Abstract
Laboratory strains of measles viruses (MV), such as Edmonston and Halle, use the complement regulatory protein CD46 as a cell surface receptor. The receptor usage of clinical isolates of MV, however, remains unclear. Receptor usage by primary patient isolates of MV was compared to isolates that had been passaged on a variety of tissue culture cell lines. All of the isolates could infect cells in a CD46-dependent manner, but their tropism was restricted according to cell type (e.g., lymphocytes versus fibroblasts). The results indicate that patient isolates that have not been adapted to tissue culture cell lines use CD46 as a receptor. In addition, passaging primary MV patient isolates in B95-8 cells selected variants that had alternate receptor usage compared to the original isolate. Thus, changes in receptor usage by MV are dependent upon the cell type used for isolation. Furthermore, our results confirm the relevance of the CD46 receptor to natural measles infection.
Measles virus (MV) is the seventh leading cause of childhood mortality worldwide (6), infecting more than 40 million children and leading to approximately one million deaths each year (4, 5, 9). In addition to causing an acute respiratory infection, measles is associated with a profound, transient suppression of cell-mediated immunity. Immunosuppression contributes to the major complications from measles: pneumonia, diarrhea, and other secondary infections (12, 20, 45). In rare cases, measles can also cause encephalitis or persistent infection of the central nervous system (20, 49). Although an effective vaccine is available, the extreme infectiousness of the agent, combined with reduced vaccine efficacy in young infants, contributes to the continuing circulation of MV in human populations (2, 14, 19, 31, 42).
The Edmonston MV was isolated in 1954 on primary human kidney cells (20). Following an extensive period of cocultivation a virus isolate that caused cytopathic effects (syncytium formation) was recovered. The Edmonston isolate was subsequently adapted to African green monkey kidney (Vero) cells, a process that attenuates MV (41). MV vaccines are attenuated in a similar manner by passage on human kidney and human amnion followed by multiple passages on chicken embryo fibroblasts (20). Laboratory strains of MV that have been grown in the same way as Edmonston have been extensively characterized and form the basis of current knowledge about MV tropism, replication, pathogenesis, and receptor usage.
Studying the interaction between a virus and its receptor(s) can provide key insights into the pathogenesis of a viral infection and can also provide targets for designing drugs that prevent infection. For MV, the viral hemagglutinin (H) glycoprotein binds directly to the cellular receptor (15, 46, 57). The viral fusion (F) glycoprotein contains a putative hydrophobic fusion peptide, which triggers fusion between the virus and host cell membranes at neutral pH (38, 57). The cellular receptor for laboratory strains of MV is membrane cofactor protein (CD46) (16, 39, 43, 44). CD46, a transmembrane glycoprotein of approximately 57 67 kDa, is a member of the regulators of complement activation (RCA) superfamily of complement-binding proteins (55). RCA proteins protect host cells from autologous complement by binding activated complement components and preventing their deposition on the host cell surface (22, 36, 37). CD46 expression allows binding, entry, and replication of laboratory strains such as Edmonston and Halle in normally nonsusceptible rodent cells (16, 39, 43).
The extracellular domain of CD46 includes four conserved modules called short consensus repeats (SCRs) that are typically found in RCA proteins (36, 37). Laboratory isolates of MV bind to regions within SCRs 1 and 2 of CD46 (10, 24, 40). Mutant CD46 proteins with deletions in SCR 1 or SCR 2 cannot bind to MV or allow MV entry (1, 40). In addition, antibodies recognizing CD46 SCRs 1 and 2 inhibit MV infection (13, 23, 40).
Following identification of the CD46 receptor for laboratory MV strains (16, 39, 43), it was suggested that CD46 does not serve as receptor for all MV strains (35, 59). While traditional isolation of MV utilizes Vero cells, recently rapid isolation of MV from patient samples has been performed using the Epstein-Barr virus (EBV)-transformed marmoset B-cell line B95-8 (34), the human immortalized B lymphoma cell line BJAB (7, 53), or the human EBV-transformed B-cell line Daikiki (M. L. Celma and R. Fernandez-Muñoz, unpublished observations). Often the resulting B-cell-adapted isolates are unable to infect CD46-expressing, nonlymphoid cell lines such as Vero or HeLa (35). In addition, some B-cell-adapted isolates are not inhibited by anti-CD46 antibody (7, 25). Together, these findings suggested that CD46, which is expressed on HeLa and Vero cells, is the receptor for vaccine or laboratory-passaged strains but not the receptor for these B-lymphotrophic isolates of MV (11, 25, 35).
To determine whether CD46 is used by strains of MV circulating in the human population and if receptor usage is affected by passage on commonly used cell lines, we studied virus tropism, interaction with CD46, and sequence changes among MVs isolated and passaged on either primary human peripheral blood lymphocytes, marmoset B lymphocytes, or Vero cells. Given that RNA viruses have relatively high mutation rates, allowing the potential for frequent generation of variants, we reasoned that laboratory culture conditions could constitute a powerful selective pressure affecting receptor usage. Our studies indicate that primary MV isolates, when taken from patients and cultured on primary human peripheral blood mononuclear cells (PBMCs), retain the use of CD46. In contrast, patient isolates adapted to B95-8 cells gain the use of an additional, unidentified receptor. We also present evidence that the binding affinity of patient isolates for target cells is low and that the strength of the interaction is influenced by amino acid 481 in the MV H.
MATERIALS AND METHODS
Viruses and cells.
Edmonston MV was obtained from the American Type Culture Collection (ATCC, Manassas, Va.) and passaged at low multiplicity on Vero (African green monkey kidney) cells as described earlier (39). MV isolates Chicago-1, Ill, Pal, 1086, and NJ and were grown on Vero cells. The isolates JW and IV were obtained by cocultivating PBMCs, taken during acute measles when patients displayed the characteristic rash, with human umbilical cord PBMCs (18). Further passages were performed on fresh adult human PBMCs. PBMC cultures were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 10 μg of phytohemagglutinin (PHA-P; Difco, Detroit, MI) per ml. The stock JW and IV isolates were not grown in tissue culture cell lines. The isolates FV93 and BCL94 were obtained from patient throat swabs (50, 51) and were grown on B95-8 marmoset B lymphocytes obtained from the ATCC. B95-8 cells were maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine and 7% FBS. CHO cells expressing the BC1 isoform of CD46 were grown in Ham F-12 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 500 μg of G418 sulfate (Geneticin; Gibco-BRL) per ml.
