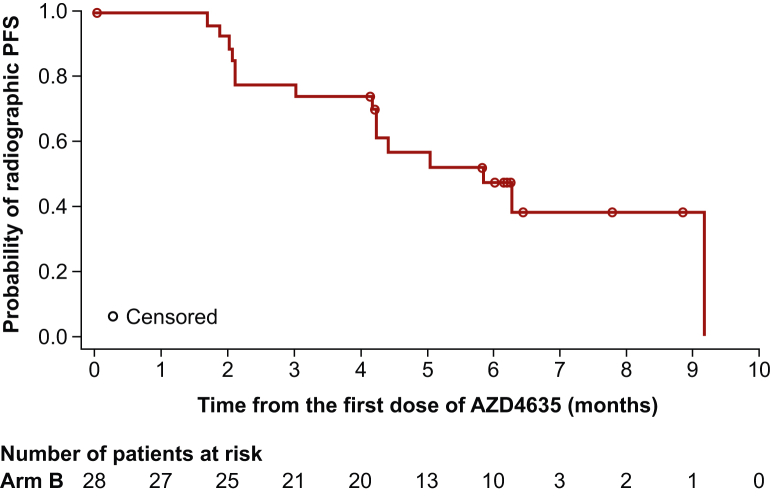
The median rPFS for patients in Arm B was 5.8 months [95% CI 4.2 months-not calculable (NC)] (Figure 1). Among the 28 patients assessed for efficacy, 12 patients showed disease progression according to RECIST v1.1, and 1 patient had bone disease progression according to PCWG3 criteria. One patient with RECIST progression also had bone disease progression per PCGW3 criteria. Three patients (SD, n = 1; NE, n = 2) died in the absence of disease progression. At the time of final data cut-off, the relatively short follow-up period precluded the calculation of median OS in Arm B (Table 1).
Figure 1.
Radiographic PFS in Arm B (assessable-for-efficacy-analysis set). For the Kaplan–Meier plot, patients who did not progress at the time of analysis were censored at their last evaluable RECIST v1.1 assessment or bone scan. If the patient had disease progression or died after two or more missed radiologic visits, the patient was censored at the time of the latest evaluable RECIST v1.1 or bone scan assessment before the two missed visits. PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria In Solid Tumors version 1.1.
Table 1.
Disease response, as-treated population
| Parameter | Arm B (N = 28) |
|---|---|
| Median radiographic progression-free survivala | |
| Months | 5.8 |
| 95% CI | 4.2–NC |
| Radiographic PFS,bn (%) | 15 (53.6) |
| RECIST progression | 12 (42.9) |
| Target lesions | 7 (25.0) |
| Nontarget lesions | 2 (7.1) |
| New lesions | 7 (25.0) |
| PCWG3 bone progression | 1 (3.6) |
| Death in the absence of progression | 3 (10.7) |
| Median OSc | |
| Months | NC |
| 95% CI | 7.9–NC |
| Best objective response,dn (%) | |
| Confirmed ORRe | 2 (10.0) |
| 95% CI | 1.23-31.70 |
| CR | 0 |
| PR | 2 (10.0) |
| SD | 11 (55.0) |
| RECIST progression | 5 (25.0) |
| Not evaluable | 1 (5.0) |
| No valid baseline assessment | 1 (5.0) |
| Confirmed PSA50 response | |
| n | 5 |
| Estimate | 0.18 |
| 95% CI | 0.06-0.37 |
CI, confidence interval; CR, complete response; NC, not calculable; NE, not evaluable; ORR, objective response rate; OS, overall survival; PCWG3, Prostate Cancer Clinical Trials Working Group 3; PD, progression of disease; PFS, progression-free survival; PR, partial response; PSA, prostate-specific antigen; PSA50, proportion of patients achieving a ≥50% decrease in PSA from baseline to the lowest post-baseline PSA; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
PFS was calculated using the Kaplan–Meier method. CI for median radiographic PFS was calculated using the Brookmeyer–Crowley method.
Only included progression events or death in the absence of progression events that occurred within two missed visits of the last evaluable assessment assessed by RECIST version 1.1 and PCWG3.
