Abstract
Background:
Histological healing is closely associated with improved long-term clinical outcomes and lowered relapses in patients with ulcerative colitis (UC). Here, we developed a novel diagnostic criterion for assessing histological healing in UC patients.
Methods:
We conducted a retrospective cohort study in UC patients, whose treatment was iteratively optimized to achieve mucosal healing at Shanghai Tenth People’s Hospital of Tongji University from January 2017 to May 2022. We identified an inflammatory cell enumeration index (ICEI) for assessing histological healing based on the proportions of eosinophils, CD177+ neutrophils, and CD40L+ T cells in the colonic lamina propria under high power field (HPF), and the outcomes (risks of symptomatic relapses) of achieving histological remission vs. persistent histological inflammation using Kaplan–Meier curves. Intrareader reliability and inter-reader reliability were evaluated by each reader. The relationships to the changes in the Nancy index and the Geboes score were also assessed for responsiveness. The ICEI was further validated in a new cohort of UC patients from other nine university hospitals.
Results:
We developed an ICEI for clinical diagnosis of histological healing, i.e., Y = 1.701X1 + 0.758X2 + 1.347X3 − 7.745 (X1, X2, and X3 represent the proportions of CD177+ neutrophils, eosinophils, and CD40L+ T cells, respectively, in the colonic lamina propria under HPF). The receiver operating characteristics curve (ROC) analysis revealed that Y <−0.391 was the cutoff value for the diagnosis of histological healing and that an area under the curve (AUC) was 0.942 (95% confidence interval [CI]: 0.905–0.979) with a sensitivity of 92.5% and a specificity of 83.6% (P <0.001). The intraclass correlation coefficient (ICC) for the intrareader reliability was 0.855 (95% CI: 0.781–0.909), and ICEI had good inter-reader reliability of 0.832 (95% CI: 0.748–0.894). During an 18-month follow-up, patients with histological healing had a substantially better outcome compared with those with unachieved histological healing (P <0.001) using ICEI. During a 12-month follow-up from other nine hospitals, patients with histological healing also had a lower risk of relapse than patients with unachieved histological healing.
Conclusions:
ICEI can be used to predict histological healing and identify patients with a risk of relapse 12 months and 18 months after clinical therapy. Therefore, ICEI provides a promising, simplified approach to monitor histological healing and to predict the prognosis of UC.
Registration:
Chinese Clinical Trial Registry, No. ChiCTR2300077792.
Keywords: Ulcerative colitis, Histological healing, Inflammatory cell enumeration index, Prognosis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) primarily affecting the rectum, sigmoid colon, and in severe cases, the entire colon.[1,2] The etiology and pathology of UC remain not fully understood; however, recent studies indicate that abnormal immune system response to microbiota, intestinal persistent infection, intestinal mucosal defect, and genetic and environmental factors may all contribute to its development.[3,4,5,6] Recent studies have emphasized the importance of achieving mucosal healing (MH) as a key therapeutic goal for UC patients,[7] as it has been consistently associated with favorable outcomes, including prolonged clinical remission and reduced rates of hospitalization and surgery.[8] However, approximately 30% of UC patients who reach clinical remission still have histological activity after comprehensive therapy, showing persistent histological inflammation in the colonic mucosa.[9] Histological healing is linked to lowered rates of hospitalization and neoplasia,[10,11] and mucin depletion is also associated with clinical relapse in UC patients with Mayo endoscopic subscore 0.[12] Therefore, histological healing seems even further to benefit disease prognosis.[13]
Histological healing, characterized by histological normalization and the absence of inflammation, neutrophils, erosion, and ulceration in the intestine, is a critical goal. Various indices, such as the Robarts histopathology index, Nancy histological index, or Geboes score, have been used for quantitative assessment.[14,15] Previous studies have demonstrated significant associations between specific components of histological activity, such as basal plasmacytosis, neutrophilic infiltrations, mucin depletion, and cryptarchitectural irregularities, with higher rates of clinical relapse.[16] Notably, neutrophil infiltrations have shown particular utility in assessing disease activity when MH is achieved.[17] Inflammatory cells, such as eosinophils, plasma cells, and T cells also play a pivotal role in the development of UC.[10,13,18] CD177 and CD64 are remarkably expressed in activated neutrophils, which play a crucial role in host defense through the recognition, phagocytosis, and elimination of invading pathogens.[19,20] Accordingly, CD177, as the gene with the highest expression level in activated neutrophils, plays a critical role in the initiation and advancement of IBD, thereby making it a promising target for drug therapy.[21] Another neutrophil biomarker CD64 has been described to be associated with disease activity and correlates with endoscopic activity.[22] CD40L and CD69 are mainly expressed in recently activated T cells (particularly in CD4+ T cells), which facilitate germinal center formation and antibody production.[23,24] CD69 is typically expressed at low levels in T cells in peripheral blood. However, upon stimulation by intestinal microbial antigens, T cells are prompted to migrate to the intestinal mucosa, where they subsequently upregulate the expression of CD69. Further investigation of molecular mechanisms underlying the CD40L signaling axis in these cells would provide important insight and novel therapeutic targets in IBD.[25] The relationship between the expression of immune cell activation markers in peripheral blood and disease activity is well-established. However, the expression of these markers in the intestinal tract and their association with histological healing remain unclear. Therefore, it is necessary to utilize CD177, CD64, CD40L, and CD69 molecules to judge the histological remission of UC. Importantly, the assessment of activated immune cell infiltrations in the colonic lamina propria may provide a rationale for the precision diagnosis of histological healing in UC patients.