Infection of cells and titration of wild-type MVs.
For MV isolates that had been adapted to Vero cells, standard plaque assays were performed on Vero cells in six-well dishes as described elsewhere (39). For viruses that did not form plaques on Vero cells, the lower limit of detection in the plaque assay was <30 PFU/ml.
For viruses that grew only on B95-8 or PBMCs, titration was performed by 50% tissue culture infectious dose (TCID50) assay as described earlier (18, 30). For experiments that compared Edmonston or other Vero-adapted viruses directly to PBMC or B95-8 adapted viruses, the TCID50 of all the viruses was determined on human PBMCs, and that value was used to determine the amount of virus inoculum used in the experiment.
For infection of Vero or CHO-CD46 cells (Table 1), cells were plated on glass coverslips and inoculated at a multiplicity of infection (MOI) of 0.1 and allowed to incubate at 37°C for 2 to 4 days. Cells were stained for immunofluorescence by using a human polyclonal antiserum as described earlier (39).
TABLE 1.
Isolation and passage history of clinical MV isolates
| Isolate | Description | Genotypea | Groupb | Yr/location | Source | Isolation | Passage | Replication inc:
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| PBMC | Vero | CHO-CD46i | ||||||||
| Edmonston (wild type) | Mvi/Edmonston/USA/54 | A | 1 | 54/Boston | Throat swab | HKg | Vero | Yes | Yes | Yes |
| Chicago-1 | Mvi/Chicago.USA/89/1 | D3 | 2 | 89/Chicago | NP-aspd | CV-1 | Vero | Yes | Yes | Yes |
| Ill | Mvi/Illinois.USA/94 | C2 | 2 | 94/Illinois | Urine sede | B95-8 | Vero | Yes | Yes | Yes |
| Pal | Mvi/Palau.BLN/93 | D5 | 2 | 93/Palau | NP-asp | B95-8 | Vero | Yes | Yes | Yes |
| NJ | Mvi/New Jersey.USA/94/1 | D6 | 2 | 94/New Jersey | PBMCf | B95-8 | Vero | Yes | Yes | Yes |
| 1086 | Mvi/Banjul.GAM/91 | B1 | 2 | 91/Gambia | PBMC | B95-8 | Vero | Yes | Yes | Yes |
| JW | Mvi/JW/USA/91 | D3 | 3 | 91/Orange Co. | PBMC | Cord PBMCh | PBMC | Yes | No | No |
| IV | Mvi/IV/USA/91 | D3 | 3 | 91/Orange Co. | PBMC | Cord PBMC | PBMC | Yes | No | No |
| FV93 | Mvi/Ma93F/Madrid.Spain/93 | C2 | 4 | 92/Madrid | Throat swab | B95-8 | B95-8 | Yes | No | No |
| BCL94 | Mvi/Ma94B/Madrid.Spain/94 | D6 | 4 | 94/Madrid | Throat swab | B95-8 | B95-8 | Yes | No | No |
Standard World Health Organization genotype assignment for measles virus (6).
Viruses are grouped according to their laboratory passage history.
Tested in this study; see Materials and Methods.
NP-asp, nasopharyngeal aspirate.
Urine sed, virus isolated from lymphocytes in urine sample.
HK, human kidney cells.
CHO-CD46, CHO fibroblasts expressing human CD46.
PBMC, human PBMCs.
Cord PBMC, human umbilical cord blood mononuclear cells.
MLR and infection of CD46+ mouse splenocytes.
CD46-transgenic mice (FVB/N [H-2q]) were used that contained a genomic copy of the human CD46 gene (47). Spleens from CD46 transgenic or nontransgenic littermates were harvested, and single-cell suspensions prepared by passage through a 70-μm (pore-size) sterile mesh. Erythrocytes were removed by a 5-min incubation in 0.83% ammonium chloride–1 mM HEPES. The remaining cells were stimulated in a mixed lymphocyte reaction (MLR) by cocultivation with an equal number of gamma-irradiated (3,000 R) splenocytes from C57BL/6J (H-2b) mice (21). After 1 day of stimulation, splenocytes were infected with MV at 0.1 TCID50/cell. After 3 days at 37°C, splenocytes were analyzed for expression of MV antigens by flow cytometry using human polyclonal antiserum recognizing MV, followed by use of fluorescein isothiocyanate (FITC)-conjugated anti-human F(ab′)2 secondary antibody (39). Samples were analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, Mountain View, Calif.).
Blocking MV infection with CD46 MAb.
Monoclonal antibodies (MAbs) against CD46 were prepared from hybridoma tissue culture supernatants. Serial 10-fold dilutions of MAb in RPMI medium containing 10% FBS were incubated with 105 PBMCs for 30 min on ice in a round-bottom 96-well plate. Cells were incubated with 10-fold dilutions of MVs also diluted in RPMI–10% FBS at 37°C for 1 h. Cells were washed three times in phosphate-buffered saline (PBS). After the last wash, cells were resuspended in RPMI–10% FBS with 10 μg PHA-P per ml. The TCID50 was calculated following a 4-day incubation at 37°C. All assays were performed in triplicate.
Adaptation of clinical MV isolates to B95-8 and Vero cells.
The JW and IV isolates of MV were adapted from PBMCs to B95-8 cells by serial low-MOI passage. Cells were incubated with virus at an MOI of 0.01 TCID50/cell for 1 h, followed by incubation at 37°C for 3 days or until a cytopathic effect was observed. Virus was isolated from the supernatant and stored in aliquots at −70°C. Passaged virus was titered by TCID50 assay on B95-8 cells.
JW, IV, FV93, and BCL94 isolates were adapted to Vero cells using the following procedure. Viruses were incubated with Vero cells in T25 flasks at an MOI of 0.01 TCID50/cell. After 7 days, cells were harvested, frozen, and thawed, and the clarified supernatant was stored in aliquots at −70°C (passage 1). An aliquot (0.5 ml) was added to 7 × 105 fresh Vero cells plated in a T25 flask. After another 7 days, the cells were again harvested and frozen (passage 2). This procedure was repeated up to 10 successive times. For some viruses cytopathicity was observed after a few passages, in which case cultures were harvested earlier than 7 days.
