Summary
Background
Reducing cigarette addictiveness has the potential to avert millions of yearly tobacco-related deaths worldwide. Substantially reducing nicotine in cigarettes decreases cigarette consumption, but no large clinical trial has determined the effects of reduced-nicotine cigarettes when other nicotine-containing products are available. The aim of this study was to examine the effects of reduced-nicotine cigarettes in the context of the availability of alternative nicotine delivery systems.
Methods
In a U.S. six-site, open-label, parallel-arm study, smokers were randomized for twelve weeks to an experimental marketplace containing cigarettes with either 0.4 mg or 15.8 mg nicotine per gram of tobacco; all had access to non-combusted alternative nicotine delivery systems (e.g., e-cigarettes; medicinal nicotine). Group differences in the primary outcomes (cigarettes per day, number of smoke-free days) were examined using linear and negative binomial regression, respectively (Trial Registration: NCT03272685).
Findings
Among 438 randomized participants (mean [standard deviation (SD), range] age, 44.5 [11.9, 20–73] years, 225 [51.4%] women, 282 [64.4%] White and 339 [77.4%] trial completers), those in the 0.4 mg vs. 15.8 mg nicotine cigarette condition experienced significantly lower cigarettes per day at the end of intervention (mean [SD], 7.05 [7.88] vs. 12.95 [9.07], adjusted mean difference, −6.21 [95% CI, −7.66 to −4.75], P < 0.0001) and greater smoke-free days during intervention (mean [SD], 18.59 [27.97] vs. 5.06 [13.77], adjusted rate ratio, 4.25 [95% CI, 2.58–6.98], P < 0.0001).
Interpretation
A reduced-nicotine cigarette standard in the context of access to other non-combusted nicotine products has the potential to benefit public health.
Funding
U.S. NIH/FDA U54DA03165.
Keywords: Reduced nicotine cigarettes, Tobacco, Tobacco policy, Tobacco control, Cigarettes, Electronic cigarettes, Nicotine delivery systems, Nicotine, Tobacco product regulations, Smoking cessation
Research in context.
Evidence before this study
For decades scientific evidence has demonstrated that nicotine is the addictive chemical in cigarettes and other tobacco products. As such, reducing nicotine in cigarettes to very low levels is likely to reduce their addictiveness and facilitate reduction in cigarette consumption among people who smoke. Systematic searches using PubMed and Scopus were conducted for reviews written on the effects of reduced nicotine content cigarettes on smoking-related behaviors. An additional search was conducted for studies that occurred after the most recent review was published. The results from the existing studies demonstrate that cigarettes that are reduced in nicotine content to 2.4 mg nicotine per g of tobacco compared to normal nicotine content (NNC) cigarettes which contain approximately 16 mg/g lead to significantly fewer cigarettes smoked per day, lower levels of exposure to carcinogens and other toxicants, and lower levels of cigarette dependence and satisfaction. Cigarettes that are reduced in nicotine content to 0.4 mg/g (aka very low nicotine content cigarettes, VLNC) are associated with significantly higher number of quit attempts, number of smoke-free days, and rates of point-prevalence abstinence compared to NNC cigarettes. These findings are pronounced when smokers are asked to switch to VLNC cigarettes immediately vs. a gradual step-down approach.
Added value of this study
The current tobacco landscape and retail markets have a diversity of tobacco and nicotine products. However, in prior studies participants were typically instructed to only use their assigned study cigarettes. No large clinical trial has examined the effects of VLNC vs. NNC cigarettes in the context of the availability of non-combusted alternative nicotine delivery systems (ANDS), reflecting a more real-world situation. The questions that need to be addressed include: 1) how are smoking-related outcomes (e.g., cigarette consumption) affected in the VLNC vs. NNC cigarette condition when non-combusted ANDS are available; 2) does the availability of only VLNC cigarettes increase the use of non-combusted ANDS compared to when NNC cigarettes are available; 3) what is the pattern of use of ANDS products in VLNC vs. NNC cigarette conditions; and 4) how do these patterns of use affect biomarkers of exposure to carcinogens and toxicants? This study demonstrates that VLNC cigarettes result in better smoking-related outcomes than NNC cigarettes, that the uptake of non-combusted ANDS is greater in the VLNC cigarette condition and with a higher rate of switching completely to ANDS, and that toxicant and carcinogen exposure is lower in the VLNC vs. NNC condition.
Implications of all the available evidence
The results from this and the previous studies support a regulatory policy that would require tobacco manufacturers to substantially reduce nicotine in cigarettes and most likely other combusted tobacco products with the goal of reducing their addictiveness. A policy that establishes a nicotine reduction standard for combusted products is likely to accelerate smoking cessation compared to the status quo of maintaining the availability of highly addictive and toxic combusted products. This study also suggests that the availability of regulated non-combusted ANDS may be important for those people who smoke and are not ready to completely quit the use of nicotine. The majority of people in the VLNC cigarette condition who achieved 7-day point prevalence abstinence used ANDS, predominantly e-cigarettes. Implementation of a nicotine reduction standard will need to entail a concerted effort to provide smoking cessation resources but also consideration of the role that ANDS can play to maximize benefits to public health.
Introduction
Reducing nicotine to minimally addictive levels in all cigarettes and other combusted products (e.g., little cigars) sold in the United States (U.S.) is predicted to avert an estimated 8.5 million tobacco-caused deaths by 2100.1 Reducing the addictiveness of cigarettes has the potential to prevent youth from becoming addicted and to facilitate cigarette abstinence among people who smoke.2 On June 21, 2022, the U.S. Food and Drug Administration (FDA) announced future plans to issue a Proposed Rule for a nicotine reduction standard for all cigarettes and most other combusted tobacco products sold in the U.S.3 In New Zealand, with a modeling study showing that “denicotization” of cigarettes can result in dramatic health benefits,4 the government passed legislation to reduce the addictiveness of cigarettes as part of the Smokefree Aotearoa 2025 plan,5 which was later repealed by the newly elected government.
These regulatory efforts are based on a large body of scientific evidence demonstrating that compared to smoking normal nicotine content (NNC) cigarettes, smoking cigarettes reduced in nicotine content by about 95% consistently leads to significantly fewer cigarettes smoked per day, lower exposure to cigarette-related toxicants, lower cigarette dependence, and higher rates of quit attempts and smoking cessation.6,7 Smoking reductions are observed regardless of age, race, sex/gender, socio-economic status, mental and physical health and extent of smoking.6, 7, 8, 9, 10, 11 One study also showed that immediate reduction of nicotine content to a minimally addictive level rather than gradually stepping down over time is associated with more rapid and greater reductions in smoking and exposure to cigarette-related toxicants while avoiding the risk of compensatory smoking at intermediate dose reductions.12 To date, limited studies have examined the effects of very low nicotine content (VLNC) cigarettes in the context of a marketplace with access to other nicotine containing products (e.g., e-cigarettes, medicinal nicotine), even though prior modeling of population impact included access to these products.1,4 To address this gap in the literature, people who smoke were randomized to use either VLNC or NNC cigarettes while also having access to non-combusted alternative nicotine delivery systems. These products were available in an experimental marketplace, wherein participants were provided points that could be exchanged for products during the study or cash at the end of the study. The overall goal was to examine the effects of VLNC vs. NNC on smoking behavior and toxicant exposure in a marketplace simulating the real-world environment. We hypothesized that a greater reduction in smoking behavior and toxicant exposure would be observed in the VLNC condition.
Methods
Study design
Randomized, parallel, open-label six-site trial in which people who smoked daily were recruited from the community and enrolled by research coordinators.
Study cigarettes and alternative nicotine delivery systems
Study cigarettes offered in the Experimental Marketplace were Spectrum brand, with participant choice of menthol or non-menthol, obtained from the National Institute on Drug Abuse.13 The median nicotine content, averaged across menthol and non-menthol cigarettes, was 0.4 mg nicotine/g tobacco for the VLNC cigarette and 15.8 mg nicotine/g tobacco for the NNC cigarette. Other constituent yields in these cigarettes have been described previously.14 Study cigarette packs had no nicotine dose information, but participants were informed about whether they were assigned VLNC or NNC cigarettes.
