Abstract
Drugs such as WIN51711 that inhibit picornavirus replication are thought to block poliovirus infectivity by binding to the capsid and preventing structural transitions required for uncoating. We examined the activity of WIN51711 at temperatures where capsid flexibility is thought to be decreased. Below 37°C, WIN51711 inhibits the binding of wild-type poliovirus to cells but does not affect the binding of a poliovirus mutant which is believed to undergo structural transitions more readily. These results suggest that the poliovirus capsid must undergo structural changes to bind to its cellular receptor.
To initiate the replication cycle, viruses must first attach to a cell surface receptor and deliver their nucleic acid to the correct cellular compartment. For some viruses the cell receptor is simply a hook which concentrates the virus on the cell surface, and uncoating is triggered by a low pH or the action of proteinases. In other cases the interaction of the virus with a cell receptor also initiates conformational changes that prime the capsid for uncoating. Poliovirus, a member of the family Picornaviridae, appears to fall into the latter category. This small, nonenveloped RNA virus initiates infection by binding to the poliovirus receptor (Pvr), a member of the immunoglobulin superfamily (13). After binding to Pvr on the cell surface, the virus undergoes a major structural change which results in the formation of altered particles. While the intact native virion sediments at 160S, the altered particle sediments at 135S and has lost the capsid protein VP4 and extruded the hydrophobic N terminus of VP1. The altered particle has been proposed as an obligate intermediate step in the viral entry pathway (5, 7). Less dramatic structural changes in the capsids of picornaviruses occur in the absence of receptors. Antibodies against internal epitopes of the poliovirus capsid proteins VP1 and VP4 can bind to the virus (11), and VP4 of rhinovirus is transiently exposed on the surface of the virus (10). The functional significance of these structural changes, called breathing, is unknown.
Breathing of the rhinovirus particle is blocked by WIN compounds (10). These antiviral drugs, originally developed by the Sterling-Winthrop Research Institute, bind in a hydrophobic pocket in the viral capsid, replacing a hydrocarbon molecule that normally occupies this site (1, 8, 9, 17). WIN compounds are thought to block poliovirus infectivity by preventing the transition to 135S particles (6, 18). It is believed that the release of lipid from the viral capsid during entry permits the structural changes required for uncoating of the genome (14). WIN compounds replace lipid in the hydrophobic pocket, preventing structural transitions which are required for uncoating.
Hypothesizing that breathing of the capsid might play an important role in viral entry, we examined the activity of the capsid-binding antiviral compound WIN51711 at temperatures where the transition to 135S particles does not occur at detectable levels. Here we demonstrate that, at temperatures below 37°C, the antiviral compound WIN51711 prevents wild-type (WT) virus from binding to Pvr. In contrast, this drug does not affect the binding of a poliovirus variant which is believed to undergo structural transitions more readily during entry. These findings suggest that the viral capsid must undergo structural changes to bind to the receptor.
Poliovirus strains were propagated in HeLa S3 cells as described previously (12). Antiviral compound WIN51711 was a gift from the Sterling-Winthrop Corporation (Rensselaer, N.Y.). Stocks were prepared as 10-mg/ml solutions in dimethyl sulfoxide (DMSO). 35S-methionine-labeled virus stocks were prepared as described previously (4). For equilibrium binding assays, aliquots of 35S-methionine-labeled WT P1/Mahoney virus stocks were preincubated for 0 or 1 h at 4, 25, or 37°C with 0 (0.05% DMSO), 0.5, or 5 μg of WIN51711 per ml. Virus was then added at a multiplicity of infection (MOI) of 10 to tubes containing 5 × 106 cells each in 350 μl of Joklik medium with the above-mentioned concentrations of WIN51711. Samples were agitated at 37 or 25°C for 1 h or at 4°C overnight, and samples were taken in triplicate from each tube to determine the total quantity of label present and quantity of label bound to cells. Results are expressed as the percentage of label bound, determined by dividing the number of counts in the bound fraction by the number of counts in the total fraction and multiplying by 100. Counts bound at different temperatures were comparable (data not shown). The effect of WIN51711 on binding of P1/Mahoney mutants Q3178R/I2231M and P1095S/V1160I was determined by performing saturation binding assays substantially as described above, but at a MOI of 5 and without preincubation of the virus with drug. To determine sensitivity of poliovirus to WIN51711, virus was preincubated at 25°C with or without 10 μg of WIN51711 per ml before infection of monolayers. Virus yields were determined, and the fold increase in titer was calculated (fold increase = titer at 8 h/titer at 0 h).