Sequence determination of H gene from wild-type MVs.
The sequence of the JW and IV isolates H gene was determined by reverse transcription (RT)-PCR from RNA prepared from infected PBMCs. For sequencing MV H gene from Vero or B95-8-adapted MVs, total RNA was prepared from cells infected with adapted JW, IV, FV93, and BCL94. The H gene sequence was determined as described above. RNA was harvested at day 4 postinfection by using Tri-reagent (MRC, Inc., Cincinnati, Ohio). cDNA was synthesized using total RNA (500 ng), Moloney murine leukemia virus-RT, and random hexamer oligonucleotide primers. PCR of the MV-H gene was performed on the cDNA template using the following primers: upstream, 5′-TCCCTCGAGGTAGTTAATTAAAACTTAGGGTGCAAG-3′; and downstream, 5′-TTCACACTAGTCGGTATGCCTGATGTCTGG-3′. Amplification conditions were as follows: 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for a total of 20 cycles. Following amplification, the 1.9-kb PCR product was separated on agarose gel, isolated, and purified by using Geneclean (Bio 101, Vista, Calif.). The purified PCR product was ligated into the TA cloning vector and transformed into the Escherichia coli Top10 strain (Invitrogen, Carlsbad, Calif.). Ten individual clones were picked, and DNA sequencing was performed using the thermal cycle sequencing kit (Amersham, Cleveland, Ohio) and 33P-labeled dideoxynucleotides. Sequencing was also performed by the Protein and Nucleic Acid Core Facility at The Scripps Research Institute. Sequences were analyzed using MacVector software. The Edmonston MV sequence used for comparison was GenBank accession number K01711. The novel GenBank accession number for the H sequence of the JW isolate is AF218821 and for IV is AF218822. Genotypes for JW and IV viruses were assigned by comparison to the World Health Organization reference panel for MV isolates (3).
Binding assays.
Fluorescence-activated cell sorting (FACS) binding assays were performed essentially as reported earlier (40), with the following modifications. Virus isolates were prepared on a discontinuous 60%–20% sucrose gradient. The titer was determined by plaque assay (for viruses grown on Vero cells) and by TCID50 assay (for viruses grown on B95-8 or PBMCs). Protein concentration (in micrograms per milliliter) for the purified viruses was determined by Bradford assay. For the binding assays, 2.5, 1.25, or 0.25 μg of purified virus was incubated overnight with 105 human PBMCs or else splenocytes from YAC-CD46-transgenic or nontransgenic mice (47) at 4°C in RPMI medium containing 10% fetal calf serum. The cells were washed four times in ice-cold medium, incubated on ice for 1 h in the presence of MAb to MV-H (48), followed by goat anti-mouse F(ab′)2 antibody conjugated to FITC. Cells were again washed in medium, fixed in 1% paraformaldehyde-PBS, and analyzed by flow cytometry using Cell Quest software (Becton Dickinson). For CD46-dependent binding to transgenic mouse splenocytes, the percentage of CD46+ cells with virus bound, minus the background binding of the same virus preparation to CD46-nontransgenic cells, was calculated and then compared to the level of CD46-specific binding seen for MV Edmonston, set at 100%. Analysis was performed by gating on the live lymphocyte population and collecting a minimum of 5,000 events.
RESULTS
Interaction of wild-type MV with CD46-expressing cells.
The Halle and Edmonston laboratory strains of MV were used to identify CD46 as the MV receptor (16, 39, 43, 44). We investigated whether CD46 receptor usage extended to wild-type isolates of MV prior to and after laboratory culture on cell lines. The MV isolates used are representatives of various MV genotypes (Table 1). For the purpose of this study the MV isolates were also divided into four groups based on their passage history (Table 1). MV Edmonston represents group 1 laboratory strains that were adapted to Vero cells for more than 50 passages. Group 2 isolates are recently isolated MV strains that have been minimally adapted to Vero cell culture (<10 passages) and include MV strains Chicago-1, Ill, Pal, 1086, and NJ. Group 3 MV isolates are recent isolates that have been passaged only on primary human peripheral blood lymphocytes and not on lymphocyte cell lines or human or primate fibroblasts. Group 4 MV isolates are recent MV isolates adapted to marmoset B95-8 cells. B95-8 lymphocytes have a marmoset CD46 homolog, and this molecule is recognized by polyclonal antibodies but not the MAbs against human CD46 that were tested (reference 25 and M.M., data not shown). In addition, the marmoset CD46 homolog expressed on B95-8 cells often lacks the SCR-1 domain, a component known to be essential for binding of Edmonston and Halle (group 1) MVs (24). Thus, it was important to determine whether viruses isolated and passaged on marmoset B95-8 lymphocytes demonstrate a receptor tropism similar to that of wild-type, PBMC-passaged MV isolates.
The isolates listed in Table 1 were first tested for their ability to infect fibroblast-like Vero cells expressing the African green monkey CD46 homologue shown to be a receptor for Edmonston and Halle MV isolates, epithelial-cell-like HeLa cells expressing human CD46, or Chinese hamster ovary (CHO) transfectants expressing the human BC1 isoform of CD46. CHO cells are permissive to Edmonston and Halle (group 1) MV infection only when they express the human CD46 receptor (16, 39, 43). The MV isolates that had been adapted to Vero cells (groups 1 and 2) were able to infect HeLa cells and CHO transfectants expressing human CD46 (Table 1). These virus strains demonstrated expression of viral proteins as measured by immunofluorescence, syncytium formation (cell-cell fusion), and production of virions. In contrast, none of the group 3 or group 4 MV isolates were able to productively infect either CHO-CD46 transfectants, HeLa cells, or Vero cells, even when their titers on human PBMCs were equal to or higher than that for MV Edmonston (Table 1). These results indicate that CD46 is not sufficient to allow a complete round of replication of group 3 and 4 clinical MV isolates in nonlymphoid cells. Several hypotheses can be advanced to explain the failure of group 3 and 4 clinical MVs to infect CD46-expressing, nonlymphoid cells. One possibility is that group 3 and 4 MVs do not use CD46 as the cellular receptor. Alternatively, group 3 and 4 MVs could use CD46 but require additional cell surface cofactors for entry into fibroblast cells. It is also possible that group 3 and 4 MV isolates utilize CD46 and enter cells but that their replication is restricted in nonlymphoid cells at a postentry step.