OS was defined as the time from the first dose until death due to any cause, regardless of whether the patient withdrew from study therapy or received another anticancer therapy. OS was calculated using the Kaplan-Meier method.
n = 20, as only dosed patients with a baseline tumor assessment and measurable disease at baseline were included. Best objective response was calculated according to RECIST v1.1 and was defined as the best response (CR, PR, SD, PD, and NE) a patient had following the first dose but before starting any subsequent cancer therapy, and up to and including RECIST progression or the last evaluable assessment in the absence of RECIST progression. Tumor response data were categorized as either: CR, PR, SD, non-CR/non-PD, PD, and NE.
Confirmed ORR was defined as the proportion of patients with a CR or PR using overall radiographic response assessed by RECIST v1.1 and PCWG3 criteria (bone) and was based on a subset of all treated patients with measurable disease at baseline per the site investigator. A confirmed response at the subsequent visit was required to confirm ORR.
In the tumor response assessable population (n = 20) for Arm B, ORR was 10.0% (95% CI 1.23%-31.70%). Two patients had confirmed PR, and no patients achieved confirmed CR. Eleven patients (55.0%) had SD; two of them had an unconfirmed PR. Nine patients had SD ≥ 56 days.
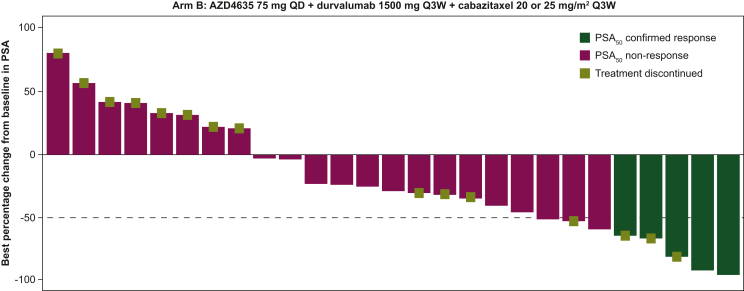
AZD4635 in combination with durvalumab and cabazitaxel showed a confirmed PSA50 response (i.e. ≥50% decline in PSA levels from baseline) in 17.9% of patients (5/28 patients; 95% CI 0.06-0.37; Table 1). The best percentage change from baseline in PSA response for Arm B is shown in Figure 2.
Figure 2.
PSA response, best percentage change from baseline for Arm B (PSA-assessable analysis set). The −50% reference line represents 50% reduction of PSA in best percentage change from baseline. PSA50 response was defined as the proportion of patients achieving a ≥50% decrease in PSA from baseline to the lowest post-baseline PSA, confirmed by a consecutive PSA ≥3 weeks later. If there was a PSA decline from baseline, progression was defined as the date of the first PSA increase (i.e. both ≥25% and ≥2 ng/ml above the nadir) and was confirmed by a second value ≥3 weeks later, even if within 12 weeks. If there was no PSA decline from baseline, progression was defined as a ≥25% increase and a ≥2 ng/ml increase from baseline beyond 12 weeks. Best percentage change from baseline in PSA is the maximum percentage reduction from baseline or the minimum percentage increase from baseline in the absence of a reduction. PSA, prostate-specific antigen; PSA50, proportion of patients achieving a ≥ 50% decrease in PSA from baseline to the lowest post-baseline PSA; QD, once daily; Q3W, every 3 weeks.
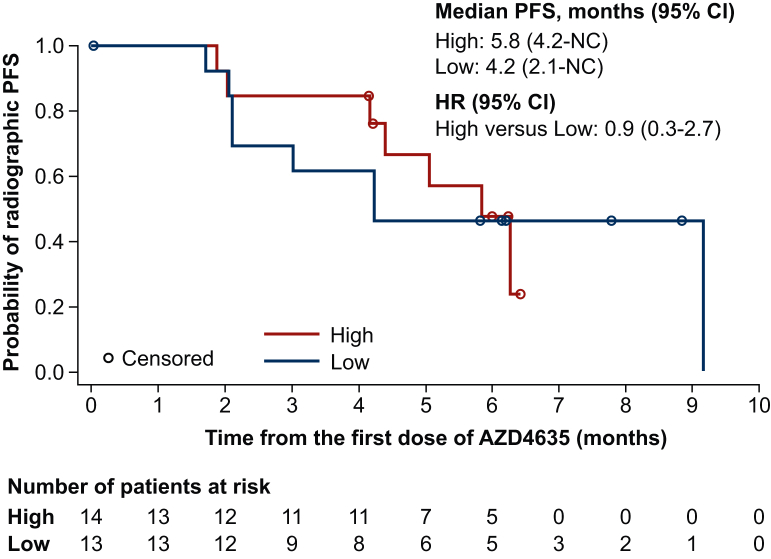
An exploratory analysis of rPFS by peripheral whole-blood adenosine-signaling signature in Arm B is shown in Figure 3. Patients with a higher blood adenosine-signaling signature had a median rPFS of 5.8 months (95% CI 4.2 months-NC). Patients with a lower blood adenosine-signaling signature were associated with a median rPFS of 4.2 months (95% CI 2.1 months-NC). The hazard ratio (HR) for high versus low was 0.9 (95% CI 0.3-2.7).