Non-combusted alternative nicotine delivery systems available in the Experimental Marketplace included e-cigarettes; oral tobacco products such as American brand moist snuff and lower tobacco specific nitrosamines snus, and nicotine pouches; and nicotine replacement therapies. The brands and flavors of these products were representative of those that had the greatest market share and/or based on a retailer survey at each study site (see Carroll et al.15). The non-combusted products in the Experimental Marketplace were updated on a yearly basis with some products added or withdrawn based on newer products that entered the marketplace or if banned by local governments or the U.S. FDA. E-cigarette systems included a cigarette-like cartridge, a pod-based system, pen-style with refillable e-liquids, and an all-in-one tank with refillable e-liquids. The flavors of e-cigarettes included tobacco, mint/menthol, fruit, and crème. For the cartridge and pod-based systems, fruit and crème flavors were eliminated during the study in response to FDA's ban of flavors other than tobacco or menthol/mint. Moist snuff and snus products were provided in both tobacco and mint flavors. Nicotine pouches were added in citrus, cinnamon, cool mint, and smooth flavors (3 and 6 mg nicotine), as they entered the marketplace. Nicotine replacement therapies included 7, 14, and 21 mg nicotine patches and 2 and 4 mg nicotine gum and lozenges provided in different flavors.
Experimental marketplace
The Experimental Marketplace was used to evaluate product choices in a systematic way while providing ecological validity. Participants were given points, worth one U.S. dollar each at every Experimental Marketplace visit. The number of points was based on their baseline daily cigarette and other nicotine product use, reflecting their typical product expenditures, plus one point per day to allow for experimentation in the Experimental Marketplace (Supplementary Material, page 60). Participants also received a single use coupon that could be applied to discount the purchase of an e-cigarette device or larger count box of nicotine replacement therapies, because of the relatively high cost of these products. Participants exchanged points for products in the Experimental Marketplace. Points for products varied by site to reflect regional differences in pricing and were updated yearly (Supplementary Material, pages 58–60). All products were discounted to 66% to discourage participants from purchasing products outside the Experimental Marketplace. Points not used at each visit could be banked and used at later visits or converted to cash (a maximum of U.S. $200) at the end of the study.
Marketplace protocol
The Experimental Marketplace was an on-line retail site, with points posted on each of the product brands/types.15 Participants were informed that they could navigate the site and exchange points for any amount or type of product up to the value of their current and banked points, also displayed on the site. Standard product information (e.g., dose, flavors) and video instruction on how to use the product were also available on this site for each product.
Participants
Participants were recruited predominantly through social media (Facebook, Instagram), internet, flyers, or other forms of advertisement at each of the six sites (see Fig. 1 for listing of sites). Participants were eligible if they were ≥ legal age of tobacco product purchase (which changed from 18 to 21 during the study at all the sites); smoked five to 40 cigarettes per day; and had an expired carbon monoxide level of ≥10 ppm or a urinary cotinine level indicative of nicotine use on a test strip. Participants were excluded if they exclusively used roll-your-own cigarettes or refused to smoke only a preferred manufactured brand of cigarettes during baseline measurements; had exposure to investigational cigarettes within the last two years; reported serious or unstable psychiatric disorder or medical disease; reported excessive alcohol drinking or problems with drinking or drug use; or were breastfeeding, pregnant or planning to become pregnant.
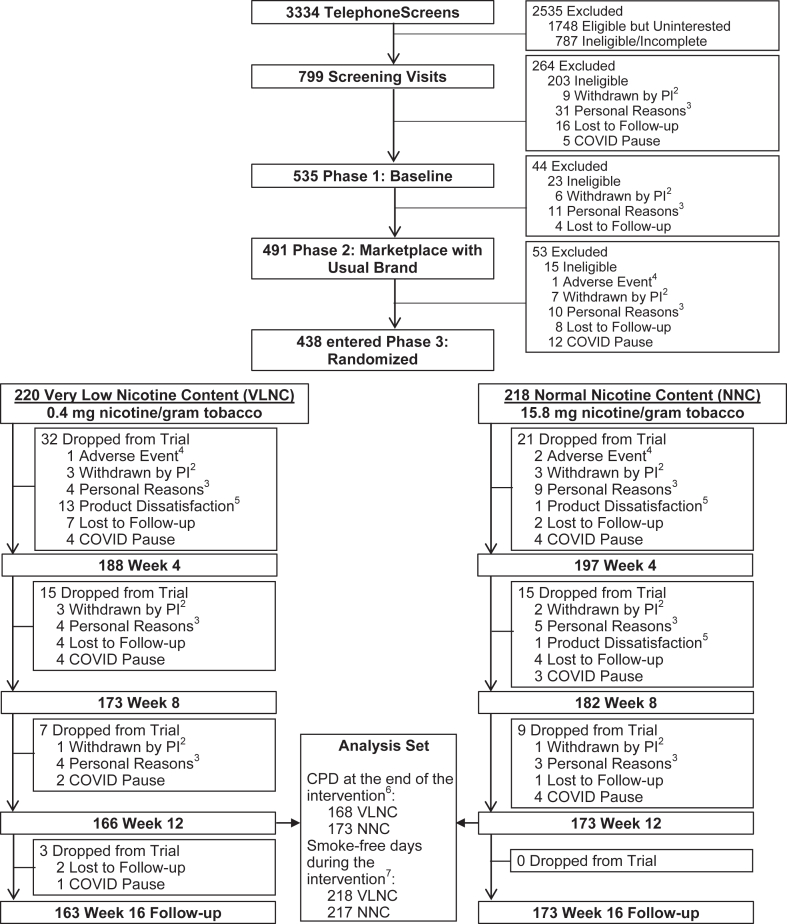
Fig. 1.
Consort Diagram1. Footnote: 1Study sites included University of Minnesota (lead; Institutional Review Board [IRB] number 00000937), University of California, San Francisco (IRB number 17–22748), Duke University (IRB number Pro00086465), Brown University, University of Pennsylvania, and Wake Forest University (IRB number 00061623, includes Brown University and University of Pennsylvania). 2Withdrawn by Principal Investigator (PI): Included unstable physical/mental health, pregnancy, low carbon monoxide, ineligible Cigarettes per Day (CPD), no internet access, non-adherence to protocol. 3Personal Reasons: Included life complications, study burden, moved out of area, lost interest, time constraints, unwilling to use non-menthol cigarettes at University of California, San Francisco site (menthol cigarettes banned in San Francisco), incarceration. 4Adverse Event: Reasons for self-withdrawal due to adverse events included difficulty tolerating withdrawal symptoms or a new illness or injury that required the individual's time and/or focus. 5Product dissatisfaction: Unwilling to smoke the study cigarette or use other Experimental Marketplace products. 6The different sample size in the CPD outcomes at the end of intervention (i.e., mean CPD over 7 days before Week 12) than the number of participants at Week 12 was due to the fact that some participants dropped out between visits but had useable Interactive Voice Response (IVR) data; all 438 participants were included in the analysis of CPD outcomes by using imputation methods. 7The sample size of smoke-free days during the intervention was based on the number of paticipants who responded to the IVR for ≥1 day during the intervention; 435 out of 438 participants were included in the analysis of the smoke-free days outcome; no imputation.
Randomization
Randomization, with a 1:1 ratio to either VLNC or NNC cigarettes, was stratified by site using the block randomization scheme with random block sizes of two, four, or six. Random numbers were computer generated using R16 by the study statistician. Participants were assigned either menthol or non-menthol study cigarettes based on their preference, except in San Francisco when a ban on menthol cigarettes was imposed.
Procedures
The protocol was divided into three phases: in Phase 1 (two weeks) participants used their usual brand cigarettes with no Experimental Marketplace; in Phase 2 (two weeks) the Experimental Marketplace contained usual brand cigarettes and non-combusted alternative nicotine delivery systems; in Phase 3 (twelve weeks) the Experimental Marketplace contained either NNC or VLNC cigarettes (randomization implemented by research coordinators) and non-combusted alternative nicotine delivery systems. Eligibility continued to be monitored after screening during Phases 1 and 2 and included following study procedures. During Phase 3, participants attended a weekly clinic visit for the first four weeks and then bi-weekly visits for the next eight weeks for a total of twelve weeks of intervention. A follow-up visit occurred at sixteen weeks post-randomization.
Due to the COVID-19 pandemic, the Week 3 visit during Phase 3 was eliminated and virtual and curbside visits were substituted for all in-person clinic visits. As a result of the virtual visits, participants were required to have internet accessible devices as an eligibility criterion. All assessments were conducted during these visits, including product accountability where opened and unopened products were shown to staff. At curbside visits, participants dropped off their biological samples and empty cigarette packs. Unopened or partially opened products could be retained by the participant and new products they chose from the Experimental Marketplace were provided.