In this study, we aimed to explore a histological healing criterion according to an alternation in proportions of inflammatory cells (i.e., CD177+ neutrophils, eosinophils, and CD40L+ T cells) in the colonic lamina propria, which allowed us to better predict UC patients to achieve histological healing, thus minimizing the overtreatment in clinical practice.
Methods
Study setting
This population-based inception cohort consisted of UC cases from the Shanghai Tenth People’s Hospital of Tongji University, the First People’s Hospital of Shangqiu City Affiliated to Xinxiang Medical University, Wuhu First People’s Hospital, Suzhou Municipal Hospital, the First Affiliated Hospital of Soochow University, the Second Affiliated Hospital of Zhengzhou University, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Affiliated Hospital of Jining Medical College, the Third Affiliated Hospital of Guangzhou Medical University, and Dongfang Hospital of Beijing University of Chinese Medicine from January 2017 to May 2022. Thereafter, a universal data collection protocol was used among all centers to facilitate the congruence of collected variables. The study was approved by the Ethics Committee of the Shanghai Tenth People’s Hospital (No. SHSY-IEC-5.0/23K17/P01) and registered with the Chinese Clinical Trial Registry (No. ChiCTR2300077792). Additionally, 300 age- and sex-matched healthy donors were recruited in our study for comparison study. All UC patients and healthy donors signed the informed consent forms.
Inclusion criteria
(1) Eligible patients had a diagnosis with UC based on standard clinical, endoscopic, and histological criteria;[26,27,28,29] (2) UC patients reached the criteria of endoscopic MH as described previously (non-inflammatory status, a modified Mayo score <3, and the ulcerative colitis endoscopic index of severity [UCEIS] ≤1);[14] (3) The relevant clinical history and endoscopic examination data were complete.
Exclusion criteria
(1) UC patients had severe complications such as toxic megacolon, intestinal perforation, colon stenosis and obstruction, massive bleeding, or colorectal cancer; (2) UC patients had received subtotal colectomy or total colectomy.
Routine clinical practice
All UC patients included in the study were closely monitored throughout the study period. This included regular outpatient visits and scheduled endoscopic evaluations at 6, 12, and 18 months after their admission to the study. The follow-up period was finished when the final UC patient completed their 18-month endoscopy. The time interval between the first and last UC patient’s endoscopic examination within the 18-month period did not exceed 6 months. For disease activity assessment, a minimum of four mucosal biopsies were obtained from the worst areas of the colon. The disease behaviors of these patients were defined by the Montreal classification.[20,30] Three pathologists who were blinded to all clinical information reviewed slides, and three gastroenterologists were blinded to other clinical data at the time of review. Endoscopic MH terms a UCEIS ≤1.[31] A universal data collection protocol was implemented across all nine IBD medical centers to ensure consistency in the variables collected.
Histopathological assessment and immunohistochemistry assessment
Histological features of UC include six parts, i.e., acute inflammatory cell infiltration, crypt abscesses, mucin depletion, surface epithelial integrity, chronic inflammatory cell infiltration, and crypt architectural irregularities in the colonic mucosa. Combining this work with our previous and other studies, we determined CD177 and CD64 as the biomarkers of activated neutrophils and CD40L and CD69 as the biomarkers of activated T cells, respectively.[19,25,32,33,34] The colonic sections of healthy donors who had a physical examination by endoscopy and UC patients were stained with hematoxylin and eosin and immunohistochemistry, respectively. Immunohistochemical analysis was performed on paraffin-embedded colon tissues using anti-CD177 mAb (Catalog number: ab255296, Abcam; Cambridge, England) at 1/2000 dilution, anti-CD69 mAb (Catalog number: ab233396, Abcam) at 1/500, anti-CD64 mAb (Catalog number: ab140779, Abcam) at 1/150 and anti-CD40L mAb (Catalog number: ab65854, Abcam) at 1/500 dilution, respectively. Ten to fifteen visual fields under high magnification (400×) were selected, and the proportions of neutrophils, eosinophils, CD177+ neutrophils, CD64+ neutrophils, CD69+ T cells, and CD40L+ T cells were calculated using a method according to previous publications under high magnification (400×).[19,32,35] Three objective researchers who were blinded to clinical data reviewed both the endoscopic and histological findings of each patient.
Outcome assessment
The recurrence of the disease, including endoscopic findings, was followed up in UC patients at 6, 12, and 18 months, respectively, after standard therapy.[28] A modified Truelove, Witts severity index (MTWSI) <4, an Ulcerative Colitis Activity Index (UCAI) <2, and calprotectin <250 μg/g indicate clinical remission of the disease.[36,37]
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software; San Diego, CA, USA) and SPSS 20.0 (SPSS Inc., Chicago, IL, USA) software were used for statistical analysis. The measurement data with normal distribution were expressed by mean ± standard deviation (SD), and the measurement data with skewness distribution were expressed by medians and interquartile ranges (IQRs). Intrareader reliability was assessed from the duplicate readings of all biopsy specimens with the computation of the intraclass correlation coefficient (ICC). For the internal consistency as recommended by Consensus-based Standards for the selection of health Measurement Instruments (COSMIN),[38] the Cronbach’s coefficient α, using partial correlation coefficients, was calculated for the overall inflammatory cell enumeration index (ICEI) and for the index with one-at-a-time item deletion to evaluate internal consistency in ICEI. Correlation analysis was used to illustrate the consistency between the ICEI and Nancy index and Geboes score. The sensitivity, specificity, positive predictive value, negative predictive value, and areas under the receiver operating characteristic curve (AUC) were used to analyze the effectiveness of the ICEI for the diagnosis of histological healing. The optimal cutoff value was determined by the Youden index. P <0.05 was considered statistically significant.
