Abstract
Many infectious disease forecasting models in the United States (US) are built with data partitioned into geopolitical regions centered on human activity as opposed to regions defined by natural ecosystems; although useful for data collection and intervention, this has the potential to mask biological relationships between the environment and disease. We explored this concept by analyzing the correlations between climate and West Nile virus (WNV) case data aggregated to geopolitical and ecological regions. We compared correlations between minimum, maximum, and mean annual temperature; precipitation; and annual WNV neuroinvasive disease (WNND) case data from 2005 to 2019 when partitioned into (a) climate regions defined by the National Oceanic and Atmospheric Administration (NOAA) and (b) Level I ecoregions defined by the Environmental Protection Agency (EPA). We found that correlations between climate and WNND in NOAA climate regions and EPA ecoregions were often contradictory in both direction and magnitude, with EPA ecoregions more often supporting previously established biological hypotheses and environmental dynamics underlying vector‐borne disease transmission. Using ecological regions to examine the relationships between climate and disease cases can enhance the predictive power of forecasts at various scales, motivating a conceptual shift in large‐scale analyses from geopolitical frameworks to more ecologically meaningful regions.
Keywords: West Nile virus, ecoregions, climate, ecosystems, health data
Key Points
While health data is collected within geopolitical boundaries, environmental disease dynamics are influenced by ecosystem characteristics
For West Nile virus, correlations between cases and climate were different depending on geopolitical or ecosystem regional groupings of data
We propose a conceptual shift from analyzing climate and health data at geopolitical boundaries to more ecologically meaningful regions
1. Introduction
Infectious disease forecasting models deal implicitly with the problem of scale, referred to as the most fundamental problem in ecology (Levin, 1992). Public health data collection and decisions are often made at institutions instated within geopolitical boundaries; in the United States (US), these are primarily state (Administration Division 1) or county (Administrative Division 2) health departments. These geopolitical boundaries were historically designated by politics, human activity, and physical geography (e.g., rivers and mountain ranges), but not similarities in ecosystems and climate. Further, patient privacy and reporting practices dictate the spatial and temporal resolution at which epidemiologic data can be shared with research scientists or made public for use in disease forecast models. For environmentally driven disease systems, models use this data and climate drivers to understand and predict broader regional or national disease risk, often across multiple ecosystems (Hahn et al., 2015; Landesman et al., 2007; Lowe et al., 2013; Paull et al., 2017). This can exacerbate the difficulty of interpreting signals in the relationships between climate variables and case incidence due to the direct mechanistic dependence of vector‐borne disease dynamics on vector and host ecology (Ellis & Wilcox, 2009; Escobar & Craft, 2016).
Instead, it may be beneficial to use regions defined by ecological dimension, where boundaries are drawn where one distinct ecosystem ends and another begins based on the combined similarity of biotic and abiotic agents and how they function in their environment (Folke, 2006; McDonnell, 1997). We speculate that organizing disease analyses around geopolitical regions could potentially mask important climate drivers of disease incidence, whereas using regions defined by ecology could reveal new insights into the natural processes important to disease dynamics and enhance the accuracy of disease forecasts.
Mosquito‐borne disease dynamics are highly influenced by environmental conditions and may serve as a good foundation for exploring the impact of using geopolitically versus ecologically based analyses. In the US, the spread of West Nile virus (WNV) and years with outbreak numbers of cases is of growing concern, and the research community is building large‐scale forecasting models to predict the local incidence of infections (Gorris et al., 2023; Holcomb et al., 2023; Keyel, Gorris, et al., 2021; Keyel, Raghavendra, et al., 2021; Kretschmer et al., 2023). WNV is the foremost cause of mosquito‐borne disease in humans in the continental US (Centers for Disease Control and Prevention, 2023). Humans may contract it when bitten by an infected mosquito. Approximately 20% of infected humans are symptomatic, and about 1 in 150 of those infected will develop a severe form of the disease that affects the nervous system, which is reported as a West Nile neuroinvasive disease (WNND) case (Centers for Disease Control and Prevention, 2022). WNND cases may be considered a more stable measure of WNV risk over time than total WNV cases. This is because non‐neuroinvasive WNV often presents as mild or flu‐like and is more subject to underreporting or misdiagnosis. In contrast, individuals with severe symptoms such as WNND are more likely to seek healthcare and be tested and confirmed as positive cases (Centers for Disease Control and Prevention, 2008; Silk et al., 2010).
Region‐specific variation in WNV dynamics has been correlated with climate variability (Hahn et al., 2015; Wimberly et al., 2014). Periods of lower‐than‐normal precipitation in typically dry areas have been correlated with lower levels of WNV incidence, whereas periods of lower‐than‐normal precipitation in typically wet areas have correlated with higher WNV incidence (Hahn et al., 2015; Landesman et al., 2007; Wimberly et al., 2014). Anomalously warm temperatures in the US, especially in winter and spring, have been associated with increased WNV (Chung et al., 2013; Hahn et al., 2015; Manore et al., 2014; Wimberly et al., 2014). Warmer temperatures may allow more mosquitoes to successfully overwinter and begin breeding earlier in the year, lengthening the disease transmission season (Gorris et al., 2023; Hahn et al., 2015; Wimberly et al., 2014). Temperature also impacts the time to adult mosquito maturation, reproduction rate, efficacy for transmitting diseases such as WNV, and other life traits (Ciota et al., 2014; Dohm et al., 2002; Moser et al., 2023). However, temperatures outside the thermotolerance of the mosquitoes may cause die‐off, especially during heat waves (Moser et al., 2023; Rueda et al., 1990). The associations reported between climate and disease incidence are likely due to environmental factors affecting the abundance and distribution of Culex mosquitoes, the primary vectors for WNV (Ciota et al., 2014; Moser et al., 2023). For example, Culex pipiens (urban‐adapted, in the eastern US), Cx. quinquefasciatus (urban‐adapted, hot and dry areas preferred), and Cx. tarsalis (primarily rural, in the western US) exhibit finely grained differences in spatial distribution due to their preferred habitats and different responses to temperature and precipitation (Bowden et al., 2011; Gorris et al., 2021). The reported associations may also be partly due to other disease transmission dynamics, like the migration of birds—the reservoir host for WNV (Bergsman et al., 2016; Marra et al., 2005).
In the US, the nine terrestrial climate regions designated by the National Oceanic and Atmospheric Association (NOAA) are one choice for regional scaling of environmentally sensitive disease forecasts. These regions group states according to climate similarity in the context of socioeconomic activity and human welfare. NOAA originally delineated the regions to help society mitigate the adverse effects of climate extremes (T. R. Karl & Koscielny, 1982; T. Karl & Koss, 1984). The NOAA climate regions marked an improvement upon previously used distinctions based on the US census by facilitating the use of historical climate data to understand both long‐ and short‐term climate trends. In the context of disease ecology, the NOAA climate regions are a balance between incorporating areas of similar climate and administrative levels of decision‐making—creating a disease forecast system for nine separate climate regions is more feasible than a forecast system modeling over 3,000 contiguous US counties separately, especially with heterogeneity in healthcare data. However, the NOAA climate regions are still grouped along state lines, organized around ecosystem goods and services rather than ecology, and therefore, more geopolitically than ecologically based regions.