During all phases, participants used an Interactive Voice Response system to complete daily questions on the number of study and non-study cigarettes smoked and other tobacco or nicotine products used on the previous day. At each clinic or virtual visit, tobacco use, other substance use, breath carbon monoxide, safety measures (e.g., vital signs, adverse events, changes in medical status and medication), psychological state (e.g., depressed mood), and subjective responses to cigarettes were reviewed or assessed. At all visits, staff conducted standardized and structured sessions in which they reviewed the importance of not smoking non-study cigarettes, problem solved any difficulties associated with study cigarette use, and supported quit smoking attempts if the participant expressed an interest in quitting. During Phases 1 and 2 and every four weeks during Phase 3, first void morning urine was collected for measurement of biomarkers of exposure. At the follow-up visit, adverse effects from use of study products were assessed.
Participants were compensated for session attendance, transportation costs, biological samples, returned unopened products that they chose not to use, completion of the Interactive Voice Response, and unspent points. In addition, a bonus payment was provided when participants reported refraining from non-study products. To increase accuracy of self-report, a bogus pipeline was used where participants were informed that compliance would be confirmed by random analysis of their biological samples. Payment was provided at the follow-up visit.
The protocol was reviewed by the U.S. FDA Center for Tobacco Products (to obtain authorization to use study cigarettes) and approved by site Institutional Review Boards (IRB, see Fig. 1 for IRB study numbers; Trial Registration: NCT03272685; See Supplementary Material for Protocol and Statistical Analysis Plan). Written informed consent was obtained from participants prior to initiation of the study.
Outcomes
Primary endpoints included mean number of cigarettes (study and non-study) smoked per day based on Interactive Voice Response data collected seven days before the Week 12 visit and number of smoke-free days during Phase 3 also based on Interactive Voice Response data. Secondary outcomes included mean study cigarettes per day based on Interactive Voice Response data collected seven days before the Week 12 visit, seven-day point-prevalence abstinence at the end of Week 12, and percent change in the mercapturic acid metabolite of the volatile organic compound acrylonitrile (CEMA), a relatively specific biomarker of tobacco smoke exposure,17 at Week 12 as compared to the last visit in Phase 2. Exploratory outcomes based on post-hoc analyses included number of non-study cigarettes smoked, the pattern of combusted product and alternative nicotine delivery systems use in Phase 3, and percent change in urinary biomarker levels including total nicotine equivalents, mercapturic acid metabolites of benzene (SPMA), acrolein (3-HPMA), propylene oxide (2-HPMA), and crotonaldehyde (HMPMA), and metabolites of a tobacco specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, NNK (total NNAL) during Phase 3 as compared to the last visit in Phase 2. Biomarker analyses were conducted as previously described for NNAL,18 3-HPMA,19 HMPMA,19 CEMA,19 2-HPMA,20 and SPMA.21
Dependence (Fagerström Test for Nicotine Dependence,22 Wisconsin Index of Smoking Dependence Motive-Primary Dependence Motives Scale23) was assessed in Phase 1, Phase 2, and Phase 3 at Weeks 4, 8 and 12. Withdrawal symptoms (Minnesota Nicotine Withdrawal Scale24) and smoking urges (Smoking Urges Questionnaire25) were assessed at every visit during each Phase.
Safety endpoints included Center for Epidemiologic Studies Depression scale,26 adverse events (assessed at each visit, rated for severity and relationship to any selected Experimental Marketplace product, and reviewed by the medical professional at each site), blood pressure, and heart rate.
Sample size and statistical analysis
The power analysis, based on a projected 20% attrition rate and nuisance parameters estimated at an interim analysis using non-comparative data, showed that 400 participants would ensure 88% power to detect an 8 cigarettes per day difference at Week 12 and > 99% power for an 11-day difference in smoke-free days during Phase 3 between the two groups, each at a 0.025 type-I error rate (based on Hatsukami et al.,12 see Supplementary Material for Statistical Analysis Plan). The retention rate at Week 12 was compared between the two groups using a Chi-square test (with Yates’ continuity correction, whenever appropriate, hereinafter). Participants who completed the Week 12 visit and those who dropped out were compared in terms of demographics and smoking history at baseline by t-test, Wilcoxon rank sum test, or Chi-square test.
The cigarettes per day variables at the Week 12 visit, the number of smoke-free days during Phase 3, and the carbon monoxide-verified (≤6 ppm) seven-day point-prevalence abstinence at Week 12 were analyzed with linear, negative binomial, and logistic regression, respectively, adjusting for their corresponding Phase 2 measurement whenever possible. Biomarkers (in both percent change relative to Phase 2 and raw values), dependence measures, and point-prevalence abstinence over time were analyzed post-hoc (except CEMA, a secondary outcome) with linear mixed model (after appropriate transformation of the dependent variable) or generalized linear mixed model with the effects of intervention condition, visit, and their interaction, adjusting for study site, whether the visit time was pre-COVID, and any baseline characteristics which were different at P < 0.20. Minimally adjusted mixed models (for only the corresponding baseline measure and the randomization stratification variable, study site) were performed as sensitivity analyses. The post-hoc analysis of pattern of product use for each two-week period (analyzed as a three-category variable: using combusted products only, using both combusted and non-combusted products, and non-combusted only or no use) compared the two conditions using Chi-square or Fisher's exact test, and its effect on key outcome variables was analyzed with linear regression. The counts of adverse events and any events (yes/no) were analyzed with negative binomial and logistic regression, respectively, adjusting for their corresponding Phase 2 measurement, whereas Centers for Epidemiological Studies Depression score was analyzed using the same method as for other repeatedly measured subjective outcomes.
Missing cigarettes per day data were imputed by the Markov Chain Monte Carlo multiple imputation method,27,28 accompanied by the baseline imputation and last observation carried forward imputation methods as sensitivity analyses. Missing abstinence values were imputed as not abstinence as a conservative estimate. Imputations were not performed elsewhere. In particular, no imputation was conducted for the Phase 3 smoke-free days outcome due to the extremely low non-response rate to Interactive Voice Response (3 out of 438 participants). All analyses were performed using the intention-to-treat principle using SAS version 9.4 (SAS Institute). All tests were 2-sided. P values less than 0.025 were considered significant for the two primary end points, and 0.05 for other end points. More details can be found in the Statistical Analysis Plan in the Supplementary Material.
Role of funding source
The funder had no role in the development of the study design, implementation, analyses, and manuscript submission.
Results
Enrollment: participant characteristics and dropouts
Participants were enrolled between June, 2018 and May, 2022, and follow up for the last participant was completed in September, 2022; 535 participants entered Phase 1 and 438 were randomized. Fig. 1 shows the consort diagram. There was no significant difference in the retention rate at the end of the intervention between the VLNC and NNC groups (166/220 [75.5%] vs. 173/218 [79.4%], respectively, P = 0.39). Table 1 shows demographic and smoking history variables by condition. Supplementary Table S1 compares completers vs. noncompleters. Completers were more highly educated (P = 0.019) and had lower cigarette dependence score on the Fagerstrom Test for Nicotine Dependence (P = 0.036) as assessed in Phase 1. Supplementary Table S2 compares Phase 1 vs. Phase 2 on tobacco use-related variables. Phase 2 was associated with greater use of alternative nicotine delivery systems (P < 0.0001) and lower breath carbon monoxide (P = 0.0013) levels.
Table 1.
Demographics and smoking history assessed at baseline.