Infection of CD46-expressing lymphoid cells by patient isolates of MV.
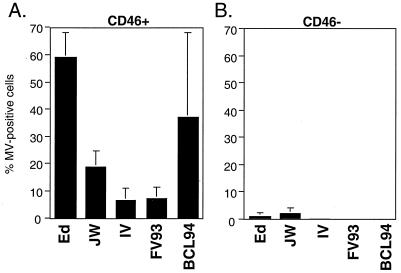
To determine whether MV isolates in groups 3 and 4 use CD46 as a receptor on lymphoid cells, splenocytes from transgenic mice expressing a human genomic copy of CD46 were examined (Fig. 1) (47). Splenocytes from the CD46-transgenic mice express CD46 at levels similar to those for human PBMC and with a similar uniform distribution within cells in the PBMC population, including B cells, T cells, and monocytes (reference 47 and data not shown). CD46-expressing or nontransgenic splenocytes were stimulated in vitro by MLR. Previous results indicate that lymphocytes from CD46-transgenic mice require stimulation by MLR in order to support maximum MV replication (21). The percentage of CD46+ or nontransgenic cells expressing MV antigens at 3 days postinfection is shown in Fig. 1. The isolates Edmonston (group 1), JW and IV (group 3), and FV93 and BCL94 (group 4) were able to infect CD46+ splenocytes to various degrees but did not infect nontransgenic lymphocytes. The group 4 strain FV93 infected fewer CD46-transgenic lymphocytes than the group 4 strain BCL94; however, this may reflect the diminished replication which has often been observed with this strain (M.M., unpublished data). To confirm that entry into the CD46-transgenic lymphocytes is via CD46 and not due to upregulation of an unrelated receptor on these transgenic cells, infection of the CD46-transgenic lymphocytes by Edmonston, group 3, and group 4 MVs was shown to be inhibited by the E4.3 MAb against CD46 (data not shown). Thus, the results of the analysis of CD46-transgenic splenocytes indicate that while group 3 and 4 isolates cannot infect fibroblasts expressing CD46, they can infect lymphoid cells in a CD46-dependent manner.
FIG. 1.
Infection of murine splenocytes with clinical MV isolates. (A) Infection of CD46 transgenic lymphocytes. (B) Infection of nontransgenic lymphocytes. Infection was detected 4 days postinoculation using a human antiserum against MV, followed by use of an anti-human FITC-conjugated secondary antibody, and then analyzed by flow cytometry. The mean ± the standard error of three samples are shown.
Inhibition of infection by anti-CD46 antibody.
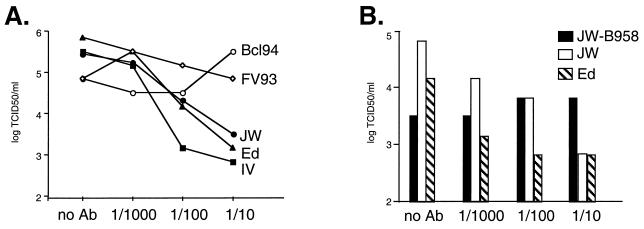
A number of studies have indicated that antibodies against CD46 cannot block infection by group 4 MV isolates adapted to B95-8 (marmoset) or BJAB (human) B-cell lines. The same antibodies, however, block infection by group 1 laboratory strains (7, 25). An anti-CD46 SCR1 antibody (E4.3) known to block Edmonston MV infection of CD46-expressing CHO cells (40) was tested for its ability to inhibit infection of human PBMCs by JW and IV (group 3), FV93 and BCL94 (group 4), and Edmonston (group 1) isolates (Fig. 2A). A 100-fold reduction of the TCID50 on human PBMCs for MV-JW and IV and MV-Edmonston was seen with the highest level of E4.3 antibody, similar to what occurs with MV-Edmonston infection of Vero or HeLa cells (39, 40). A control MAb against influenza hemagglutinin did not inhibit MV-JW infection (data not shown). In contrast, little or no inhibition of FV93 and BCL94 (group 4) MVs was detected. Additional experiments were performed with anti-SCR1 MAb Tra-2-10, which also blocks Edmonston MV infection of HeLa cells (40). Tra-2-10 inhibited the titers of Edmonston by 100-fold in the TCID50 assay and JW by 20-fold but did not inhibit FV93 or BCL94 (data not shown). These results suggest that while group 4 viruses can infect cells via CD46 as shown in Fig. 1, they may also use another receptor when CD46 is not available.
FIG. 2.
Inhibition of wild-type MV infection using MAb E4.3 against CD46. (A) Serial 10-fold dilutions of E4.3 ascites fluid were incubated with virus and then added to human PBMCs. Following 4 days of incubation, the TCID50 was determined. Data are presented as the log of the virus titer in TCID50/milliliters (determined from triplicate wells). Symbols: ▴, Edmonston MV; ■, IV; ●, JW; ○, BCL94 (Bcl94); ◊, FV93. No Ab, no antibody. (B) Inhibition of infection of human PBMCs by the MV isolate JW and an isolate of JW recovered following five passages on marmoset B95-8 cells (JW-B95-8). Striped bars, MV Edmonston; open bars, JW; black bars, JW following five successive passages on B95-8 cells.
To determine whether the failure of group 4 isolates to be inhibited by anti-CD46 antibody arose as a consequence of passaging MV on marmoset B95-8 cells, the group 3 JW isolate was passaged five successive times on marmoset B95-8 cells. The initial titer of the isolate was 104.5 TCID50/ml. Following the first passage on B95-8, the JW virus recovered had a titer of 102.83 TCID50/ml, an almost 100-fold reduction. Titers of successive passages two to five were 103.8, 104.2, 104.5, and 104.5, respectively. Similar results were seen when adapting the IV isolate to B95-8 cells, with a reduction in titer to 102.8 followed by an increase to 104.8 by the fourth passage. A sample of JW isolated from the fifth passage on B95-8 cells was tested in the TCID50 assay (Fig. 2B). Anti-CD46 MAb was no longer able to inhibit infection by B95-8-adapted JW, indicating that passage of group 3 JW on B95-8 cells selected for variants that had acquired a group 4 phenotype.