An ecologically driven approach to dividing the US is by ecoregions. Ecoregions are defined as areas with similar ecosystems and comparable quality and quantity of environmental resources (Omernik, 1987a; Omernik & Griffith, 2014). The US Environmental Protection Agency (EPA), in collaboration with other agencies, has designated a tiered system of ecological classification for ecoregions ranging from broad regions applicable for large‐scale analysis (Level I) to areas of fine detail appropriate for local monitoring and management (Level III) (McMahon et al., 2001; Omernik, 2004; Wiken et al., 1996). The ecoregion designations are based on expert analysis from map overlays, considering regional similarity in biotic (including humans), abiotic, terrestrial, and aquatic ecosystems (Omernik, 1987b). Interestingly, the use of EPA ecoregions in predicting WNV risk has previously been demonstrated for horses in Saskatchewan (Epp et al., 2011) and in developing a mechanistic model for the basic reproduction number (R 0) of WNV in Texas (Kain & Bolker, 2019), showing promise for this approach.
We partitioned climate and WNND case data in the contiguous US from 2005 to 2019 into NOAA climate regions and EPA Level I ecoregions. Our study explores the correlations between temperature, precipitation, and WNV case data and how they differ based on data aggregated to geopolitical and ecological regions. Our findings suggest that EPA ecoregions may represent patterns of causal relationships between climate and WNV described in prior literature that are not accurately represented by NOAA climate regions, motivating a conceptual shift in large‐scale disease forecasting from a geopolitical framework to an ecological one.
2. Methods
2.1. NOAA Climate Regions and EPA Ecoregions
For data collection and modeling, we chose the nine NOAA geopolitical climate regions defined as “climatically consistent regions within the contiguous United States” (Figure 1a, T. Karl & Koss, 1984; NOAA, 2023). They are the Northeast, Northern Rockies and Plains, Northwest, Ohio Valley, South, Southeast, Southwest, Upper Midwest, and West. Their boundaries follow state lines, each including at least several states (3–11). We assigned every county to its corresponding NOAA climate region.
Figure 1.
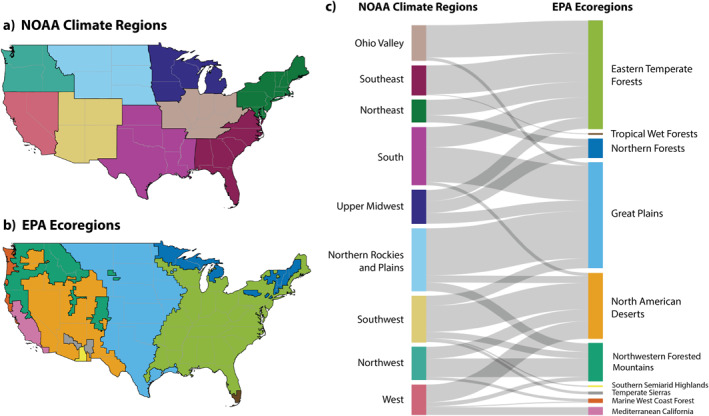
Reference maps for the NOAA climate regions (a) and EPA Level I ecoregions (b). Sankey flow diagram (c) illustrates the shared land area between the NOAA climate regions and the EPA ecoregions. The nodes in the left column represent the nine NOAA climate regions, and the nodes in the right column represent the 10 EPA ecoregions. The width of the flows is proportional to land area to show the differences and overlaps between these regional classifications.
We used the Level I EPA Ecoregions of the contiguous US for our ecological regions (Figure 1b). We chose Level I ecoregions rather than Level II for this analysis because the Level I landmass sizes are more comparable to those of the NOAA climate regions (Figure 1c, Figure S1 in Supporting Information S1). These regions are defined as “areas within which there is a relative similarity in the mosaic of all ecosystem components—biotic, abiotic, terrestrial, and aquatic, with humans being part of the biota” (Omernik & Griffith, 2014). There are 10 Level I EPA ecoregions in the contiguous US: Northern Forests, Northwestern Forested Mountains, Marine West Coast Forest, Eastern Temperate Forests, Great Plains, North American Deserts, Mediterranean California, Southern Semiarid Highlands, Temperate Sierras, and Tropical Wet Forests (US EPA, 2015). We assigned each county to an EPA ecoregion based on the majority of the landmass area within that county.
To facilitate comparisons between correlations for the geopolitical and ecoregions, we calculated the landmass area overlap in square meters between each NOAA climate region and each EPA ecoregion by merging the region boundary information with publicly available county‐state 2019 TIGER/Line shapefiles (US Census Bureau, 2019) and aggregated the county area data over the region (Figure S1 in Supporting Information S1). We calculated the overlap of each NOAA climate region to each EPA ecoregion polygon pair and visualized the relationships using a Sankey diagram with the R packages sf and networkD3 (Gandrud et al., 2017; Pebesma & Bivand, 2023; Figure 1c).
2.2. Neuroinvasive West Nile Virus Human Case Data
We obtained annual human case counts of WNND at the county level for the contiguous US from 1999 to 2021 from the US Centers for Disease Control and Prevention (CDC). Between 1999 and 2005, WNV spread westward across the US from its introduction point in the eastern US (Blitvich, 2008; Kramer et al., 2019; Ronca et al., 2021). After 2019, the COVID‐19 pandemic shifted human behavior and health reporting, possibly influencing case counts (Kretschmer et al., 2023; Melidou et al., 2020). For these reasons, we limited our analyses to the WNND cases between 2005 and 2019, when no known changes in reporting practices could bias the case counts.
To account for variable population by county, we converted WNND case counts to disease incidence (cases per 100,000 population) using annual, county‐level population estimates from the US Census Bureau (US Census Bureau, 2010, 2020). We calculated annual WNND incidence for each NOAA climate region and EPA ecoregion using the county‐level sum of WNND case counts and total population within each region.
2.3. Climate Data
We used annual averaged minimum, maximum, and mean surface air temperature and cumulative precipitation to explore climatic variation. Though there is generally a high correlation between the different temperature measures, the importance of temperature extremes on the physiology and survival of mosquitoes, as well as their ability to transmit WNV, has been shown in previous work and may be better represented by these measures (Dohm et al., 2002; Moser et al., 2023). We obtained these data from the Precipitation elevation Regressions on Independent Slopes Model (PRISM) from 2005 to 2019 at a 4 km gridded spatial resolution (Daly et al., 1994, 2008). We averaged the gridded climate data over each NOAA climate region and EPA ecoregion by calendar year to enable a meaningful comparison of climate conditions to WNND incidence for the two classification systems. For each climate variable, we first analyzed each NOAA climate region and EPA ecoregion for significant linear trends to assess whether we needed to detrend the data since temperatures in the US are already warming in response to climate change (USGCRP, 2017). Most regions did not have significant linear trends, so we did not detrend the data.
2.4. Statistical Methods
We calculated the Spearman rank correlation coefficients between the WNND incidence and climate conditions for each NOAA climate region and EPA ecoregion. We considered two significance levels: p < 0.1* and p < 0.05**. Given the aim and scope of this investigation, we chose these levels following Fisher's principle of drawing conclusions rather than making decisions (Lehmann, 1993). Since our study is exploratory and primarily aimed at comparing the effects of aggregating data according to two different regional partitions, we did not perform multiple comparisons testing, which would be necessary for examining causality (Bender & Lange, 2001). The analyses used R and Python (Python, 2023; R Core Team, 2023).
3. Results
3.1. NOAA Climate Regions Compared to EPA Ecoregions
The total land mass of the contiguous US is distributed relatively evenly among the NOAA climate regions compared to the EPA ecoregions (Figure S1 in Supporting Information S1). Each NOAA climate region included portions of two to five EPA ecoregions, meaning they are broadly ecologically heterogeneous (Figure 1; Table S1 in Supporting Information S1). For example, the Southeast NOAA climate region contains both Eastern Temperate Forests and Tropical Wet Forests, while the Southwest NOAA climate region contains five disparate EPA ecoregions ranging from North American Deserts to Northwestern Forested Mountains.