Figure 3.
Radiographic PFS in Arm B by adenosine-signaling signature high versus low level (assessable-for-efficacy-analysis set). 1 Patient did not have adenosine data. CI, confidence interval; HR, hazard ratio; NC, not calculable; PFS, progression-free survival.
Safety
The median (minimum-maximum) duration of treatment in Arm B was 23.1 weeks (1-45 weeks) for AZD4635, 25.1 weeks (3-45 weeks) for durvalumab, and 21.0 weeks (3-34 weeks) for cabazitaxel. The most frequent AEs in Arm B were nausea (60.7%), diarrhea (50.0%), and anemia (50.0%). Twenty-three (82.1%) patients had AEs possibly related to AZD4635 and 20 (71.4%) patients had AEs possibly related to durvalumab as assessed by the investigator. All patients (100%) had cabazitaxel-related AEs. A summary of treatment-related AEs (TRAEs) for Arm B is reported in Table 2. The most frequent grade ≥3 TRAEs in Arm B (occurring in ≥10% of all patients) were neutropenia (35.7%), white blood cell count decrease (10.7%), and vomiting (10.7%). No DLTs were observed in the first six DLT-assessable patients in Arm B.
Table 2.
Treatment-related AEs (any grade) occurring in ≥10% of all patientsa and corresponding number of grade ≥3 AEs
| Preferred term,bn (%) | Arm B (N = 28) |
|
|---|---|---|
| Any grade | Grade ≥ 3 | |
| Patients with any AE, n (%) | 28 (100.0) | 20 (71.4) |
| Nausea | 14 (50.0) | 2 (7.1) |
| Diarrhea | 13 (46.4) | 2 (7.1) |
| Anemia | 10 (35.7) | 2 (7.1) |
| Neutropenia | 10 (35.7) | 10 (35.7) |
| Asthenia | 9 (32.1) | 0 |
| Vomiting | 8 (28.6) | 3 (10.7) |
| Decreased appetite | 6 (21.4) | 0 |
| Dysgeusia | 6 (21.4) | 0 |
| Fatigue | 5 (17.9) | 1 (3.6) |
| Constipation | 4 (14.3) | 0 |
| Dyspepsia | 4 (14.3) | 0 |
| Amylase increased | 3 (10.7) | 1 (3.6) |
| Arthralgia | 3 (10.7) | 0 |
| Muscular weakness | 3 (10.7) | 0 |
| Edema peripheral | 3 (10.7) | 0 |
| Peripheral sensory neuropathy | 3 (10.7) | 0 |
| Weight decreased | 3 (10.7) | 0 |
| White blood cell count decreased | 3 (10.7) | 3 (10.7) |
AE, adverse event.
Number (%) of patients in Arm B with AEs, sorted in decreasing frequency by preferred term. Patients with multiple events in the same preferred term were counted only once in that preferred term. Patients with events in >1 preferred term were counted once in each of those preferred terms. Included AEs with an onset or worsen date on or after the date of first dose and up to and including the 30-day and 90-day follow-ups from the date of last dose of study medication.
Medical Dictionary for Regulatory Activities Version 24.1.