Results
Derivation cohort and verification cohort
We identified 220 UC patients (120 patients in the derivation cohort and 100 patients in the verification cohort) clinically and endoscopically who successfully achieved clinical remission and underwent the treat-to-target interventions. The derivation cohort comprised 120 patients from the Shanghai Tenth People’s Hospital and the verification cohort comprised 100 patients from other nine IBD medical centers. These patients were admitted as inpatients and had comprehensive clinical information available for long-term follow-up. MH was confirmed during the most recent endoscopy [Figure 1]. The derivation cohort included 77 males (64.2%) and 43 females (35.8%). The mean age of the patients was (50.4 ± 14.3) years. The verification cohort consisted of 37 females (37.0%) and 63 males (63.0%), with an average age of (50.1 ± 14.1) years. The clinical data of healthy donors, including the number, age, and gender, were shown in Table 1.
Figure 1.
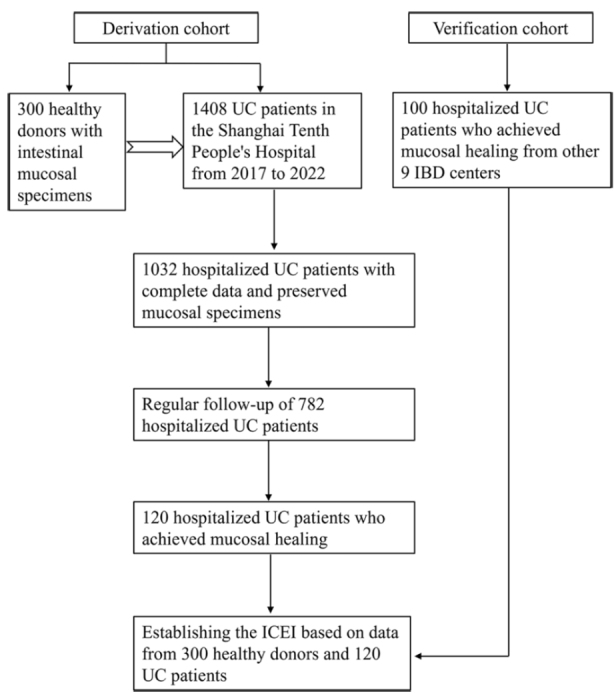
Flow chart of patient selection in the study and main study procedures. IBD: Inflammatory bowel disease; ICEI: Inflammatory cell enumeration index; UC: Ulcerative colitis.
Table 1.
Baseline of healthy donors and UC patients.
| Variables | HC | UCD | UCV |
|---|---|---|---|
| Number of patients | 300 | 120 | 100 |
| Age (years) | 51.9 ± 14.8 | 50.4 ± 14.3 | 50.1 ± 14.1 |
| Sex (female) | 110 (36.7) | 43 (35.8) | 37 (37.0) |
| Disease duration (months) | 41.2 ± 20.1 | 36.7 ± 15.7 | |
| Smoking | 10 | 8 | |
| Drinking | 6 | 7 | |
| Disease locations for UC | |||
| E1 (proctitis) | 12 | 16 | |
| E2 (left-sided colitis) | 40 | 36 | |
| E3 (pancolitis) | 68 | 48 | |
| Calprotectin (μg/g) | |||
| 0 month | 81.18 ± 36.95 | 82.02 ± 34.84 | |
| 6 months | 113.93 ± 72.00 | 100.15 ± 71.84 | |
| 12 months | 136.10 ± 88.86 | 120.50 ± 89.83 | |
| 18 months | 146.84 ± 97.32 | – |
Data were shown as n, mean ± standard deviation, orn (%). HC: Healthy donors; UC: Ulcerative colitis; UCD: Derivation cohort of UC patients; UCV: Verification cohort of UC patients.
Activation markers of inflammatory cells
We performed univariate and multivariate analyses to assess the predictive factors influencing the risk of relapse, and the results were displayed in Supplementary Tables 1 and 2, http://links.lww.com/CM9/C20. The proportions of eosinophils, CD177+ neutrophils, and CD40L+ T cells were chosen in the diagnostic criterion. Our analyses revealed that the proportions of these inflammatory cells (i.e., CD177+ neutrophils, CD40L+ T cells, and eosinophils) showed an increased trend toward the risk of relapse after an 18-month follow-up and were highly predictive of relapse.
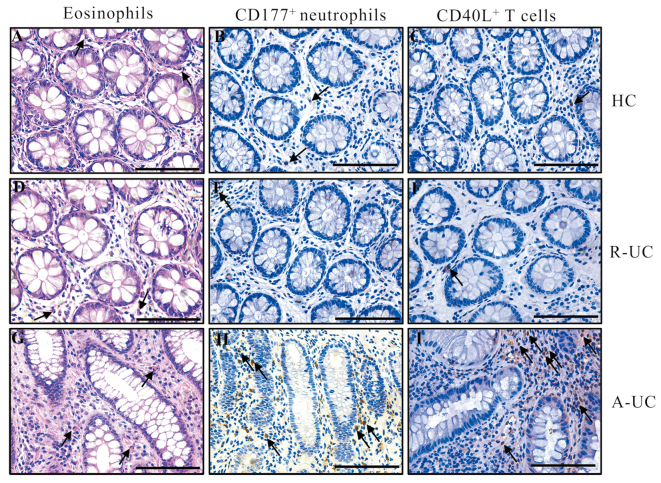
There were relatively low numbers of eosinophils, CD177+ neutrophils, or CD40L+ T cells in the colonic mucosa of healthy donors [Figure 2A–C]. Notably, UC patients who achieved histological healing exhibited a lower number of these cells [Figure 2D–F], while UC patients who failed to achieve histological healing had higher proportions of these inflammatory cells [Figure 2G–I].