The three largest NOAA climate regions together account for approximately 49% of the total land mass of the contiguous US, those being the South (19%), the Northern Rockies and Plains (16%), and the Southwest (14%; Figure S1 in Supporting Information S1); the three smallest NOAA climate regions contain approximately 22% of the total land mass, those being the Upper Midwest (8%), the Northwest (8%), and the Northeast (6%). In contrast, the three largest EPA ecoregions together account for approximately 81% of the contiguous US land mass, those being the Eastern Temperate Forests (32%), Great Plains (30%), and North American Deserts (19%); the three smallest EPA ecoregions contain just over 1% of the total, those being the Temperate Sierras (0.8%), Southern Semiarid Highlands (0.4%), and Tropical Wet Forests (0.2%).
The three largest EPA ecoregions each span five NOAA climate regions. The Eastern Temperate Forests ecoregion stretches across the following NOAA climate regions in the eastern half of the US: Ohio Valley, Southeast, Northeast, South, and Upper Midwest. The Great Plains ecoregion straddles the middle of the US with a “V” shape that reaches from Canada to Mexico, extending across the following NOAA climate regions: Ohio Valley, South, Upper Midwest, Northern Rockies and Plains, and Southwest. The North American Deserts ecoregion stretches across the following NOAA climate regions in the western half of the US: South, Northern Rockies and Plains, Southwest, Northwest, and West.
3.2. WNND Incidence
From 2005 to 2019, among the NOAA climate regions, the Northern Rockies and Plains had the highest mean annual WNND incidence (cases per 100,000 population per year; Figure 2, Table S2 in Supporting Information S1), while the Southeast had the lowest mean annual WNND incidence. The Northern Rockies and Plains region includes portions of three EPA ecoregions, while the Southeast region includes two (a portion of one and another whole region; Figure 1, Table S1 in Supporting Information S1). Among the EPA ecoregions, the mean WNND incidence was highest in the North American Deserts and lowest in the Tropical Wet Forests. The second highest mean WNND incidence was in the Great Plains ecoregion, which aligns qualitatively with the “V” shape of higher WNND incidence throughout the central US when viewed at the county level (Figure S2 in Supporting Information S1).
Figure 2.
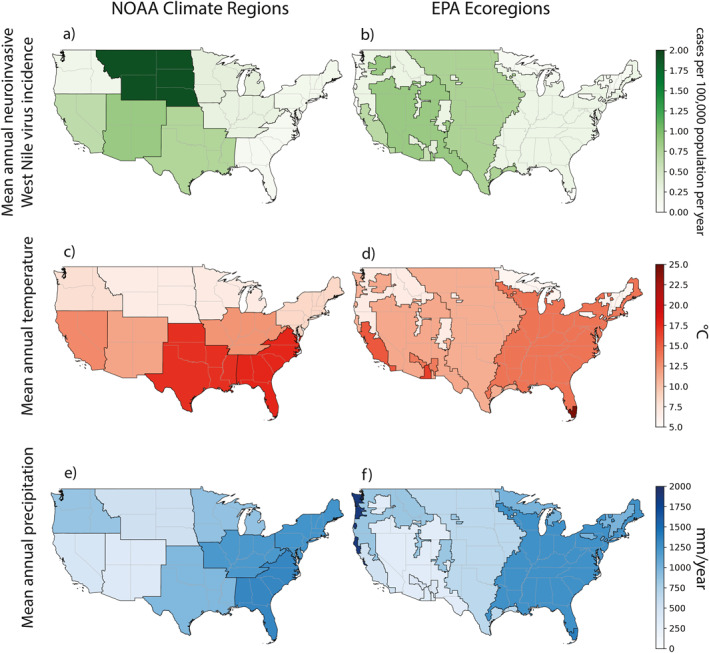
Map of mean annual WNND incidence (a, b), mean annual temperature (c, d), and mean annual precipitation (e, f) calculated for NOAA climate regions (a, c, e) and EPA ecoregions (b, d, f). Similarly, we computed the mean minimum and maximum temperatures, which showed similar patterns to mean annual temperature (not shown). Calculating the WNND case incidence by the EPA ecoregion resembles the county‐level visual distribution of incidence (Figure S3 in Supporting Information S1) more than the incidence in the NOAA climate region. Similarly, regional differences in climate conditions are apparent in EPA ecoregions and are smoothed out in the NOAA climate regions.
Over time, annual WNND incidence varied across NOAA climate regions and EPA ecoregions, with markedly different temporal patterns in the Northern Rockies and Plains NOAA climate region and South Semiarid Highlands, Great Plains, and Mediterranean California EPA ecoregions (Figure S3 in Supporting Information S1). The NOAA climate region with the highest mean WNND incidence in a single year was the Northern Rockies and Plains in 2018 (Figure S3a in Supporting Information S1). The EPA ecoregion with the highest incidence was the Southern Semiarid Highlands in 2010 (Figure S3b in Supporting Information S1).
3.3. Climate Conditions
There are apparent spatial differences in mean annual temperature and precipitation between the NOAA climate regions and EPA ecoregions, highlighting the importance of appropriately partitioning study regions. The maximum and minimum climate values between overlapping NOAA climate regions and EPA ecoregions do not always align. On average, the warmest NOAA climate region in the US from 2005 to 2019 was the Southeast (Figure 2c; Table S2 in Supporting Information S1). The Southeast also had the highest mean annual temperature within any given year from 2005 to 2019 (18.3°C, 2019; Figure S3a in Supporting Information S1). The warmest EPA ecoregion was the Tropical Wet Forests, which lies entirely within the Southeast NOAA climate region (Figures 1 and 2d). In 2015, the Tropical Wet Forests had the highest mean annual temperature within any given year among the EPA Ecoregions (25.2°C; Figure S3b in Supporting Information S1). The coolest NOAA climate region was the Northern Rockies and Plains (Figure 2c, Table S2 in Supporting Information S1).
The coolest EPA ecoregion was the Northern Forests, which stretches across the Northeast and Upper Midwest NOAA climate regions, but not the Northern Rockies and Plains (Figures 1 and 2d, Table S2 in Supporting Information S1). Within the study period, in 2014, the Upper Midwest NOAA climate region had the coolest mean annual temperature (4.9°C; Figure S3a in Supporting Information S1), and the EPA ecoregion with the coolest mean annual temperature was the Northern Forests, also in 2014 (4.0°C; Figure S3b in Supporting Information S1).
The average wettest NOAA climate region in the US from 2005 to 2019 was the Southeast (Table S2 in Supporting Information S1), which was also the wettest NOAA climate region in any given year in 2018 (1,690 mm; Figure 2e, Figure S3a in Supporting Information S1). The average wettest EPA ecoregion was the Marine West Coast Forests, which is on the opposite border of the US compared to the Southeast NOAA climate region; in 2012, the Marine West Coast Forests was also the wettest EPA ecoregion in any given year (2,371 mm; Figures 2f, Figure S3b in Supporting Information S1). The EPA ecoregions with the highest precipitation after the Marine West Coast Forests were the Tropical Wet Forests and Eastern Temperate Forests (>1,200 mm/year; Figure 2f), which overlap with the Southeast NOAA climate region (Figure 1c).
The Southwest was the average driest NOAA climate region (Table S2 in Supporting Information S1, Figure 2e). The average driest EPA ecoregion was the North American Deserts (Figure 2f), which does overlap with the Southwest NOAA climate region (Figure 1c). Precipitation was also lower in the dry EPA ecoregions of the Southern Semiarid Highlands and Temperate Sierras (<400 mm/yr; Figure 2f, Table S2 in Supporting Information S1), which both fall in the Southwest NOAA climate region. The Southwest NOAA climate region also contains portions of the rainier Northwestern Forested Mountains EPA ecoregion (Figures 1 and 2e, Table S1 in Supporting Information S1). Over our study period, the driest NOAA climate region in any given year was the West in 2013 (198 mm; Figure S3a in Supporting Information S1). This statistic was mirrored by Mediterranean California, which lies within the West NOAA climate region, being the driest EPA ecoregion in any given year, also in 2013 (151 mm; Figure S3b in Supporting Information S1).
