Key Points
Question
What is the association between endophthalmitis after cataract surgery with disinfection in combination with selective antibiotic prophylaxis vs routine prophylactic antibiotic use?
Findings
In this cohort study, among 56 598 cataract surgical procedures performed at the Rotterdam Eye Hospital, postoperative endophthalmitis incidence was comparable when using 1% povidone iodine disinfection with selective antibiotic prophylaxis vs antibiotic prophylaxis use in 37 reported studies.
Meaning
The findings indicate comparable associations between cataract surgery with endophthalmitis after disinfection with 1% povidone iodine and selective antibiotic prophylaxis as was described in the literature after routine intraocular antibiotic use.
Thie cohort study compares postoperative endophthalmitis incidence following selective use of intracameral antibiotics with 1% povidine iodine vs routine use of intracameral antibiotics with 5% povidine iodine.
Abstract
Importance
Although the effectiveness of intracameral antibiotics to prevent postoperative endophthalmitis is described, selective use of antibiotics combined with 1% povidone iodine disinfection might be equally effective and could lead to cost reduction and avoidance of unnecessary use of antibiotics.
Objective
To compare the incidence of postoperative endophthalmitis when 1% povidone iodine disinfection is applied in combination with selective intracameral antibiotics with the incidence after routine use of intracameral antibiotics in combination with 5% povidone iodine.
Design, Setting, and Participant
This was a retrospective cohort study using incidence data from the ongoing endophthalmitis register of the Rotterdam Eye Hospital, a specialized hospital providing both secondary and tertiary ophthalmological care, when intracameral antibiotics were used only during cataract procedures with occurrence of a posterior capsular tear in comparison with results from cohorts described in the literature where routine antibiotics were used. All patients who had cataract (phacoemulsification) surgery at the Rotterdam Eye Hospital between 1993 and 2022 were included. No cataract surgical procedures combined with other intraocular procedures were included.
Exposure
Povidone iodine disinfection and intracameral antibiotics during cataract surgery either routinely or only in case of posterior capsular tears.
Main Outcome and Measure
Postoperative endophthalmitis incidence.
Results
Postoperative endophthalmitis incidence after 56 598 cataract (phacoemulsification) surgical procedures in the Rotterdam Eye Hospital between 2016 and 2022 was 0.0003 (95% CI, 0.0002-0.0004). A PubMed literature search until September 2023 with respect to the incidence of postoperative endophthalmitis after routine antibiotic prophylaxis yielded 37 publications with an overall postoperative endophthalmitis incidence of 0.0003 (95% CI, 0.0003-0.0004).
Conclusions and Relevance
No difference was observed between the postoperative endophthalmitis incidence during the last 7 years in the Rotterdam Eye Hospital and the overall postoperative endophthalmitis incidence after routine intracameral antibiotics prophylaxis as described in the literature. Disinfection with 1% povidone iodine in combination with selective antibiotic prophylaxis may be equally effective as routine antibiotic use and 5% povidone iodine.
Introduction
Endophthalmitis is a serious complication of intraocular surgery that can potentially lead to a substantial loss of vision in the affected eye. There is general consensus that the causative microorganisms leading to endophthalmitis frequently originate from the periocular surfaces and cavities1,2 and enter the eye during or shortly after surgery.3 Therefore, strict adherence to preventive measures around intraocular procedures is essential.
Infection prevention generally consists of a complex combination of measures, including personnel training, preoperative preventive measures, proper patient selection, disinfection and, in selected subgroups, prophylaxis with antibiotics. To avoid postoperative endophthalmitis after cataract surgery 2 major preventive measures have been recognized through the last decades. First, disinfection with povidone iodine had a significant effect on endophthalmitis incidence in a prospective trial,4 and for a long period was considered to be the most relevant prophylactic measure.5 After publication of the results of the prospective European Society of Cataract and Refractive Surgeons (ESCRS) multicenter study of the prophylaxis of endophthalmitis after cataract surgery on endophthalmitis prophylaxis in cataract surgery,6 much attention has been given to prophylaxis with intraocular antibiotics at the end of surgery. Nevertheless, some concerns were raised about the high incidence of endophthalmitis in the control group of the ESCRS trial.7,8 Subsequent studies on this subject were usually of a case-control design, where large cohorts of operations performed in the same setting were compared during episodes before and after introduction of an intracameral antibiotic prophylaxis measure. Most of these studies confirmed the prophylactic efficacy of intraocular antibiotics. Disinfection measures during these former investigations (including the ESCRS trial) generally include the application of 5% povidone iodine. Traditionally, much attention is paid in the Rotterdam Eye Hospital (REH) to a careful preoperative disinfection with 1% povidone iodine without routine use of antibiotics. Here, an analysis of the ongoing endophthalmitis registration at the REH is presented and compared with literature on endophthalmitis prevention in cataract surgery.
Methods
From 1993 onward, all postoperative cases of endophthalmitis occurring weeks after intraocular surgery in the REH have been registered. Two input sources are used: all individuals treated for endophthalmitis at the vitreoretinal surgery department at the REH were collected together with data from the microbiology department on vitreous and anterior chamber samples. As a large tertiary center for eye surgery with a 24-hour service, it is improbable that patients with severe postoperative complications attend other hospitals for this purpose. (Personal communication with the head of the Department of Ophthalmology of the nearby tertiary Erasmus University Medical Center confirmed that patients with endophthalmitis following surgery in the REH were not treated there.)