WIN51711 inhibits virus binding at low temperatures.
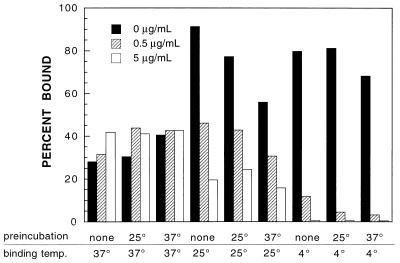
The results of previous studies demonstrate that WIN51711 prevents viral uncoating but not binding of poliovirus to its cellular receptor (6, 14, 15, 18). Equilibrium binding assays were done to determine the temperature dependence of WIN51711 activity. Poliovirus was preincubated with WIN51711 for 0 or 1 h at either 25 or 37°C, and binding assays were performed at 37, 25, or 4°C, using drug concentrations of 0 to 5 μg/ml (Fig. 1). At 37°C, virus binding to cells is unaffected by the presence of the drug, consistent with earlier results. At 25°C, the compound noticeably inhibits binding, and at 4°C, binding is virtually abolished at the highest drug concentration, regardless of whether or not a preincubation step is included. For subsequent experiments, equilibrium binding assays were performed at 25 or 4°C after a 1-h preincubation with the drug at 25°C.
FIG. 1.
WIN51711 inhibits poliovirus binding at low temperatures. 35S-methionine-labeled poliovirus (strain P1/Mahoney) was incubated for 0 or 1 h at different temperatures with WIN51711 at the indicated concentrations. Virus was added to HeLa cell suspensions (MOI = 10) in medium containing the same concentrations of WIN51711, the suspensions were agitated at the indicated temperatures overnight, and samples were taken in triplicate to determine the percentage of radioactive methionine bound to cells.
Capsid mutations can bypass WIN51711 binding inhibition.
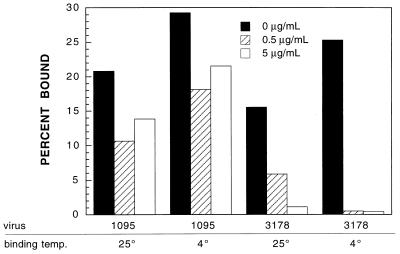
The inhibition of poliovirus binding by WIN51711 at low temperatures suggested that conformational changes in the capsid are important for receptor interaction. To test this hypothesis, the effect of WIN51711 on the binding of two variants of poliovirus which are believed to more readily undergo receptor-induced structural changes was examined. P1/Mahoney mutant Q3178R/I2231M was isolated in a screen for soluble receptor-resistant (srr) mutants and found to have an increased rate of alteration to the 135S A particle, suggesting that the mutant capsid is more flexible than that of the WT virus (2). Mutant P1095S/V1160I was selected for growth on cells expressing an altered poliovirus receptor that does not bind to the WT virus and was also believed to have greater capsid flexibility than the WT parent virus (3). Binding of P1095S/V1160I to cells was unaffected by WIN51711, while binding of Q3178R/I2231M was as sensitive to the drug as that of the WT virus (Fig. 2). The P1095S/V1160I mutations also conferred resistance to WIN51711 inhibition of infectivity (Table 1).
FIG. 2.
Poliovirus capsid mutations can bypass binding inhibition by WIN51711. 35S-methionine-labeled poliovirus mutants P1095S/V1160I (labeled 1095) and Q3178R/I2231M (labeled 3178) were added to cell suspensions (MOI = 5) in medium containing WIN51711 at the indicated concentrations, the suspensions were agitated at the indicated temperatures overnight, and samples were taken in triplicate to determine the percentage of radioactive methionine bound to cells.
TABLE 1.
WIN51711 sensitivity of WT and mutant polioviruses
| Virus | WIN51711b | Fold increase in virus titera |
|---|---|---|
| WT | − | 3,488 |
| + | 222 | |
| P1095S/V1160I | − | 1,600 |
| + | 3,000 | |
| Q3178R/I2231M | − | 611 |
| + | 142 |
Virus yield at 8 h postinfection divided by the virus yield at 0 h.
Virus was preincubated with (+) or without (−) WIN51711.
