Abstract
Arachis hypogaea is the most significant oilseed nutritious legume crop in agricultural trade across the world. It is recognized as a valued crop for its contributions to nourishing food, as a cooking oil, and for meeting the protein needs of people who are unable to afford animal protein. Currently, its production, marketability, and consumption are hindered because of Aspergillus species infection that consequently contaminates the kernels with aflatoxins. Regarding health concerns, humans and animals are affected by acute and chronic aflatoxin toxicity and millions of people are at high risk of chronic levels. Most methods used to store peanuts are traditional and serve effectively for short-term storage. Now the question for long-term storage has been raised, and this promptly finds potential approaches to the issue. It is imperative to reduce the aflatoxin levels in peanuts to a permissible level by introducing detoxifying innovations. Most of the detoxification reports mention physical, chemical, and biological techniques. However, many current approaches are impractical because of time consumption, loss of nutritional quality, or weak detoxifying efficiency. Therefore, it is crucial to investigate practical, economical, and green methods to control Aspergillus flavus that address current global food security problems. Herein, a green and economically revolutionary way is a nanotechnology that has demonstrated its potential to connect farmers to markets, elevate international marketability, improve human and animal health conditions, and enhance food quality and safety by the management of fungal diseases. Due to the antimicrobial potential of nanoparticles, they act as nanofungicides and have an incredible role in the control of aflatoxins. Nanoparticles have ultrasmall sizes and therefore penetrate the fungal body and invade the pathogen machinery, leading to fungal cell death by ROS production, mutation in DNA, disruption of organelles, and membrane leakage. This is the first mechanistic overview that unveils a comprehensive insight into aflatoxin contamination in peanuts, its prevalence, health effects, and management in addition to nanotechnological interventions that serve as a triple defense approach to detoxify aflatoxins. The optimum use of nanofungicides ensures food safety and the development of goals, especially “zero hunger”.
1. Introduction
Cultivated Arachis hypogaea, commonly known as peanut, monkey nut, groundnut, and earthnut, is recognized as a “longevity fruit” because of its nutritional health benefits. Peanut has a strategic position among the world’s cultivated cash crops. It is placed as the fourth most important oil crop and, being in the thirteenth position among food crops worldwide, is cultivated in more than 100 countries.1 The peanut crop is a key contributor to fighting malnutrition because it serves as a rich source of nutritional content, unsaturated fats, digestible proteins, carbohydrates, and minerals as well as a source of income for many underprivileged farmers in developing countries.2 Abrupt climatic conditions and adverse agroecological environments have led to a decline in plant productivity, resulting in food security challenges. In the context of addressing food security and alleviating hunger, peanuts stand out as a highly promising food source to meet nutritional needs.3 The major constituents of peanut seeds encompass carbohydrates ranging 20–105%, protein (16–36%), and oil content (36–54%), as well as medically important bioactive compounds.4 Additionally, peanuts are a rich source of beneficial minerals like calcium, magnesium, iron, zinc, phosphorus, manganese, copper, sodium, potassium, and selenium. Furthermore, peanuts are also enriched with various essential vitamins, including tocopherols, folic acid, and thiamine.5 The high antioxidant capacity of peanuts can be attributed to their rich content of various bioactive compounds, such as vitamin E, resveratrol, flavonoids, and a variety of hydroxycinnamic acids, which include caffeic, chlorogenic, coumaric, and ferulic acids.6 These bioactive compounds and multinutrients in peanuts make it a valuable functional food crop, and their occurrence in peanut seeds enhances their nutraceutical properties. Numerous studies have demonstrated that peanuts contain substantial quantities of phenolic compounds, such as flavonoids and polyphenols, that significantly contribute to their strong antioxidant capabilities.7,8 Peanut seeds contain significant amounts of phytosterols, which play a key role in various health benefits,9 including strong antitumor properties that can reduce the proliferation of different types of cancer cells.10 Additionally, peanut seeds and their food products have abundant amounts of arginine, which offers protective effects against gastrointestinal issues, also involved in spermatogenesis, mascular activity, and potential antiaging benefits.11
However, peanut production faces several challenges including drought stress, many diseases such as rosette, early and late leaf spots, blight spots, and rust, as well as pests like aphids and leaf miners.12 Biotic restraints associated with yield loss include weeds, insects, pests, and diseases. The deleterious impact of biotic stresses on the crop’s yield reflects that locally grown cultivars have low yield potential and also lack disease and insect resistance. All around the world peanut production is very limited due to several biotic and abiotic factors that lead to severe yield loss.13 Seed-borne infectious pathogens adversely influence the seedpod quality and reduce the yield. Several biotic stresses are well-known to lower peanut crop growth and agronomic production, and the disease severity and range of distribution differ with the growing season, cropping system, and region.14 A foremost limiting challenge in the profitable farming of peanuts is the mainly fungal attack of many diseases, which results in a huge loss of peanut yield at every growth stage relatively from the sowing period to harvest and then to storage. Peanut seeds are prone to harbor numerous seed- and soil-borne pathogenic fungi such as Aspergillus flavus, Aspergillus niger, Macrophomina phaseolina, Rhizoctonia solani,Fusarium oxysporum, and Fusarium solani, which were predominantly found in peanut and critically infected the peanut seed coat followed by the cotyledon and then eventually the seed axis.15 In addition to these limitations, mold infections, particularly A. flavus and Aspergillus parasiticus, in peanuts can cause the seed to become contaminated with aflatoxins.16 These aflatoxins (AFs) have the potential to make peanuts and their derived products unsuitable for both consumption and commercial trade.14 There is a wealth of literature documenting the prevalence of AFs in food commodities and their detoxification approaches.
The novelty and impactful aspect of this comprehensive review lie in the exploration of mechanistically innovative nanotechnology for AF detoxification from peanut with special reference to antimicrobial behavior, photocatalytic oxidation, and the molecular defensive role of nanoparticles that constrain A. flavus growth and control the toxin level. In addition, the current study also focuses on the prevalence, global health concerns, and control strategies along with the role of nanotechnology in the detoxification of AFs from peanut crops to ensure food security and safety for good health and socioeconomic prosperity.
2. Aflatoxins: A Major Threat to Peanut Crop
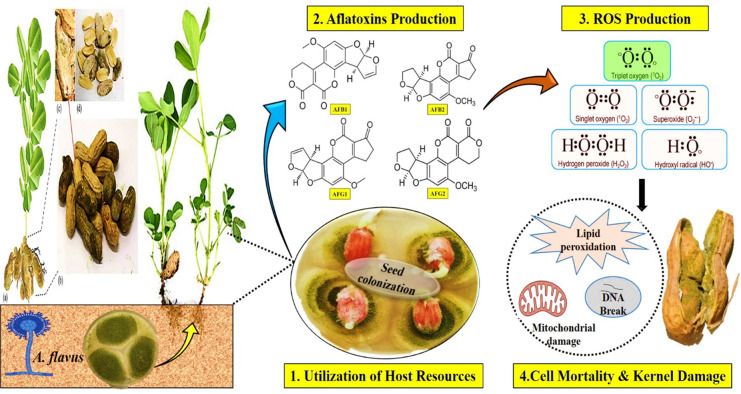
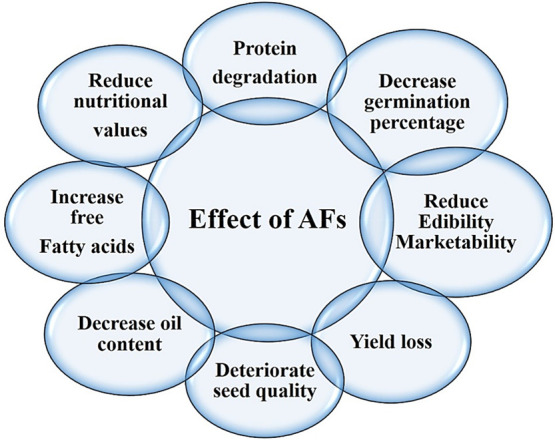
Agricultural commodities can be infrequently contaminated with mycotoxin-producing fungal agents, especially A. flavus, that produce toxins. From this attribute its name was originated: it causes sensorial changes, deteriorates nutrition, lowers seed quality attributes such as pigmentation, results in rotting and discoloration, and produces unpleasant odors and flavors. The most distinguished consequence of the presence of Aspergillus species on food and feed products is highly carcinogenic aflatoxin contamination.17 Among the other cereal and oilseed crops, the peanut crop is highly susceptible to aflatoxin contamination.18 Peanut pods produce under the soil; that is the main inoculum source for A. flavus leading to infection in peanut seeds.19 Therefore, peanut is found to be higher in aflatoxin content as compared to other food commodities and becomes a main carcinogen exposure source for humans. This is coherent with a previous study that reported in the world groundnut is highly contaminated with aflatoxin.16 To complement undernutrition, the peanut crop is produced on a large scale and is consumed in African countries for enhancing easy access to highly nutritious food. However, it is one of the crucial susceptible legumes for mold infection and is an aflatoxin contamination prone crop. In countries where peanut is widely grown, aflatoxins are a highly toxicological constraint of peanut crops. If the level of aflatoxins in the seeds is beyond the threshold level, than it significantly reduce the economic yield may be up to 100% and adversely affect the export share (Figure 1).20
Figure 1.
Deleterious effects of aflatoxins on peanut.
Asian and Western major peanut-producing countries have strict aflatoxin estimation standards of sanitary and phytosanitary for the market trade of groundnuts, which adversely affects export marketability to other countries and leads to a decrease in export potential of peanut products such as kernels, peanut butter, and oil. One of the investigations exposed that most farmers have awareness about biotic constraints such as pathogen attack, obsolete cultivars, and other environmental factors such as temperature, drought, and moisture content in soil seriously affecting the peanut crop productivity, but AF contamination is not revealed to farmers as a quick production threatening constraint.21 It has become worse because AFs are invisible, flavorless, colorless, and odorless, with long-term detrimental effects on human health and livestock.22 Moreover, mycotoxins produce Aspergillus spp. infection prevalent on peanut crops and AFs in peanut seeds are more noticeable in those areas which are prone to high moisture content, high temperatures, poor harvest management strategies, and improper storage conditions. Aflatoxin-producing fungi contaminate many food crops such as peanuts, corn, grain, legumes, and pulses by the growth of mycelium in seed tissues. Studies reported that carcinogenic A. flavus contaminates peanut shells systematically before harvesting. The incidence of aflatoxin is exacerbated in most peanut-producing countries because regular monitoring and evaluation are ineffective.13
The purpose of this comprehensive review is to offer a better understanding of aflatoxin-producing Aspergillus spp. and their impacts on peanut crop growth and quality deterioration. Moreover, the effects of aflatoxins on humans and livestock, and convenient mitigating approaches that can help to control them, are discussed. The two most toxigenic species of the Aspergillus genus, A. flavus and A. parasiticus, produce aflatoxins. Aflatoxins are decaketide-derived mycotoxins that have adverse health consequences and deteriorate several most economically significant cash crops such as peanut, maize, rice, sorghum, sugarcane, cotton, wheat, pearl millet, and some other wild fruits that ultimately cause financial implications.2,23,24 Aflatoxigenic Aspergillus fungi are present in the form of mycelium networks, conidia, and sclerotia that colonize the soil. The Aspergillus genus is the most prevalent genus among other broadly dispersed molds on earth and has 339 renowned species to date. Although Aspergillus species are not thought to be a main source of plant disorders, they are the primary source of problems in a number of crops and their food products, particularly as voracious storage molds. The prominent consequence of their proliferation is the contamination and deterioration of agricultural products by mycotoxins; out of all these, the most potent carcinogenic toxins are aflatoxins and ochratoxins, while fumonisins are less extensive. Among others in the mycotoxin family, most carcinogenic aflatoxins are classified into four different groups such as AFB1, AFB2, AFG1, and AFG2 (Figure 2) based on their mutagenic potential and teratogenic, toxicogenic, and extreme hepatocarcinogenic properties.17 Mechanical damages and climatic factors such as hot humid conditions and heat or drought stress injure the peanut pod walls, and the seed coat becomes cracked, through which fungal hyphae can invade the seeds and favor groundnut predisposed to aflatoxin contamination.25 Under drought stress, the moisture content decreases in pods causing cracks in their walls and facilitating the penetration of mycotoxigenic-producing A. flavus fungi. After colonization of seeds biotic stress causes oxidative damage which ultimately causes cell death. Low water level inhibits the production of the biogenic compound phytoalexin that is responsible for the elevation of AF level in seeds.26 Drought stress promotes aflatoxin contamination, and its tolerance cannot reduce the toxin level.27 According to the literature, many nutritional and climatic factors such as carbon, nitrogen sources, and pH levels regulate the AF synthetic gene expression and ultimately AF production.28
Figure 2.
A. flavus hijacks the host machinery, leading to cell death and kernel damage.
Aflatoxigenic fungi primarily produce AFs in a wide range of substrates, despite the fact that these are potent highly toxigenic fungi.29 Some other species of genus Aspergillus, such as A. australis, A. bombycis, A. pseudotamarii, A. novoparasiticus, A. nomius, and A. ochraceoroseus, are also responsible for AFs in foodstuff.30
3. Effect of Aflatoxins on Oil Quality of A. hypogaea
Peanut, being a king of oilseed crops, promotes the growth of pathogenic fungi that cause biodeterioration by lipase production.31 Throughout the world, aflatoxin contamination is a serious threat that is caused prominently by the A. flavus fungal pathogen. It is a critical issue of seed quality deterioration and imposed health problems that lower the market trade system and threaten the consumption of peanut.32 The oil extracted from A. flavus infected peanut seeds shows a higher concentration of aflatoxins in the peanut seed oil. Hence, it becomes an extremely toxic food product that is unhealthy for human consumption.33 It is reported that A. flavus colonization on various groundnut cultivars depicts the altered biochemical profile in infected seeds. The results also revealed that toxin-producing fungal growth on seeds resulted in decreased levels of oil content, carbohydrates, proteins, and bioactive compounds and increased unsaturated fatty acids.34 Mycotoxin-producing Aspergillus fungal species such as A. terreus cause changes in oilseed crop quality and recorded degradation of protein content. It was also noticed that saturated fatty acids in groundnut seeds and soybean are decreased due to A. flavus fungal contamination.35 The change in nutritional values of groundnut seeds starts during a storage period of about 20 weeks. An experiment was performed on stored groundnut seeds, and it was found that a total of seven pathogenic fungal species—A. flavus, A. fumigatus, A. niger, Rhizopus, Mucor, Penicillium, and Fusarium—were identified. These pathogenic fungi cause contamination and reduce the nutritional value, edibility, and marketability of groundnut, ultimately affecting the agricultural economy.36 Soil-borne opportunistic A. flavus pathogen infection deteriorates the groundnut seed quality by decreasing its nutritional value, such as lowering the oil content and increasing unsaturated fatty acids that lead to reduced edibility and marketability.37 One of the studies was performed to check the relationship between stored groundnut seed quality and A. flavus infection, and the outcomes demonstrated that the seeds that are inoculated with 0.25% fungal infection maintained their germination growth up to 71%, hence suggesting that this level could be an acceptable and permissible limit for the appropriate storage of seeds.38 According to one study, peanut seeds with a high moisture level had higher Aspergillus species fungal infections and the AF contamination level was more severe in comparison to lower moisture regimes.39 Many surveys were carried out to check the A. flavus contamination severity index and prevalence. The outcomes presented that A. flavus prevalence mainly was observed from market area collected peanut samples. Pathogen contaminated peanut seeds showed decreases in germination percentage and oil, carbohydrate, and protein contents and an increase free fatty acids.40 Preferentially aflatoxins are produced in peanut kernel cotyledons upon A. flavus infection. So, it is an alarming situation and needs attention to devise potential strategies to overcome aflatoxin contamination in peanut.