For our analysis, we identified 3 periods with distinct perioperative measures around cataract operations: 1993 to 1999, when 5% povidone iodine and no intraocular, peribulbar, or postoperative antibiotics were applied; 2000 to 2010, when 1% povidone iodine was applied preoperatively on the periocular skin and ocular surface and no prophylactic intraocular, peribulbar, or postoperative antibiotics; and 2016 to 2022, when 1% povidone iodine was applied as before and, in cases of posterior capsular rupture, ceftazidime/vancomycin was injected in the anterior chamber at the end of surgery. To avoid potential bias by suboptimal adherence (caused by an ongoing national debate about the efficacy of antibiotics prophylaxis) after introducing the selective intracameral antibiotic regimen (ceftazidime/vancomycin) in the REH, the period from 2011 to 2015 was excluded from this analysis.
As phacoemulsification was introduced early in the REH, most cataract procedures were performed with this technique in all three periods. All surgical procedures were performed in daycare and the average duration of the procedures did not change substantially over time. Phacoemulsification equipment was provided by Alcon and consisted of the Legacy (up to 2008), Infinity (2008-2017), and Centurion (from 2018) models. Incisions were mainly corneal and changed from 2.7 mm to 2.2 mm in 2016. From 1993 to 1999, mainly polymethyl methacrylate 5-mm intraocular lenses were implanted through a 5-mm scleral incision. After 1999, foldable hydrophobic acrylate implant lenses were used (Acrysof and from 2018 onward Technis ICB00/GIB00) where incisions were mainly corneal and changed from 2.7 mm to 2.2 mm in 2016. Apart from the disinfection measures mentioned above, there were no major changes in air management, operation theater design or attention toward patient related risk factors, such as lacrimal sac infections and blepharitis. No cataract surgical procedures combined with other intraocular surgery or immediately sequential bilateral cataract surgery procedures were included in this study. Vitreous samples of all individuals with endophthalmitis presenting at the REH are obtained at the operating theater after 1% povidone iodine disinfection and with the aid of a vitrectome introduced through a trocar.
A PubMed search was conducted until September 2023 and confirmed in Embase with the following combination of terms: cataract AND intracameral antibiotic AND (prophylactic OR prophylaxis OR prevention). From the results, studies were selected that reported the numbers of surgical procedures and postoperative endophthalmitis for a cohort receiving intracameral antibiotics, as well as for a control cohort that did not. After inspection for and exclusion of overlapping publications, the overall incidences were calculated for the intracameral antibiotics cohorts and for the control cohorts.9
As this was a retrospective study, ethical committee approval and informed consent were not necessary. This was confirmed by the review board of the REH.
Results
From 1993 to 1999, 36 cases of endophthalmitis occurred after 27 114 phacoemulsification procedures; from 2000 to 2010, 62 cases were recorded after 68 335 surgical procedures and from 2016 to 2022, 17 endophthalmitis cases were recorded after 56 598 phacoemulsification procedures (Table 1). Only cases occurring within 6 weeks after cataract surgery were included. The difference in incidence between the periods from 1993 to 1999 and from 2000 to 2010 was χ2, 0.0004 (95% CI, 0.000X-0.001X; P = .07), the difference between the periods from 2000 to 2010 and from 2016 to 2022 was χ2, 0.0006 (95% CI, 0.0003-0.0009; P < .001). Among the 17 endophthalmitis cases from 2016 to 2022, cultures yielded no growth in 5 cases, coagulase negative staphylococci in 8 cases and a single case each of Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus mitis, and Enterococcus faecalis. Fifteen cases occurred within 1 week after the cataract surgery and the remaining 2 within 2 postoperative weeks. All but 1 of the 17 endophthalmitis cases were uncomplicated surgical procedures where the intraocular lens was inserted in the capsular bag. The single complicated case needed anterior vitrectomy because of a posterior capsular tear with vitreous loss, and it was the only case in the endophthalmitis group where prophylactic antibiotics were applied; culture of the subsequent vitreous sample did not yield any bacterial growth.
Table 1. Incidence of Endophthalmitis After Cataract Surgery in the Rotterdam Eye Hospital.
| 1993-1999 | 2000-2010 | 2016-2022 | |
|---|---|---|---|
| Incidence (95% CI) | 0.001 (0.001-0.002) | 0.001 (0.001-0.002) | 0.0003 (0.0002-0.0004) |
The initial literature search yielded 281 hits. After selection, 37 studies remained (Table 2) for analysis and comparison with the incidence in the REH.6,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 In the Figure, the incidences of both cohorts of each study are shown, together with their corresponding 95% CIs. At the bottom of the Figure, the overall incidence calculated from the literature studies is shown for intracameral antibiotics vs no intracameral antibiotics, and the incidence in the REH from 2016 to 2022.
Table 2. Summary of Included Reference Studies.