P1095S/V1160I binds WIN51711 and Pvr.
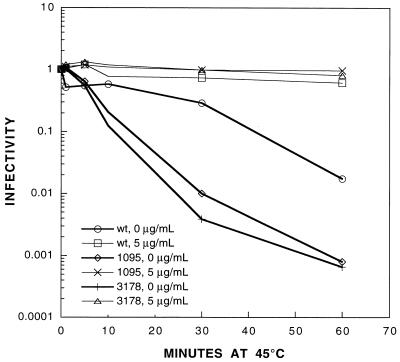
Because the loss of WIN51711 sensitivity in the equilibrium binding assay of P1095S/V1160I correlated with a loss of WIN51711 sensitivity in the virus yield experiment, the possibilities arose that the drug was unable to bind to the mutant capsid and that the mutant employed an alternative entry pathway independent of Pvr binding. To test the first possibility, we performed a thermolability assay, which takes advantage of the thermostabilizing effect of WIN51711 on the virus capsid (14, 16). P1/Mahoney WT or mutant virus was incubated at 25°C in phosphate-buffered saline (PBS) with 0.2% bovine calf serum with or without 5 μg of WIN51711 per ml for 1 h and then transferred to a 45°C water bath. Samples were taken at different times after the temperature shift, and the viral titer of each sample was determined by plaque assay. At 45°C, P1095S/V1160I is stabilized by WIN51711, indicating that the drug binds to the mutant capsid (Fig. 3). Both mutants are more thermolabile than the WT virus, consistent with the idea that they have increased capsid flexibility. Preincubation of cells with a monoclonal antibody against the poliovirus binding site on Pvr abolished binding of P1095S/V1160I, demonstrating that the mutant does not utilize an alternative receptor (data not shown).
FIG. 3.
Thermostabilization of WT and mutant poliovirus P1/Mahoney by WIN51711. WT or mutant P1/Mahoney virus was incubated at 25°C in PBS with 0.2% bovine calf serum with or without 5 μg of WIN51711 per ml for 1 h and then transferred to a 45°C water bath. Samples were taken in triplicate at 0, 1, 5, 10, 30, and 60 min after the temperature shift, and the viral titer of each sample was determined by plaque assay.
The results of structural and biochemical studies on poliovirus and rhinovirus have suggested that the viral capsid is dynamic and breathes in the absence of receptor, but their relevance to receptor binding and uncoating has not been determined (10, 11). Here, we report data which support a critical role for minor capsid transitions in receptor binding.
Initial characterization of the antiviral capsid-binding compound WIN51711 led to the conclusion that poliovirus uncoating, but not binding, is inhibited by this drug (6, 15, 18). Unfortunately, these assays were performed under conditions where saturation binding could not occur. Here, we report an analysis of saturation binding assays in which WIN51711 is found to inhibit virus binding at 25°C and abolish it at 4°C (Fig. 1).
The most likely explanation for these results is that the poliovirus capsid must undergo conformational changes in order to bind to Pvr. This hypothesis would be consistent with the finding that, in solution, the poliovirus particle is structurally dynamic (11) and would also explain the temperature dependence of the inhibition by WIN51711. At physiological temperatures, the reduction in capsid flexibility induced by the drug may be overcome by the energy available for structural transitions. At lower temperatures, where less energy is available, the effect of drug binding might become more pronounced, causing the virion to become frozen and unable to undergo changes required for receptor binding.
Our findings are consistent with observations on the reversible exposure of internal epitopes of poliovirus capsid proteins VP1 and VP4 (11). Such breathing of the capsid occurs at 37°C but not at 25°C. Furthermore, exposure of internal epitopes at 37°C is not blocked by WIN-like antiviral compounds (11). The capsid sequences that are reversibly exposed at 37°C are irreversibly exposed upon receptor binding (7). It is possible that reversible exposure of internal epitopes also produces conformational changes required for tight binding.
The hypothesis that poliovirus breathing is required for binding to Pvr was tested by examining the effects of WIN51711 on two poliovirus variants which are believed to possess a capsid that more readily undergoes structural changes. Virus mutant P1095S/V1160I was selected for growth on cells expressing a mutant poliovirus receptor that cannot bind the WT virus (3). This variant can bind to two different poliovirus receptors that have amino acid changes in well-separated regions of the molecule. Because of this property, it is believed that the mutations in this virus do not directly contact the receptor but modulate structural changes necessary for receptor binding. Binding of this mutant is not inhibited by WIN51711, even at temperatures as low as 4°C (Fig. 2). In addition, the replication of this mutant in cells is not inhibited by the drug. These data suggest that this mutant has considerably lower energy requirements for the structural changes that occur during breathing and uncoating.