Figure 2.
Representative staining images of the colon sections from UC patients and healthy donors. The arrows indicate eosinophils, CD177+, and CD40L+ cells, respectively. The scar bar represents 100 μm. (A–C) Characteristics of eosinophils, CD177+ and CD40L+ cells, respectively, in healthy donors. (D–F) Characteristics of eosinophils, CD177+, and CD40L+ cells, respectively, in UC patients who achieved histological remission. (G–I) Characteristics of eosinophils, CD177+, and CD40L+ cells, respectively, in UC patients who failed to achieve histological remission. A-UC: UC patients who stayed histological activation; HC: Healthy donors; R-UC: UC patients who achieved histological remission; UC: Ulcerative colitis.
Novel diagnostic criterion
Establishment of an ICEI for assessing histological healing
To establish a diagnostic criterion for assessing histological healing, we collected intestinal mucosal specimens from 300 healthy donors who underwent endoscopic examination routinely and counted the proportions of eosinophils under HPF, and expression of CD177 and CD40L was stained by immunohistochemistry. We found that the proportions of various types of cells conformed to a normal distribution, and then used mean + 2 SD as the cutoff of each type of immune cells [Supplementary Figure 1 and Supplementary Table 3, http://links.lww.com/CM9/C20]. In the case of 95.3% (unilateral) reliability, the setting method of cutoff was scientific and based on accurate statistical calculation. The calculated cutoffs of eosinophils, CD177+ neutrophils, and CD40L+ T cells were 5.36‰, 3.16‰, and 4.73‰, respectively [Supplementary Figure 1, http://links.lww.com/CM9/C20]. According to the ICEI established based on the data from healthy donors, UC patients achieved histological healing if the counts of each inflammatory cell in the colonic lamina propria were less than the threshold value, without crypt abscesses, mucin depletion, surface epithelial damage, and crypt architectural irregularities.
UC patients in the derivation cohort were divided into a histological remission cohort (n = 61) and a histological activity cohort (n = 59) by ICEI [Figure 3]. Binary logistic regression analysis showed that the formula for histological healing was Y = 1.701X1 + 0.758X2 + 1.347X3 − 7.745 (X1, X2, and X3 represent the proportions of CD177+ neutrophils, eosinophils, and CD40L+ T cells, respectively, in the colonic lamina propria under HPF). The receiver operating characteristics curve (ROC) analysis revealed that Y <−0.391 was the cutoff for the diagnosis of histological healing, with an AUC of 0.942 (P <0.001), indicating that the ICEI had excellent discrimination and consistency [Figure 3A and Supplementary Table 4, http://links.lww.com/CM9/C20]. Besides, the calibration was satisfactory, and the confidence interval (CI) of the observed probability covered the ideal calibration line [Figure 3B].
Figure 3.
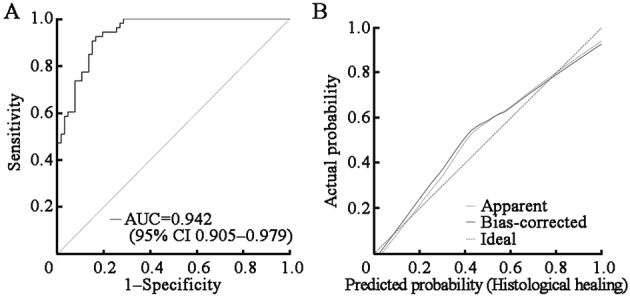
ROC analysis of the ICEI in UC patients. (A) ROC analysis of the ICEI in UC patients (n = 120); (B) Cross-validation study of the ICEI in UC patients; The dashed diagonal line represents an ideal calibration. AUC was 0.942 for the ICEI (P <0.001, 95% CI: 0.905–0.979). AUC: Area under the curve; CI: Confidence interval; ICEI: Inflammatory cell enumeration index; ROC: Receiver operating characteristics curve; UC: Ulcerative colitis.
Reliability of the ICEI for assessing histological healing
The intrareader reliability for individual items and ICEI was shown in Supplementary Table 5, http://links.lww.com/CM9/C20. The value of ICC for the intrareader reliability was 0.855 (95% CI: 0.781–0.909) in 50 randomly selected biopsies (simple random sampling). Three items constituting ICEI were scored by other three pathologists in these 50 UC patients. Additionally, inter-reader reliability was summarized in Supplementary Table 5, http://links.lww.com/CM9/C20, and the ICEI showed a good interobserver reliability of 0.832 (95% CI: 0.748–0.894).
Construct validity of the ICEI for assessing histological healing
The construct validity of the hypothesis testing was investigated with the correlation between the ICEI of reader 1 and the Nancy index scored by each reader. The correlation was strong for each reader (r = 0.809 for reader 1, 0.819 for reader 2, and 0.794 for reader 3) (P <0.001). The correlation between the Geboes score of reader 1 and the ICEI was also strong for each reader (r = 0.745 for reader 1, 0.802 for reader 2, and 0.789 for reader 3) (P <0.001).