Overall, EPA ecoregions with low mean annual precipitation (<750 mm/yr) and moderate mean annual temperatures (10.0–17.0°C) exhibited the highest WNND incidence (Figure S4 in Supporting Information S1). NOAA climate regions did not exhibit a similar clustered signal, where higher levels of WNND incidence spanned the full range of precipitation and temperature values.
3.4. Climate and WNND Incidence Correlations
Our correlation analysis structured by NOAA climate regions resulted in 42% (15/36) statistically significant correlations between climate variables and WNND incidence within five of the nine NOAA climate regions (56%), while analysis structured by EPA ecoregions resulted in 35% (14/40) statistically significant correlations within six of the 10 EPA ecoregions (60%; Figure 3; Tables S3 and S4 in Supporting Information S1). The sign and magnitude varied between the correlations. All temperature correlations for the NOAA climate regions were positive, while temperature correlations in the EPA ecoregions were both positive and negative. The negative correlations between temperature and WNND (p > 0.1) occurred in three ecoregions (Southern Semiarid Highlands, Temperate Sierras, and Tropical Wet Forests) that had relatively warmer mean annual temperatures. Correlations with precipitation were both positive and negative across both the NOAA climate regions and EPA ecoregions. Eight of the nine NOAA climate regions (89%) included at least one EPA ecoregion in its boundaries with the opposite correlation sign for precipitation, though not all precipitation correlations were statistically significant (Table S3 in Supporting Information S1).
Figure 3.
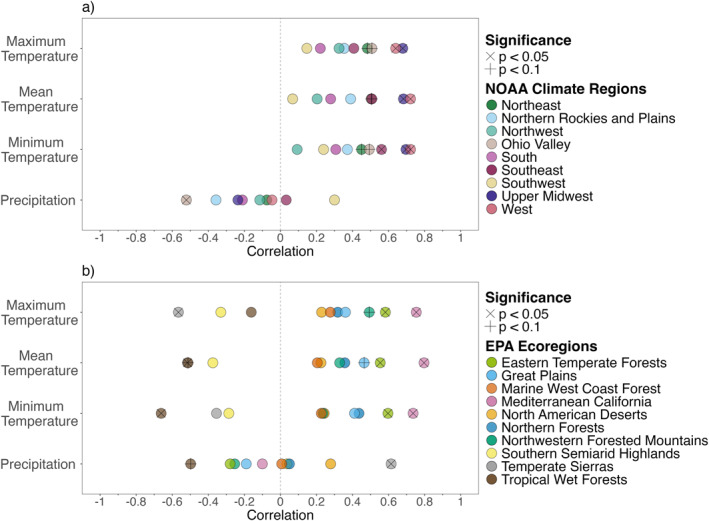
Scatterplot of Spearman rank correlations between mean annual temperature and precipitation (°C and mm/year, respectively) and annual WNND case incidence (cases per 100,000 population) for (a) NOAA climate regions and (b) EPA ecoregions. Correlation coefficient values are on the x‐axis, where the dashed line indicates a correlation coefficient of 0. Significance is indicated by a cross (p < 0.1) or X (p < 0.05) through the marker.
Several of the EPA ecoregions had correlation values contradicting those in the overlapping NOAA climate regions (Figures 1 and 4; Tables S3 and S4 in Supporting Information S1). Four EPA ecoregions lay within only one NOAA climate region in the contiguous US, allowing for direct comparisons. One of these was the Tropical Wet Forests ecoregion, which lies within the Southeast NOAA climate region (Figure 1, Table S1 in Supporting Information S1). The Tropical Wet Forests ecoregion had statistically significant negative correlations with minimum temperature and mean temperature, while the Southeast NOAA climate region had statistically significant positive correlations with minimum and mean temperature—showing opposite relationships to temperature (Table S4f in Supporting Information S1). The Tropical Wet Forests ecoregion also had a statistically significant negative correlation with precipitation (p < 0.1), while the Southeast NOAA climate region had an insignificant, near‐zero correlation with precipitation (p > 0.1). Though statistically significant via our methods, the correlations in the Tropical Wet Forests ecoregion should be interpreted cautiously since there were only seven cases total from 2005 to 2019 (Table S2 in Supporting Information S1).
Figure 4.
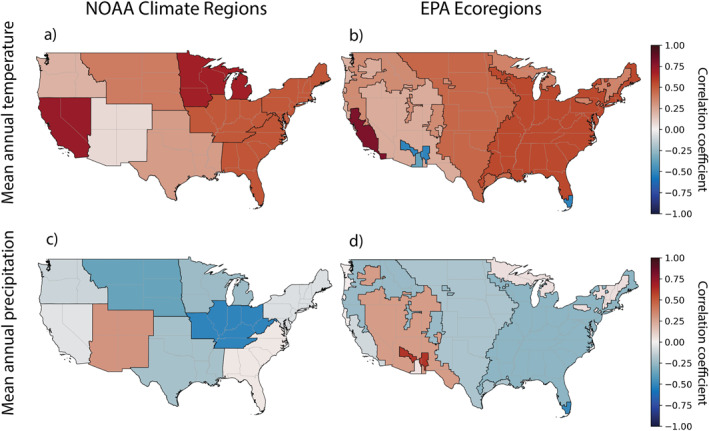
Spearman rank correlations between mean annual temperature (a, b) and precipitation (c, d) (°C and mm/year, respectively) and WNND case incidence (cases per 100,000 population), partitioned into NOAA climate regions (a, c) and EPA ecoregions (b, d). We omit maximum and minimum temperature maps to reduce redundancy.
The Southern Semiarid Highlands and Temperate Sierras EPA ecoregions lay within the Southwest NOAA climate region (Figure 1). The Southwest NOAA climate region had positive correlations between temperature and precipitation (all p > 0.1; Table S4g in Supporting Information S1). Conversely, the Southern Semiarid Highlands had negative correlations with temperature (p > 0.1). The Temperate Sierras ecoregion had statistically significant (p < 0.05) negative correlations between maximum and mean temperature and a statistically significant (p < 0.05) positive correlation with precipitation. The correlations in the Southern Semiarid Highlands and Temperate Sierras should also be interpreted cautiously since they only had 18 and 15 cases of WNND from 2005 to 2019, respectively (Table S2 in Supporting Information S1).
The last EPA ecoregion within one NOAA climate region is Mediterranean California, which lies within the West NOAA climate region. 80% (3,175/3,977) of WNND cases in the West climate region occurred in Mediterranean California (Table S2 in Supporting Information S1), though Mediterranean California only made up 26% of the landmass of the West NOAA Climate region. The correlations between maximum, minimum, and mean temperature and WNND incidence were positive (p < 0.05) for the West NOAA climate region and the Mediterranean California EPA ecoregion (Table S3 in Supporting Information S1). Similarly, there were near‐zero correlations (p > 0.1) between precipitation and WNND incidence within the two regions. This was an example of an ecoregion and climate region that were in close agreement; the three other EPA ecoregions within the West NOAA Climate region did not show as strong of agreement (Table S4i in Supporting Information S1). Two ecoregions, the North American Deserts and Marine West Coast Forest, had no statistically significant correlations. The third, Northwestern Forested Mountains, had only a statistically significant correlation (p < 0.1) with maximum temperature, which agreed with the trend in the West NOAA climate region.
The NOAA climate region with the highest WNND incidence was the Northern Rockies and Plains, which surprisingly had no statistically significant correlations between climate and WNND. Likewise, the EPA ecoregion with the highest WNND incidence was the North American Deserts, which also didn't have any statistically significant correlations.