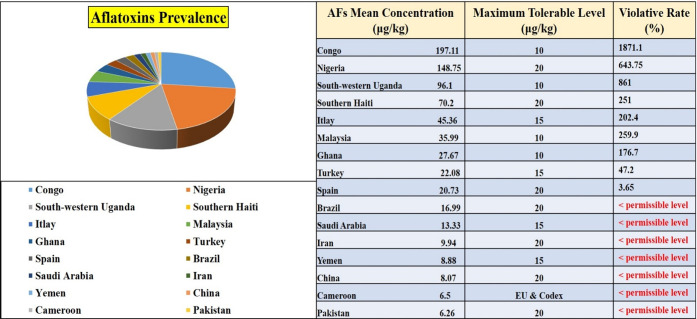
In this current mechanistic overview, we have emphasized several developments in efforts to improve the seed nutritional profile, oil quality of peanut, and crop physiology and have focused on the critical issue of aflatoxin contamination and its mitigation by significant approaches. We also highlight innovative approaches to combat aflatoxin contamination and increase the food nutritional value of peanut and income security under biotic stress. In light of rising attention about quality of life, recently food safety has emerged as a central focus in order to extend the shelf life of agricultural products and maximize their health benefits. A systematic review was conducted and reported the prevalence of aflatoxin mean concentrations in peanut samples. Researchers collected the data from different countries from 2000 to 2020. We used data from refs (41−47) and present the prevalence in a pie graph along with permissible standards of various countries and frequency rates of total AFs (Figure 3).
Figure 3.
Prevalence and violative rates of total aflatoxins in peanut among various countries.41−47
4. Adverse Biological and Health Effects of Aflatoxins
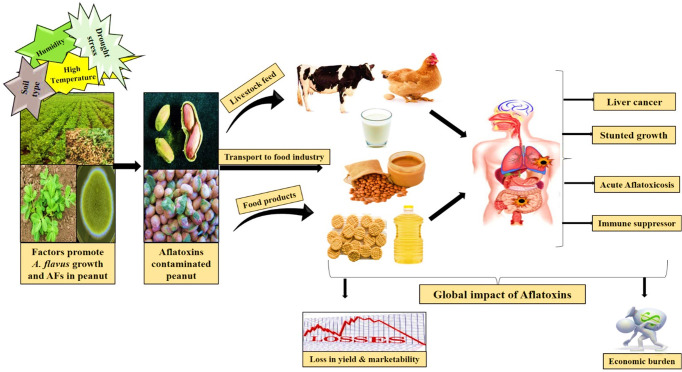
Peanut crop is highly vulnerable to aflatoxin-producing fungi and often becomes the primary cause of an outbreak of AFs in the food chain for humans (Figure 4). The prevalence of aflatoxin-producing fungi on agricultural crops and their capacity to produce toxins in edible commodities are influenced by factors such as food composition, the carcinogenicity of the fungal strain, moisture levels, humidity, drought, temperature, and presence of minerals.48 AFs are fungal secondary metabolites which are known for their toxic effects on both human health and livestock. These toxins are predominantly produced during the growth of Aspergillus species.49 Notably, in 1993 the International Agency for Research on Cancer declared that aflatoxin B1 was the most harmful toxin and potent carcinogen for human.50,51 Moreover, Aspergillus species such as A. flavus, A. niger, and A. fumigatus strains are also responsible for human and animal diseases, such as mycotoxicosis and invasive and noninvasive infections in immunosuppressed patients, and long-term fungal exposure cause hypersensitive reactions like asthma and allergic alveolitis. Numerous research findings have consistently highlighted that aflatoxin exhibits acute carcinogenic properties, suppresses the immune system, causes hepatotoxicity leading to liver damage, and demonstrates teratogenic effects by disrupting normal physiological processes. These detrimental characteristics of aflatoxin have significant repercussions on the health of both humans and animals, ultimately affecting nutritional quality and trade marketability across the world.52−54 In another study it is demonstrated that long-term exposure of aflatoxins, specifically AFB1, is strongly linked to an elevated risk of cirrhosis and liver cancer. This heightened risk arises from its conversion into a reactive 8,9-epoxide form, which has the capacity to form DNA adducts by alkylating the guanine residues.55
Figure 4.
Flow of aflatoxins through the food chain and their global impact on health and economies.
Aflatoxins are characterized by their ability to readily traverse the respiratory and gastrointestinal tracts due to their lipophilic nature.56 When AFs reach the bloodstream, these toxins widely disseminate to various tissues and accumulate primarily in the liver and some other organs. This accumulation is a key contributor to the development of hepatic cancer and aflatoxicosis.57 Prospective reports have enlightened a strong connection between aflatoxin B1 biological markers when exposed in urine or blood serum and a high risk of subsequent hepatocellular carcinoma. The synergistic impact of AFB1 and hepatitis B infection in liver cancer risk was simulated in diverse cohorts.58 Various animal experimental investigations revealed that AFB1 is an extremely potent carcinogen and highlighted it as the most toxic hepatocarcinogenic agent. Aflatoxin B1 exposure is strongly associated with a particular mutation at the 249 codon of tumor suppressor P53 gene in liver tumors, suggesting a link between aflatoxins and hepatitis B infection in the development of hepatic cancer.59 The chronic level of aflatoxin exposure is reported as a linkage with child growth stunting,60 and immunosuppression leads to AIDS.61
The most common and toxic aflatoxin is AFB1, which is produced by the filamentous fungi, mainly A. flavus and A. parasiticus, in peanut. Its continuous and medial concentration is said to become a main cause of liver cancer, and the enduring infection synergistically with the hepatitis viruses leads to exert acute aflatoxicosis.62 Lien et al.63 also reported that when animals consumed most toxic AFB1 containing feed, it caused serious issues in the respiratory and digestive systems and genital organs via various mechanistic mechanisms such as toxins interfering with macromolecular and enzymatic metabolisms and releasing AFM1 in animals. Negative effects of AFB1 on livestock depend upon the concentration and time exposure of the potent toxin, the strain, and the food products. High concentrations of this carcinogenic toxin influence health, medial concentration poses a chronic risk of many health issues, and continuous exposure targets the body organs resulting in liver and kidney failures, encephalopathy, Reye’s syndrome, and cancer.64 Since around 1/15 is from consumed aflatoxins, AFB1 is introduced as aflatoxin M1 in milk, and diverse heat treatments which are used in preparation of many milk products are unable to minimize the level of aflatoxin M1. Therefore, when aflatoxin contaminated milk is consumed, there is always a higher possibility of poisoning by this carcinogenic toxin. The capability of tumorigenesis and mutagenesis of AFM1 is lower than that of AFB1.65 After review, we concluded that consumption of aflatoxin contaminated peanut products poses serious health issues. Therefore, production of aflatoxin-free food and feed products is a major concern in agriculture by implementation of innovative strategies.
5. Aflatoxin Detoxification Approaches
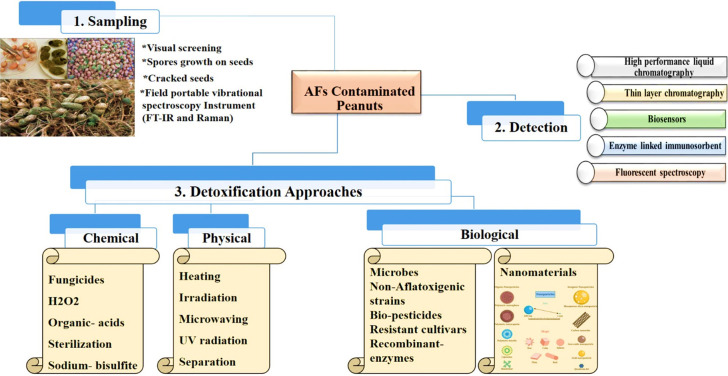
When a food commodity becomes contaminated, then it is crucial to detox the AFs to a safe level. Detoxification removes or reduces the toxic effects of AFs and can be accomplished via chemical, physical, and biological techniques. The FAO states that any detoxification process implemented on humans consuming food should inactivate, eradicate, or destroy AFs; not leave any carcinogenic, toxic, or mutagenic remnants on the treated material; and maintain the food product’s nutritional or any other quality parameters.66 Several approaches have been documented in the literature to lower the potential hazard of aflatoxin contamination in peanut crop through physical (cleaning, separating, heating, microwaving, adsorption, and UV radiation), chemical (sterilization, ozonization, chemical compounds, and acids), and biological methods (Figure 5).67
Figure 5.
Various aflatoxin detoxification approaches.
5.1. Physical Detoxification
A number of the physical approaches used to destroy or diminish AF contamination involve heating, ultrasonic, and microwaving interventions, solvent extraction, sorting, separation, UV and solar radiation, ozonization, density gradient, flotation, adsorption, γ rays, digital eye sorting, and roasting.68,69 Damaged and shattered grains have more mycotoxin contents; therefore, eliminating them reduces overall contamination.70 Manually sorting and separating grains according to their physical properties can reduce the level of AFs in agricultural commodities but not on a wide scale. However, ultraviolet light and ionization treatments can reduce the AF contamination and increase the shelf life of agricultural products because these methods destroy the fungal cells.71 Thermal methods also have a lot of potential. The concentration of AFs could be decreased by 9–100%, depending on the method of heating used and the product being treated. For instance, levels of AFs can be decreased by 9–39% when fruits are autoclaved at a temperature of 120 °C for 30 min, but when peanuts and their products are autoclaved for 90 min, the concentration of AF is lowered by 100%.72 However, AFs are resilient to heat and do not entirely disintegrate at temperatures (80–121 °C) that are routinely used during food processing. Common cooking methods like frying, roasting, boiling, and pasteurization are not likely to significantly lower the AF levels. Along with thermal processing, nonthermal techniques like cold plasma could be used to lower the levels of AFs in some nuts and seeds by as much as 95%.73 One of the efficient approaches to lower the AF content is the use of non-nutritive adsorbent in toxin contaminated feed to lower the availability of AFs.74 However, there are many disadvantages to such a technique, including the adsorbent’s insufficient specificity and its high cost. Their use is also constrained by the accumulation of absorbents in the surroundings due to animal excretion because of their nondegradable property.
5.2. Chemical Detoxification
Many chemicals are being utilized to detoxify AFs, such as reducing and oxidizing substances, fungicides, acids, bases, and chlorine-based agents. When chemical methods are coupled with physical processes, the effectiveness of detoxification is improved. Over 100 chemical substances have been discovered that hinder or reduce mold growth, which lowers levels of AFs.75 Chemical detoxification of aflatoxins from contaminated agricultural commodities has been successfully used in various contexts, but the treated substrates may contain some hazardous residues. Therefore, detoxification through natural substances becomes more demanding for consumers than utilizing synthetic chemicals. Within the limitations of the economy, these methods still need to produce sufficient levels of detoxification. All processing methods significantly reduced the level of aflatoxins to a considerable extent, even though there are certain drawbacks, such as aflatoxins are highly resistant to conventional methods. While these are heat-stable toxins, peanuts treated through this method cannot be consumed by humans, as it lowers the nutritional contents.76
In this devastating scenario, we concluded that other alternative solutions must be implemented before and after the harvest stages to reduce the contamination level from commercially available foods and their products, at least to make sure that aflatoxin levels are below permissible levels. Therefore, convenient, practical, and eco-friendly detoxifying technologies must be developed in order to reduce the aflatoxin levels in food and feedstocks to a safe level.
5.3. Biological Effective Ways to Control Aflatoxin Contamination
5.3.1. Biological Detoxification
Detoxification of aflatoxins in the field presents challenges due to multiple factors such as temperature fluctuations, insect infestations, humidity levels, soil moisture variations, and potential mineral deficiencies.77 Various approaches can be implemented to mitigate AF contamination, either before harvesting, after harvesting, or during storage.78 Prior to harvesting, the following strategies can be employed: use of proper agronomic practices, selecting resistant crop varieties, and taking measures to minimize both insect damage and mechanical harm to plants during the preharvest period.79 Compared to other methods of detoxifying aflatoxins (AFs), biological approaches are considered to be less aggressive, environmentally friendly, and cost-effective. These biological methods use microorganisms and their byproducts to remove AFs from food commodities through processes like surface adsorption, biodegradation of toxic substances to nontoxic substances, or binding to lower their availability that ultimately reduces harmful effects.80 For instance, use of the Flavobacterium aurantiacum bacterium effectively removed AFB1 from various food items, such as oil, peanut butter, milk, peanuts, and maize without any production of detrimental byproducts.81,82 Biodegradation technology offers an attractive alternative for managing or eliminating aflatoxins while regulating the quality and safety of food and feed products. In light of rising consumer concerns about the use of chemical and synthetic compounds in their food, the utilization of biological agents presents a more “natural” and demanding option.83 One of the in vitro experiments was conducted to evaluate the binding capacity of probiotic lactobacilli to AFB1.84 These probiotics were incubated with a standard range of AFB1 in phosphate-buffered saline for 2 h at a temperature of about 37 °C. The results revealed a range of AFB1 binding levels, varying from 28 to 65%, with four isolates demonstrating complete binding.
An effective and a practical biological approach to prevent aflatoxin contamination in both agricultural fields and storage facilities involves introducing naturally occurring nonaflatoxigenic strains of both A. flavus and A. parasiticus. These nonaflatoxigenic strains are applied as a conidial suspension either before planting in the soil or directly onto the seedlings.85 The effective management of aflatoxin production in agricultural fields through the use of nonaflatoxigenic Aspergillus strains also contributes to the prevention of AF contamination during subsequent storage. This approach was initially pioneered by Cotty and Bayman and has since been widely adopted for AF control worldwide.86 Some biopesticide fungal strains that are nonaflatoxigenic do not produce toxins because of a genetic deletion present within the AF biosynthetic gene cluster. In contrast, the AF36 strain does not produce aflatoxins due to a single nucleotide polymorphism that induces a premature stop codon in a gene that is responsible for the polyketide biosynthesis required for AF production.87 Besides Aspergillus, other fungal strains like Trichoderma, Penicillium, and yeast have been shown to decrease the levels of aflatoxins in agricultural fields.88,89 On a global scale, several microorganisms are still in the experimental phase for combating AF-producing fungi. The biological control of AF contamination using these microorganisms is still under development. Nevertheless, most of the existing advances are not feasible due to time limitations, nutrient loss, or limited effectiveness in detoxifying AFs.