Relationship between MV H amino acid sequence, MV replication in nonlymphoid cells, and use of CD46.
It was recently proposed that the amino acid sequence at position 481 in the MV H glycoprotein (tyrosine [Y] for group 1 MV Edmonston and vaccine strains; asparagine [N] for recent isolates in groups 2 to 4) determines whether MV can use CD46 for entry and that the sequence N481 found in group 4 isolates promotes a CD46-independent entry phenotype (25, 35). To identify whether differences in receptor usage and cell tropism between PBMC-passaged (group 3), B95-8-passaged (group 4), and Vero-passaged isolates (groups 1 and 2) correlated with the sequence of the H protein, the sequence of the entire H coding region was determined. Amino acid sequence differences between Edmonston and the other isolates are shown (Table 2). The following nucleotide sequence divergences from Edmonston MV within the H gene were found for the various isolates: Chicago-1, 1.9%; JW, 3.1%; IV, 2.6%; FV93, 1.9%; and BCL94, 1.9%. The diversity of sequences within MV H for the different isolates reflects the fact that the MV isolates used in this study are representatives of a variety of MV genotypes (Table 2). For example, at positions such as 211 and 243 the recent MV isolates (groups 2 to 4) all share an amino acid different from that of Edmonston. Changes at these positions are genotype specific and not passage history specific (50, 52). At position 481, however, mutation of N to Y indicates a specific adaptation that sometimes arises during long-term growth on Vero cells (25, 35). Table 2 shows that after 10 passages on Vero cells, JW and IV variants had converted to Y481, which was encoded by a codon change from AAC (N) to TAC (Y) at the corresponding nucleotide positions. Interestingly, both FV93 and BCL94, like Chicago-1, still retained N481 after 10 passages on Vero cells. Preliminary studies analyzing the HA sequence of the FV93-B95-8 and BCL94-B958 passaged isolates show no changes between the initial isolate and the passaged material (data not shown). Analysis of amino acid 481 demonstrates that the group 2, 3, and 4 viruses all have N481 prior to Vero cell passage and can use CD46 for infection. Thus, isolates with N481 can still utilize CD46, and the change to Y481 is not required for CD46 receptor usage.
TABLE 2.
Comparison of H sequences of clinical MV isolates
| Position | MV H sequence in virus isolate (group, genotype)a:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ed (1, A) | Chi (2, D3) | JW (3, D3) | JW Vero (2, D3) | IV (3, D3) | IV Vero (2, D3) | FV93 (4, C2) | FV93 Vero (2, C2) | BCL94 (4, D6) | BCL94 Vero (2, D6) | |
| 4 | Q | H | H | H | H | H | Q | Q | Q | Q |
| 37 | V | V | A | A | A | A | V | V | V | V |
| 174 | T | A | A | A | A | A | T | T | T | T |
| 176 | T | A | A | A | A | A | T | T | T | T |
| 211 | G | S | S | S | S | S | S | S | S | S |
| 235 | E | G | G | G | G | G | E | E | E | E |
| 243 | R | G | G | G | G | G | G | G | G | G |
| 246 | L | L | L | L | L | L | S | S | L | L |
| 247 | S | S | S | S | S | S | S | S | P | P |
| 249 | L | L | L | L | L | L | L | L | P | P |
| 252 | Y | H | H | H | H | H | Y | Y | H | H |
| 276 | L | F | F | F | F | F | F | F | F | F |
| 284 | L | F | F | F | F | F | L | L | F | F |
| 295 | K | R | R | R | K | K | K | K | K | K |
| 296 | L | F | F | F | L | L | L | L | L | L |
| 302 | G | R | R | R | G | G | G | G | G | G |
| 303 | E | E | E | E | E | E | G | G | E | E |
| 306 | I | V | V | V | I | I | I | I | I | I |
| 308 | I | V | V | V | I | I | I | I | I | I |
| 339 | L | L | L | L | L | L | F | F | L | L |
| 357 | V | V | V | V | V | V | V | V | I | I |
| 367 | V | V | V | V | V | V | I | I | V | V |
| 375 | K | K | K | K | K | K | K | K | N | N |
| 390 | I | N | N | N | N | N | I | I | I | I |
| 405 | N | N | N | N | N | N | H | H | N | N |
| 416 | D | D | N | N | D | D | D | D | D | D |
| 446 | S | T | T | T | T | T | S | S | S | S |
| 451 | V | V | V | V | V | V | E | E | V | V |
| 481 | Y | N | N | Y | N | Y | N | N | N | N |
| 484 | N | T | T | T | T | T | T | T | T | T |
| 544 | S | S | I | I | S | S | S | S | S | S |
| 546 | S | G | S | S | S | S | G | G | G | G |
| 562 | V | V | V | V | V | V | I | I | V | V |
| 600 | E | V | V | V | V | V | E | E | E | E |
| 616 | R | R | R | R | R | R | S | S | R | R |
| 617 | R | R | R | R | R | R | R | R | G | G |
Binding of wild-type MVs to target cells.
We previously showed that it was possible to detect CD46-dependent MV binding to the cell surface by flow cytometry (40). This assay was used to determine whether binding to CD46 correlated with sequence changes in the MV hemagglutinin. Figure 3 shows the binding of either 2.5, 1.25, or 0.25 μg each (x axis of each panel) of the purified MVs to primary human PBMCs in comparison to cells incubated with 2.5 μg of Edmonston MV, which was set at 100%. The binding of initial stocks and of viruses recovered after 10 successive passages on Vero cells was measured. Initial binding assays showed that Chicago-1, JW, IV, FV93, and BCL94 all had reduced binding capacity compared to Edmonston (Fig. 3, left panels). Following 10 Vero passages, the binding of JW and IV Vero variants was increased compared to the original JW and IV isolates. In contrast, adapted FV93 and BCL94 Vero variants did not show increased binding compared to the initial B95-8-adapted isolates. Viruses with N481 had reduced binding (Chicago-1, JW, IV, FV93, BCL94, FV93 Vero, and BCL94 Vero; Fig. 3 and Table 2), and variants that acquired the tyrosine 481 substitution (JW Vero and IV Vero) had increased binding (Fig. 3). To confirm that binding was CD46 dependent, binding assays were also performed comparing the binding of 1.25 μg of each purified virus to splenocytes isolated from either CD46-transgenic or nontransgenic mice. For each virus the CD46-specific binding was calculated as described in Materials and Methods and compared to that of Edmonston, which was set at 100%: JW, 10%; IV, 18%; FV93, 21%; and BCL94, 25.5%. When CD46-transgenic splenocytes were used as the target cells, similar increases in binding were seen for JW Vero (to 31.8%) and IV Vero (to 42.5%) (data not shown). Interestingly, when the purified MVs were titered on PBMCs they all had similar infectious titers per microgram of purified virus (data not shown). These results indicate that the strength of attachment to the cell surface does not correlate with the ability to infect a particular cell type. Furthermore, the N481Y mutation correlates with increased binding to the cell surface.