4. Discussion
We explored how the relationships between WNND incidence and climate may differ when the data are structured by NOAA geopolitical climate regions in contrast to the EPA ecological regions. We found that almost all NOAA climate regions (8/9) overlapped with EPA ecoregions that had contradictory correlations between climate and WNND incidence, suggesting that more refined, ecologically sensitive disease transmission dynamics may occur within the geopolitically structured regions. Overall, the correlations between temperature and WNND incidence were in closer agreement between the NOAA climate regions and EPA ecoregions than the correlations between precipitation and WNND incidence. Warmer temperatures were more often correlated with higher WNND incidence across the US. Less precipitation was more often correlated with higher WNND incidence in the eastern US and west coast, while more precipitation was correlated with higher WNND incidence in the drier southwestern portions of the US. Though our analysis didn't show overwhelming support for stronger climate‐disease correlations at the EPA ecoregion level relative to the NOAA climate region level, we found instances where the correlations within the EPA ecoregions supported previously established biological hypotheses underlying WNV disease transmission, suggesting restructuring analyses to an ecological level may be beneficial.
Surprisingly, the NOAA climate regions with the top three highest levels of WNND incidence (Northern Rockies and Plains, Southwest, South) had no statistically significant correlations with climate. However, there were statistically significant relationships in at least one of the EPA ecoregions that lay within these climate regions, signaling that there may be important biological processes influencing disease dynamics at the ecoregion level. In the Northern Rockies and Plains, two of the three ecoregions had statistically significant correlations—one with mean temperature and one with maximum temperature—demonstrating the heterogeneity between the ecoregions within one NOAA climate region. In the Southwest, three of the five EPA ecoregions had statistically significant correlations between climate and WNND incidence, and there were correlations opposing in sign compared to the Southwest NOAA climate region for both temperature and precipitation, again suggesting the ecoregion level may be more representative of ecologically sensitive disease dynamics.
The EPA ecoregions with the top three highest WNND incidences were the North American Deserts, the Great Plains, and Mediterranean California. The fact that the North American Deserts ecoregion had the highest WNND incidence was surprising, given that WNV originated from Uganda and is generally considered a tropical or temperate disease. The North American Deserts didn't have any statistically significant correlations between climate and WNND incidence, but there was a positive correlation (p > 0.1) with precipitation, supporting previous literature that found increased precipitation in arid environments results in higher WNV risk (Hahn et al., 2015; Landesman et al., 2007; Morin & Comrie, 2013). The lack of statistically significant correlations may be a reflection of most WNND cases happening in urbanized environments, where WNV transmission can persist, and Culex mosquitos can survive within the otherwise arid ecosystem (Gorris et al., 2021; Hoover & Barker, 2016). Built urban environments—especially in the desert—that inadvertently include more breeding habitats for mosquitoes and more vegetation relative to the surrounding ecosystems may bias the correlations between the larger region of interest (climate region or ecoregion) and disease incidence.
We found examples where correlations between climate and WNND at the ecoregion level were more reflective of established biological hypotheses regarding disease transmission. For example, previous work studying the effects of climate on WNV vector abundance in a county in Virginia (Southeast NOAA climate region, Eastern Temperate Forests EPA ecoregion) found vector abundance was high in years with lower rainfall (Deichmeister & Telang, 2011). This finding contradicts the near‐zero correlation (p > 0.1) we found between precipitation and WNND incidence in the Southeast NOAA climate region but is in agreement with the negative correlation we found between precipitation and WNND incidence in the Eastern Temperate Forests ecoregion (p > 0.1). The other ecoregion within the Southeast climate region was the Tropical Wet Forests, the ecoregion with the second‐highest precipitation in our study. This ecoregion, too, had a negative correlation with precipitation (p < 0.1), which supports the previously published hypothesis that less precipitation in areas otherwise known to be wet increases WNV, likely by bringing mosquitoes, birds, and humans within close proximity to each other around limited water resources (Hahn et al., 2015; Landesman et al., 2007; Shaman et al., 2005). The Temperate Sierras and Southern Semiarid Highlands ecoregions overlap with the Southwest NOAA climate region. These two ecoregions have negative correlations with temperature (mixed significance) in contrast to the positive correlations (p > 0.1) with temperature variables in the Southwest climate region. On average, these two ecoregions are warmer than the Southwest NOAA climate region. In these ecoregions, Culex quinquefasciatus is likely the important vector for WNV transmission (Godsey et al., 2012; Gorris et al., 2021; Zinser et al., 2004). These negative correlations may be reflective of temperatures in the summer months pushing the upper thermotolerance bounds of C. quinquefasciatus (July mean maximum temperature averaged from 2006 to 2019 for Temperate Sierras is 32.0°C and Southern Semiarid Highlands is 33.6°C), where relatively cooler temperatures in these ecoregions are more favorable for mosquito survival and WNV transmission (Moser et al., 2023).
There are several avenues for building upon this work. One intrinsic limitation of our study was the availability of West Nile case data. Though we eliminated much of the bias with underreporting by using WNND cases, other biases can result from misdiagnosis or under‐ascertainment due to unfamiliarity with the disease, lack of testing, or lack of access to healthcare; some of the correlations between climate and incidence may be inaccurate if excess cases in response to climate variability were unreported. Future work may consider including land use variables, which are important drivers of vector‐borne disease dynamics, although there is evidence that EPA ecoregions do effectively capture anthropogenic land use activity (Gallant et al., 2004). This study could be built upon by further analysis of the EPA Level II Ecoregions, which would help break down the larger Level I Ecoregions that have greater spatial heterogeneity in climate and land use, like the Great Plains. Further analysis may also consider the effects of same‐season or lagged seasonal climate conditions, which may act as important indicators for mosquitoes overwintering (winter), the onset of breeding and interactions with migration birds (spring), biting rate and transmission to humans (summer), and the end of the transmission season (fall), as well as lags between climate conditions and WNND.
The conceptional shift from analyzing climate‐disease relationships on an ecologically meaningful level as opposed to geopolitical levels is also applicable to other environmentally sensitive infectious diseases besides WNV, especially vector‐borne disease systems since arthropods are ectothermic and depend on temperature and water availability. Prescient examples are mosquito‐borne diseases such as dengue and malaria, tick‐borne Lyme disease, endemic fungal diseases, and zoonoses such as hantavirus or brucellosis. The transmission of these diseases is driven by the distribution and abundance of vectors, disease hosts, and/or the pathogen itself and established as climate‐sensitive disease systems (Ferro et al., 2020; Gorris et al., 2018; Head et al., 2022; Hueffer et al., 2013; Morin et al., 2013; Okuneye & Gumel, 2017; Parham & Michael, 2010; Trejo et al., 2023). Other ecologically based partitions of landmass may be more appropriate for different disease systems, such as the more regionally refined EPA Ecoregion Level II or III data, the National Ecological Observatory Network, watersheds, or Köppen‐Geiger climate classifications (Keller et al., 2008; Kottek et al., 2006; Omernik & Griffith, 2014; United States Geographical Society, 2019). Analyzing relationships this way may provide new insights into biologically driven disease processes.
