Liver transplantation (LT) was first performed over six decades ago and is currently the standard of care for patients with end-stage liver disease and life-threatening complications. There have been significant advances in the area of LT; however, it remains a highly morbid, restrictive, expensive and resource-intensive intervention (1). Early recovery for LT (ERAS4OLT.org) is a multimodal concept aimed at promoting faster recovery in patients undergoing LT. The development of a dedicated protocol for early recovery following LT has been challenging due to the complexity of developing a treatment approach, patient comorbidities associated with decompensated cirrhosis, and low case volume.
The recent titanic work by Pollok and colleagues (2) summarized the current knowledge on individual enhanced recovery by encompassing the pre-operative, intra-operative and post-operative aspects of LT (Figure 1). Using a modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, the authors provided 38 recommendations, specifically concerning several aspects of short-term complications, and peri-operative, intra-operative, and post-operative management of LT including the use of immunosuppressants, as summarized below.
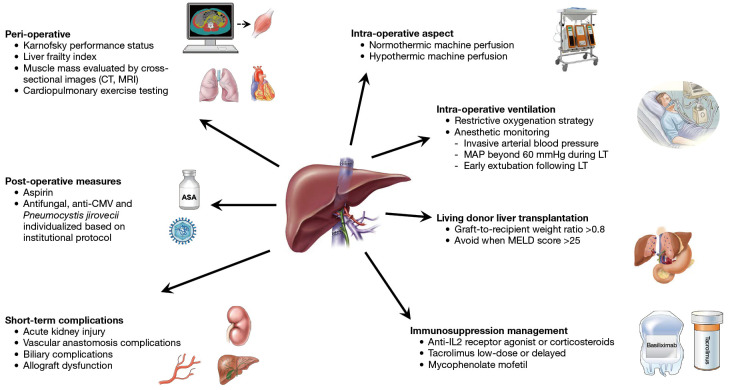
Figure 1.
The main aspects considered for early recovery for LT (ERAS4OLT.org), a multimodal concept aimed at promoting early recovery in patients undergoing LT. CT, computed tomography; MRI, magnetic resonance imaging; ASA, acetylsalicylic acid (aspirin); CMV, cytomegalovirus; MAP, mean arterial pressure; LT, liver transplantation; MELD, model for end-stage liver disease; IL2, interleukin 2.
Short-term complications are negatively associated with allograft and patient survival following LT. Clinicians should pay attention to preventing and timely managing acute kidney injury, biliary and vascular anastomosis complications, and primary allograft dysfunction.
From a peri-operative aspect, LT recipients suitable for ERASOLT.org can be identified using four assessment tools: Karnofsky performance status; Liver Frailty Index; muscle mass evaluation by cross-sectional images (computer tomography, magnetic resonance imaging); and cardiopulmonary exercise testing. These tools should be incorporated into routine pre-operative assessment as prognostic tools and candidacy suitability, in addition to enhanced recovery protocol following LT (3,4).
For example, sarcopenia or muscle wasting is common in candidates for LT and is associated with a high incidence of complications in both pre- and post-LT. The pathogenesis of sarcopenia in patients awaiting LT is multifactorial; nevertheless, chronic inflammation, insulin resistance, high levels of ammonia, increased muscle autophagy, lower levels of testosterone, growth hormones or branched-chain amino acids are considered to be the main culprit factors (5). Early and large-scale treatment interventions for sarcopenia in patients awaiting LT are needed to improve the prognosis of LT and the quality of life of patients. Daily energy intake of at least 35 kcal/kg body weight in non-obese patients and a protein intake of 1.2–1.5 g/kg, including small frequent meals and a late evening snack should be recommended in all patients awaiting LT (6).
In addition to adequate dietary intake, physical activity programs of moderate intensity that are tailored to the patient’s fitness should be recommended. Potential nutritional supplements have been evaluated in small clinical trials and pilot studies; however, large, randomized trials to support their efficacy in LT candidates are lacking (7).
Several unresolved issues are still present, including a lack of clear statements on the goals of the intervention, such as the reversal of established sarcopenia vs. maintaining or preventing further decline of muscle mass. There are also gaps regarding the specific target population (LT candidates regardless of the sarcopenic status vs. confirmed sarcopenic patients). Currently, it is not completely elucidated whether reversal or prevention of sarcopenia is feasible, and whether reversal is associated with improvement in important clinical outcomes among LT candidates, such as the risk of decompensation before LT, delisting related to deterioration of clinical status, risk of hospital admission, or mortality before and after LT.