| Characteristics | Overall (n = 438)a | VLNC cigarettes (n = 220)a | NNC cigarettes (n = 218)a |
|---|---|---|---|
| Age, mean (SD), y | 44.5 (11.9) | 44.0 (11.8) | 44.9 (12.0) |
| Median (IQR) | 44 (35–54) | 43 (34–54) | 44 (35–56) |
| Female, No. (%) | 225 (51.4%) | 123 (55.9%) | 102 (46.8%) |
| Race, No. (%) | |||
| White | 282 (64.4%) | 143 (65.0%) | 139 (63.8%) |
| Black | 99 (22.6%) | 45 (20.5%) | 54 (24.8%) |
| Otherb | 57 (13.0%) | 32 (14.5%) | 25 (11.5%) |
| Hispanic, No. (%) | 35 (8.0%) | 16 (7.3%) | 19 (8.7%) |
| Education, No. (%) | |||
| <High school | 22 (5.0%) | 10 (4.5%) | 12 (5.5%) |
| High school | 82 (18.7%) | 34 (15.5%) | 48 (22.0%) |
| >High school | 334 (76.3%) | 176 (80.0%) | 158 (72.5%) |
| Employment, No. (%) | |||
| Regular full-time work | 149 (34.0%) | 71 (32.3%) | 78 (35.8%) |
| Part-time work | 91 (20.8%) | 50 (22.7%) | 41 (18.8%) |
| Casual work (irregular or informal work) | 33 (7.5%) | 17 (7.7%) | 16 (7.3%) |
| Unemployed | 165 (37.7%) | 82 (37.3%) | 83 (38.1%) |
| Years of regular smoking, mean (SD) | 26.3 (12.3) | 25.8 (12.3) | 26.8 (12.3) |
| Median (IQR) | 25 (17–36) | 25 (17–35) | 26 (16–37) |
| Menthol cigarettes, No. (%) | 206 (47.0%) | 103 (46.8%) | 103 (47.2%) |
| cNRT use in the past 30 days, No. (%) | 13 (3.0%) | 6 (2.7%) | 7 (3.2%) |
| dUse any other tobacco product in the past 30 days, No. (%) | 154 (35.2%) | 75 (34.1%) | 79 (36.2%) |
| eUse any other combusted tobacco product in the past 30 days, No. (%) | 91 (20.8%) | 42 (19.1%) | 49 (22.5%) |
| fUse any non-combusted tobacco product in the past 30 days, No. (%) | 86 (19.6%) | 44 (20.0%) | 42 (19.3%) |
| Previous quit attempts for one day or longer, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Cigarettes per day in Phase 1, mean (SD) | 15.7 (6.9) | 15.7 (6.8) | 15.7 (7.1) |
| Median (IQR) | 15.0 (10.1–19.7) | 15.1 (10.0–20.0) | 14.5 (10.1–19.5) |
| Cigarettes per day in Phase 2, mean (SD) | 15.5 (7.3) | 15.6 (6.9) | 15.5 (7.6) |
| Median (IQR) | 14.6 (10.0–19.8) | 14.9 (10.3–19.9) | 14.2 (9.7–19.8) |
| Carbon monoxide in Phase 1, ppm, mean (SD) | 22.6 (11.2) | 22.2 (11.4) | 23.0 (11.0) |
| Median (IQR) | 20.5 (13.5–29.5) | 20.0 (13.1–29.0) | 21.5 (14.0–29.5) |
| Carbon monoxide in Phase 2, ppm, mean (SD) | 21.4 (11.1) | 21.0 (10.9) | 21.8 (11.4) |
| Median (IQR) | 18.5 (13.5–28.5) | 18.5 (13.0–27.5) | 19.5 (13.5–28.5) |
| Total nicotine equivalents in Phase 1, nmol/mg creatinine, mean (SD) | 75.2 (47.7) | 74.9 (40.6) | 75.5 (54.0) |
| Median (IQR) | 66.9 (45.2–95.0) | 67.5 (44.9–95.6) | 66.4 (45.4–94.6) |
| Total nicotine equivalents in Phase 2, nmol/mg creatinine, mean (SD) | 78.9 (108.3) | 84.9 (146.6) | 72.9 (43.4) |
| Median (IQR) | 65.3 (43.3–93.9) | 65.6 (43.2–96.7) | 64.3 (44.0–93.0) |
| gNicotine metabolite ratio in Phase 1, mean (SD) | 0.27 (0.19) | 0.29 (0.21) | 0.26 (0.16) |
| Median (IQR) | 0.23 (0.15–0.34) | 0.24 (0.15–0.36) | 0.23 (0.15–0.32) |
| hFTND in Phase 1, mean (SD) | 4.7 (2.0) | 4.6 (2.0) | 4.8 (2.0) |
| Median (IQR) | 5 (3–6) | 5 (3–6) | 5 (3–6) |
| hFTND in Phase 2, mean (SD) | 4.8 (2.1) | 4.9 (2.1) | 4.8 (2.1) |
| Median (IQR) | 5 (3–6) | 5 (3–6) | 5 (3–6) |
| iWISDM primary dependence motives subscale score in Phase 1, mean (SD) | 4.2 (1.3) | 4.2 (1.3) | 4.2 (1.4) |
| Median (IQR) | 4.2 (3.2–5.3) | 4.1 (3.1–5.2) | 4.2 (3.3–5.3) |
| iWISDM primary dependence motives subscale score in Phase 2, mean (SD) | 4.0 (1.4) | 3.9 (1.4) | 4.1 (1.4) |
| Median (IQR) | 4.0 (2.9–5.1) | 4.0 (2.8–5.1) | 4.1 (3.0–5.2) |
| Plan to quit smoking in the next month, No. (%) | 31 (7.1%) | 19 (8.7%) | 12 (5.6%) |
Acronyms: VLNC, very low nicotine content (0.4 mg nicotine/gram tobacco); NNC, normal nicotine content (15.8 mg nicotine/gram tobacco); SD, Standard Deviation; IQR, Interquartile Range.
Note: Gender was determined by self-reported response to the question, “What is your gender?” A higher percent of females was observed for the VLNC vs. NNC condition (n = 123 [55.9%] vs. n = 102 [46.8%]) as well as higher percent who had higher than high school level of education (n = 176 [80.0%] vs. n = 158 [72.5%]).
The sample size of the VLNC and NNC groups was 220 and 218, respectively, for all characteristics except for total nicotine equivalents in Phase 2 (219 and 217), NMR in Phase 1 (210 and 211), plan to quit smoking in the next month (219 and 216).
Other races include: American India/Alaska Native, Asian, Native Hawaiian, or Other Pacific Islander, and more than one race.
Nicotine replacement therapies (i.e., medicinal nicotine products) include patch, gum, and lozenge.
Other products include cigar, cigarillo, little cigar, pipe, bidis, hookah, marijuana blunts, or spliffs, chewing tobacco, moist snuff, snus, e-cig, dissolvable tobacco, and nicotine pouches.
Other combusted products include cigar, cigarillo, little cigar, pipe, bidis, hookah, and marijuana blunts or spliffs.
Noncombusted products include chewing tobacco, moist snuff, snus, e-cig, dissolvable tobacco, and nicotine pouches.
Nicotine metabolite ratio (free 3′-hydroxycotinine:free cotinine; NMR) reflects the rate of nicotine metabolism that was measured in saliva.
Fagerström Test for Nicotine Dependence; scale ranges from 0 to 10 with higher scores indicating greater nicotine dependence.
Wisconsin Index of Smoking Dependence Motives (WISDM) Primary Dependence Motives scale score ranges from 1 to 7, with higher scores indicating greater smoking dependence.
Primary outcomes: total cigarettes per day and smoke-free days
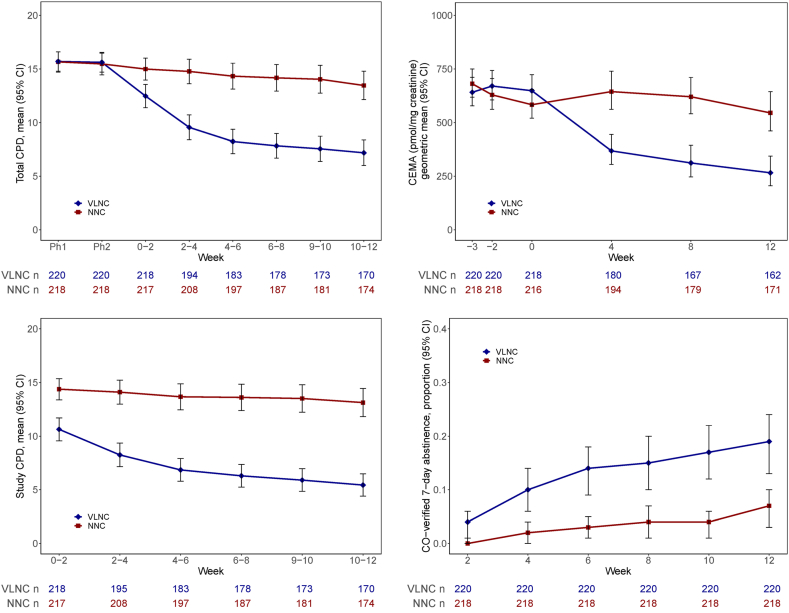
Significantly fewer total cigarettes per day were smoked in the VLNC vs. NNC condition at Week 12 (observed mean [SD], 7.05 [7.88] vs. 12.95 [9.07], adjusted Mean Difference [MD], −6.21 [95% CI, −7.66 to −4.75], P < 0.0001, Supplementary Table S3), with increasing magnitude of intervention effect over time (P < 0.0001, Fig. 2 and Supplementary Table S4). Significantly greater smoke-free days during Phase 3 were observed with VLNC vs. NNC cigarettes (observed mean [SD], 18.59 [27.97] vs. 5.06 [13.77], adjusted rate ratio [RR], 4.25 [95% CI, 2.58–6.98], P < 0.0001, Supplementary Table S5a).
Fig. 2.