5.3.2. Molecular Assisted Mechanistic Solution against Aflatoxin Control
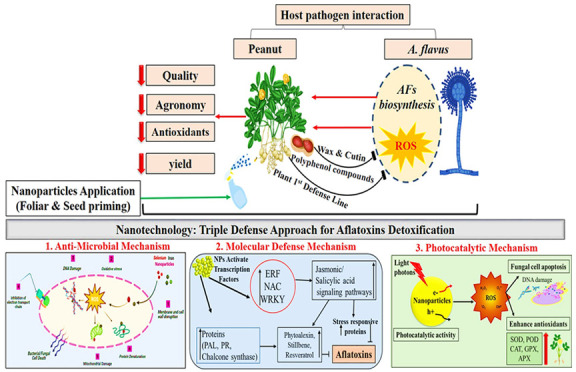
Despite extensive research efforts, molecular biologists and geneticists have not yet discovered a reliable and efficient genetic solution to combat aflatoxin contamination. Peanut has shown resistance mechanisms in three distinct categories: in vitro seed colonization (IVSC), preharvest aflatoxin contamination (PAC), and resistance to aflatoxin production within seeds are documented.90 However, A. flavus opportunistic fungi produce aflatoxins specifically in the cotyledons of peanut seeds after infection.91 Furthermore, various sources of resistance to these mechanisms have been independently identified.14 Aflatoxin contamination in peanuts is primarily associated with the initial stage of fungal infection, known as IVSC, which predominantly occurs in the seeds. Therefore, it is important to comprehend the molecular pathways, and identifying candidate genes related to IVSC resistance is crucial for potentially revolutionizing the control of fungal colonization and aflatoxin contamination in peanuts.32 Breeding efforts have thus far enabled the identification of peanut germplasm that confers resistance to both preharvest and postharvest aflatoxin contamination. In the case of peanut resistance against Aspergillus spp., a key aspect involves the synthesis of resveratrol, a natural phytoalexin, within the developing seeds. Resistance cultivars that produce more resveratrol upon fungal infection show improved resistance to IVSC.32 In response to Aspergillus infection, the host’s defense mechanisms rely on maintaining an oxidative balance to counteract the formation of reactive oxygen species (ROS). This balance is achieved through the activation of a diverse array of genes associated with ROS detoxification. These genes include resveratrol synthase, chalcone synthase, phenylalanine ammonia lyase, superoxide dismutase, catalase, glutathione-S-transferase, and senescence-associated proteins. The expression of these genes plays a crucial role in inhibiting the growth of Aspergillus and the production of aflatoxins.92 In various studies, it is highlighted that resistance-inducing genes are responsible for the production of various compounds found in the peanut seed coat, including phenylpropanoids, coumarins, stilbenes, cinnamic acid, flavonoids, and ascorbate.93,94 In addition to these compounds, transcription factors such as WRKY, ERF, and NAC are crucial for regulating genes associated with antioxidants and pathogenesis. These genes also have a significant role in the synthesis of volatile compounds like jasmonate and salicylate,15 and they govern innate immunity.95 Key controllers of A. flavus resistance include genes encoding β-1,3-glucanases, chitinases, pathogenesis-related proteins, and ribosome inactivating proteins.96
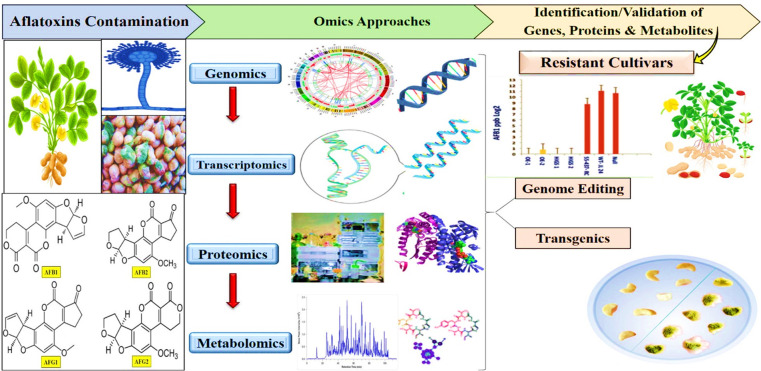
Future research is expected to increasingly focus on dissecting the traits related to aflatoxin contamination in peanuts, aiming to facilitate the development of peanut varieties free from aflatoxin without additional farmer’s costs. One potential strategy for reducing aflatoxin contamination during peanut seed storage involves the use of cultivars that are resistant to seed invasion by Aspergillus spp. (Figure 6). However, the development of such aflatoxin-resistant varieties has proved to be a challenging task for breeders. This challenge is primarily due to the limited availability of reliable sources of resistance, an incomplete understanding of plant–pathogen interactions, and the significant influence of environmental factors. In the case of peanuts, Aspergillus infection is typically influenced by several factors, including the aggressiveness of the fungus, the susceptibility of the genotype, and environmental parameters such as soil moisture and temperature.13 Notably, moisture stress, especially terminal drought, makes peanuts more susceptible to A. flavus infection and subsequent aflatoxin contamination.97 However, aflatoxin control and management strategies primarily involve inhibiting the A. flavus infection process through a combination of methods, including plant resistance or tolerance, biologically effective control, environmental factor management, good crop practices before harvest, and postharvest techniques like drying, transportation, and storage.98
Figure 6.
Omics assisted techniques to produce aflatoxin-resistant cultivars.
5.4. Limitations of Strategies for Aflatoxin Detoxification
Under the current scenarios, it is important to note that relying solely on genetic resistance is insufficient to entirely address the issue of aflatoxin contamination. Therefore, it must be complemented with a range of pre- and postharvest management measures. Gaining further insights into the prevalence of aflatoxin-producing fungi within peanut communities across the world would be beneficial to formulating effective and all-encompassing strategies to mitigate aflatoxin contamination. However, all methods have drawbacks, just as every single coin possesses two faces. The physical and chemical methods for detoxification and elimination of AFs not only affect the toxin level but also significantly impact the nutritional value.99 In one study, radiation and high temperature were used to detoxify aflatoxins from peanut samples. The findings reveled that these physical methods reduce the peanut protein content and some methods are not appropriate.100 Therefore, chemical and physical detoxifications of aflatoxins from peanut are restricted. However, chemical and physical detoxification methods have drawbacks, such as high cost and lower nutritional values.101 In real terms, biological detoxification is thought to be more environmentally friendly than the above two conventional detoxification techniques. It does not involve toxic chemical contaminants and high temperatures, or pressure. However, in this strategy when microorganisms and nonaflatoxigenic strains are used, they may consume nutrients for their growth and release unwanted metabolic byproducts, potentially affecting the nutritional status of food.102 Currently, there is a lack of efficient, practical, and straightforward methods to prevent aflatoxin contamination throughout the entire peanut supply chain, from cultivation to consumption. Nanotechnology formulates nanovehicles that can encapsulate pesticides, fungicides, antimicrobial agents, and herbicides ensuring targeted delivery to definite plant tissue sites. This strategy ensures controlled release and localized delivery, minimizing pollution and decreasing the need for chemical-based fungicides.103 This technology enhances the uptake of nutrients, improves the plant defense system, and controls the plants diseases. Although every possible way has some limitations, nanoparticles also have some disadvantages about their toxicity, cost-effectiveness, and commercial applications. Consequently, for the first time the current review is exploring green and effective methods to control aflatoxin contamination through nanotechnology.
5.5. Role of Nanotechnology in Agro-Food Industry against Aflatoxin Detoxification
Nanotechnology improves the safety of foods in a number of ways, improves the shelf life of food, and controls contamination in the field and during the processing, transportation, and storage that result in food quality enhancement. In the agriculture and food sectors mycotoxin detoxification becomes a continuous challenge.104 Therefore, it is a critical need to develop simple, economic, highly effective, safe and practical degradation technology to address mycotoxin detoxification. Moreover, it is important to discuss how nanoparticles mechanistically control other fungal diseases.
5.5.1. Green Nanotechnology: Phytomediated Nanoparticles and Their Potentialities in Plant Fungal Disease Management
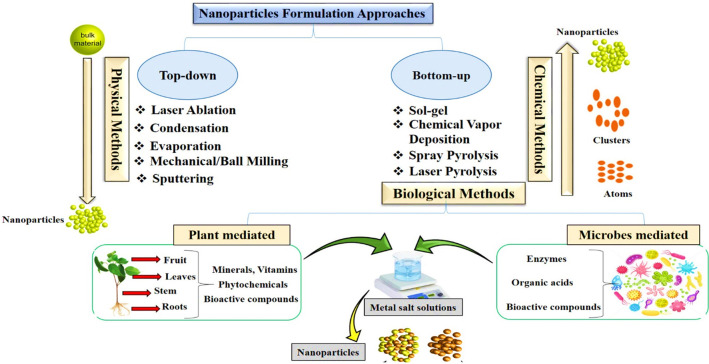
Nanobiotechnology has gained significant prominence because of its diverse applications in maintaining the agricultural ecosystem. Within the realm of agriculture, nanotechnology is increasingly gaining popularity, and researchers are actively working on the formulation of nanodevices to control agriculture at the nanoscale level.105 Metal nanoparticles (NPs) are considered as a cutting edge technology that exhibits immense potential in medical, nonmedical, and agriculture domains. Due to their remarkable high surface area, metal nanoparticles hold discriminating reactivity and possess chemical, optical, and electrical characteristics that deviate from their conventional macroscopic properties. Various methods have been followed to formulate nanoparticles including chemical, physical, and biological based (Figure 7). The chemical and physical pathways for the synthesis of nanomaterials often involve the application of forces and potentially harmful chemical reactions leading to adverse environmental impacts, degradation, low yields, instability, and costliness.106 Therefore, there is a need for a green approach that produces more stable, economical, and highly efficient metal NPs.107 Many biological entities are utilized for the formulation of stable nanoparticles. The biological approaches involve the use of microorganisms, algae, fungi, organic reducing agents, yeast, and plant material.108 Among the above-mentioned biological routes, the greener method is the most preferred biological approach for nanoparticles synthesis because the use of microorganisms is riskier due to their pathogenicity issue and because they also need cultural maintenance and care.109 Therefore, plant extracts have been preferably used to synthesize the environmentally sustainable biogenic nanoparticles that inhibit the fungal pathogens, efficiently decrease crop diseases, and ultimately promote agriculture.110 Phytosynthesized nanoparticles show promising effects, are sustainable, and are easy to produce and characterize.111 The plant extract based formulation of nanoparticles has advantages compared to conventional chemical and physical approaches because the greener method is biocompatible and plays a vital role in biologically inhibiting the proliferation of fungal species and ultimately controlling the production level of AFs.112 The plant based formulation of nanoparticles is very easy and safe. For this, the plant extract is prepared and then a metal salt is mixed with it to form extract–salt solutions at various reaction conditions.113 Plant extracts have an array of phytochemical constituents such as flavonoids, phenols, terpenoids, sulfur-containing compounds, polyphenol, and flavones, which help in the bioreduction of metal ions and stabilize the nanoparticles quickly.114 The plant extract composition is also a major factor in the formation of nanoparticles, because different plants have different concentrations and types of phytochemicals.115 For the first time alfalfa sprouts plant extract was used for the formulation of phytosynthesized metal nanoparticles.116 We conclude that plants are considered a highly promising and exceptional source for nanoparticles synthesis.
Figure 7.
Nanoparticles synthesis methods.
As a result, metal nanoparticles interact with microorganisms more intensely as compared to large particles.117 In the past two decades, nanoparticles have increasingly garnered the interest of researchers due to their multifaceted antimicrobial properties, particularly in the context of combating fungal infections. This heightened attention has been further reinforced by statements from the Food Safety Authority, which affirm that the optimal dosage of nanoparticles is safe and poses no adverse effects on humans or consumers.118,119 The advent of nanotechnology and the rapid advancement of antifungal nanomaterials have created the potential for these materials to be effectively harnessed as potent antifungal agents. At present, a wide array of nanomaterials has found extensive application in the field of antifungal treatments, as well as in the suppression of mycotoxin production.120−122 Nanomaterial based approaches for combating fungal infections can be broadly classified into two categories: In the first strategy, antifungal agents are encapsulated within polymeric nanomaterials and are subsequently released under specific conditions, such as variations in pH, elevated temperatures, or the presence of enzymes, which trigger their action against the fungi. In the second strategy, nanomaterials themselves directly contribute to the inhibition of fungal growth, without the need for additional compounds.123 This innovative approach has led to the development of nanofungicides, which effectively inhibit fungal pathogens without any severe contamination and alterations to the environment. Consequently, it is imperative to acquire high-performing fungicides that are economical and have minimal adverse effects on the environment. The current trend toward safe and economic plant fungal disease management involves the use of nanoparticles as nanofungicides.124 Therefore, nanotechnology has promising potential in agriculture for ensuring in a new era of fungicides for fungal disease control in plants.
The use of nanoparticles in disease management and plant protection can be made possible by two distinct mechanisms: (a) nanoparticles protect the plants by improving the defense system; (b) nanoparticles serve as nanowarriors and transport fungicides and biocontrol agents. Nanoparticles act as nanocarriers and have copious benefits such as (i) improved fungicide solubility, (ii) controlled release of fungicides, (iii) targeted delivery and targeted pathogen specific site, (iv) less toxicity, and (v) environmental friendliness.125 Another positive aspect of the formulation of nanofungicides is that it increases the long-term stability and efficacy of pesticides in various ecological conditions, thereby lowering the number of applications and quantity of fungicides, which in turn lessens their adverse effects and lowers their costs.126
Here we discuss some of the practical applications of antifungal potential of nanoparticles on various agriculture crops. In one study Moringa oleifera plant based synthesized titanium nanoparticles were exogenously augmented on wheat plants against Puccinia striformis fungus. The outcomes revealed that nanoparticles triggered the plant antioxidant defense system and stress-related protein upregulation was observed in the plant proteome profile.127 In another field experiment, various concentrations of biogenic silver nanoparticles were used as a foliar application on wheat plants against Bipolaris sorokiniana. The dose dependent manner of the nanoparticles demonstrated the effective results in control of spot blotch disease and elicited the biochemical profile and antioxidant enzymes and ultimately improved the wheat plant growth and yield attributes.128Melia azardica based selenium nanoparticles also showed marvelous antifungal potential against spot blotch disease of wheat. This biocompatible strategy controlled the disease incidence and showed that various levels of selenium nanoparticles enhance the plant growth by modifying its biochemical profile and control the fungal stress by disrupting the fungal cell.129 Bioinspired Chenopodium quinoa mediated cerium oxide nanoparticles showed strong antifungal potential against the fungal disease Ustilago tritici affecting wheat crop. The CeO2 nanomaterials showed astonishing results in the control of disease and improved crop yield by improving various physicochemical attributes, leading to enhanced growth.130 Researchers conducted a field experiment on tomato plant against fusarium wilt disease, concluding that iron oxide nanoparticles suppressed the fungal growth and improved the plant defense system against stress.131 In another in vivo research, iron oxide nanoparticles showed antifungal potential against F. oxysporum, a wilt-causing agent in cucumber plant. FeONPs improved the growth, yield, and physicochemical attributes in infected plants by enhancing the osmolytes, osmoprotectants, and antioxidants.132 The eco-friendly copper oxide nanoparticles also illustrate antifungal potential against root rot of cucumber. Various concentrations were applied on diseased inoculated plants, and NPs showed strong inhibitory effect against F. solani. Defense gene expression and antioxidant enzymes were elevated in treated plants.133
Based on a data review about the antifungal potential of nanomaterials, nanotechnology offers great opportunities in the formulation of nanofungicides and various active ingredients for the management of plant fungal diseases, ultimately improving the agro based food industry. There is still a gap in the application of nanotechnology in the area of agriculture due to insufficient field trial experiments and the lack of commercialization of nanofungicides. Moreover, many fungal species contaminate agricultural commodities with mycotoxins such as aflatoxins, and peanut crop is one of them. The consumption of these toxin-contaminated food products further threatens human and livestock health.134 Therefore, there is a need to produce aflatoxin-free agricultural products, and in agriculture a number of studies have concentrated on the application of nanotechnology in mycotoxin elimination and its management. The issues associated with AFs and their consequences must be addressed by implementing innovative technology. Herein, a green and economical way is nanotechnology.98 Reduction of AF contamination through nanotechnological interventions constitutes one of the initial measures in formulating a viable strategy to enhance agricultural production in a sustainable manner.
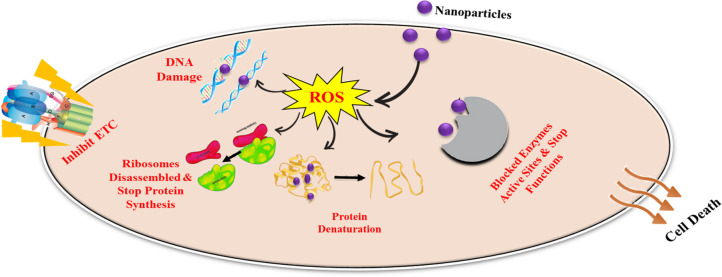
5.5.2. Role of Nanoparticles in Aflatoxin Control
Food industries and agriculture sectors encounter numerous challenges in mycotoxin detoxification, epecially of aflatoxins. Preventive measures should be implemented against aflatoxins from the field to the final product. Both field and storage fungi are linked to aflatoxins like Fusarium and Penicillium, respectively. Therefore, continuous crop observation is also crucial. Using pest- and disease-free seeds is essential for cultivating healthy plants capable of resisting aflatoxins throughout their growth cycles.135 Prosperous methods of agriculture encompass tillage, crop rotation, irrigation, and minimizing the use of chemical based pesticides in the field.136 Nanotechnology has recently garnered significant attention in agriculture because of various nanoparticles identified for their antifungal properties and potential applications as food additives, packaging materials, and storage solutions. In 2009, nanomaterials designed to eliminate mycotoxins were introduced.137 Nano based packaging materials are manufactured by embedding antifungal potent magnetic and metallic nanoparticles into jute fabrics and polypropylene bags, making them suitable for preserving food grains. These innovative packaging materials, incorporating nanomaterials with potent antifungal activity, hold promise for food storage applications.138 For instance, honey mediated silver nanoparticles have been shown to reduce aflatoxin B1 levels up to 88% from maize grains in storage conditions.139 Recently nanoparticles have been incorporated in food packaging materials and act as sensors to monitor food spoilage.140 Mycotoxin control in agriculture is a serious concern regarding human health. One strategy is the use of adsorbents in food. Certain NPs like chitosan have the ability to adsorb AFB1, AFB2, ZEN, and OTA to a significant level. Graphene oxide represents another effective adsorbent, showing high adsorption capability for mycotoxins (aflatoxin, deoxynivalenol, orchatoxin, and zearalenone).141 In practice, aflatoxin control could be mediated with the application of antifungal nanoparticles, which are used against mycotoxigenic fungi. Recent research findings showed that nanoparticles have strong antifungal potential and inhibit mold growth. When fungal cells are subjected to nanoparticles, various structural changes occur to their cell walls, including pitting and pore formation, cell clustering, and surface shrinkage.142 These alterations have been demonstrated in many studies, which shows direct NPs interaction with cell wall. This contact not only causes changes in the shape of the cell walls but also distorts the inner membranes.143 As a result, there are noticeable changes in the configuration of the internal organelles of the fungi.144 In another study, the antifungal potential of nanoparticles is demonstrated as they produce reactive oxygen species that damage the macromolecular structures, block enzyme active sites, and inhibit all metabolic processes, which ultimately leads to cell death.116 We summarize this mechanism by using the above-mentioned data in a graphic representation in Figure 8.