A total of 67.9% (19/28) of patients in Arm B had an SAE; vomiting was the most frequent (3/28; 10.7%). Twelve (42.9%) patients had an SAE possibly related to any study treatment; febrile neutropenia and vomiting were the most frequent (7.1% each). One patient had an immune-mediated SAE that was programmatically assessed (grade 3 diarrhea, resolved). Five (17.9%) patients in Arm B had an AE that led to discontinuation of AZD4635 (decreased appetite, n = 1; nausea and/or vomiting, n = 2; myositis, n = 1; hydronephrosis, n = 1); of these, nausea and vomiting in one patient were considered possibly due to AZD4635 treatment. Four (14.3%) patients in Arm B discontinued durvalumab (decreased appetite, n = 1; vomiting, n = 1; myositis, n = 1; hydronephrosis, n = 1); only myositis was considered possibly related to durvalumab. Seven (25%) patients discontinued cabazitaxel due to TEAEs (pyelitis, n = 1; peripheral sensory neuropathy, n = 2; vomiting, n = 1; myositis, n = 1; hydronephrosis, n = 1; bone contusion, n = 1). There were nine deaths in this study, (Arm A, n = 2; Arm B, n = 7). Seven deaths occurred >28 days after the drug was discontinued. Both patients in Arm A died due to disease progression, one of whom also had SAEs (i.e. hematuria and blood loss anemia) leading to discontinuation of AZD4635. Two of the deaths in Arm B were due to AEs (colitis, not related to treatment; myositis, possibly related to durvalumab). The remaining five deaths in Arm B were due to the disease under investigation.
Pharmacokinetics
The geometric mean (geometric CV%) concentration–time profiles of AZD4635, its two metabolites SSP-005173 and SSP-005174, and cabazitaxel for patients in Arm B are shown in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103446 and Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103446. Durvalumab PK analysis was precluded by a sparse sample collection schedule. Cmax and AUCinf for AZD4635 were 432.3 ng/ml and 3046 h∗ng/ml, respectively. Cmax and AUCinf for cabazitaxel (20 or 25 mg/m2) were 183.4 ng/ml and 583.0 h∗ng/ml, respectively. Cmax and AUCinf for SSP-005173 and SSP-005174 were lower compared with those of AZD4635 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103446).
Discussion
This open-label, phase II study evaluated the efficacy, safety, tolerability, and PK of AZD4635 in combination with durvalumab only (Arm A) and durvalumab and cabazitaxel (Arm B) in patients with mCRPC who were previously treated with anf NHA and taxane. A total of 30 patients received AZD4635 (2 patients in Arm A and 28 patients in Arm B). Enrollment in Arm A was stopped following a sponsor decision unrelated to safety. The very small number of patients in Arm A (n = 2) precluded meaningful interpretation of clinical results for that arm; both patients discontinued AZD4635 due to AE and disease progression. At the interim analysis for Arm B, 4 of 27 patients had a confirmed PSA50 response (14.8%); ‘no-go’ futility analysis criterion was met (<35% PSA50 responses) and enrollment in Arm B was stopped. At the final data cut-off date, treatment was still ongoing for one patient.
Limited efficacy was observed in Arm B. ORR was 10.0% (95% CI 1.23%-31.70%) and two patients had confirmed PR. The median rPFS in Arm B was 5.8 months (95% CI 4.2 months-NC). In this trial, a confirmed PSA50 response was observed in 17.9% of patients (5/28; 95% CI: 0.06-0.37) which was numerically less than the response rate of 35.7% observed in the CARD study.14 Although antagonism with the combination of AZD4635 and a checkpoint inhibitor is possible, this study had much fewer patients than the CARD trial, limiting comparison of efficacy outcomes. This is especially relevant given the wide 95% CI and the potential for patients with poorer prognosis who were enrolled in this study. However, ORR and PSA50 response rates for this triplet regimen were similar to those observed in the phase I study in the AZD4635 plus durvalumab group (16.2% ORR, 22.2% PSA50 response).25
Prostate cancer tumors are generally less responsive to immune checkpoint inhibitors due to a lack of T-cell infiltration in the TME.27,28 Suppression of adenosine within the TME can elicit antitumor immunity23 and, thus, blocking A2aR with AZD4635 may help restore the imbalance in the adenosinergic pathway in prostate cancer.25,26 However, despite the phase I results of AZD4635 in combination with durvalumab,25 the phase II study of AZD4635 in combination with durvalumab or the anti-CD73 antibody oleclumab has shown minimal clinical activity in heavily pretreated patients with mCRPC.29 Thus, available adenosine pathway inhibitors, including A2AR inhibitors, have demonstrated limited clinical activity.30,31 We hypothesized that the addition of a second-generation taxane (i.e. cabazitaxel) to the AZD4635 plus durvalumab combination may lead to increased antitumor activity in the post-docetaxel setting by decreasing the immunosuppressive environment and counteracting the negative effects of adenosine. Additionally, measurement of adenosine signature signaling score may assist with optimal patient selection for adenosine pathway modulators as there is a prognostic potential of the adenosine signature for immunotherapy.25,26 In this study, rPFS was numerically greater in patients with high adenosine signature compared with low adenosine signature (median, 5.8 versus 4.2 months, respectively). This was consistent with, although less evident than, the findings from the phase I study of AZD4635 in which patients with mCRPC with a high blood-adenosine signature score showed a numerically longer PFS (21 weeks) compared with the low blood adenosine signature score group (8.7 weeks).25 Notably, in this study, all patients had been treated with prior chemotherapy (i.e. docetaxel) unlike prior studies of AZD463525,29; this may have impacted the TME and may explain the smaller difference in rPFS observed between the two adenosine signature groups.