Observed1Total (Study and Non-Study) Cigarettes per Day (CPD)2, Study CPD2, 2-cyanoethylmercapturic acid (CEMA, Biomarker for Acrylonitrile), and Breath Carbon Monoxide (CO) Verified 7-Day Point Prevalence Abstinence Over Time. Footnote: Acronyms: VLNC, very low nicotine content (0.4 mg nicotine/gram tobacco); NNC, normal nicotine content (15.8 mg nicotine/gram tobacco); CI, confidence interval. 1No imputation for missing values in CPDs or CEMA; non-abstinence being assumed for missing values for abstinence (due to missing Interactive Voice Response [IVR] or CO data). 2Mean total CPD and mean study CPD for Phase 1 (Ph1), Phase 2 (Ph2), Weeks 0–2, 2–4, 4–6, 6–8, 8–10, and 10–12 were based on all available IVR data within each time interval.
Secondary outcomes: study cigarettes, point prevalence abstinence and CEMA
Significantly fewer study cigarettes were smoked per day in the VLNC vs. NNC condition at Week 12 (observed mean [SD], 5.33 [6.86] vs. 12.42 [9.23], adjusted MD, −7.71 [95% CI, −9.23 to −6.20], P < 0.0001. Supplementary Table S3), with increasing magnitude of intervention effect over time (P < 0.0001, Fig. 2, Supplementary Table S4). A significantly higher rate of point prevalence carbon monoxide-verified abstinence was observed at the end of 12 weeks in the VLNC vs. NNC condition (41/220 [18.6%] vs. 15/218 [6.9%], estimated odds ratio [OR] of 3.10 [95% CI, 1.69–5.96], P = 0.0004, Supplementary Table S5a); both conditions showed an increase in carbon monoxide verified cessation rates over time with no significant condition by time interaction effect (P = 0.92, Supplementary Table S5b and Fig. 2). Congruent with lower cigarettes per day with VLNC cigarettes, there was greater percent reduction of CEMA at Week 12 compared to Phase 2 (median and interquartile range [IQR] of percent change, −34% [−82% to 21%] vs. 8% [−38% to 61%], adjusted MD of percent change in a shifted-log scale [MDL], −0.75 [95% CI, −0.99 to −0.52], P < 0.0001; Table 2 and Supplementary Table S6a and b for sensitivity analysis and Supplementary Table S7 for study cigarette compliant participants).
Table 2.
Percent change from phase 2 to week 12 in biomarkers.
| Biomarker | Observed |
Model-Based |
||||
|---|---|---|---|---|---|---|
| VLNC cigarettes |
NNC cigarettes |
Estimated Mean Differencea (95% CI) | P-value | |||
| N | Median (IQR) | N | Median (IQR) | |||
| CO | 163 | −0.36 (−0.79, −0.05) | 172 | −0.17 (−0.47, 0.14) | −0.14 (−0.27, 0.00) | 0.043 |
| TNE/creatinine | 162 | −0.21 (−0.83, 0.35) | 171 | −0.07 (−0.32, 0.18) | −0.74 (−1.07, −0.40) | <0.0001 |
| Total NNAL/creatinine | 162 | −0.51 (−0.85, −0.10) | 171 | −0.05 (−0.31, 0.38) | −0.93 (−1.12, −0.74) | <0.0001 |
| CEMA/creatinine | 161 | −0.34 (−0.82, 0.21) | 170 | 0.08 (−0.38, 0.61) | −0.75 (−0.99, −0.52) | <0.0001 |
| HMPMA/creatinine | 160 | −0.28 (−0.65, 0.43) | 170 | −0.01 (−0.32, 0.75) | −0.39 (−0.57, −0.21) | <0.0001 |
| 2-HPMA/creatinine | 159 | −0.16 (−0.57, 0.38) | 170 | −0.02 (−0.43, 0.65) | −0.17 (−0.36, 0.02) | 0.087 |
| 3-HPMA/creatinine | 159 | −0.10 (−0.56, 0.55) | 170 | −0.07 (−0.45, 0.53) | −0.05 (−0.26, 0.16) | 0.65 |
| SPMA/creatinine | 162 | −0.37 (−0.74, 0.28) | 170 | −0.01 (−0.37, 0.44) | −0.52 (−0.76, −0.28) | <0.0001 |
Acronyms: VLNC, very low nicotine content (0.4 mg nicotine/gram tobacco); NNC, normal nicotine content (15.8 mg nicotine/gram tobacco); IQR, Interquartile Range; CO, carbon monoxide; TNE, total nicotine equivalents; total NNAL: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides, biomarker for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; CEMA:2-cyanoethylmercapturic acid, biomarker for acrylonitrile; HMPMA: 3-hydroxy-1-methylpropylmercapturic acid, biomarker for crotonaldehyde/methylvinyl ketone; 2-HPMA: 2-hydroxypropylmercapturic acid, biomarker for propylene oxide; 3-HPMA: 3-hydroxypropylmercapturic acid, biomarker for acrolein; SPMA: S-phenylmercapturic acid, biomarkers for benzene.
Week 12 data was extracted from Supplementary Table S6a that included biomarker values across weeks. Estimated mean difference of VLNC vs. NNC for percent change (defined as [Week 12—Phase 2]/Phase 2; for example, −0.36 means a 36% decrease) in carbon monoxide (CO) or for the shifted log-transformed percent change (defined as log [percent change + 1]) in other biomarkers, based on linear mixed model for repeated measures with fixed effects of condition, time, and their interaction, adjusting for study site, whether the visit time is pre-COVID or not, and any baseline characteristics which are different at P < 0.20 (gender, education, nicotine metabolite ratio, Wisconsin Index of Smoking Dependence Motives Primary Dependence Motives, and longest time of quit); no imputation.
Exploratory outcomes from post-hoc analyses: non-study cigarettes, uptake of alternative nicotine delivery systems, other biomarkers, and subjective responses
A slightly higher number of non-study cigarettes were smoked in the VLNC vs. NNC condition at Week 12 (observed mean [SD], 1.72 [4.67] vs. 0.53 [2.05], adjusted MD, 1.53 [95% CI, 0.68–2.37], P = 0.0005, Supplementary Table S3), with no difference in magnitude of effect over time (P = 0.18, Supplementary Table S4).
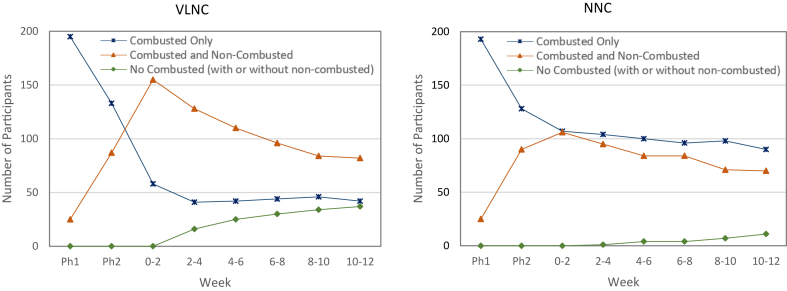
Fig. 3 shows the different patterns of exclusive combusted tobacco, dual use of combusted plus non-combusted tobacco, and non-use of combusted tobacco (with or without use of non-combusted tobacco; verified by carbon monoxide ≤6 ppm) across the VLNC and NNC conditions. Significant differences were observed in each of the two-week periods over the course of Phase 3. For example, lower combusted tobacco use and higher dual use was observed in the VLNC vs. NNC condition in the initial weeks (Weeks 4–6: 77 [35.5%] and 115 [53.0%] in VLNC, respectively, vs. 126 [58.1%] and 87 [40.1%] in NNC, respectively, P < 0.0001 across product use groups) with non-use of combusted tobacco increasing in the VLNC cigarette condition and dual use attaining similar levels as the NNC condition in later weeks (Weeks 10–12: 37 [17.2%] and 87 [40.5%] in VLNC, respectively vs. 11 [5.1%] and 75 [34.7%] in NNC, respectively, P < 0.0001 across product use groups, Supplementary Table S8). Supplementary Table S9 shows the types of non-combusted products that were used among smoke-free participants (e.g., predominantly e-cigarettes among those in the VLNC condition: 23/37 [62.2%]) and Supplementary Tables S10a and b shows effects of patterns of use on key outcome variables.
Fig. 3.
Patterns of Tobacco/Nicotine Product Use (Combusted Tobacco Only, Combusted Tobacco and Non-Combusted Products, No Combusted Tobacco1–With or Without Non-Combusted Products). Footnote: Acronyms: VLNC, very low nicotine content (0.4 mg nicotine/gram tobacco); NNC, normal nicotine content (15.8 mg nicotine/gram tobacco); Ph1, Phase 1; Ph2, Phase 2. 1Verified by carbon monoxide ≤6 ppm.