| Source | Study design | No. | Antibiotics | Disinfection |
|---|---|---|---|---|
| Montan et al,10 2002 | Historic cohort as comparator | 66 282 | Cefuroxime | Chlorhexidine |
| Endophthalmitis Study Group,6 2007 | Randomized clinical trial | 16 211 | Cefuroxime | 5% Povidone iodine |
| Lundstrom et al,11 2007 | Parallel cohort | 225 471 | Cefuroxime | Chlorhexidine |
| Yu-Wai-Man et al,12 2008 | Historic cohort as comparator | 36 743 | Cefuroxime | 5% Povidone iodine |
| Anijeet et al,13 2010 | Historic cohort as comparator | 16 606 | Vancomycin | 5% Povidone iodine |
| Arshinoff and Bastianelli14 2011 | Parallel cohort | 112 536 | Cefuroxime/moxifloxacin/vancomycin | Unclear |
| Romero-Aroca et al,15 2012 | Historic cohort as comparator | 25 001 | Cefazolin | 5% Povidone iodine |
| Barreau et al,16 2012 | Historic cohort as comparator | 5115 | Cefuroxime | 5% Povidone iodine |
| Tan et al,17 2012 | Historic cohort as comparator | 50 177 | Cefazolin | 5% Povidone iodine |
| van der Merwe et al,18 2012 | Parallel cohort | 8190 | Cefuroxime | 2.5% Povidone iodine |
| Rodriguez-Caravaca et al,19 2013 | Historic cohort as comparator | 19 463 | Cefuroxime | 5% Povidone iodine |
| Myneni et al,20 2013 | Historic cohort as comparator | 25 296 | Cefuroxime | 5% Povidone iodine |
| Matsuura et al,21 2013 | Historic cohort as comparator | 34 752 | Moxifloxacin | Unclear |
| Friling et al,22 2013 | Parallel cohort | 464 755 | Cefuroxime | Chlorhexidine |
| Beselga et al,23 2014 | Historic cohort as comparator | 15 689 | Cefuroxime | 5% Povidone iodine |
| Rudnisky et al,24 2014 | Parallel cohort | 75 318 | Moxifloxacin/vancomycin | Unclear |
| Röck et al,25 2014 | Historic cohort as comparator | 31 752 | Cefuroxime | 5% Povidone iodine |
| Sharma et al,26 2015 | Historic cohort as comparator | 15 122 | Cefuroxime | 5% Povidone iodine |
| Rahman and Murphy,27 2015 | Historic cohort as comparator | 14 043 | Cefuroxime | 5% Povidone iodine |
| Katz et al,28 2015 | Historic cohort as comparator | 56 094 | Cefuroxime | Unclear |
| Rush et al,29 2015 | Historic cohort as comparator | 20 719 | Vancomycin | 5% Povidone iodine |
| Daien et al,30 2016 | Parallel cohort | 2 432 067 | Cefuroxime | Unclear |
| Au et al,31 2016 | Historic cohort as comparator | 14 805 | Vancomycin | 5% Povidone iodine |
| Ng et al,32 2016 | Historic cohort as comparator | 30 428 | Cefuroxime | 5% Povidone iodine |
| Herrinton et al,33 2017 | Parallel cohort | 300 950 | Cefuroxime/moxifloxacin | Unclear |
| Vieira et al,34 2017 | Historic cohort as comparator | 7195 | Moxifloxacin | 5% Povidone iodine |
| Haripriya et al,35 2019 | Historic cohort as comparator | 2 062 643 | Moxifloxacin | 5% Povidone iodine |
| Li et al,36 2019 | Historic cohort as comparator | 32 526 | Cefuroxime/moxifloxacin | Unclear |
| Moser et al,37 2019 | Historic cohort as comparator | 29 275 | Cefazolin | 5% Povidone iodine |
| Melega et al,38 2019 | Randomized clinical trial | 3640 | Moxifloxacin | 5% Povidone iodine |
| Rathi et al,39 2020 | Historic cohort as comparator | 92 010 | Cefuroxime/moxifloxacin | 5% Povidone iodine |
| Ma et al,40 2020 | Historic cohort as comparator | 61 299 | Cefuroxime | 5% Povidone iodine |
| Shenoy et al,41 2021 | Historic cohort as comparator | 214 782 | Moxifloxacin | 5% Povidone iodine |
| Bhatta et al,42 2021 | Historic cohort as comparator | 111 983 | Moxifloxacin | 5% Povidone iodine |
| Dave et al,43 2021 | Historic cohort as comparator | 66 967 | Moxifloxacin | 5% Povidone iodine |
| Porwal et al,44 2021 | Historic cohort as comparator | 40 392 | Moxifloxacin | Unclear |
| da Silva Conci et al,45 2022 | Historic cohort as comparator | 16 902 | Cefuroxime | 5% Povidone iodine |
Figure. Incidence of Endophthalmitis After Cataract Surgery With and Without Intracameral Antibiotics.