The baseline clinical characteristics and demographics of the patient population in this study were generally similar to an mCRPC patient population, although this study had a relatively lower percentage of patients with liver metastasis compared with other trials.14,17,32 The AEs from this study were consistent with those observed in previous studies,14,15 and the safety profile of durvalumab was as expected. Common AEs reported with A2AR inhibitors are nausea and fatigue25,29; however, AZD4635 in combination with durvalumab or cabazitaxel was generally well tolerated. There was one immune-related AE and no DLTs in this study. Two of the deaths in Arm B were due to AEs (colitis and myositis), with myositis considered possibly due to durvalumab, a known class of AEs observed with immune checkpoint inhibitors.33,34 The overall safety profile of AZD4635, durvalumab, and cabazitaxel therapy in combination was consistent with the known safety profiles of each individual agent. However, we observed limited efficacy, particularly considering the expected single-agent activity of cabazitaxel15,35; the efficacy of durvalumab in this study was generally consistent as observed with other PD(L)-1 inhibitors.17,36 PK parameters such as Cmax, t1/2λz, and AUClast for AZD4635 in Arm B were consistent with those of AZD4635 75 mg QD monotherapy.25 Cmax and AUC of cabazitaxel, and the associated variability, were consistent with previously published results.37,38 Nevertheless, the small sample size, a limitation of the current study, makes it difficult to interpret certain clinical outcomes. Overall, AZD4635 in combination with durvalumab or in combination with cabazitaxel and durvalumab showed limited efficacy in patients with mCRPC. Although both immune- and chemotherapy-related toxicities were observed, they did not appear to occur at an increased rate. The combined toxicity profile and lack of any evidence of increased efficacy above either single agent precluded further development.
The current study was conducted with cabazitaxel in patients who had already received docetaxel and an NHA to build on the success of the CARD study by concomitant inhibition of two distinct immunosuppressive pathways, PD-L1 and A2AR.14 However, limited response was observed with durvalumab alone or durvalumab plus cabazitaxel in the current study. It is possible that the TME in this later-line patient setting was not as supportive for an observable additive benefit of an immunotherapy owing to upregulation of CD73, lack of immune cells, and/or epigenetic mutations.39, 40, 41 Various combinations with AZD4635 may be investigated to try and overcome some of these potential factors. Strengths of this study include a multicenter design and a representative patient population of mCRPC who were pretreated. Limitations of this study include small sample size, the lack of preselected patients by adenosine signature, and the lack of formal characterization of the TME at baseline.
Future trials should focus on identifying the optimal patient population and tumor characteristics where A2AR inhibition may have a larger impact. Furthermore, understanding the full impact of adenosine targeting drugs, which have broad effects on the immune system, would be beneficial.
Conclusions
AZD4635 in combination with durvalumab or in combination with cabazitaxel and durvalumab showed limited efficacy in patients with mCRPC. Although the safety profile of both combinations was consistent with known safety data of the individual agents, the results of this study do not support further clinical development of the combinations.
Acknowledgements
The authors would like to thank all patients for their participation and the Principal Investigators as well as the staff at Parexel for their assistance.
Medical writing and editorial support, conducted in accordance with Good Publication Practice 2022 (GPP 2022) and the International Committee of Medical Journal Editors (ICMJE) guidelines, were provided by Oxford PharmaGenesis Inc., Newtown, PA, USA, and funded by AstraZeneca, Gaithersburg, MD, USA.
Funding
This study was supported by AstraZeneca (no grant number), Gaithersburg, MD, USA.