FIG. 3.
Binding of sucrose gradient-purified MV isolates to human PBMCs as detected by flow cytometry. For each panel, 2.5, 1.25, or 0.25 μg of purified MV was added, respectively, as indicated on the x axis. The y axis indicates the percentage of cells with MV bound from 0 to 100%. All values are expressed relative to the percentage of cells bound to 2.5 μg of Edmonston MV, which is set at 100%. The amino acid sequence at position 481 of the MV hemagglutinin for each virus used is indicated at the top right corner of each panel. JW Vero, IV Vero, FV93 Vero, and BCL94 Vero (Bcl94 Vero) indicate the virus isolates harvested after 10 successive passages on Vero cells.
DISCUSSION
We have shown that clinical isolates of MV grown on primary human PBMCs (group 3) use CD46 as a cellular receptor, as evidenced by their ability to infect murine lymphocytes in a CD46-dependent fashion and by the ability of antibodies specific for CD46 to inhibit infection. B95-8-adapted (group 4) MV isolates also infect transgenic murine lymphocytes in a CD46-dependent manner. However, a CD46-specific MAb directed against SCR 1 did not inhibit infection of human PBMC by group 4 strains, as indicated by similar findings in previously published reports (7, 25, 35). Cultivation of group 3 isolates on B95-8 cells selected for variants whose infection of human PBMCs was no longer inhibited by anti-CD46 antibody. Our data suggest that group 4 MVs can interact with an additional receptor or interact with CD46 in a novel manner and that this is an adaptive phenotypic change that arises when patient isolates are cultivated on B95-8 cells.
Our studies confirm that MV variants with altered receptor usage can be quickly selected in vitro. Although in the field MV demonstrates marked genetic stability, it has a mutation rate similar to that of other RNA viruses such as poliovirus and vesicular stomatitis virus (54). Although B95-8 cells are more efficient for isolating MV from patient samples than Vero cells, the initial 100-fold reduction in titer seen when MV isolates are adapted from PBMCs to B95-8 cells, followed by the rebound in titer, confirms that selection occurs. Interestingly, the fact that B95-8-adapted viruses still enter mouse lymphocytes in a CD46-dependent manner indicates that dependence on CD46 is not completely lost but more likely that additional receptor usage is gained when CD46 is not available. We cannot, however, exclude the possibility that CD46 expression on murine lymphocytes increases the expression or accessibility of an unidentified coreceptor. The marmoset CD46 lacks a large portion of the MV-binding site (24, 28, 40). Thus, when MV encounters marmoset B95-8 cells, it is possible that the absence of the normal MV binding site selects for MV variants that can use an alternative receptor. Since wild-type MVs bind to the cell surface weakly, the loss of a major portion of the binding domain of CD46 on B95-8 cells would have a greater impact on wild-type MVs than on MV Edmonston.
It is likely that the natural affinity of wild-type MVs for CD46 is relatively low, and the N481Y mutation leads not to a change in receptor specificity, as had been previously suggested, but to an increased affinity for CD46. MV isolates grown on PBMCs or B95-8 cells bound very weakly to PBMCs and were marginally detectable in the FACS binding assay that easily measures the binding of MV Edmonston. Nevertheless, MVs with H sequence N481 are fully able to infect fibroblasts (Chicago-1) or lymphocytes (group 3 and 4 MV isolates) in a CD46-dependent manner. However, variants that acquire the N481Y mutation demonstrate enhanced cell surface binding. Interestingly, a recent study identified amino acids 473 to 477 of MV-H as a critical binding region for CD46 (48). Amino acids 473 to 477 are completely conserved among all MV strains used in this study. Therefore, binding of MV-H to CD46 is likely governed at least in part by interaction with the conserved region from amino acids 473 to 477, with the nearby residue 481 contributing to increased affinity. Binding of group 3 and 4 MV isolates is detectable in the FACS binding assay only in cells expressing extremely high levels of CD46, such as HeLa or human PBMCs, suggesting this is the likely reason why it is not possible to detect binding to CD46-CHO transfectants, which express 10- to 100-fold-less CD46 than human cells (M.M., data not shown). Experiments are underway to enhance the level of CD46 expression on transfected cells in order to resolve this issue.
It is common for pathogenic virus isolates from clinical specimens to have reduced cell surface binding affinity compared to their tissue culture-adapted counterparts. For example, pathogenic variants of polyomavirus bind to their receptor with lower affinity than their attenuated counterparts (8). Sindbis virus variants that cause pathogenicity in vivo bind to their receptor weakly compared to attenuated strains that bind tightly to heparan sulfate (33). For HIV-1, laboratory variants with enhanced CD4 receptor affinity arise upon in vitro culture (26, 27, 58). It has been suggested that enhanced binding affinity may inhibit efficient virus spread in vivo by preventing efficient entry and/or egress and release of virions from the cell surface, thus attenuating the virus (8). Recent data by Firsching et al. (17) suggest that relatively low levels of binding or membrane fusion are sufficient for transfer of MV genetic material into target cells. Thus, for MV, enhanced binding to the cell surface could constitute an important mechanism of attenuation for vaccine strains.
Obtaining field isolates of MV for genetic characterization is essential for accurate global surveillance and epidemiologic studies of MV. The B95-8 isolation procedure is extremely valuable for molecular epidemiologic studies as a rapid and convenient method for obtaining field isolates. The consistency of MV phylogenetic trees that examine multiple MV genes and include B95-8-adapted viruses indicates that MV isolation on B95-8 cells is appropriate for genotyping, especially when the sequences are obtained from freshly isolated virus. Nevertheless, our results indicate that even short periods of passage in B95-8 cells dramatically alter MV phenotypes and that in order to characterize phenotypes such as receptor usage and pathogenicity of MV isolates it is important to study MVs recently isolated in primary human cells.