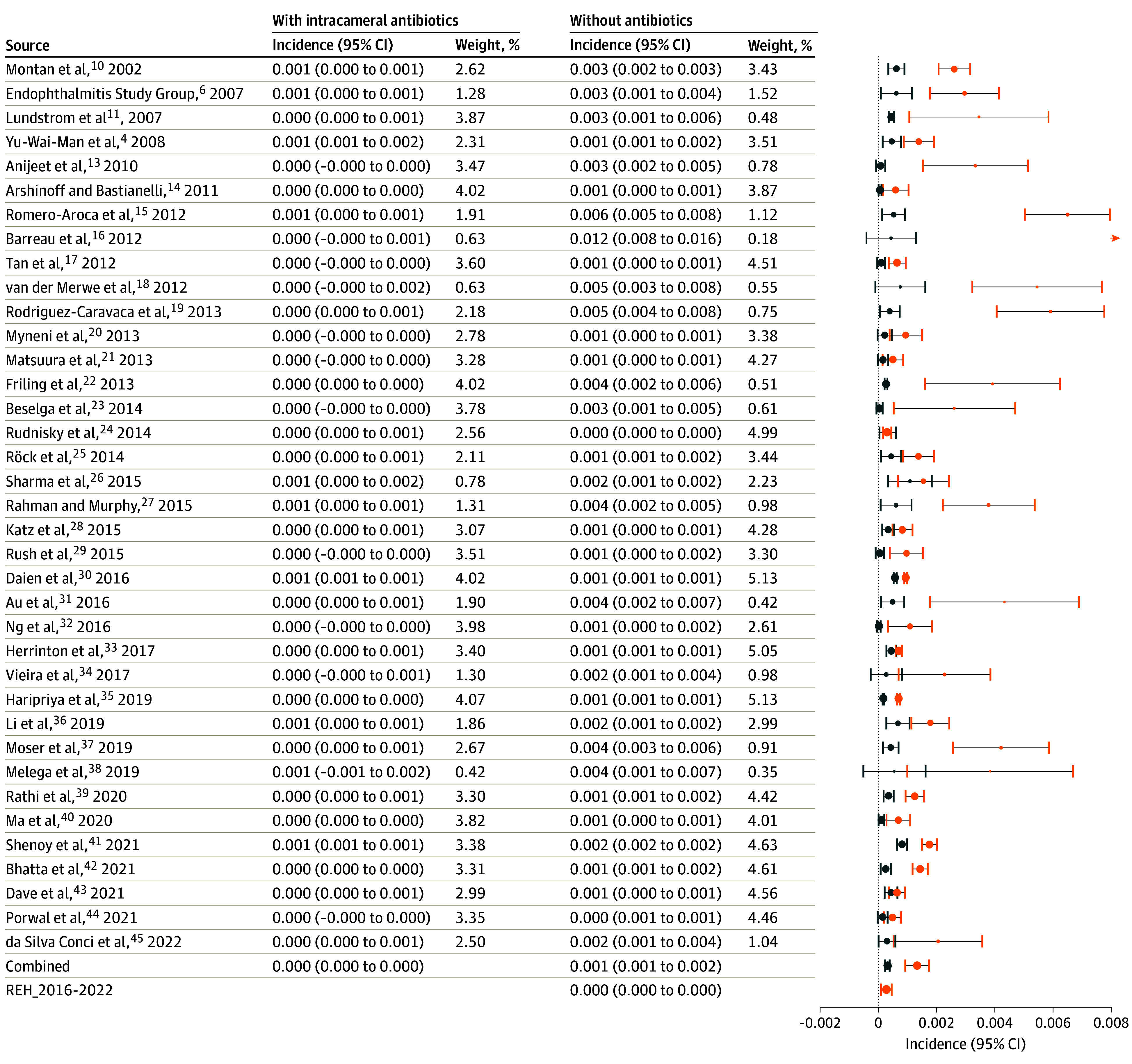
Blue indicates with antibiotics; orange, without, as reported in 37 studies, and in the Rotterdam Eye Hospital from 2016 to 2022. Postoperative endophthalmitis incidence in the Rotterdam Eye Hospital without standard prophylactic use of intracameral antibiotics did not differ from the overall mean incidence of 37 studies in which prophylactic intracameral antibiotics were used. In Rathi et al,39 reported incidence and study period were used to estimate totals of surgical procedures and postoperative endophthalmitis cases for the no antibiotic group assuming that number of surgical procedures per month was constant. For analytical purposes, in Beselga et al,23 Rush et al,29 and Ng et al,32 the incidence of 0 in the antibiotics group was replaced by 0.5.
Discussion
In this cohort study, between 2016 and 2022, when we conducted disinfection with 1% povidone iodine before cataract surgery and administered prophylactic intracameral antibiotics in complicated surgical procedures only, postoperative endophthalmitis incidence was 0.0003. This did not differ from the overall incidence calculated for the intracameral antibiotics cohorts of the 37 studies from literature (0.0003). The reliability of the reported postoperative endophthalmitis incidences of these studies was not always described in detail and, thus it cannot be excluded that some incidences were underestimated.
The introduction over time of additional preventive measures in the REH would potentially constitute a confounding factor of this study when comparing the 3 episodes. However, the only other substantial change was the replacement of HEPA filters by lamellar flow in our daycare center (ie, 2 of the 6 operating theaters) in 2013, which made no noticeable difference. As the REH has always had a very active infection prevention committee, we are confident that its postoperative endophthalmitis registry system is sound and that hardly any endophthalmitis case was missed. The reduction of postoperative endophthalmitis incidence we achieved (from 0.001 to 0.0003) by modifying the perioperative regimen between the periods from 1993 to 1999 and from 2016 to 2022 is comparable with the overall effect computed from the reports of 37 studies by the introduction of intracameral antibiotics (from 0.001 to 0.0003). Results similar to ours by means of changing perioperative disinfection over time, but without intracameral antibiotics, were reported before.46 The difference in postoperative endophthalmitis incidence we detected between the period from 2000 and 2010 and the period from 2016 to 2022 (Table 2) is an indication that intracameral antibiotics were associated with effective results in case of a posterior capsular tear during phacoemulsification surgery.
After the first randomized clinical trial on the effect of prophylactic antibiotics (cefuroxime),6 only 1 more randomized clinical trial has been performed (moxifloxacin).38 All other studies were cohort studies, 7 of which had a parallel control group and 28 of which had a historic control group. Most of the 37 studies confirm the prophylactic effect of intracameral antibiotics on postoperative endophthalmitis after cataract surgery (Figure). In most studies, either intracameral cefuroxime or moxifloxacin was administered.