Figure 8.
Antifungal mechanism of nanoparticles.
However, applications of NPs in the management of AFs in the field are in the early stage. Based on the reported data, we conclude this content as nanoparticles manage aflatoxins via various directions. Some NPs control AF levels in storage conditions, and some act as adsorbents. Practically NPs show antifungal behavior in field conditions as well, so we recommend that their application needs to be change. During storage, grain loss is one of the big factors in the food crisis, and if grains prior to storage become contaminated in the field, then its management will be more difficult. A. flavus is an opportunistic pathogen and resides in soil and agriculture waste; therefore, NPs must be applied in the field so that they could inhibit the fungal growth during germination and growth. Therefore, it is crucial for researchers to change the direction of NPs application on peanut crop, to target fungus in the field, and to carry out field experiments for the advancements in formulation of potent nanofungicides. Different nanoparticles were reported to control the aflatoxin contamination and inhibit the colonization of toxin-producing A. flavus.145 Herein we present an updated overview on a number of in vitro and a few in vivo studies about the inhibitory effects of various documented green synthesized metal nanoparticles on mycotoxin-producing fungi and aflatoxin control as summarized in Table 1.
Table 1. Inhibitory Effects of Various Biogenic Synthesized Nanoparticles on Mycotoxigenic Fungi and Aflatoxins.
| nanoparticles | biogenic extract | inhibition (%) | mycotoxigenic fungi and AFs | mode of action |
|---|---|---|---|---|
| silver | actinomycetes, mint, thyme, rosemary, eucalyptus | 100 | A. flavus, A. ochraceus, aflatoxin, and ochratoxin | leakage of proteins and DNA, disintegrate membranes146,147 |
| iron, copper | Syzygium cumini | 49 | A. flavus, AFB1 | DNA mutation148 |
| S. cumini | 80 | A. parasiticus | ||
| silver | honey | 77.5 | A. parasiticus | altered cell membrane permeability and ROS generation149 |
| honey | 58.76 | A. ochraceus | ||
| honey | 66.56 | aflatoxins | ||
| honey | 79.85 | ochratoxin | ||
| silver | A. terreus | 100 | A. flavus | produce oxidative stress that damaged the fungal cell150,151 |
| Curcuma | 98 | AFB1 | ||
| zinc oxide | lemongrass | 100 | total AFs | breakage of fungal hyphae leads to control aflatoxins152 |
| silver | Juglans regia | 100 | AFG1, G2, B1 | damaged mycelium growth153 |
| silver | pomegranate | 68 | AFs, A. flavus | oxidative stress disturbs fungal growth154,155 |
| copper oxide | Manilkara zapota | 61.5 | A. parasiticus | deactivate fungal cell machinery156 |
| zinc oxide | M. zapota | 62.4 | A. parasiticus | |
| silver | Morus nigra, A. niger | 100 | A. parasiticus, A. terreus, AFs | deactivate enzymes, proteins, and denature DNA157,158 |
| silver | Juniperus procera | 100 | A. flavus, total AFs | leakage of macromolecules from fungal cell159 |
| silver | Curcuma zedoaria | 100 | A. flavus | inhibit fungal growth160,161 |
| Moringa oleifera | 63 | A. parasiticus | ||
| zinc | S. cumini | 70 | A. flavus | ROS stress, proteolysis and cell death162 |
| zeolite | Centaurea cyanus | >99 | AFs | best adsorbent for removal of mycotoxins163 |
| C. cyanus | 55 | OTA | ||
| zinc oxide | Syzygium aromaticum | 100 | mycotoxins | lipid peroxidation and loss of membrane integrity164 |
| chitosan | Ocimum americanum | 100 | A. flavus, AFB1 | inhibit ergosterol synthesis and disrupt membranes165 |
| silver | Salvia officinalis, cinnamon | 100 | A. flavus, AFB1 | inhibit fungal growth by damaging its structure166 |
| silver–chitosan | A. terreus | 89 | A. flavus, AFB1 | damaged conidia, unusual bulges and rupture cell167 |
| copper | Magnolia kobus | 85 | A. flavus | rupture cell membrane168 |
| Mag. kobus | 86 | A. niger |
5.5.3. Representative Nanoparticles for Detoxification of Aflatoxins and Growth of A. flavus Isolates from Peanut
However, the antifungal activity of plant based metal nanoparticles on mycotoxin-producing fungal growth that affects peanut crop before and after harvest and their impact on toxin biosynthesis has not been well explored. This is the first attempt to summarize the various nanoparticles which have been used to control or degrade aflatoxins and inhibit their caused fungal strain growth isolated from peanuts. One study reported that A. flavus hyphae growth and its spore germination could be retarded after augmentation of α-Fe2O3 nanorods.98 Under sunlight exposure, α-Fe2O3 demonstrated photocatalytic capabilities that led to a substantial inhibition of A. flavus on peanuts, with an inhibitory rate of approximately 90%. Additionally, there were significant reductions of 90% in AFB1 production and 70% in AFB2 production. In other research findings, it was observed that MgO NPs exhibited an inhibitory effect on A. flavus growth at concentrations of 2 and 3%. The growth of the fungal culture was completely inhibited on potato dextrose agar at a concentration of 2%, and at a concentration of 1%, the inhibition percentage reached as high as 95%.169 In another study, Cymbopogon citratus plant extract based AgNPs were evaluated against A. flavus. Outcomes of the study show that these NPs have the capacity to restrain fungal growth and exhibited significant antifungal properties against all the tested fungi, with a minimum inhibitory concentration of 20 mg/mL. Furthermore, AgNPs exhibited remarkable antifungal potential against toxigenic strains of fungi in peanuts, suggesting their potential in effectively controlling these toxigenic pathogens.170
In another study, Acarous calamus rhizome mediated silver nanoparticles were applied on aflatoxin-producing A. flavus fungus that was isolated by damaged groundnut kernel. Various concentrations of nanoparticles (0.005, 0.01, and 0.02 g) were used by a food technique assay to test their efficiency in controlling A. flavus growth and reproduction. Outcomes negotiate that 0.02 g of synthesized silver nanoparticles significantly suppressed the A. flavus pathogen growth up to 50% and inversely correlated with spore production.171 Moreover, the aflatoxin-producing A. flavus fungus was isolated from contaminated peanut, and the impacts of mycosynthesized silver NPs, gold NPs, and their combination on inhibiting the pathogen growth and its secondary metabolite synthesis of aflatoxin B1 were noted. Different concentrations of nanoparticles (125, 250, 500, 750, 1000, 3000, 5000, 10 000) were used to test the antiaflatoxigenic efficacy on A. flavus mycelium growth and its production capability of toxin AFB1. The reduction in accumulated AFB1 levels was observed with increasing concentrations of nanoparticles, as there was a direct correlation between nanoparticle concentrations and AFB1 reduction. A. flavus growth was inhibited completely at a concentration of 10 000 μg/mL for AgNPs, AuNPs and Ag–AuNPs, and no AFB1 production was detected at the same concentrations. The highest concentration at which AFB1 production was inhibited to a significant extent (99.55% for AgNPs, 99.999% for AuNPs, and 99.59% for Ag–AuNPs) was 3000 μg/mL, while fungal growth was also significantly reduced at these concentrations.172 The antifungal efficacy of silver@silica NPs was evaluated against A. flavus culture that was isolated from peanut. The outcomes revealed that silver@silica NPs remarkably inhibit A. flavus growth in a dose dependent manner. However, a 5 mg/mL concentration of NPs completely inhibited the growth of fungus (100%). According to these results, it was suggested that these nanoparticles imposed high toxicity on A. flavus.173 One field experiment was conducted against peanut aflatoxins. Foliar application of various concentrations of zinc, copper, and magnesium oxide nanoparticles were applied on a peanut crop. The outcomes of the study revealed that nanoparticles inhibit the fungal growth and ameliorate the crop growth by eliciting the antioxidant defense system of the crop. The nanoparticles trigger the plant defense system, activating osmoprotectants and antioxidants which quench the ROS produced by biotic stress.174 Various in vitro studies were carried out to check the antiaflatoxin potential of nanoparticles against aflatoxin-producing fungi. These toxigenic fungi were isolated from contaminated peanuts. Table 2 describes all nanoparticles, their synthesis approach, and their inhibition mode of action against toxin-producing fungi.
Table 2. Antiaflatoxin Potential of Nanoparticles on Mycotoxigenic Fungi and Aflatoxins Specifically Isolates from Contaminated Peanuts.
| nanoparticles | synthesis method | mycotoxigenic fungi and AFs | mode of action |
|---|---|---|---|
| iron | physical | A. flavus, aflatoxin | oxidative stress, leakage of macromolecules, and inhibit cell growth98 |
| silver | plant synthesized | A. flavus, A. tamarii, A. niger, A. versicolor, Penicillium spp. | fungal hyphae deformation170,171 |
| gold, silver | myco synthesized | A. flavus, AFB1 | halt cell division and disturb respiratory chain172 |
| silver@silica | chemical | A. flavus, AFs | inhibit conidial growth of fungi173 |
| magnesium oxide | chemical | A. flavus, A. niger, A. ochraceus | altered cell membrane integrity6 |
| zinc oxide, copper oxide, magnesium oxide | chemical | A. flavus, AFs | growth inhibition, lipid peroxidation, and DNA denaturation174 |
Apart from bacteria and viruses, plant pathogenic fungi are significant contributors to substantial crop yield losses. Fungi inflict substantial economic damage on agriculture, leading to reduced food availability for consumption, and also result in severe, sometimes fatal, diseases in both animals and humans. Molds and other microscopic fungi exhibit remarkable adaptability in colonizing under diverse substrates and grow in challenging environmental conditions. Disk diffusion, well diffusion, and food technique methods are techniques in which nanoparticles are evaluated for their antifungal potential. In order to examine the antimicrobial activity of plant based nanoparticles, these can be analyzed by testing the microbe’s inhibition region.175 The above presented results were confirmed by the antifungal mechanisms of different nanoparticles which help to control aflatoxins and other fungal microorganisms. Based on the data review, it is crucial for researchers to change the direction of NPs application on peanut crop, to target fungus in the field, and to carry out field experiments for advancements in the formulation of potent nanofungicides.
5.5.4. Mechanistic Antiaflatoxin Potential of Phytomediated Nanoparticles
Over the past years, significant progress has been seen in the formulation of antibacterial NPs as a potential solution to combat antibiotic resistance in pathogenic bacteria. However, their effectiveness in addressing mycotoxin-related issues has been constrained by the marked differences between fungal and bacterial cellular properties. Bacteria are unicellular, are of three discrete shapes, and reproduce sexually, while most fungi are multicellular, exhibit a wide range of shapes that can lead to mycelium formation, and possess the ability to reproduce both sexually and asexually. These distinctions make fungi more vigorous and resistant to certain antibiotics.176 Research efforts have predominantly concentrated on antibacterial NPs, with limited attention given to NPs against fungal infections. The most latest advancements in the realm of antifungal NPs were summarized between 2016 and 2017.177−180 In practical terms, detoxification of mycotoxins can be achieved through the utilization of NPs that possess strong antifungal potential and which are easily synthesized on a large scale. This antifungal approach can be divided into two primary strategies. First, an antifungal potent compound is enclosed within a polymeric nanocage. Probably the drawback of this method is its susceptibility to air-induced instability; however, nanopolymers facilitate a controlled cargo release that is called “target specific”. Second, the inhibitory effect is achieved solely through the use of NPs. This method predominantly relies on stable metal NPs, which act promptly and can be formulated through eco-friendly green synthesis processes. Furthermore, the advantage of eco-friendly synthesis lies in the synthesis of nanobiocomposites through plants, microorganisms, and animal sources, which are less toxic and enhance their intrinsic characteristics.181 NPs interact with cell membrane and produce free radical species after cellular internalization that leads to causing damage in lipid bilayer cellular membranes, to lipid peroxidation, and ultimately to break the affected cell.182,183 Examination through scanning electron microscopy revealed the emergence of atypical protrusions on the fungal hyphae and a distortion in their structure after exposure to NPs. Various research findings suggested that NPs have significant inhibitory effects on fungal growth, leading to alterations in both the morphology and metabolism of the fungus.184,185 For instance, application of NPs reduced the production of organic acids such as oxalic, citric, and maleic acids, decreased mycotoxin production, and caused significant changes in the enzymatic profiles of pathogenic fungi that were responsible for the AF production.186 It is worth noting that the existing studies have yet to explore the interaction between NPs and the individual components within fungal cells. In addition to the inactivation of microorganisms, photocatalytic destruction of photosensitive AFs is also a possible mechanism of nanoparticles.
Over the past few years, photocatalytic based degradation of mycotoxins as an advanced oxidative technique has demonstrated significant promise in the detoxification of toxins from contaminated food samples. This is primarily attributed to its advantages, such as it has an environmentally friendly nature, it is practical, it is economical, it is easy to use under mild pressure and temperature, and it does not cause any secondary pollution.187,188 Remarkably, small sized nanoparticles have assumed a pivotal role in advancing the photocatalytic eradication of mycotoxins, gradually emerging as a captivating area of research in the detoxification of toxins.189,190 To date, various nanomaterials, nanohybrids, and nanocomposites such as UCNP@TiO2, graphene/ZnO, WO3/RGO/g-C3N4, g-C3N4, Fe2O3, and TiO2 have been extensively used in the photocatalytic eradication of mycotoxins.191 Photocatalytic degradation is a process that takes place when a photocatalyst is exposed to photons with energy levels equal to or greater than its band gap. This absorption of light leads to a chemical reaction, resulting in the creation of pairs of electrons in the conduction band and holes in the valence band. These generated electrons and holes can subsequently initiate reduction and oxidation reactions in molecules, such as mycotoxins, that have adhered to the surface of the photocatalyst.192 However, very few researchers have looked into the use of photocatalysts for mycotoxin detoxification from food products. Various studies support the observations and have documented that there is a correlation between fungal hypha growth and AF production.193 In another experiment, it is also confirmed that fungal mycelium formation is directly linked with AF production.194 Another hypothesis put forth by researchers suggests that the lysis or rupture of hyphae leads to a decrease in aflatoxin production. This is believed to occur because the constituents responsible for degrading aflatoxins are typically found within the hyphal cells. When the hyphal integrity is disrupted, these AF-degrading factors are released into the surrounding environment and subsequently reduce the synthesis of aflatoxins.132
Moreover, nanotechnology has emerged as a promising and innovative technology in the domain of plant science that offers novel insights about the adaptive mechanisms of plants in environmentally harsh conditions. Nanoparticles are recognized as regulatory entities for plants that are capable of regulating the diverse physiological and biochemical attributes and trigger the antioxidant defensive system, modulate hormonal responses, and activate or knock out the genes and overexpressed stress-related proteins that confer the resistance or tolerance against stress.195 In one study, the role of selenium NPs in the sesame plant were evaluated against A. flavus stress. These NPs enhanced the physicochemical, enzymatic, and nonenzymatic antioxidant defense system and metabolites of the sesame plant against biotic (A. flavus) stress. Outcomes of this experiment proved that Se NPs have the potential to enhance the sesame plant defense system to overcome the stress conditions.196 Another research experiment was performed to check the antifungal efficacy of Moringa oleifera extract mediated silver NPs on rice plant physiology and of biochemical, antioxidant, and phenolic compounds against aflatoxin-producing fungi (A. flavus). Outcomes revealed that AgNPs elicited the rice plant defense system to control A. flavus stress.197
This review mechanistically highlighted that nanoparticles have excellent ability to restrain A. flavus growth and its AF production. Therefore, this current review analysis could represent a significant advancement in the potential use of nanoparticles in plant protection and food safety and in serving as effective antifungal agents, particularly against aflatoxin/mycotoxin-producing fungi. NPs may also serve as a means of preserving food products, preventing contamination by A. flavus and the poisonous effects of aflatoxins.