Like the biomarker CEMA, there were significantly greater (all Ps < 0.0001) percent reductions in the VLNC vs. NNC condition at Week 12 compared to Phase 2 for total nicotine equivalents, total NNAL, HMPMA, and SPMA and for carbon monoxide values (P = 0.043). No significant differences were observed for 2-HPMA (P = 0.087) or 3-HPMA (P = 0.65; see Table 2 for Week 12 biomarkers, Supplementary Table S6a for biomarkers over time, S6b for sensitivity analysis, and S7 for participants who self-reported only using study cigarettes).
For measures of cigarette dependence at Week 12 (see Supplementary Table S11), significantly lower scores were observed for VLNC vs. NNC cigarettes on Fagerstrom Test for Nicotine Dependence (mean [SD], 3.72 [2.09] vs. 4.36 [2.22], MD, −0.63 [95% CI, −1.00 to −0.25], P = 0.0010) and the Wisconsin Index of Smoking Dependence Motives-Primary Dependence Motives Scale (mean [SD], 2.65 [1.63] vs. 3.55 [1.70], MD, −0.75 [95% CI, −0.97 to −0.52], P < 0.0001), with significant interaction effects (Ps < 0.0001) showing a greater reduction of scores over time observed in the VLNC vs. NNC condition (see Supplementary Figure S1). Significant increases were observed for cigarette withdrawal symptoms among those assigned to the VLNC vs. NNC cigarettes, but only at Week 1 (mean [SD], 8.44 [6.86] vs. 5.89 [4.15], MD, 2.55 [95% CI, 1.56–3.55], P < 0.0001). By Week 2, no significant differences were observed (mean [SD], 7.21 [5.97] vs. 6.22 [4.78], MD, 0.79 [95% CI, −0.22 to 1.80], P = 0.13). For Questionnaire on Smoking Urges Factor 1, significantly lower scores were observed for VLNC vs. NNC at Week 12 (mean [SD], 2.55 [1.81] vs. 3.24 [1.82], MD, −0.50 [95% CI, −0.86 to −0.15], P = 0.0050). No significant differences were observed for Questionnaire on Smoking Urges Factor 2 at Week 12 (mean [SD], 1.71 [1.16] vs. 2.00 [1.34], MD, −0.16 [95% CI, −0.41 to 0.10], P = 0.23).
Adverse events
Table 3 shows number of people who experienced adverse events (Definitely Related/Possibly Related/Relationship Unknown) by VLNC vs. NNC condition over time and Table 4 shows the adverse events that were experienced by two percent or greater of participants in either of the two conditions. Supplementary Table S12 shows the statistical analyses of the adverse events. Significant higher total adverse event counts as assessed by density of prevalence were observed for VLNC vs. NNC condition during Phase 3 (Density, 0.066 vs. 0.029, adjusted RR, 2.62 [95% CI, 1.65–4.17], P < 0.0001), primarily driven by adverse event counts during Week 1 (Density, 0.355 vs. 0.060, adjusted RR, 5.89 [95% CI, 3.06–11.34], P < 0.0001), likely resulting from withdrawal-like symptoms (See Table 3 footnote). Furthermore, significantly more people experienced any adverse event in the VLNC vs. NNC condition (78 [36.3%] vs. 38 [17.9%], adjusted OR, 2.81 [95% CI, 1.77–4.46], P < 0.0001). Depressive symptoms were not significantly different in participants switching to VLNC vs. NNC cigarettes (Week 12 mean [SD] Centers for Epidemiological Studies Depression, 7.90 [7.60] vs. 6.69 [6.82], MD, 1.37 [95% CI, −0.19 to 2.93], P = 0.086). Supplementary Table S13 shows all the adverse events by group. Two remotely (unlikely) serious adverse events were observed in the VLNC condition (Supplementary Table S13).
Table 3.
Relateda Adverse Events (AEs) Across Visits by Very Low Nicotine Content (VLNC) vs. Normal Nicotine Content (NNC) Condition and by Study Product Within Each Condition.
| Visits | All Relateda Adverse Events |
Related to Study Cigarettesb |
Related to ANDSb |
|||||
|---|---|---|---|---|---|---|---|---|
| VLNC |
NNC |
VLNC |
NNC |
VLNC |
NNC |
|||
| Nc | n (%)d | Nc | n (%)d | n (%)d | n (%)d | n (%)d | n (%)d | |
| Phase 2 (Week −1) | 220 | 5 (2.3%) | 218 | 5 (2.3%) | 0 (0.0%) | 0 (0.0%) | 4 (1.8%) | 4 (1.8%) |
| Phase 2 (Week 0) | 220 | 4 (1.8%) | 218 | 6 (2.8%) | 0 (0.0%) | 0 (0.0%) | 4 (1.8%) | 5 (2.3%) |
| Week 1e | 215 | 51 (23.7%) | 212 | 10 (4.7%) | 46 (21.4%) | 10 (4.7%) | 4 (1.9%) | 1 (0.5%) |
| Week 2 | 202 | 14 (6.9%) | 208 | 4 (1.9%) | 8 (4.0%) | 2 (1.0%) | 6 (3.0%) | 2 (1.0%) |
| Weeks 3–4f | 189 | 16 (8.5%) | 200 | 16 (8.0%) | 12 (6.3%) | 12 (6.0%) | 7 (3.7%) | 4 (2.0%) |
| Weeks 6 | 179 | 11 (6.1%) | 188 | 3 (1.6%) | 5 (2.8%) | 1 (0.5%) | 4 (2.2%) | 1 (0.5%) |
| Weeks 8 | 173 | 4 (2.3%) | 182 | 4 (2.2%) | 2 (1.2%) | 2 (1.1%) | 2 (1.2%) | 2 (1.1%) |
| Weeks 10 | 168 | 4 (2.4%) | 175 | 9 (5.1%) | 3 (1.8%) | 4 (2.3%) | 2 (1.2%) | 4 (2.3%) |
| Weeks 12 | 166 | 1 (0.6%) | 173 | 2 (1.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) | 1 (0.6%) |
| Follow-up Week 16 | 163 | 1 (0.6%) | 173 | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) |
Acronyms: ANDS, Alternative Nicotine Delivery Systems.
AE deemed Definitely Related, Possibly Related, or Relationship Unknown to study product as assessed by the site's licensed medical professional. All related AEs also include AEs attributed to study procedures.
Some AEs were deemed related to both study cigarettes and ANDS if products were used concurrently (during Phase 3). ANDS included e-cigarettes/vaping devices, nicotine replacement therapies (gum, lozenge, or patches), moist snuff, snus, or nicotine pouches.
Number of participants active on protocol at the clinic visit. If an active participant missed a visit, adverse events would have been attributed to the visit window closest to the start of the adverse event.
n = Number of participants with adverse event(s) reported since the previous visit; % = (n/N) × 100%.
Participants assigned to the VLNC cigarettes had an excess of AEs attributed to study cigarettes consistent with withdrawal symptoms. The eight most common AEs related to study cigarettes were (VLNC/NNC): Irritability (frustration) (13/2), Headache (incl. migraine) (8/3), Anxious (nervous) mood (8/0), Sore/itchy/irritated throat (3/4), Cough (5/1), Depressed (sad) mood (4/1), Insomnia (4/0) and Shortness of breath (3/1).
AEs reported at either Week 3 and/or Week 4 were combined as Week 3 was discontinued after COVID pause to reduce participant contact.
Table 4.