Preoperative disinfection of the periorbital skin, eyelashes, and conjunctival sac with 5% povidone iodine was used in 25 studies and probably also in another 3 studies; 1 study used 2.5% povidone iodine, 3 studies used chlorhexidine, and 5 studies did not report their disinfection method. In this respect it seems noteworthy that the postoperative endophthalmitis incidence in the cohort without antibiotics of some studies was rather high (see Figure), which was also the case for the 2 randomized clinical trials. The frequent occurrence of staphylococci (20% to 66%) in endophthalmitis cases in studies providing details on causative strains10,11,20,47,48,49,50 appears to indicate a suboptimal preoperative disinfection of the periocular surface, which may have led to an unnecessary high incidence of postoperative endophthalmitis in some control cohorts.
More effective methods for preoperative disinfection with diluted povidone iodine have been described previously.51,52 A study on the effect of intracameral moxifloxacin in 2 million surgical procedures demonstrated a significant effect of moxifloxacin on the occurrence of coagulase negative staphylococci, but not on S aureus in culture-positive postoperative endophthalmitis cases.35 In our current study, where 1% povidone iodine was applied during the 7 years of the study period and in which 56 598 cataract surgical procedures were performed, S aureus was detected in only 1 endophthalmitis case; in 13 of the 17 cases, either no microorganism or a coagulase negative staphylococci was cultured. This is an indication that most virulent microorganisms were eradicated with our disinfection method, and the occurrence of only 1 staphylococcal endophthalmitis in this large cohort can be regarded as an indicator for effective skin disinfection. These clinical findings are in agreement with laboratory studies indicating a substantially enhanced antibacterial effect of diluted povidone iodine solutions.53,54
Previously, results have been presented from experiments subjecting some endophthalmitis strains to lower povidone iodine concentrations which showed a reduced susceptibility.55However, it is questionable whether the bacterial load used in such experiments adequately represents the exposure of the interior eye during perioperative contamination, and to what extent the organic substances present in such in vitro experiments inactivate the lower povidone iodine concentrations.56
When intravitreal injections for macular degeneration were introduced in our center, we managed to cut down an initial rise in endophthalmitis rate by means of standardization of the antiseptic protocol with 1% povidone iodine (unpublished data). Others have also advocated that endophthalmitis prophylaxis for intravitreal injections should focus on iodine disinfection rather than topical antibiotics.57,58 A comprehensive review of povidone iodine in cataract surgery that discusses the effect of different concentrations and dosing for disinfection also appears to support the use of diluted povidone iodine.59 Indications that disinfection measures with lower concentrations of povidone iodine may contribute to the avoidance of postoperative endophthalmitis are (1) theoretical considerations and experimental research,53,54 (2) previous clinical results from the REH,60 (3) results of investigations with diluted povidone iodine in Japan,61,62,63 and (4) the results of this study.
Due to the no nocere concept of medical interventions, (potential) adverse reactions should always be addressed. The dosage of intraocular antibiotics used in endophthalmitis prophylaxis is such that levels reached in the eye are well above the minimum inhibitory concentration of the targeted microorganisms and, therefore, adverse reactions are not hypothetical. In their review article on the relative efficacy of 3 intracameral antibiotics, Bowen et al50 also evaluate toxicity. Toxicity affecting visual acuity was detected in nearly 3% of 503 cases analyzed for cefuroxime, mostly attributed to local dilution errors. For moxifloxacin, such adverse events were only detected in patients with a penetrating corneal graft, and for vancomycin, in a single case series of a severe condition known as hemorrhagic occlusive retinal vasculitis. In addition, anaphylactic reactions to cefuroxime have been described in at least 4 patients,64 which may only reflect a small proportion of all cases occurring, considering the vast numbers of patients exposed to the compound and also because cataract registers do not keep track of this adverse event. On the other hand, after repeated application of povidone iodine during cataract surgery, no effect on corneal endothelial cell density was detected52 and the experimental data strongly suggest that endothelial or retinal damage after topical application of povidone iodine is improbable.65
Questions on widespread routine use of antibiotics in cataract surgery have been raised before,7 1 of the arguments being the accelerated inducement of bacterial resistance. The detection of prophylaxis escaping strains leading to endophthalmitis after cataract procedures with intracameral cefuroxime has been reported by several authors.6,11,66,67 Therefore, eradicating pathogens with an effective disinfectant before surgery appears to be a more rational approach than letting them enter the eye and subsequently applying potentially deleterious or only partially effective prophylactic antibiotics. Only in cases of complicated cataract surgery known to be associated with an increased risk of postoperative endophthalmitis6,35 would antibiotic prophylaxis be required.
Limitations
The main limitation of this study design is that it can only show associations, not causation. Furthermore, its retrospective design has a potential for selection and information bias. However, this is also the case for the retrospective studies of 35 of the studies we used for the comparison, and the 2 randomized trials6,38 from the literature showed a relatively high endophthalmitis incidence. The REH is highly specialized and the largest provider of secondary and tertiary eye care in the Netherlands, but not an academic hospital. The patient group, level of training, internal protocols, and facilities, might not be fully comparable to general hospitals nor academic centers.
Conclusions
In conclusion, the findings indicate comparable outcomes with antibiotic prophylaxis limited to complicated cataract surgery. Such indication may be preferred above routinely exposing large numbers of patients undergoing the most frequently performed surgical procedure in the world to prophylactic antibiotics. The REH data show that a low level of postoperative endophthalmitis was associated with 1% povidone iodine disinfection in combination with selective prophylactic antibiotic use and that postoperative endophthalmitis incidence did not substantially differ from routine antibiotic use. Although the routine use of prophylactic antibiotics in modern cataract surgery may still be a matter of ongoing debate, preoperative antiseptic preparation of the surgical field with diluted povidone iodine supports that discussion.