Prognosis performance of the ICEI
UC patients in the derivation cohort were divided into a histological remission cohort (n = 61) and a histological activity cohort (n = 59), respectively, by the ICEI [Figure 4]. During an 18-month follow-up period, 8 patients (13.1%) experienced a clinical relapse in the histological remission cohort, and 24 patients (40.7%) experienced a clinical relapse in the histological activity cohort (χ2 = 11.652, P <0.001) [Supplementary Figure 2, http://links.lww.com/CM9/C20]. The recurrence rate of UC patients in the histological healing group determined by the ICEI was comparable with that of the Nancy index (13.1% vs. 10.0%, χ2 = 0.008, P >0.05) and the Geboes score (13.1% vs. 14.3%, χ2 = 0.022, P >0.05). The recurrence rates of UC patients in the non-histological healing group were significantly higher than those in the histological healing group, with the Nancy index (10.0% vs. 32.2%, χ2 = 4.602, P <0.05), the Geboes score (14.3% vs. 31.8%, χ2 = 3.873, P <0.05), and the ICEI (13.1% vs. 40.7%, χ2 = 11.652, P <0.001), respectively [Supplementary Figure 2, http://links.lww.com/CM9/C20]. Moreover, we also found only eight UC patients achieving histological healing with the above three scoring criteria, and these patients did not relapse during the 18-month follow-up. Altogether, the combination of multiple scores helps us to achieve a more accurate prediction.
Figure 4.
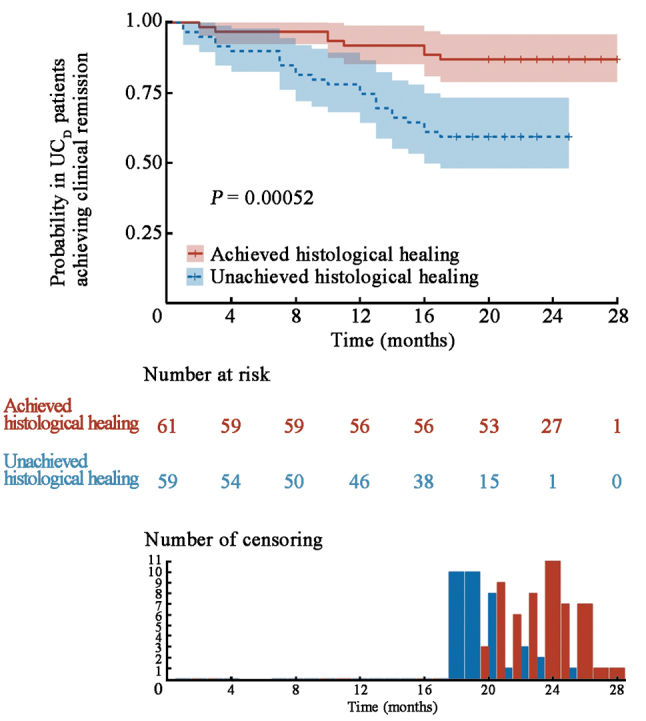
Cumulative probability in UCD patients achieving clinical remission using ICEI over an 18-month follow-up period. UCD (n = 120) (P <0.001). ICEI: Inflammatory cell enumeration index; UC: Ulcerative colitis; UCD: Derivation cohort of UC patients.
With the aim of assessing the discriminative ability of the ICEI, the predicted probability of recurrence-free survival was then plotted as Kaplan–Meier curves, stratified by the predicted probability that was calculated according to the ICEI [Figure 4]. Patients with histological remission had a significantly better outcome compared with patients with histological activity (P <0.001). Similar conclusions were also observed over a period of 12-month follow-up [Supplementary Figure 3, http://links.lww.com/CM9/C20]. Therefore, these findings indicated that the ICEI could accurately evaluate the prognosis of patients with clinical remission. We also used ROC curve analyses to evaluate the relationship between the Nancy index, the Geboes score, and the ICEI for the prediction of clinical remission during a period of 18-month follow-up [Supplementary Table 6, http://links.lww.com/CM9/C20]. The ICEI exhibited an excellent discriminative ability in predicting clinical remission, as indicated by the area under the ROC curve of 0.855 (95% CI: 0.772–0.939). This performance surpassed that of the Geboes score (AUC: 0.808, 95% CI: 0.709–0.907) and the Nancy index (AUC: 0.834, 95% CI: 0.739–0.929) (P <0.001).
External verification of the ICEI for histological healing
To validate the ICEI, we collected 100 UC patients who had achieved endoscopic healing from other nine hospitals and evaluated histological healing in these patients using the ICEI. According to the ICEI, we divided 100 UC patients into the histological healing group (n = 52) and the unachieved histological healing group (n = 48). During a 12-month follow-up observation, the ICEI predicted the recurrence probability effectively, showing that patients with histological healing had a significantly lower one-year recurrence rate (9.6%) compared to those with unachieved histological healing (20.8%) [Figure 5].
Figure 5.