5. Conclusions
We presented evidence through a correlation analysis between climate and WNND incidence that motivates a conceptual shift in analyzing climate‐disease connections at an ecological level instead of geopolitical level. Here, we analyzed correlations between temperature, precipitation, and WNND incidence within NOAA climate regions and EPA ecoregions. We found that almost all NOAA climate regions overlapped with EPA ecoregions that had contradictory correlations between climate and WNND incidence, suggesting there may be more refined, ecologically sensitive disease transmission dynamics happening within the geopolitically structured regions. We also found instances where the correlations within the EPA ecoregions supported previously established biological hypotheses underlying WNV disease transmission and mosquito dynamics. The boundaries of the EPA ecoregions are based on conditions of the natural environment that support disease vector populations; therefore, they can provide insight into the heterogeneity of those conditions and help to understand the biological dynamics underlying disease transmission risk. EPA ecoregions may be a powerful tool for vector‐borne disease forecasters to understand disease transmission patterns. Our results provide an incentive for exploring an approach to a unified nationwide forecast system that incorporates ecoregion‐based analysis for a more comprehensive and scalable framework to reduce disease burden in the US.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
The authors gratefully acknowledge support from the Los Alamos National Laboratory, Laboratory Directed Research and Development (LDRD) program (Grants 20210061DR, 20200682PRD1, 20220209ER). We thank Karen Holcomb and the US Centers for Disease Control and Prevention team who organized the 2022 West Nile Virus Forecast Challenge for inspiring this project and providing WNV case data. This work is approved for distribution under LA‐UR‐23‐30999. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of their affiliations, including Los Alamos National Laboratory. Los Alamos National Laboratory, an affirmative action/equal opportunity employer, is managed by Triad National Security, LLC, for the National Nuclear Security Administration of the US Department of Energy under contract 89233218CNA000001.
Moser, S. K. , Spencer, J. A. , Barnard, M. , Hyman, J. M. , Manore, C. A. , & Gorris, M. E. (2024). Exploring climate‐disease connections in geopolitical versus ecological regions: The case of West Nile virus in the United States. GeoHealth, 8, e2024GH001024. 10.1029/2024GH001024
Data Availability Statement
Additional tables and figures supporting the findings of this article are included within the Supporting Information S1 file. The West Nile virus case data we used in our study are not accessible to the public due to a data use agreement with the US Centers for Disease Control and Prevention. However, the CDC has now released public West Nile virus data on a website where users can download county‐level case counts by year (https://www.cdc.gov/west‐nile‐virus/data‐maps/historic‐data.html). The PRISM Climate data we used in this study, annually averaged to the NOAA climate regions and EPA ecoregions, are available on Zenodo (Gorris, 2024).
References
- Bender, R. , & Lange, S. (2001). Adjusting for multiple testing—When and how? Journal of Clinical Epidemiology, 54(4), 343–349. 10.1016/S0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- Bergsman, L. D. , Hyman, J. M. , & Manore, C. A. (2016). A mathematical model for the spread of West Nile virus in migratory and resident birds. Mathematical Biosciences and Engineering, 13(2), 401–424. 10.3934/mbe.2015009 [DOI] [PubMed] [Google Scholar]
- Blitvich, B. J. (2008). Transmission dynamics and changing epidemiology of West Nile virus. Animal Health Research Reviews, 9(1), 71–86. 10.1017/S1466252307001430 [DOI] [PubMed] [Google Scholar]
- Bowden, S. E. , Magori, K. , & Drake, J. M. (2011). Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. The American Journal of Tropical Medicine and Hygiene, 84(2), 234–238. 10.4269/ajtmh.2011.10-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2008). West Nile virus activity—United States, 2007. Morbidity and Mortality Weekly Report. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5726a2.htm [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2022). Symptoms, diagnosis, & treatment. Retrieved from https://www.cdc.gov/westnile/symptoms/index.html
- Centers for Disease Control and Prevention . (2023). West Nile virus. CDC. Retrieved from https://www.cdc.gov/westnile/index.html [Google Scholar]
- Chung, W. M. , Buseman, C. M. , Joyner, S. N. , Hughes, S. M. , Fomby, T. B. , Luby, J. P. , & Haley, R. W. (2013). The 2012 West Nile encephalitis epidemic in Dallas, Texas. Journal of the American Medical Association, 310(3), 297–307. 10.1001/jama.2013.8267 [DOI] [PubMed] [Google Scholar]
- Ciota, A. T. , Matacchiero, A. C. , Kilpatrick, A. M. , & Kramer, L. D. (2014). The effect of temperature on life history traits of Culex mosquitoes. Journal of Medical Entomology, 51(1), 55–62. 10.1603/ME13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, C. , Halbleib, M. , Smith, J. I. , Gibson, W. P. , Doggett, M. K. , Taylor, G. H. , et al. (2008). Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. International Journal of Climatology, 28(15), 2031–2064. 10.1002/joc.1688 [DOI] [Google Scholar]
- Daly, C. , Neilson, R. P. , & Phillips, D. L. (1994). A statistical‐topographic model for mapping climatological precipitation over mountainous terrain. Journal of Applied Meteorology and Climatology, 33(2), 140–158. [DOI] [Google Scholar]
- Deichmeister, J. M. , & Telang, A. (2011). Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. Journal of Vector Ecology, 36(1), 75–85. 10.1111/j.1948-7134.2011.00143.x [DOI] [PubMed] [Google Scholar]
- Dohm, D. J. , O’Guinn, M. L. , & Turell, M. J. (2002). Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. Journal of Medical Entomology, 39(1), 221–225. 10.1603/0022-2585-39.1.221 [DOI] [PubMed] [Google Scholar]
- Ellis, B. R. , & Wilcox, B. A. (2009). The ecological dimensions of vector‐borne disease research and control. Cadernos de Saúde Pública, 25, S155–S167. 10.1590/S0102-311X2009001300015 [DOI] [PubMed] [Google Scholar]
- Epp, T. Y. , Waldner, C. , & Berke, O. (2011). Predictive risk mapping of West Nile virus (WNV) infection in Saskatchewan horses. Canadian Journal of Veterinary Research, 75(3), 161–170. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3122974/ [PMC free article] [PubMed] [Google Scholar]
- Escobar, L. E. , & Craft, M. E. (2016). Advances and limitations of disease biogeography using ecological niche modeling. Frontiers in Microbiology, 7, 1174. 10.3389/fmicb.2016.01174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro, I. , Bellomo, C. M. , López, W. , Coelho, R. , Alonso, D. , Bruno, A. , et al. (2020). Hantavirus pulmonary syndrome outbreaks associated with climate variability in Northwestern Argentina, 1997‐2017. PLoS Neglected Tropical Diseases, 14(11), e0008786. 10.1371/journal.pntd.0008786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folke, C. (2006). Resilience: The emergence of a perspective for social–ecological systems analyses. Global Environmental Change, 16(3), 253–267. 10.1016/j.gloenvcha.2006.04.002 [DOI] [Google Scholar]
- Gallant, A. L. , Loveland, T. R. , Sohl, T. L. , & Napton, D. E. (2004). Using an ecoregion framework to analyze land‐cover and land‐use dynamics. Environmental Management, 34(S1), S89–S110. 10.1007/s00267-003-0145-3 [DOI] [PubMed] [Google Scholar]
- Gandrud, C. , Allaire, J. , Russell, K. , & Yetman, C. (2017). networkD3: D3 JavaScript network graphs from R [Computer software]. https://christophergandrud.github.io/networkD3/