5.6. Limitations and Grand Challenges in Application of Nanotechnology
Green nanotechnology offers environmentally friendly solutions for inhibiting the growth of toxigenic fungi. It is critical to introduce techniques that can help in the elevation of toxic fungi in agricultural crops in order to limit mycotoxin production. However, the safety and environmental impact of nanomaterials are major concerns in the implementation of nanotechnology in agriculture. It is vital to thoroughly assess the potential risks of the use of nanomaterials and confirm their proper usage and disposal. Implementing appropriate regulations is necessary to safely develop, produce, and properly use nanomaterials in agriculture as nanofungicides.198 The use of encapsulated nanofungicides and a thorough examination of toxicological studies, the biological behavior of nanoparticles such as adsorption, delivery behavior, and release in environment could significantly enhance safety for the health, environment, and food sectors in the future. There is a pressing need to update nanotoxicological assays by integrating advanced tools such as genomics, metabolomics, transcriptomic, proteomics, and phenomics to expedite and endorse the toxicity of nanoparticles.199 Due to their small size, nanoparticles can penetrate into the body through ingestion, inhalation, or skin contact. The extensive use of nanomaterials in food packaging raises concerns about their potential release.200 Still, not much information is known about how nanomaterials from packaging materials migrate into food and what effects they ultimately have on human health.201 It is essential to acknowledge and address these challenges, including the associated costs and risks. Addressing these issues requires collaborative efforts among academics, researchers, government and nongovernment organizations, and industries to engage in dialogue and develop solutions. Additionally, it can be challenging to extend nanotechnology on a large scale to fulfill the demands of agriculturists. There is a critical need to carefully assess the cost-effectiveness of nanotechnology applications compared to their potential advantages in disease management and crop yield improvement.202 Further research is required to elucidate the mechanisms underlying the uptake and transportation of nanoparticles via plant roots and their effects on plant growth. The practical use of nanotechnological interventions against aflatoxin detoxification may encounter challenges regarding formulation, stability, optimization, concentration, and toxicity.
6. Conclusion and Future Perspectives
This repertoire gives new perspectives on potential directions for future research to address the issues associated with the application of nanotechnology for detoxification of AFs from agricultural commodities. Aflatoxins inevitably and unpredictably produced in peanut crops exert highly toxic effects on animals and humans, leading to significant adverse health consequences and substantial economic losses within the agriculture sector. The contamination of carcinogenic toxins poses a severe global threat to public health and hinders the growth of the agricultural economy. As a result, to control AF contamination is a matter of significant global concern across the world. The detoxification of AFs is currently the subject of intense research, and incredible progress has been made in this area. Conventionally various approaches have been used against aflatoxin detoxification. Remarkably, the emergence of nanotechnology has garnered widespread attention due to its substantial potential in the management of fungal diseases, leading to remarkable achievements.
In this review, first of all, AF occurrences in peanut, their toxic effects on health, and detoxification approaches are deeply discoursed. Then, main attention is concentrated on the emerging nanotechnological interventions that serve as parallel to molecular techniques in the control of AF production by inhibition of fungal growth, photocatalytic degradation, activation of the molecular defense system, and upregulation of the expression of stress-related transcription factors of peanut to control contamination and ultimately enhance plant production. Although there have been significant advancements in the use of nanoparticles in the aflatoxin detoxification, based on a data review several following direction and concerns still need to be further taken into account in future research: The application of nanomaterials for aflatoxin contamination control is currently at a preliminary stage and most of the studies are in vitro. Therefore, it is important to perform in vivo studies as a future perspective to comprehend how pathogen and nanoparticles behave in the field, because practical application opens numerous prospects for the commercial use of nanopesticides in agriculture. Furthermore, effective aflatoxin detection methods are crucial for managing mycotoxin contamination. However, there is still a gap for enhancing nanomaterial-based aflatoxin detection techniques. Moreover, there is a need to address safety concerns and establish clear guidelines regarding the cytotoxicity of nanomaterials before commercialization. As we mechanistically presented, the antifungal behavior of nanomaterials is in control of fungal pathogen growth. We recommended that the nano based fungicides are cost-effective, environmentally friendly, and competitive alternatives to chemical based fungicides and act as nonwarriors for efficient control of various fungal diseases. Furthermore, there is a need for further research and collaboration among various researchers, nanotechnology experts, and peanut pathologists who can exchange information and lead to significant innovations in pathogen detoxification, resistant varieties development, diagnostic tools, and improvement in peanut production and quality. Therefore, the practical application of nanotechnology on peanut crop against A. flavus during field conditions is still needed. As based on the literature, nanoparticles showed promising antifungal behavior against aflatoxin-producing fungi; therefore, its application will be fruitful.
Acknowledgments
The authors wish to thank the Research Center College of Pharmacy and Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, for financial support.
Author Contributions
T.S. wrote and designed the manuscript. K.M., N.I.R., and Z.-U-.R.M. supervised and formally analyzed. A.H. helped in the editing; S. critically reviewed and revised the manuscript. R.U. and A.S.A. contributed to funding acquisition. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Kankam F.; Larbi-Koranteng S.; Sowley E. N. K. Aflatoxin contamination in groundnut (Arachis hypogaea L.); its causes and management. Ghana Journal of Science, Technology and Development 2021, 7 (2), 102–121. 10.47881/264.967x. [DOI] [Google Scholar]
- Akram N. A.; Shafiq F.; Ashraf M. Peanut (Arachis hypogaea L.): A prospective legume crop to offer multiple health benefits under changing climate. Comprehensive reviews in food science and food safety 2018, 17 (5), 1325–1338. 10.1111/1541-4337.12383. [DOI] [PubMed] [Google Scholar]
- Asare Bediako K.; Ofori K.; Offei S. K.; Dzidzienyo D.; Asibuo J. Y.; Adu Amoah R. Aflatoxin contamination of groundnut (Arachis hypogaea L.): Predisposing factors and management interventions. Food Control 2019, 98, 61–67. 10.1016/j.foodcont.2018.11.020. [DOI] [Google Scholar]
- Singh A.; Raina S. N.; Sharma M.; Chaudhary M.; Sharma S.; Rajpal V. R.. Functional uses of peanut (Arachis hypogaea L.) seed storage proteins. Grain and Seed Proteins Functionality; Jimenez-Lopez J. C., Ed.; IntechOpen: 2021; pp 121–142. 10.5772/intechopen.96871. [DOI] [Google Scholar]
- Toomer O. T. Nutritional chemistry of the peanut (Arachis hypogaea). Critical reviews in food science and nutrition 2018, 58 (17), 3042–3053. 10.1080/10408398.2017.1339015. [DOI] [PubMed] [Google Scholar]
- Hussein H. Z.; Al Wahbe A. A. Assessing the efficacy of certain nano, natural and chemical materials in fungal inhibition and afb1 toxin reduction of Aspergillus flavus isolated from peanut on PDA media. Plant Archives 2020, 20 (1), 1051–1057. [Google Scholar]
- de Camargo A. C.; Regitano-d’Arce M. A. B.; Rasera G. B.; Canniatti-Brazaca S. G.; do Prado-Silva L.; Alvarenga V. O.; Shahidi F.; Sant'Ana A. S. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chemistry 2017, 237, 538–544. 10.1016/j.foodchem.2017.05.046. [DOI] [PubMed] [Google Scholar]
- Tedesco M. P.; Monaco-Lourenco C. A.; Carvalho R. A. Characterization of oral disintegrating film of peanut skin extract—Potential route for buccal delivery of phenolic compounds. Int. J. Biol. Macromol. 2017, 97, 418–425. 10.1016/j.ijbiomac.2017.01.044. [DOI] [PubMed] [Google Scholar]
- Arya S. S.; Salve A. R.; Chauhan S. Peanuts as functional food: a review. Journal of food science and technology 2016, 53, 31–41. 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. S.; Salve A. R.; Chauhan S. Peanuts as functional food: a review. Journal of food science and technology 2016, 53, 31–41. 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aninbon C.; Jogloy S.; Vorasoot N.; Nuchadomrong S.; Holbrook C. C.; Kvien C.; Patanothai A.; Puppala N. Variability of arginine content and yield components in Valencia peanut germplasm. Breeding Science 2017, 67 (3), 207–212. 10.1270/jsbbs.16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello D. K.; Monyo E.; Deom C. M.; Inindo J.; Oloka H. K.. Groundnut Production Guide for Uganda: Recommended Practices for Farmers; National Agricultural Research Organisation: Entebbe, Uganda, 2013. [Google Scholar]
- Pandey M. K.; Kumar R.; Pandey A. K.; Soni P.; Gangurde S. S.; Sudini H. K.; Varshney R. K.; et al. Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post-harvest management practices. Toxins 2019, 11 (6), 315. 10.3390/toxins11060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waliyar F.; Kumar K. V. K.; Diallo M.; Traore A.; Mangala U. N.; Upadhyaya H. D.; Sudini H. Resistance to pre-harvest aflatoxin contamination in ICRISAT’s groundnut mini core collection. European Journal of Plant Pathology 2016, 145, 901–913. 10.1007/s10658-016-0879-9. [DOI] [Google Scholar]
- Korani W.; Chu Y.; Holbrook C. C.; Ozias-Akins P. Insight into genes regulating postharvest aflatoxin contamination of tetraploid peanut from transcriptional profiling. Genetics 2018, 209 (1), 143–156. 10.1534/genetics.118.300478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guchi E. Aflatoxin contamination in groundnut (Arachis hypogaea L.) caused by Aspergillus species in Ethiopia. Journal of Applied & Environmental Microbiology 2015, 3 (1), 11–19. 10.12691/jaem-3-1-3. [DOI] [Google Scholar]
- Perrone G.; Gallo A. Aspergillus species and their associated mycotoxins. Mycotoxigenic fungi: Methods and protocols 2017, 1542, 33–49. 10.1007/978-1-4939-6707-0_3. [DOI] [PubMed] [Google Scholar]
- Mohammed A.; Chala A.; Dejene M.; Fininsa C.; Hoisington D. A.; Sobolev V. S.; Arias R. S. Aspergillus and aflatoxin in groundnut (Arachis hypogaea L.) and groundnut cake in Eastern Ethiopia. Food Additives & Contaminants: Part B 2016, 9 (4), 290–298. 10.1080/19393210.2016.1216468. [DOI] [PubMed] [Google Scholar]
- Bishnu P.; De P.; Hayat A.; Roy D.; Das S.; Mahato M. K. Modern Approaches in Aflatoxin Free Groundnut Production. Advances in Agriculture Sciences 2022, 37, 24. 10.22271/ed.book.1896. [DOI] [Google Scholar]
- Okello D. K.; Kaaya A. N.; Bisikwa J.; Were M.; Oloka H. K.. Management of Aflatoxins in Groundnuts: A Manual for Farmers, Processors, Traders and Consumers in Uganda; National Agricultural Research Organisation: Entebbe, Uganda, 2010. [Google Scholar]
- Banla E. M.; Dzidzienyo D. K.; Beatrice I. E.; Offei S. K.; Tongoona P.; Desmae H. Groundnut production constraints and farmers’ trait preferences: a pre-breeding study in Togo. Journal of Ethnobiology and Ethnomedicine 2018, 14 (1), 75. 10.1186/s13002-018-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appels K.; Kooijmans R.. Aflatoxins - how a toxin causes liver cancer. Food Safety Experts, January 17, 2017. https://www.foodsafety-experts.com/aflatoxin-detection/.