Relateda Adverse Events (AEs) Reported at ≥2%; AEs by Very Low Nicotine Content (VLNC) vs. Normal Nicotine Content (NNC) Condition and by Study Product Within Each Condition During Phase 3.
| All Related AEsa |
Related: Study Cigarettesb |
Related: ANDSb |
|||||
|---|---|---|---|---|---|---|---|
| VLNC (N = 220) |
NNC (N = 218) |
% Differenced (95% CI) |
VLNC |
NNC |
VLNC |
NNC |
|
| n (%)c | n (%)c | n (%)c | n (%)c | n (%)c | n (%)c | ||
| Gastrointestinal disorders | |||||||
| Vomiting | 5 (2.27%) | 2 (0.92%) | 1.35 (−1.00, 3.70) | 1 (0.45%) | 2 (0.92%) | 3 (1.36%) | 1 (0.46%) |
| Stomach upset/pain | 9 (4.09%) | 5 (2.29%) | 1.80 (−1.49, 5.09) | 5 (2.27%) | 2 (0.92%) | 5 (2.27%) | 2 (0.92%) |
| Nervous system disorders | |||||||
| Headache (incl. migraine) | 15 (6.82%) | 3 (1.38%) | 5.44 (1.75, 9.13) | 10 (4.55%) | 2 (0.92%) | 4 (1.82%) | 1 (0.46%) |
| Psychiatric disorders | |||||||
| Anger | 7 (3.18%) | 0 (0%) | 3.18 (0.84, 5.52) | 7 (3.18%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anxious (nervous) mood | 10 (4.55%) | 5 (2.29%) | 2.26 (−1.14, 5.66) | 10 (4.55%) | 3 (1.38%) | 0 (0%) | 1 (0.46%) |
| Insomnia (change/disturbance in sleep) | 6 (2.73%) | 1 (0.46%) | 2.27 (−0.08, 4.62) | 5 (2.27%) | 0 (0%) | 1 (0.45%) | 0 (0%) |
| Irritability (frustration) | 16 (7.27%) | 8 (3.67%) | 3.60 (−0.65, 7.85) | 16 (7.27%) | 6 (2.75%) | 0 (0%) | 1 (0.46%) |
| Respiratory, Thoracic, Mediastinal disorders | |||||||
| Cough | 9 (4.09%) | 2 (0.92%) | 3.17 (0.25, 6.09) | 6 (2.73%) | 1 (0.46%) | 2 (0.91%) | 1 (0.46%) |
| Phlegm increase | 5 (2.27%) | 2 (0.92%) | 1.35 (−1.00, 3.70) | 3 (1.36%) | 2 (0.92%) | 2 (0.91%) | 0 (0%) |
| Sore throat (incl. Itchy/irritated throat) | 7 (3.18%) | 4 (1.83%) | 1.35 (−1.58, 4.28) | 5 (2.27%) | 3 (1.38%) | 2 (0.91%) | 1 (0.46%) |
Acronyms: ANDS, Alternative Nicotine Delivery Systems; CI, Confidence Interval.
AE deemed Definitely Related, Possibly Related, or Relationship Unknown to study product as assessed by the site's licensed medical professional. All related AEs also include AEs attributed to study procedures (nine participants and one participant's AE attributed to both study procedure and study cigarette).
Some AEs were deemed related to both study cigarettes and ANDS if products were used concurrently. ANDS included e-cigarettes/vaping devices, nicotine replacement therapies (gum, lozenge, or patches), moist snuff, snus, or nicotine pouches.
n = Number of participants with adverse event(s) reported; % = (n/N) × 100%.
The percent of participants reporting a specific adverse event in the VLNC condition minus that in the NNC condition. Differences are bolded if confidence interval does not include zero.
Discussion
The results of this study demonstrated that substantially reducing the nicotine content in cigarettes (in this case about a 98% reduction compared to cigarettes that are commercially available) decreased smoking behavior and toxicant exposures and increased biochemically verified smoking abstinence. These findings are concordant with findings from prior studies.6,7 This reduction in cigarette smoking has been observed even in individuals who experience the greatest health inequities10 and among youth.9 Therefore, reducing nicotine in cigarettes has the potential to result in substantial public health benefit for all populations who smoke.
This study was unique in that it was the first large, randomized control trial to examine the potential effects of a cigarette nicotine reduction standard in the context of availability of a variety of non-combusted nicotine products. The post-hoc analyses of exploratory outcomes suggest that having only VLNC cigarettes in the marketplace might accelerate switching to non-combusted nicotine delivery systems and increase the likelihood of only using these alternative products compared to the current marketplace. This observation was made in a population that was predominantly not interested in quitting smoking in the short term. Furthermore, in an additional post-hoc analysis, we observed that planning to quit smoking in the next month reported at baseline was not a significant predictor of smoking abstinence at 12 weeks (OR = 1.53, 95% CI, 0.54–3.77, P = 0.38). These suggestive findings support the U.S. FDA's comprehensive nicotine strategy described in 2017 in which the goal was to eliminate the use of combusted tobacco and for those people who smoke who need or want nicotine, to make available less harmful non-combusted alternative nicotine delivery systems.29 Thus, these results also suggest how the marketplace might shift if a nicotine reduction policy is implemented.
The availability of less harmful nicotine-containing products is likely to be an important component of a nicotine reduction standard because use of such products might minimize the discomfort experienced by reducing nicotine in cigarettes, reduce seeking nicotine products via the illegal market and improve support of a nicotine reduction standard among consumers. Furthermore, we observed that although the odds ratio for quitting smoking in the current 12-week intervention study (3.10, 95% CI, 1.69–5.96) was similar to a prior 20-week study that did not provide alternative nicotine delivery systems (3.22, 95% CI, 1.34–7.73), the actual rate of quitting was higher in the current study (41/220, 18.6% vs. 37/503, 7.4%).12 The abstinence rate at 12 weeks for the prior study was 4.4% (22/503). Thus, the availability of alternative nicotine delivery systems may have facilitated an exit away from smoking, although no direct comparisons were made.
Although this study does not specifically address the added value of e-cigarettes in the context of a nicotine reduction standard, the observed pattern of product use and post-hoc analyses could be cautiously interpreted to suggest that solely relying on nicotine replacement therapies may not be sufficient. Nearly two-thirds of participants who were assigned to the VLNC condition and who attained seven-day abstinence from combusted tobacco at the end of the intervention used e-cigarettes. A recent Cochrane report supports the use of e-cigarettes for quitting NNC cigarettes, stating that there is high certainty of evidence from randomized clinical trials demonstrating that e-cigarettes with nicotine are more effective than nicotine replacement therapies for achieving smoking abstinence.30 Of some concern, however, is the persistent use of e-cigarettes because they are not harmless. Although significant reductions are observed in exposure to toxicants and carcinogens with e-cigarettes, the long-term health effects are unknown.31 Therefore, moving the person who smokes down the continuum of product risk and/or eliminating the use of all tobacco products for individuals able to achieve this, should continue to be the optimal goal. It was also notable that in exploratory analyses exclusive use of a non-combusted product led to lower levels of CEMA and total NNAL (See Supplementary Table S10b) than use of combusted and non-combusted products for both NNC and VLNC conditions. Furthermore, among those assigned to VLNC cigarettes, dual users of combusted tobacco and non-combusted products had no differences in CEMA and total NNAL levels than exclusive combusted tobacco users (see Supplementary Table S10a), suggesting the importance of minimizing dual use to maximize public health benefit. Further characterization of dual use in future studies would be informative.
There are limitations to this study. This study did not include other combusted tobacco products. It is critical that any nicotine reduction standard would include other combusted tobacco products such as little cigars, cigarillos, pipe tobacco, bidis, roll-your-own tobacco, and tobacco used in water pipes. In a prior pilot study, those assigned to an experimental marketplace with VLNC cigarettes and only non-combusted tobacco products showed a reduction in toxicant exposure whereas those participants whose marketplace also included non-cigarette NNC combusted tobacco products showed no exposure reductions.32 The goal is to reduce the addictiveness of all the deadliest combusted products so that people who smoke will quit, or, if need be, migrate to regulated less harmful products. Second, the study was restricted to participants who were medically stable. However, adverse effects from VLNC cigarettes are not anticipated in less-medically-stable people who smoke because smoking would be reduced compared to smoking cigarettes with conventional levels of nicotine. A prior study showed that people who smoke with chronic health conditions experienced the same responses to reduced nicotine cigarettes as individuals without these conditions.33 Third, we were not able to verify adherence to only smoking study cigarettes and in fact, participants reported using approximately one additional non-study cigarette per day throughout the VLNC vs. NNC condition. It is possible that a more profound effect of reducing nicotine in cigarettes might be observed if conventional nicotine cigarettes were unavailable. Nonetheless, these findings might suggest a potential demand for illicit NNC cigarettes. In a National Research Council report issued on this topic, the illegal marketplace could be minimized if several regulatory steps were taken, including a track and trace system.34 Fourth, despite the attempts to make this study ecologically valid, it was not a natural experiment and relatedly, although the dropout rates were similar across conditions, the people who smoke who remained in the VLNC may have contributed to bias in the results. Fifth, we did not disaggregate the analyses by self-identified gender or by racial/ethnic groups for the purposes of this paper. Finally, the pattern of uptake of non-combusted nicotine products and biomarker analyses were exploratory utilizing post-hoc analyses and should be interpreted with caution.
In summary, reducing nicotine in cigarettes has the potential to improve individual and public health among people who smoke by reducing consumption. The availability of evidence-based less harmful alternative nicotine delivery systems might be an important component of this standard along with easy access to smoking cessation resources. The current tobacco-caused deaths of seven million worldwide each year,35 predominantly from cigarette smoking and among disadvantaged groups, call for urgent action; implementing a nicotine reduction standard may be an important step to protect the health of current and future generations.