- Godsey, M. S. , Burkhalter, K. , Young, G. , Delorey, M. , Smith, K. , Townsend, J. , et al. (2012). Entomologic investigations during an outbreak of West Nile virus disease in Maricopa County, Arizona, 2010. The American Journal of Tropical Medicine and Hygiene, 87(6), 1125–1131. 10.4269/ajtmh.2012.11-0700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris, M. E. (2024). lanl/climate_disease_connections: Meanannualclimatedata_v1.0 (climatedata) [Dataset]. Zenodo. 10.5281/zenodo.11403631 [DOI]
- Gorris, M. E. , Bartlow, A. W. , Temple, S. D. , Romero‐Alvarez, D. , Shutt, D. P. , Fair, J. M. , et al. (2021). Updated distribution maps of predominant Culex mosquitoes across the Americas. Parasites & Vectors, 14(1), 547. 10.1186/s13071-021-05051-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris, M. E. , Cat, L. A. , Zender, C. S. , Treseder, K. K. , & Randerson, J. T. (2018). Coccidioidomycosis dynamics in relation to climate in the southwestern United States. GeoHealth, 2(1), 6–24. 10.1002/2017GH000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris, M. E. , Randerson, J. T. , Coffield, S. R. , Treseder, K. K. , Zender, C. S. , Xu, C. , & Manore, C. A. (2023). Assessing the influence of climate on the spatial pattern of West Nile virus incidence in the United States. Environmental Health Perspectives, 131(4), 047016. 10.1289/EHP10986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, M. B. , Monaghan, A. J. , Hayden, M. H. , Eisen, R. J. , Delorey, M. J. , Lindsey, N. P. , et al. (2015). Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. The American Journal of Tropical Medicine and Hygiene, 92(5), 1013–1022. 10.4269/ajtmh.14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, J. R. , Sondermeyer‐Cooksey, G. , Heaney, A. K. , Yu, A. T. , Jones, I. , Bhattachan, A. , et al. (2022). Effects of precipitation, heat, and drought on incidence and expansion of coccidioidomycosis in western USA: A longitudinal surveillance study. The Lancet Planetary Health, 6(10), e793–e803. 10.1016/S2542-5196(22)00202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb, K. M. , Mathis, S. , Staples, J. E. , Fischer, M. , Barker, C. M. , Beard, C. B. , et al. (2023). Evaluation of an open forecasting challenge to assess skill of West Nile virus neuroinvasive disease prediction. Parasites & Vectors, 16, 11. 10.1186/s13071-022-05630-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover, K. C. , & Barker, C. M. (2016). West Nile virus, climate change, and circumpolar vulnerability. WIREs Climate Change, 7(2), 283–300. 10.1002/wcc.382 [DOI] [Google Scholar]
- Hueffer, K. , Parkinson, A. J. , Gerlach, R. , & Berner, J. (2013). Zoonotic infections in Alaska: Disease prevalence, potential impact of climate change and recommended actions for earlier disease detection, research, prevention and control. International Journal of Circumpolar Health, 72(1), 19562. 10.3402/ijch.v72i0.19562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain, M. P. , & Bolker, B. M. (2019). Predicting West Nile virus transmission in North American bird communities using phylogenetic mixed effects models and eBird citizen science data. Parasites & Vectors, 12, 395. 10.1186/s13071-019-3656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl, T. , & Koss, W. J. (1984). Regional and national monthly, seasonal, and annual temperature weighted by area, 1895‐1983 [Institutional Repository]. National Oceanic and Atmospheric Administration. Retrieved from https://repository.library.noaa.gov/view/noaa/10238 [Google Scholar]
- Karl, T. R. , & Koscielny, A. J. (1982). Drought in the United States: 1895–1981. Journal of Climatology, 2(4), 313–329. 10.1002/joc.3370020402 [DOI] [Google Scholar]
- Keller, M. , Schimel, D. S. , Hargrove, W. W. , & Hoffman, F. M. (2008). A continental strategy for the national ecological observatory network. The Ecological Society of America, 6(5), 282–284. 10.1890/1540-9295(2008)6[282:acsftn]2.0.co;2 [DOI] [Google Scholar]
- Keyel, A. C. , Gorris, M. E. , Rochlin, I. , Uelmen, J. A. , Chaves, L. F. , Hamer, G. L. , et al. (2021). A proposed framework for the development and qualitative evaluation of West Nile virus models and their application to local public health decision‐making. PLoS Neglected Tropical Diseases, 15(9), e0009653. 10.1371/journal.pntd.0009653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel, A. C. , Raghavendra, A. , Ciota, A. T. , & Elison Timm, O. (2021). West Nile virus is predicted to be more geographically widespread in New York State and Connecticut under future climate change. Global Change Biology, 27(21), 5430–5445. 10.1111/gcb.15842 [DOI] [PubMed] [Google Scholar]
- Kottek, M. , Grieser, J. , Beck, C. , Rudolf, B. , & Rubel, F. (2006). World Map of the Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15(3), 259–263. 10.1127/0941-2948/2006/0130 [DOI] [Google Scholar]
- Kramer, L. D. , Ciota, A. T. , & Kilpatrick, A. M. (2019). Introduction, spread, and establishment of West Nile virus in the Americas. Journal of Medical Entomology, 56(6), 1448–1455. 10.1093/jme/tjz151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer, M. , Ruberto, I. , Townsend, J. , Zabel, K. , Will, J. , Maldonado, K. , et al. (2023). Unprecedented outbreak of West Nile virus—Maricopa County, Arizona, 2021. American Journal of Transplantation, 23(6), 848–853. 10.1016/j.ajt.2023.05.004 [DOI] [PubMed] [Google Scholar]
- Landesman, W. J. , Allan, B. F. , Langerhans, R. B. , Knight, T. M. , & Chase, J. M. (2007). Inter‐annual associations between precipitation and human incidence of West Nile virus in the United States. Vector Borne and Zoonotic Diseases, 7(3), 337–343. 10.1089/vbz.2006.0590 [DOI] [PubMed] [Google Scholar]
- Lehmann, E. L. (1993). The Fisher, Neyman‐Pearson theories of testing hypotheses: One theory or two? Journal of the American Statistical Association, 88(424), 1242–1249. 10.1080/01621459.1993.10476404 [DOI] [Google Scholar]
- Levin, S. A. (1992). The problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology, 73(6), 1943–1967. 10.2307/1941447 [DOI] [Google Scholar]
- Lowe, R. , Bailey, T. C. , Stephenson, D. B. , Jupp, T. E. , Graham, R. J. , Barcellos, C. , & Carvalho, M. S. (2013). The development of an early warning system for climate‐sensitive disease risk with a focus on dengue epidemics in Southeast Brazil. Statistics in Medicine, 32(5), 864–883. 10.1002/sim.5549 [DOI] [PubMed] [Google Scholar]
- Manore, C. A. , Davis, J. K. , Christofferson, R. C. , Wesson, D. M. , Hyman, J. M. , & Mores, C. N. (2014). Towards an early warning system for forecasting human West Nile virus incidence. PLoS Currents, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra, P. P. , Francis, C. M. , Mulvihill, R. S. , & Moore, F. R. (2005). The influence of climate on the timing and rate of spring bird migration. Oecologia, 142(2), 307–315. 10.1007/s00442-004-1725-x [DOI] [PubMed] [Google Scholar]
- McDonnell, M. J. (1997). A paradigm shift. Urban Ecosystems, 1(2), 85–86. 10.1023/A:1018598708346 [DOI] [Google Scholar]
- McMahon, G. , Gregonis, S. M. , Waltman, S. W. , Omernik, J. M. , Thorson, T. D. , Freeouf, J. A. , et al. (2001). Developing a spatial framework of common ecological regions for the conterminous United States. Environmental Management, 28(3), 293–316. 10.1007/s0026702429 [DOI] [PubMed] [Google Scholar]