- Kachapulula P. W.; Akello J.; Bandyopadhyay R.; Cotty P. J. Aflatoxin contamination of groundnut and maize in Zambia: observed and potential concentrations. J. Appl. Microbiol. 2017, 122 (6), 1471–1482. 10.1111/jam.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachapulula P. W.; Bandyopadhyay R.; Cotty P. J. Aflatoxin contamination of non-cultivated fruits in Zambia. Frontiers in Microbiology 2019, 10, 1840. 10.3389/fmicb.2019.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera S. K.; Rani K.; Kamdar J. H.; Pandey M. K.; Desmae H.; Holbrook C. C.; Varshney R. K.; et al. Genomic Designing for Biotic Stress Resistant Peanut. In Genomic Designing for Biotic Stress Resistant Oilseed Crops; Kole C., Ed.; Springer: 2022; pp 137–214. 10.1007/978-3-030-91035-8_4. [DOI] [Google Scholar]
- Latha P.; Sudhakar P.; Sreenivasulu Y.; Naidu P. H.; Reddy P. V. Relationship between total phenols and aflatoxin production of peanut genotypes under end-of-season drought conditions. Acta Physiologiae Plantarum 2007, 29, 563–566. 10.1007/s11738-007-0068-8. [DOI] [Google Scholar]
- Hamidou F.; Rathore A.; Waliyar F.; Vadez V. Although drought intensity increases aflatoxin contamination, drought tolerance does not lead to less aflatoxin contamination. Field Crops Research 2014, 156, 103–110. 10.1016/j.fcr.2013.10.019. [DOI] [Google Scholar]
- Wang W.; Liang X.; Li Y.; Wang P.; Keller N. P. Genetic Regulation of Mycotoxin Biosynthesis. Journal of Fungi 2023, 9 (1), 21. 10.3390/jof9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A. M.; Barros G. G.; Palacios S. A.; Chulze S. N.; Battilani P. Review on pre-and post-harvest management of peanuts to minimize aflatoxin contamination. Food Research International 2014, 62, 11–19. 10.1016/j.foodres.2014.02.023. [DOI] [Google Scholar]
- Abdallah M. F.; Audenaert K.; Lust L.; Landschoot S.; Bekaert B.; Haesaert G.; De Saeger S.; De Boevre M. Risk characterization and quantification of mycotoxins and their producing fungi in sugarcane juice: A neglected problem in a widely-consumed traditional beverage. Food Control 2020, 108, 106811. 10.1016/j.foodcont.2019.106811. [DOI] [Google Scholar]
- Opio P.; Photchanachai S. Modified atmosphere influences aflatoxin B1 contamination and quality of peanut (Arachis hypogaea L.) kernels cv. Khon Kaen 84–8. Journal of Stored Products Research 2018, 78, 67–73. 10.1016/j.jspr.2018.06.005. [DOI] [Google Scholar]
- Nayak S. N.; Agarwal G.; Pandey M. K.; Sudini H. K.; Jayale A. S.; Purohit S.; Varshney R. K.; et al. Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization. Sci. Rep. 2017, 7 (1), 9659. 10.1038/s41598-017-09260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuagela M. O.; Iqdiam B. M.; Baker G. L.; MacIntosh A. J. Temperature-controlled pulsed light treatment: impact on aflatoxin level and quality parameters of peanut oil. Food and bioprocess technology 2018, 11, 1350–1358. 10.1007/s11947-018-2105-6. [DOI] [Google Scholar]
- Nakrani B. R.; Patel D. B. Effect of different storage period of groundnut seeds of different varieties for biochemical changes due to Aspergillus flavus. Journal of Pharmacognosy and Phytochemistry 2018, 7 (4), 2572–2575. [Google Scholar]
- Chavan A. M. Nutritional changes in oilseeds due to Aspergillus spp. Journal of Experimental Sciences 2011, 2 (4), 29–31. [Google Scholar]
- Fagbohun E. D.; Faleye O. S. The nutritional and mycoflora changes during storage of groundnut (Arachis hypogea). International Journal of Agronomy and Agricultural Research 2012, 2 (6), 15–22. [Google Scholar]
- Ojiewo C. O.; Janila P.; Bhatnagar-Mathur P.; Pandey M. K.; Desmae H.; Okori P.; Varshney R. K.; et al. Advances in crop improvement and delivery research for nutritional quality and health benefits of groundnut (Arachis hypogaea L.). Frontiers in Plant Science 2020, 11, 29. 10.3389/fpls.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum M. A. J.; Venudevan B.; Jayanthi M. Storage fungi in groundnut and the associate seed quality deterioration-a review. Plant Pathology Journal (Faisalabad) 2013, 12 (3), 127–134. 10.3923/ppj.2013.127.134. [DOI] [Google Scholar]
- Bhushan G.; Sharma S.; Kumar S.; Sagar P. Effect of moisture content on seed microflora, nutritive value deteriorate and aflatoxin contamination of Sorghum vulgare seeds during storage. Asian Journal of Biochemical and Pharmaceutical Research 2013, 1 (4), 202–206. [Google Scholar]
- Adiver S. S.; Kumar M. G. K. Incidence of Aspergillus flavus L. Ex. Fries in groundnut (Arachis hypogaea L.) samples and its impact on nutritive value of kernels. Karnataka Journal of Agricultural Sciences 2015, 28 (2), 224–227. [Google Scholar]
- Ebrahimi A.; Emadi A.; Arabameri M.; Jayedi A.; Abdolshahi A.; Yancheshmeh B. S.; Shariatifar N. The prevalence of aflatoxins in different nut samples: A global systematic review and probabilistic risk assessment. AIMS Agriculture & Food 2022, 7 (1), 130–148. 10.3934/agrfood.2022009. [DOI] [Google Scholar]
- Chen T.; Liu J.; Li Y.; Wei S. Burden of disease associated with dietary exposure to aflatoxins in China in 2020. Nutrients 2022, 14 (5), 1027. 10.3390/nu14051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingenbleek L.; Sulyok M.; Adegboye A.; Hossou S. E.; Koné A. Z.; Oyedele A. D.; Krska R.; et al. Regional Sub-Saharan Africa total diet study in Benin, Cameroon, Mali and Nigeria reveals the presence of 164 mycotoxins and other secondary metabolites in foods. Toxins 2019, 11 (1), 54. 10.3390/toxins11010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbord J. R.; Brown D. L. Aflatoxin contamination in Haitian peanut products and maize and the safety of oil processed from contaminated peanuts. Food control 2015, 56, 114–118. 10.1016/j.foodcont.2015.03.014. [DOI] [Google Scholar]
- Ahmed Abdullah Murshed S.; Bacha N.; Alharazi T. Detection of total aflatoxins in groundnut and soybean samples in Yemen using enzyme-linked immunosorbent assay. Journal of food quality 2019, 2019, 1–7. 10.1155/2019/1614502. [DOI] [Google Scholar]
- Wu F.; Stacy S. L.; Kensler T. W. Global risk assessment of aflatoxins in maize and peanuts: are regulatory standards adequately protective?. Toxicological sciences 2013, 135 (1), 251–259. 10.1093/toxsci/kft132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P.; Imbimbo S.; Alvino S.; Castellano V.; Arace O.; Soprano V.; Sansone D.; et al. Contamination by Aflatoxins B/G in food and commodities imported in southern Italy from 2017 to 2020: A risk-based evaluation. Toxins 2021, 13 (6), 368. 10.3390/toxins13060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F.; Shi H.; Wang S.; Ma L.; Wang M. Safety evaluation of Semen Sojae Preparatum based on simultaneous LC-ESI-MS/MS quantification of aflatoxin B1, B2, G1, G2 and M1. Biomedical Chromatography 2019, 33 (8), e4541 10.1002/bmc.4541. [DOI] [PubMed] [Google Scholar]
- Ji J.; Xie W. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils. Journal of hazardous materials 2020, 381, 120915. 10.1016/j.jhazmat.2019.120915. [DOI] [PubMed] [Google Scholar]
- Gell R. M.; Carbone I. HPLC quantitation of aflatoxin B1 from fungal mycelium culture. J. Microbiol. Methods 2019, 158, 14–17. 10.1016/j.mimet.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Eom T.; Cho H. D.; Kim J.; Park M.; An J.; Kim M.; Han S. B.; Kim S. H. Multiclass mycotoxin analysis in edible oils using a simple solvent extraction method and liquid chromatography with tandem mass spectrometry. Food Additives & Contaminants: Part A 2017, 34 (11), 2011–2022. 10.1080/19440049.2017.1363416. [DOI] [PubMed] [Google Scholar]
- Njoroge S. M.; Matumba L.; Kanenga K.; Siambi M.; Waliyar F.; Maruwo J.; Monyo E. S. A case for regular aflatoxin monitoring in peanut butter in sub-Saharan Africa: Lessons from a 3-year survey in Zambia. Journal of food protection 2016, 79 (05), 795–800. 10.4315/0362-028X.JFP-15-542. [DOI] [PubMed] [Google Scholar]
- Njoroge S. M.; Matumba L.; Kanenga K.; Siambi M.; Waliyar F.; Maruwo J.; Monyo E. S.; Machinjiri N. Aflatoxin B1 levels in groundnut products from local markets in Zambia. Mycotoxin Research 2017, 33, 113–119. 10.1007/s12550-017-0270-5. [DOI] [PubMed] [Google Scholar]
- Agbetiameh D.; Ortega-Beltran A.; Awuah R. T.; Atehnkeng J.; Cotty P. J.; Bandyopadhyay R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: population structure, distribution, and toxigenicity of the causal agents. Plant disease 2018, 102 (4), 764–772. 10.1094/PDIS-05-17-0749-RE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. J.; Yang H. I.; Wu H. C.; Liu J.; Wang L. Y.; Lu S. N.; Chen C. J.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. International Journal of Cancer 2017, 141 (4), 711–720. 10.1002/ijc.30782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi Khaneghah A.; Eş I.; Raeisi S.; Fakhri Y. Aflatoxins in cereals: State of the art. Journal of Food Safety 2018, 38 (6), e12532 10.1111/jfs.12532. [DOI] [Google Scholar]
- Heshmati A.; Zohrevand T.; Khaneghah A. M.; Mozaffari Nejad A. S.; Sant’Ana A. S. Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: Dietary exposure risk assessment. Food Chem. Toxicol. 2017, 106, 202–208. 10.1016/j.fct.2017.05.046. [DOI] [PubMed] [Google Scholar]
- Matumba L.; Kimanya M.; Chunga-Sambo W.; Munthali M.; Ayalew A. Probabilistic dietary based estimation of the burden of aflatoxin-induced hepatocellular carcinoma among adult Malawians. World mycotoxin journal 2019, 12 (4), 409–419. 10.3920/WMJ2018.2346. [DOI] [Google Scholar]
- Muhie O. A.; Bayisa A. B. Is Aflatoxin a Threat to Human-Health in Ethiopia? A Systematic Review. International Journal of Collaborative Research on Internal Medicine & Public Health 2020, 12 (4), 1007–1015. [Google Scholar]
- Andrews-Trevino J. Y.; Webb P.; Shively G.; Kablan A.; Baral K.; Davis D.; Ghosh S.; et al. Aflatoxin exposure and child nutrition: measuring anthropometric and long-bone growth over time in Nepal. American Journal of Clinical Nutrition 2021, 113 (4), 874–883. 10.1093/ajcn/nqaa397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P. E. Aflatoxin: does it contribute to an increase in HIV viral load?. Future microbiology 2014, 9 (2), 121–124. 10.2217/fmb.13.166. [DOI] [PubMed] [Google Scholar]
- Wild C. P.; Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer letters 2009, 286 (1), 22–28. 10.1016/j.canlet.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Lien K. W.; Wang X.; Pan M. H.; Ling M. P. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins 2019, 11 (2), 80. 10.3390/toxins11020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkerroum N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. International journal of environmental research and public health 2020, 17 (2), 423. 10.3390/ijerph17020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash D. A review of aflatoxin: occurrence, prevention, and gaps in both food and feed safety. Journal of Applied Microbiological Research 2018, 1 (1), 35–43. [Google Scholar]
- Ajmal M.; Bedale W.; Akram A.; Yu J. H. Comprehensive review of aflatoxin contamination, impact on health and food security, and management strategies in Pakistan. Toxins 2022, 14 (12), 845. 10.3390/toxins14120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A.; Gonçalves B. L.; de Neeff D. V.; Ponzilacqua B.; Coppa C. F.; Hintzsche H.; Oliveira C. A.; et al. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Research International 2018, 113, 74–85. 10.1016/j.foodres.2018.06.067. [DOI] [PubMed] [Google Scholar]
- Kutasi K.; Recek N.; Zaplotnik R.; Mozetič M.; Krajnc M.; Gselman P.; Primc G. Approaches to inactivating aflatoxins—a review and challenges. International Journal of Molecular Sciences 2021, 22 (24), 13322. 10.3390/ijms222413322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z.; Zhang Y.; Ai Z.; Pandiselvam R.; Guo J.; Kothakota A.; Liu Y. Current physical techniques for the degradation of aflatoxins in food and feed: Safety evaluation methods, degradation mechanisms and products. Comprehensive Reviews in Food Science and Food Safety. 2023, 22, 4030. 10.1111/1541-4337.13197. [DOI] [PubMed] [Google Scholar]
- Xu Y. A.; Doel A.; Watson S.; Routledge M. N.; Elliott C. T.; Moore S. E.; Gong Y. Y. Study of an educational hand sorting intervention for reducing aflatoxin B1 in groundnuts in rural Gambia. Journal of Food Protection 2017, 80 (1), 44–49. 10.4315/0362-028X.JFP-16-152. [DOI] [PubMed] [Google Scholar]
- Karlovsky P.; Suman M.; Berthiller F.; De Meester J.; Eisenbrand G.; Perrin I.; Dussort P.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Research 2016, 32, 179–205. 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Dong M.; Yang Q.; Apaliya M. T.; Li J.; Zhang X. Biodegradation of zearalenone by Saccharomyces cerevisiae: Possible involvement of ZEN responsive proteins of the yeast. Journal of Proteomics 2016, 143, 416–423. 10.1016/j.jprot.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Sipos P.; Peles F.; Brassó D. L.; Béri B.; Pusztahelyi T.; Pócsi I.; Győri Z. Physical and chemical methods for reduction in aflatoxin content of feed and food. Toxins 2021, 13 (3), 204. 10.3390/toxins13030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. K.; Swain B. K.. Aflatoxin Occurrence, Detection, and Novel Strategies to Reduce Toxicity in Poultry Species. In Aflatoxins-Occurrence, Detection and Novel Detoxification Strategies; IntechOpen: 2022. [Google Scholar]
- Anjum T.; Iram W.; Iqbal M.; Ghaffar A.; Abbas M. Identification of degradation products of aflatoxin B1 and B2 resulting after their biodetoxification by aqueous extracts of Acacia nilotica. World Mycotoxin Journal 2020, 13 (4), 499–514. 10.3920/WMJ2018.2411. [DOI] [Google Scholar]
- Prietto L.; Moraes P. S.; Kraus R. B.; Meneghetti V.; Fagundes C. A. A.; Furlong E. B. Post-harvest operations and aflatoxin levels in rice (Oryza sativa). Crop Prot. 2015, 78, 172–177. 10.1016/j.cropro.2015.09.011. [DOI] [Google Scholar]
- Murphy P. A.; Hendrich S.; Landgren C.; Bryant C. M. Food mycotoxins: an update. J. Food Sci. 2006, 71 (5), R51–R65. 10.1111/j.1750-3841.2006.00052.x. [DOI] [Google Scholar]
- Kabak B.; Dobson A. D.; Var I. I. L. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Critical reviews in food science and nutrition 2006, 46 (8), 593–619. 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- Xu F.; Baker R. C.; Whitaker T. B.; Luo H.; Zhao Y.; Stevenson A.; Zhang G.; Boesch C. J. Review of good agricultural practices for smallholder maize farmers to minimise aflatoxin contamination. World Mycotoxin Journal 2022, 15 (2), 171–186. 10.3920/WMJ2021.2685. [DOI] [Google Scholar]
- Tian F.; Chun H. S.. Natural products for preventing and controlling aflatoxin contamination of food. Aflatoxin-Control, Analysis, Detection and Health Risks; Abdulra’uf L., Ed.; IntechOpen: 2017; pp 13–44. 10.5772/intechopen.68413. [DOI] [Google Scholar]
- Piotrowska M.; Slizewska K.; Biernasiak J.. Mycotoxins in cereal and soybean-based food and feed. Soybean-Pest Resistance; El-Shemy H., Ed.; IntechOpen: 2013; pp 185–230. 10.5772/54470. [DOI] [Google Scholar]
- Umar A.; Bhatti H. S.; Honey S. F. A call for aflatoxin control in Asia. CABI Agriculture and Bioscience 2023, 4 (1), 27. 10.1186/s43170-023-00169-z. [DOI] [Google Scholar]