With the exception of amino acid 481 in MV H, no single amino acid has been correlated with differential receptor binding affinity or receptor usage phenotypes (7, 25, 35, 56). To support this, our preliminary studies of the H sequences from JW and IV viruses adapted to B95-8 cells do not reveal any adaptive changes (data not shown). One possible explanation is that nongenotypic changes such as glycosylation patterns may alter receptor usage. In support of this, differences in glycosylation patterns occur in transformed B-cell lines (for example, BJAB), in that the degree of sialic acid addition on glycoproteins correlates with the extent of cellular transformation (32). Furthermore, small differences in sialic acid addition can dramatically influence glycoprotein functions such as virus-receptor interactions, antibody binding, and specificity (32). An alternative possibility is that the MV F glycoprotein may play a role in determining cell tropism, as recently suggested by Johnston et al. (29). Investigation of the F sequences from the JW and IV B95-8-adapted viruses is in progress. Finally, we cannot exclude the possibility that receptor usage and cell tropism in MVs grown in immortalized B cells might be influenced by host cell proteins derived from B cells and incorporated into the MV envelope.
Wild-type and laboratory MV isolates appear to be very different in their cell tropism; however, these differences are not manifested solely at the level of receptor usage. Postentry events in the MV replication cycle can also play an important role in determining the tropism of a given isolate (21). That Edmonston laboratory MV can replicate in additional cell types indicates that it has adapted to use the cellular machinery of those cells without losing the ability to replicate in lymphoid cells. Determining how these new functions relate to virulence and attenuation will be an important step in understanding the mechanisms of measles pathogenesis.
ACKNOWLEDGMENTS
We thank I. Schulman, J. C. de la Torre, D. Naniche, J. Patterson, and W. Cao for helpful discussions and critical reading of the manuscript. We thank Mayra Estrada for technical assistance and M. L. Celma for reading the manuscript and sharing unpublished results. We thank J. Atkinson for antibodies and Priscilla Crisler and the General Clinical Research Center of Green Hospital, Scripps Clinic, for human blood samples.
This work was supported by the World Health Organization (M.M.), National Institutes of Health grants AI41514 (M.M.) and AI36222 and AI39466 (M.B.A.O.), Consejeria de Educacion y Cultura de la Comunidad de Madrid grant 08.2/002/97 (R.F.-M.), and Fondo de Investigacion Sanitaria grant 96/1591 (R.F.-M.).
Footnotes
Publication number 11694-NP from The Scripps Research Institute.
REFERENCES
- 1.Adams E M, Brown M C, Nunge M, Krych M, Atkinson J P. Contribution of the repeating domains of membrane cofactor protein (MCP; CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991;147:3005–3011. [PubMed] [Google Scholar]
- 2.Albrecht P, Ennis F A, Saltzman E J, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:775–778. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. AIDS in Africa. World Health Forum. 1998;19:214. [PubMed] [Google Scholar]
- 4.Anonymous. Measles, United States, 1st 26 weeks 1989. Morbid Mortal Weekly Rep. 1989;38:863–871. [PubMed] [Google Scholar]
- 5.Anonymous. Measles–United States. Morbid Mortal Weekly Rep. 1989;38:601–605. [PubMed] [Google Scholar]
- 6.Anonymous. Measles. Morbid Mortal Weekly Rep. 1995;45:305–307. [PubMed] [Google Scholar]
- 7.Bartz R, Firsching R, Rima B, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 8.Bauer P H, Bronson R T, Fung S C, Freund R, Stehle T, Harrison S C, Benjamin T L. Genetic and structural analysis of a virulence determinant in polyomavirus VP1. J Virol. 1995;69:7925–7931. doi: 10.1128/jvi.69.12.7925-7931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom B R. Vaccines for the third world. Nature. 1989;342:115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 11.Buckland R, Wild T F. Is CD46 the cellular receptor for measles virus? Virus Res. 1997;48:1–9. doi: 10.1016/s0168-1702(96)01421-9. [DOI] [PubMed] [Google Scholar]
- 12.Burnet F M. Measles as an index of immunological function. Lancet. 1968;ii:610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- 13.Cho S-W, Oglesby T J, Hsi B-L, Adams E M, Atkinson J P. Characterization of three monoclonal antibodies to membrane cofactor protein (MCP) of the complement system and quantitation of MCP by radioassay. Clin Exp Immunol. 1991;83:257–261. doi: 10.1111/j.1365-2249.1991.tb05624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements C J, Cutts F T. The epidemiology of measles: thirty years of vaccination. Curr Top Microbiol Immunol. 1995;191:13–33. doi: 10.1007/978-3-642-78621-1_2. [DOI] [PubMed] [Google Scholar]
- 15.Devaux P, Loveland B, Christiansen D, Milland J, Gerlier D. Interactions between the ectodomains of haemagglutinin and CD46 as a primary step in measles virus entry. J Gen Virol. 1996;77:1477–1481. doi: 10.1099/0022-1317-77-7-1477. [DOI] [PubMed] [Google Scholar]
- 16.Dorig R, Marcel A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 17.Firsching R, Buchholz C J, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forthal D N, Aarnaes S, Blanding J, de la Maza L, Tilles J G. Degree and length of viremia in adults with measles. J Infect Dis. 1992;166:421–424. doi: 10.1093/infdis/166.2.421. [DOI] [PubMed] [Google Scholar]
- 19.Gellin B G, Katz S L. Measles: state of the art and future directions. J Infect Dis. 1994;170(Suppl. 1):3–14. doi: 10.1093/infdis/170.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 20.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Vol. 3. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 21.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor provide cells exhibiting different permissivities to MV infection. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hourcade D, Holers V M, Atkinson J P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;6:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 23.Hsi B-L, Yeh C-J G, Penichel P, Samson M, Grivaux C. Monoclonal antibody GB24 recognizes a trophoblast-lymphocyte cross-reactive antigen. Am J Reprod Immunol Microbiol. 1988;18:21–27. doi: 10.1111/j.1600-0897.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu E C, Dorig R E, Sarangi F, Marcil A, Iorio C, Richardson C D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of envelope V3 loop as the major determinant of CD4 neutralization sensitivity of HIV-1. Science. 1992;257:535–537. doi: 10.1126/science.1636088. [DOI] [PubMed] [Google Scholar]