Data sharing statement
References
- 1.Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1-17. doi: 10.1016/S0002-9394(14)71959-2 [DOI] [PubMed] [Google Scholar]
- 2.Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98(5):639-649. doi: 10.1016/S0161-6420(91)32239-5 [DOI] [PubMed] [Google Scholar]
- 3.Hughes DS, Hill RJ. Infectious endophthalmitis after cataract surgery. Br J Ophthalmol. 1994;78(3):227-232. doi: 10.1136/bjo.78.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98(12):1769-1775. doi: 10.1016/S0161-6420(91)32052-9 [DOI] [PubMed] [Google Scholar]
- 5.Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109(1):13-24. doi: 10.1016/S0161-6420(01)00899-5 [DOI] [PubMed] [Google Scholar]
- 6.Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons . Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988. doi: 10.1016/j.jcrs.2007.02.032 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz SG, Flynn HW Jr, Grzybowski A, Relhan N, Ferris FL III. Intracameral antibiotics and cataract surgery: endophthalmitis rates, costs, and stewardship. Ophthalmology. 2016;123(7):1411-1413. doi: 10.1016/j.ophtha.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 8.George NK, Stewart MW. The routine use of intracameral antibiotics to prevent endophthalmitis after cataract surgery: how good is the evidence? Ophthalmol Ther. 2018;7(2):233-245. doi: 10.1007/s40123-018-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537-553. doi: 10.1002/jrsm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28(6):977-981. doi: 10.1016/S0886-3350(01)01269-X [DOI] [PubMed] [Google Scholar]
- 11.Lundström M, Wejde G, Stenevi U, Thorburn W, Montan P. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology. 2007;114(5):866-870. doi: 10.1016/j.ophtha.2006.11.025 [DOI] [PubMed] [Google Scholar]
- 12.Yu-Wai-Man P, Morgan SJ, Hildreth AJ, Steel DH, Allen D. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34(3):447-451. doi: 10.1016/j.jcrs.2007.10.041 [DOI] [PubMed] [Google Scholar]
- 13.Anijeet DR, Palimar P, Peckar CO. Intracameral vancomycin following cataract surgery: an eleven-year study. Clin Ophthalmol. 2010;4:321-326. doi: 10.2147/OPTH.S9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011;37(12):2105-2114. doi: 10.1016/j.jcrs.2011.06.036 [DOI] [PubMed] [Google Scholar]
- 15.Romero-Aroca P, Méndez-Marin I, Salvat-Serra M, Fernández-Ballart J, Almena-Garcia M, Reyes-Torres J. Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmol. 2012;12:2. doi: 10.1186/1471-2415-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreau G, Mounier M, Marin B, Adenis JP, Robert PY. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38(8):1370-1375. doi: 10.1016/j.jcrs.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 17.Tan CS, Wong HK, Yang FP. Epidemiology of postoperative endophthalmitis in an Asian population: 11-year incidence and effect of intracameral antibiotic agents. J Cataract Refract Surg. 2012;38(3):425-430. doi: 10.1016/j.jcrs.2011.09.040 [DOI] [PubMed] [Google Scholar]
- 18.van der Merwe J, Mustak H, Cook C. Endophthalmitis prophylaxis with intracameral cefuroxime in South Africa. J Cataract Refract Surg. 2012;38(11):2054. doi: 10.1016/j.jcrs.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Caravaca G, García-Sáenz MC, Villar-Del-Campo MC, Andrés-Alba Y, Arias-Puente A. Incidence of endophthalmitis and impact of prophylaxis with cefuroxime on cataract surgery. J Cataract Refract Surg. 2013;39(9):1399-1403. doi: 10.1016/j.jcrs.2013.03.031 [DOI] [PubMed] [Google Scholar]
- 20.Myneni J, Desai SP, Jayamanne DG. Reduction in postoperative endophthalmitis with intracameral cefuroxime. J Hosp Infect. 2013;84(4):326-328. doi: 10.1016/j.jhin.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Matsuura K, Miyoshi T, Suto C, Akura J, Inoue Y. Efficacy and safety of prophylactic intracameral moxifloxacin injection in Japan. J Cataract Refract Surg. 2013;39(11):1702-1706. doi: 10.1016/j.jcrs.2013.05.036 [DOI] [PubMed] [Google Scholar]
- 22.Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39(1):15-21. doi: 10.1016/j.jcrs.2012.10.037 [DOI] [PubMed] [Google Scholar]
- 23.Beselga D, Campos A, Castro M, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur J Ophthalmol. 2014;24(4):516-519. doi: 10.5301/ejo.5000417 [DOI] [PubMed] [Google Scholar]
- 24.Rudnisky CJ, Wan D, Weis E. Antibiotic choice for the prophylaxis of post-cataract extraction endophthalmitis. Ophthalmology. 2014;121(4):835-841. doi: 10.1016/j.ophtha.2013.08.046 [DOI] [PubMed] [Google Scholar]
- 25.Röck T, Bramkamp M, Bartz-Schmidt KU, et al. [Using intracameral cefuroxime reduces postoperative endophthalmitis rate: 5 years experience at the University Eye Hospital Tübingen]. Klin Monbl Augenheilkd. 2014;231(10):1023-1028. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Sahu SK, Dhillon V, Das S, Rath S. Reevaluating intracameral cefuroxime as a prophylaxis against endophthalmitis after cataract surgery in India. J Cataract Refract Surg. 2015;41(2):393-399. doi: 10.1016/j.jcrs.2014.05.038 [DOI] [PubMed] [Google Scholar]