Contributors
DKH was primarily responsible for the literature search, study conceptualization and methodology, determination of the primary, secondary, and exploratory measures, implementation and oversight of the study, data acquisition, drafting the manuscript and submitting the final version. DMC and LGS were responsible for developing and implementing the Experimental Marketplace. JAJ and RD-A were responsible for the initiation and implementation of the study. JAJ was responsible for the regulatory and day-to-day operation of the study and LGS created electronic data collection platform and monitored data quality. SSH, SGC and SEM conducted all the biomarker analyses. XL created the statistical analyses plan and she and QC analyzed the data. DKH, XL, QC, JAJ, and LGS were responsible for data verification. JT, SC, AAS, FJM, NLB, and ECD were site principal investigators. All authors provided feedback on the methods and edited the draft of the manuscript. All authors have full access to all the data and accept responsibility to submit the manuscript. DKH and ECD were responsible for the acquisition of funding for the study and approved the final submission of the manuscript.
Data sharing statement
De-identified data will be made available with a data dictionary and the protocol after the investigators have published their respective papers. Requests for the data should be made to DKH (hatsu001@umn.edu). The submission of an abstract that describes the purpose of the request, data needed, and analysis plan will be requested for record keeping. No financial support will be provided to the requestor.
Declaration of interests
DKH serves on the World Health Organization, Study Group on Tobacco Product Regulation, is a Special Government Employee for the U.S. Food and Drug Administration and serves on the Scientific Advisory Board for the Medical University of South Carolina Program Project Grant related to the use of nicotine products in a rapidly evolving nicotine marketplace. NLB serves as a consultant to Achieve Life Sciences and Qnovia, companies that are developing a new smoking cessation medications and has been an expert witness in litigation against tobacco companies. RDA serves as a consulting reviewer for Versar, a technology and management support company for the federal government, and provides advice to the Ministry of Health New Zealand. SC served on the Data Safety Monitoring Board for Truth Initiative and is an External Advisory Board for a tobacco research center grant at Yale University. JT serves on the External Advisory Board of a Center of Biomedical Research Excellence (COBRE) award at the University of Vermont Center on Behavior and Health, a Scientific Advisory Committee for a Wake University research project related to nicotine reduction (R01DA058264), a Data Safety Monitoring Board for the Yale University Center for Tobacco Regulatory Science (TCORS) and was a Deputy Editor for the journal Nicotine and Tobacco Research. ECD is a Special Government Employee for the U.S. Food and Drug Administration and a member of a technical advisory group for the Ministry of Health in New Zealand. All other authors have nothing to declare.
Acknowledgements
This work was supported by the National Institute of Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products, grant number U54DA03165 (to ECD and DKH) and the National Institute on Minority Health and Health, grant number K01MD014795 (to DMC). REDCap (Research Electronic Data Capture) services were provided by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. All statistical analyses were carried out in the Biostatistics Shared Resource of the Masonic Cancer Center, supported in part by National Cancer Institute Cancer Center Support grant P30CA077598. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Food and Drug Administration.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100796.
Appendix A. Supplementary data
References
- 1.Apelberg B.J., Feirman S.P., Salazar E., et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. doi: 10.1056/NEJMsr1714617. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz N.L., Henningfield J.E. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. doi: 10.1056/nejm199407143310212. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration FDA announces plans for proposed Rule to reduce addictiveness of cigarettes and other combusted tobacco products. 06/24/2022. 2022. https://www.fda.gov/news-events/press-announcements/fda-announces-plans-proposed-rule-reduce-addictiveness-cigarettes-and-other-combusted-tobacco
- 4.Ait Ouakrim D., Wilson T., Waa A., et al. Tobacco endgame intervention impacts on health gains and Māori:non-Māori health inequity: a simulation study of the Aotearoa/New Zealand Tobacco Action Plan. Tob Control. 2023 doi: 10.1136/tc-2022-057655. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.New Zealand Ministry of Health Smokefree Aotearoa 2025 action plan. 3/8/2023. https://www.health.govt.nz/our-work/preventative-health-wellness/tobacco-control/smokefree-aotearoa-2025-action-plan
- 6.Donny E.C., White C.M. A review of the evidence on cigarettes with reduced addictiveness potential. Int J Drug Policy. 2022;99 doi: 10.1016/j.drugpo.2021.103436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatsukami D.K., Xu D., Ferris W.G. Regulatory approaches and implementation of minimally addictive combusted products. Nicotine Tob Res. 2022;24(4):453–462. doi: 10.1093/ntr/ntab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulds J., Veldheer S., Pachas G., et al. The effects of reduced nicotine content cigarettes on biomarkers of nicotine and toxicant exposure, smoking behavior and psychiatric symptoms in smokers with mood or anxiety disorders: a double-blind randomized trial. PLoS One. 2022;17(11) doi: 10.1371/journal.pone.0275522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy R.N., Tidey J.W., Jackson K.M., et al. The impact of reducing nicotine content on adolescent cigarette smoking and nicotine exposure: results from a randomized controlled trial. Nicotine Tob Res. 2023;25(5):918–927. doi: 10.1093/ntr/ntac279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tidey J.W., Snell L.M., Colby S.M., Cassidy R.N., Denlinger-Apte R.L. Effects of very low nicotine content cigarettes on smoking across vulnerable populations. Prev Med. 2022;165(Pt B) doi: 10.1016/j.ypmed.2022.107099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins S.T., Tidey J.W., Sigmon S.C., et al. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 randomized clinical trials. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.19311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatsukami D.K., Luo X., Jensen J.A., et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. doi: 10.1001/jama.2018.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute on Drug Abuse . 2016. Nicotine research cigarettes drug supply Program.https://www.drugabuse.gov/nicotine-research-cigarette-drug-supply-program [Google Scholar]
- 14.Donny E.C., Denlinger R.L., Tidey J.W., et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll D.M., Strayer L., Nardone N., et al. Development and piloting testing of an experimental tobacco and nicotine product marketplace. Nicotine Tob Res. 2020;22(7):1230–1234. doi: 10.1093/ntr/ntz195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: a language and environment for statistical computing. [Google Scholar]
- 17.Luo X., Carmella S.G., Chen M., et al. Urinary cyanoethyl mercapturic acid, a biomarker of the smoke toxicant acrylonitrile, clearly distinguishes smokers from nonsmokers. Nicotine Tob Res. 2020;22(10):1744–1747. doi: 10.1093/ntr/ntaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmella S.G., Ming X., Olvera N., Brookmeyer C., Yoder A., Hecht S.S. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26(8):1209–1217. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmella S.G., Chen M., Zarth A., Hecht S.S. High throughput liquid chromatography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers' urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;935:36–40. doi: 10.1016/j.jchromb.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarth A.T., Carmella S.G., Le C.T., Hecht S.S. Effect of cigarette smoking on urinary 2-hydroxypropylmercapturic acid, a metabolite of propylene oxide. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;953–954:126–131. doi: 10.1016/j.jchromb.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmella S.G., Chen M., Han S., et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22(4):734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith S.S., Piper M.E., Bolt D.M., et al. Development of the brief Wisconsin inventory of smoking dependence motives. Nicotine Tob Res. 2010;12(5):489–499. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes J.R., Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 25.Cox L.S., Tiffany S.T., Christen A.G. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 26.Radloff L.S. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 27.Little R.J.A., Rubin D.B. 2nd ed. Wiley; Hoboken, NJ: 2002. Statistical analysis with missing data; pp. 200–220. [Google Scholar]
- 28.Schafer J.L. Chapman and Hall/CRC; New York: 1997. Analysis of incomplete multivariate data. [Google Scholar]
- 29.Gottlieb S., Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann-Boyce J., Lindson N., Butler A.R., et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2022;11(11):Cd010216. doi: 10.1002/14651858.CD010216.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Academies of Sciences, Engineering, and Medicine . The National Academies Press; Washington, D.C.: 2018. Public health consequences of e-cigarettes. [PubMed] [Google Scholar]
- 32.Hatsukami D.K., Luo X., Dick L., et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction. 2017;112(1):156–167. doi: 10.1111/add.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streck J.M., Bergeria C.L., Parker M.A., et al. Response to reduced nicotine content cigarettes among smokers with chronic health conditions. Prev Med Rep. 2018;12:321–329. doi: 10.1016/j.pmedr.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Research Council . The National Academies Press; Washington, DC: 2015. Understanding the U.S. Illicit tobacco market: characteristics, policy context, and lessons from international experiences. [Google Scholar]
- 35.World Health Organization . World Health Organization; Geneva: 2017. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.