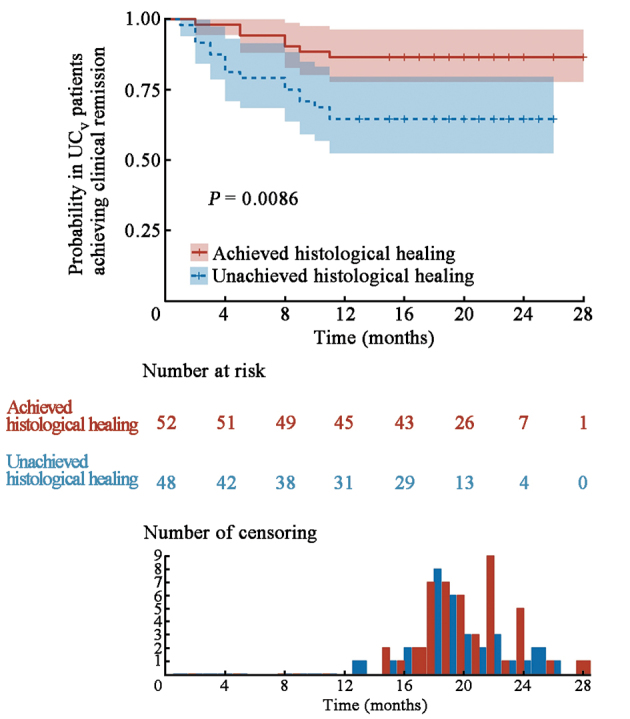
Cumulative probability in UCV patients achieving clinical remission by ICEI over a 12-month follow-up period. UCV (n = 100) (P <0.01). ICEI: Inflammatory cell enumeration index; UC: Ulcerative colitis; UCV: Verification cohort of UC patients.
Discussion
Our study has represented the pioneering multicenter population-based study in China to assess the predictors of histological healing in UC patients and the impact on clinical outcomes. The finding showed that UC patients achieving ICEI histological healing had an annual risk of clinical relapse of 8% aligned with previous research.[39,40] The recurrence probability in UC patients who still had histological activity was significantly higher than that in the histological healing group. When the Geboes score was used to predict the results of an 18-month relapse in our study, an area under the ROC curve was 0.808, which was similar to the previous study and a little bit lower than that of the ICEI.[41] Moreover, an external verification of the ICEI in cohorts from other nine hospitals further confirmed its competence in distinguishing between patients with histological healing and those with histological activity. Therefore, the ability of the ICEI to predict the recurrence probabilities provides a rationale for precise diagnosis and has an important prognostic implication.
While previous reports have indicated the positive association between histological remission and improved clinical outcomes, few studies have undertaken a longitudinal assessment of the benefits of attaining histological remission in patients who had previously experienced active disease within the context of a treat-to-target approach emphasizing MH.[40,42] Moreover, there has been a limited exploration of the feasibility and predictive factors related to achieving histological remission in patients who receive effective treatment to achieve the conventional target of MH.[43] In this study, we pioneered the inclusion of immune cell activation markers as elements of a histological scoring system. Specifically, we evaluated the proportions of eosinophils, CD177+ neutrophils, CD64+ neutrophils, CD40L+ T cells, and CD69+ T cells, along with the incidence of disease relapse during the follow-up period in patients with UC. Utilizing the univariate and multivariate logistic regression analyses, we determined the predictors of disease recurrence, optimizing for the best-fitting model. Our selection process led us to incorporate eosinophils, CD177+ neutrophils, and CD40L+ T cells into the construction of ICEI. Our findings reveal that CD177+ neutrophils serve a dual role, being both indicative of disease activity in UC patients and a prognostic parameter for disease outcome.[44] Moreover, CD40L, a T cell activation marker, has been identified as a significant indicator of histological activity in IBD, similar to its heightened expression in CD4+ T cells of patients with rheumatoid arthritis.[45]
Currently, histological healing in UC has been defined as the normalization of the microscopic image of the colonic mucosa.[46] However, a standardized and uniform system for grading intestinal mucosal inflammation is still lacking and to date, approximately 30 different histologic scoring systems for UC have been described but have never been fully validated currently,[47,48] which brings massive conundrums in clinical practice. In this study, the novel ICEI focuses on the proportions of inflammatory cells, which is different from previous histologic scoring systems with complex statistics.[11,46] Upon assessing histological healing in the same cohort of UC patients, no marked difference was observed in recurrence rates between the ICEI histological healing group and those categorized by the Nancy index. Notably, the cohort adhering to the ICEI for histological healing was twice the size of the group meeting the Nancy index for histological healing. Concurrently, patients in the ICEI group who failed to achieve histological healing exhibited the highest rate of recurrence. Besides, the ICEI can accurately evaluate the prognosis of patients with clinical remission. In conclusion, it appears a good choice to predict the prognosis and avoid overtreatment for UC patients.
There are still some limitations in our study. First, the cohorts consisted of patients from different provinces but mainly from Eastern China. As the healthcare systems are getting more easily overwhelmed, the choice of therapeutic strategies may be daunting. Second, the retrospective analysis of intestinal mucosal inflammation based on colonoscopy and histology reports might be associated with a bias. Third, we have developed and verified the ICEI in patients with MH alone. Although the ICEI showed a better prediction value of 18-month relapse for UC patients than the Nancy index and the Geboes score, it should be verified among patients with moderately to severely active UC in order to make a better comparison with the Nancy index and the Geboes score.[41,49]
As clinical therapy goals in UC continue to evolve, from endoscopic MH to histopathological healing, the ICEI offers a valuable tool for assessing and predicting the prognosis of UC patients. This study paves the way for future research aiming at further validating the ICEI, particularly among patients with moderately to severely active UC, to enhance its comparability with established scoring systems like the Nancy index and the Geboes score. These advancements will definitely contribute to a deeper understanding of UC and offer opportunities for more tailored and effective management strategies.