- 27.Rahman N, Murphy CC. Impact of intracameral cefuroxime on the incidence of postoperative endophthalmitis following cataract surgery in Ireland. Ir J Med Sci. 2015;184(2):395-398. doi: 10.1007/s11845-014-1127-y [DOI] [PubMed] [Google Scholar]
- 28.Katz G, Blum S, Leeva O, et al. Intracameral cefuroxime and the incidence of post-cataract endophthalmitis: an Israeli experience. Graefes Arch Clin Exp Ophthalmol. 2015;253(10):1729-1733. doi: 10.1007/s00417-015-3009-z [DOI] [PubMed] [Google Scholar]
- 29.Rush SW, Vu D, Rush RB. The safety and efficacy of routine administration of intracameral vancomycin during cataract surgery. J Ophthalmol. 2015;2015:813697. doi: 10.1155/2015/813697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daien V, Papinaud L, Gillies MC, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmol. 2016;134(7):810-816. doi: 10.1001/jamaophthalmol.2016.1351 [DOI] [PubMed] [Google Scholar]
- 31.Au CP, White AJ, Healey PR. Efficacy and cost-effectiveness of intracameral vancomycin in reducing postoperative endophthalmitis incidence in Australia. Clin Exp Ophthalmol. 2016;44(9):803-811. doi: 10.1111/ceo.12789 [DOI] [PubMed] [Google Scholar]
- 32.Ng AL, Tang WW, Li PS, Li KK. Intracameral cefuroxime in the prevention of postoperative endophthalmitis: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1987-1992. doi: 10.1007/s00417-016-3473-0 [DOI] [PubMed] [Google Scholar]
- 33.Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294. doi: 10.1016/j.ophtha.2015.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieira IV, Boianovsky C, Saraiva TJ, Godoy RB, Lake J. Safety and efficacy of intracameral moxifloxacin injection for prophylaxis of endophthalmitis after phacoemulsification. Arq Bras Oftalmol. 2017;80(3):165-167. doi: 10.5935/0004-2749.20170040 [DOI] [PubMed] [Google Scholar]
- 35.Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin in eyes with and without surgical complications: results from 2 million consecutive cataract surgeries. J Cataract Refract Surg. 2019;45(9):1226-1233. doi: 10.1016/j.jcrs.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 36.Li A, Shao J, Gans R, Bena J, Goshe J. Postoperative endophthalmitis before and after preferred utilization of prophylactic intracameral antibiotics for phacoemulsification cataract surgeries at Cole Eye Institute. Eye Contact Lens. 2019;45(5):306-309. doi: 10.1097/ICL.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 37.Moser CL, Lecumberri Lopez M, Garat M, Martín-Baranera M. Prophylactic intracameral cefazolin and postoperative topical moxifloxacin after cataract surgery: endophthalmitis risk reduction and safety results in a 16-year study. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2185-2191. doi: 10.1007/s00417-019-04417-9 [DOI] [PubMed] [Google Scholar]
- 38.Melega MV, Alves M, Cavalcanti Lira RP, et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: Randomized controlled clinical trial. J Cataract Refract Surg. 2019;45(3):343-350. doi: 10.1016/j.jcrs.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 39.Rathi VM, Sharma S, Das T, Khanna RC. Endophthalmitis prophylaxis study. report 1: intracameral cefuroxime and moxifloxacin prophylaxis for the prevention of postcataract endophthalmitis in rural India. Indian J Ophthalmol. 2020;68(5):819-824. doi: 10.4103/ijo.IJO_1400_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Xie L, Huang Y. Intraoperative cefuroxime irrigation prophylaxis for acute-onset endophthalmitis after phacoemulsification surgery. Infect Drug Resist. 2020;13:1455-1463. doi: 10.2147/IDR.S252674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenoy P, Goh EJH, Kashikar R, et al. Impact of prophylactic intracameral moxifloxacin on post-cataract surgery endophthalmitis: data from a tertiary eye care facility in rural India. Int Ophthalmol. 2021;41(8):2729-2736. doi: 10.1007/s10792-021-01830-0 [DOI] [PubMed] [Google Scholar]
- 42.Bhatta S, Pant N, Poudel M. Postoperative endophthalmitis with and without intracameral moxifloxacin prophylaxis in a high volume surgery setting. BMJ Open Ophthalmol. 2021;6(1):e000609. doi: 10.1136/bmjophth-2020-000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dave VP, Singh VM, Reddy JC, Sharma S, Joseph J, Das T. Clinical features and microbiology of post-cataract surgery endophthalmitis with and without intracameral moxifloxacin prophylaxis: endophthalmitis prophylaxis study report 3. Indian J Ophthalmol. 2022;70(1):158-163. doi: 10.4103/ijo.IJO_1405_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porwal AC, Patel A, Mathew BC, Jethani JN. Incidence of postoperative endophthalmitis with and without use of intracameral moxifloxacin. Indian J Ophthalmol. 2021;69(5):1353-1354. doi: 10.4103/ijo.IJO_365_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conci LDS, Favarato AP, Pinheiro AG. Cost effectiveness of intracameral cefuroxime prophylaxis and its efficacy in preventing endophthalmitis after cataract surgery in a referral hospital. Arq Bras Oftalmol. 2023;86(4):308-313. doi: 10.5935/0004-2749.20230052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nentwich MM, Ta CN, Kreutzer TC, et al. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J Cataract Refract Surg. 2015;41(1):58-66. doi: 10.1016/j.jcrs.2014.04.040 [DOI] [PubMed] [Google Scholar]