Despite strong recommendations on pre-transplant nutritional supplementation and pre-operative psychological assessment, the evidence supporting these recommendations remained limited. On a positive note, increasing evidence has emerged in the pre-habilitation (8) since the recommendation of ERAS4LT.
From an intra-operative aspect, machine perfusion decreases reperfusion injury, thereby improving short-term outcomes such as lowering the risk of early allograft dysfunction; thus, it should be considered for marginal grafts (based on available resources). This recommendation was supported by a recent systematic review of seven randomized trials showing that both normothermic and hypothermic machine perfusion were associated with lower rates of early allograft dysfunction. In addition, both perfusion strategies were found to “likely” reduce overall biliary complications and non-anastomotic biliary strictures; however, data on long-term outcomes following LT remain limited (9).
For intra-operative ventilation, a restrictive oxygenation strategy with low tidal volume, tailored administration of positive end-expiratory pressure and recruitment manoeuvre are strongly recommended. Optimal anesthetic monitoring during LT should include invasive arterial blood pressure as well as the involvement of clinicians with expertise in pulmonary artery catheter or transoesophageal echocardiography. Routine use of venovenous bypass and temporary portocaval shunt is not recommended. In addition, standard use of surgical drains is not recommended due to the potential risk of biliary leakage and infection. The use of hydroxyethyl was not recommended given its association with a higher incidence of acute kidney injury in LT recipients. Meanwhile, the consensus recommends routine use of viscoelastic testing, moderately restrictive fluid regimen (especially during the dissection phase of LT) and maintaining mean arterial pressure beyond 60 mmHg during LT. Early extubation following LT was associated with improved short-term outcomes and therefore should be attempted in most patients.
As for post-operative measures, the use of aspirin is recommended to prevent hepatic artery thrombosis. Routine use of prophylactic thromboprophylaxis to prevent de-novo portal vein thrombosis is not recommended. However, the decision for prophylactic thromboprophylaxis to prevent deep vein thrombosis and pulmonary embolism should be considered. Antifungal prophylaxis is recommended for LT recipients with a high risk of developing invasive fungal infections. Antiviral prophylaxis for cytomegalovirus and Pneumocystis jirovecii should be individualized based on institutional protocols. Early abdominal drain removal and active inpatient rehabilitation are strongly recommended; however, evidence remained limited in these areas.
Regarding immunosuppression management, several strategies are aimed at preventing early rejection (10). Tacrolimus should be recommended as the standard immunosuppression after LT. A low-dose or delayed tacrolimus is recommended to reduce acute kidney injury following LT. The combination of tacrolimus with mycophenolate, anti-interleukin 2 (IL2) receptor agonist or corticosteroids induction are recommended as they do not significantly increase the risk of infections. With regards to living donor LT (LDLT), higher model for end-stage liver disease (MELD) score (>25) and renal dysfunction were associated with an increased risk of morbidity, mortality and hospitalization following LT. Recently treated infections in recipients are not contraindications for LT. The presence of muscle wasting or sarcopenia should be considered when assessing the candidacy of LDLT. A graft-to-recipient weight ratio >0.8 should be recommended. To reduce relative portal hypertension when transplanting smaller grafts in recipients, portal flow modulation should be considered to decrease the risk of post-LT complications such as acute kidney injury, sepsis, and overall mortality.
So far, the studies evaluating enhanced recovery after surgery (ERAS) implementation included relatively low-risk patients with MELD scores below 25 and those without a prior history of LT. In addition, a considerable proportion of LT were performed for hepatocellular carcinoma (HCC) within criteria, ranging from 33.3% to 90% (11-13). One study specifically excluded patients with hepatopulmonary syndrome and those requiring intubation prior to LT (14). While arguably the better outcomes in ERAS, may be partially attributed to patients with “favourable characteristics”, thus these insights remain important for patient selection so clinicians can individualize treatment approaches in LT recipients.
ERAS is a multimodal approach. Delineating whether adherence to specific elements independently correlates with outcome is a nuance, particularly given the inherent correlation between variables and challenges in controlling for confounders. Outcomes of complex surgery such as LT could be influenced by both donor and recipient factors (i.e., patient selection) beyond peri-operative care.