- Hamdan N.; Lee C. H.; Wong S. L.; Fauzi C. E. N. C. A.; Zamri N. M. A.; Lee T. H. Prevention of enzymatic browning by natural extracts and genome-editing: a review on recent progress. Molecules 2022, 27 (3), 1101. 10.3390/molecules27031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeem N.; Nawaz M.; Anjum A. A.; Saeed S.; Sana S.; Mustafa A.; Yousuf M. R. Activity and anti-aflatoxigenic effect of indigenously characterized probiotic lactobacilli against Aspergillus flavus—A common poultry feed contaminant. Animals 2019, 9 (4), 166. 10.3390/ani9040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H. K.; Accinelli C.; Shier W. T. Biological control of aflatoxin contamination in US crops and the use of Bioplastic formulations of Aspergillus flavus biocontrol strains to optimize application strategies. J. Agric. Food Chem. 2017, 65 (33), 7081–7087. 10.1021/acs.jafc.7b01452. [DOI] [PubMed] [Google Scholar]
- Lewis M. H.; Carbone I.; Luis J. M.; Payne G. A.; Bowen K. L.; Hagan A. K.; Ojiambo P. S.; et al. Biocontrol strains differentially shift the genetic structure of indigenous soil populations of Aspergillus flavus. Frontiers in Microbiology 2019, 10, 1738. 10.3389/fmicb.2019.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisha L. C.; Cotty P. J. Genetic analysis of the Aspergillus flavus vegetative compatibility group to which a biological control agent that limits aflatoxin contamination in US crops belongs. Applied and environmental microbiology 2015, 81 (17), 5889–5899. 10.1128/AEM.00738-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa A. A.; Al-Rahmah A. N.; Abdel-Megeed A.; Sayed S. R.; Hatamleh A. A. Antagonistic activities of some fungal strains against the toxigenic Aspergillus flavus isolate and its aflatoxins productivity. J. Pure Appl. Microbiol 2013, 7, 169–178. [Google Scholar]
- Ren X.; Zhang Q.; Zhang W.; Mao J.; Li P. Control of aflatoxigenic molds by antagonistic microorganisms: Inhibitory behaviors, bioactive compounds, related mechanisms, and influencing factors. Toxins 2020, 12 (1), 24. 10.3390/toxins12010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni P.; Gangurde S. S.; Ortega-Beltran A.; Kumar R.; Parmar S.; Sudini H. K.; Varshney R. K.; et al. Functional biology and molecular mechanisms of host-pathogen interactions for aflatoxin contamination in groundnut (Arachis hypogaea L.) and maize (Zea mays L.). Frontiers in Microbiology 2020, 11, 227. 10.3389/fmicb.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolev V. S.; Walk T. E.; Arias R. S.; Massa A. N.; Orner V. A.; Lamb M. C. Transformation of Major Peanut (Arachis hypogaea) Stilbenoid Phytoalexins Caused by Selected Microorganisms. J. Agric. Food Chem. 2022, 70 (4), 1101–1110. 10.1021/acs.jafc.1c06122. [DOI] [PubMed] [Google Scholar]
- Fountain J. C.; Khera P.; Yang L.; Nayak S. N.; Scully B. T.; Lee R. D.; Guo B.; et al. Resistance to Aspergillus flavus in maize and peanut: Molecular biology, breeding, environmental stress, and future perspectives. Crop Journal 2015, 3 (3), 229–237. 10.1016/j.cj.2015.02.003. [DOI] [Google Scholar]
- Wang H.; Lei Y.; Wan L.; Yan L.; Lv J.; Dai X.; Liao B.; et al. Comparative transcript profiling of resistant and susceptible peanut post-harvest seeds in response to aflatoxin production by Aspergillus flavus. BMC Plant Biology 2016, 16 (1), 54. 10.1186/s12870-016-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L.; Li B.; Lei Y.; Yan L.; Huai D.; Kang Y.; Liao B.; et al. Transcriptomic profiling reveals pigment regulation during peanut testa development. Plant Physiology and Biochemistry 2018, 125, 116–125. 10.1016/j.plaphy.2018.01.029. [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S.; Solano R. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Frontiers in Plant Science 2013, 4, 72. 10.3389/fpls.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htoon W.; Kaewpradit W.; Vorasoot N.; Toomsan B.; Akkasaeng C.; Puppala N.; Jogloy S.; Wongkaew S. Relationships between nutrient uptake and nitrogen fixation with aflatoxin contamination in peanut under terminal drought. Agronomy 2019, 9 (8), 419. 10.3390/agronomy9080419. [DOI] [Google Scholar]
- Mendu L.; Cobos C. J.; Tengey T. K.; Commey L.; Balasubramanian V. K.; Williams L. D.; Mendu V. Seed coat mediated resistance against Aspergillus flavus infection in peanut. Plant Gene 2022, 32, 100381. 10.1016/j.plgene.2022.100381. [DOI] [Google Scholar]
- Sun D.; Mao J.; Wang Z.; Li H.; Zhang L.; Zhang W.; Li P.; Zhang Q. Inhibition of Aspergillus flavus growth and aflatoxins production on peanuts over α-Fe2O3 nanorods under sunlight irradiation. International Journal of Food Microbiology 2021, 353, 109296. 10.1016/j.ijfoodmicro.2021.109296. [DOI] [PubMed] [Google Scholar]
- Čolović R.; Puvača N.; Cheli F.; Avantaggiato G.; Greco D.; Đuragić O.; Pinotti L.; Kos J. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11 (11), 617. 10.3390/toxins11110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aygün Ş. G.Detoxification of Aflatoxin in Peanut Meal by Heating and Gamma Irradiation. PhD. Thesis, Middle East Technical University, 2015. [Google Scholar]
- Mao J.; He B.; Zhang L.; Li P.; Zhang Q.; Ding X.; Zhang W. A structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins 2016, 8 (11), 332. 10.3390/toxins8110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z.; Chen L.; Zhu Y.; Huang Y.; Hu X.; Wu Q.; Yang W.; et al. Current major degradation methods for aflatoxins: A review. Trends in Food Science & Technology 2018, 80, 155–166. 10.1016/j.tifs.2018.08.009. [DOI] [Google Scholar]
- Sembada A. A.; Lenggoro I. W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14 (2), 131. 10.3390/nano14020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky P.; Skalickova S.; Baholet D.; Skladanka J. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials 2018, 8 (9), 727. 10.3390/nano8090727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M. A.; Zahir E.; Shahid S. M.; Khan M. N.; Asghar M. A.; Iqbal J.; Walker G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. Lwt 2018, 90, 98–107. 10.1016/j.lwt.2017.12.009. [DOI] [Google Scholar]
- Javed B.; Ikram M.; Farooq F.; Sultana T.; Mashwani Z. U. R.; Raja N. I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. 10.1007/s00253-021-11171-8. [DOI] [PubMed] [Google Scholar]
- Ehsan M.; Waheed A.; Ullah A.; Kazmi A.; Ali A.; Raja N. I.; Li H. Plant-Based Bimetallic Silver-Zinc Oxide Nanoparticles: A Comprehensive Perspective of Synthesis, Biomedical Applications, and Future Trends. BioMed Research International 2022, 2022, 1215183. 10.1155/2022/1215183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffo Roberto S.; Youssef K.; Hashim A. F.; Ippolito A. Nanomaterials as alternative control means against postharvest diseases in fruit crops. Nanomaterials 2019, 9 (12), 1752. 10.3390/nano9121752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal G.; Rai P.; Pandey A. Green synthesis of nanoparticles: A greener approach for a cleaner future. Green synthesis, characterization and applications of nanoparticles 2019, 1–26. 10.1016/B978-0-08-102579-6.00001-0. [DOI] [Google Scholar]
- Omran B. A.; Baek K. H. Control of phytopathogens using sustainable biogenic nanomaterials: recent perspectives, ecological safety, and challenging gaps. Journal of Cleaner Production 2022, 372, 133729. 10.1016/j.jclepro.2022.133729. [DOI] [Google Scholar]
- Singh J.; Dutta T.; Kim K. H.; Rawat M.; Samddar P.; Kumar P. ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol. 2018, 16 (1), 84. 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M.; Raja N. I.; Mashwani Z. U. R.; Wattoo F. H.; Hussain M.; Ejaz M.; Saira H. Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L) under heat stress. IET nanobiotechnology 2019, 13 (2), 230–236. 10.1049/iet-nbt.2018.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A. K.; Chisti Y.; Banerjee U. C. Synthesis of metallic nanoparticles using plant extracts. Biotechnology advances 2013, 31 (2), 346–356. 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Dhaka A.; Chand Mali S.; Sharma S.; Trivedi R. A review on biological synthesis of silver nanoparticles and their potential applications. Results in Chemistry 2023, 6, 101108. 10.1016/j.rechem.2023.101108. [DOI] [Google Scholar]
- Sorbiun M.; Shayegan Mehr E.; Ramazani A.; Mashhadi Malekzadeh A. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochemistry Research 2018, 3 (1), 1–16. 10.22036/NCR.2018.01.001. [DOI] [Google Scholar]
- Parveen J.; Sultana T.; Kazmi A.; Malik K.; Ullah A.; Ali A.; Rehman S. U. Phytosynthesized Nanoparticles as Novel Antifungal Agent for Sustainable Agriculture: A Mechanistic Approach, Current Advances, and Future Directions. Journal of Nanotechnology 2023, 2023, 8011189. 10.1155/2023/8011189. [DOI] [Google Scholar]
- Jamkhande P. G.; Ghule N. W.; Bamer A. H.; Kalaskar M. G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. Journal of drug delivery science and technology 2019, 53, 101174. 10.1016/j.jddst.2019.101174. [DOI] [Google Scholar]
- Ogar A.; Tylko G.; Turnau K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521, 305–314. 10.1016/j.scitotenv.2015.03.101. [DOI] [PubMed] [Google Scholar]
- Kasithevar M.; Periakaruppan P.; Muthupandian S.; Mohan M. Antibacterial efficacy of silver nanoparticles against multi-drug resistant clinical isolates from post-surgical wound infections. Microbial pathogenesis 2017, 107, 327–334. 10.1016/j.micpath.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh Davarani F.; Ashrafizadeh M.; Saberi Riseh R.; Ghasemipour Afshar E.; Mohammadi H.; Razavi S. H.; Mohammadinejad R. Antifungal nanoparticles reduce aflatoxin contamination in pistachio. Pistachio and Health Journal 2018, 1 (2), 26–33. 10.22123/PHJ.2018.130469.1007. [DOI] [Google Scholar]
- Niemirowicz K.; Durnaś B.; Piktel E.; Bucki R. Development of antifungal therapies using nanomaterials. Nanomedicine 2017, 12 (15), 1891–1905. 10.2217/nnm-2017-0052. [DOI] [PubMed] [Google Scholar]
- Roque L.; Molpeceres J.; Reis C.; Rijo P.; Pinto Reis C. Past, recent progresses and future perspectives of nanotechnology applied to antifungal agents. Current Drug Metabolism 2017, 18 (4), 280–290. 10.2174/1389200218666170201152000. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Li G.; Wu D.; Liu J.; Wu Y. Recent advances on emerging nanomaterials for controlling the mycotoxin contamination: From detection to elimination. Food Frontiers 2020, 1 (4), 360–381. 10.1002/fft2.42. [DOI] [Google Scholar]
- Duhan J. S.; Kumar R.; Kumar N.; Kaur P.; Nehra K.; Duhan S. Nanotechnology: The new perspective in precision agriculture. Biotechnology reports 2017, 15, 11–23. 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M. K.; Mishra A. K.; Mohanta Y. K.; Mahanta S.; Chakrabartty I.; Kungwani N. A.; Pudake R. N.; et al. Nanotechnology as a promising tool against phytopathogens: A futuristic approach to agriculture. Agriculture 2023, 13 (9), 1856. 10.3390/agriculture13091856. [DOI] [Google Scholar]
- Balaure P. C.; Gudovan D.; Gudovan I.. Nanopesticides: a new paradigm in crop protection. In New Pesticides and Soil Sensors; Academic Press: 2017; pp 129–192. [Google Scholar]
- Satti S. H.; Raja N. I.; Ikram M.; Oraby H. F.; Mashwani Z. U. R.; Mohamed A. H.; Omar A. A.; Singh A. Plant-based titanium dioxide nanoparticles trigger biochemical and proteome modifications in Triticum aestivum L. under biotic stress of Puccinia striiformis. Molecules 2022, 27 (13), 4274. 10.3390/molecules27134274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi S.; Raza M.; Shahbaz M.; Ajmal M.; Mehak A.; Fatima N.; Maimaiti Y.; et al. Biosynthesized silver nanoparticles enhanced wheat resistance to Bipolaris sorokiniana. Plant Physiology and Biochemistry 2023, 203, 108067. 10.1016/j.plaphy.2023.108067. [DOI] [PubMed] [Google Scholar]
- Shahbaz M.; Akram A.; Mehak A.; Haq E. U.; Fatima N.; Wareen G.; Sabullah M. K.; et al. Evaluation of selenium nanoparticles in inducing disease resistance against spot blotch disease and promoting growth in wheat under biotic stress. Plants 2023, 12 (4), 761. 10.3390/plants12040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi M. O.; Alotaibi N. M.; Ghoneim A. M.; ul Ain N.; Irshad M. A.; Nawaz R.; Ali S.; et al. Effect of green synthesized cerium oxide nanoparticles on fungal disease of wheat plants: A field study. Chemosphere 2023, 339, 139731. 10.1016/j.chemosphere.2023.139731. [DOI] [PubMed] [Google Scholar]
- Elbasuney S.; El-Sayyad G. S.; Attia M. S.; Abdelaziz A. M. Ferric oxide colloid: Towards green nano-fertilizer for tomato plant with enhanced vegetative growth and immune response against fusarium wilt disease. Journal of Inorganic and Organometallic Polymers and Materials 2022, 32 (11), 4270–4283. 10.1007/s10904-022-02442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Batal A. I.; El-Sayyad G. S.; Al-Shammari B. M.; Abdelaziz A. M.; Nofel M. M.; Gobara M.; Attia M. S.; et al. Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microbial Pathogenesis 2023, 180, 106131. 10.1016/j.micpath.2023.106131. [DOI] [PubMed] [Google Scholar]
- Kamel S. M.; Elgobashy S. F.; Omara R. I.; Derbalah A. S.; Abdelfatah M.; El-Shaer A.; Elsharkawy M. M.; et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. Journal of Fungi 2022, 8 (9), 911. 10.3390/jof8090911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed R. A.; Jebur A. B.; Kang W.; El-Demerdash F. M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. Journal of Future Foods 2022, 2 (2), 91–102. 10.1016/j.jfutfo.2022.03.002. [DOI] [Google Scholar]
- Jard G.; Liboz T.; Mathieu F.; Guyonvarc’h A.; Lebrihi A. Review of mycotoxin reduction in food and feed: from prevention in the field to detoxification by adsorption or transformation. Food Additives & Contaminants: Part A 2011, 28 (11), 1590–1609. 10.1080/19440049.2011.595377. [DOI] [PubMed] [Google Scholar]
- Das S.; Ray M. K.; Panday D.; Mishra P. K. Role of biotechnology in creating sustainable agriculture. PLOS Sustainability and Transformation 2023, 2 (7), e0000069 10.1371/journal.pstr.0000069. [DOI] [Google Scholar]
- Kaushik A.; Solanki P. R.; Ansari A. A.; Ahmad S.; Malhotra B. D. A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology 2009, 20 (5), 055105. 10.1088/0957-4484/20/5/055105. [DOI] [PubMed] [Google Scholar]
- Pietrzak K.; Twarużek M.; Czyżowska A.; Kosicki R.; Gutarowska B. Influence of silver nanoparticles on metabolism and toxicity of moulds. Acta Biochimica Polonica 2015, 62 (4), 851. 10.18388/abp.2015_1146. [DOI] [PubMed] [Google Scholar]
- Nile S. H.; Baskar V.; Selvaraj D.; Nile A.; Xiao J.; Kai G. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano-Micro Letters 2020, 12, 45. 10.1007/s40820-020-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytesnikova Z.; Adam V.; Richtera L. Graphene oxide as a novel tool for mycotoxin removal. Food Control 2021, 121, 107611. 10.1016/j.foodcont.2020.107611. [DOI] [Google Scholar]
- Thirugnanasambandan T.; Gopinath S. C. Nanomaterials in food industry for the protection from mycotoxins: an update. 3 Biotech 2023, 13 (2), 64. 10.1007/s13205-023-03478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin Y. N.; Bach H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials 2022, 12 (24), 4470. 10.3390/nano12244470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Ma G.; Wei W. Simulation of nanoparticles interacting with a cell membrane: probing the structural basis and potential biomedical application. NPG Asia Materials 2021, 13 (1), 52. 10.1038/s41427-021-00320-0. [DOI] [Google Scholar]
- Huang T.; Li X.; Maier M.; O’Brien-Simpson N. M.; Heath D. E.; O’Connor A. J. Using inorganic nanoparticles to fight fungal infections in the antimicrobial resistant era. Acta Biomaterialia 2023, 158, 56–79. 10.1016/j.actbio.2023.01.019. [DOI] [PubMed] [Google Scholar]