Disclosure
TAG: research funding, honoraria, and nonfinancial or other support from IPSEN, Adacap, Pfizer, Sanofi, EISAI, Lilly, Bayer, Janssen, BMS, Astellas, Novartis, Roche. MG: honoraria from Sanofi/Aventis and Alexion Pharmaceuticals for consulting or advisory role; travel, accommodations, expenses provided by AstraZeneca and Genentech; research funding paid to institution from Janssen, AstraZeneca, Genentech. CV: research funding paid to institution from Merck MSD; consulting fees from GSK, Astellas Pharma, Merck MSD, BMS, Leo-Pharma, Janssen, Cilag, Bayer, and AstraZeneca. GR: research funding paid to institution from Bayer; consulting fees from AAA, Astellas, Bayer, Sanofi, Janssen, AstraZeneca, and Pfizer. JZ: honoraria from AstraZeneca for advisory board and speaker bureau; advisory board for Bayer, Pfizer, and Dendreon; speaker bureau for Sanofi. MP: research funding paid to institution from Karyopharm; consulting fees from AstraZeneca, Exelixis, Oncocyte, Signatera, and Janssen. JMP: grants paid to institution from MSD, BMS, Janssen, Merk Serono, BeiGene; consulting fees from Novartis, Sanofi, Janssen, Astellas, BMS, MSD, and Roche. AA: employee of AstraZeneca and may own stock or stock options. GDJ: contractor for AstraZeneca. RC: employee of AstraZeneca and may own stock or stock options. ETG: employee of AstraZeneca and may own stock or stock options. JT: employee of AstraZeneca and may own stock or stock options. GP: employee of AstraZeneca and may own stock or stock options. RK: employee of AstraZeneca and may own stock or stock options. CS: research funding paid to institution by: Janssen, Astellas, Sanofi, Bayer, Sotio, and Dendreon; patents, consulting, or advisory role: Sanofi, Janssen, Astellas, Bayer, Genentech, Pfizer, Lilly; royalties and other intellectual property: Parthenolide (Indiana University); dimethylamino parthenolide (Leuchemix); Exelixis: abiraterone plus cabozantinib combination; FRAS1 SNP and tristetraprolin as biomarkers of lethal prostate cancer; stock or other ownership: Leuchemix.
Data Sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Supplementary data
References
- 1.Bray F, Laversanne M, Sung H, et al. CA Cancer J Clin. 2024:1-35.
- 2.Vellky J.E., Ricke W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia. 2020;22:566–575. doi: 10.1016/j.neo.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayegh N., Swami U., Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract. 2022;18:45–55. doi: 10.1200/OP.21.00206. [DOI] [PubMed] [Google Scholar]
- 4.Tannock I.F., de Wit R., Berry W.R., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Beer T.M., Armstrong A.J., Rathkopf D.E., et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K., Scher H.I., Molina A., et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 7.Miller K., Carles J., Gschwend J.E., et al. The phase 3 COU-AA-302 study of abiraterone acetate plus prednisone in men with chemotherapy-naive metastatic castration-resistant prostate cancer: stratified analysis based on pain, prostate-specific antigen, and Gleason score. Eur Urol. 2018;74:17–23. doi: 10.1016/j.eururo.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Scher H.I., Fizazi K., Saad F., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 9.Sartor O., de Bono J., Chi K.N., et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker C., Nilsson S., Heinrich D., et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.de Bono J., Mateo J., Fizazi K., et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M., Mateo J., Fizazi K., et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383:2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 13.Fizazi K., Piulats J.M., Reaume M.N., et al. Rucaparib or physician's choice in metastatic prostate cancer. N Engl J Med. 2023;388:719–732. doi: 10.1056/NEJMoa2214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wit R., de Bono J., Sternberg C.N., et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 15.de Bono J.S., Oudard S., Ozguroglu M., et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 16.Jevtana®Cabazitaxel [package insert]. Bridgewater, NJ: Sanofi-aventis; 2020.
- 17.Yu E.Y., Kolinsky M.P., Berry W.R., et al. Pembrolizumab plus docetaxel and prednisone in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort B study. Eur Urol. 2022;82:22–30. doi: 10.1016/j.eururo.2022.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Sambi M., Bagheri L., Szewczuk M.R. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 2019;2019 doi: 10.1155/2019/4508794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang A., Tong D. Immunotherapy in patients with metastatic castration-resistant prostate cancer: a meta-analysis of data from 7 phase III studies and 3 phase II studies. Exp Hematol Oncol. 2022;11:63. doi: 10.1186/s40164-022-00312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MERCK. Merck provides update on phase 3 KEYNOTE-921 trial evaluating Keytruda® (pembrolizumab) plus chemotherapy in patients with metastatic castration-resistant prostate cancer. 2022.