- 27.Ivey-Hoyle M, Culp J S, Chaikin M A, Hellmig B D, Matthews T J, Sweet R W, Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata K, Seya T, Yanagi Y, Pesando J, Johnson P, Okabe M, Ueda S, Ariga H, Nagasawa S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J Biol Chem. 1995;270:15148–15152. doi: 10.1074/jbc.270.25.15148. [DOI] [PubMed] [Google Scholar]
- 29.Johnston I, ter Meulen V, Schneider-Schaulies J, Schneider-Schaulies S. A recombinant measles vaccine virus expressing wild-type glycoproteins: consequences for viral spread and cell tropism. J Virol. 1999;73:6903–6915. doi: 10.1128/jvi.73.8.6903-6915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenverusche. Arch Exp Pathol Pharmakol. 1931;162:480–483. [Google Scholar]
- 31.Katz S L, Gellin B G. Measles vaccine: do we need new vaccines or new programs? Science. 1994;265:1391–1392. doi: 10.1126/science.8073281. [DOI] [PubMed] [Google Scholar]
- 32.Keppler O T, Peter M E, Hinderlich S, Moldenhauer G, Stehling P, Schmitz I, Schwartz-Albiez R, Reutter W, Pawlita M. Differential sialylation of cell surface glycoconjugates in a human B lymphoma cell line regulates susceptibility for CD95 (APO-1/Fas)-mediated apoptosis and for infection by a lymphotropic virus. Glycobiology. 1999;9:557–569. doi: 10.1093/glycob/9.6.557. [DOI] [PubMed] [Google Scholar]
- 33.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobune F, Sakata H, Sugiura G. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M, Fernandez-Munoz R, Wild T, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liszewski M K, Atkinson J P. Membrane cofactor protein. Curr Top Microbiol Immunol. 1992;178:45–60. doi: 10.1007/978-3-642-77014-2_4. [DOI] [PubMed] [Google Scholar]
- 37.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 38.Malvoisin E, Wild T F. Measles virus glycoproteins: studies on the structure and interaction of the hemagglutinin and fusion proteins. J Gen Virol. 1993;74:2365–2372. doi: 10.1099/0022-1317-74-11-2365. [DOI] [PubMed] [Google Scholar]
- 39.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Atkinson J P, Lublin D M, Oldstone M B A. Measles virus and C3 binding sites are distinct on membrane cofactor protein (MCP; CD46) Proc Natl Acad Sci USA. 1995;92:2303–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McChesney M, Miller C, Rota P, Zhu Y, Antipa L, Lerche N, Ahmed R, Bellini W. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- 42.McLean A. After the honeymoon in measles control. Lancet. 1995;345:272. doi: 10.1016/s0140-6736(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 43.Naniche D, Varior-Krishnan G, Cervino F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naniche D, Wild T F, Rabourdin-Combe C, Gerlier D. A monoclonal antibody recognized a human cell surface glycoprotein involved in measles virus binding. J Gen Virol. 1993;73:2617–2624. doi: 10.1099/0022-1317-73-10-2617. [DOI] [PubMed] [Google Scholar]
- 45.Norrby E, Oxman M. Measles virus. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven; 1990. pp. 1013–1045. [Google Scholar]
- 46.Nussbaum O, Broder C C, Moss B, Bar-Lev Stern L, Rozenblatt S, Berger E A. Functional and structural interaction between measles virus hemagglutinin and CD46. J Virol. 1995;69:3341–3349. doi: 10.1128/jvi.69.6.3341-3349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldstone M B A, Lewicki H, Thomas D, Tishon A, Dales S, Patterson J, Manchester M, Homann D, Naniche D, Holz A. Measles virus infection in a transgenic model: virus-induced central nervous system disease and immunosuppression. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 48.Patterson J B, Scheiflinger F, Manchester M, Yilma T, Oldstone M B A. Structural and functional studies of the measles virus hemagglutinin: identification of a novel site required for CD46 interaction. Virology. 1999;256:142–151. doi: 10.1006/viro.1999.9644. [DOI] [PubMed] [Google Scholar]
- 49.Rall G F, Manchester M, Daniels L R, Callahan E, Belman A, Oldstone M B A. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rima B K, Earle J A P, Baczko K, terMeulen V, Liebert U G, Carstens C, Carabana J, Caballero M, Celma M L, Fernandez-Munoz R. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78:97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- 51.Rima B K, Earle J A P, Yeo R P, Herlihy L, Baczko K, ter Meulen V, Carabana J, Caballero M, Celma M L, Fernandez-Munoz R. Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76:5773–5783. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- 52.Rota J S, Hummel K B, Rota P A, Bellini W J. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology. 1992;188:135–142. doi: 10.1016/0042-6822(92)90742-8. [DOI] [PubMed] [Google Scholar]
- 53.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schrag S J, Rota P A, Bellini W J. Spontaneous mutation rate of measles virus: direct estimation based on mutations conferring monoclonal antibody resistance. J Virol. 1999;73:51–54. doi: 10.1128/jvi.73.1.51-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seya T, Turner J, Atkinson J P. Purification and characterization of a membrane protein (gp45-70) which is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda M, Kato A, Kobune F, Sakata H, Li T, Shioda T, Sakai Y, Asakawa M, Nagai Y. Measles virus attenuation associated with transcriptional impediment and a few amino acid changes in the polymerase and accessory proteins. J Virol. 1998;72:8690–8696. doi: 10.1128/jvi.72.11.8690-8696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wild T F, Malvoisin E, Buckland R. Measles virus: both the hemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72:439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 58.Willey R L, Theodore T S, Martin M A. Amino acid substitutions in the human immunodeficiency virus type 1 gp120 V3 loop that change viral tropism also alter physical and functional properties of the virion envelope. J Virol. 1994;69:4409–4419. doi: 10.1128/jvi.68.7.4409-4419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagi Y, Hu H, Seya T, Yoshikura H. Measles virus infects mouse fibroblast cell lines, but its multiplication is severely restricted in the absence of CD46. Arch Virol. 1994;138:39–53. doi: 10.1007/BF01310037. [DOI] [PubMed] [Google Scholar]