- Abd-Elsalam K. A.; Hashim A. F.; Alghuthaymi M. A.; Said-Galiev E.. Nanobiotechnological strategies for toxigenic fungi and mycotoxin control. In Food Preservation; Academic Press: 2017; pp 337–364. [Google Scholar]
- Abd El-Ghany M. N.; Hamdi S. A.; Korany S. M.; Elbaz R. M.; Emam A. N.; Farahat M. G. Biogenic Silver Nanoparticles Produced by Soil Rare Actinomycetes and Their Significant Effect on Aspergillus-derived mycotoxins. Microorganisms 2023, 11 (4), 1006. 10.3390/microorganisms11041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad M. M.; Attiya H. J.; Al-Zubaidi L. A. Evaluation of detoxification of aflatoxin-b1 by using Ag nanoparticles of oil extracts user prepared by using some medical herbs. Herba Polonica 2022, 68 (4), 11–19. 10.2478/hepo-2022-0020. [DOI] [Google Scholar]
- Asghar M. A.; Zahir E.; Asghar M. A.; Iqbal J.; Rehman A. A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: as an effective antimicrobial and aflatoxin B1 adsorption agents. PloS one 2020, 15 (7), e0234964 10.1371/journal.pone.0234964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Desouky T. A.; Ammar H. A. Honey mediated silver nanoparticles and their inhibitory effect on aflatoxins and ochratoxin A. Journal of Applied Pharmaceutical Science 2016, 6 (6), 083–090. 10.7324/JAPS.2016.60615. [DOI] [Google Scholar]
- Al-Othman M. R.; Abd El-Aziz A. R. M.; Mahmoud M. A.; Eifan S. A.; El-Shikh M. S.; Majrashi M. Application of silver nanoparticles as antifungal and antiaflatoxin B1 produced by Aspergillus flavus. Dig. J. Nanomater. Biostruct. 2014, 9 (1), 151–157. [Google Scholar]
- Al-zubaidi L. A.; Wsain S. M.; Ibrahim S. M. Evaluate the Antifungal and detoxification activity of silver nanoparticles prepared with the Curcuma plant extract against Aflatoxin B1 in broiler feed. IOP Conference Series: Earth and Environmental Science 2021, 779 (1), 012076. 10.1088/1755-1315/779/1/012076. [DOI] [Google Scholar]
- Kumari P.; Kumar H.; Kumar J.; Sohail M.; Singh K. P.; Prasad K. Biosynthesized Zinc Oxide nanoparticles control the growth of Aspergillus flavus and its aflatoxin production. International Journal of Nano Dimension 2019, 10 (4), 320–329. [Google Scholar]
- Naqvi S. I. Z.; Kausar H.; Afzal A.; Hashim M.; Mujahid H.; Javed M.; Anjum S.; Hano C. Antifungal Activity of Juglans-regia-Mediated Silver Nanoparticles (AgNPs) against Aspergillus-ochraceus-Induced Toxicity in In Vitro and In Vivo Settings. Journal of Functional Biomaterials 2023, 14 (4), 221. 10.3390/jfb14040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasim J. Y.; Al-Taee S. K. Evaluation of the role of green synthesis silver nanoparticles as adsorbents and protective agents for broilers tissue treated with aflatoxin. Iraqi Journal of Veterinary Sciences 2023, 37 (3), 675–681. 10.33899/ijvs.2023.136771.2614. [DOI] [Google Scholar]
- Al-Othman M. R.; Abd El-Aziz A. R. M.; Mahmoud M. A.; Hatamleh A. A. Green biosynthesis of silver nanoparticles using pomegranate peel and inhibitory effects of the nanoparticles on aflatoxin production. Pakistan Journal of Botany 2017, 49 (2), 751–756. [Google Scholar]
- Sohail Y.; Raza N.; Shakeel N.; Raza H.; Manzoor S.; Yasmin G.; Mohammad Wabaidur S.; et al. Polyaniline-coated nanoparticles of zinc oxide and copper oxide as antifungal agents against Aspergillus parasiticus. Frontiers in Plant Science 2022, 13, 925451. 10.3389/fpls.2022.925451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez R. A.; Abdel-Wahhab M. A.; Sehab A. F.; El-Din A. Z. A. K. Green synthesis of silver nanoparticles using Morus nigra leave extract and evaluation their antifungal potency on phytopathogenic fungi. Journal of Applied Pharmaceutical Science 2017, 7 (2), 041–048. [Google Scholar]
- Haider A. A.; Hussein H. Z. Efficiency of biologically and locally manufactured silver nanoparticles from Aspergillus niger in preventing Aspergillus flavus to produce aflatoxin B1 on the stored maize grains. Caspian Journal of Environmental Sciences 2022, 20 (4), 765–773. 10.22124/CJES.2022.5760. [DOI] [Google Scholar]
- Abdelghany T. M.; Hassan M. M.; El-Naggar M. A.; Abd El-Mongy M. GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnology reports 2020, 27, e00496 10.1016/j.btre.2020.e00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wsain S. M.; Attiya H. J.; Al-Zubaidi L. A. Antifungal activity of turmeric phenolic extract and turmeric silver nanoparticles on aflatoxin b 1 producing fungi. Biochemical & Cellular Archives 2020, 20 (1), 637. [Google Scholar]
- Ejaz M.; Raja N. I.; Khan S. A.; Mashwani Z. U. R.; Hanif A.; Iqbal M.; Rauf A.; et al. Biosynthesized silver nanoparticles ameliorate biotic stress in rice (oryza sativa) by intricating biochemical and mineral profile. Pak. J. Bot 2023, 55 (6), 2019–2028. 10.30848/PJB2023-6(21). [DOI] [Google Scholar]
- Raj N. B.; PavithraGowda N. T.; Pooja O. S.; Purushotham B.; Kumar M. A.; Sukrutha S. K.; Boppana S. B.; et al. Harnessing ZnO nanoparticles for antimicrobial and photocatalytic activities. J. Photochem. Photobiol. 2021, 6, 100021. 10.1016/j.jpap.2021.100021. [DOI] [Google Scholar]
- Karami-Osboo R.; Maham M.; Nasrollahzadeh M. Synthesised magnetic nano-zeolite as a mycotoxins binder to reduce the toxicity of aflatoxins, zearalenone, ochratoxin A, and deoxynivalenol in barley. Iet Nanobiotechnology 2020, 14 (7), 623–627. 10.1049/iet-nbt.2020.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmeesha T. R.; Kalagatur N. K.; Mudili V.; Mohan C. D.; Rangappa S.; Prasad B. D.; Niranjana S. R.; et al. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Frontiers in Microbiology 2019, 10, 1244. 10.3389/fmicb.2019.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. K.; Tiwari S.; Maurya A.; Das S.; Singh V. K.; Dubey N. K. Chitosan-based nanoencapsulation of Ocimum americanum essential oil as safe green preservative against fungi infesting stored millets, aflatoxin B1 contamination, and lipid peroxidation. Food and Bioprocess Technology 2023, 16, 1851. 10.1007/s11947-023-03008-1. [DOI] [Google Scholar]
- Ibrahim S. M.; Al-Zubaidi L. A.; Hamodi S. J. Evaluation of the activity of the alcohol extracts, nanomolecules and green silver nanoparticles of the plant salvia officinalis and cinnamon in treating aflatoxin b1 from aspergillus flavus in contaminated poultry feed. Plant Archives 2020, 20 (1), 1890–1896. [Google Scholar]
- Mahmoud M. A. Characterization and Antifungal Efficacy of Biogenic Silver Nanoparticles and Silver-Chitosan Nanocomposites. Egyptian Journal of Phytopathology 2021, 49 (2), 68–79. 10.21608/ejp.2021.97374.1043. [DOI] [Google Scholar]
- Devipriya D.; Roopan S. M. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Materials Science and Engineering: C 2017, 80, 38–44. 10.1016/j.msec.2017.05.130. [DOI] [PubMed] [Google Scholar]
- Hussein H. Z.; Al-wahab A. A. Assessing the efficacy of certain nano, natural and chemical materials in fungal inhibition and afb1 toxin reduction of Aspergillus flavus isolated from peanut on PDA media. Plant Archives 2020, 20 (1), 1051–1057. [Google Scholar]
- Jogee P. S.; Ingle A. P.; Rai M. Isolation and identification of toxigenic fungi from infected peanuts and efficacy of silver nanoparticles against them. Food Control 2017, 71, 143–151. 10.1016/j.foodcont.2016.06.036. [DOI] [Google Scholar]
- Kanagasabai V.; Pakeerathan K.; Sashikesh G.. Nano-based formulation of Acarous calamus rhizome extract and its efficacy on Aspergillus flavus. 4th International Conference of Agricultural Sciences; Sabaragamuwa University of Sri Lanka: 2022; Vol. 1 ( (1), ), pp 9–12. [Google Scholar]
- Sheikh H.; Awad M. F. Biogenesis of nanoparticles with inhibitory effects on aflatoxin B1 production by Aspergillus flavus. Electronic Journal of Biotechnology 2022, 60, 26–35. 10.1016/j.ejbt.2022.09.003. [DOI] [Google Scholar]
- Diagne A.; Diop B. N.; SoukTounkara L.; Andreazza C.; Sembène M. Antifungal activity of silver@silica nanoparticles against aflatoxigenic Aspergillus flavus. Global Advanced Research Journal of Agricultural Science 2018, 7 (12), 377–382. [Google Scholar]
- Hegazy A.; Abdel Hay J. A. D. H.; Mesbah A. S. A. A. S.; Abou Tahoun A. Impact of foliar spray treatments on yield, quality traits and total aflatoxin content of some peanut cultivars in sandy soils. Scientific Journal of Agricultural Sciences 2022, 4 (1), 84–91. 10.21608/sjas.2022.123372.1197. [DOI] [Google Scholar]
- Kharissova O. V.; Dias H. R.; Kharisov B. I.; Pérez B. O.; Pérez V. M. J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31 (4), 240–248. 10.1016/j.tibtech.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Sureka S.; Chakravorty A.; Holmes E. C.; Spassibojko O.; Bhatt N.; Wu D.; Turgeon B. G. Standardization of functional reporter and antibiotic resistance cassettes to facilitate the genetic engineering of filamentous fungi. ACS Synth. Biol. 2014, 3 (12), 960–962. 10.1021/sb5000143. [DOI] [PubMed] [Google Scholar]
- Niemirowicz K.; Durnaś B.; Piktel E.; Bucki R. Development of antifungal therapies using nanomaterials. Nanomedicine 2017, 12 (15), 1891–1905. 10.2217/nnm-2017-0052. [DOI] [PubMed] [Google Scholar]
- Roque L.; Molpeceres J.; Reis C.; Rijo P.; Pinto Reis C. Past, recent progresses and future perspectives of nanotechnology applied to antifungal agents. Current Drug Metabolism 2017, 18 (4), 280–290. 10.2174/1389200218666170201152000. [DOI] [PubMed] [Google Scholar]
- Soliman G. M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. International journal of pharmaceutics 2017, 523 (1), 15–32. 10.1016/j.ijpharm.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Voltan A. R.; Quindós G.; Alarcón K. P. M.; Fusco-Almeida A. M.; Mendes-Giannini M. J. S.; Chorilli M. Fungal diseases: could nanostructured drug delivery systems be a novel paradigm for therapy?. Int. J. Nanomed. 2016, 11, 3715–3730. 10.2147/IJN.S93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelere I. A.; Lateef A. A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5 (6), 567–587. 10.1515/ntrev-2016-0024. [DOI] [Google Scholar]
- Sultana T.; Malik K.; Raja N. I.; Sohail; Hameed A.; Ali A.; Alrefaei A. F.; et al. Phytofabrication, characterization, and evaluation of novel bioinspired selenium-iron (Se-Fe) nanocomposites using Allium sativum extract for bio-potential applications. Green Processing and Synthesis 2023, 12 (1), 20230049. 10.1515/gps-2023-0049. [DOI] [Google Scholar]
- Cruz-Luna A. R.; Cruz-Martínez H.; Vásquez-López A.; Medina D. I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. Journal of Fungi 2021, 7 (12), 1033. 10.3390/jof7121033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussin J.; Giusiano G. Biogenic silver nanoparticles as antifungal agents. Frontiers in Chemistry 2022, 10, 1023542. 10.3389/fchem.2022.1023542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorowicz A.; Margarita V.; Fais G.; Pantaleo A.; Manca A.; Concas A.; Cao G.; et al. Characterization of nanomaterials synthesized from Spirulina platensis extract and their potential antifungal activity. PLoS One 2022, 17 (9), e0274753 10.1371/journal.pone.0274753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X.; Sun C.; Liu D.; Luo X.; Li D.; Wang J.; Zhu Y.; et al. Photocatalytic degradation of deoxynivalenol using graphene/ZnO hybrids in aqueous suspension. Applied Catalysis B: Environmental 2017, 204, 11–20. 10.1016/j.apcatb.2016.11.010. [DOI] [Google Scholar]
- Jamil T. S.; Abbas H. A.; Nasr R. A.; El-Kady A. A.; Ibrahim M. I. Detoxification of aflatoxin B1 using nano-sized Sc-doped SrTi0. 7Fe0. 3O3 under visible light. J. Photochem. Photobiol., A 2017, 341, 127–135. 10.1016/j.jphotochem.2017.03.023. [DOI] [Google Scholar]
- Wu S.; Wang F.; Li Q.; Wang J.; Zhou Y.; Duan N.; Wang Z.; Niazi S. Photocatalysis and degradation products identification of deoxynivalenol in wheat using upconversion nanoparticles@ TiO2 composite. Food chemistry 2020, 323, 126823. 10.1016/j.foodchem.2020.126823. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wu S.; Wang F.; Li Q.; He C.; Duan N.; Wang Z. Assessing the toxicity in vitro of degradation products from deoxynivalenol photocatalytic degradation by using upconversion nanoparticles@ TiO2 composite. Chemosphere 2020, 238, 124648. 10.1016/j.chemosphere.2019.124648. [DOI] [PubMed] [Google Scholar]
- Mao J.; Li P.; Wang J.; Wang H.; Zhang Q.; Zhang L.; Peng T.; et al. Insights into photocatalytic inactivation mechanism of the hypertoxic site in aflatoxin B1 over clew-like WO3 decorated with CdS nanoparticles. Applied Catalysis B: Environmental 2019, 248, 477–486. 10.1016/j.apcatb.2019.01.057. [DOI] [Google Scholar]
- Sun S.; Zhao R.; Xie Y.; Liu Y. Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst. Food Control 2019, 100, 183–188. 10.1016/j.foodcont.2019.01.014. [DOI] [Google Scholar]
- Murugesan P.; Brunda D. K.; Moses J. A.; Anandharamakrishnan C. Photolytic and photocatalytic detoxification of mycotoxins in foods. Food Control 2021, 123, 107748. 10.1016/j.foodcont.2020.107748. [DOI] [Google Scholar]
- Kumar A.; Pathak H.; Bhadauria S.; Sudan J. Aflatoxin contamination in food crops: causes, detection, and management: a review. Food Production, Processing and Nutrition 2021, 3, 17. 10.1186/s43014-021-00064-y. [DOI] [Google Scholar]
- Tian F.; Chun H. S.. Natural products for preventing and controlling aflatoxin contamination of food. Aflatoxin-Control, Analysis, Detection and Health Risks; Abdulra’uf L. B., Ed.; InTechOpen: 2017; pp 13–44. 10.5772/intechopen.68413. [DOI] [Google Scholar]
- Khan M.; Khan A. U.; Hasan M. A.; Yadav K. K.; Pinto M. M.; Malik N.; Sharma G. K.; et al. Agro-nanotechnology as an emerging field: A novel sustainable approach for improving plant growth by reducing biotic stress. Applied Sciences 2021, 11 (5), 2282. 10.3390/app11052282. [DOI] [Google Scholar]
- Ahmad I.; Younas Z.; Mashwani Z. U. R.; Raja N. I.; Akram A. Phytomediated Selenium Nanoparticles Improved Physio-morphological, Antioxidant, and Oil Bioactive Compounds of Sesame under Induced Biotic Stress. ACS omega 2023, 8 (3), 3354–3366. 10.1021/acsomega.2c07084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana T.; Javed B.; Raja N. I.; Mashwani Z. U. R. Silver nanoparticles elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus. Green processing and synthesis 2021, 10 (1), 314–324. 10.1515/gps-2021-0034. [DOI] [Google Scholar]
- Onyeaka H.; Passaretti P.; Miri T.; Al-Sharify Z. T. The safety of nanomaterials in food production and packaging. Current Research in Food Science 2022, 5, 763–774. 10.1016/j.crfs.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S.; Singh B. K.; Dubey N. K. Aflatoxins in food systems: recent advances in toxicology, biosynthesis, regulation and mitigation through green nanoformulations. Journal of the Science of Food and Agriculture 2023, 103 (4), 1621–1630. 10.1002/jsfa.12265. [DOI] [PubMed] [Google Scholar]
- Han C.; Zhao A.; Varughese E.; Sahle-Demessie E. Evaluating weathering of food packaging polyethylene-nano-clay composites: Release of nanoparticles and their impacts. NanoImpact 2018, 9, 61–71. 10.1016/j.impact.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathakoti K.; Manubolu M.; Hwang H. M. Nanostructures: Current uses and future applications in food science. Journal of food and drug analysis 2017, 25 (2), 245–253. 10.1016/j.jfda.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Henríquez L.; Alfaro-Aguilar K.; Ugalde-Álvarez J.; Vega-Fernández L.; Montes de Oca-Vásquez G.; Vega-Baudrit J. R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10 (9), 1763. 10.3390/nano10091763. [DOI] [PMC free article] [PubMed] [Google Scholar]