- 47.García-Sáenz MC, Arias-Puente A, Rodríguez-Caravaca G, Bañuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery—ten-year comparative study. J Cataract Refract Surg. 2010;36(2):203-207. doi: 10.1016/j.jcrs.2009.08.023 [DOI] [PubMed] [Google Scholar]
- 48.Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308. doi: 10.1016/j.ophtha.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 49.Galvis V, Tello A, Sánchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmol Eye Dis. 2014;6:1-4. doi: 10.4137/OED.S13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowen RC, Zhou AX, Bondalapati S, et al. Comparative analysis of the safety and efficacy of intracameral cefuroxime, moxifloxacin and vancomycin at the end of cataract surgery: a meta-analysis. Br J Ophthalmol. 2018;102(9):1268-1276. doi: 10.1136/bjophthalmol-2017-311051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koerner JC, George MJ, Kissam EA, Rosco MG. Povidone-iodine concentration and in vitro killing time of bacterial corneal ulcer isolates. Digit J Ophthalmol. 2018;24(4):24-26. doi: 10.5693/djo.01.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimada H, Arai S, Nakashizuka H, Hattori T, Yuzawa M. Reduced anterior chamber contamination by frequent surface irrigation with diluted iodine solutions during cataract surgery. Acta Ophthalmol. 2017;95(5):e373-e378. doi: 10.1111/aos.13390 [DOI] [PubMed] [Google Scholar]
- 53.Rackur H. New aspects of mechanism of action of povidone-iodine. J HospInfect. 1985;6 (Suppl A):13-23. doi: 10.1016/s0195-6701(85)80041-4 [DOI] [PubMed] [Google Scholar]
- 54.Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15(4):635-639. doi: 10.1128/jcm.15.4.635-639.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosseini H, Ashraf MJ, Saleh M, et al. Effect of povidone-iodine concentration and exposure time on bacteria isolated from endophthalmitis cases. J Cataract Refract Surg. 2012;38(1):92-96. doi: 10.1016/j.jcrs.2011.06.030 [DOI] [PubMed] [Google Scholar]
- 56.Zamora JL, Price MF, Chuang P, Gentry LO. Inhibition of povidone-iodine’s bactericidal activity by common organic substances: an experimental study. Surgery. 1985;98(1):25-29. [PubMed] [Google Scholar]
- 57.Wykoff CC, Flynn HW Jr, Rosenfeld PJ. Prophylaxis for endophthalmitis following intravitreal injection: antisepsis and antibiotics. Am J Ophthalmol. 2011;152(5):717-9.e2. doi: 10.1016/j.ajo.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 58.Chen RW, Rachitskaya A, Scott IU, Flynn HW Jr. Is the use of topical antibiotics for intravitreal injections the standard of care or are we better off without antibiotics? JAMA Ophthalmol. 2013;131(7):840-842. doi: 10.1001/jamaophthalmol.2013.2524 [DOI] [PubMed] [Google Scholar]
- 59.Koerner JC, George MJ, Meyer DR, Rosco MG, Habib MM. Povidone-iodine concentration and dosing in cataract surgery. Surv Ophthalmol. 2018;63(6):862-868. doi: 10.1016/j.survophthal.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 60.van Rooij J, Boks AL, Sprenger A, et al. The concentration of povidone-iodine for preoperative disinfection: relation to endophthalmitis incidence. Am J Ophthalmol. 2011;152(2):321; author reply −2. doi: 10.1016/j.ajo.2011.03.036 [DOI] [PubMed] [Google Scholar]
- 61.Shimada H, Arai S, Nakashizuka H, Hattori T, Yuzawa M. Reduction of anterior chamber contamination rate after cataract surgery by intraoperative surface irrigation with 0.25% povidone-iodine. Am J Ophthalmol. 2011;151(1):11-17.e1. doi: 10.1016/j.ajo.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 62.Shimada H, Nakashizuka H, Hattori T, et al. Reducing bacterial contamination inside fluid catch bag in 25-gauge vitrectomy by use of 0.25 % povidone-iodine ocular surface irrigation. Int Ophthalmol. 2013;33(1):35-38. doi: 10.1007/s10792-012-9621-6 [DOI] [PubMed] [Google Scholar]
- 63.Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Reduction of vitreous contamination rate after 25-gauge vitrectomy by surface irrigation with 0.25% povidone-iodine. Retina. 2013;33(1):143-151. doi: 10.1097/IAE.0b013e318261a6ce [DOI] [PubMed] [Google Scholar]
- 64.Mahiat C, Robaye S, Levecq L, et al. Anaphylactic shock following cataract surgery: a documented intracameral cefuroxime allergy. J Investig Allergol Clin Immunol. 2021;32(3):236-238. doi: 10.18176/jiaci.0741 [DOI] [PubMed] [Google Scholar]
- 65.Whitacre MM, Crockett RS. Tolerance of intravitreal povidone-iodine in rabbit eyes. Curr Eye Res. 1990;9(8):725-732. doi: 10.3109/02713689008999567 [DOI] [PubMed] [Google Scholar]
- 66.Friling E, Montan P. Bacteriology and cefuroxime resistance in endophthalmitis following cataract surgery before and after the introduction of prophylactic intracameral cefuroxime: a retrospective single-centre study. J Hosp Infect. 2019;101(1):88-92. doi: 10.1016/j.jhin.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 67.Mesnard C, Beral L, Hage R, Merle H, Farès S, David T. Endophthalmitis after cataract surgery despite intracameral antibiotic prophylaxis with licensed cefuroxime. J Cataract Refract Surg. 2016;42(9):1318-1323. doi: 10.1016/j.jcrs.2016.06.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data sharing statement