The mutant Q3178R/I2231M, which was isolated in a screen for soluble receptor-resistant (srr) mutants (2), has an increased rate of alteration to the 135S A particle. The Q3178R mutation is located at the interface between promoters; it was hypothesized that it increases the flexibility of the interface, facilitating conversion to 135S particles. However, binding of this mutant to cells at low temperatures is inhibited by WIN51711 to the same degree as that of the WT virus. It is possible that the mutation selectively increases conversion to 135S particles, without affecting structural changes required for receptor binding.
We find that WIN compounds, which bind to the viral capsid and prevent structural changes required for uncoating, can block poliovirus attachment to cells at low temperatures. These findings indicate that the binding of poliovirus to its cellular receptor requires structural changes in the capsid.
Acknowledgments
This work was supported by a grant (AI20017) to V. Racaniello from the National Institutes of Health.
REFERENCES
- 1.Chapman M S, Minor I, Rossman M G, Diana G D, Andries K. Human rhinovirus 14 complexed with antiviral compound R 61837. J Mol Biol. 1991;217:455–463. doi: 10.1016/0022-2836(91)90749-v. [DOI] [PubMed] [Google Scholar]
- 2.Colston E, Racaniello V R. Soluble receptor-resistant poliovirus mutants identify surface and internal capsid residues that control interaction with the cell receptor. EMBO J. 1994;13:5855–5862. doi: 10.1002/j.1460-2075.1994.tb06930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colston E M, Racaniello V R. Poliovirus variants selected on mutant receptor-expressing cells identify capsid residues that expand receptor recognition. J Virol. 1995;69:4823–4829. doi: 10.1128/jvi.69.8.4823-4829.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dove A W, Racaniello V R. Cold-adapted poliovirus mutants bypass a postentry replication block. J Virol. 1997;71:4728–4735. doi: 10.1128/jvi.71.6.4728-4735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flore O, Fricks C E, Filman D J, Hogle J M. Conformation changes in poliovirus assembly and cell entry. Semin Virol. 1990;1:429–438. [Google Scholar]
- 6.Fox M P, Otto M J, McKinlay M A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986;30:110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricks C E, Hogle J M. The cell-induced conformational change of poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant R A, Hiremath C N, Filman D J, Syed R, Andries K, Hogle J M. Structures of poliovirus complexes with anti-viral drugs: implications for viral stability and drug design. Curr Biol. 1994;4:784–797. doi: 10.1016/s0960-9822(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim K H, Willingmann P, Gong Z X, Kremer M J, Chapman M S, Minor I, Oliveira M A, Rossmann M G, Andries K, Diana G D, Dutko F J, McKinlay M A, Pevear D C. A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A. J Mol Biol. 1993;230:206–227. doi: 10.1006/jmbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 10.Lewis J K, Bothner B, Smith T J, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proc Natl Acad Sci USA. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Yafal A G, Lee Y M-H, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao S, Racaniello V. Allele-specific adaptation of poliovirus VP1 B-C loop variants to mutant cell receptors. J Virol. 1997;71:9770–9777. doi: 10.1128/jvi.71.12.9770-9777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelsohn C, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 14.Mosser A G, Rueckert R R. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993;67:1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto M J, Fox M P, Fancher M J, Kuhrt M F, Diana G D, McKinlay M A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985;27:883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rombaut B, Andries K, Boeyé A. A comparison of WIN 51711 and R 78206 as stabilizers of poliovirus virions and procapsids. J Gen Virol. 1991;72:2153–2157. doi: 10.1099/0022-1317-72-9-2153. [DOI] [PubMed] [Google Scholar]
- 17.Smith T J, Kremer M J, Luo M, Vriend G, Arnold E, Kamer G, Rossmann M G, McKinlay M A, Diana G D, Otto M J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986;233:1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- 18.Zeichhardt H, Otto M J, McKinlay M A, Willingmann P, Habermehl K O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711) Virology. 1987;160:281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]