- 21.Ventola C.L. Cancer Immunotherapy, Part 3: challenges and Future Trends. P T. 2017;42:514–521. [PMC free article] [PubMed] [Google Scholar]
- 22.Sek K., Molck C., Stewart G.D., Kats L., Darcy P.K., Beavis P.A. Targeting adenosine receptor signaling in cancer immunotherapy. Int J Mol Sci. 2018;19:3837. doi: 10.3390/ijms19123837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borodovsky A., Barbon C.M., Wang Y., et al. Small molecule AZD4635 inhibitor of A2AR signaling rescues immune cell function including CD103(+) dendritic cells enhancing anti-tumor immunity. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Velasco M.A., Kura Y., Sako N., et al. Abstract 1071: Targeting A2aR in mouse Pten-deficient prostate cancer. Cancer Res. 2020;80:1071. [Google Scholar]
- 25.Lim E.A., Bendell J.C., Falchook G.S., et al. Phase Ia/b, Open-label, multicenter study of AZD4635 (an adenosine A2A receptor antagonist) as monotherapy or combined with durvalumab, in patients with solid tumors. Clin Cancer Res. 2022;28:4871–4884. doi: 10.1158/1078-0432.CCR-22-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidders B., Zhang P., Goodwin K., et al. Adenosine signaling is prognostic for cancer outcome and has predictive utility for immunotherapeutic response. Clin Cancer Res. 2020;26:2176–2187. doi: 10.1158/1078-0432.CCR-19-2183. [DOI] [PubMed] [Google Scholar]
- 27.Kwon J.T.W., Bryant R.J., Parkes E.E. The tumor microenvironment and immune responses in prostate cancer patients. Endocr Relat Cancer. 2021;28:T95–T107. doi: 10.1530/ERC-21-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subudhi S.K., Siddiqui B.A., Aparicio A.M., et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naive metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falchook G.S., Reeves J., Gandhi S., et al. A phase 2 study of AZD4635 in combination with durvalumab or oleclumab in patients with metastatic castration-resistant prostate cancer. Cancer Immunol Immunother. 2024;73:72. doi: 10.1007/s00262-024-03640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai Y., Zhang X., Zheng J., et al. Overcoming high level adenosine-mediated immunosuppression by DZD2269, a potent and selective A2aR antagonist. J Exp Clin Cancer Res. 2022;41:302. doi: 10.1186/s13046-022-02511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun C., Wang B., Hao S. Adenosine-A2A receptor pathway in cancer immunotherapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.837230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal N., McGregor B., Maughan B.L., et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021) Lancet Oncol. 2022;23:899–909. doi: 10.1016/S1470-2045(22)00278-9. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Casals M., Brahmer J.R., Callahan M.K., et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira A., Loquai C., Pfohler C., et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer. 2019;106:12–23. doi: 10.1016/j.ejca.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberger M., Hardy-Bessard A.C., Kim C.S., et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198–3206. doi: 10.1200/JCO.2016.72.1076. [DOI] [PubMed] [Google Scholar]
- 36.Antonarakis E.S., Piulats J.M., Gross-Goupil M., et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Center for drug evaluation and research. Pharmacology review(s): Jevtana®. 2010.
- 38.Dieras V., Lortholary A., Laurence V., et al. Cabazitaxel in patients with advanced solid tumours: results of a Phase I and pharmacokinetic study. Eur J Cancer. 2013;49:25–34. doi: 10.1016/j.ejca.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Leclerc B.G., Charlebois R., Chouinard G., et al. CD73 expression is an independent prognostic factor in prostate cancer. Clin Cancer Res. 2016;22:158–166. doi: 10.1158/1078-0432.CCR-15-1181. [DOI] [PubMed] [Google Scholar]
- 40.Vitkin N., Nersesian S., Siemens D.R., et al. The tumor immune contexture of prostate cancer. Front Immunol. 2019;10:603. doi: 10.3389/fimmu.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yegnasubramanian S., De Marzo A.M., Nelson W.G. Prostate cancer epigenetics: from basic mechanisms to clinical implications. Cold Spring Harb Perspect Med. 2019;9:a030445. doi: 10.1101/cshperspect.a030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.