ERAS implementation should be viewed as the first step, with improving patient recovery as the primary endpoint. Moving forward, regular audit on adherence is a crucial step for continual improvement. Infrastructures such as electronic medical records could facilitate tracking key outcomes of ERAS4OLT implementation (i.e., length of stay, re-admissions to the hospital, quality of life, and survival). Nevertheless, one must understand that length of stay is only one of the surrogate markers of patients’ recovery. The goal will be to focus on enhancing the recovery for all patients receiving LT. More importantly, it is not about “how well we do”, but rather we should focus on how early and safely we could extubate, withdraw cardiorespiratory monitoring, or discharge our patients.
To conclude, ERAS4OLT.org provided an overview of current evidence in promoting better outcomes in LT. Given that LT outcomes are multifactorial, these recommendations should be individualized to each patient and the specific LT centre when developing and implementing ERAS4LT protocol.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-7/coif). The authors have no conflicts of interest to declare.
References
- 1.Lucey MR, Furuya KN, Foley DP. Liver Transplantation. N Engl J Med 2023;389:1888-900. 10.1056/NEJMra2200923 [DOI] [PubMed] [Google Scholar]
- 2.Pollok JM, Tinguely P, Berenguer M, et al. Enhanced recovery for liver transplantation: recommendations from the 2022 International Liver Transplantation Society consensus conference. Lancet Gastroenterol Hepatol 2023;8:81-94. 10.1016/S2468-1253(22)00268-0 [DOI] [PubMed] [Google Scholar]
- 3.Bhanji RA, Takahashi N, Moynagh MR, et al. The evolution and impact of sarcopenia pre- and post-liver transplantation. Aliment Pharmacol Ther 2019;49:807-13. 10.1111/apt.15161 [DOI] [PubMed] [Google Scholar]
- 4.Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122-39. 10.1002/lt.24958 [DOI] [PubMed] [Google Scholar]
- 5.Ebadi M, Burra P, Zanetto A, et al. Current treatment strategies and future possibilities for sarcopenia in cirrhosis. J Hepatol 2023;78:889-92. 10.1016/j.jhep.2023.01.031 [DOI] [PubMed] [Google Scholar]
- 6.Ebadi M, Bhanji RA, Mazurak VC, et al. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol 2019;54:845-59. 10.1007/s00535-019-01605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey EJ, Lai JC, Sonnenday C, et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019;70:1816-29. 10.1002/hep.30828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serper M, Jones LS, Clement T, et al. A randomized, controlled, prehabilitation intervention to maximize early recovery (PRIMER) in liver transplantation. Liver Transpl 2024;30:10-9. 10.1097/LVT.0000000000000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parente A, Tirotta F, Pini A, et al. Machine perfusion techniques for liver transplantation - A meta-analysis of the first seven randomized-controlled trials. J Hepatol 2023;79:1201-13. 10.1016/j.jhep.2023.05.027 [DOI] [PubMed] [Google Scholar]
- 10.Montano-Loza AJ, Rodríguez-Perálvarez ML, Pageaux GP, et al. Liver transplantation immunology: Immunosuppression, rejection, and immunomodulation. J Hepatol 2023;78:1199-215. 10.1016/j.jhep.2023.01.030 [DOI] [PubMed] [Google Scholar]
- 11.Rao JH, Zhang F, Lu H, et al. Effects of multimodal fast-track surgery on liver transplantation outcomes. Hepatobiliary Pancreat Dis Int 2017;16:364-9. 10.1016/S1499-3872(17)60020-1 [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Zhu M, Li Z, et al. Enhanced recovery after surgery protocols in patients undergoing liver transplantation: A retrospective comparative cohort study. Int J Surg 2020;78:108-12. 10.1016/j.ijsu.2020.03.081 [DOI] [PubMed] [Google Scholar]
- 13.Brustia R, Monsel A, Conti F, et al. Enhanced Recovery in Liver Transplantation: A Feasibility Study. World J Surg 2019;43:230-41. 10.1007/s00268-018-4747-y [DOI] [PubMed] [Google Scholar]
- 14.Findlay JY, Jankowski CJ, Vasdev GM, et al. Fast track anesthesia for liver transplantation reduces postoperative ventilation time but not intensive care unit stay. Liver Transpl 2002;8:670-5. 10.1053/jlts.2002.34678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as