Acknowledgements
We would like to express our gratitude to physicians, nurses, and PhD students from the Center for IBD Research and Department of Pathology, the Shanghai Tenth People’s Hospital of Tongji University. We thank Dr. Xianyong Gui (Department of Pathology, University of Washington School of Medicine, Seattle, WA, USA) for helpful comments on the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 82370532, and 82341219).
Conflicts of interest
None.
Data available
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Footnotes
Han Gao, Kangsheng Peng, and Yadi Shi contributed equally to this work.
How to cite this article: Gao H, Peng KS, Shi YD, Zhu SS, Sun RC, Xu CJ, Liu P, Pang Z, Zhu LX, Chen WC, Feng BS, Wu HL, Zhou GX, Li MS, Li JX, Ding BJ, Liu ZJ. Development and validation of a novel criterion of histologic healing in ulcerative colitis defined by inflammatory cell enumeration in lamina propria mucosa: A multicenter retrospective cohort in China. Chin Med J 2024;137:1316–1323. doi: 10.1097/CM9.0000000000003154
References
- 1.Gros B, Kaplan GG. Ulcerative colitis in adults: A review. JAMA 2023;330:951–965. doi: 10.1001/jama.2023.15389. [DOI] [PubMed] [Google Scholar]
- 2.Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet 2023;402:571–584. doi: 10.1016/s0140-6736(23)00966-2. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z Liu R Gao H Jung S Gao X Sun R, et al. Genetic architecture of the inflammatory bowel diseases across East Asian and European ancestries. Nat Genet 2023;55:796–806. doi: 10.1038/s41588-023-01384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao H, Liu R, Huang H, Liu Z. Susceptibility gene profiling elucidates the pathogenesis of inflammatory bowel disease and provides precision medicine. Clin Transl Med 2023;13:e1404. doi: 10.1002/ctm2.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao H, Liu Z. The latest breakthrough on genetic characteristics of inflammatory bowel disease in Chinese and other East Asian ancestries. Precis Clin Med 2023;6:bad017. doi: 10.1093/pcmedi/pbad017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai X, Jiang L, Ruan G, Liu T, Yang H. Helicobacter pylori may participate in the development of inflammatory bowel disease by modulating the intestinal microbiota. Chin Med J 2022;135:634–638. doi: 10.1097/CM9.0000000000002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 8.Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: A Meta-analysis. Inflamm Bowel Dis 2016;22:1859–1869. doi: 10.1097/MIB.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 9.Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: Systematic review and meta-analysis. Am J Gastroenterol 2016;111:1692–1701. doi: 10.1038/ajg.2016.418. [DOI] [PubMed] [Google Scholar]
- 10.Travis SP Higgins PD Orchard T Van Der Woude CJ Panaccione R Bitton A, et al. Review article: Defining remission in ulcerative colitis. Aliment Pharmacol Ther 2011;34:113–124. doi: 10.1111/j.1365-2036.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RB Harpaz N Itzkowitz S Hossain S Matula S Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: A cohort study. Gastroenterology 2007;133:1099–1105; quiz1340–1. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki R Kobayashi T Okabayashi S Nakano M Morinaga S Hara A, et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J Crohns Colitis 2018;12:1288–1294. doi: 10.1093/ecco-jcc/jjy092. [DOI] [PubMed] [Google Scholar]
- 13.Bessissow T Lemmens B Ferrante M Bisschops R Van Steen K Geboes K, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol 2012;107:1684–1692. doi: 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]
- 14.Magro F Doherty G Peyrin-Biroulet L Svrcek M Borralho P Walsh A, et al. ECCO position paper: Harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis 2020;14:1503–1511. doi: 10.1093/ecco-jcc/jjaa110. [DOI] [PubMed] [Google Scholar]
- 15.Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut 2015;64:1412–1418. doi: 10.1136/gutjnl-2014-307477. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Yu A, Peyrin-Biroulet L, Ananthakrishnan AN. Treat to target: The role of histologic healing in inflammatory bowel diseases: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;19:1800–1813.e1804. doi: 10.1016/j.cgh.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Wu X, Xu C, Lin J, Liu Z. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis Clin Med 2021;4:246–257. doi: 10.1093/pcmedi/pbab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthas D Reznichenko A Balendran CA Böttcher G Clausen IG Kärrman Mårdh C, et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand J Gastroenterol 2017;52:125–135. doi: 10.1080/00365521.2016.1235224. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G Yu L Fang L Yang W Yu T Miao Y, et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018;67:1052–1063. doi: 10.1136/gutjnl-2016-313535. [DOI] [PubMed] [Google Scholar]
- 20.Minar P Haberman Y Jurickova I Wen T Rothenberg ME Kim M-O, et al. Utility of neutrophil Fcγ receptor I (CD64) index as a biomarker for mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis 2014;20:1037–1048. doi: 10.1097/mib.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak JK Adams AT Kalla R Lindstrøm JC Vatn S Bergemalm D, et al. Characterisation of the circulating transcriptomic landscape in inflammatory bowel disease provides evidence for dysregulation of multiple transcription factors including NFE2, SPI1, CEBPB, and IRF2. J Crohns Colitis 2022;16:1255–1268. doi: 10.1093/ecco-jcc/jjac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colman RJ Tsai YT Jackson K Boyle BM Noe JD Hyams JS, et al. Achieving target infliximab drug concentrations improves blood and fecal neutrophil biomarkers in Crohn’s disease. Inflamm Bowel Dis 2021;27:1045–1051. doi: 10.1093/ibd/izaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z Geboes K Colpaert S Overbergh L Mathieu C Heremans H, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J Immunol 2000;164:6005–6014. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Geboes K, Colpaert S, D’Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local t cell-dependent cytokine production. J Immunol 2000;164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 25.Karnell JL, Rieder SA, Ettinger R, Kolbeck R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv Drug Deliv Rev 2019;141:92–103. doi: 10.1016/j.addr.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X Sun R Jiao N Liang X Li G Gao H, et al. Integrative multi-omics deciphers the spatial characteristics of host-gut microbiota interactions in Crohn’s disease. Cell Rep Med 2023;4:101050. doi: 10.1016/j.xcrm.2023.101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maaser C Sturm A Vavricka SR Kucharzik T Fiorino G Annese V, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 29.Sturm A Maaser C Calabrese E Annese V Fiorino G Kucharzik T, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 2019;13:273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 30.Mowat C Cole A Windsor A Ahmad T Arnott I Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 31.Travis SP Schnell D Krzeski P Abreu MT Altman DG Colombel JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The ulcerative colitis endoscopic index of severity (UCEIS). Gut 2012;61:535–542. doi: 10.1136/gutjnl-2011-300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z Colpaert S D’Haens GR Kasran A de Boer M Rutgeerts P, et al. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol 1999;163:4049–4057. doi: 10.4049/jimmunol.163.7.4049. [PubMed] [Google Scholar]
- 33.Gorabi AM Hajighasemi S Kiaie N Gheibi Hayat SM Jamialahmadi T Johnston TP, et al. The pivotal role of CD69 in autoimmunity. J Autoimmun 2020;111:102453. doi: 10.1016/j.jaut.2020.102453. [DOI] [PubMed] [Google Scholar]
- 34.Calzetti F Finotti G Tamassia N Bianchetto-Aguilera F Castellucci M Canè S, et al. CD66b−CD64dimCD115− cells in the human bone marrow represent neutrophil-committed progenitors. Nat Immunol 2022;23:679–691. doi: 10.1038/s41590-022-01189-z. [DOI] [PubMed] [Google Scholar]
- 35.Arganda-Carreras I Kaynig V Rueden C Eliceiri KW Schindelin J Cardona A, et al. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017;33:2424–2426. doi: 10.1093/bioinformatics/btx180. [DOI] [PubMed] [Google Scholar]
- 36.Plevy S Salzberg B Van Assche G Regueiro M Hommes D Sandborn W, et al. A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis. Gastroenterology 2007;133:1414–1422. doi: 10.1053/j.gastro.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: Clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohns Colitis 2012;6:412–418. doi: 10.1016/j.crohns.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Mokkink LB Terwee CB Patrick DL Alonso J Stratford PW Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual Life Res 2010;19:539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodsall TM, Costello SP, Bryant RV. Histological healing in ulcerative colitis: Near enough is not good enough. J Crohns Colitis 2020;14:1341–1342. doi: 10.1093/ecco-jcc/jjaa095. [DOI] [PubMed] [Google Scholar]
- 40.Yoon H Jangi S Dulai PS Boland BS Prokop LJ Jairath V, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: A systematic review and meta-analysis. Gastroenterology 2020;159:1262–1275.e7. doi: 10.1053/j.gastro.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narula N Wong ECL Colombel JF Riddell R Marshall JK Reinisch W, et al. Early change in epithelial neutrophilic infiltrate predicts long-term response to biologics in ulcerative colitis. Clin Gastroenterol Hepatol 2022;20:1095–1104.e9. doi: 10.1016/j.cgh.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Peyrin-Biroulet L Sandborn W Sands BE Reinisch W Bemelman W Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 43.Jangi S Yoon H Dulai PS Valasek M Boland BS Jairath V, et al. Predictors and outcomes of histological remission in ulcerative colitis treated to endoscopic healing. Aliment Pharmacol Ther 2020;52:1008–1016. doi: 10.1111/apt.16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Planell N Masamunt MC Leal RF Rodríguez L Esteller M Lozano JJ, et al. Usefulness of transcriptional blood biomarkers as a non-invasive surrogate marker of mucosal healing and endoscopic response in ulcerative colitis. J Crohns Colitis 2017;11:1335–1346. doi: 10.1093/ecco-jcc/jjx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maggi J Carrascal M Soto L Neira O Cuéllar MC Aravena O, et al. Isolation of HLA-DR-naturally presented peptides identifies T-cell epitopes for rheumatoid arthritis. Ann Rheum Dis 2022;81:1096–1105. doi: 10.1136/annrheumdis-2021-220371. [DOI] [PubMed] [Google Scholar]
- 46.Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: The ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol 2014;12:929–934.e922. doi: 10.1016/j.cgh.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Rath T, Atreya R, Neurath MF. Is histological healing a feasible endpoint in ulcerative colitis? Expert Rev Gastroenterol Hepatol 2021;15:665–674. doi: 10.1080/17474124.2021.1880892. [DOI] [PubMed] [Google Scholar]
- 48.Iacucci M Parigi TL Del Amor R Meseguer P Mandelli G Bozzola A, et al. Artificial intelligence enabled histological prediction of remission or activity and clinical outcomes in ulcerative colitis. Gastroenterology 2023;164:1180–1188.e2. doi: 10.1053/j.gastro.2023.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Sands BE Peyrin-Biroulet L Loftus EV Jr. Danese S Colombel JF Törüner M, et al. Vedolizumab vs. Adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–1226. doi: 10.1056/NEJMoa1905725. [DOI] [PubMed] [Google Scholar]