- Melidou, A. , Pereyaslov, D. , Hungnes, O. , Prosenc, K. , Alm, E. , Adlhoch, C. , et al. (2020). Virological surveillance of influenza viruses in the WHO European Region in 2019/20—Impact of the COVID‐19 pandemic. Euro Surveillance, 25(46), 2001822. 10.2807/1560-7917.ES.2020.25.46.2001822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, C. W. , & Comrie, A. C. (2013). Regional and seasonal response of a West Nile virus vector to climate change. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15620–15625. 10.1073/pnas.1307135110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, C. W. , Comrie, A. C. , & Ernst, K. (2013). Climate and dengue transmission: Evidence and Implications. Environmental Health Perspectives, 121(11–12), 1264–1272. 10.1289/ehp.1306556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, S. K. , Barnard, M. , Frantz, R. M. , Spencer, J. A. , Rodarte, K. A. , Crooker, I. K. , et al. (2023). Scoping review of Culex mosquito life history trait heterogeneity in response to temperature. Parasites & Vectors, 16, 200. 10.1186/s13071-023-05792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA . (2023). Geographical reference maps: U.S. climate regions. National Centers for Environmental Information (NCEI). Retrieved from https://www.ncei.noaa.gov/access/monitoring/reference‐maps/us‐climate‐regions [Google Scholar]
- Okuneye, K. , & Gumel, A. B. (2017). Analysis of a temperature‐ and rainfall‐dependent model for malaria transmission dynamics. Mathematical Biosciences, 287, 72–92. 10.1016/j.mbs.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Omernik, J. M. (1987a). Aquatic ecoregions of the conterminous United States. In Aquatic ecoregions of the conterminous United States [Dataset]. U.S. Geological Survey. 10.3133/70046266 [DOI] [Google Scholar]
- Omernik, J. M. (1987b). Map supplement: Ecoregions of the conterminous United States. Annals of the Association of American Geographers, 77(1), 118–125. 10.1111/j.1467-8306.1987.tb00149.x [DOI] [Google Scholar]
- Omernik, J. M. (2004). Perspectives on the nature and definition of ecological regions. Environmental Management, 34(1), S27–S38. 10.1007/s00267-003-5197-2 [DOI] [PubMed] [Google Scholar]
- Omernik, J. M. , & Griffith, G. E. (2014). Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environmental Management, 54(6), 1249–1266. 10.1007/s00267-014-0364-1 [DOI] [PubMed] [Google Scholar]
- Parham, P. E. , & Michael, E. (2010). Modeling the effects of weather and climate change on malaria transmission. Environmental Health Perspectives, 118(5), 620–626. 10.1289/ehp.0901256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, S. H. , Horton, D. E. , Ashfaq, M. , Rastogi, D. , Kramer, L. D. , Diffenbaugh, N. S. , & Kilpatrick, A. M. (2017). Drought and immunity determine the intensity of West Nile virus epidemics and climate change impacts. Proceedings of the Royal Society B: Biological Sciences, 284(1848), 20162078. 10.1098/rspb.2016.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebesma, E. , & Bivand, R. (2023). Spatial data science: With applications in R. Chapman and Hall/CRC. 10.1201/9780429459016 [DOI] [Google Scholar]
- Python . (2023). Welcome to Python [Computer software]. Python Software Foundation. Retrieved from https://www.python.org/
- R Core Team . (2023). R: A language and environment for statistical computing [Computer software]. Retrieved from https://www.R‐project.org/
- Ronca, S. E. , Ruff, J. C. , & Murray, K. O. (2021). A 20‐year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Neglected Tropical Diseases, 15(5), e0009190. 10.1371/journal.pntd.0009190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda, L. M. , Patel, K. J. , Axtell, R. C. , & Stinner, R. E. (1990). Temperature‐dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology, 27(5), 892–898. 10.1093/jmedent/27.5.892 [DOI] [PubMed] [Google Scholar]
- Shaman, J. , Day, J. F. , & Stieglitz, M. (2005). Drought‐induced amplification and epidemic transmission of West Nile virus in southern Florida. Journal of Medical Entomology, 42(2), 134–141. 10.1093/jmedent/42.2.134 [DOI] [PubMed] [Google Scholar]
- Silk, B. J. , Astles, J. R. , Hidalgo, J. , Humes, R. , Waller, L. A. , Buehler, J. W. , & Berkelman, R. L. (2010). Differential West Nile Fever ascertainment in the United States: A Multilevel analysis. The American Journal of Tropical Medicine and Hygiene, 83(4), 795–802. 10.4269/ajtmh.2010.10-0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo, I. , Barnard, M. , Spencer, J. A. , Keithley, J. , Martinez, K. M. , Crooker, I. , et al. (2023). Changing temperature profiles and the risk of dengue outbreaks. PLOS Climate, 2(2), e0000115. 10.1371/journal.pclm.0000115 [DOI] [Google Scholar]
- United States Geographical Society . (2019). Watersheds and Drainage Basins. Water Science School. Retrieved from https://www.usgs.gov/special‐topics/water‐science‐school/science/watersheds‐and‐drainage‐basins#overview [Google Scholar]
- US Census Bureau . (2010). County intercensal datasets: 2000‐2010. Census.Gov. Retrieved from https://www.census.gov/data/datasets/time‐series/demo/popest/intercensal‐2000‐2010‐counties.html [Google Scholar]
- US Census Bureau . (2020). County population totals: 2010‐2020. Census.Gov. Retrieved from https://www.census.gov/programs‐surveys/popest/technical‐documentation/research/evaluation‐estimates/2020‐evaluation‐estimates/2010s‐counties‐total.html [Google Scholar]
- US Census Bureau . (2019). TIGER/Line shapefiles. Census.Gov. Retrieved from https://www.census.gov/geographies/mapping‐files/time‐series/geo/tiger‐line‐file.html [Google Scholar]
- US EPA . (2015). Ecological regions of North America [Data and Tools]. Retrieved from https://www.epa.gov/eco‐research/ecoregions
- USGCRP . (2017). In Climate science special report: Fourth national climate Assessment, Volume I (Vol. 1, pp. 1–470). U.S. Global Change Research Program. Retrieved from https://science2017.globalchange.gov/ [Google Scholar]
- Wiken, E. , Gauthier, D. , Marshall, I. , Lawton, K. , & Hirvonen, H. (1996). Perspective on Canada’s ecosystems: An overview of the terrestrial and marine ecozones. Canadian Council on Ecological Areas. Occasional paper 14. [Google Scholar]
- Wimberly, M. C. , Lamsal, A. , Giacomo, P. , & Chuang, T.‐W. (2014). Regional variation of climatic influences on West Nile virus outbreaks in the United States. The American Journal of Tropical Medicine and Hygiene, 91(4), 677–684. 10.4269/ajtmh.14-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser, M. , Ramberg, F. , & Willott, E. (2004). Scientific Note: Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: Blood meal analysis indicates feeding on both humans and birds. Journal of Insect Science, 4(1), 20. 10.1093/jis/4.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gandrud, C. , Allaire, J. , Russell, K. , & Yetman, C. (2017). networkD3: D3 JavaScript network graphs from R [Computer software]. https://christophergandrud.github.io/networkD3/
- Gorris, M. E. (2024). lanl/climate_disease_connections: Meanannualclimatedata_v1.0 (climatedata) [Dataset]. Zenodo. 10.5281/zenodo.11403631 [DOI]
- Python . (2023). Welcome to Python [Computer software]. Python Software Foundation. Retrieved from https://www.python.org/
- R Core Team . (2023). R: A language and environment for statistical computing [Computer software]. Retrieved from https://www.R‐project.org/
Supplementary Materials
Supporting Information S1
Data Availability Statement
Additional tables and figures supporting the findings of this article are included within the Supporting Information S1 file. The West Nile virus case data we used in our study are not accessible to the public due to a data use agreement with the US Centers for Disease Control and Prevention. However, the CDC has now released public West Nile virus data on a website where users can download county‐level case counts by year (https://www.cdc.gov/west‐nile‐virus/data‐maps/historic‐data.html). The PRISM Climate data we used in this study, annually averaged to the NOAA climate regions and EPA ecoregions, are available on Zenodo (Gorris, 2024).


